Abstract
Aims/Introduction
Sulfonylurea‐related hypoglycemia increases the risk of cardiovascular sequela, such as cardiac arrhythmia. This study aimed to clarify the relationship between the level of glycated hemoglobin (HbA1c) and the duration of hypoglycemia in type 2 diabetes patients treated with sulfonylureas.
Materials and Methods
Glucose levels in the enrolled patients (n = 300) were investigated with a professional continuous glucose monitoring device in the outpatient setting at six diabetes centers in Japan.
Results
A total of 269 participants completed the study. The duration of hypoglycemia with glucose values of <54 mg/dL was significantly longer in patients with an HbA1c level of ≤6.4% than in those with an HbA1c level of ≥8.0%, and that of hypoglycemia with glucose values of <70 mg/dL was significantly longer in patients with an HbA1c level of ≤6.4%, 6.5–6.9% or 7.0–7.4% than in those with an HbA1c level of ≥8.0%. Patients with an HbA1c level of ≤6.4% were exposed to glucose values of <70 mg/dL for >10% of the time in daily life (6.8 ± 5.6 min/h). The duration of hypoglycemia with glucose values of <70 mg/dL was longer at night than during the daytime, and the nadir of glucose values occurred between 03.00 and 05.00 hours irrespective of HbA1c level. The duration of hypoglycemia was associated with the duration of diabetes and sulfonylurea dose.
Conclusions
The duration of hypoglycemia was inversely correlated with HbA1c level and was longer during the night‐time than daytime in type 2 diabetes patients treated with sulfonylureas.
Keywords: Continuous glucose monitoring, Hypoglycemia, Sulfonylureas
The mean 24‐h glucose profiles over 24 h according to glycated hemoglobin level. The nadir of glucose levels was apparent between 03.00 and 05.00 hours irrespective of glycated hemoglobin level.

Introduction
Hypoglycemia is one of the most frequent adverse events in the treatment of diabetes. It not only triggers unpleasant symptoms and, in severe cases, disturbed consciousness, but also exerts exacerbating effects on various conditions associated with diabetes, including microvascular complications, cardiovascular events, cognitive disorders and shortening of lifespan1, 2, 3.
Sulfonylureas, the oldest class of oral glucose‐lowering drugs, are still widely administered around the world because of their potent glucose‐lowering effect and low cost. These drugs frequently trigger hypoglycemia, however, particularly in individuals with low levels of glycated hemoglobin (HbA1c)4, 5. Evidence suggests that sulfonylurea‐related hypoglycemia increases the risk of cardiovascular sequela, such as cardiac arrhythmia6. A large database analysis showed that the lowest hazard ratio for mortality was apparent at an HbA1c level of approximately 7.5% for patients with type 2 diabetes treated with sulfonylureas, with lower levels of HbA1c being associated with increased mortality7, suggesting that sulfonylurea‐induced hypoglycemia occurs more frequently in individuals with lower levels of HbA1c and leads to the increase in mortality. Information on the relationship between HbA1c levels and the frequency of hypoglycemia in patients treated with sulfonylureas is limited, however.
Continuous glucose monitoring (CGM), which provides a comprehensive picture of glycemic profile, has brought great advantages for the evaluation and control of glycemia in diabetes patients8. For the evaluation of hypoglycemia, CGM allows quantitative assessment of the frequency, duration and severity of the event. Furthermore, such devices are useful for the detection of unrecognized or nocturnal episodes of hypoglycemia9, 10, which are likely related to unfavorable outcomes in the treatment of diabetes11, 12. As far as we are aware, however, the relationship between HbA1c levels and the duration of hypoglycemia in individuals treated with sulfonylureas has not been examined with the use of CGM. We therefore designed a multicenter, cross‐sectional study with a large number of study participants (n = 300) to gain insight into the relationship between HbA1c levels and the duration of hypoglycemia in sulfonylurea‐treated type 2 diabetes patients with a professional CGM system.
Methods
Study design and participants
This multicenter, cross‐sectional study was carried out at six diabetes centers in Japan. Potentially eligible patients at each study site were invited to participate in the study. A total of 300 participants who had visited the study sites as outpatients between September 2017 and January 2018, been diagnosed with type 2 diabetes, and treated with sulfonylureas, but not with insulin, were enrolled. Patients were excluded if they were aged <20 years or had an HbA1c level of ≥12%. The protocol for the study was approved by the institutional review boards of the participating centers, and the study was carried out in accordance with the Declaration of Helsinki and its amendments. Written informed consent was obtained from all participants. The study was registered with the University Hospital Medical Information Network (UMIN000025284).
Procedures
All participants wore a FreeStyle Libre Pro sensor (Abbott Diabetes Care, Alameda, CA, USA) for 14 days. We analyzed CGM data of patients for whom at least 7 days of recorded data were available. We excluded data for the first day of wearing the device from analysis because of concerns about the accuracy of the CGM system during this initial period13. The CGM data were analyzed with the use of the device software (FreeStyle Libre Software, Abbott Diabetes Care; https://www.myfreestyle.com/provider, accessed 11 April 2016). All participants were asked to report hypoglycemic events and to evaluate the pain or itching associated with sensor insertion or wear according to a 5‐point scale (1, none; 2, almost none; 3, mild; 4, moderate; 5, severe).
