Abstract
Background
Anthocyanins, one of the major plant bioactive substances, possess anti-oxidative and anti-inflammatory capacity. However, their dose–response relationship has remained unclear. The present study investigated the dose–response relationship of anthocyanins with oxidative stress and inflammation in subjects with dyslipidemia.
Design
and Participants: A total of 169 participants with dyslipidemia were randomly assigned to placebo (n = 43), anthocyanins 40 mg/day (n = 44), 80 mg/day (n = 40), or 320 mg/day (n = 42) groups. Urine 8-iso-prostaglandin F2α (8-iso-PGF2α), 8-hydroxy-2′-deoxyguanosine (8-OHdG) and serum malonaldehyde (MDA), total superoxide dismutase (T-SOD), UA (uric acid), interleukin (IL)-6, IL-10, tumor necrosis factor-α (TNF-α), and C-reactive protein (CRP) were measured at baseline, at 6 weeks, and at 12 weeks.
Results
Anthocyanin supplementation (320 mg/day) for 6 weeks significantly improved T-SOD versus baseline (P < 0.05). A slight reduction in serum IL-6, TNF-α, and urine 8-iso-PGF2α from the baseline was observed at 12 weeks in the group receiving 40 mg/day anthocyanins. Anthocyanins (80 mg/day) significantly reduced serum IL-6 (−20%), TNF-α (−11%) and urine 8-iso-PGF2α (−27%) versus baseline (P < 0.05). Moreover, 320 mg/day anthocyanin supplementation reduced serum IL-6 (−40%), TNF-α (−21%), MDA (−20%) and urine 8-iso-PGF2α (−37%) and 8-OHdG (−36%) than 80 mg/day and 40 mg/day anthocyanins, P value < 0.05. Anthocyanin supplementation has dose-response relationships with decreased inflammatory cytokines IL-6, TNF-α and oxidative stress biomarkers 8-iso-PGF2α, 8-OHdG and MDA (P for trend, <0.05). Furthermore, a strong positive correlation was observed between the changes in the urine 8-iso-PGF2α , 8-OHdG levels and serum IL-6 levels in subjects from anthocyanin groups after 12 weeks of treatment.
Conclusions
Supplementation of anthocyanins for 12 weeks positively improved the anti-oxidative and anti-inflammatory capacity in a dose–response manner in individuals with dyslipidemia.
Keywords: Anthocyanins, 8-iso-prostaglandinF2α, 8-Hydroxy-2′-deoxyguanosine, Interleukin-6, Randomized controlled trial
Abbreviations: 8-iso-PGF2, 8-iso-prostaglandin F2; 8-OHdG, 8-hydroxy-2′-deoxyguanosine; MDA, malonaldehyde; T-SOD, total superoxide dismutase; UA, uric acid; IL-6, interleukin-6; CRP, C-reactive protein; TC, total cholesterol; TG, triglyceride; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; CHD, coronary heart disease; BW, body weight; BH, body height; NC, neck circumference; WC, waist circumference; HC, hip circumference; BMI, body mass index; WHR, Waist Hip Ratio; BP, blood pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; FBG, fasting blood glucose; FINS, fasting insulin; HOMA-IR, homoeostasis model assessment of insulin resistance; VCAM-1, vascular cell adhesion molecule-1; IL-1β, interleukin-1β; IFN-γ, interferon gamma; ROS, reactive oxygen species
Graphical abstract
Highlights
-
•
Anthocyanins improve oxidative stress in a dose-response manner.
-
•
Anthocyanins improve anti-inflammatory capacity in a dose-response manner.
-
•
80 mg/day anthocyanin could reach the threshold level for producing beneficial functions after 12-week intervention.
-
•
Anthocyanins prevent the progression of metabolic diseases in subjects with dyslipidemia.
1. Introduction
Dyslipidemia, including increased blood total cholesterol (TC), triglycerides (TG), and low-density lipoprotein cholesterol (LDL-C), and decreased high-density lipoprotein cholesterol (HDL-C), is a global health problem. Recent epidemiologic surveys have revealed that the prevalence of dyslipidemia reached up to 34.0% in Chinese [1,2] and up to 16.58% in Korean adults [3]. Accumulation of these risk factors significantly increases the risk of coronary heart disease (CHD) and its equivalents such as type 2 diabetes and obesity [[4], [5], [6]].
It has been well documented that dyslipidemia results in elevated levels of low-grade inflammatory cytokines, such as interleukin (IL)-6, tumor necrosis factor (TNF)-α, and C-reactive protein (CRP) [7,8]. Moreover, serum CRP, IL-6, and TNF-α concentrations have been repeatedly reported to be directly associated with several cardiovascular as well as metabolic diseases [9,10]. Meanwhile, evidence from experimental work indicated a strong association between dyslipidemia and oxidative stress [[11], [12], [13]]. Dyslipidemia lead to the generation of excess free radicals via several biochemical pathways and induce oxidative stress [14]. Oxidative stress can be defined as an imbalance between the production and elimination of reactive oxygen species (ROS). It may favor the oxidation of biomolecules such as lipids, proteins, and DNA, resulting in cell damage and loss of biological function [15]. In this sense, oxidative stress may play a decisive role in the pathogenesis and progression of chronic metabolic diseases [16,17]. Among the many types of ROS-induced oxidative modifications, urine 8-hydroxy-2′-deoxyguanosine (8-OHdG) and 8-iso-prostaglandin F2α (8-iso-PGF2α) have been widely used as sensitive markers of oxidative stress [18,19]. In addition, other biomarkers have been studied to monitor the production of ROS, such as malonaldehyde (MDA) as a surrogate marker of oxidative stress [20]. Furthermore, elevated oxidative stress and inflammation are highly interrelated and cross-promote each other in a vicious cycle, resulting in the progression of CHD outcomes [21]. Therefore, for subjects with dyslipidemia, anti-inflammatory and anti-oxidative therapy has been proposed as a promising and effective strategy for preventing metabolic diseases.
Anthocyanins, a subgroup of flavonoids, are responsible for the production of red-orange to blue-violet pigments in plants (fruits, vegetables, flowers, and grains) [22,23]. Previous human studies have demonstrated that anthocyanin-rich foods or anthocyanin extracts inhibited metabolic diseases owing to their anti-oxidative and anti-inflammatory capacity [24,25]. Among most intervention studies, only a single dose of anthocyanins was used to observe their effect on oxidation and inflammation as well as lipid profile. Thus, the effective dose of anthocyanin supplementation to induce beneficial functions has remained unclear. The aim of this randomized controlled trial was to determine the dose–response effect of anthocyanin supplementation on oxidative and inflammatory response in individuals with dyslipidemia.
2. Subjects and methods
2.1. Subjects
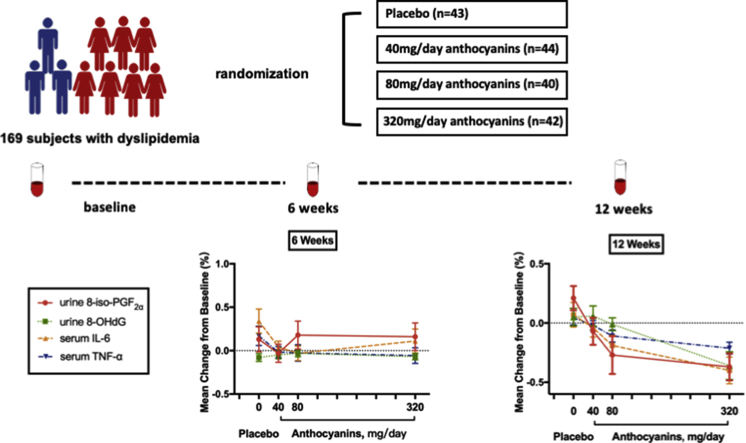
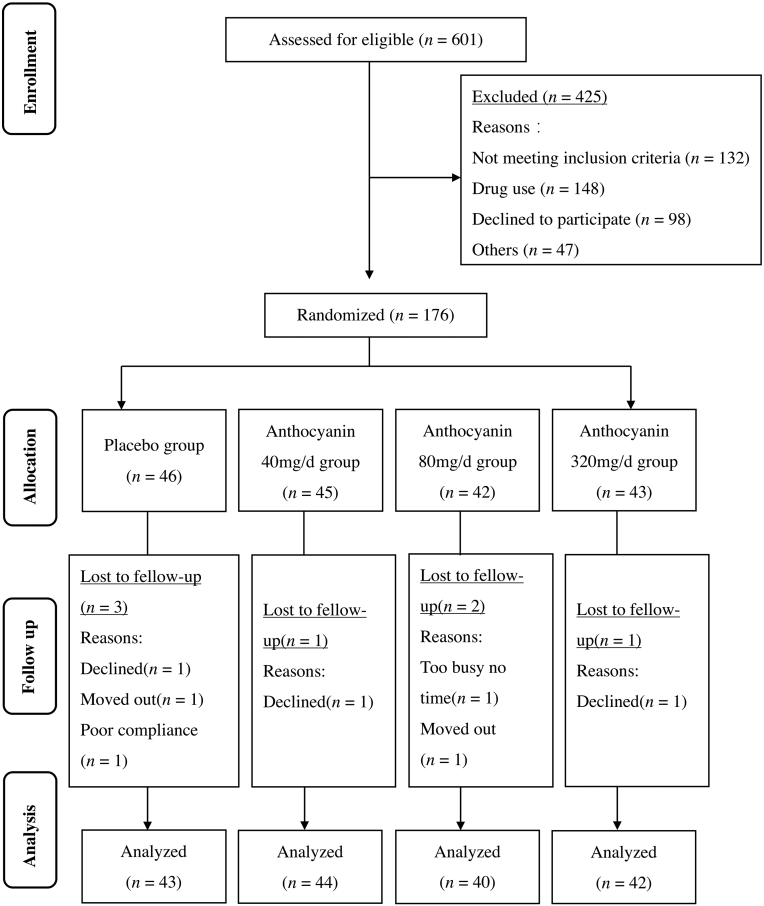
Participants were recruited from local communities in Guangzhou, China, through advertising flyers and clinicians’ recommendations at three community hospitals. Potential participants were interviewed by trained research staff over the telephone or in person with a structured screening questionnaire. Participants with a medical record of hyperlipidemia were further invited to a clinical visit to undergo a serum lipid measurement test to confirm their eligibility (Fig. 1). The inclusion criteria were: (1) men and women aged 35–70 years; (2) dyslipidemia comprising either two or more of the following four criteria [26]: fasting serum TG ≥ 150 mg/dL (1.70 mmol/L), TC ≥ 200 mg/dL (5.20 mmol/L), LDL-C ≥ 120 mg/dL (3.12 mmol/L), or HDL-C ≤ 35 mg/dL (0.91 mmol/L); and (3) less eating out and weight stability in the past 3 months. The exclusion criteria included: (1) taking any medications known to affect lipid metabolism such as statins, currently or within the past 6 months; (2) taking any anthocyanin supplements or anthocyanin-rich foods currently or within the past 2 months; (3) lactating or pregnant women; (4) and suffering from severe acute or chronic illness. The study was approved by the Ethics Committee of Sun Yat-sen University, and informed consent was obtained from each participant before conducting any experiment. The trial was registered at ClinicalTrials.gov (NCT03415503).
