Abstract
Aims/Introduction
Resistin is an adipocyte‐derived polypeptide that leads to the progression of insulin resistance and subsequent atherosclerosis. Some studies have reported an association between self‐reported intake of n−3 polyunsaturated fatty acids (PUFAs) and serum resistin levels. However, no studies have investigated the association between the ratio of serum levels of n−3 to serum n−6 PUFAs and the serum resistin concentration in the general population.
Materials and Methods
We carried out a cross‐sectional study of 3,200 community‐dwelling Japanese individuals aged ≥40 years in 2002–2003. The ratios of serum eicosapentaenoic acid or docosahexaenoic acid to arachidonic acid (AA) were categorized into quartiles. The associations of serum eicosapentaenoic acid/AA and docosahexaenoic acid/AA with the serum resistin concentration were assessed using linear regression models with adjustment for potential confounding factors.
Results
The geometric mean of serum resistin was 10.3 ng/mL. The age‐ and sex‐adjusted geometric mean of serum resistin decreased significantly with increased levels of serum eicosapentaenoic acid/AA (quartile 1: 11.3 ng/mL; quartile 2: 10.6 ng/mL; quartile 3: 10.3 ng/mL; quartile 4: 9.3 ng/mL; P for trend <0.001). A similar association was observed for serum docosahexaenoic acid/AA (quartile 1: 11.1 ng/mL; quartile 2: 10.6 ng/mL; quartile 3: 10.1 ng/mL; quartile 4: 9.7 ng/mL; P for trend <0.001). An adjustment for potential confounding factors did not change these associations.
Conclusions
Higher ratios of serum n−3 to n−6 PUFAs were associated with lower serum resistin levels. Consumption of a large amount of n−3 PUFAs might have desirable effects on resistin‐mediated diseases.
Keywords: Cross‐sectional study, n−3 polyunsaturated fatty acids, Resistin
Resistin is a polypeptide that leads to the progression of insulin resistance and inflammation. The present study showed the inverse association of the ratios of serum eicosapentaenoic acid to arachidonic acid, docosahexaenoic acid to arachidonic acid, and serum resistin levels in a general population. Higher intakes of eicosapentaenoic acid and docosahexaenoic acid would lower serum resistin levels.
Introduction
Resistin was first discovered as an adipokine in a screen for adipocyte gene products in rodents, and was later shown to mediate insulin resistance1. In the human body, resistin is mainly expressed in monocytes and macrophages, and promotes both inflammation and insulin resistance2, 3, 4. Accordingly, several previous population‐based studies showed the relationship between the serum concentrations of resistin and the development of type 2 diabetes, atherosclerosis, and cardiovascular disease5, 6, 7, 8. Furthermore, administration of resistin to mice induced glucose intolerance, whereas inhibition of resistin through the administration of anti‐resistin immunoglobulin‐γ decreased blood glucose in obese mice and also improved insulin sensitivity1. Thus, it would be of value to identify factors with the ability to modify serum resistin levels.
n−3 Polyunsaturated fatty acids (PUFAs), such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), and n−6 PUFAs, such as arachidonic acid (AA), are essential fatty acids, and thus humans cannot naturally produce them in the body. n−3 PUFAs, which are mainly obtained from marine fish and fish oil, exert anti‐inflammatory effects by inhibiting a number of aspects of inflammatory pathways, and show anti‐atherogenic and cardio‐protective effects9, 10, 11. In contrast, n−6 PUFAs are metabolically and functionally distinct from n−3 PUFAs, as they have inflammatory and atherogenic effects12, although they have a structure similar to n−3 PUFAs. Thus, the balance of n−3 and n−6 PUFAs; that is, the ratio of EPA or DHA to AA, might affect the circulating levels of inflammatory mediators and the subsequent incidence of diseases related to systemic inflammation, such as cardiovascular diseases and other diseases13, 14, 15. Several epidemiological studies found that the self‐reported intake of PUFAs, especially n−3 PUFAs, was inversely associated with serum resistin levels16, 17. In a recent systematic review, however, there was a weak‐to‐moderate correlation between self‐reported dietary intake and serum concentrations of n−3 PUFAs, as the reproducibility for the dietary assessment of fatty acids is limited18. Epidemiological studies with measurements of serum n−3 PUFAs levels could provide the further evidence required to clarify these associations. Therefore, we carried out a cross‐sectional survey to shed light on the influence of the balance between serum levels of n−3 and n−6 PUFAs (i.e., EPA/AA and DHA/AA), as well as the influence of the serum proportions of each n−3 PUFA to total free fatty acids, on serum resistin levels in a general Japanese population.
Methods
Study population
The Hisayama Study is a population‐based study of cardiovascular disease and its risk factors that has been ongoing in the town of Hisayama, a suburb of Fukuoka City in southwestern Japan. An annual health examination has been repeated since 1961. Details of this study have been described elsewhere19. In brief, a total of 3,328 residents aged ≥40 years participated in the health examination in 2002 (participation rate: 77.6%). We excluded individuals who did not consent to enter the study (n = 30), those without fasting blood‐sampling data (n = 86), those without serum fatty acid measurement (n = 2) and those without serum resistin data (n = 10), and eventually enrolled the remaining 3,200 participants in the present study.
Measurements of serum fatty acids and resistin
At the examination, blood samples were drawn from study participants after at least 12 h of fasting. The serum specimens were separated, frozen and stored at −80°C until used. Serum fatty acids were measured in 2010, and serum resistin levels were measured in 2007.