Outcomes
The primary outcome of the study was the difference in the duration of hypoglycemia (<54 or <70 mg/dL) between HbA1c levels of ≥8.0% and either ≤6.4%, 6.5–6.9%, 7.0–7.4% or 7.5–7.9%. We also analyzed the 24‐h glucose profile for each HbA1c group, the difference in the duration of hypoglycemia (<54 or <70 mg/dL) between daytime (between 07.00 and 23.00 hours) and night‐time (between 23.00 and 07.00 hours) for each HbA1c group, and the association between the duration of hypoglycemia and age, the duration of diabetes, renal function, body mass index (BMI), diabetic complications, dose of sulfonylurea, and concomitant antidiabetic medications. Furthermore, we analyzed the differences in the mean glucose level, duration of hyperglycemia (≥180 mg/dL), time in the target glucose range (70–<180 mg/dL), standard deviation (SD) of 24‐h glucose values, mean amplitude of glycemic excursions (MAGE), and the coefficient of variation of glucose levels between an HbA1c level of ≥8.0% and the other HbA1c groups. Safety end‐points were the frequency of severe hypoglycemia (requiring third‐party assistance) during CGM wear and symptoms related to sensor insertion or wear.
Statistical analysis
We calculated that a sample size of 285 was required to provide 80% power for detection of a difference between the highest HbA1c group and the other groups for the primary end‐point with a two‐sided significance level of 0.0125 (=0.05/4), as previously described4, 14. Allowing for a dropout rate of 5%, a total of 300 participants was adopted for recruitment. We assessed the primary end‐point with the unpaired Student's t‐test, with a P‐value <0.0125 being considered significant after application of Bonferroni's correction. Comparison of daytime and nocturnal hypoglycemia, as well as comparison of the mean glucose level, duration of hyperglycemia, time in the target glucose range, SD of 24‐h glucose values, MAGE and CV of glucose levels among HbA1c groups were also carried out with the unpaired Student's t‐test, and with a P‐value of <0.05 and <0.0125, respectively, being considered significant. Analysis of covariance was applied with adjustment for HbA1c level to compare the duration of hypoglycemia between subgroups based on age (≥75 vs <75 years), duration of diabetes (≥15 vs <15 years), renal function (estimated glomerular filtration rate [eGFR] of ≥60 vs <60 mL/min/1.73 m2), BMI (≥25 vs <25 kg/m2), dose of sulfonylurea (high vs low dose relative to the median) or concomitant antidiabetic medications (plus vs minus dipeptidyl peptidase‐4 [DPP‐4] inhibitors or glucagon‐like peptide‐1 [GLP‐1] receptor agonists; biguanides; thiazolidinediones; sodium–glucose cotransporter 2 inhibitors; or alpha‐glucosidase inhibitors). Mean glucose levels and glycemic variability (SD of 24‐h glucose values, MAGE and CV) were calculated with the use of EASY GV software (Nuffield Department of Primary Care Health Sciences, The University of Oxford, Oxford, UK)15. All statistical analysis was carried out with SPSS software version 22 (IBM SPSS Statistics, IBM Corp, Armonk, NY, USA).
Results
Among the 300 enrolled patients, 25 individuals had CGM data for <7 days, and six lost their CGM sensors. A total of 269 participants who completed the study were thus included in the analysis. The baseline characteristics of the participants are shown in Table 1. A total of 38 (14%), 73 (27%), 69 (26%), 38 (14%) and 51 (19%) patients had HbA1c levels of ≤6.4%, 6.5–6.9%, 7.0–7.4%, 7.5–7.9% and ≥8.0%, respectively. A total of 85, 168 and 16 patients were treated with gliclazide, glimepiride and glibenclamide, respectively, with total (mean ± SD) daily doses of 27.3 ± 18.4 mg, 1.1 ± 0.76 mg and 3.3 ± 2.0 mg, respectively.
Table 1.
Baseline characteristics of the study patients
| Characteristic | |
|---|---|
| Male/female | 182/87 |
| Age (years) | 69.0 ± 10.7 |
| BMI (kg/m2) | 24.6 ± 4.4 |
| HbA1c (%) | 7.2 ± 0.8 |
| eGFR (mL/min/1.73 m2) | 67.1 ± 22.5 |
| Duration of diabetes (years) | 17.5 ± 9.1 |
| Diabetic retinopathy (none/SDR/PPDR/PDR/unknown) | 173/55/7/26/8 |
| Diabetic nephropathy (stage 1/2/3/4) | 166/75/22/ 6 |
| Diabetic neuropathy | 102 |
| Macrovascular complications | 69 |
| Gliclazide/glimepiride/glibenclamide | 85/168/16 |
| Other antidiabetic agents (DPP‐4 inhibitors/biguanides/ thiazolidinediones/α‐glucosidase inhibitors/SGLT‐2 inhibitors/GLP‐1 receptor agonists) | 224/183/40/78/36/18 |
Total n = 269. Data are n or mean ± standard deviation values. BMI, body mass index; DPP‐4, dipeptidyl peptidase‐4; eGFR, estimated glomerular filtration rate; GLP‐1, glucagon‐like peptide‐1; HbA1c, glycated hemoglobin; PDR, proliferative diabetic retinopathy; PPDR, preproliferative diabetic retinopathy; SDR, simple diabetic retinopathy; SGLT‐2, sodium–glucose cotransporter 2.
