Abstract
NaCl and PEG stresses have negative impacts on seed germination and early seedling establishment in Oryza sativa. The present study was designed to ascertain the influence of different priming techniques (Hydro priming-HyP, Halo priming-HP, UV-B priming-UP) in enhancing oxidative and anti-oxidative mechanisms during seed germination phase in response to NaCl and PEG stresses tolerance of three rice varieties (Neeraja, Vaisakh and Vyttila 6). NaCl and PEG stresses caused delayed germination rate, enhanced reactive oxygen species content and thereby increased lipid peroxidation rate. Different priming techniques significantly hastened the metabolites/non enzymatic antioxidant contents (total sugars, total phenolics, free amino acids, proline, ascorbate and glutathione) as well as activities of antioxidant enzymes (superoxide dismutase, catalase, ascorbate peroxidase and guaiacol peroxidase), and thus reduced oxidative stress damages caused by NaCl and PEG stresses in rice seedlings. Seed priming techniques imparted abiotic stress tolerance not only to sensitive varieties but also additional tolerance potential to tolerant varieties. All three priming techniques protects the plants from toxicity caused by NaCl and PEG stresses but halo priming had proved to be more successful.
Electronic supplementary material
The online version of this article (10.1007/s12298-019-00750-9) contains supplementary material, which is available to authorized users.
Keywords: Seed priming, NaCl stress, PEG stress, Hydro priming, Halo priming, UV-B priming
Introduction
Global food security faces major threats due to increasing population and climatic changes. More than half of the world’s population depends on rice as their staple food which is considered as the second-largest cereal crop in the world (Mondal et al. 2011). Wide array of abiotic stresses cause reduced productivity and increased crop losses, thereby severely effecting biochemical and physiological mechanisms in plants (Petrov et al. 2015). The effect of stresses on plants would be directly related to the intensity of stress stimuli and the growth stage of plants at which they are exposed to stress. The seed germination stage is the first and foremost susceptible stage of plant growth and therefore more prone to stress. Any unfavorable changes during seed germination influence the further growth and development of plants (Ibrahim 2016). Among the various abiotic stresses, drought and salinity are the most prevalent ones that considerably reduce the plant productivity and quality of crops. Salinity and drought interrupt the absorption of water by plants leading to membrane destabilization, nutrient imbalance, protein denaturation and inhibition of photosynthetic and biosynthetic processes (Coskum et al. 2016).
Water plays a crucial role in seed germination, stimulating hydrolytic breakdown and solubilization of food reserves, osmotic adjustment and enzymatic reactions which leads to the successful seedling establishment (Biju et al. 2017). Drought is one of the major limitations that considerably reduces plant growth and crop yield (Ullah et al. 2018). Water deficiency results in growth retardation, reduction in photosynthetic efficiency and imbalance of ion homeostasis (Rangani et al. 2018). During salinity stress, the presence of various ions in water and uptake of the same quickly affects the cellular osmoticum of plants (Ibrahim 2016).
Although reactive Oxygen Species (ROS) are known to play a vital role in various events of seed life, they are intensified with different abiotic stress stimuli which can bring about adverse physiological and biochemical changes in germinating seeds. In orthodox seeds, ROS are produced from embryogenesis to germination, i.e. in metabolically active cells. The ROS in seeds is involved in the regulation of cellular growth, providing protection against pathogens and controlling the cell redox status signifying its importance for seed germination and seedling growth. However, uncontrolled accumulation of ROS ultimately leads to oxidative damage of biomolecules leading to necrosis and cell death (El-Maarouf-Bouteau and Bailly 2008).
The technique of seed priming has proved to be an effective method to impart stress tolerance in plants and it is a very promising strategy for modern crop management (Wojtyla et al. 2016). Seed priming can increase the rate, percentage, and uniformity of seed germination or seedling emergence, mainly under unfavorable environmental conditions. Priming activates ROS scavenging machinery (SOD, CAT, and APX) which facilitate morphological, physiological and biochemical changes leading to enhanced stress tolerance potential of plants (Bussotti et al. 2014). The advantageous outcome of seed priming with different priming agents have already been established in many crop plants, such as lentil (Biju et al. 2017), wheat (Farooq et al. 2013), mustard (Srivastava et al. 2010), tomato (Iseri et al. 2014) and sugarcane (Patade et al. 2012) under salinity and drought. Even though priming-induced salinity and drought tolerance have been shown in earlier reports, the basic mechanisms including physiological and biochemical aspects of priming induced beneficial effects specifically during seed germination phase under stress are not well understood. Moreover, the differential efficiency of various priming agents, such as NaCl, a low dose of UV-B radiation and deionized water in enhancing the tolerance during the seed germination phase towards abiotic stresses has not been comparatively studied. The present study focuses on the analysis of comparative potential of water (hydro-priming), NaCl (halo-priming), and UV-B (radiation-priming) in enhancing the antioxidative responses during the germination of Oryza sativa varieties (with varied tolerance potential towards drought and salinity) when exposed to different concentrations of polyethylene glycol (PEG) and NaCl.
Materials and methods
Plant material and growth condition
The seeds of three commonly cultivated varieties of Oryza sativa L. [Neeraja (stress-sensitive), Vaisakh (drought tolerant) and Vyttila 6 (NaCl tolerant)] were collected from RARS Pattambi and Vyttila, Kerala, India. Fifty healthy and randomly selected seeds were surface sterilized with 0.1% HgCl2 solution for 5 min and further seeds were washed thoroughly with distilled water. Further, these seeds were subjected to different priming treatments as detailed in 2.2. Seeds subjected to different priming treatments and non-primed seeds were placed in separate culture bottles (11 × 22 cm) containing absorbent cotton soaked with double distilled water (control), NaCl (75 mM-Neeraja and Vaisakh, 100 mM-Vyttila 6) and polyethylene glycol-6000 (15%-Neeraja and 20%-Vaisakh and Vyttila 6) and incubated in a plant growth chamber under controlled conditions of temperature (24 ± 2 °C), light intensity (300 μmol m−2 s−1) and relative humidity (55 ± 5%) with a 14/10 h photoperiod. Seeds were considered to be germinated when the radicle extended for at least 2 mm. The growth and biochemical attributes of seedlings raised from primed and non-primed seeds were recorded on 3 d of germination.
Seed priming techniques
The surface-sterilized seeds were treated with a low dose of UV-B (4 kJ m−2) for UV-B priming (UP). For hydro priming (HyP), rice seeds were immersed in deionized water for 12 h. Rice seeds were immersed in 50 mM NaCl (Neeraja and Vaisakh), 75 mM NaCl (Vyttila 6) for 12 h for halo priming (HP). After the priming treatments, seeds were rinsed thrice with distilled water and then dried to their original moisture content at room temperature as determined by changes in seed weight.
Germination percentage
Germination percentage (GP) was measured according to the method of Yan (2016).
Estimation of reactive oxygen species and MDA content
Hydrogen peroxide content was estimated according to the protocol of Junglee et al. (2014) using hydrogen peroxide as standard. Superoxide content was determined as per the protocol of Doke (1983) using sodium nitrate (NaNO2) as standard. The malondialdehyde (MDA) content was determined according to Heath and Packer (1968). The MDA content was calculated using its molar extinction coefficient of 155 mM−1 cm−1.
Determination of electrolyte leakage and membrane stability index (MSI)
Electrolyte leakage was estimated by the method of Agarie et al. (1995) and membrane stability was estimated as per the method of Sairam et al. (1997).
