Abstract
Objective
Retinol binding protein 4 (RBP4) is a member of the lipocalin family and a vitamin A carrier in the blood. More recently, RBP4 has been described as an adipokine that is involved in insulin resistance and metabolic syndrome (MetS). As obesity, MetS and some adipokines contribute to the pathogenesis of osteoarthritis (OA), we investigated RBP4 in patients with OA.
Materials and methods
Cartilage, synovial fluid and blood samples were collected from 100 OA patients undergoing total knee replacement surgery. Primary chondrocytes and cartilage tissue were cultured to measure the RBP4 expression. The concentrations of RBP4, other adipokines (adipsin, adiponectin, leptin and resistin) and biomarkers of OA (COMP, MMP-1, MMP-3 and YKL-40) were measured by immunoassay, and gene expression was measured by next-generation RNA sequencing.
Results
The OA cartilage samples released RBP4 into the culture medium, and the levels correlated positively with the expression of the adipokines adipsin, adiponectin, leptin and resistin. RBP4 was the most prominently expressed of these adipokines in the OA chondrocytes, and the expression of the RBP4 receptors STRA6 (stimulated by retinoic acid gene homologue 6) and TLR4 (Toll-like receptor 4) was also detected. Within the cartilage culture medium, RBP4 showed a positive correlation with MMP-1, MMP-3 and YKL-40. RBP4 was also present in the synovial fluid from the OA patients and correlated positively with the concentrations of RBP4 found in the plasma and the cartilage culture medium. Plasma RBP4 concentrations also showed a positive correlation with MMP-3 and adipsin.
Conclusions
We show here, for the first time, that RBP4 is produced within OA joints and that it is associated with increased levels of adipokines and MMPs. The results suggest a role for RBP4 in the pathogenesis of OA and as a possible target for the disease-modifying drugs for the treatment of OA.
Keywords: Adipokines, Chondrocytes, Cartilage, Matrix metalloproteinases, Osteoarthritis
Introduction
Osteoarthritis (OA) is the most prevalent joint disease and a leading cause of disability that affects an estimated 10% of the world’s population over the age of 60 years [1, 2]. It is a chronic disease that commonly affects the entire joint structure [3]. Degradation of the articular cartilage, formation of osteophytes, subchondral bone sclerosis and synovial inflammation are the principal changes in OA-affected joints. Accumulating evidence supports the contention that low-grade inflammation is critical to the pathogenesis of OA [4]. OA is related to ageing, but it is also associated with a variety of risk factors, including genetic predisposition, trauma, gender and, in particular, obesity.
Adipose tissue produces cytokine-like hormones known as ‘adipokines’. Adipokines not only regulate energy metabolism and appetite but also other functions in the human body. Several studies have reported an important role for adipokines in cartilage and bone homeostasis, metabolism and inflammation and suggest that these molecules serve as a link between obesity and OA [5]. Retinol binding protein 4 (RBP4) was first identified as an adipocyte-derived factor that contributes to the pathogenesis of type 2 diabetes [6]. RBP4 is most prevalently expressed in the liver, followed by adipose tissue [7]. In the circulation, RBP4 is the sole retinol (vitamin A) transport protein that moves vitamin A from the liver to the peripheral tissues where it is metabolized to retinoic acid [8]. Increased levels of RBP4 in obese and insulin-resistant humans and in mouse models have been reported [6, 9] as has a strong correlation of serum RBP4 levels with obesity and insulin resistance [10, 11]; however, these findings were not found in all studies [12, 13]. Several studies have also shown a correlation between RBP4 and other components of human metabolic syndrome (MetS), such as dyslipidaemia [14], hypertension [15] and cardiovascular diseases [16].
RBP4 acts by binding to the receptor ‘stimulated by retinoic acid gene homologue 6′ (STRA6) [17, 18]. In addition, some of its effects are mediated by Toll-like receptor 4 (TLR4) [19–21], which is a major pathway that induces the expression of inflammatory and catabolic factors in chondrocytes and other cells. Therefore, we aimed to investigate whether the adipokine RBP4 is associated with OA; we analysed RBP4 levels in the plasma, synovial fluid (SF) and cartilage from OA patients and determined the correlation of RBP4 with other adipokines and biomarkers implicated in OA.
Materials and methods
Subjects
One hundred OA patients [body mass index (BMI) 29.7 (8.3) kg/m2; age 72 (14) years, median (interquartile range, IQR); 62/38 females/males] undergoing total knee replacement surgery at Coxa Hospital for Joint Replacement, Tampere, Finland, participated in the study. All patients fulfilled the American College of Rheumatology classification criteria for OA [22]. The study was approved by the Ethics Committee of Tampere University Hospital, and the patients gave their written informed consent to participate in the study.