The duration of hypoglycemia with glucose levels <54 mg/dL was 1.6 ± 2.2, 0.6 ± 2.3, 0.4 ± 1.3, 0.5 ± 1.5 and 0.1 ± 0.2 min/h for patients with HbA1c levels of ≤6.4%, 6.5–6.9%, 7.0–7.4%, 7.5–7.9% and ≥8.0%, respectively, and was significantly (P < 0.0125) longer in the HbA1c ≤6.4% group than in the ≥8.0% group (Figure 1a). The duration of hypoglycemia with glucose values of <70 mg/dL was 6.8 ± 5.6, 2.6 ± 4.1, 1.8 ± 3.2, 1.6 ± 3.3 and 0.4 ± 0.9 min/h for HbA1c levels of ≤6.4%, 6.5–6.9%, 7.0–7.4%, 7.5–7.9% and ≥8.0%, respectively, and was significantly (P < 0.0125) longer in the HbA1c ≤6.4%, 6.5–6.9% and 7.0–7.4% groups than in the ≥8.0% group (Figure 1b). The duration of hypoglycemia with glucose values of <54 mg/dL (r = −0.198, P < 0.01) or <70 mg/dL (r = −0.381, P < 0.001) was inversely correlated with HbA1c levels after adjustment for age, duration of diabetes, BMI and eGFR (Figure 2).
Figure 1.
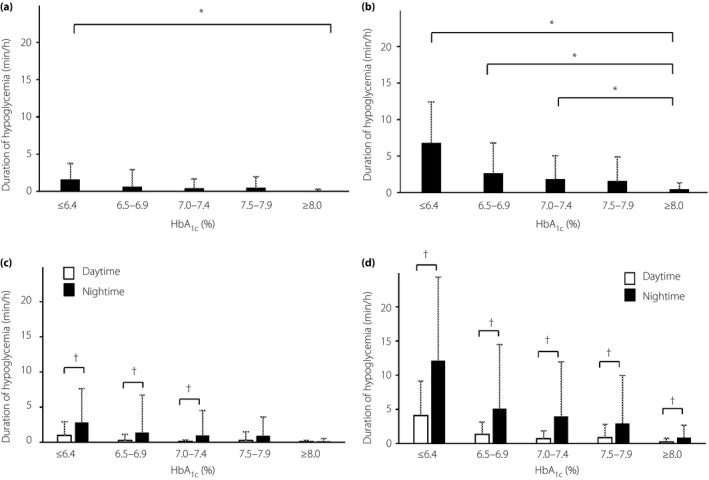
Duration of hypoglycemia according to glycated hemoglobin (HbA1c) level. (a) Duration of hypoglycemia with glucose values of <54 mg/dL. (b) Duration of hypoglycemia with glucose values of <70 mg/dL. (c) Duration of hypoglycemia with glucose values of <54 mg/dL during the daytime and night‐time. Daytime: between 07.00 and 23.00 hours. Night‐time: between 23:00 and 07:00 hours. (d) Duration of hypoglycemia with glucose levels of <70 mg/dL during the daytime and night‐time. All data are the mean ± standard deviation. *P < 0.0125, † P < 0.05 (unpaired Student's t‐test).
Figure 2.
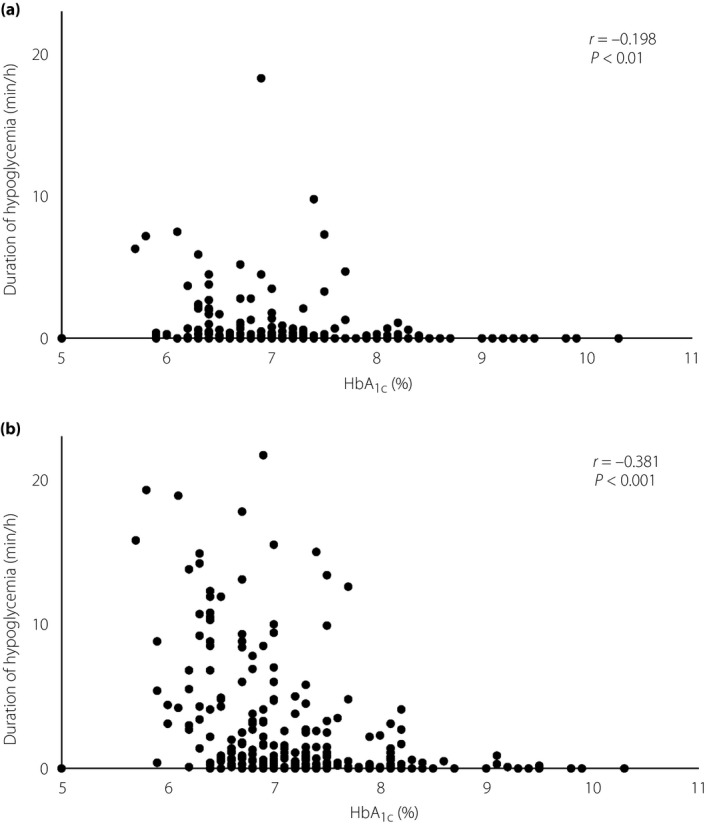
Correlation between glycated hemoglobin (HbA1c) level and duration of hypoglycemia. There was an inverse correlation between the duration of hypoglycemia with glucose levels of (a) <54 mg/dL or (b) <70 mg/dL and HbA1c level after adjustment for age, duration of diabetes, body mass index and estimated glomerular filtration rate.