The gas chromatography method (SRL, Tokyo, Japan) was used for the assay of serum fatty acids as we reported previously14, 20. The serum EPA/AA were divided into quartiles: ≤0.28, 0.29–0.41, 0.42–0.59 and 0.60–3.31. The serum DHA/AAs were also divided into quartiles: ≤0.75, 0.76–0.93, 0.93–1.15 and 1.15–4.05. We also analyzed PUFAs as follows: the proportions of EPA in total free fatty acid as EPA (%), of DHA in total free fatty acid as DHA (%) and of AA in total free fatty acid as AA (%). The serum EPA (%), DHA (%) and AA (%) were also respectively divided into the following quartiles. For EPA (%): ≤1.40, 1.41–2.06, 2.07–2.95 and 2.96–12.56; for DHA (%): ≤3.79, 3.80–4.57, 4.58–5.49 and 5.50–12.73; and for AA (%): ≤4.26, 4.27–4.94, 4.95–5.65 and 5.66–9.51.
Serum resistin concentrations were measured following the manufacturer's protocol using a human resistin enzyme‐linked immunosorbent assay kit (R&D Systems, Minneapolis, MN, USA)8.
Other risk factors
The information on medical history, medications for hypertension and diabetes, smoking habits, alcohol intake, and physical activity was obtained by means of a self‐administered questionnaire completed by each participant. Trained interviewers checked the questionnaire at the examination. Smoking and drinking habits were categorized by whether the participant currently smoked or drank or not. We defined any sports or other exercise (≥3 times/week) in the participants’ spare time as regular exercise. Height and weight were measured in light clothes, and body mass index (BMI) was calculated as weight (kg) divided by the square of the height (m) (kg/m2). Blood pressure was taken with an automated sphygmomanometer (BP‐203RV III; Colin, Tokyo, Japan) in a seated position, and in the analysis, we used the average of three consecutive measurements. We defined hypertensive participants as those with blood pressure ≥140/90 mmHg and/or those who currently used antihypertensive medications. The hexokinase method was used for the measurement of plasma glucose levels, and a commercial double antibody solid‐phase radioimmunoassay (Phadeseph Insulin; Pharmacia Diagnostics AB, Uppsala, Sweden) was used to measure serum insulin levels. Diabetes mellitus was defined as fasting plasma glucose levels ≥7.0 mmol/L, 2‐h post‐load glucose levels ≥11.1 mmol/L, casual glucose levels ≥11.1 mmol/L, or participants who currently used oral glucose‐lowering medications or insulin. The homeostasis model assessment of insulin resistance (HOMA‐IR) values, which were calculated as HOMA‐IR = fasting plasma glucose (mg/dL) × fasting serum insulin (lU/mL) / 405 were used to estimate insulin resistance21. Participants having insulin resistance were defined as those in the top quartile of HOMA‐IR distribution (HOMA‐IR = 2.6)22. We measured serum high‐sensitivity C‐reactive protein (hs‐CRP) levels by a latex‐enhanced nephelometric assay (Behring Diagnostics, Westwood, MA, USA), and hs‐CRP ≥1.0 mg/L was defined as high hs‐CRP23. The enzymatic method was used for the measurement of serum creatinine. The Chronic Kidney Disease Epidemiology Collaboration equation with a Japanese coefficient was used to estimate the estimated glomerular filtration rate (eGFR)24. White blood cells were counted using the impedance method (STKS; Beckman‐Coulter Inc., Hialeah, FL, USA). A self‐administered diet history questionnaire was used in estimating daily total energy intake.25
Statistical analysis
The characteristics of participants were summarized in total and by quartiles of serum EPA/AA and DHA/AA. A linear trend was tested across serum EPA/AA and DHA/AA by using the linear regression model for mean values, and the logistic regression model for frequencies. As the distributions of serum levels of resistin, EPA, DHA, AA, EPA (%), DHA (%), EPA/AA, DHA/AA, triglycerides, HOMA‐IR and hs‐CRP were skewed, they were naturally log‐transformed in the statistical analyses. We used the analysis of covariance method in calculating the adjusted geometric mean values of serum resistin, and in testing their linear trends across the quartiles of EPA/AA, DHA/AA, EPA (%), DHA (%) and AA (%). The associations of 1 standard deviation (SD) change in log‐transformed EPA/AA, DHA/AA, EPA (%) and DHA (%), as well as 1 SD change in AA (%) with serum resistin, were estimated using a linear regression model. The adjustment was made for potential confounders – namely, age, sex, white blood cells, eGFR, BMI, total energy intake, current smoking, current drinking and regular exercise (model 1). As an experimental study showed that factors related to insulin resistance, such as high glucose and glucocorticoid, upregulated resistin expression26, further adjustment was made for HOMA‐IR. The influence of EPA/AA and DHA/AA on serum resistin levels was estimated as per 1 SD change of log‐transformed EPA/AA and DHA/AA among subgroups defined as BMI ≥25 kg/m2 and by other risk factors, such as sex, age (<65 vs ≥65 years), hypertension, diabetes, current smoking, current drinking, regular exercise, eGFR (<60 vs ≥60 mL/min/1.73 m2), hs‐CRP (<1.0 vs ≥1.0 mg/L), HOMA‐IR (<2.6 vs ≥2.6) and total energy intake (<2,137 vs ≥2,137 kcal in men, <1,736 vs ≥1,736 kcal in women, median value for men and women, respectively), and the heterogeneity was tested between groups by adding a multiplicative interaction term to the relevant statistical model for each factor. The variables relevant to the subgroup were excluded from the corresponding model. All statistical analyses were carried out using SAS software package version 9.4 (SAS Institute, Cary, NC, USA). Statistical significance was considered at a two‐tailed P‐value <0.05.