Assay of various metabolites/non-enzymatic antioxidants
Proline content was estimated based on the procedure of Bates et al. (1973) and l-proline was used as the standard. Total soluble sugar content was extracted using 80% ethanol and determined according to Dubois et al. (1951) and d-glucose was used as the standard. Total phenolic content was measured based on the method of Folin and Denis (1915) and catechol was used as standard. Free amino acids were determined according to Moore and Stein (1948) and leucine was used as standard. Ascorbate and glutathione content was measured as per the protocol of Chen and Wang (2002), l-ascorbic acid and reduced glutathione were used as respective standards.
Assay of enzymatic antioxidants
The enzyme extracts were prepared by following the protocol of Yin et al. (2009). Protein concentrations in the enzyme extract were determined by the method of Bradford (1986), using defatted bovine serum albumin (fraction V) as the standard. The activity of catalase (CAT, EC 1.11.1.6) was determined based on a decrease in absorbance at 240 nm for 1 min following the decomposition of H2O2 (Kar and Mishra 1976). One unit of the enzyme activity was defined as μmoles H2O2 decomposed per minute per mg protein. The activity of superoxide dismutase (SOD, EC 1.15.1.1) was assayed by measuring its ability to inhibit the photochemical reduction of nitro blue tetrazolium (NBT), according to the method of Giannopolitis and Ries (1977). One unit of SOD activity was defined as the enzyme required for 50% inhibition of the photochemical reduction of NBT. Guaiacol peroxidase (GPX, EC 1.11.1.7) activity was measured by following the change of absorption at 420 nm due to guaiacol oxidation (Gaspar et al. 1975). One unit of the enzyme was defined as micromoles of guaiacol oxidized per minute per mg protein. Ascorbate peroxidase (APX, EC 1.11.1.11) activity was assayed as described by Nakano and Asada (1981). One unit of the enzyme was defined as micromoles of ascorbate oxidized per minute per mg protein.
Cumulative stress response index (CSRI)
The cumulative stress response index (CSRI) was calculated as the sum of relative individual component responses at each treatment. CSRI was calculated to evaluate the physiological responses of Neeraja, Vaisakh and Vyttila 6 subjected to different seed priming treatments (HP, HyP and UP) and further exposed to NaCl and PEG stresses in comparison to control treatment. The CSRI was calculated according to Dai et al. (2006).
Statistical analysis
Split plot design was used for the data analysis and it was carried out using SPSS software. Statistical analysis of physiological and biochemical parameters was carried out according to Tukey’s studentized range (HSD) test at 5% probability level. One-way ANOVA, pearson’s correlation and calculations were done using SPSS software (Version 16.0, SPSS Inc., Chicago, USA) to evaluate the significant difference in the traits among rice varieties. The data is an average observation from three independent experiments, each with three replications and 50 seeds per replicate.
Results
In rice varieties, Neeraja, Vaisakh and Vyttila 6, the concentrations of NaCl and PEG which caused a ~ 50% reduction in total chlorophyll content and increase in carotenoid content was determined. Stress sensitive variety Neeraja showed 50% growth retardation at 75 mM NaCl or 15% PEG; whereas, 50% growth retardation was observed at 75 mM NaCl or 20% PEG for Vaisakh (known drought tolerant) and 100 mM NaCl or 20% PEG for Vyttila 6 (known NaCl tolerant) (supplementary Table 1 and 2).
Selection of optimal concentration/dosage of priming agent for imparting effective priming was fixed from various concentrations of NaCl (0, 25, 50, 75 and 100 mM) and dosages of UV-B (0, 2, 4, 6 and 8 kJ m−2). A gradual increase of total chlorophyll content occurs up to a particular concentration/dose followed by rapid reduction due to stress effect. The concentration/dose at which maximum total chlorophyll: carotenoid ratio got maintained was fixed as priming concentration/dosage. The Halo priming concentration that was fixed for Neeraja and Vaisakh was 50 mM NaCl for 12 h whereas in the case of Vyttila, it was 75 mM for 12 h and for all the varieties distilled water was used for 12 h to impart hydro priming. For UV- B priming, 4 kJ m−2 was fixed as an effective priming dose for all the three rice varieties studied (supplementary Table 3 and 4).
The germination percentage of Neeraja, Vaisakh and Vyttila 6 seeds grown in distilled water, different concentrations of NaCl and PEG is given in Table 1. Different priming treatments improved the germination percentage of all the rice varieties subjected to different concentrations of NaCl, PEG, and unstressed conditions. Among different priming techniques, it was found that HyP had high positive impacts on germination.
Table 1.
Germination percentage of primed (UP-UV-B priming, HP-halo priming and HyP-hydro priming) rice varieties exposed to NaCl and PEG stresses
| Germination percentage | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Seed priming | Neeraja | Vaisakh | Vyttila 6 | ||||||
| UP | HP | HyP | UP | HP | HyP | UP | HP | HyP | |
| C | 80 ± 0.25c | 80 ± 0.25c | 80 ± 0.25f | 82 ± 0.04f | 82 ± 0.04d | 82 ± 0.04f | 80 ± 0.02f | 80 ± 0.02f | 80 ± 0.02f |
| CP | 84 ± 0.05bc | 86 ± 0.24d | 96 ± 0.04e | 86 ± 0.13e | 86 ± 0.29c | 98 ± 0.16e | 86 ± 0.06e | 88 ± 0.01e | 98 ± 0.29e |
| N | 70 ± 0.14b | 70 ± 0.14b | 70 ± 0.14d | 74 ± 0.20d | 74 ± 0.20b | 74 ± 0.20d | 78 ± 0.07d | 78 ± 0.07d | 78 ± 0.07d |
| NP | 78 ± 0.26ab | 80 ± 0.11c | 92 ± 0.20c | 80 ± 0.19c | 82 ± 0.15d | 92 ± 0.14c | 88 ± 0.16c | 98 ± 0.05c | 94 ± 0.18c |
| P | 56 ± 0.15a | 56 ± 0.15a | 56 ± 0.15b | 66 ± 0.17b | 66 ± 0.17a | 66 ± 0.17b | 64 ± 0.09b | 64 ± 0.09b | 64 ± 0.09b |
| PP | 70 ± 0.05b | 72 ± 0.09bc | 82 ± 0.19a | 84 ± 0.19a | 82 ± 0.28d | 90 ± 0.22a | 82 ± 0.27a | 94 ± 0.01a | 92 ± 0.16a |
C Control, CP primed seeds subjected to unstressed condition, N non-primed seeds subjected to NaCl stress, NP primed seeds subjected to NaCl stress, P non-primed seeds subjected to PEG stress, PP primed seeds subjected to PEG stress
ROS formation and MDA content
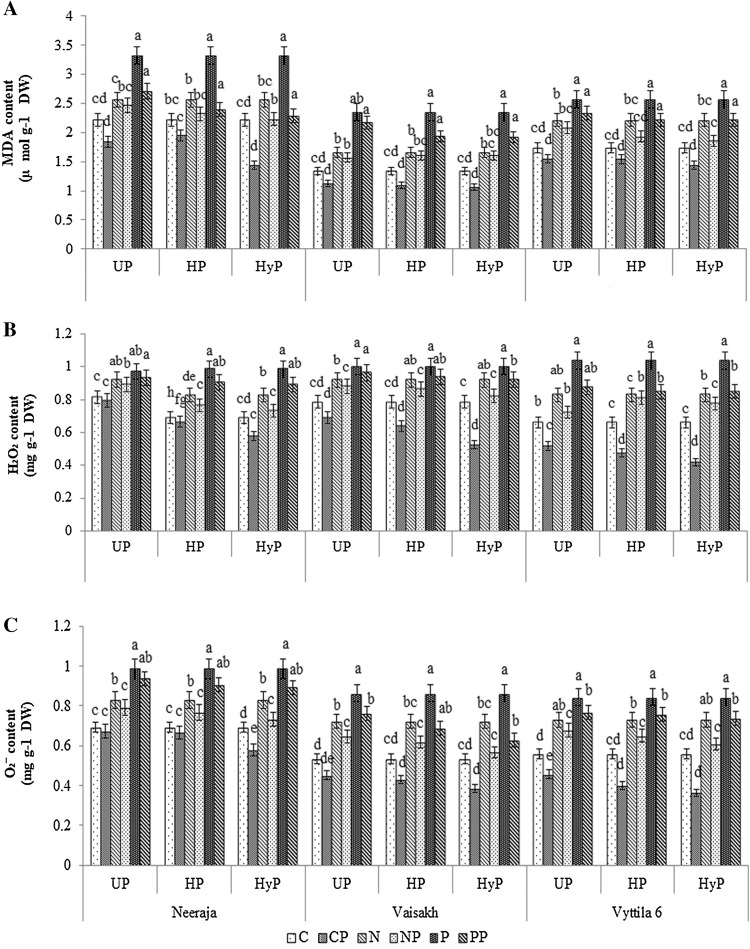
Upon exposure to different concentrations of NaCl and PEG, H2O2 and O2− content was increased in all the treatments and a higher rate of accumulation occurred in all the three rice varieties. However, the seedlings emerging from the primed (HyP, UP and HP) seeds recorded a reduction in the rate of ROS production. HyP treatment had a more positive impact on the reduction rate of ROS than UP and HP as compared to stressed seedlings that emerged from non-primed seeds (Fig. 1a–c). All of the three priming treatments could reduce the O2− content in three different varieties of rice seedlings under NaCl (NP) and PEG (PP) stresses and the extent of reduction was 14–43% as compared to the non-primed and unstressed seedlings.
