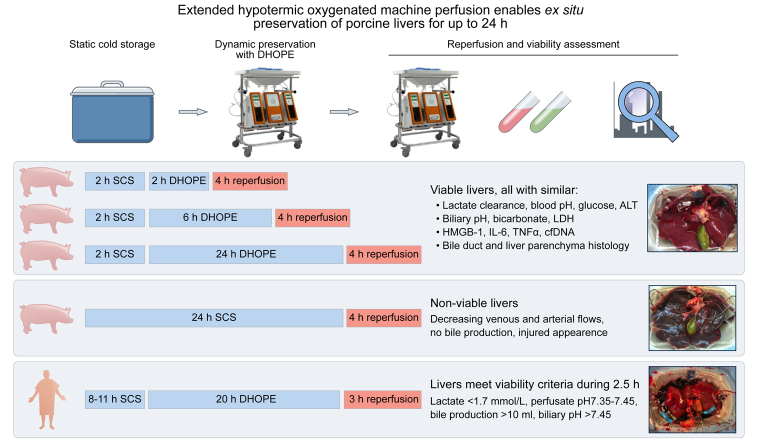
Abstract
Background & Aims
End-ischemic hypothermic oxygenated machine perfusion (HOPE) of the donor liver for 1–2 h mitigates ischemia-reperfusion injury during subsequent liver transplantation. Extended preservation time may be preferred to facilitate difficult recipient hepatectomy or to optimize logistics. We therefore investigated whether end-ischemic dual HOPE (DHOPE) could extend preservation time for up to 24 h using a porcine liver reperfusion model.
Methods
Following 30 min warm ischemia, porcine livers were subjected to 2 h static cold storage (SCS), followed by 2 h, 6 h, or 24 h DHOPE (n = 6 per group). Subsequent normothermic reperfusion was performed for 4 h using autologous blood. Two livers preserved by 24 h SCS served as additional controls. A proof of principle confirmation was carried out in 2 discarded human livers subjected to extended DHOPE. Hepatocellular and cholangiocyte injury and function were assessed. Oxidative stress levels and histology were compared between groups.
Results
Perfusion flows remained stable during DHOPE, regardless of duration. After normothermic reperfusion, livers perfused for 24 h by DHOPE had similar lactate clearance, blood pH, glucose, and alanine aminotransferase levels, and biliary pH, bicarbonate, and LDH levels, as livers perfused for 2 h and 6 h. Levels of malondialdehyde and high-mobility group box 1 in serum and liver parenchyma were similar for all groups. Histological analysis of bile ducts and liver parenchyma revealed no differences between the groups. Extended DHOPE in discarded human livers preserved hepatocellular and cholangiocyte function and histology after reperfusion. In contrast, livers preserved by 24 h SCS were non-functioning.
Conclusion
Extended end-ischemic DHOPE enabled successful preservation of porcine and discarded human donor livers for up to 24 h. Extended DHOPE enables safe extension of preservation time, which may facilitate allocation and transplantation from a logistical perspective, and further expand the donor pool.
Lay summary
It has been suggested that preserving liver grafts with a technique called (dual) hypothermic oxygenated machine perfusion ([D]HOPE) leads to better outcomes after transplantation than if livers are stored on ice, especially if an organ is of lesser quality. In this study, we showed that DHOPE could be used to preserve liver grafts for up to 24 h. This extended procedure could be used globally to facilitate transplantation and expand the donor pool.
Keywords: hypothermic machine perfusion, liver preservation, extended preservation, donation after circulatory death
Abbreviations: 8-OHdG, 8-hydroxydeoxyguanosine; ALT, alanine aminotransferase; cfDNA, cell-free DNA; DCD, donation after circulatory death; DHOPE, dual hypothermic oxygenated machine perfusion; ECD, extended criteria donor; HMGB-1, high-mobility group box 1; HMP, hypothermic machine perfusion; HOPE, hypothermic oxygenated machine perfusion; HPF, high-powered field; IL-6, interleukin 6; LDH, lactate dehydrogenase; MDA, malondialdehyde; NMP, normothermic machine perfusion; SCS, static cold storage; SEM, standard error of the mean; sTM, soluble thrombomodulin; TNFα, tumor necrosis factor-alpha; UW, University of Wisconsin; VWF, von Willebrand factor
Graphical abstract
Highlights
-
•
DHOPE can be extended for up to 24 h to prolong donor liver preservation time.
-
•
Hepatocellular and biliary function is maintained after ex situ preservation by 24 h DHOPE.
-
•
Extension of DHOPE for up to 24 h does not induce more injury compared to shorter preservation times.
-
•
The initial results of extended preservation by DHOPE for discarded human livers are promising.
Introduction
Preservation using ex situ machine perfusion has gained considerable interest as a method of increasing the utilization of liver grafts for transplantation, albeit with several technical variations.1 While normothermic machine perfusion (NMP) enables hepatobiliary viability assessment and therapeutic interventions prior to transplantation, hypothermic oxygenated machine perfusion (HOPE) reconditions the graft by inducing a hypometabolic state whilst restoring mitochondrial function through delivery of oxygen.1 End-ischemic HOPE is a relatively simple approach. Livers are preserved by static cold storage (SCS) after procurement and are then subjected to machine perfusion upon arrival at the recipient center. Dynamic preservation by HOPE for 1-2 h is sufficient to mitigate organ damage and restore mitochondrial function and cellular energy stores, resulting in postoperatively reduced graft failure.2
Previously, we have shown that a short period (2 h) of dual HOPE (DHOPE) improved hepatobiliary function and decreased injury to the liver graft and biliary tree in discarded human livers.3,4 In a prospective cohort study, 2 h of end-ischemic DHOPE was shown to be feasible and safe for resuscitating donation after circulatory death (DCD) liver grafts with 100% 1-year graft and patient survival after transplantation.5 Another important finding was that DHOPE attenuates injury of the biliary tree after transplantation of DCD liver grafts.6 In 2015, our center initiated a large international multicenter randomized controlled trial comparing DHOPE to SCS for DCD liver transplantation, with the first results expected by mid-2020.7
In addition to graft reconditioning, viability assessment, and potential therapeutic interventions, machine perfusion may also have the potential to prolong preservation time. Traditionally, graft preservation using SCS may keep good quality livers viable for transplantation for several hours. Machine perfusion, however, may extend preservation times, particularly in extended criteria donor (ECD) organs which are more vulnerable to cold ischemia.[8], [9], [10] Extended preservation by HOPE, for example, could facilitate logistics for allocation and transplantation. As a proof-of-concept, a human discarded liver has been preserved for 86 h using ex situ NMP,11 and an initially declined human liver has successfully been transplanted after preservation for 26 h, of which 17.5 h was SCS and 8.5 h was NMP.12 However, few data are available regarding extended graft preservation by DHOPE, and, currently, the maximum reported preservation time using HOPE is 8 h.10,13,14
We investigated the effects of extended preservation by applying end-ischemic DHOPE in a porcine liver ischemia-reperfusion injury model. Our endpoints included graft viability after warm reperfusion and various markers of injury during machine perfusion, and after warm reperfusion. In addition, a proof of principle confirmation of end-ischemic DHOPE preservation was carried out in discarded human livers.