The duration of hypoglycemia with glucose values of <54 mg/dL at night‐time was significantly (P < 0.05) longer than that in the daytime for patients with HbA1c levels of ≤6.4%, 6.5–6.9% or 7.0–7.4% (Figure 1c), whereas the duration of hypoglycemia with glucose values of <70 mg/dL at night‐time was significantly longer than that in the daytime for all HbA1c groups (Figure 1d). The mean 24‐h glucose profiles determined for each HbA1c group are shown in Figure 3. The nadir of glucose levels was apparent between 03.00 and 05.00 hours irrespective of HbA1c level.
Figure 3.
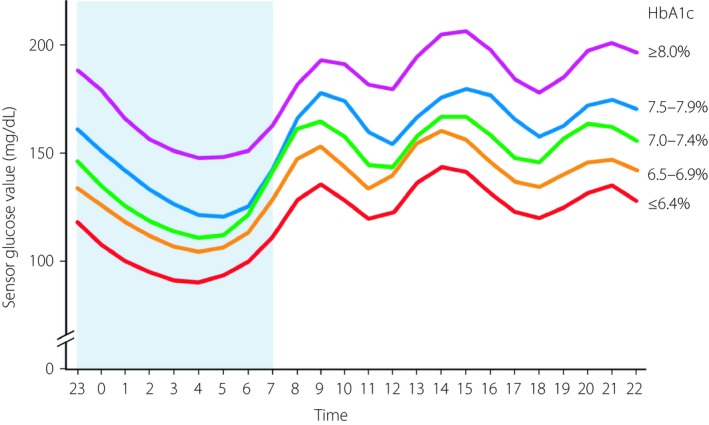
The mean 24‐h glucose profiles over 24 h according to glycated hemoglobin (HbA1c) level. The nadir of glucose levels was apparent between 03.00 and 05.00 hours irrespective of HbA1c level.
The duration of hypoglycemia with glucose values <70 mg/dL tended to be longer in patients aged ≥75 years than in those who were younger (3.0 vs 2.2 min/h, P = 0.09) after adjustment for HbA1c levels (Table 2). The duration of hypoglycemia with glucose values of <54 or <70 mg/dL was significantly longer in patients with a diabetes duration of ≥15 years than in those with a shorter disease duration (0.80 vs 0.29 min/h, P < 0.05, and 3.0 vs 1.7 min/h, P < 0.01, respectively). The duration of hypoglycemia with glucose values of <70 mg/dL tended to be longer in patients with an eGFR of <60 mL/min/1.73 m2 than in those with an eGFR of ≥60 mL/min/1.73 m2 (3.1 vs 2.1 min/h, P = 0.06). The duration of hypoglycemia with glucose values of <54 or <70 mg/dL was similar between patients with a BMI of <25 or ≥25 kg/m2. There were no significant differences in the duration of hypoglycemia between patients with or without diabetic retinopathy, nephropathy and neuropathy, as well as macrovascular complications (Table 2).
Table 2.
Duration of hypoglycemia according to age, duration of diabetes, estimated glomerular filtration rate, body mass index, sulfonylurea dose or diabetic complications
| Duration (min/h) | Duration (min/h) | P | |
|---|---|---|---|
| Hypoglycemia of <54 mg/dL | |||
| Age (years) | <75 | ≥75 | |
| 0.45 (n = 178) | 0.81 (n = 91) | 0.11 | |
| Duration of diabetes (years) | <15 | ≥15 | |
| 0.29 (n = 111) | 0.80 (n = 151) | <0.05 | |
| eGFR (mL/min/1.73 m2) | <60 | ≥60 | |
| 0.64 (n = 94) | 0.55 (n = 171) | 0.68 | |
| BMI (kg/m2) | <25 | ≥25 | |
| 0.65 (n = 68) | 0.45 (n = 199) | 0.38 | |
| Gliclazide (mg/day) | ≤20 | >20 | |
|
0.23 (n = 58) |
0.84 (n = 27) |
<0.05 | |
| Glimepiride (mg/day) | ≤1 | >1 | |
| 0.42 (n = 130) | 1.5 (n = 38) | <0.01 | |
| (+) | (−) | ||
| Diabetic retinopathy | 0.78 (n = 88) | 0.48 (n = 173) | 0.18 |
| Diabetic nephropathy | 0.50 (n = 103) | 0.61 (n = 66) | 0.58 |
| Diabetic neuropathy | 0.81 (n = 102) | 0.42 (n = 167) | 0.07 |
| Macrovascular complications | 0.57 (n = 69) | 0.54 (n = 200) | 0.89 |
| Hypoglycemia of <70 mg/dL | |||
| Age (years) | <75 | ≥75 | |
| 2.2 (n = 178) | 3.0 (n = 91) | 0.09 | |
| Duration of diabetes (years) | <15 | ≥15 | |
| 1.7 (n = 111) | 3.0 (n = 151) | <0.01 | |
| eGFR (mL/min/1.73 m2) | <60 | ≥60 | |
| 3.1 (n = 94) | 2.1 (n = 171) | 0.06 | |
| BMI (kg/m2) | <25 | ≥25 | |
| 2.6 (n = 68) | 2.2 (n = 199) | 0.32 | |
| Gliclazide (mg/day) | ≤20 | >20 | |
|
1.6 (n = 58) |
4.2 (n = 27) |
<0.01 | |
| Glimepiride (mg/day) | ≤1 | >1 | |
| 1.8 (n = 130) | 4.2 (n = 38) | <0.01 | |
| (+) | (−) | ||
| Diabetic retinopathy | 2.9 (n = 88) | 2.3 (n = 173) | 0.26 |
| Diabetic nephropathy | 2.8 (n = 103) | 2.2 (n = 166) | 0.26 |
| Diabetic neuropathy | 2.9 (n = 102) | 2.2 (n = 167) | 0.12 |
| Macrovascular complications | 2.5 (n = 69) | 2.4 (n = 200) | 0.74 |
Data are adjusted by glycated hemoglobin level. P < 0.05 was considered significant. BMI, body mass index; eGFR, estimated glomerular filtration rate.
