Abstract
To understand high temperature tolerance, Heliotropium thermophilum, a flowering plant thriving in a geothermal field with a soil temperature ranging between 55 and 65 °C, was grown in controlled laboratory conditions and two different soil temperatures were applied to the plants. One of them was the control group (CT 25 ± 3 °C) and the other was the high temperature group (HT 60 ± 4 °C). Water potential, dry weight, cell membrane injury (CMI), lipid peroxidation, hydrogen peroxide, chlorophylls, carotenoids, flavonoids, anthocyanins, proline and total soluble sugar contents were measured. Contents of total soluble sugars, phenolics, flavonoids, anthocyanins, proline were found to be higher in HT group than CT while CMI was opposite. Moreover, no difference was determined in water potential, dry weight, lipid peroxidation, total chlorophyll and carotenoids between CT and HT. H. thermophilum plants adapted to high temperature under laboratory conditions through changing membrane lipid saturation, accumulating osmotically active compounds to save water or increase its uptake and inducing antioxidants such as phenolic compounds to keep reactive oxygen species under control. In conclusion, this study showed that H. thermophilum plant was highly resistant to high soil temperature under optimized laboratory conditions. Moreover, a plant that can withstand 60 °C for a long period of time up to 60 days under laboratory conditions was reported for the first time.
Keywords: Heliotropium thermophilum, High temperature, Adaptation, Thermotolerance
Introduction
The average temperature of the world tends to increase continuously. Global air temperature is estimated to be 1.8–4.0 °C higher than the current level by 2100 (Stamatakis et al. 2013). Due to the global warming, the growth and development of plants are adversely affected. Plants with limited tolerance to high temperature lost their yields more than thermotolerant plants. It was suggested that the temperature increase of 3–4 °C will cause a decrease in cereal production in Africa and Asia by 15–35% and in the Middle East by 25–35%. High temperature stress on plants will result in nutritional problem of the world population in the future. For this reason, studies to improve the high temperature stress tolerances of the plants are important (Ahanger et al. 2017) and it is necessary to know the mechanisms that underlie the high temperature stress tolerance. As known, the plants can survive under high temperatures by developing some mechanisms such as changes in leaf location, transpiration cooling, leaf rolling, early ripening, accumulation of osmoprotectants and some secondary metabolites and changes in membrane lipid composition (Fitter and Hay 2002; Giday et al. 2015). Lipid saturation of membranes is a very important feature for high temperature tolerance. Increasing the saturation level of fatty acids in plants maintains membrane stability and plays an important role in temperature tolerance (Larkindale and Huang 2004). Moreover, secondary metabolites such as flavonoids, anthocyanins, steroids and phenolics are accumulated in response to high temperature stress and contribute significantly to high temperature tolerance (Rivero et al. 2001; Wahid 2007). Increased secondary metabolite synthesis in plant metabolism under high temperature stress has been found to play a protective role against oxidative stress damage. In addition, carotenoids play important protective roles in plants against high temperature and light (Camejo et al. 2006). For example, xanthophylls and some other terpenoids (isoprene and tocopherol) stabilize the lipid phase of thylakoid membranes.
All the above mechanisms provide tolerance to plants to various degrees against high temperature. However, the number of plants that can survive above 45 °C is quite low. One of these plants, Agrostis scabra, can grow in geothermal areas with soil temperatures up to 45 °C (Tercek et al. 2003). Another example is the dwarf swan-neck moss (Campylopus pyriformis) which has been found in soil where temperatures reached 72 °C. Kunzea robusta, a flowering plant, grows in the soil temperature about 68 °C (Chaffey 2017). Heliotropium thermophilum is a flowering plant which can grow naturally in a geothermal field with a temperature range of 55–65 °C (Tan et al. 2008). Therefore, it is very important to investigate the high temperature tolerance properties of H. thermophilum. On the other hand, this plant must cope with many stress factors such as grazing, fungal or bacterial infections and herbivory. Moreover, it is difficult to determine the exact age of plants grown in natural conditions. The age of the plants can also affect their responses to environmental factors. Therefore, the results obtained from comparative studies in natural environments may sometimes not be reliable enough. For this reason, it is essential to adapt H. thermophilum to controlled laboratory conditions and to conduct high temperature experiments in order to better understand how the plant is accustomed to high temperature conditions. In summary, this study was carried out to (1) adapt H. thermophilum to controlled laboratory conditions and to (2) better understand the mechanisms of adaptation of this plant to high soil temperature. In this study, H. thermophilum was grown under laboratory conditions for the first time and subjected to controlled long-term high soil temperature (60 days). To test the adaptation ability of the plant to high soil temperature treatment, water potential, cell membrane injury, leaf area, lipid peroxidation, contents of chlorophyll and carotenoid pigments and some phenolic compounds were determined.
Materials and methods
Plant material, growth and treatments
The seeds of the thermophile H. thermophilum (Boraginacae) were obtained from Aydın-Buharkent Turkey which was a geothermal region with a soil temperature of 55–65 °C. Two special adjustable heating units were used for heat treatments to plants in a controlled manner (Fig. 1). The seeds were sown in a soil, peat and perlite mixture (Compo Sana, Germany) in the heating units in a walk-in growth chamber (DigiTech PG42 http://www.digitech-ltd.com/en/store/pg42-iklim-dolabi). The plants were kept in the controlled conditions (16 h light/8 h dark, 25 °C day/20 °C night temperature, 400 µmol m−2 s−1 light current density, fluorescent lamps, Philips Master TL5 HE 28 W/840, 65% relative humidity) in the growth chamber, including the heating units. Temperature of the soil was monitored continuously by a digital thermometer (Trotec BT20). The soil temperature was set to 25 ± 3 °C and all plants were grown at this temperature for 20 days. At the end of this period, two different groups were formed as control (CT 25 ± 3 °C) and high temperature (HT 60 ± 4 °C) to apply two different temperatures. The temperature of the HT was increased gradually. Firstly, it was set to 40 ± 3 °C on the 20th day, then it was raised to 60 ± 4 °C on the 30th day to facilitate gradual adaptation depending on time. Temperature of the control group was kept at 25 ± 3 °C throughout the study (90 days). All plants were watered with tap water every day to 100% container capacity. The plants were harvested after 60 days of temperature applications when they were 3 months old (90 days). Leaf samples were used for following analysis.
Fig. 1.
