Abstract
To study the possibility of increasing the drought tolerance of common bean with the exogenous application of 24-epibrassinolide (EBL), an experiment was conducted in 2016 and 2017. In this experiment, two irrigation levels (optimal irrigation and drought stress) were applied to the main plots and two common bean genotypes (Kusha cultivar and COS16 genotype) and four EBL concentrations (0, 2, 4, and 6 μM) were allocated to sub-plots as factorial. In the flowering stage, drought stress was applied and plants were sprayed with EBL. The results showed that drought stress reduced relative water content (RWC) and increased proline content, malondialdehyde (MDA) content, and antioxidant enzymes activity. However, exogenous application of EBL reduced the seed yield loss and increased the drought stress tolerance in both common bean genotypes by decreasing the MDA content and increasing the RWC, proline content, antioxidant enzymes activity, and nitrate reductase activity. It can be concluded that foliar spray of 4 µM EBL as the best concentration may increase the seed yield and enhance the drought stress tolerance of common bean. Also, Cu/Zn-SOD was up-regulated in response to the drought stress and exogenous EBL. The COS16 genotype showed better response to the drought stress and exogenous EBL than the Kusha cultivar, because of the higher up-regulation of Cu/Zn-SOD in this genotype compared to the Kusha cultivar. Therefore, EBL can be used as a plant growth regulator to enhance drought stress tolerance and minimize the seed yield loss of common bean caused by water deficit.
Keywords: Antioxidant enzymes activity, Gene expression, NR activity, Phylogenetic analysis, Seed yield reduction
Introduction
Drought stress is one of the most important environmental stresses that limits the production of plants around the world and has adverse effects on plant growth and other metabolic processes. More than 50% of the yield of many plants decreases due to drought stress (Zlatev and Lidon 2012). Beans are one of the main sources of protein in arid and semi-arid regions of the world, and they are very important in the agricultural economy of these regions (Tuba Bicer et al. 2004). Common bean is susceptible to drought stress and its yield is harmed even in short periods of stress. Drought stress reduces the water content, decreases the leaf water potential, and decreases the cell growth. Extreme drought stress can also result in interfering with photosynthesis, metabolism disturbance, and eventually plant death (Jaleel et al. 2009).
One of the reasons that environmental stresses such as drought, reduces growth and plant photosynthesis ability is the disturbance in the balance between the production of reactive oxygen species and the protective mechanisms that remedy these radicals, which results in the accumulation of reactive oxygen species, induction of oxidative stress, damage to proteins, membrane lipids, and other cellular components (Mittler 2002). In adverse environmental conditions, the role of the antioxidant defense system is very important in protecting against oxidative damage to the cell membrane and other growing plant organs (Al-Ghamdi 2009). The plants use both enzymatic and non-enzymatic methods to eliminate oxidative stress (Gupta et al. 2005). The enzymatic antioxidant defense system contains enzymes such as superoxide dismutase (SOD), catalase (CAT), guaiacol peroxidase (POD), ascorbate peroxidase (APX), and glutathione reductase (GR) which are key antioxidant enzymes for the elimination of reactive oxygen species. SOD is considered as a first defense line against reactive oxygen species, which eliminates radical superoxide and converts it into oxygen and hydrogen peroxide, and thus stabilizes the membrane of plant cells in the stress (Nazari et al. 2012; Sharma et al. 2012). In the next step, the produced hydrogen peroxide is cleaved by CAT, POD, and APX enzymes (Zeid and Shedeed 2006). Plant tolerance to environmental stresses, including drought, has a positive and significant correlation with the increase in antioxidant contents (Malik et al. 2010). Thus, knowledge of the changes in proteins and enzymes under drought stress conditions may be useful to identify effective physiological traits in producing tolerant genotypes.