Fig. 1.
Study participants’ flow diagram.
2.2. Experimental design
This was a 12-week, randomized, double-blind, placebo-controlled trial with supplementation of multiple doses of anthocyanin. A total of 169 eligible subjects were randomly assigned to one of the four dosing groups: placebo (n = 43), or anthocyanins at 40 (n = 44), 80 (n = 40), or 320 (n = 42) mg/day by a list of random numbers generated by SPSS v22.0 (SPSS Inc., Chicago, IL, USA). Oral capsules with the same weight, appearance, and package were used in three types as 40 or 80 mg anthocyanins (Medox), and placebo capsules. Subjects in four groups were all instructed to consume two capsules twice daily preferably 30-min after breakfast and supper for 12 weeks. In details, subjects in placebo group consumed four placebo capsules, subjects in 40 mg Antho group consumed one 40 mg Medox and three placebo capsules, subjects in 80 mg Antho group consumed one 80 mg Medox and three placebo capsules, and subjects in 320 mg Antho group consumed four 80 mg Medox capsules per day. Subject compliance was assessed by counting the number of returned packages when they received their supplements once every 2 weeks. They were asked to maintain their usual dietary intake and physical activities. The 24-h dietary recall data on 3 consecutive days were collected in the first week, intermediate stage (after 6 weeks), and at the endpoint of our intervention (after 12 weeks). Nutrient intakes were calculated using a computer-aided nutritional analysis program for professionals (Chinese Food Composition Table) [27].
2.3. Study supplements
Medox is a vegetable-encapsulated anthocyanin extracts from wild Norwegian bilberries and blackcurrants manufactured in Norway by MedPalett Pharmaceuticals and the Biolink Group. The Medox capsules contain 80 mg or 40 mg anthocyanins, both of which comprise 17 different natural anthocyanins purified from bilberry (Vaccinium myrtillus) and blackcurrant (Ribes nigrum; refer to Supplemental Tables S1–S3 for the ingredients of anthocyanins capsules). Anthocyanin capsules also contain 4% pullulan, maltodextrin, and citric acid to maintain the stability, whereas the placebo capsules contain only pullulan and maltodextrin [28]. The anthocyanin and placebo capsules have identical weight, appearance, and package.
2.4. Anthropometric analyses
Anthropometric measurements were performed by a trained examiner. Body weight (BW), body height (BH), neck circumference (NC), waist circumference (WC), and hip circumference (HC) were measured according to the standard protocols. Body mass index (BMI) was calculated based on BW and BH: BMI (kg/m2) = BW (kg)/BH2 (m2). The waist hip ratio (WHR) was calculated based on WC and HC: WHR (%) = WC (cm)/HC (cm) × 100%. Heart rate and blood pressure (BP), including systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured using a validated oscillometric technique (Omron U30 Intellisense, JPN). BP and heart rate were determined in the non-dominant upper arm after a 20-minute resting period on each arm twice. Four values were recorded at 2-minute intervals, and the average of these measures was considered. All measurements were obtained using standardized procedures and periodically calibrated instruments. Individual information was collected by the trained staff via face-to-face interview based on a structured questionnaire on socio-demographic data, dietary habits, and living habits.
2.5. Determination of biochemical biomarkers
Overnight fasting (8–10 hour) venous blood samples were collected between 8:00 am and 9:00 am at baseline, and after 6 and 12 weeks. Samples were immediately centrifuged at 3000 rpm for 15 min at 4 °C. Each participant's serum sample was divided into several aliquots and stored at −80 °C until analysis. First-morning urine sample was collected in two containers and stored at −80 °C until subsequent analyses.
Fasting blood samples were subjected to a complete lipid profile, including LDL-C, HDL-C, TG, TC, apolipoprotein A-1, and apolipoprotein B. Other biochemical analyses included fasting blood glucose (FBG), insulin (FINS), uric acid (UA), and CRP concentrations that were measured using the Cobas c311 automated assay analyzer (c311, Roche Diagnostics, Switzerland). The homoeostasis model assessment of insulin resistance (HOMA-IR) was calculated based on FBG and FINS: HOMA-IR = FINS (mU/mL) × FBG (mmol/L)/22.5. Serum IL-6 was analyzed using commercial kits (catalog no. HS600C, R&D Systems, Minneapolis, USA). Serum IL-10 was assessed using a cytometric cytokine bead array human enhanced sensitivity master buffer kit (catalog no.558274, BD Biosciences, San Jose, CA, USA). Serum TNF-α was determined using a commercial enzyme-linked immunosorbent assay (ELISA) kit (catalog no. DP1000, SIEMENS, Munich, Germany). Serum MDA was determined using a commercial ELISA kit (catalog no. S0131, Beyotime, Shanghai, China). The serum total superoxide dismutase (T-SOD) activities were measured using a T-SOD assay kit (catalog no. A001-1-2, Jiancheng, Nanjing, China) and the hydroxylamine method. Urine 8-iso-PGF2α was determined in spot urine samples, because Helmersson and Basu reported that urinary F2-isoprostane isomers concentrations in spot urines showed no significant variation from concentrations measured in 24-hour urine samples in the same healthy individuals by radioimmunoassay [29]. Urine 8-iso-PGF2α was analyzed by competitive enzyme immunoassay (EIA) kit (catalog no.516351, Cayman Chemical Company, Ann Arbor, MI, USA) [[30], [31], [32]]. Urine 8-OHdG was measured using an ELISA Kit (catalog no.CSB-E10140h, CUSABIO, Wuhan, China). Values for 8-iso-PGF2α and 8-OHdG were normalized by per milligram of creatinine in urine, which was measured using the Mindray UA-6800 automated assay analyzer (Shenzhen, China).
2.6. Sample size planning
Sample size estimation was conducted using the PASS software (version 11.0, NCSS Inc.). In our previous clinical trial [33], supplementation with 320 mg/day of anthocyanins resulted in a 0.34 mmol/L (13.1 mg/dL) decrease in LDL-C levels relative to the levels in the group treated with placebo. Based on the conventional assumption of a two-tailed α level of 0.05 and β level of 0.10, it was determined that 38 subjects should be recruited per group. Allowing for a 10% drop-out rate, at least 42 subjects were required in each group.
2.7. Statistical analysis
The data are expressed as mean ± standard error of mean (SEM) unless otherwise stated. Variables with skewed distributions were logarithmically transformed to achieve a normal distribution. The percent change after 6 weeks and 12 weeks were calculated as follows: (value after intervention-value at baseline)/value at baseline × 100%. Comparability of the four groups at baseline was assessed by a one-way analysis of variance (ANOVA) and post hoc analysis with Bonferroni correction for multiple comparisons. Student's t tests for paired data were used accordingly to assess changes within the group from baseline to follow-up. Differences in the percent change in biomarkers between the intervention groups were analyzed by ANOVA, after logarithmic transformation on the ratio. An extension of the Wilcoxon rank sum test was used to test for linear trends across groups. Pearson correlation coefficients (r) were calculated to assess the associations between the changes in oxidative stress and inflammatory biomarkers over the 12-week study period. All statistical analyses were two-tailed and performed using SPSS v22.0 (SPSS Inc., Chicago, IL, USA). Statistical significance was set at an α level of 0.05.
3. Results
3.1. Baseline characteristics and diet monitoring
The baseline characteristics of individuals with dyslipidemia are shown in Table 1. Participants were predominantly females (73.4%) with their age ranging from 35 to 70 years and a BMI of 24.1 kg/m2 (range: 17.1–33.5 kg/m2). Participants in the four groups were comparable in terms of age, gender, smoking status, BW, BMI, NC, WC, WHR, HR, and SBP, except DBP (P < 0.05). Moreover, the baseline parameters for lipids, glucose, and insulin were comparable among the four study groups.
Table 1.
Baseline characteristics.
| Placebo (n=43) | 40mg Antho (n=44) | 80mg Antho (n=40) | 320mg Antho (n=42) | P value[1] | |
|---|---|---|---|---|---|
| Age, y | 56.21±1.01a | 57.52±1.37 | 58.28±1.17 | 57.41±1.37 | 0.607 |
| Gender (M/F) | 13/30 | 11/33 | 10/30 | 11/31 | 0.939 |
| Weight (kg) | 59.17±1.79 | 62.41±1.78 | 59.08±1.64 | 64.92±1.68 | 0.052 |
| CS (%) | 3(7.1%)a | 4(9.3%) | 2(5.1%) | 2(4.8%) | 0.827 |
| BMI (kg/m2) | 24.17±0.39 | 23.77±0.48 | 23.77±0.49 | 24.67±0.57 | 0.514 |
| NC (cm) | 34.07±0.48 | 33.95±0.46 | 33.49±0.49 | 34.24±0.58 | 0.766 |
| WC (cm) | 84.16±1.85 | 85.14±1.49 | 84.51±1.40 | 86.81±1.49 | 0.642 |
| WHR (%) | 0.88±0.02 | 0.89±0.01 | 0.89±0.01 | 0.90±0.01 | 0.674 |
| SBP (mmHg) | 126.49±2.63 | 118.69±2.59 | 117.80±2.38 | 119.85±1.98 | 0.050 |
| DBP (mmHg) | 80.55±1.34 | 75.73±1.38 | 74.81±1.39 | 75.97±1.32 | 0.015 |
| HR | 78.45±1.57 | 75.32±1.65 | 77.78±1.46 | 75.81±1.49 | 0.413 |
| TC (mmol /L) | 6.11±0.14 | 6.26±0.15 | 6.16±0.14 | 6.35±0.14 | 0.653 |
| HDL-c (mmol /L) | 1.38±0.05 | 1.43±0.06 | 1.54±0.07 | 1.47±0.06 | 0.346 |
| LDL-c (mmol /L) | 4.16±0.15 | 4.28±0.18 | 4.21±0.14 | 4.35±0.13 | 0.838 |
| TG (mmol /L) | 2.18±0.19 | 2.19±0.27 | 1.95±0.14 | 2.07±0.27 | 0.863 |
| APOA1 (g/L) | 1.42±0.03 | 1.42±0.03 | 1.50±0.05 | 1.44±0.03 | 0.430 |
| APOB (g/L) | 1.31±0.03 | 1.35±0.04 | 1.30±0.03 | 1.32±0.03 | 0.784 |
| FBS (mmol/L) | 5.53±0.28 | 5.38±0.11 | 5.26±0.09 | 5.28±0.13 | 0.710 |
| FINS (mmol/L) | 11.05±0.96 | 9.56±0.75 | 9.45±0.71 | 10.51±1.14 | 0.544 |
| HOMA-IR | 2.78±0.36 | 2.31±0.19 | 2.20±0.16 | 3.14±0.71 | 0.329 |
1P values are for comparison between the four groups (Either a one-way analysis of variance or chi-square test for independent data).
