Abstract
Aims/Introduction
This longitudinal study aimed to explore whether distinct developmental trajectories of body mass index (BMI) would be predictive of diabetes risk in general Chinese adults.
Materials and Methods
A total of 4,519 participants aged >18 years who were free of diabetes in 2011 (baseline of the current analysis) were enrolled in this study. All participants completed a medical examination every year during 2011–2016, and BMI levels were measured two to six (average 5.6) times. Group‐based trajectory modeling was applied to identify BMI trajectories over time. New‐onset diabetes was confirmed in 2016.
Results
During 2011–2016, four distinct BMI trajectories were identified according to BMI range and changing pattern over time: “low” (19.6%), “moderate” (33.4%), “moderate‐high” (33.4%) and “high” (13.6%). A total of 168 (3.7%) new‐onset diabetes cases were confirmed in 2016. Compared with the “low” BMI trajectory, participants in the “high” BMI trajectory were at significantly higher risk for new‐onset diabetes (adjusted relative risk 3.24, 95% confidence interval 1.27–8.24). Notably, BMI trajectories based on the first four or three annual BMI tests yielded similar results. By contrast, no significant correlation was found between categories of baseline BMI and new‐onset diabetes in 2016 after multivariate adjustment.
Conclusions
The present results show that distinct BMI trajectories, even identified using just four or three annual BMI tests, are significantly associated with new‐onset diabetes. Monitoring BMI trajectories over time might provide an important approach to identify subpopulations at higher risk for developing diabetes.
Keywords: Body mass index, Diabetes, Trajectory
Whether distinct body mass index (BMI) trajectories would be predictive of diabetes risk in general Chinese adults is still unknown. Our results show that distinct BMI trajectories, even identified using just four or three annual BMI tests, are significantly associated with new‐onset diabetes. The findings suggest that monitoring BMI trajectories over time might provide an important approach to identify subpopulations at higher risk for diabetes in general Chinese adults.

Introduction
Diabetes is a major and growing health concern worldwide1. In China, the prevalence of diabetes has increased sharply, from 0.67% in 1980, to 10.9% in 2013, as a result of considerable changes in lifestyles and aging2. Meanwhile, the overall mortality, disability‐adjusted life‐years and economic costs attributable to diabetes are showing rapid growth in China3, 4. Previous studies suggested that early detection and treatment of diabetes would substantially reduce the related morbidity and mortality5, 6. However, current diagnostic procedures, including fasting blood glucose test, oral glucose tolerance test and hemoglobin A1c test, are not suitable for large‐scale population screening in a developing country, such as China. Therefore, using a simple method to identify subpopulations at higher risk for diabetes would critically inform prevention efforts.
Obesity, commonly classified using a simple index – body mass index (BMI) – is well recognized as the major risk factor for diabetes. However, previous studies were often based on BMI at a certain point, regardless of the effect of long‐term BMI changes on diabetes risk7, 8. Hence, understanding the heterogeneity of diabetes risk by exploring the distinct patterns of BMI changes might carry new insights into diabetes prevention. In addition, in China, the incidence of diabetes is high, despite a relatively low prevalence of obesity, implying that the effect of BMI on diabetes might differ across various racial/ethnic groups9.
Group‐based trajectory modeling (GBTM) is a type of latent class growth analysis that can identify distinct clusters of individuals who are following similar BMI trajectories10. This method assumes that individual differences in trajectories can be summarized by a finite set of different polynomial functions of time11. In the past years, this method has been successfully used to examine the association between BMI trajectories and the risk of hypertension12, cancer13, 14, 15 and all‐cause mortality16, 17. However, to date, just a few studies have explored the association between BMI trajectories identified by GBTM and diabetes risk18, 19. Whether distinct BMI trajectories would be predictive of diabetes risk in general Chinese adults is still unknown. To address the gap, by using repeated measurements of BMI in a longitudinal cohort of Chinese adults, we aimed to examine the association of distinct BMI trajectories with new‐onset diabetes.
Methods
Study population
The Health Management Center of Third Xiangya Hospital, Changsha, Hunan, China is one of the largest medical examination centers in China, mainly servicing employees from hundreds of institutions in Changsha. Of these institutions, 127 continuously chose the center for their annual employee medical examinations during 2011–2016. In the present study, we retrospectively recruited 5,004 participants from the 127 institutions. All participants were aged >18 years and had completed a medical examination every year during 2011–2016 (Figure 1). The present study was in accordance with the guidelines of the Declaration of Helsinki and was approved by the Medical Ethics Committee of Third Xiangya Hospital. All participants signed an informed consent form and agreed to share their health information for medical research.
Figure 1.
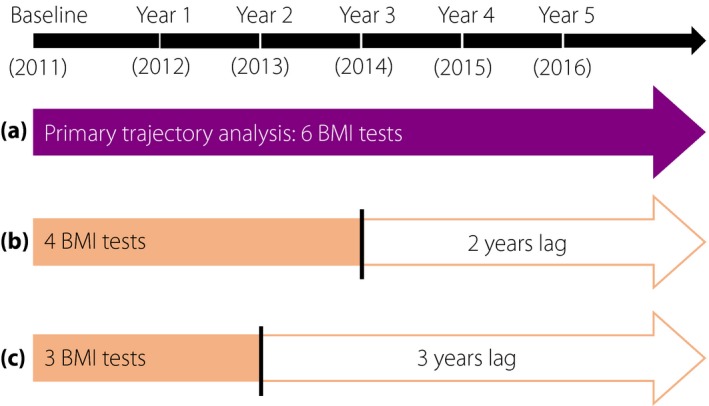
Timeline of annual visits and study design. At baseline, all participants were free of diabetes. New‐onset diabetes was confirmed in 2016. (a) The primary analysis identified distinct body mass index (BMI) trajectories using six BMI tests (during 2011–2016). Regression models were then constructed to examine the association between distinct BMI trajectories and new‐onset diabetes. (b,c) Secondary analyses reconstructed the regression models after identifying distinct BMI trajectories using four and three BMI tests.