Various environmental factors that cause oxidative stress in plants usually activate antioxidant enzymes at the transcriptional level (Jithesh et al. 2006). The relationship between SOD and drought stress has been extensively studied at the gene level. For example, to investigate the expression of Caragana Cu/Zn-SOD under drought stress, 7-week-old seedlings were treated with Hoagland medium containing 5, 15, or 25% PEG, and RNA isolated from treated plants was used to investigate the expression level of Cu/Zn-SOD. Reverse transcription PCR (RT-PCR) revealed that expression of Caragana Cu/Zn-SOD was induced by PEG-simulated drought stress, and results showed that the Caragana Cu/Zn-SOD plays an important role in degrading reactive oxygen species and increasing drought stress tolerance (Zhang et al. 2014). Transgenic experiments also show that SOD plays an important role in response to water stress in plants. For example, overexpression of a cytosolic Cu/Zn-SOD, cloned from Potentilla atrosanguinea (PaSOD), in potato (cv. Kufri Sutlej) resulted in an increase in photosynthesis and stomatal conductance compared with these traits in wt plants under both irrigation and drought stress conditions (Pal et al. 2013).
Brassinosteroids (BRs) are a group of polyhydroxysteroids that have been implicated in a wide range of physiological processes and morphological responses in plants, such as cell division and stem elongation, growth of pollen tubes, activation of enzymes, induction of ethylene biosynthesis, leaf aging, proton pump activation, nucleic acid and protein synthesis, regulation of assimilation and carbon allocation, photosynthesis activation, flower initiation, flower and fruit development, the regulation of gene expression, and response to different stresses (Talaat and Shawky 2012). In addition, BRs can protect against the effects of abiotic stresses such as drought, salinity, high temperatures, and heavy metals by activating various mechanisms in plants and increasing the activity of enzymatic antioxidants such as CAT, SOD, POD, and GR (Sytar et al. 2019; Zhang et al. 2008). BRs can use some of the reactive oxygen species, such as H2O2, as intermediates for inducing transcription of antioxidant genes (Kang and Guo 2011). The effect of BRs on the increasing of the activity of antioxidant systems in plant cells and the activation of plant resistance to oxidative damage caused by environmental conditions such as drought, salinity, heavy metals, and high and low temperatures have been reported in some studies (Bajguz and Hayat 2009; Ding et al. 2012; Li et al. 2012). Different studies have shown that the external use of BRs increase the tolerance of drought to plants by affecting the protein contents, NR activity, ethylene production, polyamines storage, and RWC (Anjum et al. 2011; Talaat and Shawky 2016; Yuan et al. 2010).
Since the effects of EBL can be monitored at both antioxidant and molecular levels and considering the importance of BRs in increasing the tolerance to environmental stresses, the aim of this study was to investigate the effect of EBL on some of the physiological traits and Cu/Zn-SOD expression of two common bean genotypes, under optimal irrigation and drought stress conditions and the possibility of increasing the tolerance of common bean under drought stress with the use of EBL. We further examined the effects of EBL at different concentrations to see whether or not the effects of this hormone are concentration-dependent.
Materials and methods
Growth conditions, treatments, and application of treatments
This two-year field experiment was carried out at the Research Farm of the Faculty of Agriculture, University of Zanjan (36° 41′ N, 48° 29′ E), Zanjan, Iran in 2016 and 2017. The precipitation, average temperature, average minimum and maximum temperature, and average relative humidity during 2016 and 2017 are shown in Table 1.
Table 1.
