Abstract
Wildlife interaction with humans increases the risk of potentially infected ticks seeking an opportunistic blood meal and consequently leading to zoonotic transmission. In the United States, human babesiosis is a tick-borne zoonosis most commonly caused by the intraerythrocytic protozoan parasite, Babesia microti. The presence of Babesia microti and other species of Babesia within Texas has not been well characterized, and the molecular prevalence of these pathogens within wildlife species is largely unknown. Small (e.g. rodents) and medium sized mammalian species (e.g. racoons) represent potential reservoirs for specific species of Babesia, though this relationship has not been thoroughly evaluated within Texas. This study aimed to characterize the molecular prevalence of Babesia species within small and medium sized mammals at two sites in East Texas with an emphasis on detection of pathogen presence in these two contrasting wild mammal groups at these sites. To that end, a total of 480 wild mammals representing eight genera were trapped, sampled, and screened for Babesia species using the TickPath layerplex qPCR assay. Two sites were selected for animal collection, including The Big Thicket National Preserve and Gus Engeling Wildlife Management Area. Molecular analysis revealed the prevalence of various Babesia and Hepathozoon species at 0.09% each, and Sarcocystis at 0.06% . Continued molecular prevalence surveys of tick-borne pathogens in Texas wild mammals will be needed to provide novel information as to which species of Babesia are most prevalent and identify specific wildlife species as pathogen reservoirs.
Keywords: Babesia microti, Babesia spp., Hepatozoon spp., Mesocarnivores, Sarcocystis spp., Small-mammals
Graphical abstract
Highlights
-
•
Babesia microti was identified for the first time in Texas and only within raccoons.
-
•
3 Hepatozoon spp. were found within Texas wild mammals.
-
•
Co-infections of Babesia microti, Babesia spp. and Hepatozoon spp. were detected in sampled mammals.
1. Introduction
Within the United States, human babesiosis is primarily caused by the piroplasm Babesia microti (Baniecki et al., 2019). In contrast, the causative agents of babesiosis parasitizing wildlife (e.g. raccoons, opossums, rodents) can vary drastically and encompass multiple species (Yabsley and Shock, 2013). At this time, the only proven competent vector of B. microti in the U.S. is the black-legged tick Ixodes scapularis (Nelder et al., 2016). Transmission of other Babesia species occur mostly through tick vectors or direct contact with blood (Clark et al., 2012). The pathogenic landscape of Babesia species within Texas wildlife species has not been fully explored, leaving the zoonotic concern and role of wildlife in the Babesia transmission cycle unclear. As more habitats shift from sylvan to disturbed environments, human-wildlife interaction will continue to increase, subsequently leading to an increased potential for Babesia-related zoonoses (Goethert et al., 2018).
Other tick-borne piroplasms, such as species within the Hepatozoon genus, have also been noted to circulate within wildlife of southern states (Cummings et al., 2005). North American hepatozoonosis was first identified in Texas, which included reports of the organism circulating in raccoons and white-tailed deer of Texas (Clark et al., 1973). Hepatozoonosis has now been reported in several other states where recent surveillance reports have identified additional novel host specific species of Hepatozoon parasitizing wildlife that differ from previously known Piroplasmida parasites of wildlife (e.g. Hepatozoon procyonis) (Allen et al., 2011).
While infection with hepatozoonosis is also reliant on a tick vector, transmission occurs through tick ingestion rather than a result of tick feeding (Allen et al., 2011). Amblyomma americanum ticks are associated with Hepatozoon canis infection in dogs, though tick species responsible for transmitting non-canine hepatozoonosis have not yet been fully uncovered (Allen et al., 2011). However, it is important to note that A. americanum ticks have been collected from wildlife species such as raccoons in the past, and may represent a viable tick vector for other species of Hepatozoon (Allen et al., 2011).
Past surveillance studies aimed at characterizing Hepatozoon species within wildlife have been conducted in southern states such as Oklahoma, Texas, Georgia, and Missouri (Allen et al., 2011), though corroborating surveillance studies in these southern states have not yet been conducted. Notably, the study in Oklahoma by Allen and colleagues presented numerous novel Hepatozoon species circulating within various wildlife species. Although Hepatozoon species have been detected within wildlife in past studies, the genus is ultimately under-studied and lacks proper classification, leading to a majority of detected isolates referred to with host specific nomenclature (e.g. Hepatozoon sp. ex Procyon lotor) (Allen et al., 2011).
This study aimed to evaluate the molecular prevalence of Babesia species and other parasitic protozoan species responsible for hepatozoonosis in medium and small sized mammals from Texas. To our knowledge, this is the first study of its kind in Texas and can provide baseline data for future research and surveillance studies on this group of parasites.