Natural habitat of Helitropium thermophilum plants (a), images of the plant in its natural habitat (b, c), temperature adjustable heating unit (d), plants grown in the heating unit (e–h), soil-free control (i), high temperature (j) plants after treatments
Measurement of leaf water potential, leaf dry weight, leaf area, specific leaf area and root length
Leaf water potentials were measured according to Savage and Cass (1984). To evaluate the water status of plant leaves, the water potential of leaf discs was measured by using the sample chamber C-52. Psychrometers were simultaneously connected to a water potential data logger Psypro P2-132 (Wescor, Inc.).
To calculate the leaf dry weight, leaf samples of equal size from 10 plants were selected and weighed after drying at 65 °C for 48 h.
Area calculation was carried out with the image analysis software (ImageJ). For area calculation, first a scale was set by using the line drawer option of ImageJ. A line was drawn on the ruler in the image, and then the length in pixels and the known distance on the ruler were entered in the set scale menu. Two plugins were used for the further calculation. One of the plugins, “Threshold Color” allowed to threshold a color RGB image in the HSB, RGB, CIE Lab or YUV color spaces, isolating only green colored areas in the images. After thresholding, images were converted to 8-bit greyscale images. To remove background pixels from a thresholded image, first a dilation operation was applied, followed by an erosion (or a closing operation, combining the above in one step). The area calculator plugin was used to measure the thresholded leaf area of 8-bit images (Lenk et al. 2007; Koubouris et al. 2018). Specific leaf area (SLA) was calculated according to following formulae SLA = leaf area (cm2)/leaf dry weight (g) according to Demir and Celikel (2019).
To measure primary root length, the plants were separated from the soil in a manner that would not harm the primary roots. Remaining soil particles and other residues on the roots were removed by washing them with water without damaging them. The plants were photographed on a white background with a ruler. Photos were taken with a Nikon D70 digital camera fixed on a tripod at a distance of 30 cm. The images were then analyzed with commercially available software (WinRHIZO Pro 2019a, Regent Instruments, Canada). The software allowed length measurement of traced roots. An outline was traced around each root therefore, invididual measurements were taken. The length of 1 cm on the ruler in each photograph was set to the software. The set scale was used as a reference to determine the length of the roots (Su et al. 2019).
Determination of cell membrane injury, lipid peroxidation and hydrogen peroxide
Cell membrane injury (CMI) was determined by conductometric method of Tripathy et al. (2000). Fifteen leaf discs with a diameter of 1 cm were washed and incubated for 24 h in deionized water at 25 °C. The electrical conductivity (EC) of the discs was then determined using a conductivity meter (Delta Ohm). EC values at this stage was named as C1 then, the same discs were boiled in the water bath for 15 min and cooled to 25 °C. EC was measured again, this was named as C2. Cellular injury was determined by using the formula: (C1/C2) × 100 where C refers to conductivity one and two.
Lipid peroxidation was determined according to Heath and Packer (1968). Leaf samples of 0.5 g was pulverized in liquid nitrogen and then homogenized with 10 ml 0.1% trichloroacetic acid (TCA) by a homogenizer (TissueLyser LT, Qiagen). The homogenate was centrifuged at 15.000 g for 5 min (MPW-260R Centrifuge, MPW Med Instruments). Supernatant was added to 0.5% thiobarbituric acid prepared in 20% TCA. The mixture was then heated at 95 °C for 30 min in an oven then the reaction was stopped in an ice-bath. The mixture was centrifuged at 10.000 g for 10 min and measured at 532 and 600 nm (Evolution 201 UV–Vis Spectrophotometer, Thermo Scientific). Thiobarbituric acid reactive substances (TBARS) content was calculated by following formula: ΔA532–A600 = Ɛ.Ɩ.c (Ɛ: Absorption coefficient, 155 mmol−1 cm−1, c: TBARS concentration and Ɩ: the pathlength of cuvette of 1 cm).
The content of hydrogen peroxide was determined according to the method developed by Velikova et al. (2000). Fresh leaf samples of 0.1 g were homogenized in 0.1% TCA. The homogenate was centrifuged at 10.000 rpm for 10 min, then 10 mM potassium phosphate buffer and 1 M potassium iodide (KI) were added. Absorbance of the mixture at 390 nm were read on the spectrophotometer. These results were expressed as nmol per gram.
Determination of total chlorophyll and carotenoid contents
Fresh leaf samples of 0.5 g was ground to powder in liquid nitrogen then homogenized in 80% acetone solution at 4 °C by mortar and pestle. The resulting homogenate was centrifuged at 3.000 rpm for 10 min at room temperature and measured at 450, 645 and 663 nm. The total chlorophyll content was determined according to Arnon (1949). Total carotenoid content was calculated according to Witham et al. (1971)
Determination of total phenolic, flavonoid and anthocyanin contents
Total phenolic content was determined spectrophotometrically according to Singleton et al. (1999). Fresh leaf samples of 0.1 g were homogenized with 95% methanol. This homogenate was centrifuged at 10.000 rpm for 10 min. The supernatant was diluted with water in 1/3 ratio. The diluted supernatant was added to 3900 µl of 7.5% Na2CO3 and 100 µl of 2 N Folin–Ciocalteu’s Phenol Reagent. The samples were kept in dark for 30 min and measured at 765 nm on a spectrophotometer according to a standard curve of gallic acid.
Total flavonoid content was determined by colorimetric method according to Kim et al. (2003). Fresh leaf samples of 0.1 g were weighed and homogenized in 1% acidic methanol (1% v/v HCl). The homogenate was centrifuged at 10.000 rpm for 15 min and concentrated by evaporator at 45 °C. Then, 150 µl of 5% NaNO2 was added to the extracts. After 6 min, the mixture was treated with 150 µl of 10% AlCl3 and incubated for 6 min. 2 ml of 1 N NaOH was added to the mixture. Final volume of the mixture was made 5 ml by the addition of distilled water. It was measured at 510 nm after 15 min. Total flavonoid content was expressed as mg quercetin in 100 g fresh sample.
Total anthocyanin content was performed spectrophotometrically according to Mancinelli et al. (1988). Fresh leaf samples of 0.1 g were weighed and homogenized with acidic methanol (1% v/v HCl) for 24 h at 4 °C in a shaker (Thermo Shaker Incubator MS-100) in darkness. The homogenate was centrifuged at 5.000 g for 15 min. The absorbance of the supernatant was read on the spectrophotometer at 530 and 657 nm. In order to compensate for the absorption of chlorophyll degradation products in A530, the total anthocyanins were calculated using the formula A530 − 0.25A657. Cyanidine-3-glycoside was used as standard.