Ethical considerations
This study was approved by the Kyushu University Institutional Review Board for Clinical Research (approval No. 30‐460), and it conforms to the provisions of the Declaration of Helsinki. All participants provided written informed consent.
Results
Characteristics of participants
Table 1 shows the characteristics of the total study population. The mean age of the study participants was 61.6 years, and the proportion of men was 43.2%. The median values of serum EPA (%), DHA (%), EPA/AA and DHA/AA were 2.1%, 4.6%, 0.42 and 0.94, respectively. The mean value of AA (%) was 5.0%. There were positive associations between the levels of serum EPA/AA and: (i) the mean values of age, serum total cholesterol, BMI and total energy intake; (ii) the geometric mean values of HOMA‐IR and serum hs‐CRP; and (iii) the frequencies of male sex, hypertension, diabetes mellitus, use of lipid‐lowering agents, current drinking and regular exercise. In contrast, increased serum levels of EPA/AA were associated with lower levels of the mean values of eGFR (Table S1). There were similar associations for serum DHA/AA levels and each factor, except for the use of lipid‐lowering agents, which showed no trend across the quartiles of serum DHA/AA levels (Table S2). In these unadjusted analyses, upward trends were observed in the association of the quartiles of serum EPA/AA or DHA/AA with the geometric mean values of HOMA‐IR or serum hs‐CRP levels. However, in the case of HOMA‐IR, these upward trends disappeared after adjusting for age, sex and BMI (1.7, 95% confidence interval [CI] 1.6–1.8 in quartile 1 [Q1]; 1.8, 95% CI 1.7–1.8 in Q2; 1.7, 95% CI 1.6–1.8 in Q3; and 1.8 95% CI 1.7–1.8 in Q4 for serum EPA/AA levels; P for trend = 0.72), whereas the geometric mean values of serum hs‐CRP levels decreased with higher quartiles of serum EPA/AA (0.59, 95% CI 0.55–0.64 mg/L in Q1; 0.56, 95% CI 0.51–0.60 mg/L in Q2; 0.49, 95% CI 0.46–0.54 mg/L in Q3; and 0.49, 95% CI 0.45–0.53 mg/L in Q4, P for trend = 0.007). Similar associations were observed for DHA/AA.
Table 1.
Characteristics of the study population
| No. participants | 3,200 |
| Age (years) | 61.6 (12.4) |
| Men (%) | 43.2 |
| Serum resistin (ng/mL) | 10.0 (7.2–14.8) |
| Serum EPA (μg/mL) | 62.0 (42.2–90.2) |
| Serum DHA (μg/mL) | 138.0 (108.2–172.8) |
| Serum AA (μg/mL) | 148.0 (125.3–172.6) |
| Serum EPA (%) | 2.1 (1.4–3.0) |
| Serum DHA (%) | 4.6 (3.8–5.5) |
| Serum AA (%) | 5.0 (1.0) |
| Serum EPA/AA | 0.42 (0.29–0.60) |
| Serum DHA/AA | 0.94 (0.76–1.15) |
| Systolic blood pressure (mmHg) | 132 (21) |
| Hypertension (%) | 43.9 |
| Fasting plasma glucose (mmol/L) | 6.1 (1.3) |
| Diabetes mellitus (%) | 17.6 |
| Serum total cholesterol (mmol/L) | 5.3 (0.9) |
| Serum HDL cholesterol (mmol/L) | 1.6 (0.4) |
| Serum LDL cholesterol (mmol/L) | 3.2 (0.8) |
| Serum triglycerides (mmol/L) | 1.1 (0.8–1.6) |
| Use of lipid‐lowering agents (%) | 10.0 |
| Use of EPA drugs (%) | 0.5 |
| Obesity (%) | 26.2 |
| Estimated glomerular filtration rate (mL/min/1.73 m2) | 78.6 (13.2) |
| HOMA‐IR | 1.7 (1.1–2.6) |
| White blood cell count (/μL) | 5801 (1579) |
| High‐sensitivity CRP (mg/L) | 0.47 (0.23–1.03) |
| Body mass index (kg/m2) | 23.1 (3.4) |
| Total energy intake (kcal/day) | 1979 (577) |
| Current smoking (%) | 21.8 |
| Current drinking (%) | 43.7 |
| Regular exercise (%) | 10.8 |
Values are the mean (standard deviation), percentages, or median (interquartile range). The proportions of eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) or arachidonic acid (AA) in total free fatty acid were defined as EPA (%), DHA (%) and AA (%), respectively. HDL, high‐density lipoprotein; HOMA‐IR, homeostasis model assessment of insulin resistance; LDL, low‐density lipoprotein.
Association of serum EPA/AA and DHA/AA with serum resistin
The age‐ and sex‐adjusted or multivariable‐adjusted geometric mean values of serum resistin levels according to the quartiles of serum EPA/AA are shown in Figure 1a and Table 2. The age‐ and sex‐adjusted geometric means of serum resistin decreased significantly with increasing serum EPA/AA levels (P for trend <0.001). This inverse association remained significant, even after adjustment for age, sex, white blood cells, eGFR, BMI, total energy intake, current smoking, current drinking and regular exercise (P for trend <0.05), and after further adjustment for HOMA‐IR (P for trend <0.05). Multivariable‐adjusted geometric mean values of serum resistin levels decreased by 3 ng/dL per 1‐SD increment of log‐transformed serum EPA/AA (Table 3). In addition, we compared the associations of 1‐SD increment in log‐transformed serum EPA/AA with serum resistin levels between the subgroups. The influence of 1‐SD increment in log‐transformed serum EPA/AA on serum resistin levels was greater in non‐obese than obese participants, with a marginal heterogeneity in the association between subgroups (P for heterogeneity = 0.06), whereas no such difference was observed for the other risk factors (all P for heterogeneity >0.1; Table S3). Similar associations were observed for the analysis of serum DHA/AA, as shown in Figure 1b, and Tables 2, 3 and S3. Inverse associations were found between DHA/AA and serum resistin in the age‐ and sex‐adjusted model, and in the multivariable‐adjusted models (all P for trend <0.05; Table 2), and multivariable‐adjusted geometric mean values of serum resistin levels decreased by 2 ng/dL per 1‐SD increment of log‐transformed serum DHA/AA (Table 3). When compared between the presence and absence of obesity, there was a greater influence of DHA/AA on serum resistin levels in non‐obese than obese participants (P for heterogeneity = 0.047).