Because diabetes is our primary outcome variable, 94 participants who had missing data on diabetes in 2011, and 288 participants who were diagnosed with diabetes in 2011 were excluded from this study. Meanwhile, 56 participants who had missing data on the diagnosis of diabetes in 2016 were also excluded. In addition, as the calculation of BMI trajectories required at least two BMI tests20, 21, we further excluded 47 participants who could not meet this criterion. Finally, 4,519 participants were deemed eligible for the present study.
Diagnosis of diabetes
Venous blood samples were collected in the morning after overnight fasting for 8–12 h and then transfused into ethylenediaminetetraacetic acid‐containing vacuum tubes. Blood samples were stored at −20°C until analyzed. The concentration of fasting blood glucose was determined by enzymatic colorimetric assay using an automated analyzer (Hitachi 7600‐110; Hitachi, Tokyo, Japan) at the central laboratory of Third Xiangya Hospital. The intra‐assay coefficients of variation for blood glucose were <2.5%. Diabetes was defined by the presence of any of the following: (i) self‐reported doctor‐diagnosed diabetes; (ii) current use of insulin or oral hypoglycemic agents; and (iii) fasting blood glucose ≥7.0 mmol/L22.
Assessment of BMI
Bodyweight, height and waist circumference were measured to the nearest 0.1 kg or 0.1 cm, with participants wearing light clothes and no shoes. BMI was calculated as bodyweight in kilograms (kg) divided by the square of height in meters (m2).
Assessment of potential covariates
Information on demographic variables (age, sex, race, occupation and marital status), lifestyle factors (cigarette smoking and alcohol consumption) and medical history were obtained by well‐trained interviewers using standardized questionnaires. A total of 11 racial groups, including Han, Miao, Tujia, Hui, Zhuang, Bai, Dong, Man, Yao, Mongolian and Xibo, were identified in our study sample. For the analyses, just two categories were considered: Han and all other minorities. Occupation was classified into seven groups according to the types of institutions.
Blood pressure (BP) was measured to the nearest 0.1 mmHg in a sitting position after a 10‐min rest. Using a corrected mercury sphygmomanometer, two readings were obtained for both systolic and diastolic BP, with a 30‐s interval. The mean of the two readings was considered as the participant's BP. If the two readings differed by >5 mmHg, BP was re‐measured, and the participant's BP was finally calculated as the average of the three readings. Hypertension was defined as systolic BP ≥140 mmHg and/or diastolic BP ≥90 mmHg, or self‐reported doctor‐diagnosed hypertension, or current use of antihypertensive agents23.
Other biochemical measurements, including total cholesterol, triglycerides, hemoglobin, creatinine and uric acid, were also taken at the central laboratory of Third Xiangya Hospital. Details about the measurement methods were published previously23, 24.
Statistical analysis
All analyses were carried out using Stata version 14.0 (Stata Corp., College Station, TX, USA). The BMI trajectories were determined by GBTM, fitted using the user‐written “traj” procedure in Stata10, 25. We used censored normal distribution to model BMI. We chose the number and shape (i.e., intercept, linear, quadratic and cubic) of BMI trajectories following the criteria: lowest Bayesian information criterion, high posterior probabilities (>0.7) and ≥5% of total sample in a trajectory group. In a step‐by‐step manner, we finally decided on the best‐fitting model with four linear trajectories (Figure 2).
Figure 2.
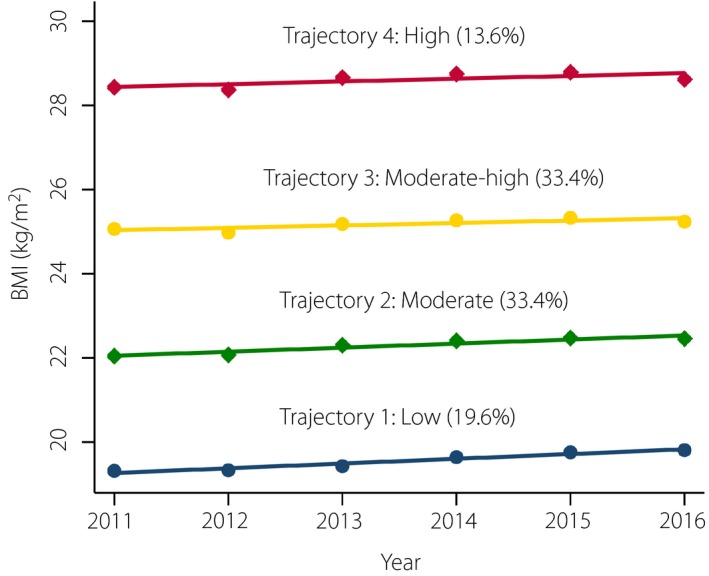
Group‐based trajectory modeling used to determine distinct body mass index (BMI) trajectories. Distinct BMI trajectories were identified using six BMI tests (during 2011–2016).
