Abstract
Recent progress in research on glucagon and α‐cells highlights their pathophysiological roles in diabetes. We previously showed that plasma glucagon levels measured by newly developed enzyme‐linked immunosorbent assay were dysregulated in patients with type 1 diabetes with respect to plasma glucose levels, suggesting dysregulated secretion. In the current study, the annual change in plasma glucagon levels was assessed in these same patients. We found that the plasma glucagon levels in the 66 Japanese patients involved in the study were significantly correlated between both years. In addition, they were significantly associated with serum blood urea nitrogen levels in a multivariate linear regression analysis, as reported in our previous study. The statistical correlation in glucagon levels between annual checkups and the sustained significant correlation between glucagon and blood urea nitrogen suggest a constant dysregulation of glucagon in association with altered amino acid metabolism in patients with type 1 diabetes.
Keywords: Amino acids, Glucagon, Type 1 diabetes
The annual plasma glucagon levels in 66 Japanese patients with type 1 diabetes were significantly correlated between both years. The plasma glucagon levels were significantly associated with serum blood urea nitrogen levels. The results suggest constant dysregulation of glucagon in association with altered amino acid metabolism in patients with type 1 diabetes.

Introduction
Recent progress in glucagon and α‐cell research, both basic and clinical, highlights their pathophysiological roles in diabetic hyperglycemia and hypoglycemia1, 2. We previously showed that plasma glucagon levels measured by the dual‐antibody sandwich enzyme‐linked immunosorbent assay (ELISA) were dysregulated in patients with type 1 diabetes with respect to plasma glucose levels, suggesting dysregulated secretion3. In addition, the glucagon levels were correlated significantly with serum blood urea nitrogen (BUN) levels, suggesting their association with amino acid metabolism4, 5. In the current study, the change in plasma glucagon levels from one year to the next was assessed in the same patients with type 1 diabetes in order to investigate the significance of glucagon dysregulation in the disease.
Methods
Japanese patients with type 1 diabetes, who received the annual checkup in 2018, were enrolled in the study after providing written informed consent. The study was carried out in accordance with the tenets of the Declaration of Helsinki, and the study protocol was approved by the local ethics committee of Osaka University Hospital (no. 12372). The methodology is the same as previously reported3, 6 and currently described in the Methods S1. Venous blood samples were randomly obtained with no restriction on meals and insulin injections. Plasma glucagon levels were evaluated by the specific dual‐antibody sandwich ELISA (Mercodia AB, Uppsala, Sweden/Cosmic Corporation Co., Ltd., Tokyo, Japan)7. The laboratory analyses were carried out at SRL Inc. (Tokyo, Japan). Statistical analyses were carried out using JMP Pro version 14.0.0 (SAS Institute, Cary, NC, USA). A P‐value <0.05 was considered statistically significant.
Results
The clinical characteristics of the 77 participants in 2018 are presented in Table 1. The plasma glucagon values were widely spread, and weakly but significantly correlated with plasma glucose levels (Pearson's correlation coefficient r = 0.265, P = 0.020; Figure 1a). The glucagon levels also showed significant correlations with serum BUN (r = 0.325, P = 0.004; Figure 1b), alanine aminotransferase (ALT; r = 0.253, P = 0.03; Figure S1a), total cholesterol (r = 0.236, P = 0.04; Figure S1b) and low‐density lipoprotein cholesterol (r = 0.250, P = 0.03; Figure S1c) levels, but not with other clinical parameters listed in Table 1, including diabetic complications. Among these parameters, only the serum BUN levels were significantly associated with the glucagon levels (P = 0.022) in a multivariate linear regression analysis that included parameters showing a significant correlation in the univariate analyses. We further compared the data of those patients (66 of 77) who had also received the medical checkups in 2017. In those cases, the plasma glucagon levels correlated significantly between both years (r = 0.407, P = 0.0007; Figure 1c), whereas the levels of 2018 (15.5 ± 9.41 pg/mL) were significantly lower than those of 2017 (26.1 ± 17.4 pg/mL; P < 0.001). Statistical analyses for other clinical parameters between 2017 and 2018 are presented in the Supporting Information (Figure S2; Table S1).
Table 1.
