Abstract
The present experiment was set-up to appraise protective role of ALA in sunflower cultivars (FH-1581 and FH-1572) under water scarcity stress. The ameliorative role of ALA in sunflower under water stress is not fully understood. Results showed significant decline in growth parameters, ascorbic acid and chlorophyll but marked increase in MDA, H2O2, total soluble proteins, flavonoids, proline, phenolics, total free amino acids as well as enzymes activities namely CAT, POD and SOD in plants under water scarcity. ALA application reduced oxidative damage by lowering H2O2 and MDA contents. ALA application differentially affected two cultivars under stress. Higher biomass accumulation was manifested in cv. FH-1581, while cv. FH-1572 was inferior in this context. Greater drought tolerance in cv. FH-1581 was related to higher cellular levels of proline, total free amino acids and efficient antioxidant system.
Electronic supplementary material
The online version of this article (10.1007/s12298-019-00756-3) contains supplementary material, which is available to authorized users.
Keywords: Secondary metabolite, Phenolics, Ascorbic acid, Flavonoids, Oxidative damage, Photosynthetic pigments
Introduction
Drought occurs in different regions around the globe (Li et al. 2011) and it causes substantial decline in growth and yield related attributes in plant (Mao et al. 2003). Consequently, plant scientists are studying important physiological and biochemical mechanisms conferring water stress tolerance in plants (Yordanov et al. 2000). Plants face drought more frequently owing to shortage of water resources and warm climates (Jury and Vaux 2005). Resultantly, the incidence of longer drought periods is the major set-back for farmers, particularly in arid and semi-arid parts (Shao et al. 2008). Water is major limiting aspect affecting several metabolic, biochemical, physiological, morphological, proteomics and transcriptomics processes that principally govern plant development, survival and yield production (Alam et al. 2017). Effective strategies are required to counteract the undesirable effects of warm climates. The response of plant to drought stress varies with plant organ and therefore, research dealing with whole plant is mandatory in spite of studying the response of individual plant organ (Chaves et al. 2003). Plants display active osmotic adjustments in response to osmotic stress. Plants accumulate many compatible solutes and inorganic ions to sustain turgor pressure since loss of turgor leads to plant death (Dutta et al. 2018). Plants spend large sum of energy to synthesize organic osmolytes. The buildup and translocation of organic osmolytes intervene important metabolic events and ultimately enhance plant growth and yield (Sun et al. 2015).
Drought increases reactive oxygen species (ROS) generation such as hydrogen peroxide H2O2 and O·−2 (superoxide radical) (Mahawar et al. 2018a, b; Nguyen et al. 2019). The accumulation of ROS affects normal functioning of cells through damage to proteins and lipids thereby resulting in cell death (Hussain et al. 2019). Plants contain well-defined antioxidant system for the detoxification of ROS. Ascorbic acid, anthocyanins, phenolics and flavonoids detoxify ROS non-enzymatically whereas SOD, POD, APX and CAT carry out enzymatic detoxification of ROS (Shekhawat et al. 2008; Li et al. 2011; Mahawar and Shekhawat 2019).
Drought stress is known to induce substantial accumulation of proline, an important organic osmolyte for improving plant stress tolerance. The biosynthesis of ALA and proline share the same precursor, glutamic acid. Exogenous application of ALA may increase cellular levels of ALA and the precursor is then consumed for proline biosynthesis. Proline buffers cytosolic pH, protects subcellular compartments and scavenges ROS (Averina et al. 2010). 5-Aminolevulinic acid (ALA) functions as essential precursors for the biosynthesis of various tetrapyrroles such as chlorophyll, heme, billins and vitamin B12 (Li et al. 2011). ALA supplementation has significant potential in agriculture sector as ALA enhances abiotic stress tolerance of plants (Sun et al. 2009). The supplementation of ALA improves photosynthesis, chlorophyll contents, growth and crop yield under abiotic stresses (Hotta et al. 1997). Addition of ALA as foliar applications improves oxidative defense and plant tolerance to abiotic stress (Memon et al. 2008; Akram et al. 2012; Kosar et al. 2015). Low doses of ALA enhanced oxidative defense system and drought tolerance in oilseed rape (Liu et al. 2011). ALA increased performance of Brassica under salinity (Wang et al. 2005), chilling tolerance in maize (Wang et al. 2018), water stress in Brassica napus (Naeem et al. 2011), heat stress in cucumber (Zhang et al. 2012), herbicide toxicity in Brassica (Zhang et al. 2008), and lead toxicity in Brassica (Ali et al. 2014). Sunflower plants when supplied with foliar ALA improved salinity tolerance (Akram et al. 2012). In this context, very few reports are available on protective role of ALA in sunflower plants under water limited conditions.
Sunflower is considered an important oilseed crop throughout the world. Besides, its use as oil source, sunflower is used in the preparation of textile dyes, rubber, latex and serves as rich source of proteins. Moreover, sunflower plants also bear important medicinal properties (Akram et al. 2012). Approximately, 0.323 Mha area is under sunflower cultivation with 0.407 million tons of production (Akram et al. 2012). The growth and production of sunflower is significantly affected by drought stress (Hussain et al. 2018). Therefore, the present research was organized to investigate whether or not exogenous ALA could enhance the growth and development of sunflower plants under drought, and decrease the degree of oxidative injury by the increasing antioxidant enzyme activities as well as secondary metabolites accumulation.
Materials and methods
Seeds of sunflower cultivars (FH-1581 and FH-1572) were obtained from Ayub Agricultural Research Institute (AARI), Faisalabad, Pakistan. A completely randomized three factorial pot experiment was performed with six replications of each treatment. The climatic conditions during experiment were as average day and night temperature 29.5 ± 6.2 and 17.5 ± 6.7, respectively, average relative humidity 32.11 ± 3.84 and 10 h photoperiod. Plastic pots were filled with 8 kg of sandy-loam soil. Eight seeds were sown in each pot. After 1 week of germination, two plants per pot were left by hand hoeing of other plants. Two weeks after germination, plants were given two different water levels, i-e., 100% field capacity (control) and 70% field capacity (water stress). Three weeks after the onset of water deficit conditions, plants were sprayed with different levels of 5-aminolevulinic acid (ALA), i.e., untreated control (NT), water spray (WS), 10, 20 and 30 mg/L of ALA. Distilled water containing 0.1% Tween-20 was used to make different concentrations of ALA. Plants were harvested 2 weeks after foliar spray of ALA and changes in growth and key biochemical attributes were recorded. Plant fresh and dry weights were measured. For dry weights, plants were put in an oven at 65 °C for 1 week.