Figure 1.
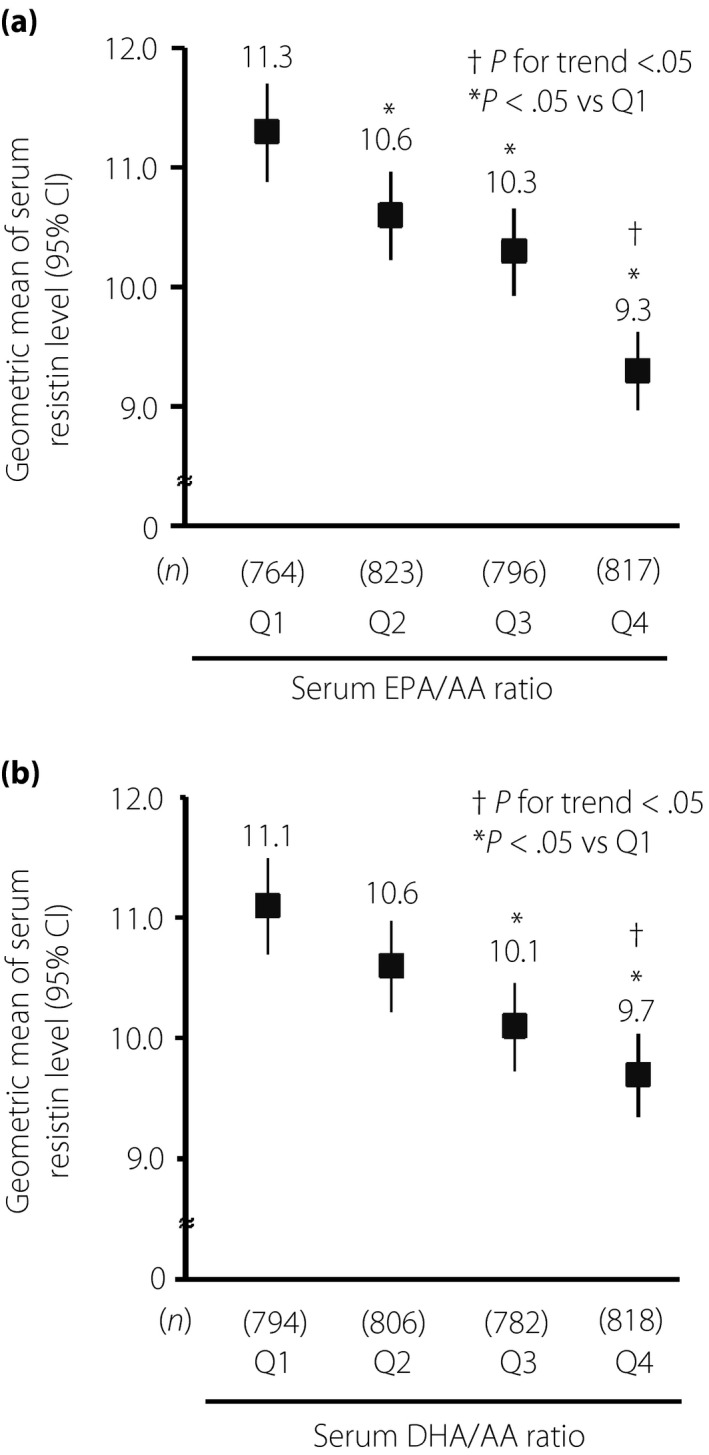
(a) The age‐ and sex‐adjusted geometric mean values of serum resistin versus serum eicosapentaenoic acid (EPA)/arachidonic acid (AA). Age‐ and sex‐adjusted geometric means of serum resistin decreased with rising serum EPA/AA (P for trend < 0.001). (b) The age‐ and sex‐adjusted geometric mean values of serum resistin against serum docosahexaenoic acid (DHA)/AA. Age‐ and sex‐adjusted geometric means of serum resistin decreased with rising serum DHA/AA (P for trend < 0.001). *P < 0.05 versus quartile 1 (Q1), † P for trend < 0.05. CI, confidence interval.
Table 2.