Baseline characteristics of the study sample were summarized as the median (interquartile range) for continuous variables, and as the number (percentage) for categorical variables. Comparisons of baseline characteristics by distinct BMI trajectories were assessed using Kruskal–Wallis test for continuous variables, and the χ2‐test for categorical variables. Trends in baseline characteristics across distinct BMI trajectories were assessed using Spearman's correlation for continuous variables, and the Cochran–Armitage trend test for binary variables. Modified Poisson regression models, using the robust error variance26, were used to estimate the relative risks (RRs) for new‐onset diabetes in 2016. All potential covariates in the regression models were collected at baseline. Missing values of potential covariates were assumed to be random, and were handled using multiple imputation with chained equations27. As approximately 30% of the study sample had missing values on at least one study variable, we generated 30 imputed datasets in the imputation models27. To optimize the imputation, we added all study variables into the imputation models27.
Statistical interactions between BMI trajectories and sex (male vs female), age (<45 vs ≥45 years) and impaired fasting glucose (fasting blood glucose <5.6 vs ≥5.6 mmol/L) in relation to risks of new‐onset diabetes were examined using likelihood ratio tests in the regression models. To compare with traditional methods based on baseline BMI, we excluded 196 participants who had missing values on baseline BMI in a secondary analysis. To explore whether BMI trajectories based on fewer BMI tests could predict new‐onset diabetes in 2016, we further examined the association of BMI trajectories identified using just four or three annual BMI tests with new‐onset diabetes in 2016 (Figure 1).
Results
Baseline characteristics of study participants by distinct BMI trajectories
Of the 4,519 participants, 2,438 (53.9%) were men, and the median age at baseline was 42.0 years (interquartile range 32.0–57.0 years). Based on the BMI range and changing pattern during 2011–2016, four linear BMI trajectories were identified in these participants (Figure 2). Table 1 shows the baseline characteristics of the study participants by distinct BMI trajectories. Participants belonging to the “high” BMI trajectory were more likely to be older, male, current smoker and current alcohol drinker at baseline, and they tended to have an increased prevalence of hypertension, as well as higher levels of BMI, waist circumference, fasting blood glucose, total cholesterol, triglycerides, hemoglobin, creatinine and uric acid at baseline. In addition, the percentages of occupation, married status and use of antihypertensive at baseline were also significantly different across distinct BMI trajectories. However, there was no significant difference in race and family history of diabetes by distinct BMI trajectories.
Table 1.
Baseline characteristics of study participants by distinct body mass index trajectories
| Variables | Total (n = 4,519) | Trajectory 1: Low (n = 884) | Trajectory 2: Moderate (n = 1,511) | Trajectory 3: Moderate‐high (n = 1,510) | Trajectory 4: High (n = 614) | P‐value* | P for trend† |
|---|---|---|---|---|---|---|---|
| Age (years) | 42.0 (32.0–57.0) | 32.0 (27.0–43.0) | 41.0 (31.0–55.0) | 47.0 (37.0–61.0) | 47.0 (37.0–63.0) | <0.001 | <0.001 |
| Male (%) | 2,438 (53.9) | 194 (22.0) | 616 (40.8) | 1,115 (73.8) | 513 (83.6) | <0.001 | <0.001 |
| BMI (kg/m2) | 23.4 (20.9–25.6) | 19.4 (18.5–20.1) | 22.0 (21.1–22.9) | 25.1 (24.2–25.9) | 28.2 (27.3–29.2) | <0.001 | <0.001 |
| WC (cm) | 80.0 (71.0–87.0) | 67.0 (64.0–71.0) | 75.0 (71.0–80.0) | 85.0 (80.0–88.0) | 93.0 (88.0–96.0) | <0.001 | <0.001 |