Fig. 1.
MDA (a), hydrogen peroxide (b) and superoxide (c) content of primed (UP- UV-B priming, HP- halo priming, HyP- hydro priming) rice varieties exposed to NaCl and PEG stresses. C (Control), CP (primed seeds subjected to unstressed condition), N (non-primed seeds subjected to NaCl stress), NP (primed seeds subjected to NaCl stress), P (non-primed seeds subjected to PEG stress) and PP (primed seeds subjected to PEG stress)
MDA content was found to be highest in non-primed seedlings exposed to NaCl and PEG, this increase was reduced in seedlings emerging from seeds subjected to HyP, HP, and UP. UP and HP caused a maximum reduction in MDA content in the variety Vaisakh followed by the other two varieties (Vyttila 6 and Neeraja). The reduction in MDA content under NaCl (NP) and PEG (PP) stresses in the variety Vaisakh was 19 and 43% in HyP, 19 and 44% in HP and 16 and 62% in UP respectively (Fig. 1a).
Electrolyte leakage and membrane stability index
Upon exposure to NaCl and PEG, the electrolyte leakage increased in all the varieties, however, the increase was significantly reduced in seedlings that emerged from seeds subjected to HyP when compared with UP and HP (Fig. 2a). Membrane stability in the seeds of Neeraja, Vaisakh and Vyttila 6 significantly decreased under NaCl and PEG stresses; however, HyP significantly enhanced membrane stability even under the influence of these stresses (Fig. 2b). HyP treatment significantly increased the membrane stability under NaCl (NP) and PEG (PP) stresses in Vaisakh by 71 and 65% respectively, followed by Vyttila 6 and Neeraja. HP and UP also increased the membrane stability in all the varieties but to a lesser extent than that recorded in the variety Vaisakh subjected to HyP.
Fig. 2.
Electrolyte leakage (a) and membrane stability index (MSI) (b) of primed (UP- UV-B priming, HP- halo priming, HyP- hydro priming) rice varieties exposed to NaCl and PEG stresses. C (Control), CP (primed seeds subjected to unstressed condition), N (non-primed seeds subjected to NaCl stress), NP (primed seeds subjected to NaCl stress), P (non-primed seeds subjected to PEG stress) and PP (primed seeds subjected to PEG stress)
Metabolites/non-enzymatic antioxidants
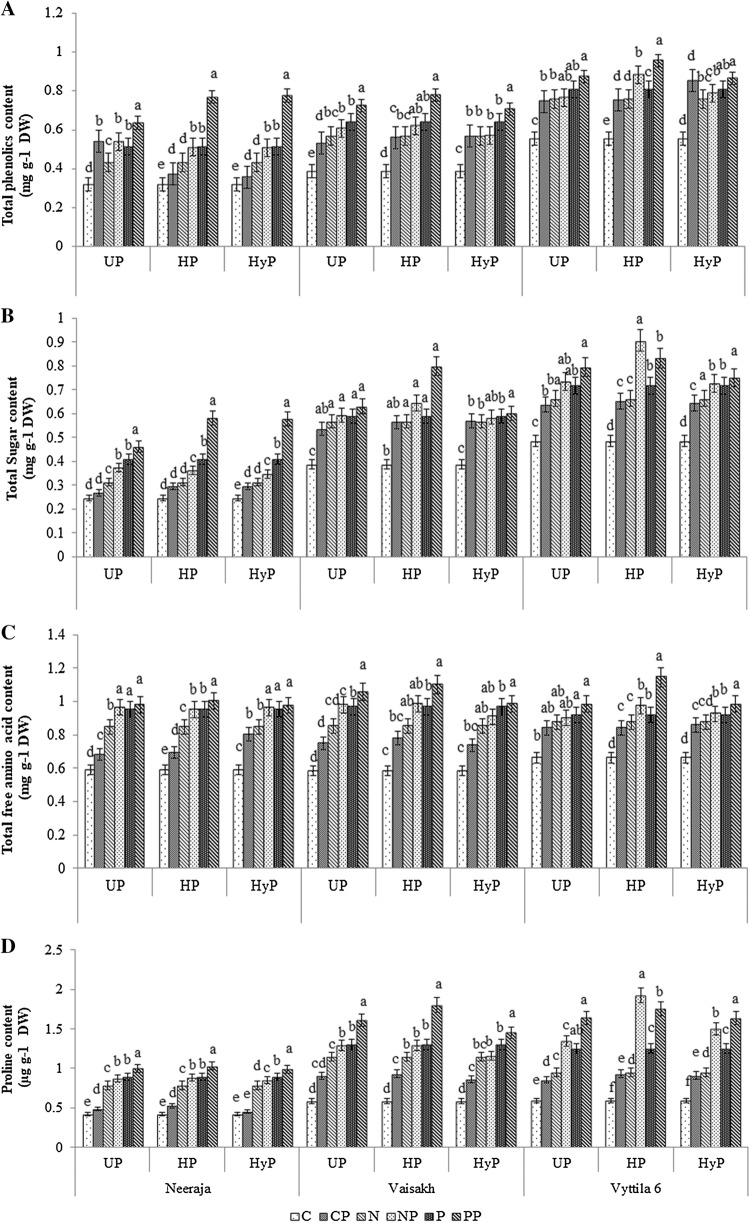
The three different priming treatments also enhanced the total phenolic content in all three rice varieties studied under NaCl and PEG stresses and it was particularly very high in HP primed seedlings. Moreover, HP recorded the highest increase in phenolic content in Vaisakh than the other two varieties subjected to NaCl (NP) and PEG (PP) and the increase was 61 and 101% respectively (Fig. 3a).
Fig. 3.
