Abstract
This study was conducted to evaluate the influence of brassinosteroid (24-epibrassinolide, EBL) seed priming and optimal nitrogen (N) supply in improving salt tolerance in soybean. The experimental treatments were (a) control (nutrient solution without N and without EBL priming), (b) nutrient solution without N and EBL seed priming, (c) N supplemented nutrient solution without EBL priming and (d) EBL seed priming + N supplemented nutrient solution under optimal (0 mM NaCl) and salt stress (0 mM NaCl) conditions. Salt stress caused significant reduction in growth and biomass accumulation of soybean. However, EBL seed priming and application of N improved the soybean performance under optimal and salt stress conditions. In this regard, treatments receiving both EBL and N were more effective. EBL priming and N, alone and in combination, triggered the accumulation of osmolytes including proline, glycine betaine and sugars resulting in better photo-protection through maintenance of tissue water content. Antioxidant activity and osmolyte accumulation significantly increased due to combined treatment of N and EBL under normal as well as salt stress conditions. In conclusion, salt stress caused reduction in growth and biomass soybean due to oxidative damage and osmotic stresses. However, soybean performance was improved by seed priming with EBL. Supplementation of N further improved the effectiveness of EBL treatment in improving salt tolerance in soybean.
Keywords: Antioxidants, Secondary metabolites, Osmolytes, Oxidative damage, NaCl, Glycine max
Introduction
Salinity stress is one of the most prevalent abiotic stress factors restricting the growth and productivity of crop plants in arid and semi-arid regions of the world. Salt tolerance is a complex phenomenon integrating several morphological and developmental alterations controlled at physiological and biochemical levels (Munns 2002; Farooq et al. 2015). During the stress exposure and subsequent response by plants, vital plant processes like nutrient uptake and assimilation, ion compartmentalization, photosynthesis, and protein synthesis, etc., are affected (Munns 2002; Morton et al. 2018).
Salt stress affects plant growth in a bi-phasic manner causing osmotic stress followed by specific ion toxicity (Munns 2002; Hanin et al. 2016). This specific ion toxicity disturbs the ion homeostasis with increase in tissue Na+ and Cl− concentration and decrease in tissue K+ (Hanin et al. 2016; Ahanger and Agarwal 2017). However, re-establishing the ion homeostasis is very important to for survival under salt stress (Munns 2002).
Survival and growth of plants on saline soils can be depicted by the efficiency of adaptive mechanisms including selective ion transport and compartmentalization, increased synthesis and accumulation of compatible solutes, and induction of reactive oxygen species (ROS) scavenging mechanisms (Farooq et al. 2017). Among the compatible solutes, many are nitrogen-containing compounds like amino acids, amides, and betaines, therefore, adequate nitrogen supply can help in improving the salt tolerance (Farooq et al. 2015). Salt stress triggers the accumulation of osmolytes as a part of the main adaptive mechanisms to contribute stress tolerance by scavenging the free radicals and protecting enzymes (Farooq et al. 2015). Accumulation of excess Na+ in plants triggers the generation of toxic ROS. In this regard, the up regulation of ROS scavenging mechanism is required as a defense mechanism (Farooq et al. 2019a). ROS scavenging mechanism is constituted of both enzymatic and non-enzymatic components which work in coordination (Farooq et al. 2017).
Phytohormones play a vital role as systemic signaling molecules for the establishment of various developmental programs aimed in shaping the plant growth and development in parallel with plant adaptation to environmental stresses (Farooq et al. 2009; Divi et al. 2016). During the last decade, significant advances have been made in elucidating the regulatory role of brassinosteroids (BRs) in improving plant tolerance against abiotic stresses (Farooq et al. 2009; Anjum et al. 2011). It is interesting to note that almost every response mediated by molecules like BRs is controlled by its crosstalk between other phytohormones or any cellular molecules that integrate hormonal signaling pathways during stressful conditions (Li and Jin 2006). The interplay among different hormones or molecules is ubiquitous for proper stress response elicitation (Halliday 2004). The active involvement of BRs in promoting the tolerance of plants against different stresses like temperature (high and low), drought, salinity, and metals is correlated with the increased expression of marker genes in them (Divi et al. 2016). BRs upregulate the protein translation machinery during heat stress leading to increased accumulation of heat-shock proteins and rapid resumption of cellular protein synthesis in Brassica napus (Dhaubhadel et al. 2002).
Inadequate supply of nitrogen limits the plant growth and development whereas adequate supply, uptake, and assimilation affect the storage, mobilization and the growth regulatory mechanisms in plants (Cánovas et al. 2018). Nitrogen is absorbed from the soil solution as nitrate (NO3−) and ammonium (NH4+) using a variety of transporters including AMT (ammonium transporter) or NRT (nitrate transporter) protein family transporters. Within plants, NO3− is reduced to NH4+ by sequential activity of nitrate and nitrite reductase. Soil absorbed NH4+ as well as that formed from reduction reactions is assimilated into amino acid by glutamine synthetase and glutamate synthase or by glutamate dehydrogenase pathway (Miflin and Habash 2002). Nitrogen availability has, thus, great influence on the processes like photosynthesis, amino acid synthesis and hence the stress tolerance (Xu and Zhou 2005). The link between photosynthesis and the adequate supply of nitrogen containing metabolites to photosynthesizing organelles is met by the glutamine synthetase in them (Sibout and Guerrier 1998). Stress-mediated reduction in nitrogen metabolism has been reported to affect the photosynthetic capacity and the yield significantly (Xu and Zhou 2005). Appropriate availability of N in soil prevents water stress-induced growth inhibition in Sophora davidii through improved photosynthesis (Wu et al. 2008).
Although, the roles of seed priming BRs and N nutrition in improving stress tolerance in crop plants are well-known (Wu et al. 2008; Divi et al. 2016; Farooq et al. 2019b). To the best of our knowledge, the integrated effect of N and BRs on soybean under salt stress has never been reported. For this study, it was hypothesized that adequate nitrogen availability and BRs interact with each other to regulate salt tolerance in soybean. The study was conducted to evaluate the interaction of EBL pretreatment with nitrogen in mitigating the salinity stress-induced oxidative damages in soybean.