Cartilage, synovial fluid and blood samples
The cartilage samples (n = 97) were processed as previously described [23] and cultured in Dulbecco’s modified Eagle’s medium (DMEM) with Glutamax I containing 10% heat-inactivated foetal bovine serum and penicillin (100 units/ml), streptomycin (100 µg/ml)***, and amphotericin B (250 ng/ml) (all obtained from Invitrogen Carlsbad, CA, USA). The cartilage samples were cultured in a six-well plate and after 42 h, the culture medium was collected and stored at − 20 °C.
Synovial fluid samples were collected by joint puncture at the beginning of the arthroplasty and centrifuged at 4000g for 15 min at 4 °C, and the supernatants were stored at − 70 °C until analysed. The SF samples were available from 68 OA patients for this study.
The blood samples were obtained from all patients just prior to their operation, and the plasma was separated by centrifugation at 1200 rpm for 10 min at 4 °C and stored at − 70 °C until analysed.
Enzyme-linked immunosorbent assay (ELISA)
Concentrations of the adipokines RBP4, adipsin, adiponectin, leptin and resistin, as well as those of the OA biomarkers cartilage oligomeric matrix protein (COMP), matrix metalloproteinase 1 (MMP-1), matrix metalloproteinase 3 (MMP-3) and chitinase-3-like protein 1 (CHI3L1, also known as YKL-40), were measured by immunoassay (all obtained from R&D Systems Europe Ltd, Abingdon, UK, except COMP which was obtained from BioVendor Research and Diagnostic Products, Modřice, Czech Republic).
Next-generation sequencing (NGS) and data analysis
Analysis of mRNA expression was performed using chondrocytes isolated from the knee cartilages from ten additional OA patients whose samples were not used in the experiments described above [n = 10; BMI 27.3 (5.8) kg/m2; age 70 (15) years, median (IQR); 4/6 females/males] undergoing knee replacement surgery at Coxa Hospital for Joint Replacement, Tampere, Finland. The cells were isolated as described [24] and cultured for 24 h. Total RNA was isolated, and next-generation sequencing (NGS) was carried out with Illumina HiSeq2500 according to the manufacturer’s instructions (Illumina Inc., CA, USA) at the Finnish Institute of Molecular Medicine (FIMM) sequencing core, Helsinki, Finland.
The sequencing depth was 20 million paired-end reads of 100 bp. The read quality was first assessed using FastQC [25], and the reads were trimmed using Trimmomatic [26]. The trimmed reads were aligned to the reference human genome with STAR [27]. The count matrices were prepared with the featureCounts program [28]. Gene counts were normalized with the DESeq2 method [29] implemented in the Chipster software package [30]. For each gene, a geometric mean of count values across all samples was calculated. Then, the count value in each sample was divided by this mean. For each sample, a median of values obtained in the previous step for all genes was determined, giving the normalization factor for each sample. The raw count value for each gene in each sample was then divided by this normalization factor, giving the normalized count value for each gene in each sample. Expression levels of genes are given as mean and SEM of DESeq2-normalized counts (n = 10).
Statistical analysis
The data are reported as the mean ± SEM, unless stated otherwise. The statistical analysis was performed using Spearman's rank correlation coefficient (r) (IBM SPSS Statistics 23, IBM Corporation, NY, USA) and t tests (GraphPad Instat version 3.1 and GraphPad Prism version 5.02, GraphPad Software Inc., San Diego, CA, USA). A p value less than 0.05 was considered significant.
Results
RBP4 is present in the plasma and synovial fluid of the patients with osteoarthritis
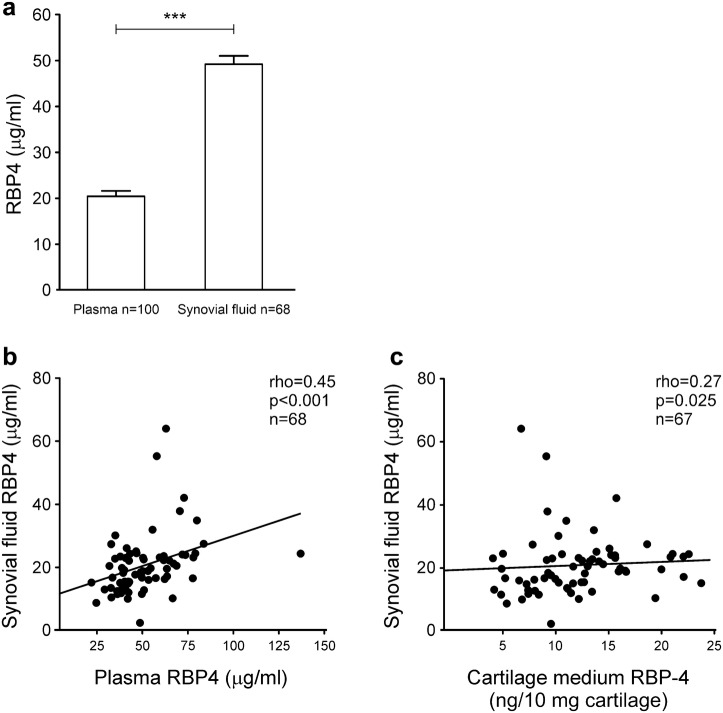
We measured the levels of RBP4 in the plasma and synovial fluid obtained from the OA patients. RBP4 was present at significant concentrations in the plasma and in the synovial fluid from the OA patients. RBP4 levels in plasma (49.2 ± 1.8 μg/ml) were higher than those in synovial fluid (20.4 ± 1.2 μg/ml, Fig. 1a), and there was a positive correlation between them (r = 0.45, p < 0.0001, Fig. 1b). The RBP4 levels in the synovial fluid also correlated with the amount of RBP4 released from the cultured cartilage obtained from the same patients (r = 0.27, p = 0.025, Fig. 1c).