Precipitation, average temperature, average minimum and maximum temperature, and average relative humidity during 2016 and 2017
| Parameters | January | February | March | April | May | June | July | August | September | October | November | December |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2016 | ||||||||||||
| Precipitation (mm) | 15.9 | 31.8 | 31.6 | 83.2 | 6.6 | 0.5 | 1.1 | 0 | 2.9 | 16.6 | 73.6 | 14.6 |
| Average temperature (°C) | 2.4 | 2.3 | 8.8 | 10.8 | 16.7 | 22.3 | 26.6 | 25.9 | 21.5 | 17.3 | 9.5 | 2.5 |
| Average minimum temperature (°C) | − 2.8 | − 3.2 | 2.6 | 4.2 | 8.4 | 12.9 | 18.5 | 16.1 | 12.7 | 9.1 | 4.6 | − 2.8 |
| Average maximum temperature (°C) | 7.7 | 7.9 | 15.0 | 17.4 | 25.0 | 31.8 | 34.6 | 35.8 | 30.3 | 25.4 | 14.3 | 7.9 |
| Average relative humidity (%) | 64 | 63 | 56 | 56 | 46 | 44 | 41 | 36 | 50 | 52 | 66 | 65 |
| 2017 | ||||||||||||
| Precipitation (mm) | 9.7 | 27.2 | 42 | 62 | 28.1 | 15.9 | 1.6 | 0 | 0 | 0.1 | 22.6 | 21.9 |
| Average temperature (°C) | 1.7 | − 0.5 | 4.3 | 9.5 | 16.5 | 19.9 | 24.7 | 25.6 | 22.6 | 15.6 | 11.3 | 1.81 |
| Average minimum temperature (°C) | − 4.5 | -5.2 | − 2.4 | 3.7 | 8.6 | 11.1 | 16.5 | 16.3 | 12.8 | 6.1 | 4.3 | − 4.4 |
| Average maximum temperature (°C) | 7.8 | 4.1 | 11 | 15.3 | 24.4 | 28.6 | 32.9 | 34.8 | 32.5 | 25.1 | 18.2 | 8 |
| Average relative humidity (%) | 62 | 67 | 60 | 61 | 55 | 47 | 46 | 42 | 42 | 46 | 55 | 54 |
Four EBL concentrations consisting of 0 or control, 2, 4, and 6 µM 24-epibrassinolide (C28H48O6, Molar mass 480.68 g mol−1, Sigma-Aldrich, USA) and two irrigation levels including optimal irrigation and drought stress were applied on two common bean genotypes (Kusha cultivar and COS16 genotype). The Kusha cultivar was an indeterminate upright cultivar (Type II); however, the COS16 genotype was a determinate upright genotype (Type I). A three-replicate split factorial design based on randomized complete block design (RCBD) was conducted, in which main plots consisted of the two irrigation levels, subplots consisted of the two common bean genotypes and four foliar EBL application concentrations.
In the flowering stage, simultaneously for both genotypes (in July), drought stress was applied, and simultaneously with drought stress, different concentrations of EBL were sprayed on the leaves of the common bean plants three times every 4 days in the final daylight h to avoid hormone damage caused by intense light. In order to apply drought stress, at the flowering stage, irrigation was cut, and continued till the soil water potential was reached − 1.5 MPa (Contour-Ansel et al. 2010), and then re-irrigation was performed. In both years, each sub-plot consisted of four rows, each measuring 3 m long and 0.5 m apart and planted at a rate of 40 seeds m−2.
Measurements
Estimation of RWC
The RWC was measured at the peak of the drought stress and after hormone spraying, using the following formula (Hayat et al. 2007):
| 1 |
Determination of proline content
The proline content was measured in fresh leaves at the peak of the drought stress and after hormone spraying (Bates et al. 1973).
Determination of MDA content
The MDA content was measured in fresh leaves at the peak of the drought stress and after hormone spraying (Heath and Packer 1968).
Determination of antioxidant enzymes activity
The CAT, POD, APX, and SOD activities were measured in three technical replicates at the peak of the drought stress and after hormone spraying. For analysis of CAT, POD, and SOD activities, fresh leaves were homogenized in 50 mM phosphate buffer (pH 7.0) containing 1% (w/v) polyvinylpyrrolidone (PVP) and centrifuged at 10,000×g for 20 min at 4 °C. For analysis of APX activity, 2.0 mM ascorbate was added to the homogenizing medium. The supernatant was collected for assay of antioxidant enzymes activity.
The CAT reaction mixture contained 50 mM phosphate buffer (pH 7.0), 10 mM H2O2, and enzyme extract. The specific activity of the enzyme was expressed in terms of micromolar H2O2 in min per mg of soluble protein (Chance and Maehly 1955).
The POD reaction mixture contained 50 mM phosphate buffer (pH 7.0), 0.25% (v/v) guaiacol, 0.75% (v/v) H2O2, and enzyme extract. The specific activity of the enzyme was expressed in terms of micromolar tetraguaiacol in min per mg of soluble protein (Chance and Maehly 1955).