Materials and methods
Porcine donation after circulatory death liver procurement
Livers from 5-month-old white female landrace pigs were retrieved after circulatory death. Pigs were sacrificed by a standardized procedure of electrocution followed by exsanguination. Two liters of autologous blood was collected in a container with 25,000 IU of heparin (heparin LEO 5000 IU/ml, LEO Pharmaceutical Products, Denmark). Blood was then stored in bags supplemented with the anticoagulant citrate-phosphate-dextrose (Sanquin, Amsterdam, the Netherlands) and cold stored (4°C) until subsequent use. Within 30 min after circulatory death, livers were flushed by gravity via the portal vein with 1 L of cold (4°C) NaCl 0.9% (Baxter BV, Utrecht, the Netherlands) supplemented with 25,000 IU of heparin, followed by 2 L of cold University of Wisconsin (UW) solution (Bridge to Life, Ltd, London, United Kingdom). After portal flush, the aorta was cannulated, and side branches were clipped followed by cold arterial flush out with UW solution using a syringe. The cystic duct was ligated, and the common bile duct was cannulated with a bile cannula (8 Fr, Organ Assist, Groningen, the Netherlands). Livers were static cold stored (4°C) in UW solution for 2 h. The porcine livers used in the present study were retrieved from a slaughterhouse (Kroon, Groningen, the Netherlands) where humane circumstances are applied according to national legislation. According to the Dutch law, no institutional approval is needed when using a slaughterhouse model.
Dual hypothermic oxygenated machine perfusion
After SCS, grafts were randomly assigned to either 2 h DHOPE (DHOPE-2), 6 h (DHOPE-6), or 24 h DHOPE (DHOPE-24) (n = 6 per group). Machine perfusion was performed using the Liver Assist device (Organ Assist, Groningen, the Netherlands).15 Livers were perfused with 2 L Belzer UW machine perfusion solution (Bridge to Life, Ltd, London, United Kingdom), oxygenated with 100% O2. Temperature was maintained between 8–10°C. Pressures were limited to 25 mmHg in the hepatic artery and 3 mmHg in the portal vein. Perfusate samples were collected from the arterial inflow cannula at the end of preservation. Liver tissue samples were obtained after procurement, at the end of DHOPE preservation, and at the end of warm reperfusion.
Ex situ normothermic oxygenated whole blood reperfusion
After extended DHOPE preservation for 2 h, 6 h, or 24 h, liver grafts were flushed by gravity with 1 L of cold (4°C) saline solution and 1 L of saline at room temperature. Livers were then transferred to a second Liver Assist device and reperfused at 37°C with autologous whole blood. Whole blood was used during this phase to simulate clinical transplantation. Sodium bicarbonate (B. Braun Medical, Melsungen, Germany) was added in the first hour after reperfusion to adjust to a physiological pH. The blood was oxygenated with a carbogen mixture of 95% O2 and 5% CO2 at 1 L/min. The portal vein was perfused continuously at a pressure of 11 mmHg, and the hepatic artery was perfused with a pulsatile flow at a mean pressure of 70 mmHg. Perfusate samples were taken before reperfusion, and at 5, 60, 120, 180, and 240 min after reperfusion. Arterial blood gas samples were taken before reperfusion, and every 30 min thereafter. Partial oxygen pressure, Hb, pH, glucose, and lactate were measured using the i-STAT clinical analyzer (Abbot Point of Care Inc., Princeton, NJ). Bile production was measured gravimetrically throughout reperfusion. Every hour, bile was collected under mineral oil, as described previously,16 and biliary pH, bicarbonate and lactate dehydrogenase (LDH) levels were measured.
As controls, 2 porcine livers were preserved with SCS (4°C) in UW-solution for 24 h, followed by 4 h of normothermic reperfusion with whole blood as described above.
Outcomes
Hepatocellular injury after warm reperfusion was assessed by alanine aminotransferase (ALT) levels, using a standardized laboratory method. Nuclear subcellular injury was measured by release of high mobility group box-1 protein (HMGB-1) using a specific ELISA (IBL International GmbH, Fujioka, Japan). Cell-free DNA (cfDNA) was used as a marker of cell damage and necrosis and measured as described previously.17 Tumor necrosis factor alpha (TNFα) and interleukin 6 (IL-6) levels were measured by ELISA (Biotechne, Abingdon, UK). Lipid peroxidation, a marker of oxidative stress, was quantified by measurement of malondialdehyde (MDA) in the perfusate and liver biopsies.15 MDA levels in liver parenchyma were corrected for the amount of protein (Bradford assay, Bio-Rad, Hercules, USA). In addition, 8-hydroxy-2-deoxy guanosine (8-OHdG) was measured (FineTest, Wuhan, China) as a product of oxidative damage of DNA by reactive oxygen and nitrogen species. Soluble thrombomodulin (sTM) was determined using a commercially available ELISA (Biotechne, Abingdon, UK).18 Hepatic content of ATP was used as an indicator of energy status of grafts before and after DHOPE. For this purpose, liver biopsies were immediately frozen in liquid nitrogen and later processed for ATP measurement, as described previously.19
Histological analysis
Liver biopsies were fixed in formalin and embedded in paraffin. We performed H&E staining to assess necrosis and von Willebrand factor (VWF) staining to assess endothelial activation. Quantification of necrosis and VWF-positive endothelial cells was determined in 3 random visual fields (20x). Biopsies of the extrahepatic bile ducts were taken after procurement and at the end of reperfusion. After H&E staining, biliary injury was assessed using a modified scoring system as described by Op den Dries et al.20 Scoring was conducted in a blinded fashion by 2 independent investigators.
Human protocol
Human application of extended DHOPE was performed as a preclinical study using discarded human liver grafts. The donors' families gave consent for research. Donor livers were static cold stored on ice after procurement and transferred to our hospital. To mimic clinical practice at our center as closely as possible, grafts were subjected to our clinically used DHOPE-COR-NMP protocol.21 Extended 20 h end-ischemic DHOPE preservation was applied using UW machine perfusion solution. The timeframe of 20 h instead of 24 h was chosen for logistical reasons. After DHOPE preservation, UW was changed to a perfusion solution containing a hemoglobin-based oxygen carrier, as described previously.22 In accordance with our clinical protocol, discarded human livers subsequently underwent 1 h of controlled oxygenated rewarming (10°C to 37°C) followed by normothermic (37°C) reperfusion and liver viability testing for an additional 4 h.21
Statistics
All values are expressed as means ± standard error of the mean (SEM) for n = 6 animals per group. Differences between the groups were tested using the Kruskal-Wallis test unless stated otherwise. A 2-sided p value less than 0.05 was considered to be significant. Analyses were performed using IBM SPSS software version 25 for windows.