Abbreviation: SEM, standard error of mean; Antho, anthocyanin group; M, male; F, female; CS: current smoking; BMI: body mass index; NC: neck circumference; WC: waist circumference; WHR: waist-hip ratio; SBP: systolic blood pressure; DBP: diastolic blood pressure; HR: heart rate; TC: total cholesterol; HDL-c: high density lipoprotein cholesterol; LDL-c: low density lipoprotein cholesterol; TG: total triglyceride; APOA1, apolipoprotein A-1; APOB, apolipoprotein B; FBS: fasting blood glucose; FINS: fasting insulin; HOMA-IR: homoeostasis model assessment of insulin resistance.
Mean ± SEM or n (%).
Dietary intake data at baseline and at the end of 12 weeks are shown in Table 2. There were no significant differences in energy or nutrient intake among four groups. No significant differences were observed between the groups in the daily intake of anthocyanins and vitamins, with anti-oxidative capacity, at baseline and after a 12-week intervention.
Table 2.
Daily dietary intakes of total energy and nutrients at baseline and 12 weeks after treatment.
| Placebo (n=43) | 40mg Antho (n=44) | 80mg Antho (n=40) | 320mg Antho (n=42) | P value[1] | |
|---|---|---|---|---|---|
| Total energy (kcal/d) | |||||
| Baseline | 1516.65±99.07a | 1445.86±83.61 | 1580.97±113.86 | 1625.68±100.70 | 0.599 |
| 12 wk | 1718.84±93.65 | 1501.20±96.56 | 1535.59±142.07 | 1552.08±86.34 | 0.458 |
| Total protein (g/d) | |||||
| Baseline | 68.74±4.09 | 70.53±3.46 | 74.00±5.06 | 81.30±5.26 | 0.208 |
| 12 wk | 79.42±4.20 | 72.91±4.17 | 74.69±10.36 | 76.94±3.99 | 0.880 |
| Carbohydrates (g/d) | |||||
| Baseline | 199.37±19.68 | 185.36±13.46 | 194.02±15.34 | 200.35±13.18 | 0.899 |
| 12 wk | 218.61±15.18 | 194.74±14.73 | 184.83±10.89 | 209.41±14.46 | 0.340 |
| Total lipids (g/d) | |||||
| Baseline | 49.28±3.37 | 45.62±3.09 | 56.55±6.02 | 55.43±5.80 | 0.299 |
| 12 wk | 58.67±4.24 | 46.36±3.90 | 54.12±8.14 | 45.61±3.84 | 0.219 |
| Cholesterol (mg/d) | |||||
| Baseline | 440.30±40.08 | 434.78±30.82 | 421.51±38.26 | 553.32±40.59 | 0.054 |
| 12 wk | 471.33±31.90 | 412.46±40.05 | 533.72±140.43 | 460.35±41.86 | 0.717 |
| Dietary fiber (g/d) | |||||
| Baseline | 10.64±0.93 | 11.43±1.43 | 11.49±1.06 | 12.73±1.06 | 0.639 |
| 12 wk | 10.80±0.82 | 11.53±1.16 | 10.88±1.34 | 11.94±1.20 | 0.875 |
| Vitamin C (mg/d) | |||||
| Baseline | 105.24±9.55 | 128.03±9.41 | 129.32±11.31 | 134.48±10.47 | 0.180 |
| 12 wk | 134.99±11.26 | 123.17±10.26 | 126.59±11.47 | 158.69±14.69 | 0.155 |
| Vitamin A (ug retinol equivalent/d) | |||||
| Baseline | 601.48±69.69 | 765.54±88.48 | 741.67±93.03 | 756.20±65.92 | 0.420 |
| 12 wk | 693.39±71.92 | 1037.74±188.56 | 718.08±124.48 | 810.75±88.54 | 0.338 |
| Vitamin E (mg/d) | |||||
| Baseline | 13.92±1.53 | 10.41±0.88 | 12.95±1.34 | 15.13±1.71 | 0.100 |
| 12 wk | 16.04±2.27 | 15.81±2.45 | 17.74±5.20 | 14.31±2.06 | 0.903 |
| Anthocyanins (mg/d) | |||||
| Baseline | 11.02±0.48 | 11.63±1.03 | 8.55±0.32 | 8.04±0.26 | 0.900 |
| 12 wk | 19.06±0.92 | 7.21±0.41 | 19.03±1.03 | 18.72±1.02 | 0.109 |
1P values are for comparison between the four groups at baseline and after 12 weeks of intervention (A one-way analysis of variance for independent data).
Abbreviation: SEM, standard error of mean; Antho, anthocyanin group.
Mean ± SEM (all such values).
3.2. Effects of anthocyanin on serum inflammatory cytokines
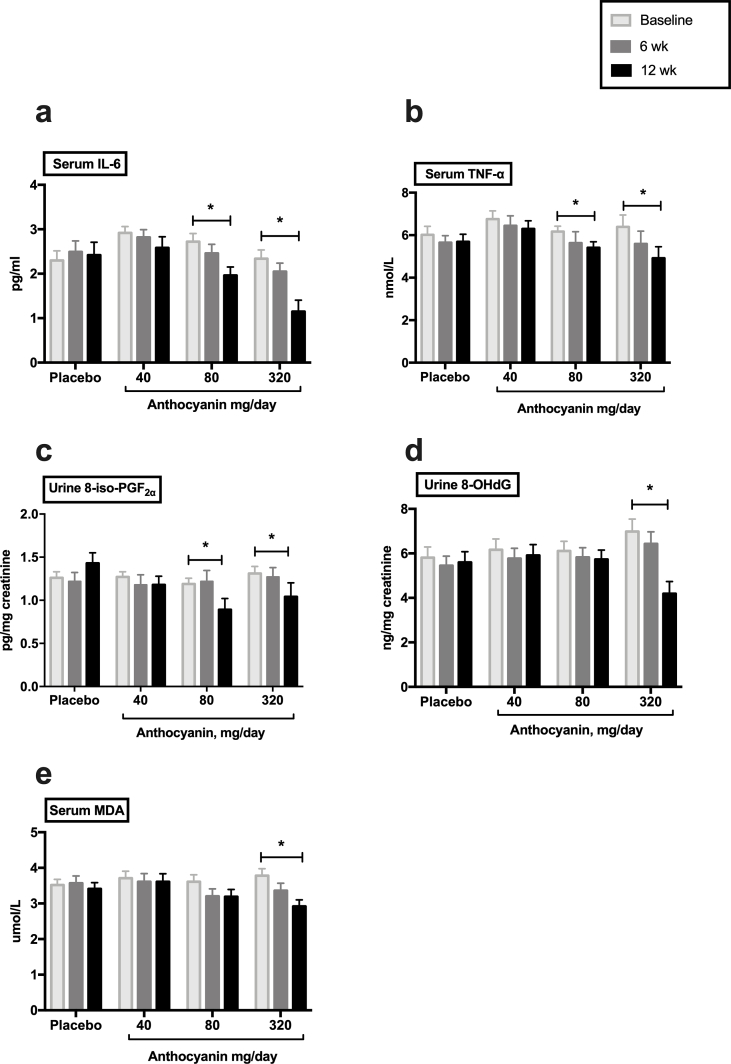
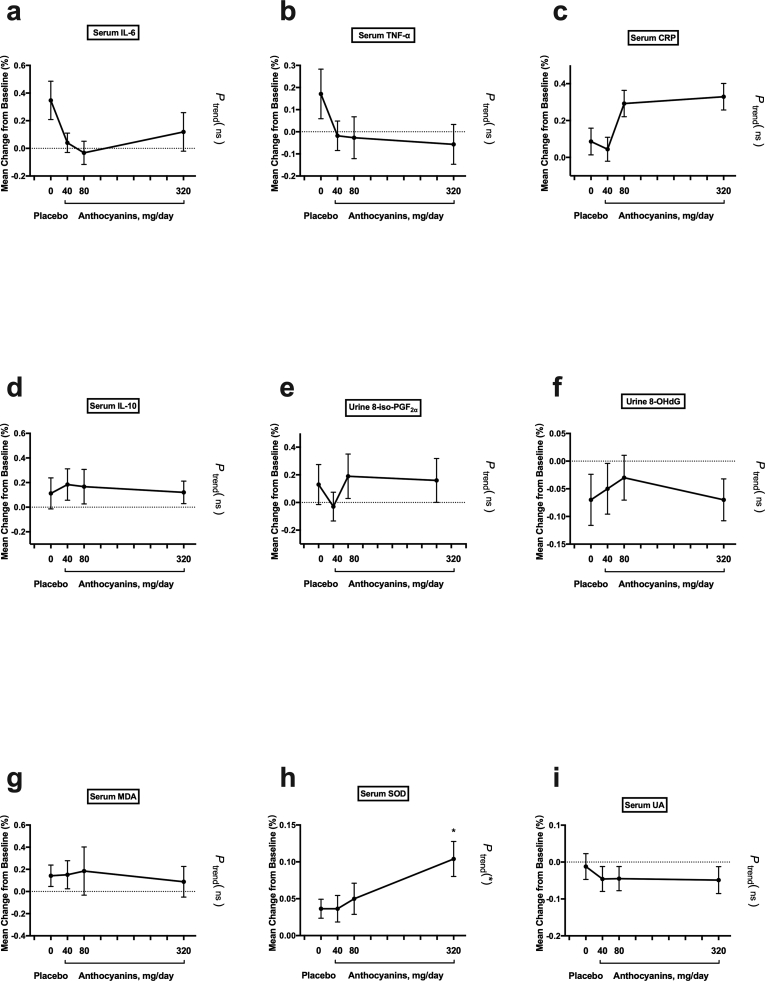
The absolute changes in inflammatory cytokines concentration from baseline to follow-up (at 6 and 12 weeks) were assessed within the groups (Fig. 2, Table 3). After 6 weeks, the inflammatory cytokines did not significantly change. After 12 weeks, 40 mg/day anthocyanins moderately reduced the serum IL-6 and TNF-α from the baseline, but without significance (Fig. 2a and b). However, a 12-week anthocyanin supplementation at 80 mg/day and 320 mg/day significantly decreased the serum IL-6, with baseline 2.71 ± 0.18 pg/mL and 2.34 ± 0.19 pg/mL to final 1.98 ± 0.18 pg/mL and 1.06 ± 0.18 pg/mL, respectively (P < 0.05, Fig. 2a, Table 3), as well as TNF-α, with baseline 6.17 ± 0.25 nmol/L and 6.39 ± 0.55 nmol/L to final 5.39 ± 0.40 nmol/L and 4.92 ± 0.53 nmol/L, respectively (P < 0.05, Fig. 2b, Table 3).
Fig. 2.
Absolute changes in inflammation and oxidative stress cytokines within groups. Data represent the mean (SEM) measurements for each group. *P < 0.05. SEM, standard error of mean; IL-6, interleukin-6; TNF-α, tumor necrosis factor α; 8-iso-PGF2α, 8-iso-prostaglandin F2α; 8-OHdG, 8-hydroxy-2′-deoxyguanosine; MDA, malonaldehyde.