Total sugar (a), total phenolics (b) total free amino acid (c) and proline (d) content of primed (UP- UV-B priming, HP- halo priming, HyP- hydro priming) rice varieties exposed to NaCl and PEG stresses. C (Control), CP (primed seeds subjected to unstressed condition), N (non-primed seeds subjected to NaCl stress), NP (primed seeds subjected to NaCl stress), P (non-primed seeds subjected to PEG stress) and PP (primed seeds subjected to PEG stress)
Total sugars content showed an increased pattern of accumulation in all the three varieties subjected to HyP, HP and UP as compared with non-primed seeds in response to NaCl and PEG stresses (Fig. 3b). Under NaCl (NP) and PEG (PP) stresses, total sugar content was increased in all primed set, with a maximum increase of 67 and 106% in halo primed Vaisakh as compared with respective control and this increase was even higher when compared to seedlings raised from seeds subjected to other priming techniques (Fig. 3b).
The content of total free amino acids were also found to be enhanced in all rice seedlings upon stress exposure. The amino acid content in seedlings of Neeraja, Vaisakh and Vyttila 6 as a response to all the three priming techniques increased on exposure to NaCl and PEG stresses as compared with respective controls. Seedlings that emerged from halo primed seeds of Vaisakh showed a significant increase of 69 and 89% when compared to control and it was the highest recorded as compared to other priming treatments (Fig. 3c).
A significant accumulation of proline was also observed in rice seedlings in response to NaCl and PEG stresses, while a negligible accumulation of the same occurred in controls. The proline accumulation was significantly increased in the seedlings by different means of priming in all the three varieties subjected to NaCl (NP) and PEG (PP) stresses. Maximum proline accumulation was observed in response to HP and UP, followed by HyP.
In response to HP, proline content increased to the extent of 120 and 208% (Vaisakh) and 225 and 196% (Vyttila 6) when exposed to NaCl (NP) and PEG (PP) stresses respectively. Interestingly, upon HP treatment, elevated proline content was observed in drought-tolerant variety as compared with sensitive variety exposed to PEG than NaCl. Meanwhile, HP treatment significantly increased the proline in NaCl tolerant variety when exposed to NaCl followed by PEG (Fig. 3d).
Antioxidant enzymes
Non-enzymatic antioxidants
A considerable increase in the content of non-enzymatic antioxidants, glutathione (GSH) and ascorbate was found in seedlings emerged from seeds subjected to HP followed by UP and HyP. On exposure of seedlings emerged from HP primed seeds to NaCl (NP) and PEG (PP) stresses, ascorbate content increased in Vaisakh to the extent of 74 and 144% relative to the control. Whereas the glutathione content in Vaisakh was found to be increased by 256 and 267% as compared with control in response to HP and it was much higher than that recorded in Vyttila 6 and Neeraja (Fig. 4a, b).
Fig. 4.
Ascorbate (a) and glutathione (b) content of primed (UP- UV-B priming, HP- halo priming, HyP- hydro priming) rice varieties exposed to NaCl and PEG stresses. C (Control), CP (primed seeds subjected to unstressed condition), N (non-primed seeds subjected to NaCl stress), NP (primed seeds subjected to NaCl stress), P (non-primed seeds subjected to PEG stress) and PP (primed seeds subjected to PEG stress)
Enzymatic antioxidants
Priming significantly enhanced superoxide dismutase (SOD), catalase (CAT), APX (ascorbate peroxidase) and GPOX (guaiacol peroxidase) activities in primed as compared to non-primed seeds of different rice varieties studied. In seedlings subjected to NaCl and PEG stresses, there was an increase in the activity of SOD, however a prominent increase was observed in seedlings of Neeraja, Vaisakh and Vyttila 6 in response to HP under NaCl and PEG stresses (Fig. 5a). Halo priming induced a higher increase in the SOD activities in Vyttila 6 (340 and 325%) followed by vaisakh (305 and 376%) in response to NaCl (NP) and PEG (PP) stresses respectively. Like SOD, CAT activity was also found to increase in response to NaCl and PEG stresses; and different priming methods increased the CAT activity in all varieties under stress. The CAT activity was hastened to the extent of 293 and 337% in Vaisakh subjected to HP and it was higher than that recorded in Vyttila 6 and Neeraja on exposure to NaCl (NP) and PEG (PP) stresses, when compared with the seedlings emerged from non-primed seeds (Fig. 5b).
Fig. 5.
SOD (a), CAT (b), APX (c) and GPX (d) activities of primed (UP- UV-B priming, HP- halo priming, HyP- hydro priming) rice varieties exposed to NaCl and PEG stresses. C (Control), CP (primed seeds subjected to unstressed condition), N (non-primed seeds subjected to NaCl stress), NP (primed seeds subjected to NaCl stress), P (non-primed seeds subjected to PEG stress) and PP (primed seeds subjected to PEG stress)
Moderate level of APX and GPOX activities were detected in all seedlings of Neeraja, Vaisakh and Vyttila 6 emerged from primed seeds on exposure to NaCl and PEG stresses, when compared with their respective untreated ones. The activities of APX and GPOX were triggered with more UV-B and halo priming. It was found that APX activity increased by 271 and 234% in Vyttila 6 as a result of halo priming as compared to control and it was higher than other two priming methods. GPOX activity was higher in response to UV-B priming in Vaisakh and there was 202 and 203% increase in NaCl (NP) and PEG (PP) stresses than Vyttila 6 and Neeraja when compared to the stressed seedlings emerged from non-primed seeds (Fig. 5c, d).
Cumulative stress response index (CSRI) and correlation studies
From the Table 2, it is clear that the CSRI was higher for seedlings of Neeraja, Vaisakh and Vyttila 6 subjected to PEG stress than NaCl stress. Among different priming treatments, it was found that HP recorded high CSRI for all the rice varieties studied.
Table 2.
Cumulative stress response index (CSRI) of primed (UP-UV-B priming, HP-halo priming and HyP-hydro priming) rice varieties exposed to NaCl and PEG stresses
| Cumulative stress response index (CSRI) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Rice varieties | NaCl | PEG | UP | HP | HyP | |||
| NaCl | PEG | NaCl | PEG | NaCl | PEG | |||
| Neeraja | 1926.2 ± 0.03h | 2550.5 ± 0.19b | 2963.8 ± 0.03f | 3162.8 ± 0.04e | 3438.9 ± 0.02d | 3319.9 ± 0.15c | 2531.9 ± 0.21g | 3060.4 ± 0.03a |
| Vaisakh | 2848.1 ± 0.02h | 3418.6 ± 0.17g | 4245.4 ± 0.02f | 4919.8 ± 0.03e | 4406.3 ± 0.03d | 5190.2 ± 0.08c | 3855.8 ± 0.02b | 4241.4 ± 0.25a |
| Vyttila 6 | 3627.7 ± 0.06g | 4023.3 ± 0.01f | 4430.3 ± 0.07e | 4922.3 ± 0.02b | 5169.8 ± 0.02c | 5085.3 ± 0.01d | 4328.3 ± 0.21e | 4457.2 ± 0.11a |
C Control, CP primed seeds subjected to unstressed condition, N non-primed seeds subjected to NaCl stress, NP primed seeds subjected to NaCl stress, P non-primed seeds subjected to PEG stress, PP primed seeds subjected to PEG stress
Under both NaCl and PEG stresses, superoxide content and hydrogen peroxide had positive correlation with MDA content while MDA content negatively correlated with membrane stability index. Membrane stability index had negative correlation with electrolyte leakage whereas the electrolyte leakage was negatively correlated with sugar, total phenolics, proline and amino acids contents in all the three rice varieties subjected to NaCl and PEG stresses. Hydrogen peroxide content was positively correlated with ascorbate, glutathione, CAT, GPX and APX activities. Whereas the superoxide content had positive correlation with SOD alone (Tables 3 and 4).
Table 3.