For further details regarding materials and methods used, please refer to the CTAT table.
Results
Ischemia times
There were no significant differences in warm and cold ischemia times between the groups. Mean warm ischemia time was 24 ± 2 min for DHOPE-2, 22 ± 2 min for DHOPE-6, and 22 ± 1 min for DHOPE-24 livers (p = 0.847). Mean cold ischemia time was 129 ± 11 min for DHOPE-2 livers, 122 ± 5 min for DHOPE-6 livers, and 122 ± 5 min for DHOPE-24 livers (p = 0.890).
Flows during DHOPE
The perfusate temperature was maintained at 10°C throughout all perfusions. In addition, portal venous and hepatic arterial flows remained stable in all livers throughout the preservation period. Mean arterial flow at the end of DHOPE was 104.8 ± 29.2 ml/min in the DHOPE-2 group, 104.3 ± 35.2 ml/min in the DHOPE-6 group, and 149.3 ± 41.7 ml/min in the DHOPE-24 group (p = 0.676). Mean portal flow at the end of DHOPE was 311.7 ± 49.6 ml/min in the DHOPE-2 group, 282.0 ± 72.2 ml/min in the DHOPE-6 group, and 193.3 ± 16.3 ml/min in the DHOPE-24 group (p = 0.219).
Liver graft and bile duct viability after extended DHOPE
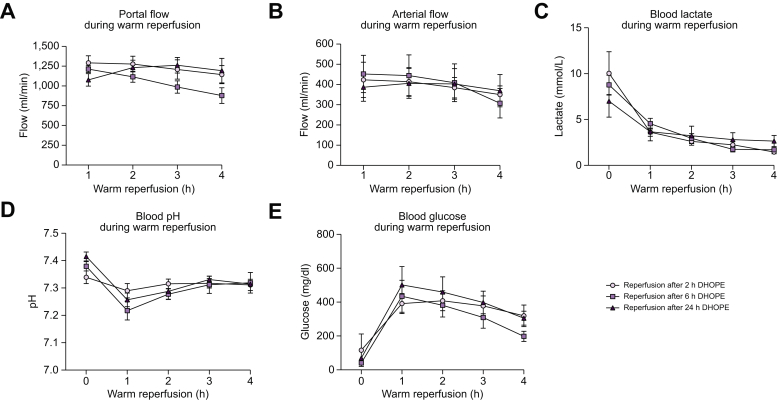
Warm reperfusion was used to assess liver graft viability after extended preservation by 24 h DHOPE. Portal (Fig. 1A) as well as arterial (Fig. 1B) flows steadily increased in all liver grafts upon reperfusion. There were no significant differences between the groups. In all groups, all livers cleared lactate (Fig. 1C). Perfusate pH was adjusted within the first hour after reperfusion by adding bicarbonate. There were no significant differences in the amount of added bicarbonate between the groups. After the first hour, no further corrections were needed, and pH remained stable during all liver perfusions (Fig. 1D). Starting 1 h after reperfusion, perfusate glucose levels gradually decreased in all livers (Fig. 1E).
Fig. 1.
Perfusion flows and markers of liver function during 4 h of normothermic reperfusion after either 2 h, 6 h, or 24 h of DHOPE.
(A) Portal venous flow, (B) hepatic artery flow, (C) lactate concentration, (D) perfusate pH, (E) glucose levels. All livers show good liver function during reperfusion after preservation for either 2 h, 6 h, or 24 h of DHOPE. Shown here are the mean and SEM for n = 6 livers per group. There were no significant differences between the groups in all markers shown (Kruskal-Wallis test). DHOPE, dual hypothermic oxygenated machine perfusion.
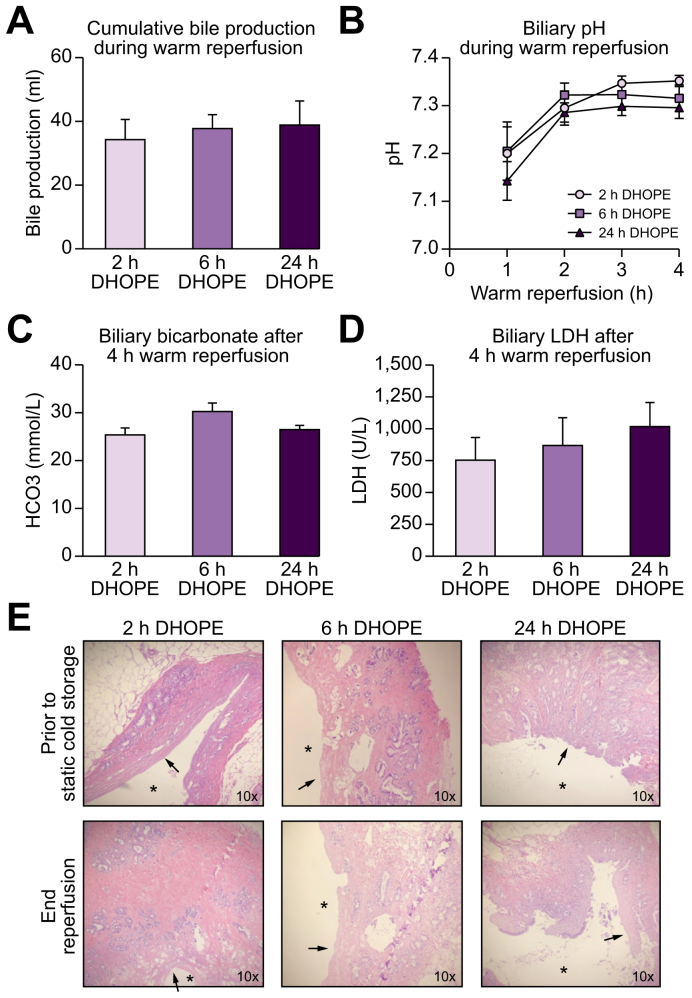
All livers produced sufficient quantities of bile, without differences in cumulative bile production (Fig. 2A). In addition, bile duct viability was assessed during 4 h of NMP. In all groups, biliary pH and bicarbonate increased during 4 h of warm reperfusion. Levels of biliary pH, bicarbonate and LDH were similar in all groups at the end of reperfusion (Fig. 2B-D). In this porcine model of DCD livers, we observed extensive loss of biliary epithelial lining immediately after procurement (30 min of warm ischemia). This was consistent with previous findings.20 At the end of warm reperfusion, bile ducts of all livers displayed signs of bile duct injury. Histological comparison of bile ducts revealed similar injury at the end of reperfusion in all groups (Fig. 2E, Fig. S1).
Fig. 2.
Biliary function during 4 h of normothermic reperfusion.