[Correction added on 2 October, after first online publication: Some alignments in the table have been amended.]
We also analyzed the relationship between sulfonylurea dose and the duration of hypoglycemia after adjustment for HbA1c level (Table 2). Given that the median dose for gliclazide and glimepiride was 20 and 1 mg/day, respectively, we defined low and high doses of these drugs as ≤20 and >20 mg/day for gliclazide, and as ≤1 and >1 mg/day for glimepiride, respectively. We did not analyze the data for glibenclamide because of the small number of participants taking this drug. The duration of hypoglycemia with glucose values <54 or <70 mg/dL was significantly longer in patients treated with the high dose of each drug than in those treated with the low dose (gliclazide and <54 mg/dL, 0.84 vs 0.23 min/h, P < 0.05; gliclazide and <70 mg/dL, 4.2 vs 1.6 min/h, P < 0.01; glimepiride and <54 mg/dL, 1.5 vs 0.42 min/h, P < 0.01; and glimepiride and <70 mg/dL, 4.2 vs 1.8 min/h, P < 0.01).
The mean glucose level over a 24‐h period was significantly (P < 0.0125) higher, the duration of hyperglycemia (≥180 mg/dL) was significantly longer and the time in the target glucose range (70 to <180 mg/dL) was significantly shorter in the HbA1c ≥8.0% group than in each of the other four HbA1c groups (Table 3). The SD of 24‐h glucose values and MAGE were significantly higher, and the CV of glucose levels was significantly smaller in the HbA1c ≥8.0% group than in the ≤6.4% and 6.5–6.9% groups (Table 3).
Table 3.
Mean glucose levels and other glycemic parameters according to glycated hemoglobin level
| Parameter | ≤6.4% | 6.5–6.9% | 7.0–7.4% | 7.5–7.9% | ≥8.0% |
|---|---|---|---|---|---|
| Mean glucose level (mg/dL) | 118.3 ± 18.9* | 134.0 ± 16.9* | 144.4 ± 17.1* | 155.7 ± 30.0* | 179.4 ± 29.2 |
| Duration of hyperglycemia (min/h) | 5.5 ± 5.5* | 9.7 ± 6.4* | 13.3 ± 7.0* | 17.5 ± 12.1* | 27.6 ± 12.1 |
| Duration of euglycemia (min/h) | 47.7 ± 6.4* | 47.7 ± 7.1* | 44.9 ± 7.2* | 40.9 ± 11.4* | 31.9 ± 11.8 |
| SD (mg/dL) | 38.2 ± 8.4* | 42.6 ± 10.7* | 43.2 ± 10.5 | 46.1 ± 9.9 | 51.0 ± 13.1 |
| MAGE (mg/dL) | 95.8 ± 23.6* | 107.6 ± 30.4* | 110.3 ± 25.7 | 118.8 ± 26.8 | 123.6 ± 36.8 |
| CV (%) | 32.7 ± 7.2* | 31.9 ± 7.2* | 30.3 ± 7.6 | 30.0 ± 6.0 | 28.6 ± 6.7 |
Data are mean ± standard deviation (SD). *P < 0.0125 versus glycated hemoglobin level of ≥8.0% (unpaired Student's t‐test). CV, coefficient of variation; MAGE, mean amplitude of glycemic excursions.
After adjustment for HbA1c levels, the duration of hypoglycemia with glucose values of <70 mg/dL was significantly shorter in patients taking incretin‐related agents (DPP‐4 inhibitors or GLP‐1 receptor agonists) than in those not taking these drugs (2.3 vs 3.8 min/h, P < 0.05). When DPP‐4 inhibitors and GLP‐1 receptor agonists were analyzed independently, no significant reduction of the duration of hypoglycemia with these drugs was observed. There were no significant differences in the duration of hypoglycemia between patients taking or not taking other concomitant antidiabetic medications (Table 4).
Table 4.