Table 3.
Effects of anthocyanin on inflammatory biomarkers.
| Placebo (n=43) |
40mg Antho (n=44) |
80mg Antho (n=40) |
320mg Antho (n=42) |
|||||
|---|---|---|---|---|---|---|---|---|
| value | MCb (%) | value | MC (%) | value | MC (%) | value | MC (%) | |
| IL-6 (pg/ml) | ||||||||
| Baseline | 2.30±0.21a | 2.92±0.14 | 2.71±0.18 | 2.34±0.19 | ||||
| 6 wk | 2.50±0.25 | 0.34±0.13 | 2.83±0.17 | 0.04±0.07 | 2.46±0.20 | -0.03±0.08 | 2.05±0.18 | 0.12±0.13 |
| 12 wk | 2.29±0.28 | 0.08±0.10 | 2.77±0.25 | -0.04±0.08 | 1.98±0.18#[1] | -0.20±0.08∗[2] | 1.06±0.18# | -0.40±0.11∗∗[2] |
| TNF-α (nmol/L) | ||||||||
| Baseline | 6.02±0.39 | 6.76±0.38 | 6.17±0.25 | 6.39±0.55 | ||||
| 6 wk | 5.65±0.39 | 0.17±0.11 | 6.39±0.47 | -0.02±0.06 | 5.63±0.53 | -0.06±0.09 | 5.60±0.60 | 0.01±0.05 |
| 12 wk | 5.69±0.35 | 0.05±0.06 | 6.52±0.42 | -0.02±0.05 | 5.39±0.40# | -0.11±0.06 | 4.92±0.53# | -0.21±0.05∗∗ |
| IL-10 (pg/ml) | ||||||||
| Baseline | 1.23±0.10 | 1.11±0.09 | 1.14±0.07 | 0.99±0.06 | ||||
| 6 wk | 1.01±0.09 | 0.11±0.12 | 0.99±0.09 | 0.18±0.12 | 1.13±0.12 | 0.16±0.14 | 1.01±0.08 | 0.12±0.09 |
| 12 wk | 1.09±0.09 | 0.12±0.09 | 1.04±0.08 | 0.13±0.08 | 1.20±0.06 | 0.19±0.07 | 1.03±0.06 | 0.13±0.08 |
| CRP (mg/L) | ||||||||
| Baseline | 1.42±0.16 | 1.46±0.17 | 1.52±0.25 | 1.52±0.16 | ||||
| 6 wk | 1.33±0.14 | 0.08±0.07 | 1.38±0.17 | 0.04±0.06 | 1.29±0.17 | 0.03±0.07 | 1.40±0.13 |
0.03±0.07 |
| 12 wk | 1.53±0.25 | 0.15±0.11 | 1.52±0.21 | 0.12±0.09 | 1.62±0.30 | 0.10±0.12 | 1.23±0.20 | -0.13±0.09 |
1 Compared within the group from baseline to follow-up (Either Wilcoxon signed rank test or Students’ t test for paired data), #P<0.05.
2 Compared between the placebo group and anthocyanin groups (ANOVA for independent data), ∗P<0.05, ∗∗P<0.01.
Abbreviation: SEM, standard error of mean; MC, mean change; Antho, anthocyanin group; IL-6, interleukin-6; TNF-α, tumor necrosis factor α; IL-10: interleukin-10; CRP: C-reactive protein.
Mean ± SEM (all such values).
Calculated as (value after intervention-value before intervention) / value before intervention ×100 %.
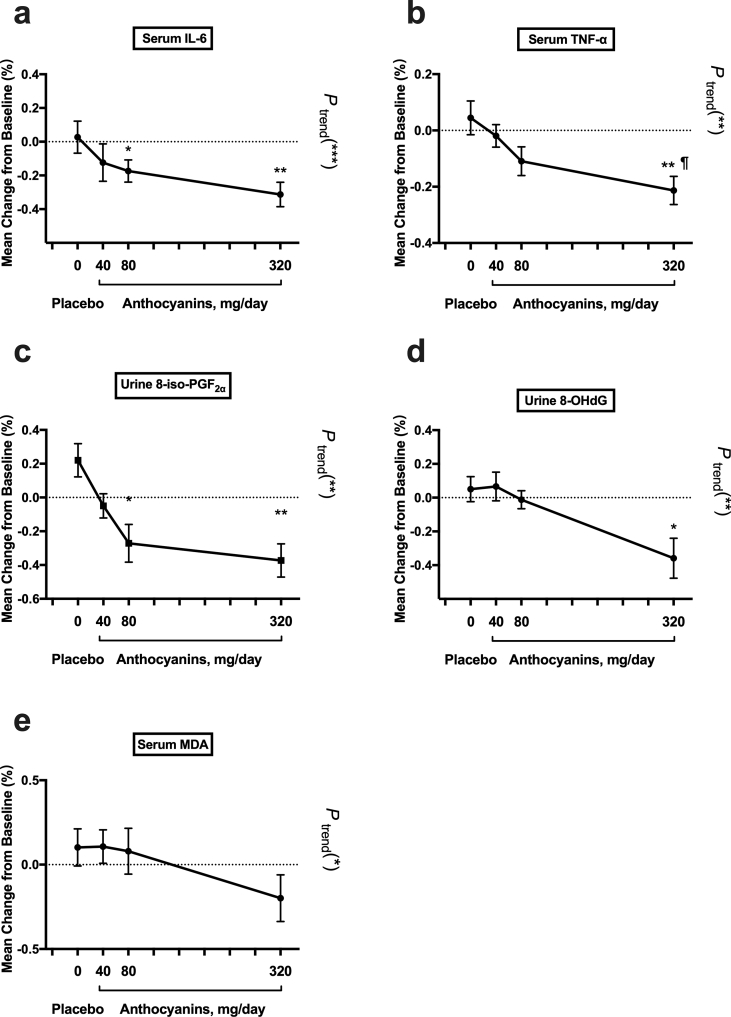
The percent change from baseline to 12 weeks in inflammatory cytokines was compared between the groups (Table 3). Next, we checked whether the serum cytokines levels decreased linearly with the dose of anthocyanin (Fig. 3). The reduction in serum IL-6 from baseline to 12 weeks in both the 80 mg anthocyanin (−20%, P < 0.05) and 320 mg anthocyanin (−40%, P < 0.01) groups was significantly different from the change in the placebo group (8%) (Table 3). Similarly, the reduction in serum TNF-α from the baseline to 12 weeks in the 320 mg anthocyanin group (−21%) was different from the alteration in the placebo group (5%; P < 0.01) (Table 3). The reduction in IL-6 (P for trend < 0.001) and TNF-α (P for trend < 0.01) was further found to be significantly dependent on the dose of anthocyanin supplementation (Fig. 3a and b). The percent changes from baseline to 12 weeks in IL-10 and CRP were not significantly different between the groups (Table 3). Interestingly, the alteration of TNF-α between the 40 mg and 320 mg anthocyanin groups was significantly different at the end of 12 weeks (P < 0.05, Fig. 3b). In addition, the percent change from baseline to 6 weeks in these inflammatory cytokines was not significant between the groups or dependent on the dose of anthocyanin supplementation (Table 3 and Supplemental Fig. 2).
Fig. 3.
Percentage change in biomarker concentration after the 12-week intervention. Percentage change was compared between groups and linear trend analysis was further assessed. Data represent the mean (SEM) measurements for each group. *P < 0.05; **P < 0.01 and ***P < 0.001 between anthocyanins groups and placebo group as well as linear trend analysis; ¶P < 0.05 between 320 mg/day and 40 mg/day anthocyanins group. SEM, standard error of mean; IL-6, interleukin-6; TNF-α, tumor necrosis factor α; 8-iso- PGF2α, 8-iso-prostaglandin F2α; 8-OHdG, 8-hydroxy-2′-deoxyguanosine; MDA, malonaldehyde; Antho, anthocyanin group.
3.3. Effects of anthocyanin on oxidative stress biomarkers
Within groups, the absolute changes in the concentration of oxidative stress biomarkers from baseline to follow-up were assessed. After 6 weeks, a significant increase was observed in the T-SOD activity from 162.68 ± 4.30 U/mL to 178.79 ± 5.21 U/mL (P < 0.05) in 320 mg anthocyanin group (Table 4). After 12 weeks, subjects receiving 40 mg/day anthocyanins reported moderately reduce of urine 8-iso-PGF2α, but without significance (Fig. 2c, Table 4). The 12-week anthocyanin supplementation at 80 mg/day produced a significant reduction in urine 8-iso-PGF2α from 1.19 ± 0.07 pg/mg creatinine to 0.89 ± 0.13 pg/mg creatinine (P < 0.05, Fig. 2c, Table 4). Furthermore, 12-week anthocyanin supplementation at 320 mg/day yielded further reduction than 80 mg/day and 40 mg/day dosages for improving urine 8-iso-PGF2α, 8-OHdG, and serum MDA, with baseline 1.31 ± 0.08 pg/mg creatinine, 6.98 ± 0.56 ng/mg creatinine, and 3.78 ± 0.20 μmol/L to post-treatment 1.04 ± 0.16 pg/mg creatinine, 4.25 ± 0.53 ng/mg creatinine, and 2.92 ± 0.18 μmol/L, respectively (P < 0.05; Fig. 2c, d, and 2e, Table 4).
Table 4.