2. Materials and methods
2.1. Ethical statement
All animal related procedures were performed and approved in accordance with the Institutional Biosafety Committee (IBC 2013-039 and 2016–051) and the Institutional Animal Care and Use Committee (IACUC) at Texas A&M University as detailed in the Animal Use Protocols (AUP 2014–0227 and 2016–0243). In addition, the collections were performed under Texas State University San Marcos IACUC permit AUP#201598223. Furthermore, collections were approved by the Big Thicket National Preserved under the scientific research and collecting permit BITH-2015-SCI-0016 and the Department of Texas Parks and Wildlife scientific permit SPR-1112-1052. The animals were maintained in an animal facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) during tick collection and euthanasia.
2.2. Sample collections
As part of larger study of tick-borne pathogens two areas in east Texas were selected based on suitable habitat for the black legged tick I. scapularis (Feria-Arroyo et al., 2014). Gus Engeling Wildlife Management Area was selected as the study site to represent the Post Oak Savannah ecoregion (UTM Zone 15 R 227000 3535000), and Big Thicket National Preserve was selected to represent the Piney Woods ecoregion (UTM Zone 15 R 352391 3374283). Two distinct groups (guilds) of wild mammals conformed the potential capture species that varied in size, diet, and home range. Three of the species, are referred here as medium sized mammals based on their moderate body size (1 Kg - 20 Kg), omnivorous to carnivorous diet, and large home range (>1 Km2). The remaining 10 mammal species, referred together as small mammals, consisted of species of small body size (<1 Kg), small home range (<1Km2), and granivorous, insectivorous, or frugivorous diets.
Sampling occurred over two trapping seasons: October 2015–January 2016, and December 2016–April 2017. Each site was sampled for small and medium sized mammals 6 and 8 times during the first and second seasons, respectively. Small mammals were captured with Sherman live traps (Model: LFATDG; Sherman Traps, Inc., Tallahassee, FL). During each sampling 400 traps, placed 5–10 m apart, were set in a curvilinear transect. Traps were set 1–2 h prior to dusk and checked the following morning within 2 h of sunrise. Weather conditions permitting, traps were re-baited with rolled oats and peanut butter and remained open during the diurnal hours dependent upon ambient temperatures. Similarly, meso-mammals were captured with Tomahawk live traps (sizes 20 × 7 × 7 inches and 32 × 10 × 12 inches; Tomahawk Live Trap Inc., Hazelhurst, WI) set in a curvilinear transect of 50 traps, placed 100 m apart, at each site. Tomahawk traps were baited with one of the following attractants: sardines, wet/dry cat food, boiled eggs, and marshmallows. Both sets of transects were ran for 3 nights, until cage availability ran out for meso-mammals (n = 5), or until permitted take limitations were reached. Total trapping effort consisted of 14,690 Sherman trap-nights and 1,460 Tomahawk trap-nights (Milholland et al., 2017).
Mammal trap mortalities from the field were processed on site. Tissue samples were aseptically removed, stored separately, and transferred to long-term storage (−80 °C). Tissues collected included the spleen, liver, kidney, heart, lung, bladder, and articulating joint.
A total of 480 heart and spleen samples were collected from 53 medium (15 raccoons, 37 opossums, 1 gray fox) and 427 small sized mammals that included: 209 cotton mice (Peromyscus gossypinus); 73 eastern woodrats (Neotoma floridana); 59 golden mice (Ochrotomys nuttalli); 28 white-footed mice (P. leucopus); 23 fulvous harvest mice (Reithrodontomys fulvescens); 20 hispid cotton rats (Sigmodon hispidus); 12 North American least shrews (Cryptotis parva); 1 pygmy mouse (Baiomys taylori); 1 rice rat (Oryzomys palustris) and 1 southern flying squirrel (Glaucomys volans).
2.3. DNA extractions and PCR analysis
DNA was extracted from frozen tissue samples using High Pure PCR Template Preparation Kit (Roche) and stored at −20 °C until PCR analysis. The samples were screened for the presence of Babesia species and closely related species pathogen DNA using the patent pending (Patent Application Serial No. 16/130,177) TickPath layerplex real-time PCR (qPCR) assay as described previously (Modarelli et al., 2019) with modifications to adapt the protocol for use in the CFX96 Touch™ Real-Time PCR Detection System (Bio-Rad Laboratories). The specificity and sensitivity of this technology to detect Babesial protozoans was 100% (47.8–100%) and 100% (99.7–100%) respectively. The following PCR conditions were used: an initial denaturation at 95 °C for 3 min, followed by 40 cycles of denaturation at 95 °C for 10 s and annealing-extension at 60 °C for 45 s.