Chlorophyll and carotenoids
Chlorophyll and carotenoids contents were measured from fresh leaf material following the protocol of Arnon (1949). Fresh leaf tissue (0.25 g) was ground in 5 mL of 80% aqueous acetone. The supernatant was used to measure the absorbance at 663, 645, and 480 nm.
Non-enzymatic antioxidants
Fresh leaf material was used for the determination of anthocyanin following the procedure of Kubo et al. (1999). Leaf total phenolics were measured with the help of Folin Ciocalteu reagent (Wolfe et al. 2003). The leaf phenolic levels were expressed in mg g−1 where standard curve for phenolics was made with gallic acid. The ascorbic acid levels of fresh leaf material were determined following the protocol described earlier (Mukherjee and Choudhuri 1983). Flavonoids levels of fresh leaf material was estimated with the help of method described by Marinova et al. (2005).
Antioxidant enzyme assays
Fresh leaf material was ground in 10 mL of 50 mM chilled potassium phosphate buffer (pH 7.5). The ground material was centrifuged at 10,000×g for 20 min to collect supernatant. This supernatant was used to measure enzyme activities. CAT and POD activities were measured following the earlier described procedure (Chance and Maehly 1955). The activity of SOD was measured following the protocol described by Van Rossum et al. (1997). The activities of antioxidant enzymes were expressed in Units mg g−1 proteins.
Total soluble proteins and total free amino acids
Total soluble proteins were determined from fresh leaf material ground in chilled buffer (50 mM potassium phosphate with pH 7.5). The procedure earlier described (Hamilton et al. 1943) was used to determine total free amino acids from fresh leaf material. One mL of the sample (the one used for total soluble proteins) is reacted with 1 mL of 10% pyridine and the mixture was heated in water bath at 95 °C for 30 min. Distilled water was used to make volume to 7.5 mL. The absorbance was read at 570 nm.
Proline
Plant material 0.25 g (fresh leaf) was crushed in 5 mL sulfosalicylic acid (3%) and the homogenate was then filtered. The filtrate (1 mL) was reacted with equal volume of glacial acetic acid and acid ninhydrin. The reaction mixture was heated in water bath at 95 °C for 50 min. After cooling the mixture, toluene (2 mL) was added to the reaction. The reading of absorbance was measured at 520 nm using spectrophotometer (Bates et al. 1973).
Hydrogen peroxide (H2O2) determination
Fresh leaf tissue (0.5 g) was homogenized in chilled TCA (0.1%). After centrifugation, supernatant 0.5 mL was reacted with 0.5 mL of potassium phosphate buffer (pH 7.0; 50 mM) and 1 mL of potassium iodide (KI; 1 M). The samples were incubated for 30 min at room temperature and absorbance of the sample was recorded at 390 nm (Velikova et al. 2000).
Malondialdehyde (MDA) determination
Fresh leaf tissue (0.5 g) was homogenized in chilled TCA (5%). After centrifugation of the sample, 0.5 mL of the supernatant was added to thiobarbituric acid (0.5%; 2 mL). The reaction mixture was incubated at 95 °C for 45 min, and immediately put on ice bath. The reading of the mixture was taken at 600 and 532 nm with the help of a spectrophotometer (Heath and Packer 1968).
Statistical analysis
Experiment was conducted in completely randomized three factor factorial design with six replications of each treatment. Analysis of variance (ANOVA) of data was computed with the help of statistical software (Costat windows version 6.2, CoHort, Monterey, CA, USA). The difference among means was compared with least significant difference test at 5% probability (P ≤ 0.05).
Results
Growth characteristics
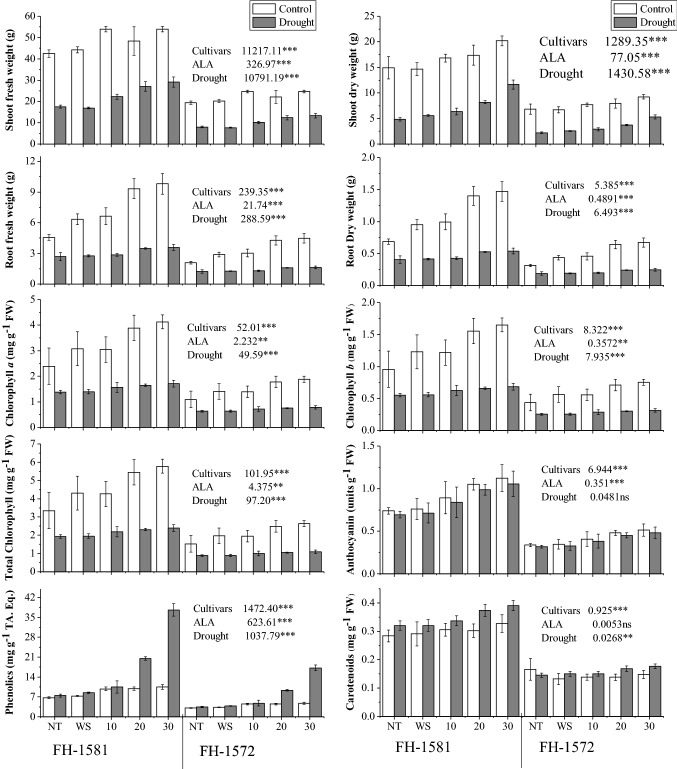
Water deficit stress significantly (P ≤ 0.001) decreased shoot and root fresh and dry masses in two sunflower cultivars. Drought-induced reduction in different growth attributes was maximal in cv. FH-1572 than that of cv. FH-1582. Foliar-applied ALA (30 mg/L) improved (P ≤ 0.001) growth attributes under water stress. We found a − 58.57 and − 61.80% decrease in shoot fresh weight in non-treated plants (NT) and water spray plants (WS), respectively in cv. FH-1581 under drought. The application of foliar ALA (30 mg/L) improved shoot fresh weight as − 46.06% decrease was seen under drought. Similarly, drought-mediated decrease in shoot fresh weight in cv. FH-1572 was 59.90, 63.70 and 44.35% in NT, WS and ALA (30 mg/L) treatments, respectively. The decrease in shoot dry of cv. FH-1581 was − 67.73, − 61.84 and − 42.25% in NT, WS and ALA (30 mg/L) treatments, respectively, under water deficit conditions. In cv. FH-1572, drought resulted in − 65.95, − 72.83 and − 50.25% decrease in shoot dry weight in NT, WS and ALA (30 mg/L) treatments, respectively. Drought decreased root fresh weight by − 41.02, − 56.50 and − 63.48% in NT, WS and ALA (30 mg/L) treatments, respectively, in cv. FH-1581. The cv. FH-1572 manifested − 49.15% (NT), − 59.50% (WS) and 68.88% (30 mg/L ALA) decrease in root fresh weight under drought. The plants of cv. FH-1581 had − 41.02% (NT), − 59.50% (WS) and − 62.63% (30 mg/L ALA) decrease in root dry weight under water deficit conditions. The drought resulted in − 40.11% (NT), 57.33% (WS) and 59.89% (30 mg/L ALA) decrease in root dry weight cv. FH-1572 (Fig. 1; Table 1S).