Multivariable‐adjusted geometric mean of serum resistin levels according to quartiles of eicosapentaenoic acid/ arachidonic acid, docosahexaenoic acid/arachidonic acid, eicosapentaenoic acid (%), docosahexaenoic acid (%) and arachidonic acid (%)
| Serum EPA/AA, DHA/AA, EPA (%), DHA (%) or AA (%) levels | No. participants | Geometric mean and 95% CI of serum resistin (ng/mL) | |||||
|---|---|---|---|---|---|---|---|
| Age‐ and sex‐adjusted | P‐value | Model 1 | P‐value | Model 1 + HOMA‐IR | P‐value | ||
| Serum EPA/AA ratio | |||||||
| Q1 (<0.28) | 764 | 11.3 (10.9–11.7) | (Reference) | 10.5 (10.2–10.9) | (Reference) | 10.5 (10.2–10.9) | (Reference) |
| Q2 (0.29–0.41) | 823 | 10.6 (10.3–11.0) | 0.02 | 10.4 (10.0–10.7) | 0.53 | 10.4 (10.1–10.7) | 0.58 |
| Q3 (0.42–0.59) | 796 | 10.3 (9.9–10.6) | <0.001 | 10.2 (9.8–10.5) | 0.16 | 10.2 (9.8–10.5) | 0.17 |
| Q4 (0.60–3.31) | 817 | 9.4 (9.0–9.7) | <0.001 | 9.5 (9.2–9.8) | <0.001 | 9.5 (9.1–9.8) | <0.001 |
| P for trend | <0.001 | <0.001 | <0.001 | ||||
| Serum DHA/AA ratio | |||||||
| Q1 (<0.75) | 794 | 11.1 (10.7–11.5) | (Reference) | 10.4 (10.0–10.7) | (Reference) | 10.4 (10.0–10.7) | (Reference) |
| Q2 (0.76–0.93) | 806 | 10.6 (10.3–11.0) | 0.14 | 10.4 (10.1–10.8) | 0.79 | 10.4 (10.1–10.8) | 0.78 |
| Q3 (0.94–1.14) | 782 | 10.1 (9.7–10.4) | <0.001 | 10.1 (9.7–10.4) | 0.22 | 10.1 (9.7–10.4) | 0.22 |
| Q4 (1.15–4.05) | 818 | 9.7 (9.3–10.0) | <0.001 | 9.7 (9.3–10.0) | 0.007 | 9.7 (9.3–10.0) | 0.007 |
| P for trend | <0.001 | 0.01 | 0.01 | ||||
| Serum EPA (%) | |||||||
| Q1 (≤1.40) | 788 | 11.3 (10.9–11.7) | (Reference) | 10.6 (10.2–11.0) | (Reference) | 10.6 (10.2–11.0) | (Reference) |
| Q2 (1.41–2.06) | 800 | 10.9 (10.5–11.3) | 0.15 | 10.5 (10.1–10.8) | 0.64 | 10.5 (10.1–10.8) | 0.59 |
| Q3 (2.07–2.95) | 808 | 10.0 (9.6–10.3) | <0.001 | 10.0 (9.6–10.3) | 0.02 | 10.0 (9.7–10.3) | 0.02 |
| Q4 (2.96–12.56) | 804 | 9.3 (9.0–9.7) | <0.001 | 9.6 (9.2–9.9) | <0.001 | 9.5 (9.2–9.9) | <0.001 |
| P for trend | <0.001 | <0.001 | <0.001 | ||||
| Serum DHA (%) | |||||||
| Q1 (≤3.79) | 792 | 11.5 (11.1–11.9) | (Reference) | 10.6 (10.2–11.0) | (Reference) | 10.6 (10.2–11.0) | (Reference) |
| Q2 (3.80–4.57) | 801 | 10.7 (10.3–11.1) | 0.005 | 10.4 (10.1–10.8) | 0.51 | 10.4 (10.1–10.8) | 0.49 |
| Q3 (4.58–5.49) | 804 | 10.1 (9.7–10.4) | <0.001 | 10.0 (9.7–10.4) | 0.02 | 10.0 (9.7–10.4) | 0.03 |
| Q4 (5.50–12.73) | 803 | 9.2 (8.9–9.6) | <0.001 | 9.5 (9.2–9.8) | <0.001 | 9.5 (9.2–9.8) | <0.001 |
| P for trend | <0.001 | <0.001 | <0.001 | ||||
| Serum AA (%) | |||||||
| Q1 (≤4.26) | 796 | 10.7 (10.4–11.1) | (Reference) | 10.3 (9.9–10.6) | (Reference) | 10.3 (10.0–10.7) | (Reference) |
| Q2 (4.27–4.94) | 796 | 10.6 (10.2–10.9) | 0.50 | 10.4 (10.1–10.8) | 0.56 | 10.4 (10.0–10.8) | 0.72 |
| Q3 (4.95–5.65) | 806 | 10.0 (9.7–10.4) | 0.008 | 9.9 (9.5–10.2) | 0.12 | 9.8 (9.5–10.2) | 0.07 |
| Q4 (5.66–9.51) | 802 | 10.0 (9.7–10.4) | 0.009 | 10.0 (9.6–10.3) | 0.22 | 10.0 (9.7–10.3) | 0.21 |
| P for trend | 0.01 | 0.10 | 0.09 | ||||
The proportions of eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) or arachidonic acid (AA) in total free fatty acid were defined as EPA (%), DHA (%) and AA (%), respectively. Model 1 includes age, sex, estimated glomerular filtration rate, body mass index, white blood cell count, total energy intake, current smoking, current drinking and regular exercise. CI, confidence interval; HOMA‐IR, homeostasis model assessment of insulin resistance; SD, standard deviation.
Table 3.