(A) Cumulative bile production during reperfusion, (B) biliary pH, (C) biliary bicarbonate at the end of reperfusion, (D) biliary LDH at the end of reperfusion. All livers show good biliary function after preservation for either 2 h, 6 h, or 24 h of DHOPE and there were no significant differences between the groups in all parameters shown (Kruskal-Wallis test). (E) Histology of extrahepatic bile ducts, ∗ = bile duct lumen, arrow = absence of luminal epithelium. Shown here are the mean and SEM for n = 6 livers per group. DHOPE, dual hypothermic oxygenated machine perfusion; LDH, lactate dehydrogenase.
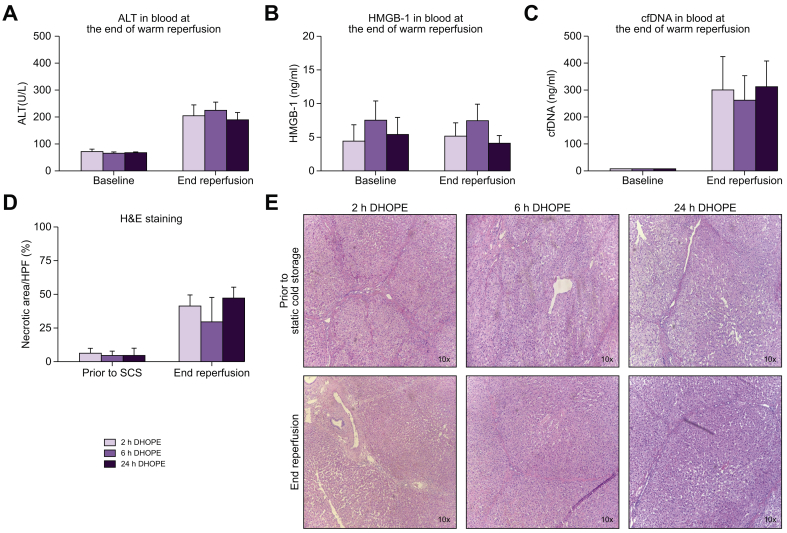
Next, we investigated the degree of hepatocellular injury after DHOPE preservation for 2 h, 6 h and 24 h. We analyzed several markers for cellular injury at the end of 4 h of reperfusion. Levels of ALT (Fig. 3A; p = 0.687), HMGB-1 (Fig. 3B; p = 0.690), or cfDNA (Fig. 3C; p = 0.229) in the perfusate were not elevated by extended DHOPE preservation. Histological analysis of liver parenchyma revealed that DHOPE preservation for up to 24 h did not result in more necrosis after reperfusion compared to shorter preservation times (p = 0.396) (Fig. 3D and E).
Fig. 3.
Markers for cellular injury at the end of normothermic reperfusion.
Perfusate levels of (A) ALT (p = 0.687), (B) HMGB-1 (p = 0.690), (C) and cfDNA (p = 0.229). There were no statistically significant differences between the groups at the end of reperfusion (Kruskal-Wallis test). (D) percentage of necrosis on histological analysis of liver parenchyma after H&E staining, (p = 0.396) (E) H&E staining of liver parenchyma. Shown here are the mean and SEM for n = 6 livers per group. ALT, alanine aminotransferase; cfDNA, cell-free DNA; DHOPE, dual hypothermic oxygenated machine perfusion; HMGB-1, high-mobility group 1; SCS, static cold storage.
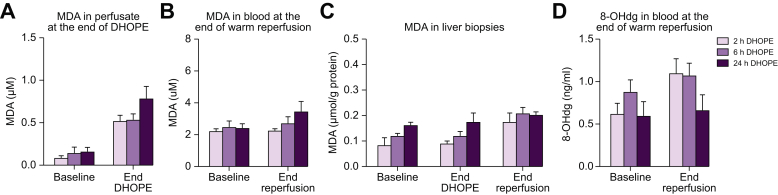
Since livers were perfused for up to 24 h with an oxygenated solution, we investigated markers for oxidative stress at the end of DHOPE preservation and after 4 h warm reperfusion. Extended preservation was not associated with increased levels of MDA in the perfusate at the end of DHOPE (Fig. 4A; p = 0.312), nor in blood at the end of warm reperfusion (Fig. 4B; p = 0.308). Extended DHOPE preservation was also not associated with increased levels of MDA in liver biopsies at the end of DHOPE (p = 0.125), nor at the end of reperfusion (p = 0.604) (Fig. 4C). Perfusate 8-OHdG measured at the end of warm reperfusion was similar in all groups (p = 0.172) (Fig. 4D).
Fig. 4.
Markers related to oxidative stress at the end of DHOPE and normothermic reperfusion.
(A) MDA in perfusate at the end of DHOPE (p = 0.312), (B) MDA in liver parenchyma at the end of DHOPE (p = 0.125) and reperfusion (p = 0.604), (C) MDA in perfusate at the end of normothermic reperfusion (p = 0.308), (D) 8-OHdg in the perfusate at the end of normothermic reperfusion (p = 0.172). There were no statistically significant differences between the groups on all parameters (Kruskal-Wallis test). 24 h of DHOPE preservation does not induce oxidative injury compared to shorter preservation times. Shown here are the mean and SEM for n = 6 animals per group. 8-OHdG, 8-hydroxydeoxyguanosine; DHOPE, dual hypothermic oxygenated machine perfusion; MDA, malondialdehyde.
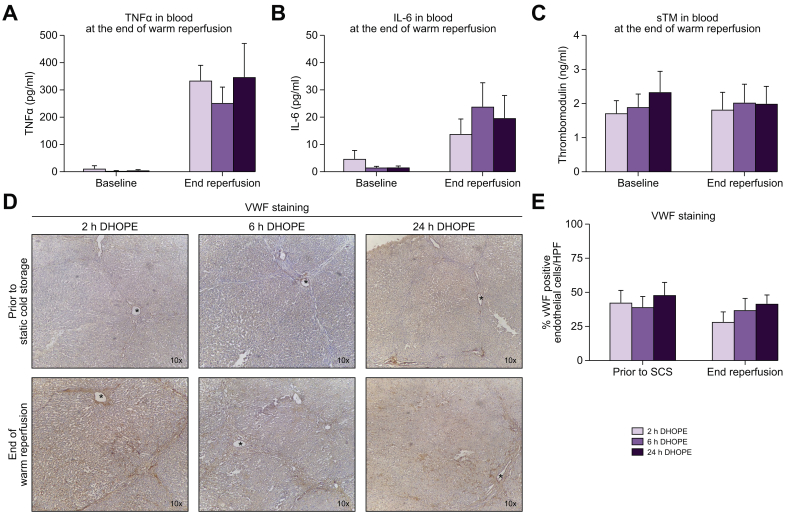
Extended DHOPE preservation was not associated with increased release of inflammatory cytokines TNF-a and IL-6. Mean TNF-a was 334.4 ± 55.8 pg/ml for DHOPE-2, 252.3 ± 57.8 pg/ml for DHOPE-6, and 347.3 ± 123.2 pg/ml for DHOPE-24 preserved livers, respectively (p = 0.692) (Fig. 5A). Mean IL-6 was 13.9 ± 5.5 pg/ml for DHOPE-2, 23.9 ± 8.7 pg/ml for DHOPE-6, and 19.7 ± 8.2 pg/ml for DHOPE-24 preserved livers, respectively (p = 0.578) (Fig. 5B).