Pearson’s correlation coefficients among various parameters from three rice varieties exposed to control, NaCl and PEG stresses
| O2−×MDA | H2O2 × MDA | H2O2 × ASA | H2O2 × GSH | O2−×SOD | H2O2 × CAT | H2O2 × GPX | H2O2 × APX | |
|---|---|---|---|---|---|---|---|---|
| Control | ||||||||
| Neeraja | ||||||||
| C | 0.915b | 0.975b | 0.742 | 0.780 | 0.652 | 0.792a | 0.632 | 0.872a |
| N | 0.947b | 0.972b | 0.791a | 0.731 | 0.854a | 0.972b | 0.973b | 0.885a |
| P | 0.986b | 0.344 | 0.864a | 0.973b | 0.853a | 0.551 | 0.944a | 0.753 |
| Vaisakh | ||||||||
| C | 0.772 | 0.782 | 0.922b | 0.872a | 0.772a | 0.815a | 0.962b | 0.965b |
| N | 0.713 | 0.884a | 0.956b | 0.954a | 0.596a | 0.982b | 0.861a | 0.752 |
| P | 0.874a | 0.863a | 0.633 | 0.778a | 0.834a | 0.997b | 0.750 | 0.841a |
| Vyttila 6 | ||||||||
| C | 0.782 | 0.755 | 0.809a | 0.874a | 0.962b | 0.927a | 0.964b | 0.833a |
| N | 0.973b | 0.882a | 0.932b | 0.865a | 0.745 | 0.982b | 0.795a | 0.872a |
| P | 0.824a | 0.824a | 0.993b | 0.991b | 0.922a | 0.891a | 0.921a | 0.981b |
C Control, N non-primed seeds subjected to NaCl stress, P non-primed seeds subjected to PEG stress
aCorrelation is significant at the 0.05 level (2-tailed)
bCorrelation is significant at the 0.01 level (2-tailed)
Table 4.
Pearson’s correlation coefficients among various parameters from primed seeds of three rice varieties grown in control, NaCl and PEG stresses
| O2−×MDA | H2O2 × MDA | H2O2 × ASA | H2O2 × GSH | O2−×SOD | H2O2 × CAT | H2O2 × GPX | H2O2 × APX | |
|---|---|---|---|---|---|---|---|---|
| Neeraja | ||||||||
| UP | ||||||||
| CP | 0.862a | 0.975b | 0.815a | 0.867a | 0.776 | 0.862a | 0.917a | 0.934a |
| NP | 0.722 | 0.951b | 0.921a | 0.988b | 0.815 | 0.896a | 0.485a | 0.765 |
| PP | 0.756 | 0.650 | 0.722 | 0.865b | 0.984b | 0.553 | 0.836a | 0.890a |
| HP | ||||||||
| CP | 0.915a | 0.668 | 0.876a | 0.924a | 0.722 | 0.791a | 0.765 | 0.811a |
| NP | 0.944b | 0.845a | 0.873a | 0.885a | 0.844a | 0.994b | 0.872a | 0.995b |
| PP | 0.362 | 0.676 | 0.812 | 0.964b | 0.865a | 0.988 | 0.921b | 0.991b |
| HyP | ||||||||
| CP | 0.832a | 0.445 | 0.810 | 0.968b | 0.775 | 0.743 | 0.592 | 0.867a |
| NP | 0.945b | 0.676 | 0.752 | 0.855a | 0.532 | 0.922a | 0.896a | 0.618 |
| PP | 0.786 | 0.942b | 0.934b | 0.826a | 0.670 | 0.754 | 0.734b | 0.852a |
| Vaisakh | ||||||||
| UP | ||||||||
| CP | 0.873a | 0.895a | 0.921a | 0.906a | 0.992b | 0.914b | 0.972b | 0.972b |
| NP | 0.765 | 0.824a | 0.784 | 0.985b | 0.891a | 0.988b | 0.950b | 0.873a |
| PP | 0.854a | 0.972b | 0.865a | 0.574 | 0.832a | 0.991b | 0.941a | 0.894a |
| HP | ||||||||
| CP | 0.822a | 0.835a | 0.927a | 0.988b | 0.723 | 0.987b | 0.835a | 0.935a |
| NP | 0.813 | 0.985b | 0.938a | 0.997b | 0.991a | 0.845a | 0.991b | 0.845a |
| PP | 0.921a | 0.694 | 0.789 | 0.975b | 0.842a | 0.832a | 0.972b | 0.867a |
| HyP | ||||||||
| CP | 0.868a | 0.942a | 0.986b | 0.822a | 0.772 | 0.683 | 0.832a | 0.86a |
| NP | 0.889a | 0.841 | 0.715 | 0.987b | 0.592 | 0.954b | 0.811a | 0.95b |
| PP | 0.728 | 0.862a | 0.916a | 0.981b | 0.834a | 0.782 | 0.921a | 0.911a |
| Vyttila 6 | ||||||||
| UP | ||||||||
| CP | 0.913a | 0.832 | 0.815a | 0.866a | 0.775 | 0.868a | 0.872a | 0.860a |
| NP | 0.871a | 0.951b | 0.756 | 0.989b | 0.816 | 0.898a | 0.976b | 0.612 |
| PP | 0.865a | 0.652 | 0.938b | 0.867a | 0.986b | 0.942b | 0.594 | 0.852a |
| HP | ||||||||
| CP | 0.875a | 0.542 | 0.791 | 0.811 | 0.924a | 0.733 | 0.941b | 0.931a |
| NP | 0.846 | 0.874a | 0.840 | 0.868a | 0.881 | 0.972b | 0.875a | 0.842 |
| PP | 0.882a | 0.985b | 0.857a | 0.996b | 0.983b | 0.915a | 0.898a | 0.874a |
| HyP | ||||||||
| CP | 0.881a | 0.813 | 0.812 | 0.747 | 0.634 | 0.837 | 0.915a | 0.772 |
| NP | 0.792 | 0.775 | 0.835 | 0.895a | 0.798 | 0.877a | 0.495 | 0.843a |
| PP | 0.872a | 0.881a | 0.914a | 0.869a | 0.898a | 0.864a | 0.832 | 0.882a |
UP UV-B priming, HP halo priming, HyP hydro priming CP primed seeds subjected to unstressed condition, N non-primed seeds subjected to NaCl stress, NP primed seeds subjected to NaCl stress, P non-primed seeds subjected to PEG stress, PP primed seeds subjected to PEG stress
aCorrelation is significant at the 0.05 level (2-tailed)
bCorrelation is significant at the 0.01 level (2-tailed)
Discussion
Seed germination and seedling establishment are the most crucial and sensitive phases of plant life. Metabolically active cells of seeds continuously produce ROS during germination which plays a vital role in important processes such as dormancy alleviation and seed germination. Phase II of germination involves activation of a regulatory system controlled by intrinsic and extrinsic factors leading to the increased production of ROS and its accumulation (Bailly et al. 2008). During germination, although hydration results in the resumption of mitochondrial activity, there are occasions when electron-transport chain (ETC) transfers electrons directly to O2 and reduced O2 serve as an unavoidable primary source of mitochondrial ROS (Rhoads et al. 2006; Noctor et al. 2007). The successful seed germination depends on the rate of ROS accumulation and the activities of the ROS neutralizing system (Pedrosa and Souza 2013). Thus, a stringently regulated accumulation of ROS is considered essential for germination. In this study, it was found that the HP, HyP, and UP alleviated the PEG and NaCl stresses effects in the seed germination stage itself which indicates that the three different priming treatments could effectively regulate the ROS content in germinating seeds. Therefore, the low level of ROS produced as a result of priming could effectively involve in the germinative process, instead of being a destructive agent.