Duration of hypoglycemia according to concomitant antidiabetic medications
| Duration (min/h) | Duration (min/h) | P | |
|---|---|---|---|
| (+) | (−) | ||
| Hypoglycemia of <54 mg/dL | |||
| Incretin‐related agents (DPP‐4 inhibitors or GLP‐1 receptor agonists)† | 0.54 (n = 241) | 0.82 (n = 28) | 0.42 |
| DPP‐4 inhibitors | 0.55 (n = 224) | 0.67 (n = 45) | 0.67 |
| GLP‐1 receptor agonists | 0.40 (n = 18) | 0.58 (n = 251) | 0.66 |
| Biguanides | 0.63 (n = 183) | 0.43 (n = 86) | 0.38 |
| Thiazolidinediones | 0.44 (n = 40) | 0.59 (n = 229) | 0.60 |
| SGLT‐2 inhibitors | 0.37 (n = 36) | 0.6 (n = 233) | 0.47 |
| Alpha‐glucosidase inhibitors | 0.41 (n = 78) | 0.63 (n = 191) | 0.33 |
| Hypoglycemia of <70 mg/dL | |||
| Incretin‐related agents (DPP‐4 inhibitors or GLP‐1 receptor agonists)† | 2.3 (n = 241) | 3.8 (n = 28) | <0.05 |
| DPP‐4 inhibitors | 2.3 (n = 224) | 3.2 (n = 45) | 0.15 |
| GLP‐1 receptor agonists | 2.1 (n = 18) | 2.5 (n = 251) | 0.69 |
| Biguanides | 2.7 (n = 183) | 1.9 (n = 86) | 0.13 |
| Thiazolidinediones | 2.6 (n = 40) | 2.4 (n = 229) | 0.85 |
| SGLT‐2 inhibitors | 2.3 (n = 36) | 2.5 (n = 233) | 0.77 |
| Alpha‐glucosidase inhibitors | 1.8 (n = 78) | 2.7 (n = 191) | 0.07 |
Data are adjusted by glycated hemoglobin level. †One participant administered both a dipeptidyl peptidase‐4 (DPP‐4) inhibitor and a glucagon‐like peptide‐1 (GLP‐1) receptor agonist. SGLT‐2, sodium–glucose cotransporter 2
No episodes of severe hypoglycemia were reported by the study patients. Unrecognized hypoglycemia (glucose of <70 mg/dL without a self‐report of hypoglycemia) occurred in all patients for whom a glucose value of <70 mg/dL was recorded at least once (n = 205, 76%). A total of 18 of these patients also reported hypoglycemia with symptoms.
The wearing time for the sensor was 13.4 ± 1.5 days (mean ± SD). A total of 228 (85%) patients reported no pain or almost no pain during sensor insertion, and 240 (89%) patients reported no itching or almost no itching during sensor wear.
Discussion
By CGM analysis with a large number of participants, we have shown here that the duration of hypoglycemia was inversely correlated with HbA1c level in patients with type 2 diabetes treated with sulfonylureas. Whereas previous CGM‐based studies with smaller numbers of participants showed that hypoglycemia occurs more frequently in individuals treated with sulfonylureas than in those not taking these drugs, the relationship between HbA1c level and the duration of hypoglycemia was not analyzed in these studies4, 16. A meta‐analysis found that the incidence of hypoglycemia was inversely correlated with baseline HbA1c level in type 2 diabetes patients who initiated medications, including sulfonylureas, with the development of hypoglycemia being detected by symptoms or self‐monitoring of blood glucose17, which does not allow quantitative assessment. A CGM‐based study with 101 type 1 diabetes patients treated with basal–bolus insulin therapy showed that the HbA1c level was inversely correlated with the duration of hypoglycemia18. The present study is thus the first to investigate the relationship between HbA1c level and the duration of hypoglycemia, as determined by CGM in type 2 diabetes patients treated with sulfonylureas.
Previous studies with CGM showed that hypoglycemia occurred more frequently during the night than during the daytime in insulin‐treated patients with type 1 or type 2 diabetes18, 19. In contrast, daytime hypoglycemic events were more frequent than nocturnal hypoglycemic events in a study based on self‐reporting of such events20. In the present study, we found that the duration of nocturnal hypoglycemia was longer than that of daytime hypoglycemia, and became longer as the HbA1c level decreased. A meta‐analysis in insulin‐treated patients with type 2 diabetes also showed that the incidence of nocturnal hypoglycemia was inversely correlated with HbA1c level21. The 24‐h glucose profiles of patients in the present study showed that glucose levels were lowest in the early hours of the morning irrespective of HbA1c level. Of note, patients were not aware of most nocturnal hypoglycemic events, suggesting that healthcare providers should pay careful attention to night‐time hypoglycemia, particularly in the early hours of the morning, in patients treated with sulfonylureas.
We found that diabetes duration, but not age, was directly related to the duration of hypoglycemia in patients treated with sulfonylureas. The duration of diabetes and age have previously been shown to be associated with the incidence of hypoglycemia in patients with type 2 diabetes in other treatment settings21, 22, 23. Furthermore, we found that the dose of sulfonylureas was associated with the duration of hypoglycemia. A study in which hypoglycemia was detected on the basis of a questionnaire administered to patients showed a weak positive association between sulfonylurea dose and hypoglycemia24. Treatment with DPP‐4 inhibitors or with GLP‐1 receptor agonists is associated with a reduced risk of self‐reported hypoglycemia25. We have now shown that the duration of hypoglycemia with glucose values of <70 mg/dL was shorter for patients taking these drugs. Impairment of renal function (eGFR of <60 mL/min/m2) tended to be related to the duration of hypoglycemia in the present study, with such impairment having previously been associated with an increased rate of severe hypoglycemic events in sulfonylurea‐treated patients with type 2 diabetes26.