Fig. 5.
Endothelial cell activation and levels of soluble thrombomodulin during reperfusion.
(A) TNFα in perfusate (p = 0.692), (B) IL-6 in perfusate (p = 0.578), (C) soluble thrombomodulin in perfusate (p = 0.985) (D) VWF staining, (E) percentage VWF positive endothelial cells per HPF. There were no statistically significant differences between the groups at the end of reperfusion (Kruskal-Wallis test). Preservation by 24 h of DHOPE did not induce more injury to endothelial cells compared to shorter preservation times. DHOPE, dual hypothermic oxygenated machine perfusion; HPF, high-power field; IL-6, interleukin 6; TNFα, tumor necrosis factor alpha; SCS, static cold storage; sTM, soluble thrombomodulin; VWF, von Willebrand factor.
Finally, we investigated the degree of endothelial injury after prolonged DHOPE preservation. Levels of sTM were not affected by prolonged DHOPE (p = 0.985) (Fig. 5C). Intensity of VWF staining did not reveal more activation of endothelial cells on histological analysis (Fig. 5D and E).
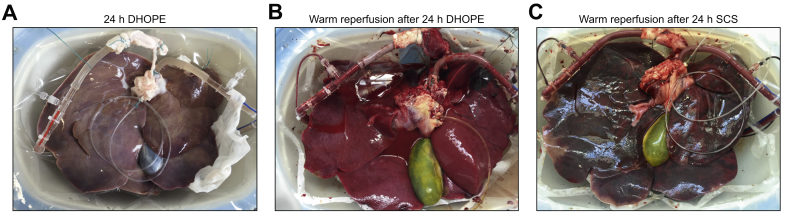
24 h SCS preserved livers
As a control group, 2 porcine livers were preserved by SCS for 24 h. Subsequent warm reperfusion was performed for 4 h to investigate viability. Macroscopically, both livers preserved by 24 h SCS depict large, patchy dark and greyish areas. During 4 h of warm reperfusion of these grafts, perfusion flow rates gradually decreased, bile production was absent, pH was decreased to <7.0, and lactate in the perfusate reached >8mmol/L at the end of reperfusion. Fig. 6A and B depict a porcine liver with macroscopically normal appearance after 24 h of DHOPE preservation and 4 h of subsequent NMP. In contrast, Fig. 6C shows normothermic reperfusion after 24 h preservation by SCS, demonstrating severe hemorrhagic injury. Because of the severe degree of injury after 24 h SCS preservation (i.e., non-functioning grafts), a further increase in the number of livers in the 24 h SCS group was deemed futile.
Fig. 6.
Representative pictures.
(A) porcine liver at the end of 24 h of DHOPE preservation, (B) porcine liver during reperfusion after 24 h of DHOPE preservation, (C) porcine liver during reperfusion after 24 h of SCS preservation. Clearly, livers preserved by 24 h of SCS depict large patchy dark and greyish areas, suggestive of a failing graft. DHOPE, dual hypothermic oxygenated machine perfusion; SCS, static cold storage.
Human preclinical experience in extended DHOPE
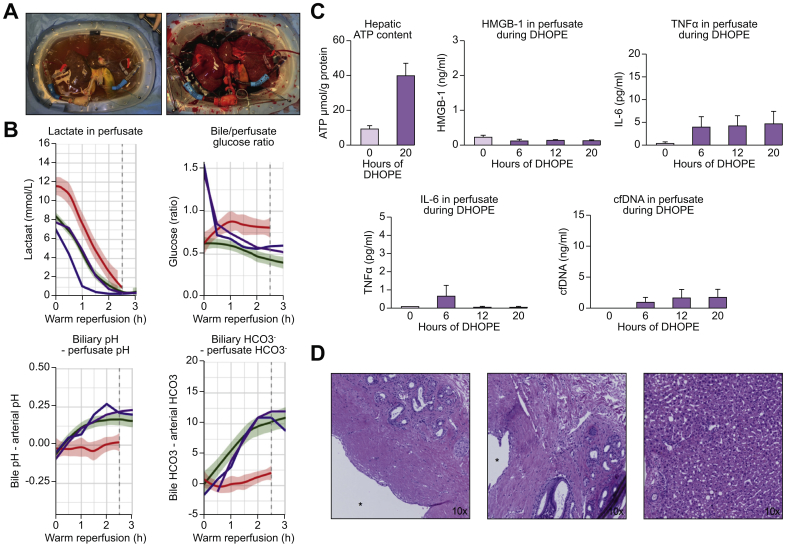
Finally, we investigated whether the observations in our porcine model could also be applied to clinical liver transplantation. Therefore, we perfused 2 discarded human donor livers using a protocol currently used at our center (Fig. 7A).21 The first liver was from a 71-year old DCD donor, with a BMI of 29, history of smoking, macroscopic hepatic steatosis of 40%, 121 min between withdrawal of life support and death, a warm ischemia time of 16 min, and 668 min of SCS. The second graft was from a 59-year old donation after brain death donor, with a BMI of 31, a history of smoking and hypertension, and a SCS time of 466 min. This liver was discarded by another transplant center because of a macroscopic appearance of poor perfusion, and subsequently rejected for transplantation nationwide. These human liver grafts were preserved by traditional SCS during transportation from the donor center and then subjected to extended DHOPE for 20 h. After 1 h of gradual controlled oxygenated rewarming followed by an additional 4 h of normothermic reperfusion, both grafts met the viability criteria that we use in our practice (Fig. 7B). Fig. 7B shows data from our prospective clinical trial, in which the green margins depict livers that were deemed viable after 2.5 h normothermic reperfusion.21 The purple and blue lines represent both human livers included in this study. After 2.5 h of reperfusion, both livers met the viability criteria, based on lactate clearance, the delta of biliary and arterial pH, the biliary/arterial glucose ratio, and the delta of biliary and arterial bicarbonate. Hepatic ATP content was increased 3–5-fold during 20 h of DHOPE preservation (Fig. 7C). Perfusate levels of HMGB-1, TNF-a, IL-6, and cfDNA remained low in both livers during the entire period of DHOPE (Fig. 7C). At the end of reperfusion, histology of the extrahepatic bile ducts, the intrahepatic bile ducts as well as the liver parenchyma revealed remarkable preservation with only minor cholangiocellular and hepatocellular injury (Fig. 7D).
Fig. 7.