Methods
Experimental design and treatments
Healthy seeds of soybean (Glycine max L.) cultivar Giza 22, obtained from Agriculture Research Center, Giza, Egypt, were used in this study. The seeds were surface sterilized using 0.001% HgCl2 for 5 min and were then thoroughly rinsed with distilled water. Seeds were soaked in aerated distilled water (control) or 100 mM 24-epibrassinolide (EBL) for 10 h. Aeration was provided by a simple aquarium pump. Seeds were then surface dried under shade for 6 h and were sown in earthen pots (ten seeds per pot) containing reconstituted soil (7 kg) composed of peat, compost, and sand (4:1:1). At sowing, each pot received 250 mL full-strength nutrient solution (Hoagland and Arnon 1950). After uniformity of germination, three plants were maintained in each pot. The pots were divided into two groups; (i) receiving 3 mM 100 mL KNO3 solution or (ii) not receiving N. Ten days after sowing, salt stress was imposed in one set of pots in two increments each of 50 mM NaCl to reach the target level of 100 mM NaCl, while the second group didn’t receive the salinity treatment. Thus, the experimental treatments were (a) control (nutrient solution without N and without EBL priming), (b) nutrient solution without N and EBL seed priming, (c) N supplemented nutrient solution without EBL priming, (d) EBL seed priming + N supplemented nutrient solution. All these treatments were maintained with (100 mM NaCl) and without salt stress (0 mM NaCl). The experiment was conducted in completely randomized design with five replicates for each treatment. Thirty-five days after germination, plants were carefully uprooted and analyzed for osmolytes, secondary metabolites, oxidative stress markers, and antioxidants. During the entire experimental period, pots were kept in the greenhouse under natural climatic conditions.
Observations and measurements
Photosynthetic pigments
Extraction of chlorophyll and carotenoids was carried by macerating fresh leaf tissue using pestle and mortar in 80% acetone (Arnon 1949). Homogenate was centrifuged at 3000 × g for 20 min, and the volume of supernatant was made to 10 mL using acetone. Optical density was recorded at 480, 645 and 663 nm using UV–visible spectrophotometer (UV-1601PC; Shimadzu, Tokyo, Japan). Total chlorophyll and carotenoids were determined following Arnon (1949).
Gas exchange parameters
Portable photosynthetic system (CID-340, Photosynthesis System, Bio-Science, USA) was used for the measurement of net photosynthetic rate, intercellular CO2 concentration and stomatal conductance in a fully expanded leaf.
Relative leaf water contents
Relative leaf water contents (RWC) were determined following the method of Smart and Bingham (1974). An equal number of leaf discs were punched from each treatment, and their fresh weight (FW) was recorded. The same leaf discs were allowed to gain turgidity by floating them on distilled water in petri dishes for 1 h, and the turgid weight (TW) was recorded followed by drying of discs for 24 h at 80 °C to record dry weight (DW). The RWC was calculated using the following formula:
Soluble sugars, leaf free proline, and glycine betaine
Soluble sugar contents were measured by the anthrone method after extracting in 80% ethanol and measuring the absorbance at 585 nm (Shields and Burnett 1960). For estimation of leaf free proline, method of Bates et al. (1973) was employed and proline content was determined after reacting the extract with ninhydrin reagent. Glycine betaine was extracted in distilled water and concentration was determined by dissolving the periodide crystals in 1, 2-dichloroethane following Grieve and Grattan (1983).
Lipid peroxidation and hydrogen peroxide
Lipid peroxidation was estimated by determining the formation of malonaldehyde (MDA) in leaves as described by Heath and Packer (1968). Hydrogen peroxide was estimated by macerating 500 mg fresh sample in 0.1% TCA following the protocol of (Velikova et al. 2000).
Nitrate reductase
For assaying nitrate reductase (E.C. 1.6.6.1.) activity, fresh leaf tissue (500 mg) from each treatment was cut into pieces and kept in polythene vials containing 2.5 mL of phosphate buffer (pH 7.5) supplemented with 20 mM potassium nitrate and 5% isopropanol. Nitrate reductase activity was determined following Jaworski (1971).
Antioxidant enzymes
For extraction of antioxidant enzymes, fresh tissue (1 g) was homogenized in chilled pestle and mortar in liquid nitrogen. Superoxide dismutase (SOD, EC 1.15.1.1) activity was assayed, as the ability of the enzyme to inhibit the photochemical reduction of nitroblue tetrazolium chloride (NBT), following the method of Bayer and Fridovich (1987).
The activity of ascorbate peroxidase (APX, EC 1.11.1.11) was determined following the method of Nakano and Asada (1981). Glutathione reductase (GR; EC 1.6.4.2) activity was measured following Foyer and Halliwell (1976), and glutathione-dependent oxidation of NADPH. The activity of catalase (CAT, EC 1.11.1.6) was assayed following Luck (1974) Activities of antioxidant enzymes are expressed as EU mg−1 protein (Lowry et al. 1951).
Ascorbate, tocopherol and reduced glutathione
Ascorbate contents were determined from fresh plant material by homogenizing leaf tissue TCA (6%) following Mukherjee and Choudhuri (1983). Tocopherol contents were determined following the method of Backer et al. (1980) from the fresh leaf tissue extracted in ethanol and petroleum ether (5 mL, 1.6:2). For estimation of reduced glutathione (GSH), fresh leaf tissue (100 mg) was extracted in phosphate buffer (pH 8.0), and GSH contents were determined as described by Ellman (1959).
Phenols and flavonoids
Total phenol content in leaves was extracted in methanol and estimation was done by reacting aliquot with Folin–Ciocalteu reagent (Singleton and Rossi 1965). Flavonoid contents were determined in the leaf tissue extracted in methanol following Zhishen et al. (1999).
Nitrogen and sodium contents
Nitrogen was estimated following the method of Subbaiah and Asija (1956). Digested plant samples are distilled with KMNO4 (0.3%) and NaOH (2.5%), and ammonium liberated is collected in boric acid (2%) containing an indicator. The distillate was titrated with 0.02 N H2SO4. Sodium was estimated by digesting dried plant samples in acid using H2SO4 and HClO4. Na was estimated by digesting dried plant samples in acid using H2SO4 and HClO4. Digested samples were read on flame photometer (Jenway Flame Photometer, UK) using Na filter.
Statistical analysis
Data were analyzed statistically by analysis of variance (ANOVA) technique. Duncan’s New Multiple Range test was used, at P < 0.05, for mean separation.