Effects of anthocyanin on oxidative stress biomarkers.
| Placebo (n=43) |
40mg Antho (n=44) |
80mg Antho (n=40) |
320mg Antho (n=42) |
|||||
|---|---|---|---|---|---|---|---|---|
| value | MCb (%) | value | MC (%) | value | MC (%) | value | MC (%) | |
| Urine 8-iso-PGF2α (pg/mg creatinine) | ||||||||
| Baseline | 1.26±0.07a | 1.27±0.06 | 1.19±0.07 | 1.31±0.08 | ||||
| 6 wk | 1.22±0.10 | 0.13±0.15 | 1.18±0.12 | -0.02±0.10 | 1.22±0.13 | 0.19±0.16 | 1.27±0.11 | 0.16±0.16 |
| 12 wk | 1.44±0.12 | 0.21±0.10 | 1.18±0.09 | -0.05±0.07 | 0.89±0.13#[1] | -0.27±0.11∗[2] | 1.04±0.16# | -0.37±0.10∗∗[2] |
| Urine 8-OHdG (ng/mg creatinine) | ||||||||
| Baseline | 5.81±0.48 | 6.16±0.49 | 6.12±0.43 | 6.98±0.56 | ||||
| 6 wk | 5.31±0.45 | -0.08±0.05 | 5.63±0.47 | -0.05±0.05 | 5.82±0.44 | -0.03±0.04 | 6.55±0.55 | -0.07±0.04 |
| 12 wk | 5.61±0.47 | 0.05±0.07 | 5.91±0.49 | 0.07±0.08 | 5.74±0.42 | -0.01±0.05 | 4.25±0.53# | -0.36±0.08∗ |
| MDA (μmol/L) | ||||||||
| Baseline | 3.52±0.16 | 3.71±0.19 | 3.62±0.19 | 3.78±0.20 | ||||
| 6 wk | 3.57±0.20 | 0.14±0.10 | 3.61±0.23 | 0.15±0.13 | 3.21±0.21 | 0.18±0.22 | 3.37±0.21 | 0.09±0.14 |
| 12 wk | 3.41±0.17 | 0.10±0.11 | 3.62±0.23 | 0.11±0.10 | 3.20±0.20 | 0.08±0.14 | 2.92±0.18# | -0.20±0.05 |
| T-SOD (U/ml) | ||||||||
| Baseline | 162.80±3.61 | 166.52±3.80 | 166.56±3.35 | 162.68±4.30 | ||||
| 6 wk | 168.02±3.49 | 0.04±0.01 | 171.55±3.89 | 0.04±0.02 | 171.28±4.10 | 0.05±0.02 | 178.79±5.21# | 0.10±0.02∗ |
| 12 wk | 161.48±3.26 | -0.00±0.01 | 165.99±3.84 | 0.00±0.12 | 165.78±3.05 | -0.00±0.07 | 162.76±3.76 | 0.01±0.01 |
| UA (μmol/L) | ||||||||
| Baseline | 367.65±17.54 | 367.01±15.07 | 346.74±12.81 | 361.21±16.96 | ||||
| 6 wk | 349.80±15.44 | -0.01±0.04 | 347.21±19.27 | -0.05±0.03 | 328.73±16.96 | -0.04±0.03 | 339.73±18.38 | -0.05±0.04 |
| 12 wk | 392.70±17.22 | 0.11±0.04 | 392.97±20.53 | 0.05±0.03 | 354.62±14.53 | 0.07±0.04 | 370.43±16.90 | 0.06±0.02 |
1 Compared within the group from baseline to follow-up (Either Wilcoxon signed rank test or Students’ t test for paired data), #P<0.05.
2 Compared between the placebo group and anthocyanin groups (ANOVA for independent data), ∗P<0.05, ∗∗P<0.01.
Abbreviation: SEM, standard error of mean; MC, mean change; Antho, anthocyanin group; 8-iso-PGF2α, 8-iso-prostaglandin F2α; 8-OHdG, 8-hydroxy-2'-deoxyguanosine; MDA, malonaldehyde; T-SOD: total superoxide dismutase; UA, uric acid.
Mean ± SEM (all such values).
Calculated as (value after intervention-value before intervention) / value before intervention ×100%.
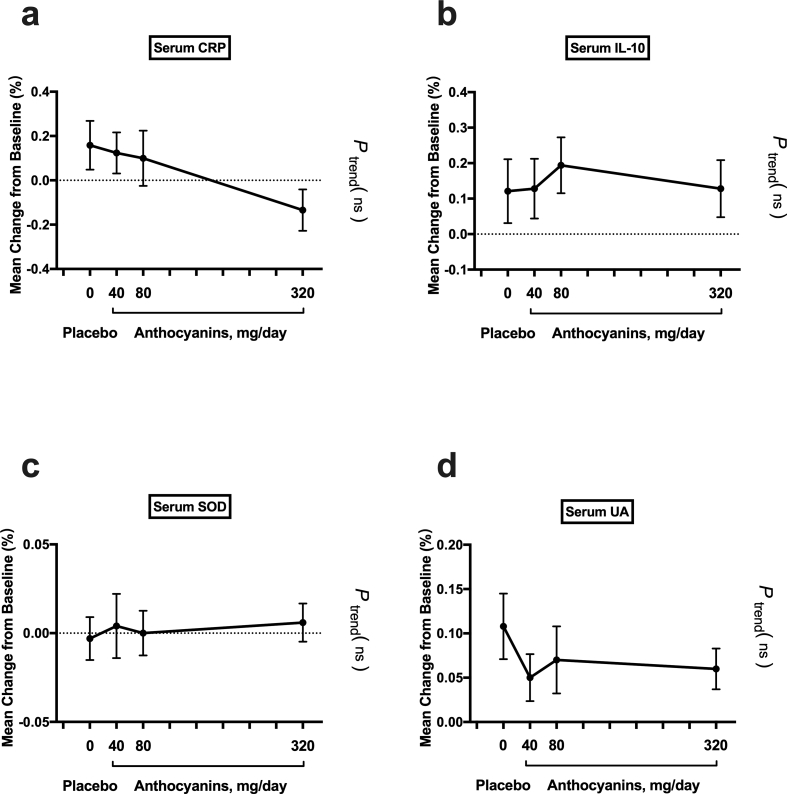
Between groups, percent change was compared and further tested whether oxidative stress biomarkers changed linearly as the dose of anthocyanin increased (Fig. 3 and Table 4). The reduction in urine 8-iso-PGF2α from baseline to 12 weeks in the 80 mg anthocyanins (−27%, P < 0.05) and 320 mg anthocyanins (−37%, P < 0.01) groups was significantly different from the change in the placebo group (21%) (Table 4). Moreover, urine 8-OHdG decreased significantly in the 320 mg anthocyanin group (−36%, P < 0.05) compared with the alteration in the placebo group (5%) after the 12-week treatment (Table 4). Furthermore, these changes were found to be significantly dependent on the anthocyanin dose (P for trend, < 0.01) (Fig. 3c and d). Although the percentage change in MDA was not significantly different between groups, there was a significant dose–response effect of the intervention (P for trend, < 0.05) (Fig. 3e). However, the percentage changes in T-SOD and UA were not significantly different between the groups, nor was there a significant dose-dependent effect of anthocyanin (Supplemental Figs. 1c and 1d, Table 4). In addition, after 6-week treatment, a significant percent change was observed in the T-SOD activity between anthocyanins group (320 mg/day) and placebo group. Moreover, the change was found to be significantly dependent on the anthocyanin dose (P for trend, < 0.01) (Supplemental Fig. 2h). However, other oxidative stress biomarkers did not show significant percent change between groups after 6 weeks (Table 4 and Supplemental Fig. 2).
3.4. Association between changes in oxidative stress and inflammation biomarkers
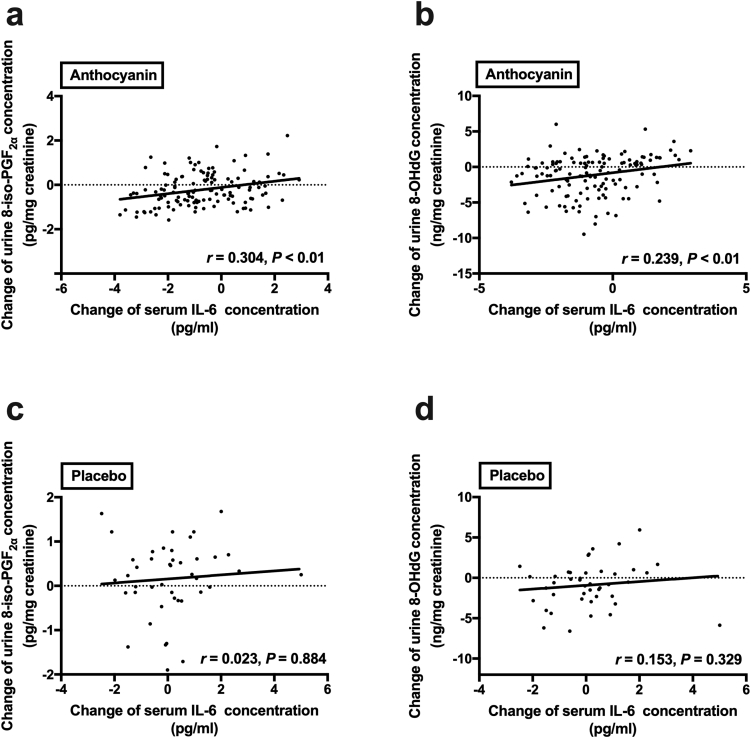
After the 12-week anthocyanin intervention, the decrease in urine 8-iso-PGF2α exhibited a positive correlation with the change in serum IL-6 (r = 0.304, P < 0.01; Fig. 4a) in subjects from the anthocyanin groups including the 40 mg, 80 mg, and 320 mg anthocyanin groups. Similarly, a positive association between the change in urine 8-OHdG and the alteration in serum IL-6 (r = 0.239, P < 0.01; Fig. 4b) was observed in subjects from the anthocyanin groups. However, these associations were not found in the placebo-treated subjects (Fig. 4c and d).
Fig. 4.
Correlation between changes in oxidative stress and inflammation biomarkers. Association between the change in serum IL-6 levels and the alteration in urine 8-iso PGF2α levels are shown in Fig. 4a and c; and the decrease in urine 8-OHdG are shown in Fig. 4b and d. Pearson's correlation coefficients are noted for each plot. Fig. 4a and b represent the anthocyanin groups (n = 126), whereas Fig. 4c and d represent the placebo group (n = 43). 8-iso- PGF2α, 8-iso-prostaglandin F2α; 8-OHdG, 8-hydroxy-2′-deoxyguanosine; IL-6, interleukin-6.
4. Discussion
In this randomized controlled trial, we observed a linear dose–response relationship for the change in inflammatory and oxidative biomarkers after anthocyanin supplementation in individuals with dyslipidemia. At 12 weeks, anthocyanin supplementation at 40 mg/day moderately reduced serum IL-6 and TNF-α and urine 8-iso-PGF2α. Supplementation of anthocyanins 80 mg/day for 12 weeks produced significant reduction in IL-6 and TNF-α and 8-iso-PGF2α. Subjects who received 320 mg/day anthocyanins for 12 weeks showed further improvement in reducing serum IL-6 and TNF-α, MDA, and urine 8-iso-PGF2α, 8-OHdG than those who received 80 mg/day and 40 mg/day anthocyanins. In addition, 6-week anthocyanin supplementation at 320 mg/day significantly improved T-SOD, but no other cytokines. These results revealed the dose–response of anthocyanin-induced anti-inflammatory and anti-oxidative effects. Moreover, these results indicated that anthocyanin supplementation at 80 mg/day could reach the threshold level for producing beneficial functions among subjects with dyslipidemia.