Determination of proline and soluble sugar contents
Proline content was performed spectrophotometrically according to Bates et al. (1973). 0.5 g of fresh leaf samples were weighed and fractionated with nitrogen. It was then homogenized with 3% sulfosalicylic acid and then filtered. After that, 2 ml of this filtrate was taken, and 1 ml of acetic acid and 1 ml of ninhydrin were added. Ninhydrin was prepared using acetic acid and ortho-phosphoric acid. The mixture was then kept in a water bath at 100 °C for 1 h. Then 3 ml of toluene was added onto the samples and vortexed. Finally, absorbance at 520 nm was read on the spectrophotometer.
Total soluble sugar content was determined spectrophotometrically according to Dubois et al. (1956). Dry leaves of 0.3 g were weighed and homogenized by adding 5 ml of 70% ethanol. The homogenate was boiled at 80 °C for 3 min then centrifuged for 5 min at 10.000 g. The supernatant of 100 µl was taken into a clean tube and 900 µl distilled water was added. This mixture was added onto 1 ml of 5% phenol and mixed with the stirring. Then 5 ml 96% sulfuric acid was added to the same mixture and vortexed. Absorbance of the mixture was measured at 490 nm. Glucose was used as standard.
Statistical analysis
Each value was expressed as the mean ± SE of the three independent experiments. All data were analyzed using SPSS 16.0 (Windows, Chicago, IL, USA). Significance was determined by independent sample t test. Asterisks in figures indicate significant differences at P < 0.05.
Results
Leaf water potential, dry weight, leaf area, specific leaf area, and root length
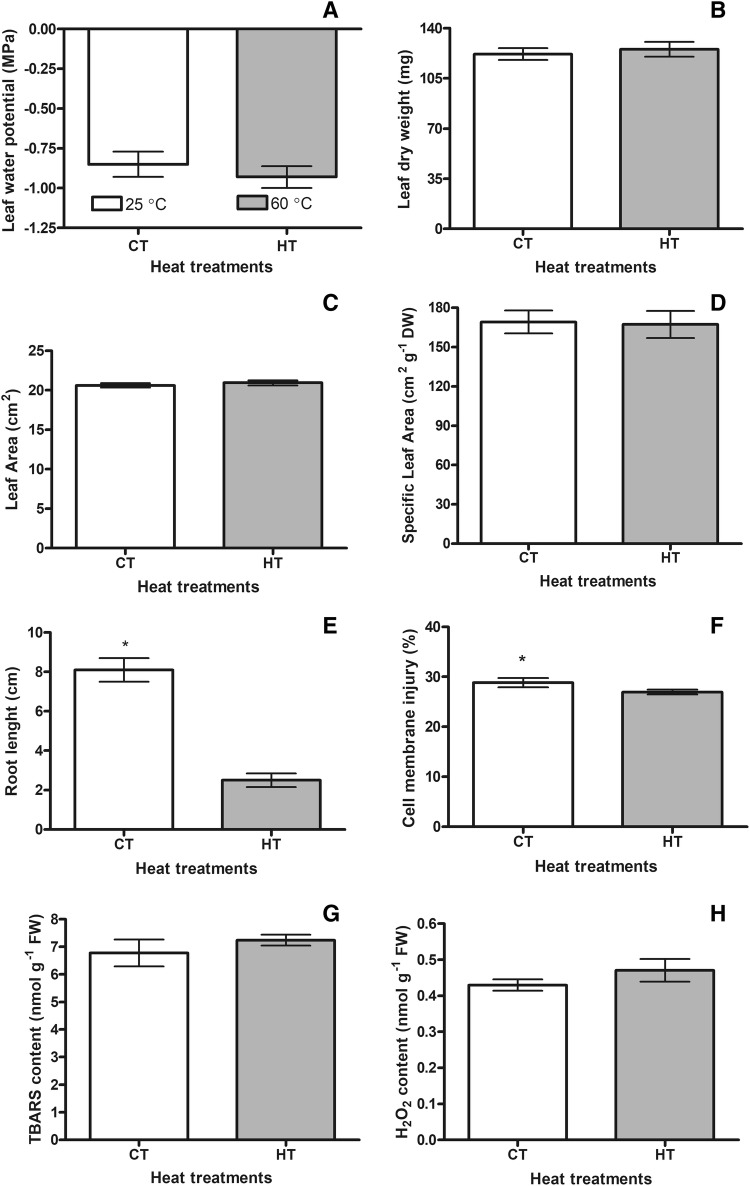
There was no statistically significant difference between control (CT 25 ± 3 °C) and high temperature (HT 60 ± 4 °C) groups in terms of leaf water potential and leaf dry weight (Fig. 2a, b). It was also understood that the plants maintained their water contents and dry matter amounts under high temperature conditions (Fig. 2). In addition, there was not any differences between CT and HT in regards of leaf area (Fig. 2c). No significant difference was detected in terms of SLA between CT and HT (Fig. 2d). However, the root lengths of the high temperature plants were decreased compared to the control group (Fig. 1i: control, j: high temperature, Fig. 2e).
Fig. 2.
Changes of leaf water potentials (a), leaf dry weights (b), leaf areas (c), specific leaf area (d), root lengths (e), cell membrane injuries (f), TBARS (g) and H2O2 (h) contents of the control (CT 25 ± 3 °C) and the high temperature (HT 60 ± 4 °C) plants. Bars on the columns are standard deviations. Statistically significant differences are indicated by asterisks
Cell membrane injury, lipid peroxidation and hydrogen peroxide
In the present study, it was determined that cell membrane injury (CMI) of the HT was 1.1-fold lower than that of the control group. This showed that plants were able to modify their membranes to adapt to high temperature (Fig. 2f). In addition, there was no statistically significant difference between the CT and the HT groups in terms of TBARS and H2O2 contents (Fig. 2g, h). These data showed that high temperature plants were not exposed to oxidative stress.