The strengths of the present study include its multicenter design and large number of participants. Furthermore, CGM analysis allowed us to quantitatively evaluate the duration, timing and severity of hypoglycemia, and the study with outpatients was informative not only of hypoglycemia, but also of glycemic variability in a real‐world setting. Here, we showed that that patients with an HbA1c level of ≤6.4% were exposed to glucose values of <70 mg/dL for >10% of the time during daily life (6.8 ± 5.6 min/h).
There were several limitations in the current study. First, the accuracy of the CGM device (FreeStyle Libre Pro) has not been fully characterized. The mean absolute relative difference of the device was reported to be 11.1%, which is not inferior to that of other conventional calibrated CGM devices8. Second, we did not collect information that might affect the duration of hypoglycemia, such as dietary and exercise habit, alcohol intake or socioeconomic status. Third, given that the present study was carried out at diabetes units of core hospitals of the community, it is unknown whether the current results are applicable to general patients cared for by general practitioners. Finally, whereas we recruited 300 patients, which is our predetermined sample size, we could only analyze the data of 269 patients because of the loss and the inappropriate length (<7 days) of available data. The sample size of 269 is, however, larger than the required number of patients with the statistical power of 75% (250).
We have shown for the first time that the duration of hypoglycemia was inversely correlated with HbA1c level in patients with type 2 diabetes treated with sulfonylureas. Healthcare providers, as well as patients themselves, should thus pay careful attention to the possibility of hypoglycemia development during treatment with sulfonylureas.
Disclosure
YH has received lecture fees from Eli Lilly, Sanofi and Takeda Pharmaceutical. YO has received lecture fees from Sanofi. KS has received research support from Astellas, AstraZeneca and Novo Nordisk Pharma, as well as lecture fees from Boehringer Ingelheim, Dainippon‐Sumitomo Pharma, Eli Lilly, Mitsubishi Tanabe Pharma, MSD, Novartis, Novo Nordisk Pharma and Sanofi. WO has received research support from Abbot, Astellas, Boehringer Ingelheim, Chugai Pharmaceutical, Dainippon‐Sumitomo Pharma, Daiichi Sankyo, Kyowa Kirin, Mitsubishi Tanabe Pharma, MSD, Novartis, Novo Nordisk Pharma, Ono Pharmaceutical, Sanofi, Taisho Toyama Pharmaceutical, Takeda Pharmaceutical and Teijin Pharma, as well as lecture fees from Abbot, Astellas, Boehringer Ingelheim, Dainippon‐Sumitomo Pharma, Mitsubishi Tanabe Pharma, MSD, Novartis and Takeda Pharmaceutical. All other authors declare no conflict of interest.
Acknowledgments
We thank Yoshiaki Kido, Tetuya Hosooka, Shun‐ichiro Asahara, Kazuhiro Nomura, Yasunori Fujita, Youichi Sakamoto, Kenji Sugawara, Tomoko Yamada, Hiroshi Miura and Yusei Hosokawa of Kobe University Hospital; Yuka Ooi, Masayuki Kanatani and Hirotaka Suzuki of Rokko Island Konan Hospital; Shin Urai and Michinori Takabe of Hyogo Brain and Heart Center; Hideyuki Fukunaga, Masahide Iwai, Makoto Koide, Sachiko Kimura, Sayaka Kashiwagi and Shinsuke Nakajima of Nishiwaki Municipal Hospital; and Yuko Koketsu and Kunie Kimata of Shinko Hospital for recruitment of patients to the study. We also thank all other investigators and participants. This study was supported by a grant from the alumni association of The Second Department of Internal Medicine, Kobe University School of Medicine, to YH.