NaCl and PEG stresses delay and reduces the germination and seedling establishment rate as a result of reducing water potential in seed/seedling tissues thereby significantly affecting plant growth. Reduced absorption of water during the imbibition phase of germination results in poor metabolic activities and unsuccessful seedling establishment. The present study shows that UV-B, hydro and halo priming techniques alleviated the effects of NaCl and PEG stresses through the activation of various biochemical processes and thereby increased seed germination percentage of Neeraja, Vaisakh and Vyttila 6 was achieved. Our results are in agreement with Neelamegam and Sutha (2015), wherein they reported that priming with UV-C enhanced the germination rate and seedling growth parameters of groundnut seeds. Similarly, Patade et al. (2012) reported that halo priming can activate seedling establishment by reducing oxidative damages through activation of antioxidant machinery. The present study reports that HyP treatments recorded higher germination percentage and seedling growth than UP and HP in Neeraja, Vaisakh and Vyttila 6 in unstressed conditions as well as upon exposure to NaCl and PEG stresses. This could be due to the controlled hydration process occurring in hydro primed seeds resulting in the increased water uptake potential and thereby exhibiting the smooth functioning of cellular activities.
ROS such as O2− and H2O2 production during seed imbibition are intensified with the stress factors NaCl and PEG in Neeraja, Vaisakh and Vyttila 6; nevertheless, HyP, UP and HP alleviated this effect by accelerated antioxidant machinery and osmo-solute synthesis. The effect of stress factors on the rice varieties was different due to the variations in tolerance potential of each variety. Although different seed priming techniques increased the stress tolerance potential of the sensitive variety Neeraja when exposed to NaCl and PEG, a higher accumulation rate of O2− and H2O2 was observed in this variety as compared with other two varieties. Increased accumulation of O2− and H2O2 resulted in the altering of membrane integrity that results in the increased electrolyte leakage and MDA content. The accumulated ROS as a result of stress is positively correlated with the MDA content, which causes enhanced membrane fluidity that finally results in the loss of membrane stability and increased electrolyte leakage. Increased electrolyte leakage is an indicator of oxidative damage of membrane lipids and macromolecules such as proteins, DNA and RNA (Kumar et al. 2016). In the present study, the decreased electrolyte leakage of the primed seeds/seedlings of Neeraja, Vaisakh and Vyttila 6 than non-primed seeds/seedlings in response to NaCl and PEG reveals the positive impact of HP, HyP and UP on enhancing the membrane stability index. This is in agreement with the studies of Manafi et al. (2015), in which they have observed that seed priming with 5-aminolevolunic acid reduced the rate of electrolyte leakage and thereby increased membrane stability in soya bean when exposed to cold stress. Increased germination rate and reduced MDA content in HyP treated seedlings reveals the occurrence of a reduced rate of oxidative stress as a result of priming accompanied by improved antioxidant mechanisms. Similar to our findings, the result of Gharahlar et al. (2010) proved that seed priming with KNO3 resulted in reduced seedling electrolyte leakage when subjected to salinity stress.
Priming of seeds induces the mobilization of seed storage proteins and activates antioxidant machinery, thereby reducing the lipid peroxidation rate (Iseri et al. 2014). Report of Farooq et al. (2013) shows that drought stress results in the imbalance of membrane stability in wheat, however, priming with ascorbic acid significantly increased the membrane stability. Improved membrane stability index could be attributed to the priming induced enhanced production of ROS scavengers and also reduction in the rate of ROS synthesis. In this study, it was found that increased electrolyte leakage was negatively associated with the contents of sugar, phenolics, proline and amino acids accumulated in response to NaCl and PEG, which clearly indicates the positive impact of these ROS scavengers cum osmo-regulators on the cell membrane stability by controlling cellular electrolyte leakage and these were highly accumulated in seeds subjected to HyP followed by HP and UP.
Priming improves osmoregulation in seeds when subjected to NaCl and PEG stresses. HP, UP and HyP techniques mediated accumulation of proline improves plant water status by regulating osmoticum of cell and thus improves membrane stability and reduces oxidative damages such as lipid peroxidation and electrolyte leakage in the seedlings of different rice varieties studied. This is in agreement with the findings of Farooq et al. (2013), wherein seed priming with ascorbic acid results in the accumulation of proline and thereby maintained tissue water status and thus reduced oxidative damage under drought stress in wheat. Also, the current study gives the evidence that sugar accumulation significantly occurs in response to HP, HyP and UP under NaCl and PEG stresses in seedlings of Neeraja, Vaisakh and Vyttila 6. The accumulated sugars play an important role in imparting stress tolerance by increasing the osmoticum of cells. During seed germination, osmotic adjustment of cells is mainly carried out by soluble sugars besides its detoxification role (Bolarin et al. 1995). In response to HP, HyP, and UP, amino acid accumulation also occurs under NaCl and PEG stresses in seedlings of Neeraja, Vaisakh and Vyttila 6. In response to stress, plants accumulate free amino acids for an osmotic adjustment (Ibrahim 2016). Accumulated osmolytes cause increased uptake of water and thereby expansion of cells, which altogether results in the increased growth of seedlings. The present study shows that all the priming techniques HyP, UP and HP results in the over-accumulation of osmolytes such as amino acids, sugars, and proline during seed germination even under the NaCl and PEG stresses and HP have higher impacts on increased osmolytes synthesis than HyP and UP on the three rice varieties studied.
Phenolics act as a non-enzymatic antioxidant during stress conditions, moreover, they are also involved in growth and development and acts as a cell wall constituent besides its antioxidant potential (Cheynier et al. 2013). Enhanced phenolics content as a result of HP, HyP and UP actively reduces membrane oxidative damage by reducing electrolyte leakage in the seedlings of Neeraja, Vaisakh and Vyttila 6 upon NaCl and PEG exposure. Similar to phenolics, it was also found that an increase in non-enzymatic antioxidants such as ascorbate and glutathione occurred in response to HyP, UP and HP when exposed to PEG and NaCl stresses. Priming with UV-B and NaCl resulted in the increased content of ascorbate and glutathione in all rice varieties than hydro priming which reveals the enhanced potential of UV-B and halo priming in imparting stress tolerance. Increased hydrogen peroxide content was positively correlated with ascorbate and glutathione content and it reveals the effective scavenging of increased ROS in response to NaCl and PEG. The accumulated ascorbate and glutathione also facilitate the enhanced activities of the enzymes APX and glutathione reductase which are involved in ascorbate—glutathione cycle, resulting in the detoxification of H2O2 to H2O (Dusart et al. 2019).
Earlier reports have revealed the importance of antioxidant machinery in imparting stress tolerance against oxidative damage (Faseela and Puthur 2019; Thomas and Puthur 2019). The present study reports that the HyP, UP and HP can significantly accelerate the activities of various antioxidant enzymes in Neeraja, Vaisakh and Vyttila 6 under NaCl and PEG stresses. It was reported that a low dose of UV-B stimulates the activities of antioxidant enzymes like SOD and non-enzymatic antioxidants such as glutathione and ascorbate (Takeuchi et al. 1996). Furthermore, activities of SOD, CAT, APX, and GPOX were reported to be stimulated by a low dose of UV-B radiation (Mishra et al. 2009). Increased synthesis of enzymatic and non-enzymatic antioxidants results in the reduction of lipid peroxidation rate. UP and HP treatment resulted in the boosting up of enzymatic and non-enzymatic antioxidant machinery due to the initial mild stress effects imparted during seed priming. Under NaCl and PEG stresses, a significant increase in SOD, CAT, GPOX, and APX activities were found in all the three varieties subjected to HP and UP, however, the prominent increase was observed in Vaisakh and Vyttila 6. Another form of priming, osmo priming with PEG significantly elevated the activities of antioxidant enzymes CAT, SOD and APX in rice seedlings under nano-ZnO stress (Salah et al. 2015). In this study, it was found that superoxide content was positively correlated with the SOD activity whereas the hydrogen peroxide content was positively correlated with CAT, GPX and APX activity, the former being responsible for the increased detoxification of superoxides into hydrogen peroxides and the latter enzymes converted it into water by the action of CAT, GPX and APX (Hasanuzzaman, et al. 2018).