The association of 1 standard deviation increment in eicosapentaenoic acid/arachidonic acid, docosahexaenoic acid/arachidonic acid, eicosapentaenoic acid (%), docosahexaenoic acid (%) and arachidonic acid (%) with log‐transformed serum resistin level
| Age‐ and sex‐adjusted | P‐value | Model 1 | P‐value | Model 1 + HOMA‐IR | P‐value | |
|---|---|---|---|---|---|---|
| β (95% CI) per 1 SD increment in log‐transformed EPA/AA | −0.06 (−0.08 to −0.04) | <0.001 | −0.03 (−0.05 to −0.01) | <0.001 | −0.03 (−0.05 to −0.01) | <0.001 |
| β (95% CI) per 1 SD increment in log‐transformed DHA/AA | −0.05 (−0.07 to −0.03) | <0.001 | −0.02 (−0.04 to −0.01) | 0.009 | −0.02 (−0.04 to −0.01) | 0.01 |
| β (95% CI) per 1 SD increment in log‐transformed EPA (%) | −0.07 (−0.09 to −0.06) | <0.001 | −0.04 (−0.06 to −0.02) | <0.001 | −0.04 (−0.06 to −0.02) | <0.001 |
| β (95% CI) per 1 SD increment in log‐transformed DHA (%) | −0.08 (−0.1 to −0.07) | <0.001 | −0.04 (−0.06 to −0.02) | <0.001 | −0.04 (−0.06 to −0.02) | <0.001 |
| β (95% CI) per 1 SD increment in AA (%) | −0.03 (−0.05 to −0.012) | 0.001 | −0.02 (−0.04 to −0.001) | 0.04 | −0.01 (−0.04 to −0.002) | 0.03 |
The proportions of eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) or arachidonic acid (AA) in total free fatty acid were defined as EPA (%), DHA (%) and AA (%), respectively. Model 1 includes age, sex, estimated glomerular filtration rate, body mass index, white blood cell count, total energy intake, current smoking, current drinking and regular exercise. CI, confidence interval; FA, fatty acid; HOMA‐IR, homeostasis model assessment of insulin resistance; SD, standard deviation.
Association of serum EPA (%), DHA (%) and AA (%) with resistin
The sensitivity analyses investigating the association between the serum ratios of each PUFA to the total free fatty acids and the serum resistin level showed that the multivariable‐adjusted geometric mean values of serum resistin decreased with higher quartiles of serum EPA (%) or DHA (%; both P for trend <0.001; Table 2). In the multivariable‐adjusted models, however, no significant association between serum AA (%) and geometric mean serum resistin was observed (P for trend = 0.09; Table 2). In the analyses taking these variables as continuous variables, there were inverse associations between serum EPA (%), DHA (%) or AA (%), and serum resistin (Table 3), although the relationship between AA (%) and serum resistin was no longer significant in the analysis excluding the 198 participants with outliers of EPA/AA, DHA/AA, EPA (%), DHA (%) and AA (%; data not shown).
Discussion
In the present population‐based study, we showed that higher serum EPA/AA and DHA/AA were significantly associated with lower serum resistin in a general Japanese population. This significant association was still observed even after adjustment for potential confounding factors and additional adjustment for HOMA‐IR, whereas the association was attenuated in obese people. Furthermore, the serum resistin levels were reduced with elevating serum EPA (%) and DHA (%). These findings highlight the possibility that higher intakes of EPA and DHA might have desirable effects on resistin‐mediated diseases.
Previous reports have described inverse associations between serum resistin and either food frequency questionnaire‐estimated intake of PUFAs16 or n−3 PUFAs17. These findings are similar to the results of the present study, which found an inverse association of n−3 PUFAs with serum resistin in an even more robust setting by evaluating the serum levels of PUFAs. In contrast, a small, randomized, single‐blind, placebo‐controlled pilot study of the effects of DHA + EPA‐enriched fish oil in Mexican patients with type 2 diabetes reported that there was no difference in the change of serum resistin between the intervention and placebo groups27. The discrepancy between these previous and the present findings might be related to the difference in the intake amount of n−3 PUFAs; based on a diet analysis, the mean intakes of n−3 PUFAs in the Mexican study were 0.89 g/day and 0.79 g/day in the two groups at baseline, and 1.32 g/day in both groups after intervention; whereas in the present study, the mean intake of n − 3 PUFAs was 2.86 g/day. These findings suggest that the dose of n−3 PUFAs received in the present randomized controlled study might have been insufficient to lower the serum resistin. Randomized controlled trials in different settings will be required, such as in other races, with large amounts of n−3 PUFAs and large numbers of participants.
n−3 PUFAs compete with AA to participate in eicosanoid metabolism, thereby reducing the production of pro‐inflammatory prostaglandins28. The present study also found that higher serum levels of EPA/AA and DHA/AA were associated with lower serum CRP levels. Hence, the serum EPA/AA ratio has been used as an indicator of the balance of pro‐inflammatory eicosanoids and anti‐inflammatory eicosanoids29. Furthermore, it has been reported that n−3 PUFAs exert anti‐inflammatory effects either directly30 or indirectly through the metabolites of EPA and DHA; that is, resolvin E1 and D131, 32. n−3 PUFAs and their metabolites could suppress the expression of resistin messenger ribonucleic acid by lowering the production of several cytokines – namely, tumor necrosis factor‐α, interferon‐γ, interleukin‐1β and interleukin‐69, 31, 32, which have been acknowledged to enhance the expression of resistin messenger ribonucleic acid33. Intriguingly, the present study found that there was no clear association of serum EPA/AA and DHA/AA with serum resistin among obese participants. The exact explanation for this finding is not clear, but a high accumulation of AA in adipose tissue might disturb the anti‐inflammatory effects of n−3 PUFAs34, 35.
The strengths of the present study include its population‐based design, high participation rate and consideration of detailed information about potential confounders in the statistical analyses. However, some limitations should also be mentioned. First, the cross‐sectional study design limits the interpretation of causality between serum PUFA levels and serum resistin levels; however, as n−3 PUFAs are essential fatty acids and their serum levels are primarily determined based on intake of food or supplements rich in n−3 PUFAs36, it might not be reasonable to consider that resistin affects the serum EPA/AA and DHA/AA. Second, as we measured each serum PUFA level only once, misclassification of the study participants into other categories might occur. Such misclassification would weaken the association shown in the present study, and bias the results toward the null hypothesis. Finally, although adjustments were made for demographic and other confounding factors within the statistical model, the presence of residual confounders could not be excluded.