20 h of DHOPE preservation of discarded human liver grafts.
(A) pictures of a human liver at the end of DHOPE preservation (left) and during normothermic reperfusion (right). (B) Markers for graft viability. The green margins depict historical data of clinically perfused livers that were deemed viable after 2.5 h of normothermic perfusion and thus accepted for transplantation. The red margins depict historical data of livers that were deemed non-viable after 2.5 h of reperfusion, which were secondarily discarded for transplantation. The purple and blue lines represent the 2 livers included in this study. Both livers follow the green margins, indicative of a viable graft. Shown are lactate clearance, the delta of biliary and arterial pH, the biliary/arterial glucose ratio, and the delta of biliary and arterial bicarbonate. After 2.5 h, both livers meet the viability criteria. (C) Hepatic ATP content at the end of DHOPE, and perfusate levels of HMGB-1, TNFα, IL-6, and cfDNA during DHOPE preservation. (D) H&E staining of extrahepatic bile ducts (left), intrahepatic bile ducts (middle), and liver parenchyma (right), ∗ = bile duct lumen. cfDNA, cell-free DNA; DHOPE, dual hypothermic oxygenated machine perfusion; HMGB-1, high-mobility group 1; IL-6, interleukin 6; TNFα, tumor necrosis factor alpha.
Discussion
In this study we have shown that in a porcine DCD model with 30 min of warm ischemia, livers preserved by DHOPE remained viable for at least 24 h, whereas 24 h preservation by SCS resulted in non-viable grafts.23,24 DHOPE for up to 24 h appears to be feasible to extend ex situ liver graft preservation times.
The results described in this paper provide evidence that DHOPE may substantially extend liver preservation times. These findings are important for several reasons. First, extended DHOPE could remove logistical constraints to organ allocation. It may be useful to extend preservation times in cases of suboptimal livers that are more difficult to allocate. Second, this technique can be favored when livers need to be transported across regions of large countries (e.g. the United States), or between countries (e.g. Eurotransplant). At the same time, it may reduce transportation costs when livers can be shipped with commercial flights instead of chartered jets. Lastly, storage time can be prolonged in cases of logistical issues at the recipient center (lack of operating rooms or medical teams), or to schedule transplantation surgery the next day instead of during the night, since the latter has been associated with a greater risk of morbidity and mortality.25
Up to now, it has been shown that short-term (1–2 h) (D)HOPE prior to transplantation is an effective approach to mitigate ischemia-reperfusion injury by slowing down mitochondrial respiration.2 The present study shows that DHOPE can also be used to extend preservation times. Only a few studies investigated extended cold perfusion of donor livers.8,26 Belzer and colleagues were the first who managed to preserve and transplant good quality canine livers after 72 h hypothermic machine perfusion (HMP).8 More than a decade later, Xu et al. published a study on 24 h preservation by HMP in a rat reperfusion model, but without very good results.26 At the end of 30 min reperfusion, LDH was higher in the HMP group than the SCS group, indocyanine green clearance was similar between the groups, and hepatic hyaluronic acid uptake showed severely impaired hepatic sinusoidal endothelial cells after 24 h of HMP. In contrast to our study, Xu and colleagues did not use an oxygenated perfusion solution. It has been shown that complete absence of oxygen during HMP triggers hepatocyte cell death via mitochondria, and fails to prevent reperfusion injury.2 The liver is a metabolically high-demanding organ, even under hypothermic conditions. Therefore, active oxygenation is needed, especially in grafts with more preservation injury, such as DCD livers.2,27,28
A potential disadvantage of HMP is the risk of undesired endothelial injury (shear stress) in the liver sinusoids caused by higher vascular resistances in the cold.29 In our experiments, we have observed stable perfusion flows during the entire preservation period of 24 h. This is in contrast to what other authors have described for extended HMP.26,[30], [31], [32] If machine settings are adjusted at portal pressures ≤3 mmHg and arterial pressures ≤25 mmHg, shear stress can be avoided.2,33 In this study, using low perfusion pressures, we did not observe activation of endothelial cells, indicated by VWF staining as well as low levels of sTM. sTM is released from the vascular endothelium when liver sinusoids are injured by, for example, graft preservation.34 Levels of sTM have also been correlated with elevated liver enzymes and increased adherence of leukocytes in liver tissue.18
Research groups world-wide are pushing the boundaries of organ preservation times. More recently, the feasibility of extended preservation by NMP was demonstrated.11,35,36 The group from Cleveland even shows 86 h liver perfusion by NMP in a non-transplantation model.11 Normothermic conditions allow testing of organ viability, but it also bears a risk of severe injury as the organ is much more metabolically active.37 In addition, normothermic perfusion requires more intensive labor, since the liver produces waist products and the composition of the perfusate needs to be continuously monitored and adjusted. Hypothermic perfusion can be advantageous since the organ is in a hypometabolic state with less production of waste products. In addition, compared to NMP, it prevents graft loss if the perfusion system fails (the graft would still be preserved in SCS). This study shows that temperature can be maintained at 10°C without interventions, it does not require an oxygen carrier (e.g. blood), and no adjustments of the perfusate needed to be done, making it a relatively easy and substantially cheaper approach compared to NMP.
Clearly these results must be assessed in the context of what is an experimental study using porcine livers. We did not confirm our findings in a transplantation model, but an ex-situ whole blood reperfusion model was used to test liver viability, as done previously by others.15,[38], [39], [40] In addition, it was recently shown that 2.5 h of normothermic reperfusion is sufficient to assess hepatobiliary viability.21 It is generally accepted that the pig liver is a very rigorous model of organ preservation, with maximum successful SCS preservation times that are substantially shorter than those regularly achieved in clinical practice.35 In combination with our preliminary results on the 2 discarded human liver grafts, these are grounds for optimism that comparable results may be achieved in the clinical setting. Although the results of the current study are encouraging, experimental work with subsequent transplantation to validate this approach remains necessary. Until then, extended DHOPE should only be performed within a research setting.
In conclusion, this is the first study to show successful extended preservation by DHOPE of DCD porcine and human livers. If confirmed to the clinical setting, extended DHOPE could be used globally to facilitate transplantation logistics and expand the donor pool.
Financial support
The authors received no financial support to produce this manuscript.
Authors’ contributions
Isabel Brüggenwirth participated in research design, performing the experiments, laboratory analysis, and writing of the paper; Otto van Leeuwen participated in performing the experiments, histological analysis, clinical perfusions, and revising the manuscript; Yvonne de Vries participated in performing the experiments, study design, and revising the manuscript; Silke Bodewes performed laboratory analysis; Jelle Adelmeijer performed laboratory analysis; Janneke Wiersema-Buist performed laboratory analysis; Ton Lisman participated in research design, interpreting the results, and revising the manuscript; Paulo Martins participated in research design, interpreting the results, and revising the manuscript; Vincent de Meijer participated in research design, performing the clinical perfusions, interpreting the results, and revising the manuscript; Robert Porte participated in research design, interpreting the results, performing the clinical perfusions, and revising the manuscript.