Significant stimulation of the antioxidant enzyme activities was observed in HyP, UP and HP treated on being subjected to PEG stress, seedlings which reveal the greater stress imparted by PEG as compared to NaCl. Increased antioxidant enzyme activities coincide with the effective detoxification of ROS, which in turn results in the reduced lipid peroxidation rate. Although SOD, CAT, APX and GPOX activities increased in NaCl and PEG stresses as compared to control, the prominent increase was observed in primed and stressed plants. Similar to these findings, it was reported that activities of SOD and glutathione reductase increased under NaCl and PEG stresses in a hydro primed set of Brassica juncea as compared to non-primed sets (Srivastava et al. 2010) Functioning of antioxidant machinery to scavenge overproduced ROS is variety specific. In the case of sensitive variety Neeraja, it was found that the rate of SOD, CAT, APX and GPX activities was lower than Vaisakh and Vyttila 6. However, priming techniques increased the rate of enzymatic activities even in Neeraja but to a lesser extent than Vaisakh and Vyttila 6. Among the three priming techniques, it was found that priming with UV-B and NaCl have a high positive impact on enhancing the activities of antioxidant enzymes in three rice varieties. Of the different priming techniques, it was found that halo priming effectively induced antioxidant machinery in Neeraja and Vaisakh under PEG stress followed by NaCl stress, whereas in the case of Vyttila 6 (a NaCl tolerant variety), halo priming results in the enhanced activation of antioxidant machinery in NaCl stress than PEG stress. While UV-B and hydro priming bestowed PEG stress tolerance followed by NaCl to all the three rice varieties studied.
Seed pretreatment with HP, HyP and UP positively influenced various parameters such as enhanced activities of SOD, CAT, APX, GPOX, increased content of glutathione, ascorbate, proline, amino acid, total phenolics, and sugar both under stress treatment and unstressed condition and thus leads to improved seed germination of different rice varieties under NaCl and PEG stresses. Based on CSRI, it was clear that HP had the highest positive effects, followed by UP than HyP as evidenced by various biochemical parameters studied in the varieties Neeraja, Vyttila 6 and Vasiakh on exposure to NaCl and PEG stresses. In summary, the tolerant varieties Vyttila 6 and Vasiakh were found to be more tolerant in response to NaCl and PEG stresses as a result of different priming techniques employed and Neeraja (a known stress-sensitive variety) showed considerable enhancement in the abiotic stress tolerance potential.
Conclusion
NaCl and PEG stresses have a negative impact on seed germination and early seedling establishment in seedlings of Neeraja, Vaisakh and Vyttila 6. Different seed priming techniques HP, HyP and UP were found to be effective strategies to impart stress tolerance to all three varieties during the seed germination stage itself. From this study, it was clear that HP had higher beneficial effects concerning the alleviation of harmful effects of NaCl and PEG stresses through boosting up of antioxidant machinery during seed germination and successive seedling establishment. Seed priming not only enhances the stress tolerance potential of sensitive varieties but also imparts enhanced stress tolerance to the tolerant varieties.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
AS is indebted to the Council of Scientific & Industrial Research (CSIR), New Delhi, for the financial assistance through award of Junior Research Fellowship (JRF). The Regional Agricultural Research Station (RARS) Pattambi and Vyttila, Kerala, India, is gratefully acknowledged for providing seeds of rice varieties. We thank Dinakar Challabathula, Department of Life Sciences, Central University of Tamil Nadu, India for helpful discussion and suggestions related to the manuscript.
Author contribution
AS wrote the main text of the manuscript and collected the necessary references. JTP edited the manuscript. All authors have read and approved the final manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Agarie S, Hanaoka N, Kubota F, Agata W, Kaufman PB. Measurement of cell membrane stability evaluated by electrolyte leakage as a drought and heat tolerance test in rice (Oryza sativa L.) J Fat Agr Kyushu Univ. 1995;40:233–240. [Google Scholar]
- Bailly C, El-Maarouf-Bouteau H, Corbineau F. From intracellular signaling networks to cell death; the dual role of reactive oxygen species in seed physiology. C R Biol. 2008;331:806–814. doi: 10.1016/j.crvi.2008.07.022. [DOI] [PubMed] [Google Scholar]
- Bates LS, Waldren RP, Teare IK. Rapid determination of free proline for water studies. Plant Soil. 1973;39:205–207. [Google Scholar]
- Biju S, Fuentes S, Gupta D. Silicon improves seed germination and alleviates drought stress in lentil crops by regulating osmolytes, hydrolytic enzymes and antioxidant defense system. Plant Physiol Biochem. 2017;119:250–264. doi: 10.1016/j.plaphy.2017.09.001. [DOI] [PubMed] [Google Scholar]
- Bolarín MC, Santa-Cruz A, Cayuela E, Pérez-Alfocea F. Short-term solute changes in leaves and roots of cultivated and wild tomato seedlings under salinity. J Plant Physiol. 1995;147:463–468. [Google Scholar]
- Bradford KJ. Manipulation of seed water relations via osmotic priming to improve germination under stress conditions. Hortic Sci. 1986;21:1105–1112. [Google Scholar]
- Bussotti F, Ferrini F, Pollastrini M, Fini A. The challenge of Mediterranean sclerophyllous vegetation under climate change: from acclimation to adaptation. Environ Exp Bot. 2014;103:80–98. [Google Scholar]
- Chen JX, Wang XF. Guide to plant physiological experiments. Guangzhou: South China University of Technology Press; 2002. pp. 123–127. [Google Scholar]
- Cheynier V, Comte G, Davies KM, Lattanzio V, Martens S. Plant phenolics: recent advances on their biosynthesis, genetics, and eco physiology. Plant Physiol Biochem. 2013;72:1–20. doi: 10.1016/j.plaphy.2013.05.009. [DOI] [PubMed] [Google Scholar]
- Coskun D, Britto DT, Huynh WQ, Kronzucker HJ. The role of silicon in higher plants under salinity and drought stress. Front Plant Sci. 2016;7:1072. doi: 10.3389/fpls.2016.01072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Q, Yan B, Huang S. Response of oxidative stress defense systems in rice (Oryza sativa) leaves with supplemental UV-B radiation. Physiol Plant. 2006;101:301–308. [Google Scholar]
- Doke N. Involvement of superoxide anion generation in the hypersensitive response of potato tuber tissues to infection with an incompatible race of Phytophtora infestans and the hyphal wall components. Physiol Plant Pathol. 1983;23:345–357. [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1951;28:350–356. doi: 10.1038/168167a0. [DOI] [PubMed] [Google Scholar]
- Dusart N, Thiec D, Collignon C, Jolivet Y, Vaultier M. Integrated analysis of the detoxification responses of two Euramerican poplar genotypes exposed to ozone and water deficit: focus on the ascorbate-glutathione cycle. Sci Total Environ. 2019;651:2365–2379. doi: 10.1016/j.scitotenv.2018.09.367. [DOI] [PubMed] [Google Scholar]