In conclusion, the present study showed that serum EPA/AA and DHA/AA levels, as well as individual values of serum EPA (%) and DHA (%), were inversely associated with serum resistin levels. As the increased production of resistin could cause insulin resistance, diabetes and subsequent atherosclerotic diseases, high intakes of EPA and DHA might have preventive effects on these diseases. Other population‐based studies in different populations, observational studies and randomized control studies will be necessary to confirm these points.
Disclosure
Toshiharu Ninomiya received research funding from Mochida Pharmaceutical Co., Ltd. The other authors declare no conflict of interest.
Supporting information
Table S1 | Characteristics of the study participants by the quartiles of serum eicosapentaenoic acid/arachidonic acid level.
Table S2 | Characteristics of the study population by the quartiles of serum docosahexaenoic acid/arachidonic acid level.
Table S3 | Multivariable‐adjusted association of 1 standard deviation increment in log‐transformed eicosapentaenoic acid/arachidonic acid and docosahexaenoic acid/arachidonic acid with serum resistin level according to risk factor levels.
Acknowledgments
This study was supported in part by Grants‐in‐Aid for Scientific Research A (JP16H02644, and JP16H02692), B (JP16H05850, JP16H05557, JP17H04126, and JP18H02737), C (JP16K09244, JP17K09114, JP17K09113, JP17K01853, JP18K07565, and JP18K09412) and Early‐Career Scientists (JP18K17925, and JP18K17382) from the Ministry of Education, Culture, Sports, Science and Technology of Japan; by Health and Labor Sciences Research Grants of the Ministry of Health, Labor and Welfare of Japan (H29‐Junkankitou‐Ippan‐003, and H30‐Shokuhin‐[Sitei]‐005); and by the Japan Agency for Medical Research and Development (JP18dk0207025, JP18ek0210082, JP18gm0610007, JP18ek0210083, JP18 km0405202, JP18ek0210080, JP18fk0108075). In addition, this study was sponsored by Mochida Pharmaceutical Co., Ltd. (Tokyo).
J Diabetes Investig 2020; 11: 482–489
References
- 1. Steppan CM, Bailey ST, Bhat S, et al The hormone resistin links obesity to diabetes. Nature 2001; 409: 307–312. [DOI] [PubMed] [Google Scholar]
- 2. Patel L, Buckels AC, Kinghorn IJ, et al Resistin is expressed in human macrophages and directly regulated by PPAR gamma activators. Biochem Biophys Res Commun 2003; 300: 472–476. [DOI] [PubMed] [Google Scholar]
- 3. Schwartz DR, Lazar MA. Human resistin: found in translation from mouse to man. Trends Endocrin Met 2011; 22: 259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Osawa H, Tabara Y, Kawamoto R, et al Plasma resistin, associated with single nucleotide polymorphism ‐420, is correlated with insulin resistance, lower HDL cholesterol, and high‐sensitivity C‐reactive protein in the Japanese general population. Diabetes Care 2007; 30: 1501–1506. [DOI] [PubMed] [Google Scholar]
- 5. Chen BH, Song Y, Ding EL, et al Circulating levels of resistin and risk of type 2 diabetes in men and women: results from two prospective cohorts. Diabetes Care 2009; 32: 329–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reilly MP, Lehrke M, Wolfe ML, et al Resistin is an inflammatory marker of atherosclerosis in humans. Circulation 2005; 111: 932–939. [DOI] [PubMed] [Google Scholar]
- 7. Muse ED, Feldman DI, Blaha MJ, et al The association of resistin with cardiovascular disease in the Multi‐Ethnic Study of Atherosclerosis. Atherosclerosis 2015; 239: 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Osawa H, Doi Y, Makino H, et al Diabetes and hypertension markedly increased the risk of ischemic stroke associated with high serum resistin concentration in a general Japanese population: the Hisayama Study. Cardiovasc Diabetol 2009; 8: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Calder PC. n‐3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr 2006; 83: 1505S–1519S. [DOI] [PubMed] [Google Scholar]
- 10. Thies F, Garry JM, Yaqoob P, et al Association of n‐3 polyunsaturated fatty acids with stability of atherosclerotic plaques: a randomised controlled trial. Lancet 2003; 361: 477–485. [DOI] [PubMed] [Google Scholar]
- 11. Mozaffarian D, Wu JH. Omega‐3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol 2011; 58: 2047–2067. [DOI] [PubMed] [Google Scholar]
- 12. Grimble RF. Dietary lipids and the inflammatory response. Proc Nutr Soc 1998; 57: 535–542. [DOI] [PubMed] [Google Scholar]
- 13. Itakura H, Yokoyama M, Matsuzaki M, et al Relationships between plasma fatty acid composition and coronary artery disease. J Atheroscler Thromb 2011; 18: 99–107. [DOI] [PubMed] [Google Scholar]
- 14. Ninomiya T, Nagata M, Hata J, et al Association between ratio of serum eicosapentaenoic acid to arachidonic acid and risk of cardiovascular disease: the Hisayama Study. Atherosclerosis 2013; 231: 261–267. [DOI] [PubMed] [Google Scholar]
- 15. Simopoulos AP. Evolutionary aspects of diet, the omega‐6/omega‐3 ratio and genetic variation: nutritional implications for chronic diseases. Biomed Pharmacother 2006; 60: 502–507. [DOI] [PubMed] [Google Scholar]