Species within the Apicomplexa phylum detected by layerplex qPCR analysis were compared with those obtained by conventional PCR by utilizing primers RLB-F and RLB-R to amplify a 460 to 520 bp fragment of the 18S rRNA V4 gene region as described previously (Gubbels et al., 1999). Positive (genomic) controls and negative controls (water) were included in all PCR assays. Consensus sequences obtained by Sanger sequencing (Eurofins Scientific, Louisville, KY) were evaluated with CLC Main Workbench (CLCbio, Aarhus, Denmark) using Maximum Likelihood analysis and the GTR substitution model, and compared with published sequences on the National Center for Biotechnology Information (NCBI) database using the Basic Local Alignment Search Tool (BLAST). All DNA extractions and PCR reactions were prepared and performed under GLP conditions to avoid potential cross-contamination among tested samples. Sequences of species detected in this study were deposited in GenBank® under the accession numbers listed in Table 1.
Table 1.
Molecular detection of Babesia, Hepatozoon, and Sarcocystis spp. in tissues of medium and small mammals. No. refers to the number of animals in which a particular pathogen was detected in. Note that some animals were co-infected with more than one pathogen.
| Species | No. | Pathogens | GenBank® Code |
|---|---|---|---|
| Raccoon | 1 |
Babesia microti, Babesia sp. Coco |
MN011931, MN013190 |
| Raccoon | 1 | Babesia microti | MN011933 |
| Raccoon | 1 |
Babesia microti, Babesia sp. AJB-2006 |
MN011935, MN013191 |
| Raccoon | 2 |
Babesia microti, Hepatozoon sp. ex Procyon lotor |
MN011932, MN011934, MN012926-2927 |
| Raccoon | 3 | Hepatozoon sp. ex Procyon lotor | MN012928-2930 |
| Cotton mouse | 2 | Hepatozoon sp. ex Peromyscus leucopus | MN012924-2925 |
| Eastern woodrat | 1 | Hepatozoon sp. ex Sigmodon hispidus | MN012931 |
| Opossums | 3 | Sarcocystis sp. Didelphis virginiana | MN013159-3169 |
Samples tested for the presence of Babesia, Hepatozoon, and Sarcocystis provided binomial data (positive or negative) presented as a proportion of the subset sampled. Using this data we calculated prevalence with Jeffreys Confidence Interval and an alpha value of 0.05 using the package “prevalence” (v0.0.4.0) in R. Although samples come from two trapping sessions, we pooled those together to represent a point prevalence in time. Given the exploratory nature of our study and that we have no indications that major ecological or climatic changes happened between sampling sessions it is reasonable to pool samples for prevalence estimations.
3. Results
A total of 480 heart and spleen samples collected from 53 medium and 427 small sized mammals were tested for parasitic protozoan species. The distribution of positives by site for each of the parasites among each wild mammal species was highly heterogeneous, with Babesia and Sarcocystis only present in a single species of medium sized mammal each (racoons and virgina opossums, respectively) whereas Hepatozoon was detected in a single species of medium sized mammal (racoons) and two species (cotton mice and eastern wood rats) of small mammals (Table 2, Table 3, Table 4). The Gus Engeling WMA had more positives among all parasitic protozoan species for both medium sized mammals (9) and small mammals (3) in contrast to the Big Thicket National Preserve that had 4 positive medium sized mammals (Table 2, Table 3, Table 4). Based on molecular analysis of all the medium sized mammals, 33.3% (5/15) raccoons were positive for Babesia microti and 20% (3/15) for a Hepatozoon sp. that matches a strain found within raccoons from Oklahoma (Allen et al., 2011). Coinfections were observed in 26.7% (4/15) of the raccoons, comprised of B. microti paired with another species of Babesia or Hepatozoon (Table 1). Within small mammals, only two distinct species of Hepatozoon were detected within two cotton mice and a single eastern woodrat (Table 1). Besides the detection of Sarcocystis sp. in three opossums, Apicomplexa DNA was not detected in any other medium or small mammal species.
Table 2.