Clinical characteristics of the study group
| Age (years) | 34.9 ± 6.4 | AST (U/L) | 20.8 ± 8.6 |
| Female, n (%) | 52 (67.5) | ALT (U/L) | 17.4 ± 11.5 |
| Duration of diabetes (years) | 25.8 ± 7.7 | GGT (U/L) | 22.8 ± 28.1 |
| BMI (kg/m2) | 23.7 ± 3.6 | BUN (mg/dL) | 13.1 ± 3.3 |
| UA (mg/dL) | 4.2 ± 1.1 | ||
| HbA1c (%) | 7.6 ± 1.0 | Cr (mg/dL) | 0.68 ± 0.11 |
| GA (%) | 22.9 ± 4.0 | LDL‐C (mg/dL) | 105 ± 22 |
| Plasma glucose (mg/dL) | 177 ± 82 | HDL‐C (mg/dL) | 73 ± 16 |
| Plasma glucagon (pg/mL) | 16.0 ± 9.8 | TG (mg/dL) | 97 ± 91 |
| TC (mg/dL) | 195 ± 26 | ||
| CVR‐R (%) | 4.79 ± 3.8 | ||
| ABI | 1.05 ± 0.08 | Daily total insulin dose (U/day) | 40.6 ± 15.0 |
| PWV (cm/s) | 1329 ± 215 | Insulin dose (U/kg bodyweight) | 0.63 ± 0.19 |
| Diabetic retinopathy (%) | 41 (55) | Proliferative retinopathy (%) | 9 (12) |
Total n = 77. Data are expressed as mean ± standard deviation or number (percentage). ABI, ankle‐brachial index; ALT, alanine transaminase; AST, aspartate transaminase; BMI, body mass index; BUN, blood urea nitrogen; Cr, creatinine; CVR‐R, coefficient of variation of R‐R intervals; GA, glycoalbumin; GGT, γ‐glutamyltransferase; HbA1c, glycated hemoglobin A1c; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; PWV, pulse wave velocity; TC, total cholesterol; TG, triglyceride; UA, uric acid.
Figure 1.
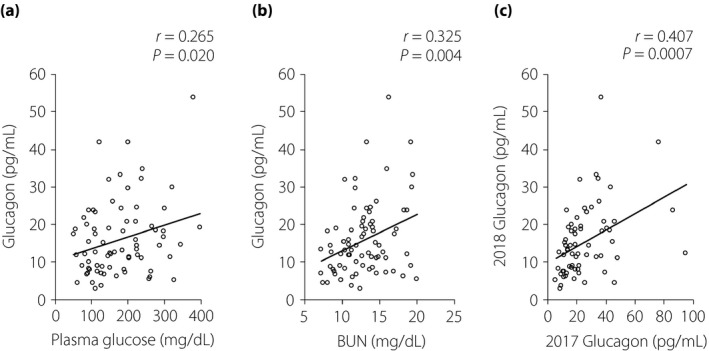
Values for plasma glucagon and (a) plasma glucose or (b) BUN in the patients with type 1 diabetes in 2018 (n = 77). Values for (c) plasma glucagon in the annual checkups of 2017 and 2018 for the patients with type 1 diabetes (n = 66). Circles indicate individual samples and the line indicates the approximate straight line. Statistical analysis for correlation was carried out using Pearson's univariate test.
Discussion
We previously showed that plasma glucagon levels were dysregulated, but significantly correlated with serum BUN levels in patients with type 1 diabetes3. In the current study, we investigated the annual change in plasma glucagon levels in these same patients and found a significant correlation between measurements in both years. In addition, we confirmed the significant association between glucagon and BUN levels. These results suggest a potentially constant dysregulation of glucagon in association with altered amino acid metabolism in patients with type 1 diabetes.
In contrast to our previous result, where the plasma glucagon levels were only significantly correlated with serum BUN levels3, in the current study the glucagon levels were also significantly correlated with plasma glucose, serum alanine transaminase, low‐density lipoprotein cholesterol and total cholesterol levels. Although there is not yet a clear explanation for the differences between both years, the correlation between glucagon and BUN, observed in 2017 and 2018, was confirmed. Indeed, only the serum BUN levels were significantly associated with the glucagon levels in a multivariate linear regression analysis. Amino acids stimulate glucagon secretion from α‐cells, whereas glucagon enhances amino acid metabolism in the liver4, 5. In hepatocytes, amino acids are metabolized and utilized to produce adenosine triphosphate, resulting in ammonia as a by‐product, which is later detoxified during the urea cycle. Indeed, glucagon is known to regulate carbamoyl phosphate synthetase 1, which is a rate‐limiting enzyme in the urea cycle8. The present data might provide clinical evidence for a functional link between these two important factors.