- 16. Fargnoli JL, Fung TT, Olenczuk DM, et al Adherence to healthy eating patterns is associated with higher circulating total and high‐molecular‐weight adiponectin and lower resistin concentrations in women from the Nurses’ Health Study. Am J Clin Nutr 2008; 88: 1213–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Noumi Y, Kawamura R, Tabara Y, et al An inverse association between serum resistin levels and n‐3 polyunsaturated fatty acids intake was strongest in the SNP‐420 G/G genotype in the Japanese cohort: the Toon Genome Study. Clin Endocrinol 2018; 88: 51–57. [DOI] [PubMed] [Google Scholar]
- 18. Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res 2008; 47: 348–380. [DOI] [PubMed] [Google Scholar]
- 19. Hata J, Ninomiya T, Hirakawa Y, et al Secular trends in cardiovascular disease and its risk factors in Japanese: half‐century data from the Hisayama Study (1961–2009). Circulation 2013; 128: 1198–1205. [DOI] [PubMed] [Google Scholar]
- 20. Ozawa A, Takayanagi K, Fujita T, et al Determination of long chain fatty acids in human total plasma lipids using gas chromatography [in Japanese]. Jpn Analyst 1982; 31: 87–91. [Google Scholar]
- 21. Matthews DR, Hosker JP, Rudenski AS, et al Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419. [DOI] [PubMed] [Google Scholar]
- 22. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications part 1: diagnosis and classification of diabetes mellitus – provisional report of a WHO consultation. Diabetic Med 1998; 15: 539–553. [DOI] [PubMed] [Google Scholar]
- 23. Arima H, Kubo M, Yonemoto K, et al High‐sensitivity C‐reactive protein and coronary heart disease in a general population of Japanese – the Hisayama study. Arterioscler Thromb Vasc Biol 2008; 28: 1385–1391. [DOI] [PubMed] [Google Scholar]
- 24. Horio M, Imai E, Yasuda Y, et al Modification of the CKD epidemiology collaboration (CKD‐EPI) equation for Japanese: accuracy and use for population estimates. Am J Kidney Dis 2010; 56: 32–38. [DOI] [PubMed] [Google Scholar]
- 25. Okubo H, Sasaki S, Rafamantanantsoa HH, et al Validation of self‐reported energy intake by a self‐administered diet history questionnaire using the doubly labeled water method in 140 Japanese adults. Eur J Clinical Nutr 2008; 62: 1343–1350. [DOI] [PubMed] [Google Scholar]
- 26. Shojima N, Sakoda H, Ogihara T, et al Humoral regulation of resistin expression in 3T3‐L1 and mouse adipose cells. Diabetes 2002; 51: 1737–1744. [DOI] [PubMed] [Google Scholar]
- 27. Jacobo‐Cejudo MG, Valdés‐Ramos R, Guadarrama‐López AL, et al Effect of n‐3 polyunsaturated fatty acid supplementation on metabolic and inflammatory biomarkers in type 2 diabetes mellitus patients. Nutrients 2017; 9: 573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Calder PC. Long‐chain n‐3 fatty acids and inflammation: potential application in surgical and trauma patients. Brazil J Med Biol Res 2003; 36: 433–446. [DOI] [PubMed] [Google Scholar]
- 29. Ohnishi H, Saito Y. Eicosapentaenoic acid (EPA) reduces cardiovascular events: relationship with the EPA/arachidonic acid ratio. J Atheroscler Thromb 2013; 20: 861–877. [DOI] [PubMed] [Google Scholar]
- 30. Haugen F, Zahid N, Dalen KT, et al Resistin expression in 3T3‐L1 adipocytes is reduced by arachidonic acid. J Lipid Res 2005; 46: 143–153. [DOI] [PubMed] [Google Scholar]
- 31. Schwab JM, Chiang N, Arita M, et al Resolvin E1 and protectin D1 activate inflammation‐resolution programmes. Nature 2007; 447: 869–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schmid M, Gemperle C, Rimann N, et al Resolvin D1 polarizes primary human macrophages toward a proresolution phenotype through GPR32. J Immunol 2016; 196: 3429–3437. [DOI] [PubMed] [Google Scholar]
- 33. Kaser S, Kaser A, Sandhofer A, et al Resistin messenger‐RNA expression is increased by proinflammatory cytokines in vitro. Biochem Biophys Res Commun 2003; 309: 286–290. [DOI] [PubMed] [Google Scholar]
- 34. Garaulet M, Pérez‐Llamas F, Pérez‐Ayala M, et al Site‐specific differences in the fatty acid composition of abdominal adipose tissue in an obese population from a Mediterranean area: relation with dietary fatty acids, plasma lipid profile, serum insulin, and central obesity. Am J Clin Nutr 2001; 74: 585–591. [DOI] [PubMed] [Google Scholar]
- 35. Li K, Huang T, Zheng J, et al Effect of marine‐derived n‐3 polyunsaturated fatty acids on C‐reactive protein, interleukin 6 and tumor necrosis factor alpha: a meta‐analysis. PLoS One 2014; 9: e88103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Block RC, Harris WS, Pottala JV. Determinants of blood cell omega‐3 fatty acid content. Open Biomark J 2008; 1: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 | Characteristics of the study participants by the quartiles of serum eicosapentaenoic acid/arachidonic acid level.
Table S2 | Characteristics of the study population by the quartiles of serum docosahexaenoic acid/arachidonic acid level.
Table S3 | Multivariable‐adjusted association of 1 standard deviation increment in log‐transformed eicosapentaenoic acid/arachidonic acid and docosahexaenoic acid/arachidonic acid with serum resistin level according to risk factor levels.