The SOD reaction mixture contained 50 mM phosphate buffer (pH 7.8), 13 mM methionine, 75 mM nitroblue tetrazolium chloride (NBT), 300 µM EDTA, 2 mM riboflavin, and 0, 50, 100, 150, and 200 µM enzyme extract. One unit of SOD activity was defined as the amount of enzyme required to cause 50% inhibition of NBT photo reduction (Beauchamp and Fridovich 1971).
The APX reaction mixture contained 50 mM phosphate buffer (pH 7.0), 1 mM ascorbate, 1 mM H2O2, and enzyme extract. The specific activity of the enzyme was expressed in terms of changes in absorbance in min per mg of soluble protein (Nakano and Asada 1981).
Determination of NR activity
The NR activity was measured at the peak of the drought stress and after hormone spraying. Approximately 0.2 g of common bean leaves were homogenized with a mortar and pestle at 4 °C in 2 ml of buffer A [50 mM Hepes–KOH (pH 7.5), 5% (v/v) glycerol, 10 mM MgCl2, 1 mM dithiothreitol (DTT), 1 mM phenylmethyl-sulfonyl fluoride (PMSF), 1 mM benzamidine, and 10 µM FAD]. After centrifugation at 15,000×g for 20 min, 1 ml of supernatant was added to 1 ml of buffer B (50 mM Hepes–KOH (pH 7.5), 10 mM MgCl2, 1 mM DTT, 2 mM KNO3, and 200 µM NADH) and pre incubated at 30 °C for 5 min. The reactions were stopped by adding 200 µl of 0.5 M zinc acetate, 600 µl of 1% (w/v) sulfanilamide, and 600 µl of 0.02% (w/v) N-(1-naphthyl) ethylenediamine dihydrochloride, the mixtures were centrifuged at 15,000×g for 2 min and nitrite content was determined at 540 nm. The specific activity of the enzyme was expressed in terms of micromolar NO2 in h per mg of soluble protein (MacKintosh et al. 1995).
Determination of the amount of soluble proteins in enzymes extracts
To measure the amount of soluble protein in extracts, 50 µl of extract was added to 2950 µl of Bradford solution and was completely stirred. After 10 min, the absorbance of the mixture at 595 nm was measured by a spectrophotometer (Lambda 25; PerkinElmer, Waltham, MA, USA). Bovine serum albumin (BSA) in the 10–100 µg ml−1 range was used as the standard for determining protein concentration (Bradford 1976).
Determination of seed yield and seed yield loss
In the physiological maturity stage, plants in an area of 2 m2 were harvested, and the seed yield were determined and the seed yield loss were calculated.
Study of Cu/Zn-SOD expression
To study the expression of Cu/Zn-SOD, after analyzing the results of the SOD activity, concentrations of EBL, in which the activity of this enzyme was the lowest and highest (0 and 6 μM, respectively), were selected and investigated in two optimal irrigation and drought stress conditions and two common bean genotypes.
Total RNA was isolated using CTAB method (Majidi et al. 2013). The quality and concentration of each of the RNA samples were determined using Nanodrop ND-2000 spectrophotometer (Thermo Scientific, Waltham, MA, USA). The 4.0 μg aliquot of DNase I (Thermo Fisher Scientific, Waltham, MA, USA) treated total RNA was reverse-transcribed using Hyperscript RT-PCR Mastermix (GeneAll, Seoul, South Korea) and oligo-dT primers according to the manufacturer’s instructions.
Gene-specific real time PCR primers were designed using Primer Premier 5 (Premier Biosoft, Palo Alto, CA, USA) and Actin was used as reference gene (Borges et al. 2012) (Table 2). The real-time quantification of the first strand cDNA was performed in three technical replicates on the Rotor-Gene Q Real-Time PCR System (Qiagen, Hilden, Germany). The reaction mixture (20 μl) contained 2 μl of cDNA, 0.5 μM of each primer, and 4 μl of HOT FIREPOL Eva Green qPCR Mix Plus (no ROX) (Solis BioDyne, Tartu, Estonia). For a negative control reaction, no cDNA was added to the reaction mixture, which resulted in non-noticeable fluorescence signal from the reaction. qRT-PCR conditions were set as follows: initial denaturation for 15 min at 95 °C, followed by 40 cycles of denaturation at 95 °C for 15 s, annealing at 56 °C for 20 s and extension at 72 °C for 20 s (Schmittgen and Livak 2008). After amplification cycles, the melting curves were evaluated for each reaction to confirm the specificity of amplified products. The Ct value and melting curve for each reaction were determined with the Rotor-Gene Software ver.2.1.0. The differential expression of target gene (CSD) was calculated using Ct and the 2−ΔΔCt formula (Livak and Schmittgen 2001).