As in our previous work3, the glucagon levels with respect to plasma glucose levels were dysregulated in the current study. Glucagon secretion is considered to be suppressed in a hyperglycemic state, whereas it is enhanced in a hypoglycemic state2. In the current study, the plasma glucagon levels of patients with type 1 diabetes were positively correlated with the plasma glucose levels (Figure 1a). This paradoxical pattern of plasma glucagon might be involved in an instability of glycemic control in the disease; that is, exacerbation of hyperglycemia as a result of increasing glucagon, and/or fragility to hypoglycemia as a result of insufficient response of glucagon. In addition, the positive correlation of annual plasma glucagon levels (Figure 1c) suggests a sustained dysregulation of plasma glucagon in the patients. Given the physiological significance of glucagon in amino acid metabolism4, 5, the dysregulated glucagon – especially inadequately elevated glucagon – could enhance amino acid metabolism followed by protein catabolism. Recent progress in glucagon research, including ours, might reveal another aspect of type 1 diabetes: amino acids and protein homeostasis are altered by dysregulated glucagon, possibly leading to protein breakdown in skeletal muscles and sarcopenia.
The mechanisms underlying dysregulation in glucagon secretion might be varied and complex. In addition to physiological regulators of glucagon secretion from α‐cells, including nutrients, nervous systems and endocrine systems, we previously revealed the intra‐islet regulatory mechanism carried out mainly by neighboring β‐cells9, 10. This intra‐islet regulation for glucagon secretion might not be operational in patients with type 1 diabetes. The experimental data showing that chemical elimination of mouse β‐cells by streptozotocin induces elevated plasma glucagon levels, together with extreme hyperglycemia and insulin shortage11, support this hypothesis.
Can we reverse this dysregulated glucagon secretion? We do not know. In the current study, the annual glucagon levels were significantly correlated between the two years. In contrast, the levels of 2018 were significantly lower than those of 2017. So far, a reasonable explanation for this decrease has not been found from our current data. It is possible that this difference is due to changes of condition at the time of blood sample collection, although the procedures for the preparation of glucagon samples and glucagon assays were exactly the same as the 2017 study. We also investigated the annual difference in plasma glucagon values in the 66 patients and analyzed possible associated factors; however, we did not find any, explaining the changes (data not shown). Possible progressive deterioration in α‐cell function, including glucagon unresponsiveness, should be taken into consideration. To the best of our knowledge, this is the first report evaluating plasma glucagon levels by a newly established ELISA and following annual changes in the same patients with type 1 diabetes. Further follow ups for measuring glucagon levels and other clinical parameters could show the pathological history of glucagon regulation in type 1 diabetes patients, and help to find potential treatments to reverse glucagon dysregulation.
As we discussed in our previous report3, these studies have limitations. All participants had type 1 diabetes; hence, restriction of meals and insulin in order to standardize plasma glucose levels were not carried out for medical and ethical reasons. In addition, blood samples were obtained randomly with respect to patient food intake; therefore, we cannot exclude the possible influence of meal ingredients, especially proteins and amino acids, on glucagon regulation. In contrast, the BUN levels, which showed a positive association with glucagon, would not change so quickly according to amino acid load, as they are end‐products of amino acid metabolism. It is necessary to carefully analyze amino acid intakes and profiles to reach definite conclusions. The clinical and technical limitations raised here should be taken into account in further analyses and studies.
To date, there have not been any clinical reports investigating annual changes in plasma glucagon levels in patients with type 1 diabetes using the sandwich ELISA. The current data emphasize the pathophysiological significance of glucagon in type 1 diabetes patients, particularly in the status of amino acid and protein metabolism, and suggest comprehensive approaches to the disease by targeting glucagon.
Disclosure
The authors declare no conflict of interest.