Fig. 1.
RBP4 is present in the synovial fluid from the OA patients, and the levels correlate positively with its concentration in the plasma and the cartilage culture medium. In a the results are expressed as the mean + SEM. t test was used to calculate statistical significance, ***p < 0.001. In b and c Spearman’s correlation analysis was used to detect any association
RBP4 correlates with adipokines and biomarkers in the cartilage from the patients with osteoarthritis
The cartilage samples from the OA patients released RBP4 protein into the culture medium (11.9 ± 0.5 ng/10 mg cartilage), and these levels correlated positively with the other adipokines measured: adiponectin (r = 0.54, p < 0.001), resistin (r = 0.38, p < 0.001), leptin (r = 0.29, p = 0.004) and adipsin (r = 0.27, p = 0.007). Interestingly, RBP4 also showed a positive correlation with the levels of MMP-1 (r = 0.26, p = 0.010), MMP-3 (r = 0.24, p = 0.017) and YKL-40 (r = 0.23, p = 0.025) released into the culture medium but did not correlate with BMI.
For the synovial fluid, no correlations between RBP4 and adipokines, MMP-1, MMP-3 or YKL-40 were found. The plasma RBP4 concentrations positively correlated with adipsin (r = 0.39, p < 0.0001) and MMP-3 (r = 0.25, p = 0.012).
RBP4 is expressed in the primary human OA chondrocytes
As the results pointed to cartilage as a source of RBP4, we explored the expression of this adipokine and its receptors in chondrocytes from the OA patients by using RNA sequencing (RNA-Seq). As shown in Table 1, RBP4 was the most prominently expressed adipokine in the OA chondrocytes. We also observed that the receptors activated by RBP4, namely stimulated by retinoic acid gene homologue 6 (STRA6) and Toll-like receptor 4 (TLR4), were expressed in the OA chondrocytes (Table 1).
Table 1.
Expression of retinol binding protein 4 (RBP4) and adipokines adipsin, adiponectin, leptin and resistin, as well as receptors activated by RBP4 in the OA chondrocytes as measured by RNA-Seq
| Gene name | Expression | SEM |
|---|---|---|
| Adipokines | ||
| Retinol binding protein 4 | 472.5 | 75.9 |
| Adipsin | 4.4 | 1.6 |
| Adiponectin | 0.8 | 0.5 |
| Leptin | 0.8 | 0.8 |
| Resistin | < 0.1 | < 0.1 |
| Receptors activated by RBP4 | ||
| Stimulated by retinoic acid gene homologue 6 | 4.9 | 37.7 |
| Toll-like receptor 4 | 234.1 | 1.9 |
The primary chondrocytes were isolated from knee cartilages of the OA patients (n = 10) undergoing knee replacement surgery and cultured for 24 h. Expression levels of genes are given as mean and SEM of DESeq2-normalized counts
Discussion
RBP4 is a retinol transport protein in blood that is prevalently expressed in the liver but is also highly present in adipose tissue [6, 7]. Because of the close correlation between obesity, MetS and OA [6, 9–11, 14, 31], we analysed here, for the first time, the potential associations of RBP4 into the pathogenesis of OA.
To date, there have been no studies on the RBP4 production in joint tissues. Here, we showed that cultured cartilage released RBP4. Furthermore, these RBP4 levels correlated positively with the other adipokines implicated in OA pathogenesis, i.e., adiponectin [24, 32–34], resistin [35, 36], leptin [23, 37–41] and adipsin [41, 42]. All these adipokines can be found in the synovial fluid of OA joints. Adipokines are produced, e.g., in adipose tissue, and from the circulation, they likely diffuse into the joint. They can also be produced intra-articularly, and the expression of leptin and adiponectin mRNA/protein has been previously detected in OA cartilage and chondrocytes [33, 43]. In the expression analysis presented here, human OA chondrocytes also expressed adipsin and RBP4, and in fact, RBP4 was the most prominently expressed of the measured adipokines in the OA chondrocytes (Table 1).