Total chlorophyll and carotenoids
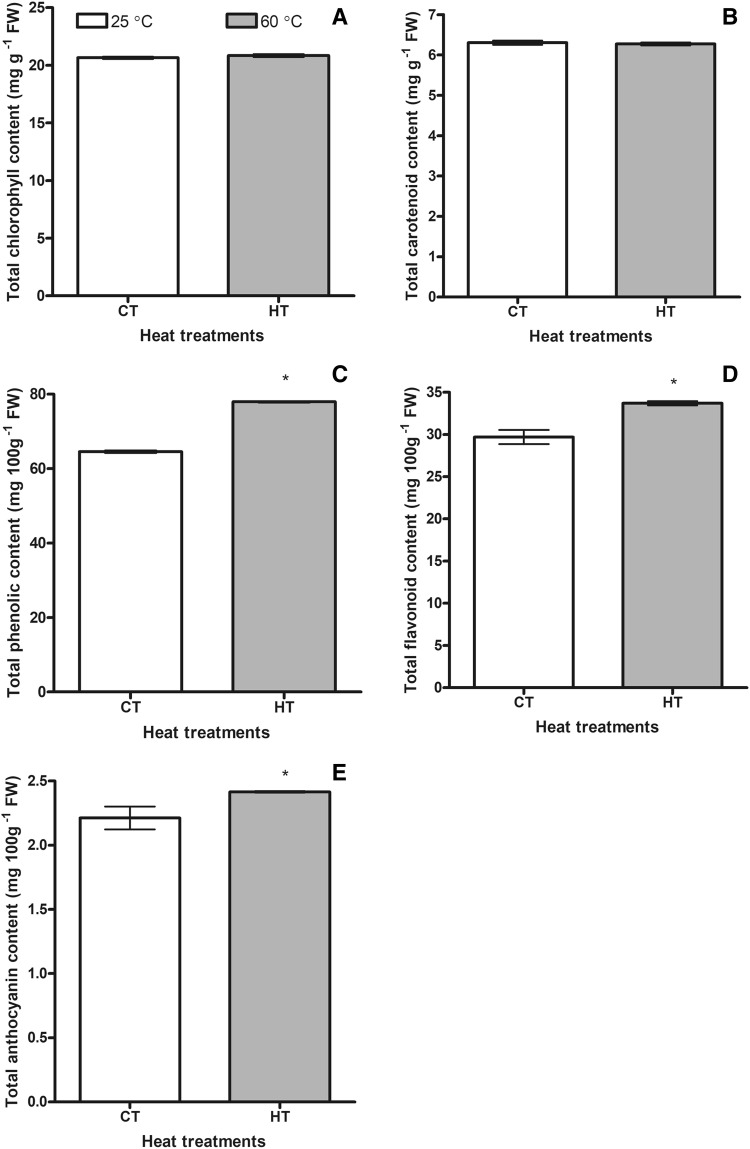
When total chlorophyll and total carotenoid contents were measured, no difference was found between CT and HT groups (Fig. 3a, b). Photosynthetic pigments were not affected by high temperature.
Fig. 3.
Changes of total chlorophyll (a), total carotenoid (b) total phenolic (c), total flavonoid (d) and total anthocyanins (e) contents of the control (CT 25 ± 3 °C) and the high temperature (HT 60 ± 4 °C) plants. Bars on the columns are standard deviations. Statistically significant differences are indicated by asterisks
Total phenolics, flavonoids and anthocyanines
High temperature treatment caused to increase in the total phenolic content of the experimental group compared to the CT group. It was 1.2-fold higher than that of the CT group (Fig. 3c). Total flavonoid content was also induced by high temperature treatments. Total flavonoid content of the HT group was 1.1-fold higher than that of the CT group (Fig. 3d). Rising soil temperature resulted in increasing total anthocyanin content in the HT group compared to the CT group. The total anthocyanin content in the experimental group was 1.1 times higher than that of the control group (Fig. 3e).
Proline and total soluble sugars
Proline content was determined to increase with rising soil temperature. The amount of proline measured in the HT group was found to be 1.3 times higher than in the CT group (Fig. 4a). Total soluble sugar content of the experimental group was also found to be higher than that of the control group (Fig. 4b). The plants seemed to adapt to high temperature by accumulating osmolytes.
Fig. 4.
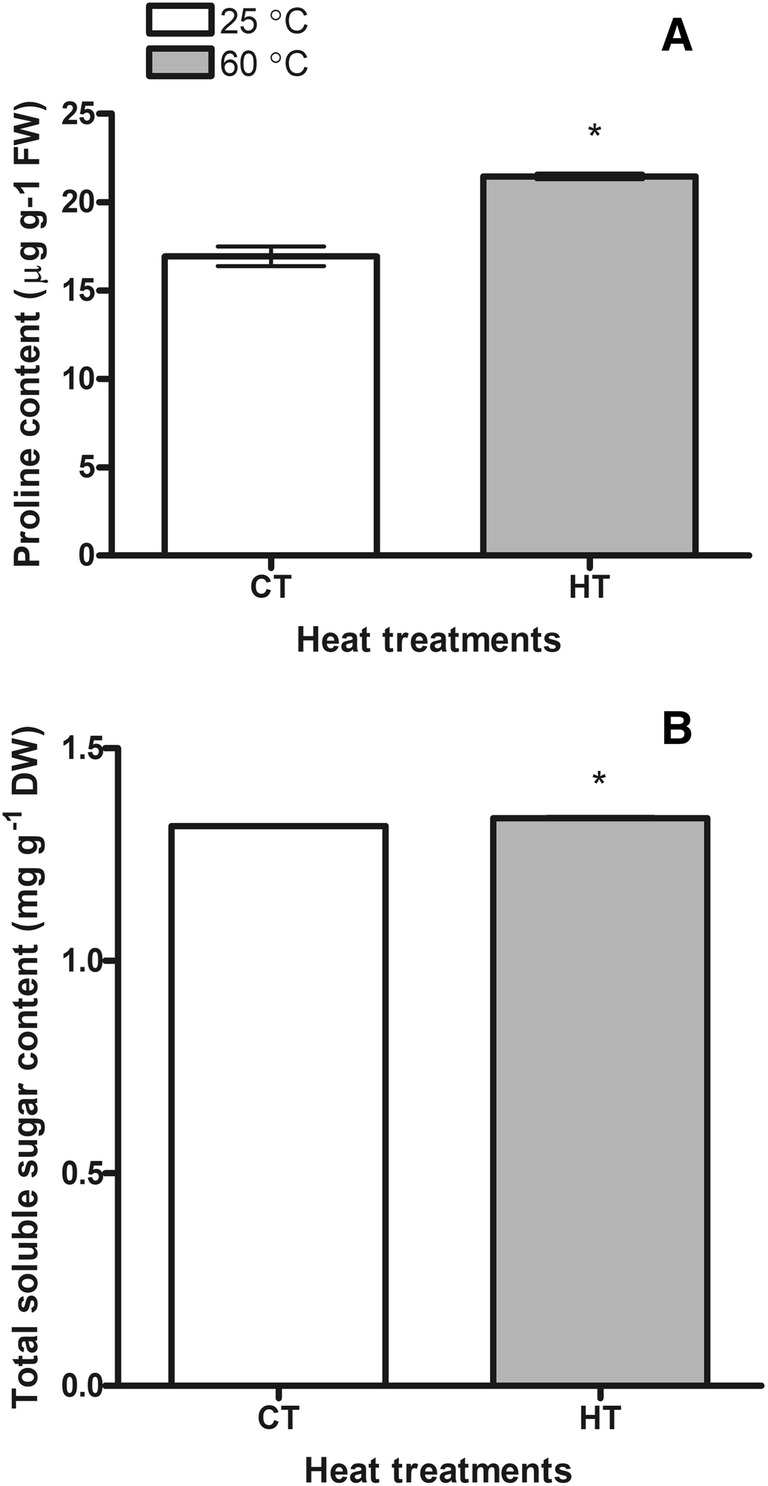
Changes of proline (a) and total soluble sugar (b) contents of the control (CT 25 ± 3 °C) and the high temperature (HT 60 ± 4 °C) plants. Bars on the columns are standard deviations. Statistically significant differences are indicated by asterisks
Discussion
Growing thermotolerant plants that are adapted to living at high soil temperatures under controlled conditions in order to understand how they survive under these conditions is very important. These plants are exposed to various stresses simultaneously in their natural habitats however, under controlled conditions, the plants are only exposed to high soil temperature, and thus it will be possible to examine their specific responses to this environmental factor in detail. Therefore, in this study, it has been tried to adapt H. thermophilum which is a thermotolerant plant to laboratory conditions and to test the responses of the plants to the high soil temperature. Thus, leaf water potential, leaf dry weight, leaf area, SLA, cell membrane stability, hydrogen peroxide, lipid peroxidation, total chlorophyll, carotenoid, phenolic, anthocyanin, flavonoid, proline and soluble sugar contents were determined.
Water potential is an important parameter to determine the water condition of the plant and the degree of stress (Romero-Trigueros et al. 2019). In order to have an idea about the water content of the plants under control and high temperature conditions, the leaf water potential was measured. In our study, there was no difference between the control and the high temperature group in regards of the leaf water potential. When plants are exposed to heat stress, their water potential and relative water content in leaves are significantly reduced (Akter and Rafiqul 2017). An et al. (2014) observed that there was a significant decrease in water potential in Medicago sativa genotypes after 72-h high temperature (38 °C) treatment and the decrease in heat stress sensitive genotypes was higher. Similarly, Duan et al. (2017) reported that there was no significant change in water potential in tomato plants under a 7-day 42 °C temperature stress application compared to control. This result showed that the plants exposed to high temperature could maintain the leaf water content at high temperature. This might be related to some mechanisms that protected the structure