Results
Plant growth and biomass production
Seed priming with EBL improved growth and biomass production over the control plants, and this beneficial impact of EBL was more pronounced in seedling supplemented with N. Relative to control, highest increase in height (32.93%) and biomass accumulation (37.49%) was observed in plants treated with EBL and N. Salt stress reduced plant height and biomass accumulation by 40.04 and 29.11% respectively over the control plants. However, seedlings raised after EBL priming and supplemented with N and exposed to salinity exhibited only 0.80 and 1.21% reduction in plant height and biomass accumulation over the control depicting the amelioration of NaCl mediated growth reduction (Table 1).
Table 1.
Influence of seed priming with 24-epibrassinolide (EBL) and nitrogen (N) application on shoot length, shoot dry weight, and leaf nitrogen and sodium contents in soybean under salt stress
| Treatments | Shoot length (cm) | Shoot dry weight (g/plant) | Nitrogen (mg/g DW) | Sodium (mg/g DW) | ||||
|---|---|---|---|---|---|---|---|---|
| 0 mM NaCl | 100 mM NaCl | 0 mM NaCl | 100 mM NaCl | 0 mM NaCl | 100 mM NaCl | 0 mM NaCl | 100 mM NaCl | |
| Control | 16.23 ± 0.115d | 9.73 ± 0.029g | 1.0338 ± 0.665d | 0.7322 ± 0.378g | 23.50 ± 2.23d | 14.50 ± 1.23f | 4.81 ± 0.556e | 13.14 ± 1.84a |
| EBL | 19.06 ± 0.061c | 11.26 ± 0.031ef | 1.279 ± 0.230bc | 0.8482 ± 0.404ef | 26.37 ± 2.26c | 18.03 ± 1.12e | 3.96 ± 0.152ef | 11.01 ± 1.04b |
| N | 20.80 ± 0.080b | 12.93 ± 0.112e | 1.389 ± 0.529b | 0.9225 ± 0.152e | 32.03 ± 2.43ab | 23.15 ± 1.66d | 3.23 ± 0.157f | 9.38 ± 0.517c |
| N + EBL | 24.20 ± 0.049a | 16.10 ± 0.400d | 1.654 ± 0.754a | 1.021 ± 0.087d | 34.39 ± 2.87a | 25.87 ± 2.47c | 2.94 ± 0.138g | 8.10 ± 0.253cd |
Values are mean of three replicates ± standard error of means
Mean sharing the same letter, for a parameter, don’t differ significantly at P ≤ 0.05
Tissue Na+ and N contents
Relative to control, EBL primed, and N supplemented seedlings showed higher N and reduced Na contents. Percent increase in N reached to a maximum of 31.66% in EBL + N treated seedlings, whereas Na+ decline by 38.87% over the control plants. Plants raised under salt stress, exhibited a decline of 38.29% in N content and 63.39% increase in Na+ accumulation over the control. Relative to NaCl stressed plants, EBL primed, or N supplemented seedlings showed significant amelioration of the negative impact of NaCl on N uptake. Percent amelioration of 19.57% in NaCl + EBL, 37.36% in NaCl + N and 43.95% in NaCl + N + EBL was observed in N content over the NaCl stressed ones. Seedlings raised after EBL priming and treated with N exhibited a decline of 38.35% in Na accumulation over the NaCl treated plants (Table 1).
Photosynthetic pigments and gas exchange
Application of N and EBL priming significantly enhanced the synthesis of chlorophylls and carotenoids alone as well as when applied together resulting in considerable increase in the photosynthetic parameters (Table 2). Relative to control, increase in total chlorophyll contents, carotenoids, photosynthetic rate, intercellular CO2 concentration, and stomatal conductance were highest in the plants treated with EBL + N and this effect was maintained under salinity as well. Percent increase in total chlorophyll, carotenoids, photosynthetic rate, intercellular CO2 concentration, and stomatal conductance was 20.54, 14.93, 37.25, 27.22 and 19.24%, respectively in EBL + N treated plants over the control. Salt stress reduced the total chlorophylls by 46.22%, carotenoids by 40.21%, photosynthetic rate by 42.11%, intercellular CO2 concentration by 26.55% and stomatal conductance by 22.13% over the control plants. Application of N or EBL pre-treatment reduced the NaCl mediated decline and maximal amelioration in total chlorophylls (42.14%), carotenoids (22.79%), photosynthetic rate (46.61%), intercellular CO2 concentration (31.59%) and stomatal conductance (20.60%) was observed in seedlings treated with N + EBL (Table 2).
Table 2.
Influence of seed priming with 24-epibrassinolide (EBL) and nitrogen (N) application on total chlorophyll contents, leaf carotenoids, net photosynthetic rate, intercellular CO2 and stomatal conductance in soybean under salt stress
| Treatments | Total leaf chlorophyll (mg/g FW) | Leaf carotenoids (mg/g FW) | Net photosynthetic rate (µmol m−2 S−1) | Intracellular CO2 (µmol mol−1) | Stomatal conductance (mmol m−2 S−1) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 mM NaCl | 100 mM NaCl | 0 mM NaCl | 100 mM NaCl | 0 mM NaCl | 100 mM NaCl | 0 mM NaCl | 100 mM NaCl | 0 mM NaCl | 100 mM NaCl | |
| Control | 1.721 ± 0.128cd | 0.9254 ± 0.026h | 0.4237 ± 0.022d | 0.2533 ± 0.025h | 15.53 ± 1.28d | 8.99 ± 0.90g | 217.3 ± 5.85e | 159.6 ± 4.50g | 326.6 ± 5.68d | 254.3 ± 4.04g |
| EBL | 1.874 ± 0.096c | 1.142 ± 0.051g | 0.4465 ± 0.013c | 0.2657 ± 0.015g | 16.63 ± 1.77c | 11.03 ± 0.97f | 248.6 ± 6.02c | 197.6 ± 4.16f | 356.3 ± 5.50c | 280.6 ± 5.03ef |
| N | 2.073 ± 0.072ab | 1.325 ± 0.052f | 0.4899 ± 0.010b | 0.2874 ± 0.012f | 19.63 ± 1.51b | 14.05 ± 0.94e | 280.0 ± 6.92ab | 210.3 ± 4.50e | 387.6 ± 7.09b | 298.6 ± 5.50e |
| N + EBL | 2.166 ± 0.116a | 1.600 ± 0.093e | 0.4981 ± 0.011a | 0.3281 ± 0.019e | 24.75 ± 1.61a | 16.84 ± 0.80c | 298.6 ± 7.02a | 233.3 ± 5.03d | 417.3 ± 6.02a | 320.3 ± 4.93d |
Values are mean of three replicates ± standard error of means
Mean sharing the same letter, for a parameter, don’t differ significantly at P ≤ 0.05
Hydrogen peroxide and lipid peroxidation
Supplementation of N and EBL priming resulted in the reduced generation of H2O2, and lipid peroxidation under salt stress (Fig. 1a, b). Generation of H2O2 increased by 39.80% under salt stress and was declined by 30.46% in EBL, 38.62% in N, and 45.95% in N + EBL treated plants, respectively over respective control. Reduced H2O2 generation in N and EBL treated plants resulted in decrease in lipid peroxidation. Under normal conditions, lipid peroxidation exhibited a decline of 12.11, 20.20 and 30.77% respectively in EBL, N, and N + EBL treated plants. However, under salt stress, N + EBL treated plants exhibited a decline of 18.60% in H2O2 reflecting 11.37% reduction in lipid peroxidation than the respective control. Seed priming with EBL, N supplementation and combination of both proved effective in reducing the H2O2 generation and lipid peroxidation under normal as well as saline conditions, however, combined application was more effective (Fig. 1a, b).