RBP4 acts as an immunomodulatory adipocytokine. Kahn et al. reported that RBP4 induces the secretion of proinflammatory cytokines in mouse dendritic cells and macrophages, causing Th1 polarization and proliferation in vivo [44]. RBP4 also activates APCs (antigen-presenting cells) in adipose tissue through the JNK pathway, promoting adipose tissue inflammation and systemic insulin resistance [44]. It has been demonstrated that immune cells play a role in the pathogenesis of OA and that OA synovium contains T cells and increased levels of Th1 cytokines [34, 45–48]. Therefore, it is reasonable to suggest that, as with leptin [49], RBP4 found within the joint could modulate the inflammatory milieu in a manner that promotes cartilage damage in OA.
In the present study, we show that OA synovial fluid contains RBP4 and that there is a significant positive correlation between the RBP4 levels in the OA synovial fluid and the cartilage culture medium from the same patients. Regarding the synovial fluid, we did not find any correlations between the RBP4 levels and the OA biomarkers. This finding is likely due to the contribution by other joint tissues than cartilage to the content of the synovial fluid, but this possibility was not explored in the present study. Instead, the findings point to cartilage as a significant target tissue that bears the destructive effects of RBP4. OA chondrocytes express STRA6 and TLR4, receptors known to be activated by RBP4 [17–21]. The positive correlation between the cartilage-derived RBP4 and the degrading enzymes MMP-1 and MMP-3 and the OA-related inflammatory marker YKL-40 could be due to the induction of these factors by the RBP4 in the cartilage, likely through the activation of the TLR4 receptor, which has been suggested to mediate some of the pro-inflammatory effects induced by RBP4 [19–21, 50]. Recent studies of other tissues have shown that RBP4 has the ability to stimulate MMP release and that the knocked-down RBP4 suppressed the expression of the matrix metalloproteinases [51, 52]. Based on these studies and the findings presented in this work, RBP4 could have similar effects in cartilage.
We also measured the levels of RBP4 in the plasma from the OA patients. These levels correlated positively with adipsin, an adipokine recently demonstrated as a factor that contributes to cartilage degradation in osteoarthritis [41, 42]. Moreover, for plasma, we found a positive correlation between RBP4 concentration and MMP-3, a classic biomarker of OA [53, 54]. The plasma from the OA patients contained more RBP4 than the SF, and we observed a positive correlation between the RBP4 concentration in these two compartments. Therefore, it is likely that, in addition to local production, RBP4 produced by the liver and adipose tissue diffuses into the joint from the circulation.
Several studies reported an increased concentration of RBP4 in obesity [9, 55–57] and in conditions related to obesity complications, including metabolic syndrome [58, 59], diabetes [9, 10, 60] and cardiovascular diseases [61–63]. Reported RBP4 levels normally range between 20 and 40 µg/ml in non-OA study populations [9–11], while RBP4 concentrations were at 50 µg/ml-level in OA patients in the present study. Direct comparisons are not advisable because of differences in the populations and the methods used. However, it is noteworthy that obesity is a major risk factor for OA, and OA is increasingly regarded as the fifth clinical feature of metabolic syndrome, which makes the adipokine RBP4 a potential novel factor linking the metabolic state, inflammation and articular degradation in OA.
In conclusion, our data demonstrated, for the first time, that RBP4 is a prominently expressed adipokine in OA chondrocytes and is present in synovial fluid and plasma from OA patients at considerable μg/ml levels. RBP4 was also found to be associated with increased levels of adipokines and matrix metalloproteinases MMP-1 and MMP-3.
The results suggest a role for RBP4 in the pathogenesis of OA and as a potential target for disease-modifying drugs for the treatment of OA, which encourages additional studies to reveal the more detailed role of RBP4 in the pathogenesis and pathology of OA. Further studies in large cohorts of patients and in cells and joint tissues are needed to confirm the implications of RBP4 in OA.
Acknowledgements
We thank Ms. Terhi Salonen for excellent technical assistance and Ms. Heli Määttä for skilful secretarial help. Competitive Research Funding of the Tampere University Hospital, Paulo Foundation, Scandinavian Rheumatology Research Foundation, Maire Lisko Foundation and Pirkanmaa Regional Fund of the Finnish Cultural Foundation are acknowledged for funding.