Conflict of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
We are grateful to Arjan van de Plaats, Martin Kuizenga, Mark Slotemaker, and Emma Offringa (Organ Assist, Groningen, The Netherlands) for their technical support and assistance during the perfusion experiments. We would also like to thank the employees of Kroon, Groningen, for their willing cooperation.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2020.100092.
Supplementary data
References
- 1.de Meijer V.E., Fujiyoshi M., Porte R.J. Ex situ machine perfusion strategies in liver transplantation. J Hepatol. 2019;70(1):203–205. doi: 10.1016/j.jhep.2018.09.019. [DOI] [PubMed] [Google Scholar]
- 2.Schlegel A., de Rougemont O., Graf R., Clavien P.-A., Dutkowski P. Protective mechanisms of end-ischemic cold machine perfusion in DCD liver grafts. J Hepatol. 2013;58(2):278–286. doi: 10.1016/j.jhep.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 3.op den Dries S., Karimian N., Sutton M.E., Westerkamp A.C., Nijsten M.W.N., Gouw A.S.H. Ex vivo normothermic machine perfusion and viability testing of discarded human donor livers. Am J Transplant. 2013;13(5):1327–1335. doi: 10.1111/ajt.12187. [DOI] [PubMed] [Google Scholar]
- 4.Westerkamp A.C., Karimian N., Matton A.P.M., Mahboub P., van Rijn R., Wiersema-Buist J. Oxygenated hypothermic machine perfusion after static cold storage improves hepatobiliary function of extended criteria donor livers. Transplantation. 2016;100(4):825–835. doi: 10.1097/TP.0000000000001081. [DOI] [PubMed] [Google Scholar]
- 5.van Rijn R., Karimian N., Matton A.P.M., Burlage L.C., Westerkamp A.C., van den Berg A.P. Dual hypothermic oxygenated machine perfusion in liver transplants donated after circulatory death. Br J Surg. 2017;104(7):907–917. doi: 10.1002/bjs.10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Rijn R., van Leeuwen O.B., Matton A.P.M., Burlage L.C., Wiersema-Buist J., van den Heuvel M.C. Hypothermic oxygenated machine perfusion reduces bile duct reperfusion injury after transplantation of donation after circulatory death livers. Liver Transpl. 2018;24(5):655–664. doi: 10.1002/lt.25023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Rijn R., van den Berg A.P., Erdmann J.I., Heaton N., van Hoek B., de Jonge J. Study protocol for a multicenter randomized controlled trial to compare the efficacy of end-ischemic dual hypothermic oxygenated machine perfusion with static cold storage in preventing non-anastomotic biliary strictures after transplantation of liver grafts donated after circulatory death: DHOPE-DCD trial. BMC Gastroenterol. 2019;19(1):40. doi: 10.1186/s12876-019-0956-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pienaar B.H., Lindell S.L., Van Gulik T., Southard J.H., Belzer F.O. Seventy-two-hour preservation of the canine liver by machine perfusion. Transplantation. 1990;49(2):258–260. doi: 10.1097/00007890-199002000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Tamaki T., Kamada N., Wight D.G., Pegg D.E. Successful 48-hour preservation of the rat liver by continuous hypothermic perfusion with haemaccel-isotonic citrate solution. Transplantation. 1987;43(4):468–471. doi: 10.1097/00007890-198704000-00002. [DOI] [PubMed] [Google Scholar]
- 10.De Carlis R., Lauterio A., Ferla F., Di Sandro S., Sguinzi R., De Carlis L. Hypothermic machine perfusion of liver grafts can safely extend cold ischemia for up to 20 hours in cases of necessity. Transplantation. 2017;101(7):e223–e224. doi: 10.1097/TP.0000000000001753. [DOI] [PubMed] [Google Scholar]
- 11.Liu Q., Nassar A., Buccini L., Grady P., Soliman B., Hassan A. Ex situ 86-hour liver perfusion: pushing the boundary of organ preservation. Liver Transpl. 2018;24(4):557–561. doi: 10.1002/lt.25007. [DOI] [PubMed] [Google Scholar]
- 12.Watson C.J.E., Randle L.V., Kosmoliaptsis V., Gibbs P., Allison M., Butler A.J. 26-hour storage of a declined liver before successful transplantation using ex vivo normothermic perfusion. Ann Surg. 2017;265(1):e1–e2. doi: 10.1097/SLA.0000000000001834. [DOI] [PubMed] [Google Scholar]
- 13.Schlegel A., Muller X., Kalisvaart M., Muellhaupt B., Perera M.T.P.R., Isaac J.R. Outcomes of DCD liver transplantation using organs treated by hypothermic oxygenated perfusion before implantation. J Hepatol. 2019;70(1):50–57. doi: 10.1016/j.jhep.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Dutkowski P., Polak W.G., Muiesan P., Schlegel A., Verhoeven C.J., Scalera I. First comparison of hypothermic oxygenated perfusion versus static cold storage of human donation after cardiac death liver transplants: an international-matched case analysis. Ann Surg. 2015;262(5):764–770. doi: 10.1097/SLA.0000000000001473. discussion 770-1. [DOI] [PubMed] [Google Scholar]
- 15.Op den Dries S., Sutton M.E., Karimian N., de Boer M.T., Wiersema-Buist J., Gouw A.S.H. Hypothermic oxygenated machine perfusion prevents arteriolonecrosis of the peribiliary plexus in pig livers donated after circulatory death. PLoS One. 2014;9(2):e88521. doi: 10.1371/journal.pone.0088521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matton A.P.M., de Vries Y., Burlage L.C., van Rijn R., Fujiyoshi M., de Meijer V.E. Biliary bicarbonate, pH and glucose are suitable biomarkers of biliary viability during ex situ normothermic machine perfusion of human donor livers. Transplantation. 2019;103(7):1405–1413. doi: 10.1097/TP.0000000000002500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Meijenfeldt F.A., Burlage L.C., Bos S., Adelmeijer J., Porte R.J., Lisman T. Elevated plasma levels of cell-free DNA during orthotopic liver transplantation are associated with activation of coagulation. Liver Transpl. 2018;24(12):1716–1725. doi: 10.1002/lt.25329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sido B., Datsis K., Mehrabi A., Kraus T., Klar E., Otto G. Soluble thrombomodulin—a marker of reperfusion injury after orthotopic liver transplantation. Transplantation. 1995;60(5):462–466. doi: 10.1097/00007890-199509000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Sutton M.E., op den Dries S., Karimian N., Weeder P.D., de Boer M.T., Wiersema-Buist J. Criteria for viability assessment of discarded human donor livers during ex vivo normothermic machine perfusion. PLoS One. 2014;9(11):e110642. doi: 10.1371/journal.pone.0110642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.op den Dries S., Westerkamp A.C., Karimian N., Gouw A.S.H., Bruinsma B.G., Markmann J.F. Injury to peribiliary glands and vascular plexus before liver transplantation predicts formation of non-anastomotic biliary strictures. J Hepatol. 2014;60(6):1172–1179. doi: 10.1016/j.jhep.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 21.van Leeuwen O.B., de Vries Y., Fujiyoshi M., Nijsten M.W.N., Ubbink R., Pelgrim G.J. Transplantation of high-risk donor livers after ex situ resuscitation and assessment using combined hypo- and normothermic machine perfusion: a prospective trial. Ann Surg. 2019;270(5):906–914. doi: 10.1097/SLA.0000000000003540. [DOI] [PubMed] [Google Scholar]