Fig. 1.

Influence of seed priming with 24-epibrassinolide (EBL) and nitrogen (N) application on a hydrogen peroxide contents and b lipid peroxidation in soybean under salt stress. Bars are mean of three replicates ± standard error of means. Bars sharing the same letter, for a parameter, don’t differ significantly at P ≤ 0.05
Antioxidant system
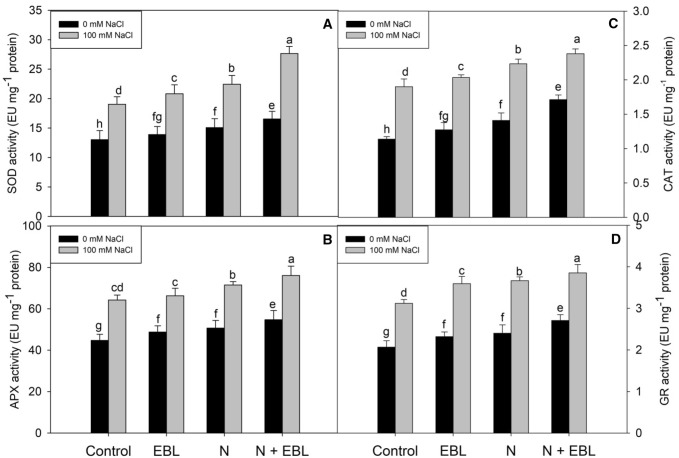
Supplementation of N and EBL priming increased the antioxidant activity by up regulating the enzymatic components and increasing the content of non-enzymatic constituents. EBL priming and N supplementation individually increased the activities of SOD, CAT, APX, and GR, and the contents of AsA, GSH, and tocopherol over the control, however, when supplied in combination, it proved much beneficial (Figs. 2, 3). Relative to control, N + EBL treated plants exhibited an increase of 21.28% in SOD, 18.20% in CAT, 33.64% in APX and 23.98% in GR. Such impact was maintained under NaCl stress also with a significant increase in SOD (31.15%), CAT (15.78%), APX (20.16%) and GR (11.16%) activities over NaCl stressed plants (Fig. 2a–d). The maximal increase in AsA, GSH and tocopherol contents was observed in N + EBL treated plants with observed increase of 17.18% in AsA, 13.04% in GSH and 42.28% in tocopherols over the control plants (Fig. 3). Under salt stress, the maximal increase in GSH (32.74%) and tocopherol (49.11%) was observed in seedlings treated with N + EBL over the control. Under salt stress, AsA (9.97%) reduced while GSH (26.24%) and tocopherol (17.39%) increased over the control. Supplementation of N + EBL ameliorated the decline in AsA by 9.05% over the NaCl stressed plants (Fig. 3).
Fig. 2.
Influence of seed priming with 24-epibrassinolide (EBL) and nitrogen (N) application on activities of a superoxide dismutase, b catalase, c ascorbate peroxidase and d glutathione reductase in soybean under salt stress. Bars are mean of three replicates ± standard error of means. Bars sharing the same letter, for a parameter, don’t differ significantly at P ≤ 0.05
Fig. 3.