Author contributions
MS was involved in the conception and design of the study, in the laboratory analyses, in calculating the results, and in interpretation of the data, and she also drafted the manuscript. AK was involved in the design of the study, in the laboratory analyses, and in revising the manuscript. AP was involved in the design of the study, in calculating the results, in the interpretation of the data, and in revising the manuscript. TL was involved in the design of the study, in the laboratory analyses, in the interpretation of the data, and in revising the manuscript. MH was involved in the design of the study, in the laboratory analyses, in the interpretation of the data, and in revising the manuscript. TM was involved in the design of the study, in selecting the patients and in acquiring the patient samples, in interpretation of the data, and in revising the manuscript. EM was involved in the conception and design of the study, in interpretation of the data, and in revising the manuscript. KV was involved in the conception and design of the study, in calculating the results, in interpretation of the data, and in writing the manuscript. All authors approved the final version of the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the Ethics Committee of Tampere University Hospital and carried out in accordance with the Declaration of Helsinki. The patients gave their written informed consent to participate in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Morena Scotece, Email: morena.scotece84@gmail.com.
Anna Koskinen-Kolasa, Email: anna.koskinen-kolasa@fimnet.fi.
Antti Pemmari, Email: antti.pemmari@tuni.fi.
Tiina Leppänen, Email: tiina.leppanen@tuni.fi.
Mari Hämäläinen, Email: mari.hamalainen@tuni.fi.
Teemu Moilanen, Email: teemu.moilanen@coxa.fi.
Eeva Moilanen, Email: eeva.moilanen@tuni.fi.
Katriina Vuolteenaho, Email: katriina.vuolteenaho@tuni.fi.
References
- 1.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheumatol. 2008;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen D, Shen J, Zhao W, Wang T, Han L, Hamilton JL, et al. Osteoarthritis: toward a comprehensive understanding of pathological mechanism. Bone Res. 2017;5:16044. doi: 10.1038/boneres.2016.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheumatol. 2012;64(6):1697–1707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robinson WH, Lepus CM, Wang Q, Raghu H, Mao R, Lindstrom TM, et al. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12(10):580–592. doi: 10.1038/nrrheum.2016.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scotece M, Conde J, Vuolteenaho K, Koskinen A, López V, Gómez-Reino J, et al. Adipokines as drug targets in joint and bone disease. Drug Discov Today. 2014;19(3):241–258. doi: 10.1016/j.drudis.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436(7049):356–362. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- 7.Tsutsumi C, Okuno M, Tannous L, Piantedosi R, Allan M, Goodman DS, et al. Retinoids and retinoid-binding protein expression in rat adipocytes. J Biol Chem. 1992;267(3):1805–1810. [PubMed] [Google Scholar]
- 8.Newcomer ME, Ong DE. Plasma retinol binding protein: structure and function of the prototypic lipocalin. Biochim Biophys Acta. 2000;1482(1–2):57–64. doi: 10.1016/s0167-4838(00)00150-3. [DOI] [PubMed] [Google Scholar]
- 9.Graham TE, Yang Q, Blüher M, Hammarstedt A, Ciaraldi TP, Henry RR, et al. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med. 2006;354(24):2552–2563. doi: 10.1056/NEJMoa054862. [DOI] [PubMed] [Google Scholar]
- 10.Cho YM, Youn BS, Lee H, Lee N, Min SS, Kwak SH, et al. Plasma retinol-binding protein-4 concentrations are elevated in human subjects with impaired glucose tolerance and type 2 diabetes. Diabetes Care. 2006;29(11):2457–2461. doi: 10.2337/dc06-0360. [DOI] [PubMed] [Google Scholar]
- 11.Lee D-C, Lee J-W, Im J-A. Association of serum retinol binding protein 4 and insulin resistance in apparently healthy adolescents. Metabolism. 2007;56(3):327–331. doi: 10.1016/j.metabol.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 12.Gómez-Ambrosi J, Rodríguez A, Catalán V, Ramírez B, Silva C, Rotellar F, et al. Serum retinol-binding protein 4 is not increased in obesity or obesity-associated type 2 diabetes mellitus, but is reduced after relevant reductions in body fat following gastric bypass. Clin Endocrinol (Oxf) 2008;69(2):208–215. doi: 10.1111/j.1365-2265.2007.03156.x. [DOI] [PubMed] [Google Scholar]