| Race (%) | |||||||
| Han | 4,350 (96.6) | 851 (96.3) | 1,466 (97.5) | 1,443 (95.9) | 590 (96.7) | 0.102 | 0.577 |
| Other minorities | 153 (3.4) | 33 (3.7) | 38 (2.5) | 62 (4.1) | 20 (3.3) | ||
| Occupation (%) | |||||||
| Civil servants | 1,887 (41.8) | 230 (26.0) | 574 (38.0) | 755 (50.0) | 328 (53.4) | <0.001 | ― |
| Educators | 166 (3.7) | 26 (2.9) | 56 (3.7) | 68 (4.5) | 16 (2.6) | ||
| Media workers | 747 (16.5) | 168 (19.0) | 244 (16.2) | 238 (15.8) | 97 (15.8) | ||
| Medical workers | 1,090 (24.1) | 353 (39.9) | 429 (28.4) | 239 (15.8) | 69 (11.2) | ||
| Bank workers | 228 (5.1) | 48 (5.4) | 91 (6.0) | 63 (4.2) | 26 (4.2) | ||
| Police officers | 132 (2.9) | 8 (0.9) | 37 (2.5) | 57 (3.8) | 30 (4.9) | ||
| Others | 269 (6.0) | 51 (5.8) | 80 (5.3) | 90 (6.0) | 48 (7.8) | ||
| Current smoker (%) | 989 (26.5) | 71 (9.5) | 239 (19.4) | 450 (36.5) | 229 (44.6) | <0.001 | <0.001 |
| Current alcohol drinker (%) | 2,034 (60.1) | 259 (38.3) | 608 (54.6) | 807 (71.6) | 360 (77.4) | <0.001 | <0.001 |
| Married status (%) | 3,415 (86.3) | 535 (70.9) | 1,108 (84.7) | 1,265 (93.9) | 507 (92.9) | <0.001 | <0.001 |
| Family history of diabetes (%) | 347 (7.7) | 65 (7.4) | 119 (7.9) | 117 (7.8) | 46 (7.5) | 0.968 | 0.923 |
| Hypertension (%) | 946 (21.6) | 49 (5.8) | 201 (13.8) | 426 (28.9) | 270 (45.5) | <0.001 | <0.001 |
| Use of antihypertensive (%) | 407 (9.0) | 24 (2.7) | 91 (6.0) | 179 (11.9) | 113 (18.4) | <0.001 | <0.001 |
| Fasting blood glucose (mmol/L) | 5.0 (4.7–5.3) | 4.8 (4.5–5.1) | 4.9 (4.7–5.2) | 5.1 (4.8–5.5) | 5.2 (4.8–5.6) | <0.001 | <0.001 |
| Total cholesterol (mmol/L) | 4.7 (4.2–5.3) | 4.4 (3.9–5.0) | 4.6 (4.1–5.3) | 4.8 (4.3–5.5) | 4.9 (4.3–5.5) | <0.001 | <0.001 |
| Triglycerides (mmol/L) | 1.13 (0.79–1.67) | 0.79 (0.62–1.06) | 1.00 (0.73–1.44) | 1.37 (0.99–1.96) | 1.62 (1.20–2.43) | <0.001 | <0.001 |
| Hemoglobin (g/L) | 133.0 (124.0–144.0) | 125.0 (119.0–132.0) | 129.0 (122.0–140.0) | 140.5 (130.0–148.0) | 143.0 (133.8–150.0) | <0.001 | <0.001 |
| Creatinine (μmol/L) | 66.0 (54.0–78.0) | 55.0 (50.0–64.0) | 61.0 (52.0–73.0) | 73.0 (60.0–81.0) | 75.0 (65.0–84.0) | <0.001 | <0.001 |
| Uric acid (μmol/L) | 282.0 (221.0–349.0) | 216.0 (183.0–268.0) | 255.0 (209.0–318.0) | 319.0 (261.8–379.0) | 350.0 (295.0–410.0) | <0.001 | <0.001 |
Data were presented as median (interquartile range) or n (%). Distinct body mass index (BMI) trajectories were identified using six BMI tests (during 2011–2016). *P‐values are calculated using Kruskal–Wallis test for continuous variables, and the χ2‐test for categorical variables. † P‐values for trend are calculated using Spearman's correlation for continuous variables, and the Cochran–Armitage trend test for binary variables. WC, waist circumference.
Association between distinct BMI trajectories and diabetes risk
A total of 168 (3.7%) new‐onset diabetes cases were identified in 2016. As shown in Table 2, the incidence of new‐onset diabetes progressively increased from the “low” to “high” BMI trajectory (0.6, 2.0, 5.1 and 9.1%, respectively). In model 1 adjusting for age and sex at baseline, the RR for new‐onset diabetes in the comparison between the “high” and “low” BMI trajectory was 9.17 (95% confidence interval [CI] 3.67–22.89). After further adjusting for all other potential covariates, the risk was attenuated and the RR for new‐onset diabetes in the comparison between the “high” and “low” BMI trajectory was 3.24 (95% CI 1.27–8.24). No significant interactions were observed between distinct BMI trajectories and sex, age and impaired fasting glucose in relation to risks of new‐onset diabetes (P‐values for interactions were 0.520, 0.389 and 0.849, respectively).
Table 2.