A number of studies reported that consumption of anthocyanin-rich foods such as berries (raspberries [34], bilberry juice [35], and blueberries [36]) as well as anthocyanin extracts [37,38] resulted in beneficial functions such as decreasing inflammatory and oxidative stress biomarkers, improving glucose and fatty acid metabolism, as well as improving vascular endothelial functions [39]. However, the effective dose of anthocyanin at which it exerts beneficial functions has not been well studied. Despite the widely variant anthocyanin doses in previous intervention studies, ranging from 59.5 mg/day [40] to 640 mg/day [41], most studies only used a single dose to observe the effect of anthocyanins. For example, Traustadottir et al. [40] reported that intake of 240 mL tart cherry juice twice daily (59.5 mg of total anthocyanins per day) for 4 weeks reduced urine 8-OHdG and plasma F2-isoprostane compared with the levels in subjects in the placebo group. However, another trial with similar dose and conducted by Estevez-Santiago [42] showed that 8-month anthocyanin supplementation at 60 mg/day improved none of the plasma CRP, IL-6, vascular cell adhesion molecule-1 (VCAM-1), or intercellular adhesion molecule-1 in postmenopausal women. Previous trial [43] with supplementation of 320 mg/day anthocyanins showed significant reduction in UA but not in CRP after 24 weeks in adults with prediabetes. The supplementation of anthocyanins 640 mg/day for 4 weeks significantly improved serum concentrations of HDL and blood glucose, but no effects on the inflammatory or oxidative stress biomarkers in pre-hypertensive men [41] were observed. Taken together, these studies with inconsistent results used a single dose of anthocyanins and their study designs largely varied, with differences in intervention duration and the studied population, which hindered the comparison of effects between different anthocyanin levels. Therefore, there exists no evidence to raise the concentration of anthocyanins to adequate levels to improve the health status and prevent or treat metabolic diseases. The present study first assessed the dose–response effect of anthocyanins and demonstrated that anthocyanins at 80 mg/day could produce beneficial functions by improving inflammation and oxidative stress among subjects with dyslipidemia, whereas 320 mg/day anthocyanins would result in obvious improvement in inflammatory and oxidative response. This evidence indicated that a daily intake of 80 mg or more of anthocyanins could be recommended as a therapeutic strategy for dyslipidemia.
Observational studies across countries showed that anthocyanin intake in daily diet was relatively low, ranging from 11.48 to 47.0 mg/day. For example, Finnish [44] consumed anthocyanin 47.0 mg/day from their daily diet, which was higher than those consumed by other adults in Korea [45] (37.0 mg/day), France [46](35.0 mg/day), China [47] (28.0 mg/day), Australia [48] (24.17 mg/day), Spain [49] (18.8 mg/day), and America [50] (11.48 mg/day). Taken together, anthocyanins intake from daily diet in these countries did not reach 80 mg, which was recommended to exert beneficial functions in our trial. Thus, supplementation with adequate amounts of anthocyanins, for example in the form of anthocyanin-rich food or anthocyanin extracts, appeared necessary. Actually, it is not difficult to meet the anthocyanin supplementation at 80 mg/day with food rich in anthocyanins, especially berries and currants. For example, anthocyanins content ranges from 295.48 mg to 1266.00 mg in every 100 g black elderberry [51,52], 125.63 mg–989.70 mg in every 100 g black chokeberry [51,53], and 85.21 mg–190.62 mg per 100 g blackberry [54]. Therefore, anthocyanin supplementation at 80 mg/day appears both clinical relevant and realizable by food.
In addition to adequate levels of anthocyanin intake, the duration of anthocyanin supplementation plays an important role in preventing metabolic diseases. In the present trial, we found that the intervention effects for 6 weeks were not as significant as those at 12 weeks, with improving only CRP and T-SOD but no other biomarkers. A linear dose–response relationship was not observed after 6 weeks either. It indicated that adequate intervention duration was important. Wright et al. [55] reported that 4-week supplementation with dried purple carrot (118.5 mg/day anthocyanins) showed no significant decrease in BMI, body composition, appetite, LDL-C, TC, or CRP in overweight and obese adults. Another short-term trial conducted by Karlsen et al. [56] showed that 3-week intake of purified anthocyanins (300 mg/day) did not result in significant alterations in CRP, TNF-α, or interleukin-1β (IL-1β) concentrations in healthy adults. However, a long-term intervention trial with similar anthocyanin levels conducted by Zhu et al. [57] reported that purified anthocyanin supplementation at 320 mg/day significantly reduced CRP, VCAM-1, and IL-1β concentrations in subjects with hypercholesterolemia at 12 and 24 weeks after intervention [69]. Similarly, another 12-week intervention study showed that anthocyanin-rich beverage significantly improved biomarkers for inflammation and oxidative stress, including plasma interferon gamma (IFN-γ) and urine 8-isoprostane concentration in individuals with metabolic syndrome. It might be possible that the efficacy of anthocyanin on inflammatory, oxidative stress, lipids, and other responses depends on, to some extent, the duration of the intervention.
Dyslipidemia is associated with elevated levels of reactive oxygen species (ROS), biomarkers of lipid peroxidation, DNA oxidation, and pro-inflammatory cytokines [8,[58], [59], [60]]. Evidence suggests that oxidative stress, as an active contributor to the early steps of metabolic diseases, is primarily attributed to the pathologic role of ROS in visceral adiposity, endothelial damage, and lipoprotein metabolism [61,62]. Pro-inflammatory cytokines, including CRP, IL-6, and TNF-α, have been repeatedly reported to be directly associated with several cardiovascular, as well as metabolic diseases [9,10], by activation of NF-κB and MAPK signaling pathways [63,64]. Meanwhile, oxidative stress can also activate NF-κB and trigger the release of pro-inflammatory cytokine CRP [65]. These findings indicate that dyslipidemia, inflammation, and oxidative stress are highly interrelated and cross-promote each other in a vicious cycle, resulting in the progression of cardiovascular and metabolic diseases [21]. Recently, some studies have revealed that anthocyanin supplementation significantly improved the inflammation and oxidative stress in individuals with hypercholesterolemia [66] or hyperlipidemia [67], consistent with the effects of anthocyanins observed in the present trial. In the present study, we revealed that anthocyanin-mediated reduction in the oxidative stress biomarkers, urine 8-iso-PGF2α, and 8-OHdG, was positively correlated with the change in the inflammatory cytokine IL-6 after 12-week treatment. However, the correlations were not observed in the placebo group. These findings indicate a potential mechanism by which anthocyanins exert protective effects on the metabolic diseases, achieved through the comprehensive regulation of oxidative stress and inflammation.
The present study has both strengths and limitations. The double-blinded, randomized controlled trial design with multi-dose interventions is the major strength of this study; it minimizes the chance that our results are attributed to confounding bias. Moreover, self-reported compliance to the intervention is high. Results of diet monitoring indicated that the daily intake of energy, nutrients, and anthocyanins was comparable among the four study groups at baseline and after a 12-week intervention, which may also strengthen our study. A limitation of the study was that the results applied to individuals with dyslipidemia. Therefore, it remains unknown whether these also apply to healthy individuals or those with more advanced stages of other metabolic diseases or CVDs. Another limitation of this work was that like most other randomized controlled trials using anthocyanins as treatment, we did not detect anthocyanins or their metabolites in serum samples as a measure of compliance, mainly owing to the short half-life of anthocyanins (4 h) [68].
Overall, results of the current study demonstrated supplementation with purified anthocyanins for 12 weeks reduced the oxidative and inflammatory response in a dose–response manner in individuals with dyslipidemia.
Funding
This work was supported by Grant 81730090 from the Key Project of National Natural Science Foundation of China and the Guangzhou Science, Technology and Innovation Committee (Grant. 201804020045).
Authors’ contributions
The authors made the following contributions: HY.Z., ZL.X. and HW.Z. carried out the human intervention experiments; J.P., Q.L., and X.W. carried out the experimental determinations; HY.Z., ZL.X., and HW.Z. conducted the statistical evaluation of the data; X.W. contributed to summarizing and calculating the raw data and revising the manuscript; and HY.Z. contributed to the experimental design and the manuscript, managed the overall project, and wrote the manuscript.
Disclosure summary
The authors have nothing to disclose.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the results presented in the manuscript entitled.
Acknowledgements
The treated capsules, including the anthocyanin (Medox) and the placebo, were kindly donated by MedPalett Pharmaceuticals and the Biolink Group in Norway.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2020.101474.
Contributor Information
Hanyue Zhang, Email: zhanghy97@mail2.sysu.edu.cn.
Zhongliang Xu, Email: xuzhliang3@mail2.sysu.edu.cn.
Huiwen Zhao, Email: zhaohw5@mail2.sysu.edu.cn.
Xu Wang, Email: wangxu25@mail2.sysu.edu.cn.
Juan Pang, Email: pangj7@mail2.sysu.edu.cn.
Qing Li, Email: liqing45@mail2.sysu.edu.cn.
Yan Yang, Email: yangyan3@mail.sysu.edu.cn.
Wenhua Ling, Email: lingwh@mail.sysu.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplemental Fig. 1.
Percentage change in biomarker concentration after the 12-week intervention. Percentage change was compared between groups and linear trend analysis was further assessed. Data represent the mean (SEM) measurements for each group. SEM, standard error of mean; CRP: C-reactive protein; IL-10: interleukin-10; T-SOD: total superoxide dismutase; UA, uric acid.
Supplemental Fig. 2.
Percentage change in biomarker concentration after the 6-week intervention. Percentage change was compared between groups and linear trend analysis was further assessed. Data represent the mean (SEM) measurements for each group. *P < 0.05 between anthocyanins groups and placebo group as well as linear trend analysis. SEM, standard error of mean; IL-6 indicates interleukin-6; TNF-α, tumor necrosis factor α; CRP: C-reactive protein; IL-10: interleukin-10; 8-iso- PGF2α, 8-iso-prostaglandin F2α; 8-OHdG, 8-hydroxy-2′-deoxyguanosine; MDA, malonaldehyde; T-SOD: total superoxide dismutase; UA, uric acid.