- 22.Vries Y., Matton A.P.M., Nijsten M.W.N., Werner M.J.M., Berg A.P., Boer M.T. Pretransplant sequential hypo- and normothermic machine perfusion of suboptimal livers donated after circulatory death using a hemoglobin-based oxygen carrier perfusion solution. Am J Transplant. 2019;19(4):1202–1211. doi: 10.1111/ajt.15228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manner M., Shult W., Senninger N., Machens G., Otto G. Evaluation of preservation damage after porcine liver transplantation by assessment of hepatic microcirculation. Transplantation. 1990;50(6):940–943. doi: 10.1097/00007890-199012000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Steininger R., Roth E., Holzmüller P., Reckendorfer H., Grünberger T., Sperlich M. Comparison of HTK- and UW-solution for liver preservation tested in an orthotopic liver transplantation model in the pig. Transpl Int. 1992;5(Suppl 1):S403–S407. doi: 10.1007/978-3-642-77423-2_119. [DOI] [PubMed] [Google Scholar]
- 25.Lonze B.E., Parsikia A., Feyssa E.L., Khanmoradi K., Araya V.R., Zaki R.F. Operative start times and complications after liver transplantation. Am J Transplant. 2010;10(8):1842–1849. doi: 10.1111/j.1600-6143.2010.03177.x. [DOI] [PubMed] [Google Scholar]
- 26.Xu H., Lee C.Y., Clemens M.G., Zhang J.X. Pronlonged hypothermic machine perfusion preserves hepatocellular function but potentiates endothelial cell dysfunction in rat livers. Transplantation. 2004;77(11):1676–1682. doi: 10.1097/01.tp.0000129644.23075.71. [DOI] [PubMed] [Google Scholar]
- 27.Lüer B., Koetting M., Efferz P., Minor T. Role of oxygen during hypothermic machine perfusion preservation of the liver. Transpl Int. 2010;23(9):944–950. doi: 10.1111/j.1432-2277.2010.01067.x. [DOI] [PubMed] [Google Scholar]
- 28.Westerkamp A.C., Mahboub P., Meyer S.L., Hottenrott M., Ottens P.J., Wiersema-Buist J. End-ischemic machine perfusion reduces bile duct injury in donation after circulatory death rat donor livers independent of the machine perfusion temperature. Liver Transpl. 2015;21(10):1300–1311. doi: 10.1002/lt.24200. [DOI] [PubMed] [Google Scholar]
- 29.Schlegel A., Dutkowski P. Role of hypothermic machine perfusion in liver transplantation. Transpl Int. 2015;28(6):677–689. doi: 10.1111/tri.12354. [DOI] [PubMed] [Google Scholar]
- 30.Jain S., Xu H., Duncan H., Jones J.W., Zhang J.X., Clemens M.G. Ex-vivo study of flow dynamics and endothelial cell structure during extended hypothermic machine perfusion preservation of livers. Cryobiology. 2004;48(3):322–332. doi: 10.1016/j.cryobiol.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 31.Minor T., Manekeller S., Sioutis M., Dombrowski F. Endoplasmic and vascular surface activation during organ preservation: refining upon the benefits of machine perfusion. Am J Transplant. 2006;6(6):1355–1366. doi: 10.1111/j.1600-6143.2006.01338.x. [DOI] [PubMed] [Google Scholar]
- 32.van der Plaats A., Maathuis M.H.J., 't Hart N.A., Bellekom A.A., Hofker H.S., van der Houwen E.B. The groningen hypothermic liver perfusion pump: functional evaluation of a new machine perfusion system. Ann Biomed Eng. 2006;34(12):1924–1934. doi: 10.1007/s10439-006-9207-4. [DOI] [PubMed] [Google Scholar]
- 33.Debbaut C., Monbaliu D., Casteleyn C., Cornillie P., Van Loo D., Masschaele B. From vascular corrosion cast to electrical analog model for the study of human liver hemodynamics and perfusion. IEEE Trans Biomed Eng. 2011;58(1):25–35. doi: 10.1109/TBME.2010.2065229. [DOI] [PubMed] [Google Scholar]
- 34.Verhoeven C.J., Farid W.R.R., de Jonge J., Metselaar H.J., Kazemier G., van der Laan L.J.W. Biomarkers to assess graft quality during conventional and machine preservation in liver transplantation. J Hepatol. 2014;61(3):672–684. doi: 10.1016/j.jhep.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 35.Vogel T., Brockmann J.G., Pigott D., Neil D.A.H., Muthusamy A.S.R., Coussios C.C. Successful transplantation of porcine liver grafts following 48-hour normothermic preservation. PLoS One. 2017;12(11):e0188494. doi: 10.1371/journal.pone.0188494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vogel T., Brockmann J.G., Quaglia A., Morovat A., Jassem W., Heaton N.D. The 24-hour normothermic machine perfusion of discarded human liver grafts. Liver Transpl. 2017;23(2):207–220. doi: 10.1002/lt.24672. [DOI] [PubMed] [Google Scholar]
- 37.Schlegel A., Muller X., Dutkowski P. Hypothermic machine preservation of the liver: state of the art. Curr Transplant Rep. 2018;5(1):93–102. doi: 10.1007/s40472-018-0183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Q., Nassar A., Farias K., Buccini L., Mangino M.J., Baldwin W. Comparing normothermic machine perfusion preservation with different perfusates on porcine livers from donors after circulatory death. Am J Transplant. 2016;16(3):794–807. doi: 10.1111/ajt.13546. [DOI] [PubMed] [Google Scholar]
- 39.von Horn C., Hannaert P., Hauet T., Leuvenink H., Paul A., Minor T. Cold flush after dynamic liver preservation protects against ischemic changes upon reperfusion - an experimental study. Transpl Int. 2019;32(2):218–224. doi: 10.1111/tri.13354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Minor T., Efferz P., Fox M., Wohlschlaeger J., Lüer B. Controlled oxygenated rewarming of cold stored liver grafts by thermally graduated machine perfusion prior to reperfusion. Am J Transplant. 2013;13(6):1450–1460. doi: 10.1111/ajt.12235. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.