J Diabetes Investig 2020; 11: 417–425
Clinical Trial Registry
UMIN Clinical Trials Registry
UMIN000025284
References
- 1. Feinkohl I, Aung PP, Keller M, et al Severe hypoglycemia and cognitive decline in older people with type 2 diabetes: the Edinburgh type 2 diabetes study. Diabetes Care 2014; 37: 507–515. [DOI] [PubMed] [Google Scholar]
- 2. Zhao Y, Campbell CR, Fonseca V, et al Impact of hypoglycemia associated with antihyperglycemic medications on vascular risks in veterans with type 2 diabetes. Diabetes Care 2012; 35: 1126–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goto A, Arah OA, Goto M, et al Severe hypoglycaemia and cardiovascular disease: systematic review and meta‐analysis with bias analysis. BMJ 2013; 347: f4533. [DOI] [PubMed] [Google Scholar]
- 4. Hay LC, Wilmshurst EG, Fulcher G. Unrecognized hypo‐ and hyperglycemia in well‐controlled patients with type 2 diabetes mellitus: the results of continuous glucose monitoring. Diabetes Technol Ther 2003; 5: 19–26. [DOI] [PubMed] [Google Scholar]
- 5. van Dijk P, Bouma A, Landman GW, et al Hypoglycemia in frail elderly patients with type 2 diabetes mellitus treated with sulfonylurea. J Diabetes Sci Technol 2017; 11: 438–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Middleton TL, Wong J, Molyneaux L, et al Cardiac effects of sulfonylurea‐related hypoglycemia. Diabetes Care 2017; 40: 663–670. [DOI] [PubMed] [Google Scholar]
- 7. Currie CJ, Peters JR, Tynan A, et al Survival as a function of HbA(1c) in people with type 2 diabetes: a retrospective cohort study. Lancet 2010; 375: 481–489. [DOI] [PubMed] [Google Scholar]
- 8. Rodbard D. Continuous glucose monitoring: a review of successes, challenges, and opportunities. Diabetes Technol Ther 2016; 18(Suppl 2): 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schiaffini R, Ciampalini P, Fierabracci A, et al The Continuous Glucose Monitoring System (CGMS) in type 1 diabetic children is the way to reduce hypoglycemic risk. Diabetes Metab Res Rev 2002; 18: 324–329. [DOI] [PubMed] [Google Scholar]
- 10. Chico A, Vidal‐Ríos P, Subirà M, et al The continuous glucose monitoring system is useful for detecting unrecognized hypoglycemias in patients with type 1 and type 2 diabetes but is not better than frequent capillary glucose measurements for improving metabolic control. Diabetes Care 2003; 26: 1153–1157. [DOI] [PubMed] [Google Scholar]
- 11. Chow E, Bernjak A, Williams S, et al Risk of cardiac arrhythmias during hypoglycemia in patients with type 2 diabetes and cardiovascular risk. Diabetes 2014; 63: 1738–1747. [DOI] [PubMed] [Google Scholar]
- 12. Graveling AJ, Frier BM. The risks of nocturnal hypoglycaemia in insulin‐treated diabetes. Diabetes Res Clin Pract 2017; 133: 30–39. [DOI] [PubMed] [Google Scholar]
- 13. Bailey T, Bode BW, Christiansen MP, et al The performance and usability of a factory‐calibrated flash glucose monitoring system. Diabetes Technol Ther 2015; 17: 787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haak T, Hanaire H, Ajjan R, et al Flash glucose‐sensing technology as a replacement for blood glucose monitoring for the management of insulin‐treated type 2 diabetes: a multicenter, open‐babel randomized controlled trial. Diabetes Ther 2017; 8: 55–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hill NR, Oliver NS, Choudhary P, et al Normal reference range for mean tissue glucose and glycemic variability derived from continuous glucose monitoring for subjects without diabetes in different ethnic groups. Diabetes Technol Ther 2011; 13: 921–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Monnier L, Colette C, Wojtusciszyn A, et al Toward defining the threshold between low and high glucose variability in diabetes. Diabetes Care 2017; 40: 832–838. [DOI] [PubMed] [Google Scholar]
- 17. Monami M, Dicembrini I, Kundisova L, et al A meta‐analysis of the hypoglycaemic risk in randomized controlled trials with sulphonylureas in patients with type 2 diabetes. Diabetes Obes Metab 2014; 16: 833–840. [DOI] [PubMed] [Google Scholar]
- 18. Tsujino D, Nishimura R, Onda Y, et al The relationship between HbA1c values and the occurrence of hypoglycemia as assessed by continuous glucose monitoring in patients with type 1 diabetes. Diabetol Metab Syndr 2016; 8: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pazos‐Couselo M, García‐López JM, González‐Rodríguez M, et al High incidence of hypoglycemia in stable insulin‐treated type 2 diabetes mellitus: continuous glucose monitoring vs. self‐monitored blood glucose. Observational prospective study. Can. J Diabetes 2015; 39: 428–433. [DOI] [PubMed] [Google Scholar]
- 20. Ratzki‐Leewing A, Harris SB, Mequanint S, et al Real‐world crude incidence of hypoglycemia in adults with diabetes: results of the InHypo‐DM Study, Canada. BMJ Open Diabetes Res Care 2018; 6: e000503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bae JP, Duan R, Fu H, et al Risk factors for nocturnal hypoglycemia in insulin‐treated patients with type 2 diabetes: a secondary analysis of observational data derived from an integrated clinical trial database. Clin Ther 2017; 39: 1790–1798. [DOI] [PubMed] [Google Scholar]
- 22. Yu O, Azoulay L, Yin H, et al Sulfonylureas as initial treatment for type 2 diabetes and the risk of severe hypoglycemia. Am J Med 2018; 131: 317.e11–317.e22. [DOI] [PubMed] [Google Scholar]
- 23. Schernthaner G, Schernthaner‐Reiter MH. Diabetes in the older patient: heterogeneity requires individualisation of therapeutic strategies. Diabetologia 2018; 61: 1503–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kalra S, Deepak MC, Narang P, et al Usage pattern, glycemic improvement, hypoglycemia, and body mass index changes with sulfonylureas in real‐life clinical practice: results from OBSTACLE Hypoglycemia Study. Diabetes Technol Ther 2013; 15: 129–135. [DOI] [PubMed] [Google Scholar]
- 25. Tschöpe D, Bramlage P, Binz C, et al Incidence and predictors of hypoglycaemia in type 2 diabetes—an analysis of the prospective DiaRegis registry. BMC Endocr Disord 2012; 12: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schloot NC, Haupt A, Schütt M, et al Risk of severe hypoglycemia in sulfonylurea‐treated patients from diabetes centers in Germany/Austria: how big is the problem? Which patients are at risk? Diabetes Metab Res Rev 2016; 32: 316–324. [DOI] [PubMed] [Google Scholar]