of membranes under high temperature conditions. Many studies point to the cell membrane as the starting site of stress damage, and the function and structure of plant cell membranes are largely damaged by environmental stress (Takahashi et al. 2013). Therefore, the assessment of cellular membrane integrity as a measure of environmental stress tolerance seems to be a related parameter (El Basyoni et al. 2017). Thermotolerance is acquired through reorganizing the lipid compositions of cell membranes and modifying biochemical processes (Perera et al. 2019). A tolerance mechanism developed by plants in response to heat stress damage is the cell membrane stability. Thermotolerant species are characterized by an increased membrane temperature stability (Bento et al. 2017). In our study, cell membrane injury (CMI) as an indicator of the cell membrane stability was measured. The plants adapted to the high temperature conditions (HT) had lower CMI compared to the control plants (CT). Similarly, Ergin et al. (2016) applied high temperature between 35 and 60 °C for 2 h to two different strawberry genotypes, which are tolerant and sensitive to high temperatures. They found that the membrane damage occurred in the temperature-tolerant genotype was lower than the temperature-sensitive genotype.
As increased stability in cell membranes brings resistance to temperature stress, changes in lipid peroxidation in order to understand the stress status of plants under high temperature has been investigated in the current study. In cases where the level of TBARS increases, the plants are regarded as sensitive to high temperatures, while the plants in which its level decreases or does not change, the plants may be accepted as thermotolerant (Awasthi et al. 2015). In the present study, there was no statistically significant difference between the HT and CT groups for TBARS. This result was consistent with the CMI determination, which is another stress parameter. Low CMI might be evidence of no destruction of membrane lipids under high temperature conditions.
It is important that the membranes remain intact during stress, in terms of continuity of the turgor. The continuity of the turgor pressure is also necessary for the growth of plant cells (Li et al. 2013). As the cells grow, dry matter contents also increase. The dry weight of the leaves is one of the main stress parameters which is highly affected by high temperature conditions (Oh-e et al. 2007). In our study, there was no significant difference between CT and HT in case of the dry weight. This suggests that the plant develops various adaptations to protect the water content. Conservation of the water content of the plant has a great importance in terms of growth, development and physiology. The fact that there was no difference between the CT and HT groups in terms of the leaf area and SLA supported this situation. On the other hand, root growth decreased under high soil temperature conditions. But stem growth was not affected. The decrease in root growth was thought to result from the promotion of ethylene biosynthesis under high temperature conditions. Our unpublished RnaSeq data belonging to H. thermophilum showed that the expression levels of some genes involved in ethylene signaling and synthesis increased in high temperature conditions (60 °C) compared to the control group (25 °C). For instance, Arabidopsis orthologs of H. thermophilum Ethylene Response Factors (ERF71 and ERF109), Ein3 Binding F-Box Protein and ACC Deaminase 1 were induced by high soil temperature. Indeed, ethylene was shown to inhibit root growth under heat stress (Koevoets et al. 2016). In addition, the expression levels of some genes related to mineral uptake and transport (Arabidopsis orthologs of H. thermophilum Sulfate Transporter1, Nitrate Transporter1, Phospate Transporter1 and Purple Acid Phosphatase) increased in high temperature conditions. This indicated that even though the growth of the roots was inhibited, mineral transport to the leaves continued.