Supporting information
Methods S1 | Study population. Preparation of plasma samples for glucagon assay.
Figure S1 | Values for clinical parameters and plasma glucagon in the patients with type 1 diabetes in 2018 (n = 77). Circles indicate individual samples and the line indicates the approximate straight line. Statistical analysis for correlation was carried out using Pearson's univariate test.
Figure S2 | Values for plasma glucose and glycated hemoglobin in the annual checkups of 2017 and 2018 for patients with type 1 diabetes (n = 66). Circles indicate individual samples and the line indicates the approximate straight line. Statistical analysis for correlation was carried out using Pearson's univariate test.
Table S1 | Results of Pearson's univariate tests in the annual checkups of 2017 and 2018 for patients with type 1 diabetes (n = 66).
Acknowledgments
The authors thank the Cosmic Corporation Co., Ltd. (Tokyo, Japan) for their assistance in the glucagon ELISA. This study is supported partly by a research grant from the Japan Diabetes Foundation (to DK).
J Diabetes Investig 2020; 11: 337–340
References
- 1. Kawamori D. Exploring the molecular mechanisms underlying alpha‐ and beta‐cell dysfunction in diabetes. Diabetol Int 2017; 8: 248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gromada J, Chabosseau P, Rutter GA. The alpha‐cell in diabetes mellitus. Nat Rev Endocrinol 2018; 14: 694–704. [DOI] [PubMed] [Google Scholar]
- 3. Kawamori D, Katakami N, Takahara M, et al Dysregulated plasma glucagon levels in Japanese young adult type 1 diabetes patients. J Diabetes Investig 2019; 10: 62–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Holst JJ, Wewer Albrechtsen NJ, Pedersen J, et al Glucagon and amino acids are linked in a mutual feedback cycle: The liver‐alpha‐cell axis. Diabetes 2017; 66: 235–240. [DOI] [PubMed] [Google Scholar]
- 5. Hayashi Y, Seino Y. Regulation of amino acid metabolism and α‐cell proliferation by glucagon. J Diabetes Investig 2018; 9: 464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Osawa S, Kawamori D, Katakami N, et al Significant elevation of serum dipeptidyl peptidase‐4 activity in young‐adult type 1 diabetes. Diabetes Res Clin Pract 2016; 113: 135–142. [DOI] [PubMed] [Google Scholar]
- 7. Miyachi A, Kobayashi M, Mieno E, et al Accurate analytical method for human plasma glucagon levels using liquid chromatography‐high resolution mass spectrometry: comparison with commercially available immunoassays. Anal Bioanal Chem 2017; 409: 5911–5918. [DOI] [PubMed] [Google Scholar]
- 8. Snodgrass PJ, Lin RC, Muller WA, et al Induction of urea cycle enzymes of rat liver by glucagon. J Biol Chem 1978; 253: 2748–2753. [PubMed] [Google Scholar]
- 9. Kawamori D, Kurpad AJ, Hu J, et al Insulin signaling in alpha cells modulates glucagon secretion in vivo. Cell Metab 2009; 9: 350–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kawamori D, Welters HJ, Kulkarni RN. Molecular pathways underlying the pathogenesis of pancreatic alpha‐cell dysfunction. Adv Exp Med Biol 2010; 654: 421–445. [DOI] [PubMed] [Google Scholar]
- 11. Kawamori D, Akiyama M, Hu J, et al Growth factor signalling in the regulation of alpha‐cell fate. Diabetes Obes Metab 2011; 13(Suppl 1): 21–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Methods S1 | Study population. Preparation of plasma samples for glucagon assay.
Figure S1 | Values for clinical parameters and plasma glucagon in the patients with type 1 diabetes in 2018 (n = 77). Circles indicate individual samples and the line indicates the approximate straight line. Statistical analysis for correlation was carried out using Pearson's univariate test.
Figure S2 | Values for plasma glucose and glycated hemoglobin in the annual checkups of 2017 and 2018 for patients with type 1 diabetes (n = 66). Circles indicate individual samples and the line indicates the approximate straight line. Statistical analysis for correlation was carried out using Pearson's univariate test.
Table S1 | Results of Pearson's univariate tests in the annual checkups of 2017 and 2018 for patients with type 1 diabetes (n = 66).