Fig. 1.
Effect of 5-aminolevulinic acid on growth characteristics, photosynthetic pigments and phenolics in two sunflower cultivars under water deficit stress (n = 6; means ± SE). ALA 5-aminolevulinic acid, ns non-significant. Mean sum of squares of ANOVA with significance at P ≤ 0.05 (*); P ≤ 0.01 (**); P ≤ 0.001 (***)
Photosynthetic pigments
When two sunflower cultivars were subjected to water deficit stress, a significant reduction (P ≤ 0.001) in both types of chlorophyll (a and b) and total chlorophyll was recorded. We found higher values of Chl. a and b as well as total chlorophyll in cv. FH-1581 over cv. FH-1572 under water deficit conditions. Addition of ALA (30 mg/L) markedly enhanced (P ≤ 0.001) chlorophyll under water deficit environment. The decrease in Chl. a was − 42.17% (NT), − 54.63% (WS) and − 57.69% (30 mg/L ALA) in cv. FH-1581 under drought. The cv. FH-1572 manifested a decrease by − 44.23% (NT), − 58.69% (WS) and − 60.71% (30 mg/L ALA) under water deficit conditions. The decline in Chl. b contents was − 43.18% (NT), − 53.61% (WS) and − 59.11% (30 mg/L ALA) in cv. FH-1581 under water deficit conditions. The drought caused − 44.50% (NT), − 55.61% (WS) and − 61.11% (30 mg/L ALA) decrease in this variable in cv. FH-1572. The total chlorophyll contents decreased by − 41.21% (NT), − 53.51% (WS) and − 58.72% (30 mg/L ALA) in cv. FH-1581 under drought. By contrast, cv. FH-1572 exhibited a decrease by − 44.21% (NT), 55.51% (WS) and 60.11% (30 mg/L ALA) in this variable under water deficit conditions (Fig. 1; Table 1S).
Non-enzymatic antioxidants
Anthocyanins
Anthocyanins levels remained unaffected under water deficit stress. Cultivar FH-1581 exhibited higher anthocyanin contents than cv. FH-1572. Increase in anthocyanin contents in response to foliar-applied ALA (30 mg/L) was significant under water deficit stress. The non-significant effect of drought is reflected in the form of decrease in anthocyanins by nearly − 6% in different treatments in both the cultivars (Fig. 1; Table 1S).
Carotenoids
Increase in carotenoids (P ≤ 0.001) contents was recorded in sunflower cultivars under water deficit conditions. Cultivar FH-1581 had higher endogenous levels of carotenoids than that of cv. FH-1572. Addition of ALA as foliar spray induced concentration dependent increase in this variable in response to water stress. The drought caused an increase of 12.74% (NT), 10.25% (WS) and 19.12% (30 mg/L ALA) in cv. FH-1581. The increase of 13.37% (WS) and 19.84% (30 mg/L ALA) was seen in cv. FH-1572 under drought except for a decrease by − 12.49% in untreated control (NT) (Fig. 1; Table 1S).
Leaf total phenolics
Exposure of sunflower plants to stressful conditions increased (P ≤ 0.001) phenolics contents. However, this increase was seen in plants treated with 30 mg/L ALA in both cultivars. Higher phenolic contents were recorded in cv. FH-1581, while cv. FH-1572 was inferior in this context. The influence of foliar spray of varying doses of ALA was significant (P ≤ 0.001) for this variable. Phenolics contents increased by 11.17% (NT), 15.78% (WS) and 258.56% (30 mg/L ALA) in cv. FH-1581 under drought. The cv. FH-1572 manifested an increase of 12.11% (NT), 14.71% (WS) and 244.66% (30 mg/L ALA) under water deficit conditions (Fig. 1; Table 1S).
Flavonoids
Water deficit conditions resulted in significant increase in flavonoids levels in sunflower cultivars. The increase in flavonoids contents was different in two cultivars under water stress. Higher flavonoid contents were present in cv. FH-1581 under water deficit stress. Foliar spray of ALA significantly enhanced (P ≤ 0.001) flavonoids in plants under water deficit stress and this increase in flavonoids was maximal in plants treated with 30 mg/L of ALA. The drought resulted in a 400% increase (NT), 500% (WS) and 900% (30 mg/L ALA) in cv. FH-1581. In cv. FH-1572, an increase of 380% (NT), 489% (WS) and 869% (30 mg/L ALA) was recorded under water deficit conditions (Fig. 2; Table 1S).
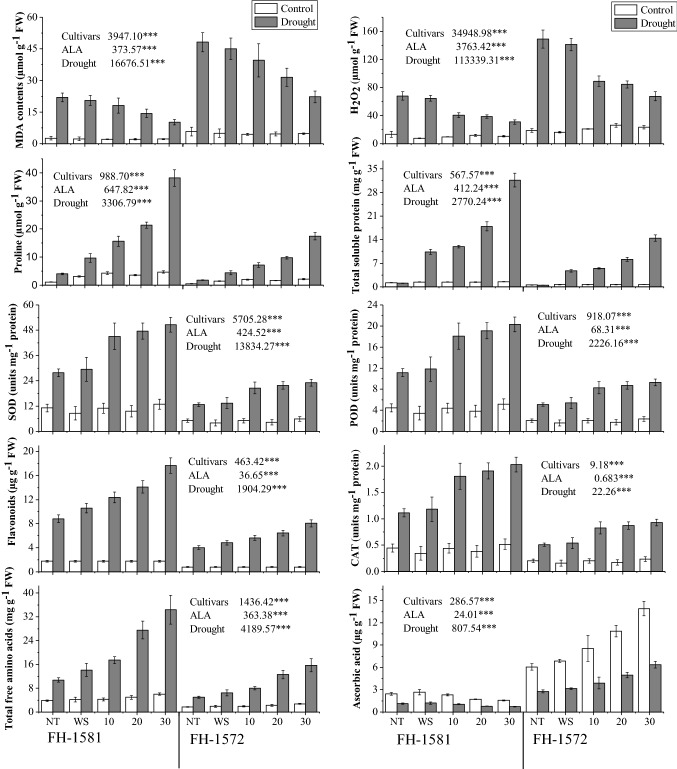
Fig. 2.