Relative risk and 95% confidence interval of new‐onset diabetes in 2016, by distinct body mass index trajectories and categories of baseline body mass index
| Variables | N | Cases (%) |
Model 1 Adjusted RR (95% CI) † |
Model 2 Adjusted RR (95% CI) ‡ |
Model 3 Adjusted RR (95% CI) § |
Model 4 Adjusted RR (95% CI) ¶ |
Model 5 Adjusted RR (95% CI) †† |
|---|---|---|---|---|---|---|---|
| BMI trajectories | 4,519 | 168 (3.7) | |||||
| Trajectory 1: Low | 884 | 5 (0.6) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Trajectory 2: Moderate | 1,511 | 30 (2.0) | 2.66 (1.04–6.81) | 2.48 (0.96–6.41) | 2.23 (0.86–5.82) | 1.66 (0.64–4.31) | 1.48 (0.56–3.88) |
| Trajectory 3: Moderate‐high | 1,510 | 77 (5.1) | 5.51 (2.24–13.54) | 5.07 (2.04–12.57) | 3.65 (1.45–9.14) | 2.47 (0.98–6.23) | 2.11 (0.84–5.32) |
| Trajectory 4: High | 614 | 56 (9.1) | 9.17 (3.67–22.89) | 8.26 (3.28–20.77) | 5.35 (2.12–13.52) | 3.24 (1.27–8.24) | 2.79 (1.09–7.11) |
| P‐value for trend | <0.001 | <0.001 | <0.001 | <0.001 | 0.001 | ||
| Baseline BMI (groups)‡‡ | 4,323 | 155 (3.6) | |||||
| First group (<20.4) | 848 | 7 (0.8) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | – |
| Second group (20.4≤ to <23.6) | 1,444 | 20 (1.4) | 1.26 (0.53–2.98) | 1.14 (0.47–2.76) | 0.94 (0.39–2.28) | 0.71 (0.30–1.68) | – |
| Third group (23.6≤ to <27.0) | 1,443 | 79 (5.5) | 3.71 (1.68–8.20) | 3.30 (1.47–7.39) | 2.16 (0.95–4.94) | 1.33 (0.57–3.08) | – |
| Fourth group (≥27.0) | 588 | 49 (8.3) | 5.34 (2.36–12.09) | 4.71 (2.07–10.72) | 2.72 (1.18–6.25) | 1.45 (0.62–3.41) | – |
| P‐value for trend | <0.001 | <0.001 | <0.001 | 0.020 | |||
| Baseline BMI (quartiles) | 4,323 | 155 (3.6) | |||||
| First quartile: (<20.9) | 1,083 | 9 (0.8) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | – |
| Second quartile: (20.9≤ to <23.4) | 1,079 | 15 (1.4) | 1.26 (0.55–2.88) | 1.16 (0.50–2.69) | 1.04 (0.45–2.43) | 0.83 (0.37–1.87) | – |
| Third quartile: (23.4≤ to <25.6) | 1,081 | 51 (4.7) | 3.38 (1.64–6.96) | 3.05 (1.46–6.38) | 2.28 (1.08–4.80) | 1.41 (0.67–2.95) | – |
| Fourth quartile (≥25.6) | 1,080 | 80 (7.4) | 4.92 (2.40–10.05) | 4.40 (2.14–9.03) | 2.76 (1.33–5.74) | 1.73 (0.84–3.55) | – |
| P‐value for trend | <0.001 | <0.001 | <0.001 | 0.008 |
Distinct body mass index (BMI) trajectories were identified using six BMI tests (during 2011–2016). †Model 1: adjusted for age and sex at baseline. ‡Model 2: further adjusted for race, occupation, current smoker, current alcohol drinker and married status at baseline. §Model 3: further adjusted for diagnosis of hypertension, use of antihypertensive, total cholesterol, triglycerides, hemoglobin, creatinine and uric acid at baseline. ¶Model 4: further adjusted for fasting blood glucose and family history of diabetes at baseline. ††Model 5: sensitivity analysis was carried out by excluding 196 participants (4,323 participants left) who had missing information on baseline BMI. ‡‡From the first to fourth group, the proportions of participants were 19.6, 33.4, 33.4 and 13.6%, respectively. The divisions were consistent with the proportions of BMI trajectories. RR, relative risk.
Comparison with traditional methods based on baseline BMI
Previous studies predicting diabetes risk usually divided baseline BMI into groups. In the present study, we first divided baseline BMI into four groups according to the proportions of BMI trajectories. Then, we divided baseline BMI into quartiles, which were in accordance with many previous studies28, 29. As shown in Table 2, the incidence of new‐onset diabetes progressively increased with higher BMI groups (0.8, 1.4, 5.5 and 8.3%, respectively), as well as with higher BMI quartiles (0.8, 1.4, 4.7 and 7.4%, respectively). However, in models adjusting for all potential covariates, no significant RRs for new‐onset diabetes were found in the comparison between the fourth and first group (P = 0.393), as well as in the comparison between the fourth and first quartile (P = 0.137).
By contrast, as described above, there was a significant RR for new‐onset diabetes in the comparison between “high” and “low” BMI trajectory (P = 0.014). In a sensitivity analysis, by excluding 196 participants who had missing data on baseline BMI, the RR for new‐onset diabetes in the comparison between the “high” and “low” BMI trajectory remained significant (P = 0.032; Table 2).
Association between BMI trajectories identified using fewer BMI tests and diabetes risk
Finally, we explored whether BMI trajectories identified using the first four or three annual BMI tests were associated with new‐onset diabetes in 2016 as well. When using the first four annual BMI tests, we also identified four linear BMI trajectories through the GBTM (Figure S1). As shown in Table 3, the incidence of new‐onset diabetes progressively increased from the “low” to “high” BMI trajectory (0.7, 2.4, 5.1 and 10.1%, respectively). In models adjusting for all potential covariates, the RR for new‐onset diabetes in the comparison between “high” and “low” BMI trajectory was 3.10 (95% CI 1.30–7.38). In a sensitivity analysis, by further excluding 114 participants who were diagnosed with diabetes or had no information about diabetes in 2014, the RR for new‐onset diabetes in the comparison between “high” and “low” BMI trajectory remained significant (P = 0.028; Table 3).
Table 3.