- 13.Promintzer M, Krebs M, Todoric J, Luger A, Bischof MG, Nowotny P, et al. Insulin resistance is unrelated to circulating retinol binding protein and protein C inhibitor. J Clin Endocrinol Metab. 2007;92(11):4306–4312. doi: 10.1210/jc.2006-2522. [DOI] [PubMed] [Google Scholar]
- 14.Ng TWK, Watts GF, Barrett PHR, Rye K-A, Chan DC. Effect of weight loss on LDL and HDL kinetics in the metabolic syndrome: associations with changes in plasma retinol-binding protein-4 and adiponectin levels. Diabetes Care. 2007;30(11):2945–2950. doi: 10.2337/dc07-0768. [DOI] [PubMed] [Google Scholar]
- 15.Qi Q, Yu Z, Ye X, Zhao F, Huang P, Hu FB, et al. Elevated retinol-binding protein 4 levels are associated with metabolic syndrome in Chinese people. J Clin Endocrinol Metab. 2007;92(12):4827–4834. doi: 10.1210/jc.2007-1219. [DOI] [PubMed] [Google Scholar]
- 16.Ingelsson E, Lind L. Circulating retinol-binding protein 4 and subclinical cardiovascular disease in the elderly. Diabetes Care. 2009;32(4):733–735. doi: 10.2337/dc08-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muenzner M, Tuvia N, Deutschmann C, Witte N, Tolkachov A, Valai A, et al. Retinol-binding protein 4 and its membrane receptor STRA6 control adipogenesis by regulating cellular retinoid homeostasis and retinoic acid receptor α activity. Mol Cell Biol. 2013;33(20):4068–4082. doi: 10.1128/MCB.00221-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelly M, Widjaja-Adhi MAK, Palczewski G, von Lintig J. Transport of vitamin A across blood-tissue barriers is facilitated by STRA6. FASEB J. 2016;30(8):2985–2995. doi: 10.1096/fj.201600446R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Norseen J, Hosooka T, Hammarstedt A, Yore MM, Kant S, Aryal P, et al. Retinol-binding protein 4 inhibits insulin signaling in adipocytes by inducing proinflammatory cytokines in macrophages through a c-Jun N-terminal kinase- and toll-like receptor 4-dependent and retinol-independent mechanism. Mol Cell Biol. 2012;32(10):2010–2019. doi: 10.1128/MCB.06193-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farjo KM, Farjo RA, Halsey S, Moiseyev G, Ma J-X. Retinol-binding protein 4 induces inflammation in human endothelial cells by an NADPH oxidase- and nuclear factor kappa B-dependent and retinol-independent mechanism. Mol Cell Biol. 2012;32(24):5103–5115. doi: 10.1128/MCB.00820-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao W, Wang H, Zhang L, Cao Y, Bao J-Z, Liu Z-X, et al. Retinol-binding protein 4 induces cardiomyocyte hypertrophy by activating TLR4/MyD88 pathway. Endocrinology. 2016;157(6):2282–2293. doi: 10.1210/en.2015-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheumatol. 1986;29(8):1039–49. [DOI] [PubMed]
- 23.Vuolteenaho K, Koskinen A, Kukkonen M, Nieminen R, Päivärinta U, Moilanen T, et al. Leptin enhances synthesis of proinflammatory mediators in human osteoarthritic cartilage—mediator role of NO in leptin-induced PGE2, IL-6, and IL-8 production. Mediat Inflamm. 2009;2009:345838. doi: 10.1155/2009/345838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koskinen A, Juslin S, Nieminen R, Moilanen T, Vuolteenaho K, Moilanen E. Adiponectin associates with markers of cartilage degradation in osteoarthritis and induces production of proinflammatory and catabolic factors through mitogen-activated protein kinase pathways. Arthritis Res Ther. 2011;13(6):R184. doi: 10.1186/ar3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andrews S. FastQC: a quality control tool for high throughput sequence data. https://www.bioinformatics.babraham.ac.uk/projects/fastqc
- 26.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30(7):923–930. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 29.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kallio MA, Tuimala JT, Hupponen T, Klemelä P, Gentile M, Scheinin I, et al. Chipster: user-friendly analysis software for microarray and other high-throughput data. BMC Genomics. 2011;12:507. doi: 10.1186/1471-2164-12-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jia W, Wu H, Bao Y, Wang C, Lu J, Zhu J, et al. Association of serum retinol-binding protein 4 and visceral adiposity in Chinese subjects with and without type 2 diabetes. J Clin Endocrinol Metab. 2007;92(8):3224–3229. doi: 10.1210/jc.2007-0209. [DOI] [PubMed] [Google Scholar]