Effect 5-aminolevulinic acid on MDA, H2O2, proline, total free amino acids, total soluble proteins, activities of SOD, POD, CAT and concentrations of flavonoids and ascorbic acid in two sunflower cultivars under water deficit stress (n = 6; means ± SE). ALA 5-minolevulinic acid. Mean sum of squares of ANOVA with significance at P ≤ 0.05 (*); P ≤ 0.01 (**); P ≤ 0.001 (***)
Ascorbic acid
The ascorbic acid level was different in two sunflower cultivars. Greater ascorbic acid contents were present in cv. FH-1572 than that of cv. 1581. Water deficit stress caused decline in ascorbic acid contents. ALA application significantly enhanced ascorbic acid levels in cv. FH-1572 under water deficit conditions. Drought mediated the decrease of − 54.33% (NT), − 54.21% (WS) and − 54.11% (30 mg/L ALA) in cv. FH-1581. The cv. FH-1572 exhibited a decrease of − 54.44% (NT), − 54.31% (WS) and − 53.11% (30 mg/L ALA) under drought (Fig. 2; Table 1S).
Activities of antioxidant enzymes
SOD activity
Drought-induced increase (P ≤ 0.001) was seen in SOD activity in sunflower plants. Cultivar FH-1581 showed higher SOD activity, while cv. FH-1572 was inferior in this regard. Exogenous application of ALA markedly (P ≤ 0.001) enhanced SOD activity in both cultivars and ALA-induced increase in this variable was maximal in plants treated with 30 mg/L concentration of ALA. SOD activity increased by 150.05% (NT), 241.15% (WS) and 292.04% (30 mg/L ALA) in cv. FH-1581 under drought. The increase in this variable was as 137.13% (NT), 238.29% (WS), and 271.13% (30 mg/L ALA) in cv. FH-1572 under drought (Fig. 2; Table 1S).
POD activity
Higher POD activity (P ≤ 0.001) was evident in plants under water deficit conditions. Cultivar FH-1581 had higher POD activity than that of cv. FH-1572 under water deficit stress. ALA application markedly raised (P ≤ 0.001) POD activity and higher POD activity was seen in plants treated with 30 mg/L ALA. The drought increased POD activity by 145.11% (NT), 239.12% (WS) and 269.14% (30 mg/L ALA) in cv. FH-1581. The cv. FH-1572 had an increase of 130.11% (NT), 225.19% (WS) and 277.13% (30 mg/L ALA) under drought (Fig. 2; Table 1S).
CAT activity
Water deficit environment induced marked (P ≤ 0.001) increase in CAT activity in two sunflower cultivars. The increase in CAT activity was more in cv. FH-1581 than that in cv. FH-1572 under water stress. ALA application induced dose-dependent increase in CAT activity and plants treated with 30 mg/L ALA displayed greater increase in CAT activity under water deficit conditions. The increase of 131.14% (NT), 210.29% (WS) and 250.24% (30 mg/L ALA) was present in cv. FH-1581 under drought. The cv. FH-1572 manifested an increase of 129.13% (NT), 201.39% (WS) and 279.11% (30 mg/L ALA) under drought (Fig. 2; Table 1S).
Oxidative stress markers
Sunflower plants grown in limited water conditions showed significant increase (P ≤ 0.001) in MDA and H2O2 contents. Higher cellular levels of MDA and H2O2 were present in cv. FH-1572 than that in cv. FH-1581. Decline in H2O2 and MDA in response to ALA was significant (P ≤ 0.001) and maximal decline in these variables were seen in plants treated with 30 mg/L ALA. The intensity of lipid peroxidation was seen as the percentage increase of 738.11% (NT), 799.13% (WS) and 369% (30 mg/L ALA) in cv. FH-1581 under drought. The increase in lipid peroxidation in cv. FH-1572 in response to different treatments under drought was as 701.15% (NT), 750% (WS) and 380% (30 mg/L ALA). H2O2 production was also many folds higher in drought-stressed-plants reflected as an increase of 412.49% (NT), 758.91% (WS) and 188.37% (30 mg/L ALA) in cv. FH-1581. The drought resulted in 419.14% (NT), 770.11% (WS) and 210.31% (30 mg/L ALA) increase in H2O2 contents in cv. FH-1572. (Fig. 2; Table 1S).
Secondary metabolites accumulation
Leaf free proline
Sunflower plants accumulated significant (P ≤ 0.001) amount of proline under water deficit conditions. There existed marked differences in two sunflower cultivars for this variable. Higher proline contents were present in cv. FH-1581, whereas cv. FH-1572 displayed lower endogenous levels of proline. ALA enhanced (P ≤ 0.001) proline contents and plants given 30 mg/L ALA exhibited maximal accumulation of proline under water deficit conditions. The drought increased proline contents by 254.86% (NT), 217.61% (WS) and 710.45% (30 mg/L ALA) in cv. FH-1581. The increase of 250.86% (NT), 225.15% (WS) and 699.13% (30 mg/L ALA) was recorded in cv. FH-1572 under water deficit conditions (Fig. 2; Table 1S).
Total soluble proteins
Plants under water deficit stress showed significant increase (P ≤ 0.001) in total soluble proteins. The increase in total soluble proteins was not the same in two sunflower cultivars. Higher total soluble proteins were evident in cv. FH-1581. Addition of ALA resulted in marked increase in this variable. Plants treated with 30 mg/L had maximal values for total soluble proteins under water deficit conditions. Total soluble proteins increased by 622.31% (WS) and 2010.72% (30 mg/L ALA) in cv. FH-1581 under drought. The increase of 610.13% (WS) and 2025.21% (30 mg/L ALA) was present in cv. FH-1572 under drought (Fig. 2; Table 1S).
Total free amino acids
Higher total free amino acid contents were present in cv. FH-1581 under water deficit conditions. Plants exhibited significant increase (P ≤ 0.001) in total free amino acids when ALA was applied as foliar spray. ALA-induced increase in this variable was concentration dependent. Maximal increase in total free amino acids was evident in plants treated with 30 mg/L ALA. The cv. FH-1581 had an increase of 183.82% (NT), 237.52% (WS) and 474.13% (30 mg/L ALA) under drought. By contrast, cv. FH-1572 displayed 170.14% increase (NT), 230.14% (WS) and 450.25% (30 mg/L ALA) under water deficit conditions (Fig. 2; Table 1S).