Babesia prevalence, with Jefferys confidence intervals (Jeff. C.I.), among medium sized mammals sampled from two sites in eastern Texas (Gus Engeling WMA and Big Thicket National Preserve). Additionally, 427 small mammals from 10 species from these sites were tested but no positives were detected. For a distribution of sample sizes tested per species and site see Table 2. Positives columns represent numbers of infected individuals (outside parenthesis) and total sample size (n, in parenthesis).
|
Species |
Overall |
Gus Engeling WMA |
Big Thicket National Preserve |
|||
|---|---|---|---|---|---|---|
| positives (n) | Prevalence (Jeff. C.I.) | positives (n) | Prevalence (Jeff. C.I.) | positives (n) | Prevalence (Jeff. C.I.) | |
| Didelphis virginiana | 0 (37) | 0.00 (0.00–0.05) | 0 (11) | 0.00 (0.00–0.16) | 0 (26) | 0.00 (0.00–0.07) |
| Procyon lotor | 5 (15) | 0.33 (0.14–0.58) | 5 (12) | 0.42 (0.18–0.69) | 0 (3) | 0.00 (0.00–0.44) |
| Urocyon cinereoargeneus | 0 (1) | 0.00 (0.00–0.77) | – | – | 0 (1) | 0.00 (0.00–0.77) |
| Overall | 5 (53) | 0.09 (0.04–0.19) | 5 (23) | 0.22 (0.09–0.41) | 0 (30) | 0.00 (0.00–0.06) |
Table 3.
Hepatozoon prevalence, with Jefferys confidence intervals (Jeff. C.I.), among medium and small sized mammals sampled from two sites in eastern Texas (Gus Engeling WMA and Big Thicket National Preserve). Positives columns represent numbers of infected individuals (outside parenthesis) and total sample size (n, in parenthesis).
| Species | Overall |
Gus Engeling WMA |
Big Thicket National Preserve |
||||
|---|---|---|---|---|---|---|---|
| positives (n) | Prevalence (Jeff. C.I.) | positives (n) | Prevalence (Jeff. C.I.) | positives (n) | Prevalence (Jeff. C.I.) | ||
| Medium sized mammal species | Didelphis virginiana | 0 (37) | 0.00 (0.00–0.05) | 0 (11) | 0.00 (0.00–0.16) | 0 (26) | 0.00 (0.00–0.07) |
| Procyon lotor | 5 (15) | 0.33 (0.14–0.58) | 3 (12) | 0.25 (0.08–0.53) | 2 (3) | 0.67 (0.18–0.96) | |
| Urocyon cinereoargeneus | 0 (1) | 0.00 (0.00–0.77) | – | – | 0 (1) | 0.00 (0.00–0.77) | |
| Overall | 5 (53) | 0.09 (0.04–0.19) | 3 (23) | 0.13 (0.04–0.31) | 2 (30) | 0.07 (0.01–0.20) | |
| Small mammal species | Baiomys taylorii | 0 (1) | 0.00 (0.00–0.77) | – | – | 0 (1) | 0.00 (0.00–0.77) |
| Cryptotis parva | 0 (12) | 0.00 (0.00–0.15) | – | – | 0 (12) | 0.00 (0.00–0.15) | |
| Glaucomys volans | 0 (1) | 0.00 (0.00–0.77) | 0 (1) | 0.00 (0.00–0.77) | – | – | |
| Neotoma floridana | 1 (73) | 0.01 (0.00–0.06) | 1 (46) | 0.02 (0.00–0.10) | 0 (27) | 0.00 (0.00–0.07) | |
| Ochrotomys nuttalli | 0 (59) | 0.00 (0.00–0.03) | 0 (32) | 0.00 (0.00–0.06) | 0 (27) | 0.00 (0.00–0.07) | |
| Oryzomys texicanii | 0 (1) | 0.00 (0.00–0.77) | 0 (1) | 0.00 (0.00–0.77) | – | – | |
| Peromyscus gossypinus | 2 (209) | 0.01 (0.00–0.03) | 2 (83) | 0.02 (0.01–0.08) | 0 (126) | 0.00 (0.00–0.02) | |
| Peromyscus leucopus | 0 (28) | 0.00 (0.00–0.07) | 0 (16) | 0.00 (0.00–0.11) | 0 (12) | 0.00 (0.00–0.15) | |
| Reithrodontomys fulvescens | 0 (23) | 0.00 (0.00–0.08) | 0 (14) | 0.00 (0.00–0.13) | 0 (9) | 0.00 (0.00–0.19) | |
| Sigmodon hispidus | 0 (20) | 0.00 (0.00–0.09) | 0 (11) | 0.00 (0.00–0.16) | 0 (9) | 0.00 (0.00–0.19) | |
| Overall | 3 (427) | 0.01 (0.00–0.02) | 3 (204) | 0.01 (0.00–0.03) | 0 (223) | 0.00 (0.00–0.01) | |
Table 4.