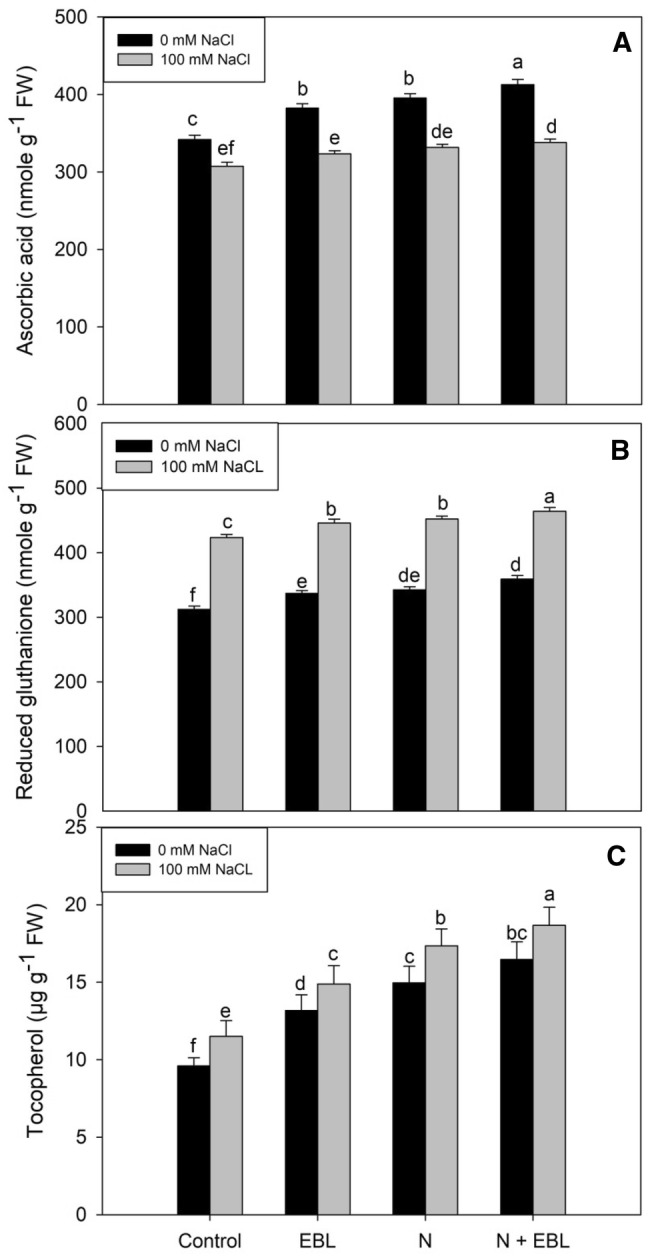
Influence of seed priming with 24-epibrassinolide (EBL) and nitrogen (N) application on a ascorbic acid, b reduced glutathione and c tocopherol in soybean under salt stress. Bars are mean of three replicates ± standard error of means. Bars sharing the same letter, for a parameter, don’t differ significantly at P ≤ 0.05
Relative water contents, soluble sugars, proline, and glycine betaine
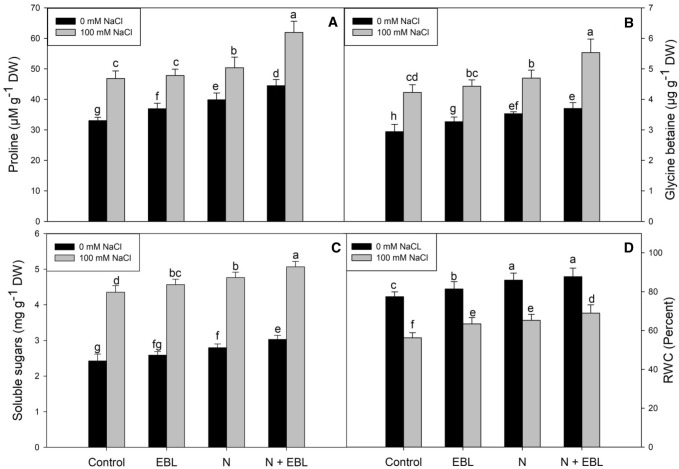
Priming with EBL and supplementation of N resulted in increased accumulation of proline, glycine betaine and soluble sugar content over the control plants. Relative to control, the maximal accumulation of proline, glycine betaine and soluble sugar was observed in NaCl + N + EBL treated plants. Relative to control, proline, glycine betaine and soluble sugar content increased by 25.71, 21.62 and 20.13% respectively in N + EBL treated plants. However, a maximal increase of 46.66% for proline, 47.46% for glycine betaine and 52.17% for soluble sugar were observed in NaCl + N + EBL treated plants over the control plants. It was obvious that NaCl stress triggered the accumulation of proline, glycine betaine and soluble sugar but was not as much as N or EBL did (Fig. 4a–c). RWC was reduced by 27.37% due to salinity over the control seedlings. It was observed that both N and EBL affectively increased RWC and also mitigated the decline caused by NaCl. Maximal (11.81%) increase in RWC was observed in N + EBL treated plants over control, and maximal (18.44%) amelioration was observed in seedlings treated with NaCl + N + EBL over the NaCl stressed plants (Fig. 4d).
Fig. 4.
Influence of seed priming with 24-epibrassinolide (EBL) and nitrogen (N) application on a proline, b glycine betaine, c soluble sugars and d relative leaf water contents (RWC) in soybean under salt stress. Bars are mean of three replicates ± standard error of means. Bars sharing the same letter, for a parameter, don’t differ significantly at P ≤ 0.05
Nitrate reductase activity
The activity of nitrate reductase was reduced by salinity stress by 39.87% over the control. However, nitrate reductase was significantly increased by EBL seed priming (9.77%), N application (16.19%), combination of both (20.87%). Under salt stress, EBL seed priming, N supplementation and combination of both caused 5.31, 16.19 and 24.32% increase respectively in nitrate reductase activity over control plants (Fig. 5).
Fig. 5.
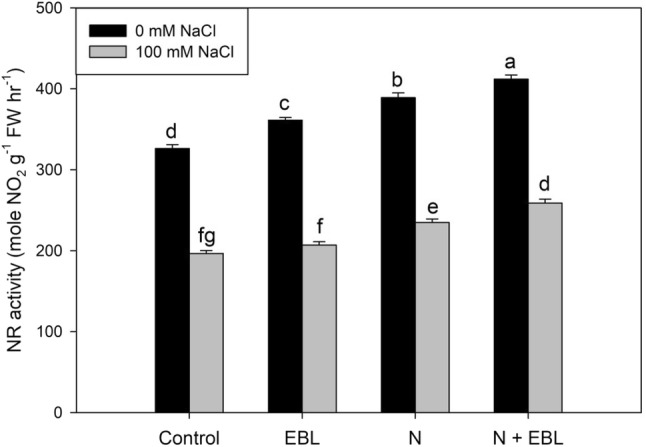
Influence of seed priming with 24-epibrassinolide (EBL) and nitrogen (N) application on nitrate reductase activity in soybean under salt stress. Bars are mean of three replicates ± standard error of means. Bars sharing the same letter, for a parameter, don’t differ significantly at P ≤ 0.05
Phenols and flavonoids
Phenols and flavonoids increased due to N and EBL treatment individually and in combination attaining a maximal enhancement of 41.96 and 50.58% in N + EBL treated plants over the control. NaCl treatment imparted a slight decline in phenols (4.81%) and flavonoids (0.92%) as compared to control plants. Application N or EBL or N + EBL to NaCl treated plants mitigated the decline in phenols and flavonoids completely and in NaCl + N + EBL treated plants, phenols and flavonoids increased by 41.12% and 49.31% over the NaCl stressed plants (Fig. 6).
Fig. 6.

Influence of seed priming with 24-epibrassinolide (EBL) and nitrogen (N) application on a total phenols and b flavonoids in soybean under salt stress. Bars are mean of three replicates ± standard error of means. Bars sharing the same letter, for a parameter, don’t differ significantly at P ≤ 0.05
Discussion
Salt stress caused significant reduction in the soybean performance. However, adequate nitrogen availability and EBL seed priming interacted to regulate salt tolerance and improved the biomass production in soybean. The study hypothesis was, therefore, accepted.