As known, high temperature can inhibit photosynthesis entirely before other symptoms of the stress are appeared (Camejo et al. 2006). The absence of a reduction in dry matter in high temperature conditions also showed that photosynthesis could continue effectively under these conditions. In addition, under high temperature stress, reduced chlorophyll amount and structural and functional degradation of chloroplasts may lead to a reduction in photosynthesis. However, in the current study no change in chlorophyll content was observed between the CT and HT groups. Thus, we may suggest here that photosynthesis may not be disrupted by high temperature treatment. In addition, chlorophyll was shown to be indicator of heat tolerance of plants under heat stress treatments. Heat-tolerant wheat varieties under increased temperature were reported to maintain a relatively high chlorophyll content (Almeselmani et al. 2006). It was determined that the chlorophyll content of the three Labiatea species which were exposed to high temperature stress was almost constant. The authors suggested that the unchanged chlorophyll content was a mechanism of resistance to temperature (Asensi-Fabado et al. 2013). Carotenoids not only act as accessory light-harvesting pigments, but also protect photosynthetic systems as nonenzymatic antioxidant compounds against reactive oxygen species produced during stress. Therefore, the plants may develop tolerance to a stress by maintaining a higher or unchanged total carotenoid level (Camejo and Torres 2001). According to our findings, there is no difference in total amount of carotenoids between CT and HT groups. These results were consistent with previous studies related to plant acclimation to stress.
High temperatures can induce lipid peroxidation and harm proteins, nucleic acids and pigments by causing overproduction of ROS at the cellular level (Xie et al. 2019). Some mechanisms developed by plants protect them from ROS damage. These mechanisms include scavenging of ROS by means of an enzymatic antioxidant system and non-enzymatic antioxidants such as carotenoids, anthocyanins. In the current study, hydrogen peroxide (H2O2) content was investigated to obtain information about the stress status of the plant. No change was observed between CT and HT plants in terms of H2O2 content. This result supported TBARS results that the plant did not experience a stress at soil temperature of 60 ± 4 °C.
Flavonoids, anthocyanins are phenolics compounds including the most important secondary metabolite class in plants, and play a variety of roles, including tolerance to abiotic stresses (Sharma et al. 2019). Therefore, total phenolic content was measured in the present study. According to our findings, the total phenolic content of the plants growing at 60 ± 4 °C temperature was higher than the plants growing at 25 ± 3 °C. It is known that phenolic compounds are expressed in large amounts in plants at high temperatures and this helps in detoxification of ROS and providing heat resistance to plants (Rivero et al. 2001). In our study, TBARS and H2O2 concentrations did not increase in high temperature conditions which may be due to ROS scavenging abilities of phenolic compounds. The content of flavonoids has also been investigated in the current study. According to our findings, the total flavonoid content of the HT plants was higher than the CT plants. Flavonoid accumulation has been reported to protect plants against various stressful conditions such as high and low temperatures and played an important role as antioxidants against ROS toxicity (Morimoto 1998; Lillo et al. 2008). As known, anthocyanins like other phenolics have an important place in plants for tolerance against abiotic stresses. In addition, the amount of anthocyanin is important in determining the level of stress. Anthocyanin is sensitive to temperature changes. It has been found that anthocyanins have a protective role in injury during high temperature. They induce antioxidant system in Arabidopsis under high temperature stress (Shao et al. 2007). However decreased anthocyanin accumulation has been reported in red-wine grapes under high temperatures (Mori et al. 2007). In the current study, the total amount of anthocyanin was investigated and the total amount of anthocyanin in high temperature plants was found to be higher than the control group. As a result, the H. thermophilum plant synthesized large amounts of flavonoids, anthocyanins and other phenolic substances at high temperatures, thus contributing to the adaptation to this extreme high temperature.
Many plants under abiotic stress accumulate osmolytes such as proline and soluble sugars to make the osmotic adjustments and promote the removal of free radicals by antioxidant enzymes (Seki et al. 2007). Proline has been recorded to have functions such as, osmotic adjustment, ROS’s scavenging, membrane integrity protection, ensuring the stability of proteins and cytosol pH (Seki et al. 2007; Hassanvand et al. 2019). According to the results obtained in our study, proline content of HT plants was found to be higher than the CT plants. This result was an indication that the plant increases the amount of internal proline and provides tolerance to temperature, and due to the increasing amount of proline, the content of water in the plant might be preserved under high soil temperature conditions. Leaf water potential and leaf dry weight findings in our study support this idea. These findings were also found to be similar to the changes in proline content of moth bean seedlings under high temperature stress in the literature (Harsh et al. 2016). Heat stress was applied to 7 days old moth bean seedlings at 42 °C for 1 h in hot air oven. They also suggested that proline functioned as an indicator of plant water status of the plants. Moreover, in a study conducted by Ergin et al. (2016), proline content in the strawberry plants increased with high temperature (43 °C/2 h). They suggested that proline might contribute to stress tolerance of the strawberry plant by serving as a ROS scavenger or protein stabilizer under high temperature stress.
The accumulation of sugars in plants is known to contribute to the protection of biomolecules and membranes by regulating the internal osmotic potential (Seki et al. 2007; Sadiq et al. 2017). Stress-induced sugar deposition has been reported in many types and is believed to play an important role in temperature tolerance. The accumulation of sugars can protect the membranes and enzymes against the harmful effects of the destabilization of the ions, as well as maintain the osmotic equilibrium (Abdallah et al. 2016). Therefore, total sugar content was measured spectrophotometrically in the present study. According to the data obtained, the total soluble sugar content of the HT plants was higher than the CT plants. Total soluble sugars might function as osmoprotectant to maintain turgor and protect cellular membranes of HT plants.
Conclusions
Heliotropium thermophilum plant was found to easily adapt to controlled laboratory conditions and was not affected from high soil temperature treatment adversely. A plant that can withstand 60 °C for a long period of time up to 60 days under laboratory conditions was reported for the first time here. H. thermophilum changed membrane lipid saturation, saved water or increased its uptake accumulating osmotically active compounds and induced antioxidants such as phenolic compounds to keep ROS under control to survive in high soil temperature conditions. In addition, the adaptation of a thermotolerant plant to laboratory conditions has enabled the possibility of more detailed studies on the responses to high temperature under controlled conditions. Moreover, considering that the plant has a structure and characteristics that can be adapted to high temperatures, it is suggested that it will be beneficial to investigate its responses to higher temperatures with further studies.