- 32.Conde J, Scotece M, López V, Gómez R, Lago F, Pino J, et al. Adiponectin and leptin induce VCAM-1 expression in human and murine chondrocytes. PLoS One. 2012;7(12):e52533. doi: 10.1371/journal.pone.0052533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Francin P-J, Abot A, Guillaume C, Moulin D, Bianchi A, Gegout-Pottie P, et al. Association between adiponectin and cartilage degradation in human osteoarthritis. Osteoarthr Cartil. 2014;22(3):519–526. doi: 10.1016/j.joca.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 34.Scotece M, Pérez T, Conde J, Abella V, López V, Pino J, et al. Adipokines induce pro-inflammatory factors in activated CD4+ T cells from osteoarthritis patient. J Orthop Res. 2017;35(6):1299–1303. doi: 10.1002/jor.23377. [DOI] [PubMed] [Google Scholar]
- 35.Koskinen A, Vuolteenaho K, Moilanen T, Moilanen E. Resistin as a factor in osteoarthritis: synovial fluid resistin concentrations correlate positively with interleukin 6 and matrix metalloproteinases MMP-1 and MMP-3. Scand J Rheumatol. 2014;43(3):249–253. doi: 10.3109/03009742.2013.853096. [DOI] [PubMed] [Google Scholar]
- 36.Philp AM, Collier RL, Grover LM, Davis ET, Jones SW. Resistin promotes the abnormal Type I collagen phenotype of subchondral bone in obese patients with end stage hip osteoarthritis. Sci Rep. 2017;7(1):4042. doi: 10.1038/s41598-017-04119-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koskinen A, Vuolteenaho K, Nieminen R, Moilanen T, Moilanen E. Leptin enhances MMP-1, MMP-3 and MMP-13 production in human osteoarthritic cartilage and correlates with MMP-1 and MMP-3 in synovial fluid from OA patients. Clin Exp Rheumatol. 2011;29(1):57–64. [PubMed]
- 38.Vuolteenaho K, Koskinen A, Moilanen T, Moilanen E. Leptin levels are increased and its negative regulators, SOCS-3 and sOb-R are decreased in obese patients with osteoarthritis: a link between obesity and osteoarthritis. Ann Rheum Dis. 2012;71(11):1912–1913. doi: 10.1136/annrheumdis-2011-201242. [DOI] [PubMed] [Google Scholar]
- 39.Vuolteenaho Katriina, Koskinen Anna, Moilanen Eeva. Leptin - A Link between Obesity and Osteoarthritis. Applications for Prevention and Treatment. Basic & Clinical Pharmacology & Toxicology. 2013;114(1):103–108. doi: 10.1111/bcpt.12160. [DOI] [PubMed] [Google Scholar]
- 40.Scotece M, Mobasheri A. Leptin in osteoarthritis: focus on articular cartilage and chondrocytes. Life Sci. 2015;140:75–78. doi: 10.1016/j.lfs.2015.05.025. [DOI] [PubMed] [Google Scholar]
- 41.Martel-Pelletier J, Raynauld J-P, Dorais M, Abram F, Pelletier J-P. The levels of the adipokines adipsin and leptin are associated with knee osteoarthritis progression as assessed by MRI and incidence of total knee replacement in symptomatic osteoarthritis patients: a post hoc analysis. Rheumatology (Oxford) 2016;55(4):680–688. doi: 10.1093/rheumatology/kev408. [DOI] [PubMed] [Google Scholar]
- 42.Valverde-Franco G, Tardif G, Mineau F, Paré F, Lussier B, Fahmi H, et al. High in vivo levels of adipsin lead to increased knee tissue degradation in osteoarthritis: data from humans and animal models. Rheumatology (Oxford) 2018;57(10):1851–1860. doi: 10.1093/rheumatology/key181. [DOI] [PubMed] [Google Scholar]
- 43.Dumond H, Presle N, Terlain B, Mainard D, Loeuille D, Netter P, et al. Evidence for a key role of leptin in osteoarthritis. Arthritis Rheumatol. 2003;48(11):3118–3129. doi: 10.1002/art.11303. [DOI] [PubMed] [Google Scholar]
- 44.Moraes-Vieira PM, Yore MM, Dwyer PM, Syed I, Aryal P, Kahn BB. RBP4 activates antigen-presenting cells, leading to adipose tissue inflammation and systemic insulin resistance. Cell Metab. 2014;19(3):512–526. doi: 10.1016/j.cmet.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sakkas LI, Scanzello C, Johanson N, Burkholder J, Mitra A, Salgame P, et al. T cells and T-cell cytokine transcripts in the synovial membrane in patients with osteoarthritis. Clin Diagn Lab Immunol. 1998;5(4):430–437. doi: 10.1128/cdli.5.4.430-437.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jasin HE. Immune mediated cartilage destruction. Scand J Rheumatol Suppl. 1988;76:111–116. doi: 10.3109/03009748809102960. [DOI] [PubMed] [Google Scholar]
- 47.Nakamura H, Yoshino S, Kato T, Tsuruha J, Nishioka K. T-cell mediated inflammatory pathway in osteoarthritis. Osteoarthr Cartil. 1999;7(4):401–402. doi: 10.1053/joca.1998.0224. [DOI] [PubMed] [Google Scholar]
- 48.Ishii H, Tanaka H, Katoh K, Nakamura H, Nagashima M, Yoshino S. Characterization of infiltrating T cells and Th1/Th2-type cytokines in the synovium of patients with osteoarthritis. Osteoarthr Cartil. 2002;10(4):277–281. doi: 10.1053/joca.2001.0509. [DOI] [PubMed] [Google Scholar]