Sarcocystis prevalence, with Jefferys confidence intervals (Jeff. C.I.), among medium sized mammals sampled from two sites in eastern Texas (Gus Engeling WMA and Big Thicket National Preserve). Additionally, 427 small mammals from 10 species from these sites were tested but no positives were detected. For a distribution of sample sizes tested per species and site see Table 2. Positives columns represent numbers of infected individuals (outside parenthesis) and total sample size (n, in parenthesis).
|
Species |
Overall |
Gus Engeling WMA |
Big Thicket National Preserve |
|||
|---|---|---|---|---|---|---|
| positives (n) | Prevalence (Jeff. C.I.) | positives (n) | Prevalence (Jeff. C.I.) | positives (n) | Prevalence (Jeff. C.I.) | |
| Didelphis virginiana | 3 (37) | 0.08 (0.02–0.20) | 1 (11) | 0.09 (0.01–0.35) | 2 (26) | 0.08 (0.02–0.22) |
| Procyon lotor | 0 (15) | 0.00 (0.00–0.12) | 0 (12) | 0.00 (0.00–0.15) | 0 (3) | 0.00 (0.00–0.44) |
| Urocyon cinereoargeneus | 0 (1) | 0.00 (0.00–0.77) | – | – | 0 (1) | 0.00 (0.00–0.77) |
| Overall | 3 (53) | 0.06 (0.02–0.14) | 1 (23) | 0.04 (0.00–0.19) | 2 (30) | 0.07 (0.01–0.20) |
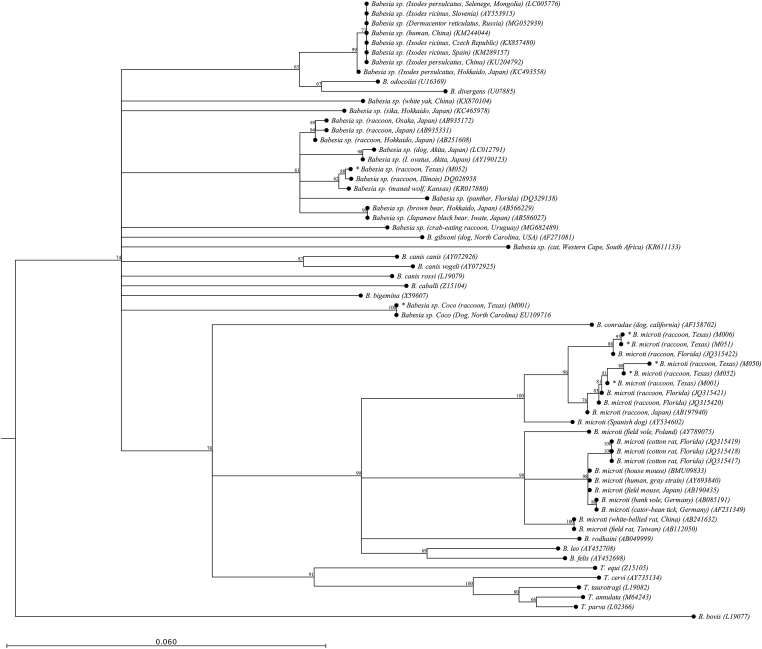
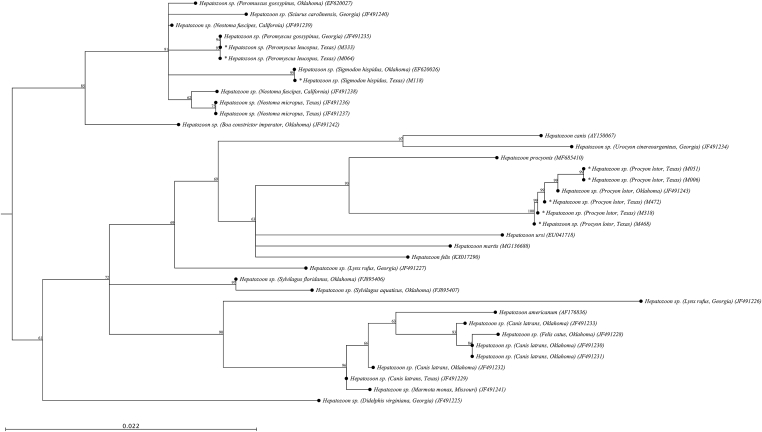
A total of 18 sequences of partial 18S rRNA gene (~500 bp) of various Apicomplexa species were analyzed. Babesia microti sequences from raccoons (GenBank® accessions MN011931-1935) showed 99–100% similarity with B. microti sequences derived from raccoons in Florida (GenBank® accession number JQ315422) (Clark et al., 2012). Therefore, we kept the same name observed in GenBank® since they sequence cluster in the same branch. Additional Babesia spp. detected in raccoons (GenBank® accessions MN013191 and MN013190) indicated 99 and 100% similarity to Babesia sp. ex. Procyon lotor from a raccoon in Illinois (GenBank® accession DQ028958) and Babesia sp. Coco (GenBank® accession EU109716) from a dog in North Carolina, respectively (Birkenheuer et al., 2006; Lehtinen et al., 2008). Hepatozoon sp. detected in raccoons (GenBank® accessions MN012926-2930) showed 100% similarity to strains of Hepatozoon sp. from raccoons in Oklahoma (GenBank® accession JF491243) (Allen et al., 2011). Comparable similarity analysis was observed from Sarcocystis sp. detected in opossums (GenBank® accessions MN013159-3161) matching 99–100% to strains from opossums in Mexico (GenBank ® accession KX470742-44) (Gallo et al., 2018). Finally, species of Hepatozoon detected in cotton mice and an eastern woodrat (GenBank® accessions MN012924–2925 and MN012931, respectively) matched 100% to strains from cotton rats and white-footed mice in Oklahoma (GenBank® accession EF620026) (Allen et al., 2011). Phylogenetic analysis of Babesia and Hepatozoon species sequences demonstrated grouping consistent with host species and geographic sampling locations (Fig. 1, Fig. 2).