Salinity suppresses the plant growth in two phases; in the phase 1, it causes osmotic affect restricting the uptake and availability of water, whereas in phase 2, salinity causes toxicity of specific ions (Munns 2002; Farooq et al. 2017). Salt stress alters cell cycle functioning by declining the cyclin-dependent kinase and CYCB1;2 promoters resulting in the production of smaller meristems (West et al. 2004). Salinity-induced osmotic stress and toxicity of specific ions cause oxidative stress resulting in over-production of ROS and increase in peroxidation of lipids (Elkelish et al. 2019; Farooq et al. 2019a). Plants exposed to salinity have higher lipoxygenase activity leading to significant reduction in the fatty acid composition which causes functional instability of membranes (Zhang et al. 2013).
Interaction of Na+ with other cations causes significant reduction in the uptake of K and other cations including Ca2+ and Mg2+ (Farooq et al. 2015) as has been noted in this study. In addition of Ca2+ and Mg2+, salt stress also suppresses uptake and translocation of nitrogen to other plant parts (Farooq et al. 2015). Increase in salinity level causes gradual decrease in the nitrogen uptake (Gadalla et al. 2007) and its metabolism (Campbell 1999) as has also been noted in this study. Salinity-induced deficiency of nitrogen causes significant reduction in carbon metabolism (Farooq et al. 2015) primarily due to degradation of pigment proteins through up-regulation of degrading enzymes like chlorophyllase (Li et al. 2015) and reduction in the activity of enzymes involved in chlorophyll synthesis (Pattanayak and Tripathy 2011).
Nitrogen application helped cope its deficiency and improve the soybean performance under optimal and stress conditions. Tolerance to salinity is directly dependent on the adequate nitrogen supply as most of the compatible solutes, including amino acids, amides, and betaines, are nitrogen-containing compounds (Farooq et al. 2015). Adequate nitrogen uptake regulates the photosynthetic system by maintaining the its supply to Rubisco protein and the synthesis of amino acids, acting as precursors for other molecules like chlorophyll (Ahanger and Agarwal 2017). With adequate nitrogen supply, plants up-regulate antioxidant system to avert the salinity-mediated oxidative damages (Iqbal et al. 2015).
Seed priming with EBL potentially reduces the ROS accumulation by up regulating the antioxidant system (Farooq et al. 2009). However, reports discussing interaction of N and EBL are not available. Increased activity of AsA-GSH cycle in plants raised from EBL primed seeds prevented the generation of toxic hydroxyl and the damaging effects of H2O2 by preventing its diffusion into other cellular organelles. Optimal functioning of AsA-GSH pathway protects the photosynthesis by maintaining the redox homeostasis and the structural integrity of chloroplasts (Foyer and Shigeoka 2011).
Adequate nitrogen supply and EBL seed priming mediated greater membrane stability was reflected in improved NR activity and maintenance of CO2 concentrations which may be probably ascribed to their impact at translational or transcriptional levels. Treatment of EBL to nitrogen supplied or nitrogen deficient plants may facilitate NO3− and CO2 uptake for the greater synthesis of NR (Mai et al. 1989) and the photosynthetic enzymes like Rubisco respectively.
Reduction in ROS generation vis-a-vis maintenance of redox homeostasis in nitrogen and EBL treated plants prevented the salinity mediated photosynthetic inhibition. Seed priming with EBL has been reported to improve the photosynthetic functioning by protecting the structural integrity of chloroplast molecules mainly by reducing the generation of toxic radicals like superoxide and H2O2 (Yuan et al. 2012).
Increase in solute accumulation in cytosol brings down tissue water potential, and osmotic and ionic effects of salinity are reduced. Osmolyte accumulation results from the differential regulation of their biosynthetis and degradation (Tabasssum et al. 2017). Proline, sugars and glycine betaine help protect the photosynthetic functioning, mediate cellular stress signalling and assist in ROS scavenging (Farooq et al. 2017, 2019a). In this study, increased accumulation of osmolytes due to nitrogen supply and/or EBL priming prevented the oxidative damage, improved redox homeostasis, and lead to maintenance of tissue water contents. Soluble sugars have a central role in plant structure and metabolism at the cellular and whole-plant levels and can act in nutrient and metabolite signalling by activating either specific or hormone crosstalk transduction pathways reflecting in the modification of the gene expression and the proteome (Couee et al. 2006). Sugar-mediated changes in gene expression under stressful conditions have been linked with the production rate of ROS and the antioxidant-functioning (Matros et al. 2015). Nitrogen supply and EBL-mediated sugar accumulation confirms the probable crosstalk between sugars-phytohormones-nutrients in the regulation of antioxidant pathways for better photosynthetic functioning. The increment in the accumulation of osmolytes influences the yield potential of crop plants by optimizing the photosynthesis. Mineral nutrients have the potentiality to integrate the cellular stress signalling for better response elicitation (Rouached and Tran 2015).
Increase in the synthesis of phenols and flavonoids in plants, raised from EBL primed seeds and supplemented with adequate nitrogen, helped in better salinity alleviation through quick removal of ROS and/or by maintaining the tissue osmotica. Accumulation of secondary metabolites can benefit plants in several ways for better amelioration of deleterious effects of stress (Pourcel et al. 2006). For instance, secondary metabolites prevent stress-triggered decline in chlorophyll and help in maintenance of nitric oxide homeostasis for better stress amelioration (Klein et al. 2015). Therefore, plants exhibiting greater accumulation of secondary metabolites show quick stress acclimation (Farooq et al. 2017).
Conclusion
Salt stress caused a significant reduction in growth and biomass production of soybean due to oxidative damage and osmotic stress. However, the soybean performance was significantly improved by seed priming with EBL. In this regard, supplementation of N further improved the effectiveness of EBL treatment in improving salt tolerance in soybean.
Author’s contribution
MS, AE and MF planned the experiment, MS and TS conducted the experiment, HA conducted statistical analysis. MS, AE, TS and HA prepared the manuscript draft. MF finalized the manuscript.
Compliance with ethical standards
Conflict of interest
Authors declare that there is no conflict of interest regarding the publication of this paper.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Amr Elkelish, Email: amr.elkelish@science.suez.edu.eg.