Acknowledgements
This work was supported by Karadeniz Technical University Scientific Research Projects Unit through Project No. FYL-2017-6918. Authors also want to thank to KERMAK Company for construction of the heating units.
Author contributions
AS and AK designed the research. KO and AS carried out the experiments. AK, AS and KO analyzed the experimental data and wrote the manuscript.
Compliance with ethical standards
Conflict of interest
All authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdallah MS, Abdelgawad ZA, El-Bassiouny HM. Alleviation of the adverse effects of salinity stress using trehalose in two rice varieties. S Afr J Bot. 2016;103:275–282. [Google Scholar]
- Ahanger MA, Akram NA, Ashraf M, Alyemeni MN, Wijaya L, Ahmad P. Plant responses to environmental stresses-from gene to biotechnology. AoB Plants. 2017;9:plx025. doi: 10.1093/aobpla/plx025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akter N, Rafiqul IM. Heat stress effects and management in wheat: a review. Agron Sustain Dev. 2017;37:37. [Google Scholar]
- Almeselmani M, Deshmukh PS, Sairam RK, Kushwaha SR, Singh TP. Protective role of antioxidant enzymes under high temperature stress. Plant Sci. 2006;171:382–388. doi: 10.1016/j.plantsci.2006.04.009. [DOI] [PubMed] [Google Scholar]
- An Y, Zhou P, Liang J. Effects of exogenous application of abscisic acid on membrane stability, osmotic adjustment, photosynthesis and hormonal status of two lucerne (Medicago sativa L.) genotypes under high temperature stress and drought stress. Crop Pasture Sci. 2014;65:274–286. [Google Scholar]
- Arnon DI. Copper enzymes in isolated chloroplasts, polyphenoxidase in Beta vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asensi-Fabado MA, Oliván A, Munné-Bosch S. A comparative study of the hormonal response to high temperatures and stress reiteration in three Labiatae species. Environ Exp Bot. 2013;94:57–65. [Google Scholar]
- Awasthi R, Bhandari K, Nayyar H. Temperature stress and redox homeostasis in agricultural crops. Front Environ Sci. 2015;3:11. [Google Scholar]
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water stress studies. Plant Soil. 1973;39:205–207. [Google Scholar]
- Bento M, Gomes Pereira S, Viegas W, Silva M. Durum wheat diversity for heat stress tolerance during inflorescence emergence is correlated to TdHSP101C expression in early developmental stages. PLoS ONE. 2017;12:e0190085. doi: 10.1371/journal.pone.0190085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camejo D, Torres W. High temperature effect on tomato (Lycopersicon esculentum) pigment and protein content and cellular viability. Cul Trop. 2001;22:13–17. [Google Scholar]
- Camejo D, Jiménez A, Alarcón JJ, Torres W, Gómez JM, Sevilla F. Changes in photosynthetic parameters and antioxidant activities following heat-shock treatment in tomato plants. Funct Plant Biol. 2006;33:177–187. doi: 10.1071/FP05067. [DOI] [PubMed] [Google Scholar]
- Chaffey N. Plant cuttings: news in botany. Ann Bot. 2017;120(6):3–6. doi: 10.1093/aob/mcx169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demir S, Celikel FG. Effects of plant growth regulators on the plant height and quantitative properties of Narcissus tazetta. Turk J Agric For. 2019;43:105–114. [Google Scholar]
- Duan H, Wu J, Huang G, Zhou Sh, Liu W, Liao Y, Yang X, Xiao Z, Fan H. Individual and interactive effects of drought and heat on leaf physiology of seedlings in an economically important crop. AoB Plants. 2017;9:plw090. doi: 10.1093/aobpla/plw090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Calorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. [Google Scholar]
- El Basyoni I, Saadalla M, Baenziger S, Bockelman H, Morsy S. Cell membrane stability and association mapping for drought and heat tolerance in a worldwide wheat collection. Sustainability. 2017;9:1–16. [Google Scholar]
- Ergin S, Gülen H, Kesici M, Turhan E, İpek A, Köksal N. Effects of high temperature stress on enzymatic and non-enzymatic antioxidants and proteins in strawberry plants. Turk J Agric For. 2016;40:908–917. [Google Scholar]
- Fitter A, Hay R. Environmental physiology of plants. San Francisco: Academic Press; 2002. [Google Scholar]
- Giday H, Kjaer KH, Ottosen CO, Fanourakis D. Cultivar differences in plant transpiration rate at high relative air humidity are not related to genotypic variation in stomatal responsiveness. Acta Hortic. 2015;1064:99–106. [Google Scholar]
- Harsh A, Sharma YK, Joshi U, Rampuria S, Singh G, Kumar S, Sharma R. Effect of short-term heat stress on total sugars, proline and some antioxidant enzymes in moth bean (Vigna aconitifolia) Ann Agric Sci. 2016;61:57–64. [Google Scholar]
- Hassanvand F, Nejad AR, Fanourakis D. Morphological and physiological components mediating the silicon-induced enhancement of geranium essential oil yield under saline conditions. Ind Crop Prod. 2019;134:19–25. [Google Scholar]
- Heath RL, Packer L. Photoperoxidation in isolated chloroplast. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Kim DO, Jeong SW, Lee CY. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003;81:321–326. [Google Scholar]
- Koevoets IT, Venema JH, Elzenga JTM, Testerink C. Roots withstanding their environment: exploiting root system architecture responses to abiotic stress to improve crop tolerance. Front Plant Sci. 2016;7:1335. doi: 10.3389/fpls.2016.01335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koubouris G, Bouranis D, Vogiatzis E, Nejad AR, Giday H, Tsaniklidis G, Ligoxigakis EK, Blazakis K, Kalaitzis P, Fanourakis D. Leaf area estimation by considering leaf dimensions in olive tree. Sci Hort. 2018;240:440–445. [Google Scholar]