Discussion
Aminolevulinic acid (ALA) is an essential precursor in the biosynthesis of chlorophyll molecule and for that reason, low doses of supplemented ALA have been suggested to improve plant growth and stress tolerance (Xiong et al. 2018). Our results displayed that addition of different doses of ALA protected sunflower plants from adverse effects of water deficit stress reflected in the form of significant increase in biomass accumulation. The ALA-mediated increase in stress tolerance is related to lower cellular levels of reactive oxygen species (H2O2), decrease in lipid peroxidation measured in the form of MDA contents, lesser degradation of photosynthetic pigments and an efficient oxidative defense system.
In present study, a significant decline in different growth characteristics was found in sunflower plants subjected to water deficit stress. Plants under water limited environment suffer from nutritional deficiency that could inhibit plant growth and development (Abbas et al. 2018). Our results are in corroboration with previous reports in maize (Naeem et al. 2018). The application of ALA markedly enhanced photosynthetic pigments and biomass accumulation under water stress. Similar findings were reported in Brassica napus L. where significant improvement in growth characteristics and photosynthesis was induced in response to ALA application under NaCl salinity (Xiong et al. 2018). The ALA-mediated improvement in growth might have been due to its significant role in the regulation of important metabolic processes (Akram et al. 2012; Farid et al. 2018).
Chlorophyll is an essential photosynthetic pigment in plants to harvest light and generation of energy in the form of reducing power (Gitelson et al. 2003). Water deficit conditions cause significant decline in chlorophyll contents (Liu et al. 2019). Our results also indicated significant decline in chlorophyll contents in plants under water stress. Enhanced chlorophyll degradation or slow biosynthesis of chlorophyll is the typical sign of oxidative stress in plants grown under limited water conditions (Smirnoff 1993). Further, it was also suggested that measure of chlorophyll contents can be taken as a good selection criterion for screening drought tolerance in different cultivars (Guerfel et al. 2009). Exogenous application of ALA resulted in significant enhancement in chlorophyll contents in both sunflower cultivars under water deficit conditions. It is documented in the literature that addition of low concentration of ALA improves protein biosynthesis, nutrient uptake, antioxidant system, photosynthesis and chlorophyll biosynthesis (Niu and Ma 2018). Efficient oxidative defense system scavenges ROS that directly degrade chlorophyll contents (Akram et al. 2012).
Plants accumulate compatible solutes such as proline as an adaptive mechanism to tolerate abiotic stress (Ashraf and Foolad 2007). In our study, we found significant increase in cellular levels of proline under water deficit conditions. Addition of ALA increased proline contents. Besides, its role as compatible solute, proline also functions as antioxidant scavenging toxic ROS (Islam et al. 2009). Similar report of ALA-mediated increase in proline content was found in Brassica napus L. under salt stress (Xiong et al. 2018). The cellular levels of proline increase with concomitant decline in endogenous levels of ALA in the barely (Averina et al. 2010) because both proline and ALA biosynthesis has common precursor, glutamic acid. Exogenous application of ALA increased ALA contents that might have reduced ALA biosynthesis along with increase in proline biosynthesis. However, whether influence of exogenous application of ALA on proline accumulation is associated with decline in ALA biosynthesis needs further research (Zhang et al. 2015).
Phenolics are important non-enzymatic antioxidant compound that readily scavenges singlet oxygen (Akram et al. 2012). Exogenous application of ALA resulted in substantial increase in phenolics levels under drought stress. Increase in endogenous levels of phenolics protect plants from oxidative damage (Caliskan et al. 2017). Among secondary metabolites, flavonoids are an important defensive secondary metabolite that protects plants from drought-induced oxidative damage (Nakabayashi et al. 2014). Our results also indicated increase in endogenous levels of flavonoids contents in plants treated with different doses of ALA. Supplementation of ALA mediated flavonoid biosynthesis in plants (Liu et al. 2016). The rise in flavonoid due to ALA application was found in apples (Chen et al. 2014). Our study also displayed increased anthocyanin contents in plants treated with exogenous ALA. Similar results on ALA-induced higher anthocyanins were reported in peach (Guo et al. 2013). Anthocyanins possess significant antioxidant activity to scavenge ROS (Stintzing and Carle 2004). Ascorbic acid is significant antioxidant compound present in plant tissues. Further, drought stress negatively affects ascorbic acid biosynthesis in plants (Seminario et al. 2017). Our results manifested large decrease in ascorbic acid contents under water stress. Similar drought-induced reduction in ascorbic acid content has been reported in soybean (Seminario et al. 2017) and wheat (Bartoli et al. 1999). ALA increased ascorbic acid in cv. FH-1572 but not in cv. FH-1581.
A common result of water deficit conditions is osmotic stress in plants. Proline is accumulated in significant amount by drought-stressed plants where proline acts as compatible solute (Akram et al. 2012). However, amino acids also perform other essential functions in plants. For example, amino acids mediate redox homeostasis, gene expression, activities of enzymes, stomatal opening and ion transport in plants under abiotic stress (Kovács et al. 2012). We also recorded significant increase in the buildup of total soluble amino acids in both sunflower cultivars under water stress. Exogenous application of ALA resulted in further increase in this variable. Similarly, Yang et al. (2014) reported increased amino acid accumulation in Creeping Bentgrass in response to ALA application.
Drought stress induces oxidative damage in plants due to incomplete destruction of ROS (Kosar et al. 2015). However, the detoxification of ROS highly relies on better oxidative defense in the form of enzymatic and non-enzymatic compounds (Ashraf 2009; Mahawar and Shekhawat 2019). Our results displayed significant increase in the activities of SOD, CAT and POD. The enzyme SOD is present in peroxisome, mitochondrion, chloroplast and cytoplasm where it reacts with superoxide radicals and converts it into H2O2 (Li et al. 2011). Exogenous ALA has the ability to induce the activity of SOD (Memon et al. 2008). Drought-induced increase in SOD activity was found in Brassica campestris (Memon et al. 2008). Higher SOD activity was seen in plants given ALA under herbicide stress (Zhang et al. 2008). Exogenous application of ALA also increased SOD activity in Brassica napus subjected to salinity (Naeem et al. 2010). The resulting enhanced generation of H2O2 can quickly diffuse across the cell membranes and damages plant growth (Foyer et al. 1997). The disintegration of H2O2 is carried out in microbody and mitochondrion, whereas POD scavenges H2O2 in cytosol and chloroplast (Shigeoka et al. 2002). In previous studies, drought increased CAT and POD activities (Türkan et al. 2005; Manivannan et al. 2008). ALA has been shown to increase the activities of CAT and POD in cucumber under drought and salinity stress (Li et al. 2011; Wu et al. 2019). Our study also indicated decline in H2O2 and MDA contents due to efficient antioxidant system in plants treated with exogenous ALA.