Muhammad Farooq, Email: farooqcp@gmail.com.
References
- Ahanger MA, Agarwal RM. Salinity stress induced alterations in antioxidant metabolism and nitrogen assimilation in wheat (Triticum aestivum L) as influenced by potassium supplementation. Plant Physiol Biochem. 2017;115:449–460. doi: 10.1016/j.plaphy.2017.04.017. [DOI] [PubMed] [Google Scholar]
- Anjum SA, Wang LC, Farooq M, Hussain M, Xue LL, Zou CM. Brassinolide application improves the drought tolerance in maize through modulation of enzymatic antioxidants and leaf gas exchange. J Agron Crop Sci. 2011;197:177–185. doi: 10.1111/j.1439-037X.2010.00459.x. [DOI] [Google Scholar]
- Arnon DI. Copper enzymes in isolated chloroplast polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backer H, Frank O, de Angells B, Feingold S. Plasma tocopherol in man at various times after ingesting free or ocetylaned tocopherol. Nutr Rep Int. 1980;21:531–536. [Google Scholar]
- Bates LS, Waldre RP, Teare ID. Rapid determination of free proline for water stress studies. Plant Sci. 1973;39:205–207. [Google Scholar]
- Bayer WF, Fridovich JL. Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem. 1987;161:559–566. doi: 10.1016/0003-2697(87)90489-1. [DOI] [PubMed] [Google Scholar]
- Campbell HW. Nitrate reductase structure, function and regulation bridging the gap between biochemistry and physiology. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:277–303. doi: 10.1146/annurev.arplant.50.1.277. [DOI] [PubMed] [Google Scholar]
- Cánovas FM, Cañas RA, de la Torre FN, Pascual MB, Castro-Rodríguez V, Avila C. Nitrogen metabolism and biomass production in forest trees. Front Plant Sci. 2018;9:1449. doi: 10.3389/fpls.2018.01449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couee I, Sulmon C, Gouesbet G, El Amrani A. Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants. J Exp Bot. 2006;57:449–459. doi: 10.1093/jxb/erj027. [DOI] [PubMed] [Google Scholar]
- Dhaubhadel S, Browning KS, Gallie DR, Krishn P. Brassinosteroid functions to protect the translational machinery and heat-shock protein synthesis following thermal stress. Plant J. 2002;29:681–691. doi: 10.1046/j.1365-313X.2002.01257.x. [DOI] [PubMed] [Google Scholar]
- Divi UK, Rahman T, Krishna P. Gene expression and functional analyses in brassinosteroid-mediated stress tolerance. Plant Biotechnol J. 2016;14:419–432. doi: 10.1111/pbi.12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkelish EE, Soliman MH, Alhaithloul HA, El-Esawi MA. Selenium protects wheat seedlings against salt stress-mediated oxidative damage by up-regulating antioxidants and osmolytes metabolism. Plant Physiol Biochem. 2019;137:144–153. doi: 10.1016/j.plaphy.2019.02.004. [DOI] [PubMed] [Google Scholar]
- Ellman GL. Tissue sulphydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Farooq M, Wahid A, Basra SMA, Din IU. Improving water relations and gas exchange with brassinosteroids in rice under drought stress. J Agron Crop Sci. 2009;195:262–269. doi: 10.1111/j.1439-037X.2009.00368.x. [DOI] [Google Scholar]
- Farooq M, Hussain M, Wakeel A, Siddique KHM. Salt stress in maize: effects, resistance mechanisms and management. A review. Agron Sustain Dev. 2015;35:461–481. doi: 10.1007/s13593-015-0287-0. [DOI] [Google Scholar]
- Farooq M, Gogoi N, Hussain M, Barthakur S, Paul S, Bharadwaj N, Migdadi HM, Alghamdi SS, Siddique KHM. Effects, tolerance mechanisms and management of salt stress in grain legumes. Plant Physiol Biochem. 2017;118:199–217. doi: 10.1016/j.plaphy.2017.06.020. [DOI] [PubMed] [Google Scholar]
- Farooq M, Usman M, Nadeem F, Rehman H, Wahid A, Basra SMA, Siddique KHM. Seed priming in field crops—potential benefits, adoption and challenges. Crop Pasture Sci. 2019;70:731–771. doi: 10.1071/CP18604. [DOI] [Google Scholar]
- Farooq MA, Niazi AK, Akhtar J, Saifullah Farooq M, Souri Z, Karimi N, Rengel Z. Acquiring control: the evolution of ROS-induced oxidative stress and redox signaling pathways in plant stress responses. Plant Physiol Biochem. 2019;141:353–369. doi: 10.1016/j.plaphy.2019.04.039. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Halliwell B. The presence of glutathione and glutathione reductase in chloroplast: a proposed role in ascorbic acid metabolism. Planta. 1976;133:21–25. doi: 10.1007/BF00386001. [DOI] [PubMed] [Google Scholar]
- Foyer C, Shigeoka S. Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol. 2011;155:93–100. doi: 10.1104/pp.110.166181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadalla AM, Hamdy A, Galal YGM, Aziz HAA, Mohamed MAA. Evaluation of maize growth under salinity stress and N application strategies using stable nitrogen isotope. Proc Afr Crop Sci Conf. 2007;8:1553–1562. [Google Scholar]
- Grieve CM, Grattan SR. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil. 1983;70:303–307. [Google Scholar]
- Halliday KJ. Plant hormones: the interplay of brassinosteroids and auxin dispatch. Curr Biol. 2004;14:1008–1010. doi: 10.1016/j.cub.2004.11.025. [DOI] [PubMed] [Google Scholar]
- Hanin M, Ebel C, Ngom M, Laplaze L, Masmoudi K. New insights on plant salt tolerance mechanisms and their potential use for breeding. Front Plant Sci. 2016;7:1787. doi: 10.3389/fpls.2016.01787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath RL, Packer L. Photoperoxidation in isolated chlorplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Hoagland DR, Arnon DI (1950) The water culture method for growing plants without soil. California Agricultural Experimental Station Circular No. 47, pp 1–32. University of California, Berkeley, CA
- Iqbal N, Umar S, Khan NA. Nitrogen availability regulates proline and ethylene production and alleviates salinity stress in mustard (Brassica juncea) J Plant Physiol. 2015;178:84–91. doi: 10.1016/j.jplph.2015.02.006. [DOI] [PubMed] [Google Scholar]