Fig. 1.
Phylogenetic tree of 18S rRNA gene sequence alignments for Babesia spp. purified from this study (*) and relevant host species extracted from GenBank®. In parenthesis are referenced all the GenBank® accession numbers of the sequences used to generate this alignment. As a note, the Babesia Spanish Dog isolate (AY534602) is now reffered to as B. vulpes. Babesia microti -like sequences from this study: M001 = GenBank® MN011931; M006 = GenBank® MN011932; M050 = GenBank®MN011933; M051 = GenBank® MN011934; M052 = GenBank® MN011935. Babesia sp Coco in this study M001 = GenBank® MN013190. Babesia sp: M052 = GenBank® MN013191.
Fig. 2.
Phylogenetic tree of 18S rRNA gene sequence alignments for Hepatozoon spp. purified from this study (*) and relevant host species extracted from GenBank®. In parenthesis are referenced all the GenBank® accession numbers of the sequences used to generate this tree. Hepatozoon sp. Sequences detected in this study: M064 = GenBank® MN012924; M333 = GenBank® MN012925; M006 = GenBank® MN012926; M051 = GenBank® MN012927; M318 = GenBank® MN012928; M468 = GenBank® MN012929; M472 = GenBank® MN012930; M118 = GenBank® MN012931.
4. Discussion
The emergence of babesiosis is a threat to humans, though the influence wildlife species may have on this relationship remains unelucidated in southern U.S. (Yabsley and Shock, 2013). Surveillance studies focusing on babesiosis in small and medium sized wildlife species in Florida revealed the presence of 2 distinct groups of Babesia microti dependent on host species (i.e. cotton rats and raccoons) (Clark et al., 2012). As Florida and Texas have been shown to share similar tick-competent ecologies based on past simulations of other tick species (Donaldson et al., 2016), it was predicted that similar wildlife species may be involved in the babesiosis life cycle.
This study revealed that B. microti was found only in raccoons within the areas sampled. As depicted in Fig. 1, DNA sequence analysis revealed that the B. microti strains resembled Florida raccoon strains as well as racoons from northern U.S. (Birkenheuer et al., 2006) and Japan (Hirata, unpubl.). It is important to note that this study revealed the first molecular identification of B. microti in Texas, and the zoonotic concern of these findings should be considered. Coinfections with B. microti in raccoons were also detected and included two different Babesia species, Babesia sp. Coco (GenBank® EU109716) and a Babesia species that most closely resembles Babesia sp. AJB-2006 (GenBank® DQ028958). Originally detected in a raccoon in Illinois (Birkenheuer et al., 2006), Babesia sp. AJB-2006 has since been detected in a maned wolf (Chrysocyon brachyurus) in a Kansas zoo (99.59% match) (Phair et al., 2012), black bears (Ursus americanus) in North Carolina (sequences unavailable for analysis) (Westmoreland et al., 2019), and now raccoons in Texas (99.79% match) as depicted in Table 1. Interestingly, the species 18S rRNA sequence also resembles Babesia species detected in Ixodes ovatus ticks (99.17% match) and dogs (98.97% match) from Akita, Japan (Inokuma et al., 2003), and a more distant match to a panther (Puma concolor) in Florida (97.73% match) (Yabsley et al., 2006). Sequence analysis of candidatus Babesia sp. AJB-2006, in respect to available host data, indicates that the organism may be a nondiscriminatory wildlife species. Though the overall pathogenicity of the organism is currently unclear, it has been shown to be pathogenic in the aforementioned Illinois raccoon and Kansas maned wolf case studies. Of further interest, the organism has demonstrated a broad geographical distribution, ranging from northeastern to southeastern U.S. states and reports in Japan.