References
- 1.Pan L., Yang Z.H., Wu Y., Yin R.X., Liao Y.H., Wang J.W., Gao B.X., Zhang L.X., Grp C.K.D.W. The prevalence, awareness, treatment and control of dyslipidemia among adults in China. Atherosclerosis. 2016;248:2–9. doi: 10.1016/j.atherosclerosis.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Yang W.Y., Xiao J.Z., Yang Z.J., Ji L.N., Jia W.P., Weng J.P., Lu J.M., Shan Z.Y., Liu J., Tian H.M., Ji Q.H., Zhu D.L., Ge J.P., Lin L.X., Chen L., Guo X.H., Zhao Z.G., Li Q., Zhou Z.G., Shan G.L., He J., Disorders C.N.D.M. Serum lipids and lipoproteins in Chinese men and women. Circulation. 2012;125:2212–2221. doi: 10.1161/CIRCULATIONAHA.111.065904. [DOI] [PubMed] [Google Scholar]
- 3.Boo S., Yoon Y.J., Oh H. Evaluating the prevalence, awareness, and control of hypertension, diabetes, and dyslipidemia in Korea using the NHIS-NSC database A cross-sectional analysis. Medicine. 2018;97 doi: 10.1097/MD.0000000000013713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Von Eynatten M., Hamann A., Twardella D., Nawroth P.P., Brenner H., Rothenbacher D. Relationship of adiponectin with markers of systemic inflammation, atherogenic dyslipidemia, and heart failure in patients with coronary heart disease. Clin. Chem. 2006;52:853–859. doi: 10.1373/clinchem.2005.060509. [DOI] [PubMed] [Google Scholar]
- 5.Mooradian A.D. Dyslipidemia in type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2009;5:150–159. doi: 10.1038/ncpendmet1066. [DOI] [PubMed] [Google Scholar]
- 6.Klop B., Elte J.W.F., Cabezas M.C. Dyslipidemia in obesity: mechanisms and potential targets. Nutrients. 2013;5:1218–1240. doi: 10.3390/nu5041218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brooks G.C., Blaha M.J., Blumenthal R.S. Relation of C-reactive protein to abdominal adiposity. Am. J. Cardiol. 2010;106:56–61. doi: 10.1016/j.amjcard.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 8.Sparks D.L., Chatterjee C. Purinergic signaling, dyslipidemia and inflammatory disease. Cell. Physiol. Biochem. 2012;30:1333–1339. doi: 10.1159/000343322. [DOI] [PubMed] [Google Scholar]
- 9.Loprinzi P.D., Edwards M.K. CVD-related Fit-Fat Index on inflammatory-based CVD biomarkers. Int. J. Cardiol. 2016;223:284–285. doi: 10.1016/j.ijcard.2016.08.194. [DOI] [PubMed] [Google Scholar]
- 10.Lumeng C.N., Saltiel A.R. Inflammatory links between obesity and metabolic disease. J. Clin. Invest. 2011;121:2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diniz Y.S., Rocha K.K.H.R., Souza G.A., Galhardi C.M., Ebaid G.M.X., Rodrigues H.G., Novelli J.L.V.B., Cicogna A.C., Novelli E.L.B. Effects of N-acetylcysteine on sucrose-rich diet-induced hyperglycaemia, dyslipidemia and oxidative stress in rats. Eur. J. Pharmacol. 2006;543:151–157. doi: 10.1016/j.ejphar.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 12.Annuzzi G., Bozzetto L., Costabile G., Giacco R., Mangione A., Anniballi G., Vitale M., Vetrani C., Cipriano P., Della Corte G., Pasanisi F., Riccardi G., Rivellese A.A. Diets naturally rich in polyphenols improve fasting and postprandial dyslipidemia and reduce oxidative stress: a randomized controlled trial(1-3) Am. J. Clin. Nutr. 2014;99:463–471. doi: 10.3945/ajcn.113.073445. [DOI] [PubMed] [Google Scholar]
- 13.Gomez-Garcia A., Torres G.M., Ortega-Pierres L.E., Rodriguez-Ayala E., Alvarez-Aguilar C. Rosuvastatin and metformin decrease inflammation and oxidative stress in patients with hypertension and dyslipidemia. Rev. Esp. Cardiol. 2007;60:1242–1249. doi: 10.1157/13113929. [DOI] [PubMed] [Google Scholar]
- 14.Mollazadeh H., Carbone F., Montecucco F., Pirro M., Sahebkar A. Oxidative burden in familial hypercholesterolemia. J. Cell. Physiol. 2018;233:5716–5725. doi: 10.1002/jcp.26466. [DOI] [PubMed] [Google Scholar]
- 15.Bresciani G., da Cruz I.B., Gonzalez-Gallego J. Manganese superoxide dismutase and oxidative stress modulation. Adv. Clin. Chem. 2015;68:87–130. doi: 10.1016/bs.acc.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Vassalle C., Bianchi S., Bianchi F., Landi P., Battaglia D., Carpeggiani C. Oxidative stress as a predictor of cardiovascular events in coronary artery disease patients. Clin. Chem. Lab. Med. 2012;50:1463–1468. doi: 10.1515/cclm-2011-0919. [DOI] [PubMed] [Google Scholar]
- 17.Schwedhelm E., Bartling A., Lenzen H., Tsikas D., Maas R., Brummer J., Gutzki F.M., Berger J., Frolich J.C., Boger R.H. Urinary 8-iso-prostaglandin F2alpha as a risk marker in patients with coronary heart disease: a matched case-control study. Circulation. 2004;109:843–848. doi: 10.1161/01.CIR.0000116761.93647.30. [DOI] [PubMed] [Google Scholar]
- 18.Wu L.L., Chiou C.C., Chang P.Y., Wu J.T. Urinary 8-OHdG: a marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics. Clin. Chim. Acta. 2004;339:1–9. doi: 10.1016/j.cccn.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 19.Kim J.Y., Lee J.W., Youn Y.J., Ahn M.S., Ahn S.G., Yoo B.S., Lee S.H., Yoon J., Choe K.H. Urinary levels of 8-iso-prostaglandin f2alpha and 8-hydroxydeoxyguanine as markers of oxidative stress in patients with coronary artery disease. Kor. Circ. J. 2012;42:614–617. doi: 10.4070/kcj.2012.42.9.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bastos A.S., Graves D.T., Loureiro A.P., Rossa Junior C., Abdalla D.S., Faulin Tdo E., Olsen Camara N., Andriankaja O.M., Orrico S.R. Lipid peroxidation is associated with the severity of periodontal disease and local inflammatory markers in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 2012;97:E1353–E1362. doi: 10.1210/jc.2011-3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bryan S., Baregzay B., Spicer D., Singal P.K., Khaper N. Redox-inflammatory synergy in the metabolic syndrome. Can. J. Physiol. Pharmacol. 2013;91:22–30. doi: 10.1139/cjpp-2012-0295. [DOI] [PubMed] [Google Scholar]
- 22.Koponen J.M., Happonen A.M., Mattila P.H., Torronen A.R. Contents of anthocyanins and ellagitannins in selected foods consumed in Finland. J. Agric. Food Chem. 2007;55:1612–1619. doi: 10.1021/jf062897a. [DOI] [PubMed] [Google Scholar]
- 23.Manach C., Scalbert A., Morand C., Remesy C., Jimenez L. Polyphenols: food sources and bioavailability. Am. J. Clin. Nutr. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 24.Zafra-Stone S., Yasmin T., Bagchi M., Chatterjee A., Vinson J.A., Bagchi D. Berry anthocyanins as novel antioxidants in human health and disease prevention. Mol. Nutr. Food Res. 2007;51:675–683. doi: 10.1002/mnfr.200700002. [DOI] [PubMed] [Google Scholar]
- 25.Kolehmainen M., Mykkanen O., Kirjavainen P.V., Leppanen T., Moilanen E., Adriaens M., Laaksonen D.E., Hallikainen M., Puupponen-Pimia R., Pulkkinen L., Mykkanen H., Gylling H., Poutanen K., Torronen R. Bilberries reduce low-grade inflammation in individuals with features of metabolic syndrome. Mol. Nutr. Food Res. 2012;56:1501–1510. doi: 10.1002/mnfr.201200195. [DOI] [PubMed] [Google Scholar]
- 26.Franssen R., Monajemi H., Stroes E.S.G., Kastelein J.J.P. Obesity and dyslipidemia. Med. Clin. N. Am. 2011;95:893. doi: 10.1016/j.mcna.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Ju L., Yu D., Fang H., Guo Q., Xu X., Li S., Zhao L. Trends and food sources composition of energy, protein and fat in Chinese residents, 1992-2012. Wei Sheng Yan Jiu. 2018;47:689–704. [PubMed] [Google Scholar]
- 28.Zhu W., Jia Q., Wang Y., Zhang Y., Xia M. The anthocyanin cyanidin-3-O-beta-glucoside, a flavonoid, increases hepatic glutathione synthesis and protects hepatocytes against reactive oxygen species during hyperglycemia: involvement of a cAMP-PKA-dependent signaling pathway. Free Radic. Biol. Med. 2012;52:314–327. doi: 10.1016/j.freeradbiomed.2011.10.483. [DOI] [PubMed] [Google Scholar]
- 29.Helmersson J., Basu S. F-2-isoprostane excretion rate and diurnal variation in human urine. Prostag Leukotr Ess. 1999;61:203–205. doi: 10.1054/plef.1999.0091. [DOI] [PubMed] [Google Scholar]
- 30.Michoulas A., Tong V., Teng X.W., Chang T.K.H., Abbott F.S., Farrell K. Oxidative stress in children receiving valproic acid. J. Pediatr. US. 2006;149:692–696. doi: 10.1016/j.jpeds.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 31.Shibata H., Nabika T., Moriyama H., Masuda J., Kobayashi S. Correlation of NO metabolites and 8-iso-prostaglandin F-2a with periventricular hyperintensity severity. Arterioscler. Thromb. Vasc. Biol. 2004;24:1659–1663. doi: 10.1161/01.ATV.0000137415.67349.3c. [DOI] [PubMed] [Google Scholar]
- 32.Devries M.C., Hamadeh M.J., Glover A.W., Raha S., Samjoo I.A., Tarnopolsky M.A. Endurance training without weight loss lowers systemic, but not muscle, oxidative stress with no effect on inflammation in lean and obese women. Free Radical Biol. Med. 2008;45:503–511. doi: 10.1016/j.freeradbiomed.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 33.Zhu Y., Xia M., Yang Y., Liu F., Li Z., Hao Y., Mi M., Jin T., Ling W. Purified anthocyanin supplementation improves endothelial function via NO-cGMP activation in hypercholesterolemic individuals. Clin. Chem. 2011;57:1524–1533. doi: 10.1373/clinchem.2011.167361. [DOI] [PubMed] [Google Scholar]
- 34.Schell J., Betts N.M., Lyons T.J., Basu A. Raspberries improve postprandial glucose and acute and chronic inflammation in adults with type 2 diabetes. Ann. Nutr. Metab. 2019;74:165–174. doi: 10.1159/000497226. [DOI] [PubMed] [Google Scholar]