- Jaworski EG. Nitrate reductase assay in intact plant tissue. Biochem Biophys Res Commun. 1971;43:1274–1279. doi: 10.1016/S0006-291X(71)80010-4. [DOI] [PubMed] [Google Scholar]
- Klein A, Keyster M, Ludidi N. Response of soybean nodules to exogenously applied caffeic acid during NaCl-induced salinity. S Afr J Bot. 2015;96:13–18. doi: 10.1016/j.sajb.2014.10.016. [DOI] [Google Scholar]
- Li J, Jin H. Regulation of brassinosteroid signaling. Trends Plant Sci. 2006;12:37–41. doi: 10.1016/j.tplants.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Li J, Hu L, Zhang L, Pan X, Hu X. Exogenous spermidine is enhancing tomato tolerance to salinity-alkalinity stress by regulating chloroplast antioxidant system and chlorophyll metabolism. BMC Plant Biol. 2015;15:303. doi: 10.1186/s12870-015-0699-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NS, Farrand AL, Randall RJ. Protein measurement with Folin phenol reagent. J Biol Chem. 1951;193:263–275. [PubMed] [Google Scholar]
- Luck H. Catalases. In: Bregmeyer HU, editor. Methods of enzymatic analysis. New York: Academic Press; 1974. [Google Scholar]
- Mai Y, Lin S, Zeng X, Ran R. Effect of brassinolide on nitrate reductase activity in rice seedlings. Plant Physiol Commun. 1989;2:50–52. [Google Scholar]
- Matros A, Peshev D, Peukert M, Mock HP, den Ende WV. Sugars as hydroxyl radical scavengers: proof-of-concept by studying the fate of sucralose in Arabidopsis. Plant J. 2015;82:822–839. doi: 10.1111/tpj.12853. [DOI] [PubMed] [Google Scholar]
- Miflin BJ, Habash DZ. The role of glutamine synthetase and glutamate dehydrogenase in nitrogen assimilation and possibilities for improvement in the nitrogen utilization of crops. J Exp Bot. 2002;53:979–987. doi: 10.1093/jexbot/53.370.979. [DOI] [PubMed] [Google Scholar]
- Morton MJL, Awlia M, Al-Tamimi N, Saade S, Pailles Y, Negrão S, Tester M. Salt stress under the scalpel—dissecting the genetics of salt tolerance. Plant J. 2018;97:148–163. doi: 10.1111/tpj.14189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee SP, Choudhuri MA. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol Plant. 1983;58:166–170. doi: 10.1111/j.1399-3054.1983.tb04162.x. [DOI] [Google Scholar]
- Munns R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002;25:239–250. doi: 10.1046/j.0016-8025.2001.00808.x. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- Pattanayak GK, Tripathy BC. Overexpression of protochlorophyllide oxidoreductase C regulates oxidative stress in Arabidopsis. PLoS ONE. 2011;6:e26532. doi: 10.1371/journal.pone.0026532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourcel L, Routaboul JM, Cheynier V, Lepiniec L, Debeaujon I. Flavonoid oxidation in plants: from biochemical properties to physiological functions. Trends Plant Sci. 2006;12:29–36. doi: 10.1016/j.tplants.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Rouached H, Tran LSP. Regulation of plant mineral nutrition: transport, sensing and signaling. Int J Mol Sci. 2015;16:29717–29719. doi: 10.3390/ijms161226198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields R, Burnett W. Determination of protein-bound carbohydrate in serum by a modified anthrone method. Anal Chem. 1960;32:885–886. doi: 10.1021/ac60163a053. [DOI] [Google Scholar]
- Sibout R, Guerrier G. Solute incompatibility with glutamine synthetase in water-stressed Populus nigra. Environ Exp Bot. 1998;40:173–178. doi: 10.1016/S0098-8472(98)00032-X. [DOI] [Google Scholar]
- Singleton VL, Rossi JA., Jr Colorimetry of total phenolics with phosphor–molybdic–phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–153. [Google Scholar]
- Smart RE, Bingham GE. Rapid estimates of relative water content. Plant Physiol. 1974;53:258–260. doi: 10.1104/pp.53.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbaiah BV, Asija GL. A rapid procedure for the estimation of available nitrogen in soil. Curr Sci. 1956;25:259. [Google Scholar]
- Tabasssum T, Farooq M, Ahmad R, Wahid A. Seed priming and transgenerational drought memory improves tolerance against salt stress in bread wheat. Plant Physiol Biochem. 2017;18:362–369. doi: 10.1016/j.plaphy.2017.07.007. [DOI] [PubMed] [Google Scholar]
- Velikova V, Yordanov I, Edreva A. Oxidative stress and some antioxidant systems in acid rain treated bean plants, protective role of exogenous polyamines. Plant Sci. 2000;151:59–66. [Google Scholar]
- West G, Inzé D, Beemster GTS. Cell cycle modulation in the response of the primary root of Arabidopsis to salt stress. Plant Physiol. 2004;135:1050–1058. doi: 10.1104/pp.104.040022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu FZ, Bao WK, Li FL, Wu N. Effects of water stress and nitrogen supply on leaf gas exchange and fluorescence parameters of Sophora davidii seedlings. Photosynthetica. 2008;46:40–48. doi: 10.1007/s11099-008-0008-x. [DOI] [Google Scholar]
- Xu ZZ, Zhou GS. Effects of water stress on photosynthesis and nitrogen metabolism in vegetative and reproductive shoots of Leymus chinensis. Photosynthetica. 2005;43:29–35. [Google Scholar]
- Yuan L, Shu S, Sun J, Guo S, Tezuka T. Effects of 24-epibrassinolide on the photosynthetic characteristics, antioxidant system, and chloroplast ultrastructure in Cucumis sativus L. under Ca(NO3)2 stress. Photosynth Res. 2012;112:205–214. doi: 10.1007/s11120-012-9774-1. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhang G, Wang Y, Zhou Z, Meng Y, Chen B. Effect of soil salinity on physiological characteristics of functional leaves of cotton plants. J Plant Res. 2013;126:293–304. doi: 10.1007/s10265-012-0533-3. [DOI] [PubMed] [Google Scholar]
- Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–559. doi: 10.1016/S0308-8146(98)00102-2. [DOI] [Google Scholar]