Additional B. microti coinfections were detected alongside species specific raccoon strains of Hepatozoon. As depicted in Fig. 2, all detected Hepatozoon sp. from raccoons were genetically similar to strains detected in Oklahoma, though this study reports the first coinfection of Hepatozoon and B. microti in raccoons. While hepatozoonosis of wildlife is generally considered nonpathogenic, coinfections with species of Babesia may introduce complications to the host. Cotton mouse and eastern woodrat strains of Hepatozoon sp. detected in this study match those reported previously in Oklahoma and corroborate the geographical locale of the wildlife species strains. Sarcocystis species detected in opossums in this study matched those reported in Mexico within the same species, and at this time are not described to pose zoonotic risk (Gallo et al., 2018). As the main aim of this study was to provide surveillance data for Babesia species within wildlife, future studies should aim to further characterize the prevalence of other Apicomplexa species with assays that are specific to each of the genera of interest.
While the overall prevalence of Babesia, Hepatozoon, and Sarcocystis species was low in the samples screened (0.09%, 0.09% and 0.06%, respectively), it is important to note the high number of B. microti in sampled Texas raccoons (5/15). The prevalence of B. microti highlights the potential role raccoons may have as reservoirs for the pathogen. This study also represents the first report of B. microti in Texas, and further studies should aim to characterize the prevalence of the pathogen in other wildlife species that may intersect with the human population, and subsequently determine the zoonotic risk of the strain. However, as was established in the 2012 study of B. microti raccoon specific strains in Florida, no human infections have been caused by the raccoon strains to date (Clark et al., 2012). Though, public health agencies should still monitor for the risk of human babesiosis in this area due to the established presence of the competent tick vector Ixodes scapularis (Feria-Arroyo et al., 2014).
The sampling sites for this study were selected based on prior studies indicating high prevalence of the I. scapularis tick vector of interest (Feria-Arroyo et al., 2014). Therefore, this study provided potentially biased surveillance data of Apicomplexa species in an area that may not be representative of the state of Texas as a whole. Future studies aiming to build upon findings in this study should include sampling ticks collected alongside wildlife specimens in additional wildlife areas.
Declaration of competing interest
The authors declare that they have no conflict of interest in the research presented in this study.
Acknowledgments
We thank Dr. Castro-Arellano undergraduate and graduate students volunteers for their assistance during animal collections and harvesting tissue samples. This work was supported by the United States National Institutes of Health [grant number 1R21AI107380-01A1].
References
- Allen K.E., Yabsley M.J., Johnson E.M., Reichard M.V., Panciera R.J., Ewing S.A., Little S.E. Novel Hepatozoon in vertebrates from the southern United States. J. Parasitol. 2011;97:648–653. doi: 10.1645/GE-2672.1. [DOI] [PubMed] [Google Scholar]
- Baniecki M.L., Moon J., Sani K., Lemieux J.E., Schaffner S.F., Sabeti P.C. Development of a SNP barcode to genotype Babesia microti infections. PLoS Neglected Trop. Dis. 2019;13 doi: 10.1371/journal.pntd.0007194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkenheuer A.J., Whittington J., Neel J., Large E., Barger A., Levy M.G., Breitschwerdt E.B. Molecular characterization of a Babesia species identified in a North American raccoon. J. Wildl. Dis. 2006;42:375–380. doi: 10.7589/0090-3558-42.2.375. [DOI] [PubMed] [Google Scholar]
- Clark K., Savick K., Butler J. Babesia microti in rodents and raccoons from northeast Florida. J. Parasitol. 2012;98:1117–1121. doi: 10.1645/GE-3083.1. [DOI] [PubMed] [Google Scholar]
- Clark K.A., Robinson R.M., Weishuhn L.L., Galvin T.J., Horvath K. Hepatozoon procyonis infections in Texas. J. Wildl. Dis. 1973;9:182–193. doi: 10.7589/0090-3558-9.2.182. [DOI] [PubMed] [Google Scholar]
- Cummings C.A., Panciera R.J., Kocan K.M., Mathew J.S., Ewing S.A. Characterization of stages of Hepatozoon americanum and of parasitized canine host cells. Vet. Pathol. 2005;42:788–796. doi: 10.1354/vp.42-6-788. [DOI] [PubMed] [Google Scholar]