- 35.Biedermann L., Mwinyi J., Scharl M., Frei P., Zeitz J., Kullak-Ublick G.A., Vavricka S.R., Fried M., Weber A., Humpf H.U., Peschke S., Jetter A., Krammer G., Rogler G. Bilberry ingestion improves disease activity in mild to moderate ulcerative colitis - an open pilot study. J. Crohns Colitis. 2013;7:271–279. doi: 10.1016/j.crohns.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 36.Curtis P.J., van der Velpen V., Berends L., Jennings A., Feelisch M., Umpleby A.M., Evans M., Fernandez B.O., Meiss M.S., Minnion M., Potter J., Minihane A.M., Kay C.D., Rimm E.B., Cassidy A. Blueberries improve biomarkers of cardiometabolic function in participants with metabolic syndrome-results from a 6-month, double-blind, randomized controlled trial. Am. J. Clin. Nutr. 2019;109:1535–1545. doi: 10.1093/ajcn/nqy380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang X., Zhu Y., Song F., Yao Y., Ya F., Li D., Ling W., Yang Y. Effects of purified anthocyanin supplementation on platelet chemokines in hypocholesterolemic individuals: a randomized controlled trial. Nutr. Metab. (Lond) 2016;13:86. doi: 10.1186/s12986-016-0146-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu Y., Ling W., Guo H., Song F., Ye Q., Zou T., Li D., Zhang Y., Li G., Xiao Y., Liu F., Li Z., Shi Z., Yang Y. Anti-inflammatory effect of purified dietary anthocyanin in adults with hypercholesterolemia: a randomized controlled trial. Nutr. Metabol. Cardiovasc. Dis. 2013;23:843–849. doi: 10.1016/j.numecd.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 39.Jin Y., Alimbetov D., George T., Gordon M.H., Lovegrove J.A. A randomised trial to investigate the effects of acute consumption of a blackcurrant juice drink on markers of vascular reactivity and bioavailability of anthocyanins in human subjects. Eur. J. Clin. Nutr. 2011;65:849–856. doi: 10.1038/ejcn.2011.55. [DOI] [PubMed] [Google Scholar]
- 40.Traustadottir T., Davies S.S., Stock A.A., Su Y., Heward C.B., Roberts L.J., Harman S.M. Tart cherry juice decreases oxidative stress in healthy older men and women. J. Nutr. 2009;139:1896–1900. doi: 10.3945/jn.109.111716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hassellund S.S., Flaa A., Kjeldsen S.E., Seljeflot I., Karlsen A., Erlund I., Rostrup M. Effects of anthocyanins on cardiovascular risk factors and inflammation in pre-hypertensive men: a double-blind randomized placebo-controlled crossover study. J. Hum. Hypertens. 2013;27:100–106. doi: 10.1038/jhh.2012.4. [DOI] [PubMed] [Google Scholar]
- 42.Estevez-Santiago R., Silvan J.M., Can-Cauich C.A., Veses A.M., Alvarez-Acero I., Martinez-Bartolome M.A., San-Roman R., Camara M., Olmedilla-Alonso B., de Pascual-Teresa S. Lack of a synergistic effect on cardiometabolic and redox markers in a dietary supplementation with anthocyanins and xanthophylls in postmenopausal women. Nutrients. 2019;11 doi: 10.3390/nu11071533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang L.P., Ling W.H., Yang Y., Chen Y.M., Tian Z.Z., Du Z.C., Chen J.Y., Xie Y.L., Liu Z.M., Yang L.L. Role of purified anthocyanins in improving cardiometabolic risk factors in Chinese men and women with prediabetes or early untreated diabetes-A randomized controlled trial. Nutrients. 2017;9 doi: 10.3390/nu9101104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ovaskainen M.L., Torronen R., Koponen J.M., Sinkko H., Hellstrom J., Reinivuo H., Mattila P. Dietary intake and major food sources of polyphenols in Finnish adults. J. Nutr. 2008;138:562–566. doi: 10.1093/jn/138.3.562. [DOI] [PubMed] [Google Scholar]
- 45.Jun S., Shin S., Joung H. Estimation of dietary flavonoid intake and major food sources of Korean adults. Br. J. Nutr. 2016;115:480–489. doi: 10.1017/S0007114515004006. [DOI] [PubMed] [Google Scholar]
- 46.Perez-Jimenez J., Fezeu L., Touvier M., Arnault N., Manach C., Hercberg S., Galan P., Scalbert A. Dietary intake of 337 polyphenols in French adults. Am. J. Clin. Nutr. 2011;93:1220–1228. doi: 10.3945/ajcn.110.007096. [DOI] [PubMed] [Google Scholar]
- 47.Li G., Zhu Y., Zhang Y., Lang J., Chen Y., Ling W. Estimated daily flavonoid and stilbene intake from fruits, vegetables, and nuts and associations with lipid profiles in Chinese adults. J. Acad. Nutr. Diet. 2013;113:786–794. doi: 10.1016/j.jand.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 48.Igwe E.O., Charlton E., Probst Y.C. Usual dietary anthocyanin intake, sources and their association with blood pressure in a representative sample of Australian adults. J. Hum. Nutr. Diet. 2019;32:578–590. doi: 10.1111/jhn.12647. [DOI] [PubMed] [Google Scholar]
- 49.Zamora-Ros R., Andres-Lacueva C., Lamuela-Raventos R.M., Berenguer T., Jakszyn P., Barricarte A., Ardanaz E., Amiano P., Dorronsoro M., Larranaga N., Martinez C., Sanchez M.J., Navarro C., Chirlaque M.D., Tormo M.J., Quiros J.R., Gonzalez C.A. Estimation of dietary sources and flavonoid intake in a Spanish adult population (EPIC-Spain) J. Am. Diet Assoc. 2010;110:390–398. doi: 10.1016/j.jada.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 50.Kim K., Vance T.M., Chun O.K. Estimated intake and major food sources of flavonoids among US adults: changes between 1999-2002 and 2007-2010 in NHANES. Eur. J. Nutr. 2016;55:833–843. doi: 10.1007/s00394-015-0942-x. [DOI] [PubMed] [Google Scholar]
- 51.Wu X.L., Gu L.W., Prior R.L., McKay S. Characterization of anthocyanins and proanthocyanidins in some cultivars of Ribes, Aronia, and Sambucus and their antioxidant capacity. J. Agric. Food Chem. 2004;52:7846–7856. doi: 10.1021/jf0486850. [DOI] [PubMed] [Google Scholar]
- 52.Kaack K., Austed T. Interaction of vitamin C and flavonoids in elderberry (Sambucus nigra L.) during juice processing. Plant Foods Hum. Nutr. 1998;52:187–198. doi: 10.1023/a:1008069422202. [DOI] [PubMed] [Google Scholar]
- 53.Zheng W., Wang S.Y. Oxygen radical absorbing capacity of phenolics in blueberries, cranberries, chokeberries, and lingonberries. J. Agric. Food Chem. 2003;51:502–509. doi: 10.1021/jf020728u. [DOI] [PubMed] [Google Scholar]
- 54.Johnson M.H., de Mejia E.G. Comparison of chemical composition and antioxidant capacity of commercially available blueberry and blackberry wines in Illinois. J. Food Sci. 2012;77:C141–C148. doi: 10.1111/j.1750-3841.2011.02505.x. [DOI] [PubMed] [Google Scholar]
- 55.Wright O.R.L., Netzel G.A., Sakzewski A.R. A randomized, double-blind, placebo-controlled trial of the effect of dried purple carrot on body mass, lipids, blood pressure, body composition, and inflammatory markers in overweight and obese adults: the QUENCH trial. Can. J. Physiol. Pharmacol. 2013;91:480–488. doi: 10.1139/cjpp-2012-0349. [DOI] [PubMed] [Google Scholar]
- 56.Karlsen A., Retterstol L., Laake P., Paur I., Kjolsrud-Bohn S., Sandvik L., Blomhoff R. Anthocyanins inhibit nuclear factor-kappa B activation in monocytes and reduce plasma concentrations of pro-inflammatory mediators in healthy adults. J. Nutr. 2007;137:1951–1954. doi: 10.1093/jn/137.8.1951. [DOI] [PubMed] [Google Scholar]
- 57.Zhu Y., Ling W., Guo H., Song F., Ye Q., Zou T., Li D., Zhang Y., Li G., Xiao Y., Liu F., Li Z., Shi Z., Yang Y. Anti-inflammatory effect of purified dietary anthocyanin in adults with hypercholesterolemia: a randomized controlled trial. Nutr. Metab. Cardiovasc. 2013;23:843–849. doi: 10.1016/j.numecd.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 58.Matsuda M., Shimomura I. Increased oxidative stress in obesity: implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obes. Res. Clin. Pract. 2013;7:e330–e341. doi: 10.1016/j.orcp.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 59.Novembrino C., Accinni R., Della Noce C., Rosina M., Lonati S., Lorenzano E., Ciani A., Bamonti F. Oxidative stress in dyslipidemia. Clin. Chem. 2006;52 doi: 10.1016/j.numecd.2005.05.006. A119-A119. [DOI] [PubMed] [Google Scholar]
- 60.Mietus-Synder M., Engler M.M., Engler M.B., Chiu E.Y., Morrow J.D., Ridker P.M., Rifai N., Malloy M.J. Obesity augments vascular oxidative stress in children with dyslipidemia. Circulation. 2004;109 E141-E141. [Google Scholar]
- 61.Vona R., Gambardella L., Cittadini C., Straface E., Pietraforte D. Biomarkers of oxidative stress in metabolic syndrome and associated diseases. Oxid. Med. Cell Longev. 2019;2019 doi: 10.1155/2019/8267234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cervantes Gracia K., Llanas-Cornejo D., Husi H. CVD and oxidative stress. J. Clin. Med. 2017;6 doi: 10.3390/jcm6020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miyake S., Takahashi N., Sasaki M., Kobayashi S., Tsubota K., Ozawa Y. Vision preservation during retinal inflammation by anthocyanin-rich bilberry extract: cellular and molecular mechanism. Lab. Invest. 2012;92:102–109. doi: 10.1038/labinvest.2011.132. [DOI] [PubMed] [Google Scholar]
- 64.Hou D.X., Yanagita T., Uto T., Masuzaki S., Fujii M. Anthocyanidins inhibit cyclooxygenase-2 expression in LPS-evoked macrophages: structure-activity relationship and molecular mechanisms involved. Biochem. Pharmacol. 2005;70:417–425. doi: 10.1016/j.bcp.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 65.Davi G., Ciabattoni G., Consoli A., Mezzetti A., Falco A., Santarone S., Pennese E., Vitacolonna E., Bucciarelli T., Costantini F., Capani F., Patrono C. In vivo formation of 8-iso-prostaglandin F-2 alpha and platelet activation in diabetes mellitus - effects of improved metabolic control and vitamin E supplementation. Circulation. 1999;99:224–229. doi: 10.1161/01.cir.99.2.224. [DOI] [PubMed] [Google Scholar]
- 66.Zhu Y.N., Huang X.W., Zhang Y.H., Wang Y., Liu Y., Sun R.F., Xia M. Anthocyanin supplementation improves HDL-associated paraoxonase 1 activity and enhances cholesterol efflux capacity in subjects with hypercholesterolemia. J. Clin. Endocrinol. Metab. 2014;99:561–569. doi: 10.1210/jc.2013-2845. [DOI] [PubMed] [Google Scholar]
- 67.Soltani R., Hakimi M., Asgary S., Ghanadian S.M., Keshvari M., Sarrafzadegan N. Evaluation of the effects of vaccinium arctostaphylos L. Fruit extract on serum lipids and hs-CRP levels and oxidative stress in adult patients with hyperlipidemia: a randomized, double-blind, placebo-controlled clinical trial. Evid. Base Compl. Alternative Med. 2014;2014 doi: 10.1155/2014/217451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McGhie T.K., Walton M.C. The bioavailability and absorption of anthocyanins: towards a better understanding. Mol. Nutr. Food Res. 2007;51:702–713. doi: 10.1002/mnfr.200700092. [DOI] [PubMed] [Google Scholar]
- 69.Kim H., Simbo S.Y., Fang C., McAlister L., Roque A., Banerjee N., Talcott S.T., Zhao H., Kreider R.B. Acai (Euterpe oleracea Mart.) beverage consumption improves biomarkers for inflammation but not glucose- or lipid-metabolism in individuals with metabolic syndrome in a randomized, double-blinded, placebo-controlled clinical trial. Food Funct. 2018;9:3097–3103. doi: 10.1039/c8fo00595h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.