Relative risk and 95% confidence interval of new‐onset diabetes in 2016, by body mass index trajectories identified using fewer body mass index tests
| Variables | N † | Cases (%) |
Model 1 Adjusted RR (95% CI) ‡ |
Model 2 Adjusted RR (95% CI) § |
Model 3 Adjusted RR (95% CI) ¶ |
Model 4 Adjusted RR (95% CI) †† |
Model 5 Adjusted RR (95% CI) ‡‡ |
|---|---|---|---|---|---|---|---|
| BMI trajectories (four tests) | 4,475 | 167 (3.7) | |||||
| Trajectory 1: Low | 1,031 | 7 (0.7) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Trajectory 2: Moderate | 1,487 | 35 (2.4) | 2.66 (1.11–6.33) | 2.47 (1.03–5.94) | 2.07 (0.85–5.00) | 1.60 (0.67–3.82) | 3.65 (0.83–16.09) |
| Trajectory 3: Moderate‐high | 1,440 | 73 (5.1) | 5.13 (2.22–11.88) | 4.75 (2.03–11.10) | 3.28 (1.39–7.79) | 2.27 (0.96–5.38) | 4.14 (0.95–18.13) |
| Trajectory 4: High | 517 | 52 (10.1) | 9.50 (4.05–22.26) | 8.59 (3.64–20.27) | 5.22 (2.20–12.43) | 3.10 (1.30–7.38) | 5.46 (1.20–24.82) |
| P‐value for trend | <0.001 | <0.001 | <0.001 | <0.001 | 0.018 | ||
| BMI trajectories (three tests) | 4,408 | 166 (3.8) | |||||
| Trajectory 1: Low | 984 | 6 (0.6) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Trajectory 2: Moderate | 1,493 | 33 (2.2) | 2.49 (1.11–5.59) | 2.32 (1.02–5.27) | 2.04 (0.89–4.66) | 1.52 (0.67–3.45) | 2.35 (0.69–8.05) |
| Trajectory 3: Moderate‐high | 1,433 | 76 (5.3) | 4.37 (2.00–9.56) | 4.06 (1.84–8.95) | 2.85 (1.27–6.39) | 1.93 (0.86–4.34) | 3.44 (1.00–11.84) |
| Trajectory 4: High | 498 | 51 (10.2) | 8.27 (3.73–18.36) | 7.51 (3.36–16.82) | 4.81 (2.13–10.87) | 2.71 (1.19–6.19) | 4.67 (1.35–16.15) |
| P‐value for trend | <0.001 | <0.001 | <0.001 | 0.001 | 0.001 |
†For body mass index (BMI) trajectories identified using four BMI tests, 44 participants were excluded from the total population due to the analysis requiring at least two BMI tests. Similarly, for BMI trajectories identified using three BMI tests, 111 participants were excluded from the total population. ‡Model 1: adjusted for age and sex at baseline. §Model 2: further adjusted for race, occupation, current smoker, current alcohol drinker and married status at baseline. ¶Model 3: further adjusted for diagnosis of hypertension, use of antihypertensive, total cholesterol, triglycerides, hemoglobin, creatinine and uric acid at baseline. ††Model 4: further adjusted for fasting blood glucose and family history of diabetes at baseline. ‡‡Model 5: Patients with diabetes might lose or control their bodyweight. To avoid the potential impact of new‐onset diabetes during follow up on BMI, sensitivity analysis was carried out. For BMI trajectories identified using four BMI tests, 114 participants who were diagnosed with diabetes or had no information about diabetes in 2014 were further excluded. For BMI trajectories identified using three BMI tests, 87 participants who were diagnosed with diabetes or had no information about diabetes in 2013 were further excluded. CI, confidence interval; RR, relative risk.
Similarly, when using the first three annual BMI tests, we identified four linear BMI trajectories through the GBTM (Figure S1). The incidence of new‐onset diabetes progressively increased from the “low” to “high” BMI trajectory (0.6, 2.2, 5.3 and 10.2%, respectively; Table 3). In models adjusting for all potential covariates, the RR for new‐onset diabetes in the comparison between the “high” and “low” BMI trajectory was 2.71 (95% CI 1.19–6.19). In a sensitivity analysis, by further excluding 87 participants who were diagnosed with diabetes or had no information about diabetes in 2013, the RR for new‐onset diabetes in the comparison between “high” and “low” BMI trajectory remained significant (P = 0.015; Table 3). These results suggest that BMI trajectories based on just four or three annual BMI tests were significantly associated with subsequent new‐onset diabetes.
Discussion
In the present longitudinal study carried out during 2011–2016, we identified four distinct developmental trajectories of BMI by the GBTM. We found that distinct BMI trajectories were significantly associated with new‐onset diabetes in 2016. In addition, we found that BMI trajectories identified using the first four or three annual BMI tests were still significantly associated with new‐onset diabetes in 2016. By contrast, no significant correlation was found between categories of baseline BMI and new‐onset diabetes in 2016 after multivariate adjustment.
Although few previous studies have explored the association between BMI trajectories determined by GBTM and diabetes risk, the majority of them focused on the effect of childhood BMI trajectories on adult diabetes30, 31, 32 or the effect of BMI trajectories before and during early pregnancy on gestational diabetes33. For example, in a longitudinal cohort consisting of 2,449 USA residents, Tao et al.31 found that childhood (4–19 years) BMI trajectories have a significant impact on adult (20–51 years) diabetes. In an analysis of 8,009 Australian women aged 18–36 years, Kakoly et al.33 identified three distinct BMI trajectories across six surveys, and found that women in a high‐rising trajectory were independently associated with gestational diabetes, as compared with those in a low‐stable trajectory (OR 2.50, 95% CI 1.80–3.48). However, these studies carried out with children or pregnant women could not provide direct evidence for the primary prevention of diabetes in general adults.