- Donaldson T.G., Perez de Leon A.A., Li A.Y., Castro-Arellano I., Wozniak E., Boyle W.K., Hargrove R., Wilder H.K., Kim H.J., Teel P.D., Lopez J.E. Assessment of the geographic distribution of Ornithodoros turicata (Argasidae): climate variation and host diversity. PLoS Neglected Trop. Dis. 2016;10 doi: 10.1371/journal.pntd.0004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feria-Arroyo T.P., Castro-Arellano I., Gordillo-Perez G., Cavazos A.L., Vargas-Sandoval M., Grover A., Torres J., Medina R.F., de Leon A.A., Esteve-Gassent M.D. Implications of climate change on the distribution of the tick vector Ixodes scapularis and risk for Lyme disease in the Texas-Mexico transboundary region. Parasites Vectors. 2014;7:199. doi: 10.1186/1756-3305-7-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo S.S.M., Lindsay D.S., Ederli N.B., Matteoli F.P., Venancio T.M., de Oliveira F.C.R. Identification of opossums Didelphis aurita (Wied-Neuweid, 1826) as a definitive host of Sarcocystis falcatula-like sporocysts. Parasitol. Res. 2018;117:213–223. doi: 10.1007/s00436-017-5695-4. [DOI] [PubMed] [Google Scholar]
- Goethert H.K., Molloy P., Berardi V., Weeks K., Telford S.R., 3rd Zoonotic Babesia microti in the northeastern U.S.: evidence for the expansion of a specific parasite lineage. PloS One. 2018;13 doi: 10.1371/journal.pone.0193837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubbels J.M., de Vos A.P., van der Weide M., Viseras J., Schouls L.M., de Vries E., Jongejan F. Simultaneous detection of bovine Theileria and Babesia species by reverse line blot hybridization. J. Clin. Microbiol. 1999;37:1782–1789. doi: 10.1128/jcm.37.6.1782-1789.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inokuma H., Yoshizaki Y., Shimada Y., Sakata Y., Okuda M., Onishi T. Epidemiological survey of Babesia species in Japan performed with specimens from ticks collected from dogs and detection of new Babesia DNA closely related to Babesia odocoilei and Babesia divergens DNA. J. Clin. Microbiol. 2003;41:3494–3498. doi: 10.1128/JCM.41.8.3494-3498.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtinen L.E., Birkenheuer A.J., Droleskey R.E., Holman P.J. In vitro cultivation of a newly recognized Babesia sp. in dogs in North Carolina. Vet. Parasitol. 2008;151:150–157. doi: 10.1016/j.vetpar.2007.10.022. [DOI] [PubMed] [Google Scholar]
- Milholland M.T., Castro-Arellano I., Arellano E., Nava-Garcia E., Rangel-Altamirano G., Gonzalez-Cozatl F.X., Suzan G., Schountz T., Gonzalez-Padron S., Vigueras A., Rubio A.V., Maikis T.J., Westrich B.J., Martinez J.A., Esteve-Gassent M.D., Torres M., Rodriguez-Ruiz E.R., Hahn D., Lacher T.E. Species identity supersedes the Dilution effect concerning hantavirus prevalence at sites across Texas and Mexico. ILAR J. 2017;58:401–412. doi: 10.1093/ilar/ily001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modarelli J.J., Ferro P.J., de Leon A.A.P., Esteve-Gasent M.D. TickPath Layerplex: adaptation of a real-time PCR methodology for the simultaneous detection and molecular surveillance of tick-borne pathogens. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-43424-y. 6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelder M.P., Russell C.B., Sheehan N.J., Sander B., Moore S., Li Y., Johnson S., Patel S.N., Sider D. Human pathogens associated with the blacklegged tick Ixodes scapularis: a systematic review. Parasites Vectors. 2016;9:265. doi: 10.1186/s13071-016-1529-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phair K.A., Carpenter J.W., Smee N., Myers C.B., Pohlman L.M. Severe anemia caused by babesiosis in a maned wolf (Chrysocyon brachyurus) J. Zoo Wildl. Med. 2012;43:162–167. doi: 10.1638/2010-0210.1. [DOI] [PubMed] [Google Scholar]
- Westmoreland L.S.H., Stoskopf M.K., Sheppard E., DePerno C.S., Gould N.P., Olfenbuttel C., Maggi R.G. Detection and prevalence of Babesia spp. in American black bears (Ursus americanus) from eastern and Western North Carolina, USA. J. Wildl. Dis. 2019;55(3):678–681. doi: 10.7589/2018-06-164. [DOI] [PubMed] [Google Scholar]
- Yabsley M.J., Murphy S.M., Cunningham M.W. Molecular detection and characterization of Cytauxzoon felis and a Babesia species in cougars from Florida. J. Wildl. Dis. 2006;42:366–374. doi: 10.7589/0090-3558-42.2.366. [DOI] [PubMed] [Google Scholar]
- Yabsley M.J., Shock B.C. Natural history of zoonotic Babesia: role of wildlife reservoirs. Int J Parasitol Parasites Wildl. 2013;2:18–31. doi: 10.1016/j.ijppaw.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]