- Larkindale J, Huang B. Changes of lipid composition and saturation level in leaves and roots for heat-stressed and heat-acclimated creeping bentgrass (Agrostis stolonifera) Environ Exp Bot. 2004;51:57–67. [Google Scholar]
- Lenk S, Chaerle L, Pfuendel EE, Langsdorf G, Hagenbeek D, Lichtenthaler HK, Van Der Straeten D, Buschmann C. Multispectral fluorescence and reflectance imaging at the leaf level and its possible applications. J Exp Bot. 2007;58:807–814. doi: 10.1093/jxb/erl207. [DOI] [PubMed] [Google Scholar]
- Li SX, Wang ZH, Stewart BA. Chapter five—responses of crop plants to ammonium and nitrate N. Adv Agron. 2013;118:205–397. [Google Scholar]
- Lillo C, Lea US, Ruoff P. Nutrient depletion as a key factor for manipulating gene expression and product formation in different branches of the flavonoid pathway. Plant Cell Environ. 2008;31:587–601. doi: 10.1111/j.1365-3040.2007.01748.x. [DOI] [PubMed] [Google Scholar]
- Mancinelli AL, Hoff AM, Cottrell M. Anthocyanin production in Chl-rich and Chl-poor seedlings. Plant Physiol. 1988;86:652–654. doi: 10.1104/pp.86.3.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K, Goto-Yamamoto N, Kitayama M, Hashizume K. Loss of anthocyanins in red-wine grape under high temperature. J Exp Bot. 2007;58:1935–1945. doi: 10.1093/jxb/erm055. [DOI] [PubMed] [Google Scholar]
- Morimoto RI. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones and negative regulators. Gene Dev. 1998;12:3788–3796. doi: 10.1101/gad.12.24.3788. [DOI] [PubMed] [Google Scholar]
- Oh-e I, Saitoh K, Kuroda T. Effects of high temperature on growth, yield and dry-matter production of rice grown in the paddy field. Plant Prod Sci. 2007;10:412–422. [Google Scholar]
- Perera RS, Cullen BR, Eckard RJ. Growth and physiological responses of temperate pasture species to consecutive heat and drought stresses. Plants. 2019;8:227. doi: 10.3390/plants8070227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivero RM, Ruiz JM, Garcia PC, López-Lefebre LR, Sánchez E, Romero L. Resistance to cold and heat stress: accumulation of phenolic compounds in tomato and watermelon plants. Plant Sci. 2001;160:315–321. doi: 10.1016/s0168-9452(00)00395-2. [DOI] [PubMed] [Google Scholar]
- Romero-Trigueros C, Gambín JMB, Tortosa PAN, Cabañero JJA, Nicolás EN. Determination of crop water stress index by infrared thermometry in grapefruit trees irrigated with saline reclaimed water combined with deficit irrigation. Remote Sens. 2019;11(7):757. [Google Scholar]
- Sadiq M, Aisha Akram N, Ashraf M. Foliar applications of alpha-tocopherol improves the composition of fresh pods of Vigna radiata subjected to water deficiency. Turk J Bot. 2017;41:244–252. [Google Scholar]
- Savage MJ, Cass A. Psychrometric field measurement of water potential changes following leaf excision. Plant Physiol. 1984;74:96–98. doi: 10.1104/pp.74.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki M, Umezawa T, Urano K, Shinozaki K. Regulatory metabolic networks in drought stress responses. Curr Opin Plant Biol. 2007;10:296–302. doi: 10.1016/j.pbi.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Shao L, Shu Z, Sun SL, Peng CL, Wang XJ, Lin ZF. Antioxidation of anthocyanins in photosynthesis under high temperature stress. J Integr Plant Biol. 2007;49:1341–1351. [Google Scholar]
- Sharma A, Shahzad B, Rehman A, Bhardwaj R, Landi M, Zheng B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules. 2019;24:2452. doi: 10.3390/molecules24132452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol. 1999;299:152–178. [Google Scholar]
- Stamatakis E, Nnoaham K, Foster C, Scarborough P. The influence of global heating on discretionary physical activity: an important and overlooked consequence of climate change. J Phys Act Health. 2013;10:765–768. doi: 10.1123/jpah.10.6.765. [DOI] [PubMed] [Google Scholar]
- Su WN, Kamran M, Xie J, Meng XP, Han QF, Liu TN, Han J. Shoot and root traits of summer maize hybrid varieties with higher grain yields and higher nitrogen use efficiency at low nitrogen application rates. Peerj. 2019;7:21. doi: 10.7717/peerj.7294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi D, Li B, Nakayama T, Kawamura Y, Uemura M. Plant plasma membrane proteomics for improving cold tolerance. Front Plant Sci. 2013;4:90. doi: 10.3389/fpls.2013.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan K, Çelik A, Gemici Y, Gemici M, Yıldırım H. Heliotropium thermophilum (Boraginaceae), a new taxon from SW Anatolia, Turkey. Adv Sci Lett. 2008;1:132–139. [Google Scholar]
- Tercek MT, Hauber DP, Darwin SP. Genetic and historical relationships among geothermally adapted Agrostis (bentgrass) of North America and Kamchatka: evidence for a previously unrecognized, thermally adapted taxon. Am J Bot. 2003;90:1306–1312. doi: 10.3732/ajb.90.9.1306. [DOI] [PubMed] [Google Scholar]
- Tripathy JN, Zhang J, Robin S, Nguyen TT, Nguyen HT. QTLs for cell-membrane stability mapped in rice (Oryza sativa L.) under drought stress. Theor Appl Genet. 2000;100:1197–1202. [Google Scholar]
- Velikova V, Yordanov I, Edreva A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants, protective role of exogenous polyamines. Plant Sci. 2000;151:59–66. [Google Scholar]
- Wahid A. Physiological implications of metabolite biosynthesis for net assimilation and heat-stress tolerance of sugarcane (Saccharum officinarum) sprouts. J Plant Res. 2007;120:219–228. doi: 10.1007/s10265-006-0040-5. [DOI] [PubMed] [Google Scholar]
- Witham FH, Blaydes BF, Devlin RM. Experiments in plant physiology. New York: Van Nostrand Reinhold; 1971. [Google Scholar]
- Xie X, He Z, Chen N, Tang Z, Wang Q, Cai Y. The roles of environmental factors in regulation of oxidative stress in plant. Biomed Res Int. 2019;2019:9732325. doi: 10.1155/2019/9732325. [DOI] [PMC free article] [PubMed] [Google Scholar]