- 49.Abella V, Scotece M, Conde J, Pino J, Gonzalez-Gay MA, Gómez-Reino JJ, et al. Leptin in the interplay of inflammation, metabolism and immune system disorders. Nat Rev Rheumatol. 2017;13(2):100–109. doi: 10.1038/nrrheum.2016.209. [DOI] [PubMed] [Google Scholar]
- 50.Du M, Martin A, Hays F, Johnson J, Farjo RA, Farjo KM. Serum retinol-binding protein-induced endothelial inflammation is mediated through the activation of toll-like receptor 4. Mol Vis. 2017;23:185–197. [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Y, Wang Y, Zhang Z. Adipokine RBP4 drives ovarian cancer cell migration. J Ovarian Res. 2018;11(1):29. doi: 10.1186/s13048-018-0397-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li H, Cao G, Zhang N, Lou T, Wang Q, Zhang Z, et al. RBP4 regulates trophoblastic cell proliferation and invasion via the PI3K/AKT signaling pathway. Mol Med Rep. 2018;18(3):2873–2879. doi: 10.3892/mmr.2018.9240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kraus VB, Burnett B, Coindreau J, Cottrell S, Eyre D, Gendreau M, et al. Application of biomarkers in the development of drugs intended for the treatment of osteoarthritis. Osteoarthr Cartil. 2011;19(5):515–542. doi: 10.1016/j.joca.2010.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lohmander LS, Brandt KD, Mazzuca SA, Katz BP, Larsson S, Struglics A, et al. Use of the plasma stromelysin (matrix metalloproteinase 3) concentration to predict joint space narrowing in knee osteoarthritis. Arthritis Rheumatol. 2005;52(10):3160–3167. doi: 10.1002/art.21345. [DOI] [PubMed] [Google Scholar]
- 55.Aeberli Isabelle, Biebinger Ralf, Lehmann Roger, l’Allemand Dagmar, Spinas Giatgen A., Zimmermann Michael B. Serum Retinol-Binding Protein 4 Concentration and Its Ratio to Serum Retinol Are Associated with Obesity and Metabolic Syndrome Components in Children. The Journal of Clinical Endocrinology & Metabolism. 2007;92(11):4359–4365. doi: 10.1210/jc.2007-0468. [DOI] [PubMed] [Google Scholar]
- 56.Reinehr T, Stoffel-Wagner B, Roth CL. Retinol-binding protein 4 and its relation to insulin resistance in obese children before and after weight loss. J Clin Endocrinol Metab. 2008;93(6):2287–2293. doi: 10.1210/jc.2007-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Friebe D, Neef M, Erbs S, Dittrich K, Kratzsch J, Kovacs P, et al. Retinol binding protein 4 (RBP4) is primarily associated with adipose tissue mass in children. Int J Pediatr Obes. 2011;6(2–2):e345–e352. doi: 10.3109/17477166.2010.491228. [DOI] [PubMed] [Google Scholar]
- 58.Gavi S, Qurashi S, Stuart LM, Lau R, Melendez MM, Mynarcik DC, et al. Influence of age on the association of retinol-binding protein 4 with metabolic syndrome. Obesity (Silver Spring) 2008;16(4):893–895. doi: 10.1038/oby.2007.138. [DOI] [PubMed] [Google Scholar]
- 59.Lim S, Yoon JW, Choi SH, Park YJ, Lee JJ, Park JH, et al. Combined impact of adiponectin and retinol-binding protein 4 on metabolic syndrome in elderly people: the Korean Longitudinal Study on Health and Aging. Obesity (Silver Spring) 2010;18(4):826–832. doi: 10.1038/oby.2009.232. [DOI] [PubMed] [Google Scholar]
- 60.Sun L, Qi Q, Zong G, Ye X, Li H, Liu X, et al. Elevated plasma retinol-binding protein 4 is associated with increased risk of type 2 diabetes in middle-aged and elderly Chinese adults. J Nutr. 2014;144(5):722–728. doi: 10.3945/jn.113.189860. [DOI] [PubMed] [Google Scholar]
- 61.Bobbert P, Weithäuser A, Andres J, Bobbert T, Kühl U, Schultheiss HP, et al. Increased plasma retinol binding protein 4 levels in patients with inflammatory cardiomyopathy. Eur J Heart Fail. 2009;11(12):1163–1168. doi: 10.1093/eurjhf/hfp153. [DOI] [PubMed] [Google Scholar]
- 62.Huang G, Wang D, Khan UI, Zeb I, Manson JE, Miller V, et al. Associations between retinol-binding protein 4 and cardiometabolic risk factors and subclinical atherosclerosis in recently postmenopausal women: cross-sectional analyses from the KEEPS study. Cardiovasc Diabetol. 2012;11:52. doi: 10.1186/1475-2840-11-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lambadiari V, Kadoglou NPE, Stasinos V, Maratou E, Antoniadis A, Kolokathis F, et al. Serum levels of retinol-binding protein-4 are associated with the presence and severity of coronary artery disease. Cardiovasc Diabetol. 2014;13:121. doi: 10.1186/s12933-014-0121-z. [DOI] [PMC free article] [PubMed] [Google Scholar]