In nutshell, cv. FH-1581 exhibited higher drought tolerance compared with cv. FH-1572 reflected in the form of lower degradation of photosynthetic pigments and efficient oxidative defense system. Exogenous application of ALA enhanced biomass accumulation by protecting chlorophyll degradation and decreasing lipid peroxidation. Furthermore, ALA-mediated decrease in H2O2 and MDA levels was due to enhanced concentration of non-enzymatic antioxidant compounds and higher activities of antioxidant enzymes. The present research opens ways and consequently, needs further research on the influence of ALA application on the expression of stress associated genes.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- POD
Peroxidase
- H2O2
Hydrogen peroxide
- SOD
Superoxide dismutase
- ALA
5-Aminolevulinic acid
- CAT
Catalase
- MDA
Malondialdehyde
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abbas T, Rizwan M, Ali S, Adrees M, Mahmood A, Zia-ur-Rehman M, Ibrahim M, Arshad M, Qayyum MF. Biochar application increased the growth and yield and reduced cadmium in drought stressed wheat grown in an aged contaminated soil. Ecotoxicol Environ Safe. 2018;148:825–833. doi: 10.1016/j.ecoenv.2017.11.063. [DOI] [PubMed] [Google Scholar]
- Akram NA, Ashraf M, Al-Qurainy F. Aminolevulinic acid-induced changes in some key physiological attributes and activities of antioxidant enzymes in sunflower (Helianthus annuus L.) plants under saline regimes. Sci Hortic. 2012;142:143–148. [Google Scholar]
- Alam SA, Huang JG, Stadt KJ, Comeau PG, Dawson A, Gea-Izquierdo G, Aakala T, Holtta T, Vesala T, Makela A, Berninger F. Effects of competition, drought stress and photosynthetic productivity on the radial growth of white spruce in western Canada. Front Plant Sci. 2017;8:1915. doi: 10.3389/fpls.2017.01915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali B, Xu X, Gill RA, Yang S, Ali S, Tahir M, Zhou W. Promotive role of 5-aminolevulinic acid on mineral nutrients and antioxidative defense system under lead toxicity in Brassica napus. Ind Crops Prod. 2014;52:617–626. [Google Scholar]
- Arnon DI. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in beta vulgaris. J Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf M. Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnol Adv. 2009;27:84–93. doi: 10.1016/j.biotechadv.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Ashraf M, Foolad MR. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot. 2007;59:206–216. [Google Scholar]
- Averina NG, Gritskevich ER, Vershilovskaya IV, Usatov AV, Yaronskaya EB. Mechanisms of salt stress tolerance development in barley plants under the influence of 5-aminolevulinic acid. Russ J Plant Physiol. 2010;57:792–798. [Google Scholar]
- Bartoli CG, Simontacchi M, Tambussi E, Beltrano J, Montaldi E, Puntarulo S. Drought and watering-dependent oxidative stress: effect on antioxidant content in Triticum aestivum L. leaves. J Exp Bot. 1999;50:375–383. [Google Scholar]
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–207. [Google Scholar]
- Caliskan O, Radusiene J, Temizel KE, Staunis Z, Cirak C, Kurt D, Odabas MS. The effects of salt and drought stress on phenolic accumulation in greenhouse-grown Hypericum pruinatum. Ital J Agron. 2017;12:271–275. [Google Scholar]
- Chance B, Maehly AC. Methods in Enzymology. Cambridge: Academic Press; 1955. Assay of catalases and peroxidases; pp. 764–775. [Google Scholar]
- Chaves MM, Maroco JP, Pereira JS. Understanding plant responses to drought—from genes to the whole plant. Funct Plant Biol. 2003;30:239–264. doi: 10.1071/FP02076. [DOI] [PubMed] [Google Scholar]
- Chen L, Guo Y, Zhang X, Mi R. Effect of 5-aminolevulinic acid on flavonoids content and expression of CHS and CHI genes of young apples. J Northwest Univ Ed. 2014;42:161–172. [Google Scholar]
- Dutta T, Neelapu NRR, Wani SH, Challa S. Compatible solute engineering of crop plants for improved tolerance toward abiotic stresses. In: Wani SH, editor. Biochemical, physiological and molecular avenues for combating abiotic stress tolerance in plants. London: Academic Press; 2018. pp. 221–254. [Google Scholar]
- Farid M, Ali S, Rizwan M, Ali Q, Saeed R, Nasir T, Abbasi GH, Rehmani MI, Ata-Ul-Karim ST, Bukhari SA, Ahmad T. Phyto-management of chromium contaminated soils through sunflower under exogenously applied 5-aminolevulinic acid. Ecotoxicol Environ Saf. 2018;151:255–265. doi: 10.1016/j.ecoenv.2018.01.017. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Lopez-Delgado H, Dat JF, Scott IM. Hydrogen peroxide-and glutathione-associated mechanisms of acclimatory stress tolerance and signalling. Physiol Plant. 1997;100:241–254. [Google Scholar]
- Gitelson AA, Gritz Y, Merzlyak MN. Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. J Plant Physiol. 2003;160:271–282. doi: 10.1078/0176-1617-00887. [DOI] [PubMed] [Google Scholar]
- Guerfel M, Baccouri O, Boujnah D, Chaïbi W, Zarrouk M. Impacts of water stress on gas exchange, water relations, chlorophyll content and leaf structure in the two main Tunisian olive (Olea europaea L.) cultivars. Sci Hortic. 2009;119:257–263. [Google Scholar]
- Guo L, Cai Z, Zhang B, Xu J, Song H, Ma R. The mechanism analysis of anthocyanin accumulation in peach accelerated by ALA. Acta Hortic Sin. 2013;40:1043–1050. [Google Scholar]
- Hamilton PB, Van Slyke DD, Lemish S. The gasometric determination of free amino acids in blood filtrates by the ninhydrin-carbon dioxide method. J Biol Chem. 1943;150:231–250. [Google Scholar]