For those studies directly carried out with general adults, three of them34, 35, 36 only showed the developmental trajectories of BMI before the diagnosis of diabetes, without examining the relative risk of diabetes across distinct BMI trajectories. In another study consisting of 24,875 Australian adults, Peter et al.37 showed that the incidence of diabetes was different across distinct BMI trajectories; however, this study did not adjust for potential covariates in the regression models. Therefore, whether distinct BMI trajectories predict diabetes risk could not be concluded from these studies. To date, there are just two studies18, 19 that have examined the relative risk of diabetes by distinct BMI trajectories in general adults. Nevertheless, both studies spanned decades. It remains uncertain whether distinct BMI trajectories have an impact on diabetes risk, even in a shorter period, such as several years. In addition, we could not apply the conclusions to inform diabetes prevention in China, as both studies were carried out in Western countries. Thus, more evidence is really needed.
To our knowledge, the present study is the first to describe the association between distinct BMI trajectories and diabetes risk in general Chinese adults. We found that distinct BMI trajectories, even identified using the first four or three annual BMI tests, were significantly associated with new‐onset diabetes. The findings suggest that distinct BMI trajectories might be an effective tool to predict future diabetes. In addition, no significant interactions between distinct BMI trajectories and sex, age and impaired fasting glucose in relation to risks of new‐onset diabetes were observed in the present study, implying that the effect of distinct BMI trajectories on diabetes risk was consistent across these factors.
The strengths of the present study included a longitudinal design, a large sample size, the availability of repeated measurements of BMI over time and the use of GBTM. However, some limitations should also be noted. First, the diagnosis of diabetes was based on fasting blood glucose without using the oral glucose tolerance test or hemoglobin A1c test, which is due to limited data collection for such a large cohort. Second, we did not collect information about the type of diabetes but, as the incident rate of type 1 diabetes is extremely low in Chinese adults38, the majority of new‐onset cases in our cohort should be type 2 diabetes. Third, approximately 30% of our study sample had missing values on at least one study variable. We thus used multiple imputation to increase the power of statistical tests. Multiple imputation is well recognized as a flexible and reliable tool for handling missing data, and has been widely used in clinical studies39. Fourth, the current study included only Chinese adults. As the BMI level and its implications for diabetes differ widely across various racial/ethnic groups9, the present findings might not be generalizable to other populations.
In conclusion, the present results show that distinct BMI trajectories, even identified using just four or three annual BMI tests, are significantly associated with new‐onset diabetes. The findings suggest that monitoring BMI trajectories over time might provide an important approach to identify subpopulations at higher risk for diabetes in general Chinese adults.
Disclosure
The authors declare no conflict of interest.
Supporting information
Figure S1 Distinct body mass index trajectories identified using (a) four body mass index tests and (b) three body mass index tests.
Acknowledgments
This study was supported by the National Science Foundation of China (81800393), the Natural Science Foundation of Hunan Province (2018JJ3783), and the Postgraduate Independent Exploration and Innovation Project of Central South University (2018zzts264).
J Diabetes Investig 2020; 11: 466–474
References
- 1. NCD Risk Factor Collaboration (NCD‐RisC) . Worldwide trends in diabetes since 1980: a pooled analysis of 751 population‐based studies with 4.4 million participants. Lancet 2016; 387: 1513–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang L, Gao P, Zhang M, et al Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA 2017; 317: 2515–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yang G, Wang Y, Zeng Y, et al Rapid health transition in China, 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet 2013; 381: 1987–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bommer C, Heesemann E, Sagalova V, et al The global economic burden of diabetes in adults aged 20–79 years: a cost‐of‐illness study. Lancet Diabetes Endocrinol 2017; 5: 423–430. [DOI] [PubMed] [Google Scholar]
- 5. Herman WH, Ye W, Griffin SJ, et al Early detection and treatment of type 2 diabetes reduce cardiovascular morbidity and mortality: a simulation of the results of the Anglo‐Danish‐Dutch study of Intensive Treatment in People With Screen‐Detected Diabetes in Primary Care (ADDITION‐Europe). Diabetes Care 2015; 38: 1449–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Simmons RK, Griffin SJ, Lauritzen T, et al Effect of screening for type 2 diabetes on risk of cardiovascular disease and mortality: a controlled trial among 139,075 individuals diagnosed with diabetes in Denmark between 2001 and 2009. Diabetologia 2017; 60: 2192–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Narayan KM, Boyle JP, Thompson TJ, et al Effect of BMI on lifetime risk for diabetes in the U.S. Diabetes Care 2007; 30: 1562–1566. [DOI] [PubMed] [Google Scholar]
- 8. Araneta MR, Kanaya AM, Hsu WC, et al Optimum BMI cut points to screen Asian Americans for type 2 diabetes. Diabetes Care 2015; 38: 814–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eriksson J. Developmental pathways and programming of diabetes: epidemiological aspects. J Endocrinol 2019; 242: T95–T104. [DOI] [PubMed] [Google Scholar]
- 10. Nagin DS, Jones BL, Passos VL, et al Group‐based multi‐trajectory modeling. Stat Methods Med Res 2018; 27: 2015–2023. [DOI] [PubMed] [Google Scholar]