- El-Maarouf-Bouteau H, Bailly C. Oxidative signaling in seed germination and dormancy. Plant Signal Behav. 2008;3(3):175–182. doi: 10.4161/psb.3.3.5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooq M, Irfan M, Aziz T, Ahmad I, Cheema SA. Seed priming with ascorbic acid improves drought resistance of wheat. J Agron Crop Sci. 2013;199:12–22. [Google Scholar]
- Faseela P, Puthur JT. Intraspecific variation in sensitivity of high yielding rice varieties towards UV-B radiation. Physiol Mol Biol Plant. 2019;25(3):727–740. doi: 10.1007/s12298-019-00646-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folin O, Denis W. A colorimetric method for the determination of phenols (and phenols derivaties) in urine. J Biol Chem. 1915;22:305–308. [Google Scholar]
- Gaspar T, Penel C, Greppin H. Peroxidase and isoperoxidation in relation to root and flower formation. Plant Biochem J. 1975;2:33–47. [Google Scholar]
- Gharahlar AS, Farhoudi R, Jaime A, Silva T. Influence of snake melon (Cucumis melo Var. flexuosus) seed priming on seedling emergence and seedling electrolyte leakage under salinity. Seed Sci Biotech. 2010;4(1):15–18. [Google Scholar]
- Giannopolitis CN, Ries SK. Superoxide dismutase-occurrence in higher plants. Plant Physiol. 1977;59:309–314. doi: 10.1104/pp.59.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasanuzzaman M, Nahar K, Rahman A, Inafuku M, Oku H, Fujita M. Exogenous nitric oxide donor and arginine provide protection against short-term drought stress in wheat seedlings. Physiol Mol Biol Plants. 2018;24(6):993–1004. doi: 10.1007/s12298-018-0531-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath RL, Packer L. Phytoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Ibrahim EA. Seed priming to alleviate salinity stress in germinating seeds. J Plant Physiol. 2016;192:38–46. doi: 10.1016/j.jplph.2015.12.011. [DOI] [PubMed] [Google Scholar]
- Iseri OD, Sahin F, Hberal M. Sodium chloride priming improves salinity responses of tomato at seedling stage. J Plant Nutr. 2014;37:374–392. [Google Scholar]
- Junglee S, Urban L, Sallanon H, Lopez-Lauri F. Optimized assay for hydrogen peroxide determination in plant tissue using potassium iodide. Am J Anal Chem. 2014;5(11):730. [Google Scholar]
- Kar M, Mishra D. Catalase, peroxidase, and polyphenoloxidase activities during rice leaf senescence. Plant Physiol. 1976;57:315–319. doi: 10.1104/pp.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar SPJ, Prasad S, Kumar M, Singh C, Sinha AK, Pathak A. Seed quality markers: a review. Res Rev. 2016;3:13–17. [Google Scholar]
- Manafi E, Sanavy SAMM, Aghaalikhan M, Dolatabadian A. Exogenous 5-aminolevulenic acid promotes antioxidative defense system, photosynthesis and growth in soyabean against cold stress. Not Sci Biol. 2015;7(4):468–494. [Google Scholar]
- Mishra V, Srivastava G, Prasad SM. Antioxidant response of bitter gourd (Momordica charantia L.) seedlings to interactive effect of dimethoate and UV-B irradiation. Sci Hortic. 2009;120(3):373–378. [Google Scholar]
- Mondal S, Viji P, Bose B. Role of seed hardening in rice variety Swarna (MTU 7029) Res J Seed Sci. 2011;4:157–165. [Google Scholar]
- Moore S, Stein WH. Photometric ninhydrin method for use in chromatography of aminoacids. J Biol Chem. 1948;176:367–388. [PubMed] [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- Neelamegam R, Sutha T. UV-C irradiation effect on seed germination, seedling growth and productivity of groundnut (Arachis hypogaea L.) Int J Curr Microbiol App Sci. 2015;4(8):430–443. [Google Scholar]
- Noctor G, De Paepe R, Foyer CH. Mitochondrial redox biology and homeostasis in plants. Trends Plant Sci. 2007;12:125–134. doi: 10.1016/j.tplants.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Patade VY, Bhargava S, Suprasanna P. Halopriming mediated salt and iso-osmotic PEG stress tolerance and gene expression profiling in sugarcane (Saccharum officinarum L.) Mol Biol Rep. 2012;39:9563–9572. doi: 10.1007/s11033-012-1821-7. [DOI] [PubMed] [Google Scholar]
- Pedrosa M, Souza Q. Reactive oxygen species and seed germination. Biologia. 2013;68(3):351–357. [Google Scholar]
- Petrov V, Hille J, Mueller-Roeber B, Gechev TS. ROS-mediated abiotic stress-induced programmed cell death in plants. Front Plant Sci. 2015;6:69–77. doi: 10.3389/fpls.2015.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangani J, Panda A, Patel M, Parida AK. Regulation of ROS through proficient modulations of antioxidative defense system maintains the structural and functional integrity of photosynthetic apparatus and confers drought tolerance in the facultative halophyte Salvadora persica L. J Photochem Photobiol B. 2018;189:214–233. doi: 10.1016/j.jphotobiol.2018.10.021. [DOI] [PubMed] [Google Scholar]
- Rhoads DM, Umbach AL, Subbaiah CC, Siedow JN. Mitochondrial reactive oxygen species. Contribution to oxidative stress and interorganellar signaling. Plant Physiol. 2006;141:357–366. doi: 10.1104/pp.106.079129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sairam RK, Deshmukh PS, Shukla DS. Tolerance of drought and temperature stress in relation to increased antioxidant enzyme activity in wheat. J Agron Crop Sci. 1997;178(3):171–178. [Google Scholar]
- Salah Mohamed S, Yajing G, Dongdong C, Jie L, Aamir N, Qijuan H, Weimin H, Mingyu N, Jin H. Seed priming with polyethylene glycol regulating the physiological and molecular mechanism in rice (Oryza sativa L.) under nano-ZnO stress. Sci Rep. 2015;5:14278. doi: 10.1038/srep14278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava AK, Lokhande VH, Patade VY, Suprasanna P, Sjahril R, Souza SF. Comparative evaluation of hydro-, chemo- and hormonal priming methods for imparting salt and PEG stress tolerance in Indian mustard (Brassica juncea L.) Acta Physiol Plant. 2010;32:1135–1144. [Google Scholar]
- Takeuchi Y, Murakami M, Nakajima N, Kondo N, Nikaido O. Induction and repair of damage to DNA in cucumber cotyledons irradiated with UV-B. Plant Cell Physiol. 1996;37:181–187. [Google Scholar]
- Thomas DT, Puthur JT. Amplification of abiotic stress tolerance potential in rice seedlings with a low dose of UV-B seed priming. Funct Plant Biol. 2019;46(5):455–466. doi: 10.1071/FP18258_AC. [DOI] [PubMed] [Google Scholar]
- Ullah H, Luc PD, Gautam A, Datta A. Growth, yield and silicon uptake of rice (Oryza sativa) as influenced by dose and timing of silicon application under water-deficit stress. Arch Agron Soil Sci. 2018;64(3):318–330. [Google Scholar]
- Wojtyla Ł, Lechowska K, Kubala S, Garnczarska M. Molecular processes induced in primed seeds-increasing the potential to stabilize crop yields under drought conditions. J Plant Physiol. 2016;203:116–126. doi: 10.1016/j.jplph.2016.04.008. [DOI] [PubMed] [Google Scholar]
- Yan M. Hydro-priming increases seed germination and early seedling growth in two cultivars of Napa cabbage (Brassica rapa subsp. pekinensis) grown under salt stress. J Hortic Sci Biotechnol. 2016;91:421–426. [Google Scholar]
- Yin D, Chen S, Chen F, Guan Z, Fang W. Morphological and physiological responses of two chrysanthemum cultivars differing in their tolerance to waterlogging. Environ Exp Bot. 2009;67(1):87–93. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.