- Heath RL, Packer L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Hotta Y, Tanaka T, Takaoka H, Takeuchi Y, Konnai M. New physiological effects of 5-aminolevulinic acid in plants: the increase of photosynthesis, chlorophyll content, and plant growth. Biosci Biotechnol Biochem. 1997;61:2025–2028. doi: 10.1271/bbb.61.2025. [DOI] [PubMed] [Google Scholar]
- Hussain M, Farooq S, Hasan W, Ul-Allah S, Tanveer M, Farooq M, Nawaz A. Drought stress in sunflower: physiological effects and its management through breeding and agronomic alternatives. Agric Water Manag. 2018;201:152–166. [Google Scholar]
- Hussain S, Rao MJ, Anjum MA, Ejaz S, Zakir I, Ali MA, Ahmad N, Ahmad S. Oxidative stress and antioxidant defense in plants under drought conditions. In: Hasanuzzaman M, Hakeem K, Nahar K, Alharby H, editors. Plant abiotic stress tolerance. Cham: Springer; 2019. pp. 207–219. [Google Scholar]
- Islam MM, Hoque MA, Okuma E, Banu MNA, Shimoishi Y, Nakamura Y, Murata Y. Exogenous proline and glycinebetaine increase antioxidant enzyme activities and confer tolerance to cadmium stress in cultured tobacco cells. J Plant Physiol. 2009;166:1587–1597. doi: 10.1016/j.jplph.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Jury WA, Vaux H. The role of science in solving the world’s emerging water problems. Proc Natl Acad Sci USA. 2005;102:15715–15720. doi: 10.1073/pnas.0506467102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosar F, Akram NA, Ashraf M. Exogenously-applied 5-aminolevulinic acid modulates some key physiological characteristics and antioxidative defense system in spring wheat (Triticum aestivum L.) seedlings under water stress. S Afr J Bot. 2015;96:71–77. [Google Scholar]
- Kovács Z, Simon-Sarkadi L, Vashegyi I, Kocsy G. Different accumulation of free amino acids during short- and long-term osmotic stress in wheat. Sci World J. 2012;2012:216521. doi: 10.1100/2012/216521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo A, Aono M, Nakajima N, Saji H, Tanaka K, Kondo N. Differential responses in activity of antioxidant enzymes to different environmental stresses in Arabidopsis thaliana. J Plant Res. 1999;112:279–290. [Google Scholar]
- Li D-M, Zhang J, Sun W-J, Li Q, Dai A-H, Bai J-G. 5-Aminolevulinic acid pretreatment mitigates drought stress of cucumber leaves through altering antioxidant enzyme activity. Sci Hortic. 2011;130:820–828. [Google Scholar]
- Liu D, Pei Z, Naeem M, Ming D, Liu H, Khan F, Zhou W. 5-Aminolevulinic acid activates antioxidative defence system and seedling growth in Brassica napus L. under water-deficit stress. J Agron Crop Sci. 2011;197:284–295. [Google Scholar]
- Liu L, Xiong L, An Y, Zheng J, Wang L. Flavonols induced by 5-aminolevulinic acid are involved in regulation of stomatal opening in apple leaves. Hortic Plant J. 2016;2:323–330. [Google Scholar]
- Liu B, Liang J, Tang G, Wang X, Liu F, Zhao D. Drought stress affects on growth, water use efficiency, gas exchange and chlorophyll fluorescence of Juglans rootstocks. Sci Hortic. 2019;250:230–235. [Google Scholar]
- Mahawar L, Shekhawat GS. EsHO 1 mediated mitigation of NaCl induced oxidative stress and correlation between ROS, antioxidants and HO 1 in seedlings of Eruca sativa: underutilized oil yielding crop of arid region. Physiol Mol Biol Plants. 2019;25(4):895–904. doi: 10.1007/s12298-019-00663-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahawar L, Khator K, Shekhawat GS. Role of Proline in mitigating NaCl induced oxidative stress in Eruca sativa Miller: an important oil yielding crop of Indian Thar Desert. Vegetos. 2018;31(special):55–63. [Google Scholar]
- Mahawar L, Kumar R, Shekhawat GS. Evaluation of heme oxygenase 1 (HO 1) in Cd and Ni induced cytotoxicity and crosstalk with ROS quenching enzymes in two to four leaf stage seedlings of Vigna radiata. Protoplasma. 2018;255(2):527–545. doi: 10.1007/s00709-017-1166-0. [DOI] [PubMed] [Google Scholar]
- Manivannan P, Jaleel CA, Somasundaram R, Panneerselvam R. Osmoregulation and antioxidant metabolism in drought-stressed Helianthus annuus under triadimefon drenching. C R Biol. 2008;331:418–425. doi: 10.1016/j.crvi.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Mao X, Liu M, Wang X, Liu C, Hou Z, Shi J. Effects of deficit irrigation on yield and water use of greenhouse grown cucumber in the North China Plain. Agric Water Manag. 2003;61:219–228. [Google Scholar]
- Marinova D, Ribarova F, Atanassova M. Total phenolics and total favonoids in Bulgarian fruits and vegetables. J Univ Chem Technol Metallurgy. 2005;40:255–260. [Google Scholar]
- Memon SA, Hou X, Wang L, Li Y. Promotive effect of 5-aminolevulinic acid on chlorophyll, antioxidative enzymes and photosynthesis of Pakchoi (Brassica campestris ssp. chinensis var. communis Tsen et Lee) Acta Physiol Plant. 2008;31:51. [Google Scholar]
- Mukherjee SP, Choudhuri MA. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol Plant. 1983;58:166–170. [Google Scholar]
- Naeem MS, Jin ZL, Wan GL, Liu D, Liu HB, Yoneyama K, Zhou WJ. 5-Aminolevulinic acid improves photosynthetic gas exchange capacity and ion uptake under salinity stress in oilseed rape (Brassica napus L.) Plant Soil. 2010;332:405–415. [Google Scholar]
- Naeem MS, Rasheed M, Liu D, Jin ZL, Ming DF, Yoneyama K, Takeuchi Y, Zhou WJ. 5-Aminolevulinic acid ameliorates salinity-induced metabolic, water-related and biochemical changes in Brassica napus L. Acta Physiol Plant. 2011;33:517–528. [Google Scholar]