- 11. Nagin DS. Group‐based trajectory modeling: an overview. Ann Nutr Metab 2014; 65: 205–210. [DOI] [PubMed] [Google Scholar]
- 12. Fan B, Yang Y, Dayimu A, et al Body mass index trajectories during young adulthood and incident hypertension: a longitudinal cohort in Chinese population. J Am Heart Assoc 2019; 8: e011937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. De Rubeis V, Cotterchio M, Smith BT, et al Trajectories of body mass index, from adolescence to older adulthood, and pancreatic cancer risk; a population‐based case‐control study in Ontario, Canada. Cancer Causes Control 2019; 30: 955–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kelly SP, Lennon H, Sperrin M, et al Body mass index trajectories across adulthood and smoking in relation to prostate cancer risks: the NIH‐AARP Diet and Health Study. Int J Epidemiol 2019; 48: 464–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang K, Chen X, Gerke TA, et al BMI trajectories and risk of overall and grade‐specific prostate cancer: an observational cohort study among men seen for prostatic conditions. Cancer Med 2018; 7: 5272–5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Murayama H, Shaw BA. Heterogeneity in trajectories of body mass index and their associations with mortality in old age: a literature review. J Obes Metab Syndr 2017; 26: 181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang M, Yi Y, Roebothan B, et al Trajectories of body mass index among Canadian seniors and associated mortality risk. BMC Public Health 2017; 17: 929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang M, Yi Y, Roebothan B, et al Body mass index trajectories among middle‐aged and elderly Canadians and associated health outcomes. J Environ Public Health 2016; 2016: 7014857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xian H, Vasilopoulos T, Liu W, et al Steeper change in body mass across four decades predicts poorer cardiometabolic outcomes at midlife. Obesity (Silver Spring) 2017; 25: 773–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li Y, Huang Z, Jin C, et al Longitudinal change of perceived salt intake and stroke risk in a Chinese population. Stroke 2018; 49: 1332–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Isakova T, Cai X, Lee J, et al Longitudinal FGF23 trajectories and mortality in patients with CKD. J Am Soc Nephrol 2018; 29: 579–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sun L, Liang L, Gao X, et al Early prediction of developing type 2 diabetes by plasma acylcarnitines: a population‐based study. Diabetes Care 2016; 39: 1563–1570. [DOI] [PubMed] [Google Scholar]
- 23. Dai H, Wang W, Tang X, et al Association between homocysteine and non‐alcoholic fatty liver disease in Chinese adults: a cross‐sectional study. Nutr J 2016; 15: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dai H, Wang W, Chen R, et al Lipid accumulation product is a powerful tool to predict non‐alcoholic fatty liver disease in Chinese adults. Nutr Metab (Lond) 2017; 14: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jones BL, Nagin DS. A note on a Stata plugin for estimating group‐based trajectory models. Sociol Method Res 2013; 42: 608–613. [Google Scholar]
- 26. Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004; 159: 702–706. [DOI] [PubMed] [Google Scholar]
- 27. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011; 30: 377–399. [DOI] [PubMed] [Google Scholar]
- 28. Wang Z, Hoy WE. Waist circumference, body mass index, hip circumference and waist‐to‐hip ratio as predictors of cardiovascular disease in Aboriginal people. Eur J Clin Nutr 2004; 58: 888–893. [DOI] [PubMed] [Google Scholar]
- 29. Song BM, Kim HC, Kim DJ, et al Aminotransferase levels, body mass index, and the risk of diabetes: a prospective cohort study. Ann Epidemiol 2018; 28: 675–680 e676. [DOI] [PubMed] [Google Scholar]
- 30. Thearle MS, Votruba SB, Piaggi P, et al The effect of differing patterns of childhood body mass index gain on adult physiology in American Indians. Obesity (Silver Spring) 2015; 23: 1872–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang T, Xu J, Li S, et al Trajectories of childhood BMI and adult diabetes: the Bogalusa Heart Study. Diabetologia 2019; 62: 70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Buscot MJ, Thomson RJ, Juonala M, et al Distinct child‐to‐adult body mass index trajectories are associated with different levels of adult cardiometabolic risk. Eur Heart J 2018; 39: 2263–2270. [DOI] [PubMed] [Google Scholar]
- 33. Kakoly NS, Earnest A, Moran LJ, et al Group‐based developmental BMI trajectories, polycystic ovary syndrome, and gestational diabetes: a community‐based longitudinal study. BMC Med 2017; 15: 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chiu CJ, Li SL, Wu CH, et al BMI trajectories as a harbinger of pre‐diabetes or underdiagnosed diabetes: an 18‐year retrospective cohort study in Taiwan. J Gen Intern Med 2016; 31: 1156–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vistisen D, Witte DR, Tabak AG, et al Patterns of obesity development before the diagnosis of type 2 diabetes: the Whitehall II cohort study. PLoS Med 2014; 11: e1001602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kuwahara K, Honda T, Nakagawa T, et al Body mass index trajectory patterns and changes in visceral fat and glucose metabolism before the onset of type 2 diabetes. Sci Rep 2017; 7: 43521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Peter RS, Keller F, Klenk J, et al Body mass trajectories, diabetes mellitus, and mortality in a large cohort of Austrian adults. Medicine (Baltimore) 2016; 95: e5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Weng J, Zhou Z, Guo L, et al Incidence of type 1 diabetes in China, 2010–13: population based study. BMJ 2018; 360: j5295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li P, Stuart EA, Allison DB. Multiple imputation: a flexible tool for handling missing data. JAMA 2015; 314: 1966–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Distinct body mass index trajectories identified using (a) four body mass index tests and (b) three body mass index tests.