- Naeem M, Naeem MS, Ahmad R, Ihsan MZ, Ashraf MY, Hussain Y, Fahad S. Foliar calcium spray confers drought stress tolerance in maize via modulation of plant growth, water relations, proline content and hydrogen peroxide activity. Arch Agron Soil Sci. 2018;64:116–131. [Google Scholar]
- Nakabayashi R, Mori T, Saito K. Alternation of flavonoid accumulation under drought stress in Arabidopsis thaliana. Plant Signal Behav. 2014;9:e29518. doi: 10.4161/psb.29518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KH, Mostofa MG, Watanabe Y, Tran CD, Rahman MM, Tran L-SP. Overexpression of GmNAC085 enhances drought tolerance in Arabidopsis by regulating glutathione biosynthesis, redox balance and glutathione-dependent detoxification of reactive oxygen species and methylglyoxal. Environ Exp Bot. 2019;161:242–254. [Google Scholar]
- Niu K, Ma H. The positive effects of exogenous 5-aminolevulinic acid on the chlorophyll biosynthesis, photosystem and calvin cycle of Kentucky bluegrass seedlings in response to osmotic stress. Environ Exp Bot. 2018;155:260–271. [Google Scholar]
- Seminario A, Song L, Zulet A, Nguyen HT, González EM, Larrainzar E. Drought stress causes a reduction in the biosynthesis of ascorbic acid in soybean plants. Front Plant Sci. 2017;8:1042. doi: 10.3389/fpls.2017.01042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao HB, Chu LY, Jaleel CA, Zhao CX. Water-deficit stress-induced anatomical changes in higher plants. CR Biol. 2008;331:215–225. doi: 10.1016/j.crvi.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Shekhawat GS, Prasad A, Verma K, Sharma A. Changes in growth, lipid peroxidation and antioxidant system in seedlings of Brassica juncea (L.) czern. under cadmium stress. Biochem Cell Arch. 2008;8:145–149. [Google Scholar]
- Shigeoka S, Ishikawa T, Tamoi M, Miyagawa Y, Takeda T, Yabuta Y, Yoshimura K. Regulation and function of ascorbate peroxidase isoenzymes. J Exp Bot. 2002;53:1305–1319. [PubMed] [Google Scholar]
- Smirnoff N. The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol. 1993;125:27–58. doi: 10.1111/j.1469-8137.1993.tb03863.x. [DOI] [PubMed] [Google Scholar]
- Stintzing FC, Carle R. Functional properties of anthocyanins and betalains in plants, food, and in human nutrition. Trends Food Sci Technol. 2004;15:19–38. [Google Scholar]
- Sun Y-P, Zhang Z-P, Wang L-J. Promotion of 5-aminolevulinic acid treatment on leaf photosynthesis is related with increase of antioxidant enzyme activity in watermelon seedlings grown under shade condition. Photosynthetica. 2009;47:347. [Google Scholar]
- Sun CX, Gao XX, Fu JQ, Zhou JH, Wu XF. Metabolic response of maize (Zea mays L.) plants to combined drought and salt stress. Plant Soil. 2015;388:99–117. [Google Scholar]
- Türkan İ, Bor M, Özdemir F, Koca H. Differential responses of lipid peroxidation and antioxidants in the leaves of drought-tolerant P. acutifolius Gray and drought-sensitive P. vulgaris L. subjected to polyethylene glycol mediated water stress. Plant Sci J. 2005;168:223–231. [Google Scholar]
- van Rossum MWPC, Alberda M, van der Plas LHW. Role of oxidative damage in tulip bulb scale micropropagation. Plant Sci J. 1997;130:207–216. [Google Scholar]
- Velikova V, Yordanov I, Edreva A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci J. 2000;151:59–66. [Google Scholar]
- Wang LJ, Jiang WB, Liu H, Liu WQ, Kang L, Hou XL. Promotion by 5-aminolevulinic acid of germination of pakchoi (Brassica campestris ssp. chinensis var. communis Tsen et Lee) seeds under salt stress. J Integr Plant Biol. 2005;47(9):1084–1091. [Google Scholar]
- Wang Y, Li J, Gu W, Zhang Q, Tian L, Guo S, Wei S. Exogenous application of 5-aminolevulinic acid improves low-temperature stress tolerance of maize seedlings. Crop Pasture Sci. 2018;69(6):587–593. [Google Scholar]
- Wolfe K, Wu X, Liu RH. Antioxidant activity of apple peels. J Agric Food Chem. 2003;51:609–614. doi: 10.1021/jf020782a. [DOI] [PubMed] [Google Scholar]
- Wu Y, Hu L, Liao W, Dawuda MM, Lyu J, Xie J, Feng Z, Calderón-Urrea A, Yu J. Foliar application of 5-aminolevulinic acid (ALA) alleviates NaCl stress in cucumber (Cucumis sativus L.) seedlings through the enhancement of ascorbate-glutathione cycle. Sci Hort. 2019;257:108761. [Google Scholar]
- Xiong J-L, Wang H-C, Tan X-Y, Zhang C-L, Naeem MS. 5-aminolevulinic acid improves salt tolerance mediated by regulation of tetrapyrrole and proline metabolism in Brassica napus L. seedlings under NaCl stress. Plant Physiol Biochem. 2018;124:88–99. doi: 10.1016/j.plaphy.2018.01.001. [DOI] [PubMed] [Google Scholar]
- Yang Z, Chang Z, Sun L, Yu J, Huang B. Physiological and metabolic effects of 5-aminolevulinic acid for mitigating salinity stress in creeping bentgrass. PLoS ONE. 2014;9:e116283. doi: 10.1371/journal.pone.0116283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yordanov I, Velikova V, Tsonev T. Plant responses to drought, acclimation, and stress tolerance. Photosynthetica. 2000;38:171–186. [Google Scholar]
- Zhang WF, Zhang F, Raziuddin R, Gong HJ, Yang ZM, Lu L, Ye QF, Zhou WJ. Effects of 5-aminolevulinic acid on oilseed rape seedling growth under herbicide toxicity stress. J Plant Growth Regul. 2008;27:159–169. [Google Scholar]
- Zhang J, Li D-M, Gao Y, Yu B, Xia C-X, Bai J-G. Pretreatment with 5-aminolevulinic acid mitigates heat stress of cucumber leaves. Biol Plantarum. 2012;56:780–784. [Google Scholar]
- Zhang Z-P, Miao M-M, Wang C-L. Effects of ALA on photosynthesis, antioxidant enzyme activity, and gene expression, and regulation of proline accumulation in tomato seedlings under NaCl stress. J Plant Growth Regul. 2015;34:637–650. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.