Table 2.
Primer sequences of reference (ACT) and target (CSD) genes used in qRT-PCR
| Gene name | Accession number | Primer sequence (5′ → 3′) | Primer length (nt) | Ta (°C) | Product size | |
|---|---|---|---|---|---|---|
| CSD | XM_007147050.1 | Forward | ACAGCCAGATTCCTCTTACT | 20 | 56 | 153 |
| Reverse | GCAGACCAATGATACCACAG | 20 | ||||
| ACT | KF033476.1 | Forward | GAGACCTTCAACACTCCTGCTA | 22 | 56 | 131 |
| Reverse | CCTTCATAGATAGGAACCGTGT | 22 |
Phylogenetic analysis
Phylogenetic analysis was performed using MEGA7 software and Neighbor-joining method. Finally, the Softberry online tool was used to confirm the prediction of the probable place of the Cu/Zn-SOD activity (www.softberry.com). Bootstrap resampling was used to estimate branch-length standard errors and the support values of nodes (Efron and Gong 1983).
Investigation of cis-regulatory elements in the upstream regions of CSD
Identifying the factors controlling the expression of genes plays an important role in examining how plants respond to stress. So far, many efforts have been made to identify molecular factors and genes involved in response to stress. However, the identification of genes alone is not enough and how expression of genes are regulated and how genes respond is one of the most important questions in this regard. Therefore, studying the promoter region of these genes is very important for the presence of different regulatory elements that induce gene expression. After identification of the CSD in common bean, the sequence of 500 upstream nucleotides of this gene was extracted from NCBI and investigated to identify cis-regulatory elements (www.ncbi.nlm.nih.gov). Then, the upstream regions of this gene were studied using web promoter review tools to investigate the cis-regulatory elements, motifs, and sites accepting elements that are attached to the motifs (Higo et al. 1999).
Data analysis
A combined analysis of variances over the data obtained from the two-year study was done using the general linear model (GLM) in SAS software (Version 9.1), and means were compared using Duncan’s multiple range tests (p ≤ 0.05). Also, Excel (2013) software was used to draw charts.
Results
RWC
The Kusha cultivar in optimal irrigation had the highest RWC and the Kusha cultivar and the COS16 genotype—both of them in drought stress—had the lowest RWC (Fig. 1). Drought stress decreased the RWC in the COS16 genotype and the Kusha cultivar by 28.70% and 34.26% respectively in contrast with the optimal irrigation (Fig. 1). Moreover, the application of 2, 4, and 6 µM EBL in the optimal irrigation increased the RWC by 7.12, 6.01, and 3.68% compared with the control respectively, but in the drought stress, it increased the RWC by 20.10, 25.79, and 16.77% respectively compared with the control (Fig. 2).
Fig. 1.
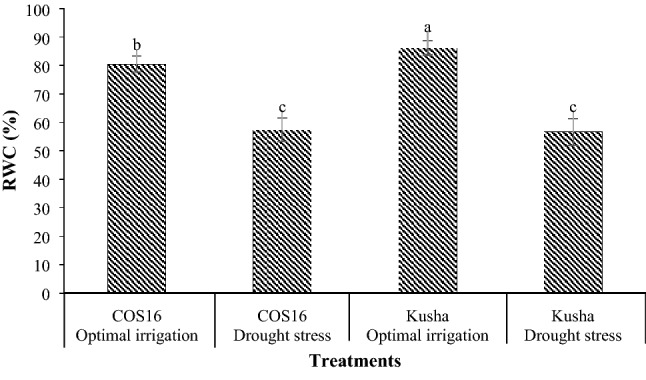
Mean comparison for interaction effects of irrigation levels × genotypes on RWC. Mean values sharing similar letter(s) are not significant at p ≤ 0.05, according to Duncan’s multiple range tests
Fig. 2.
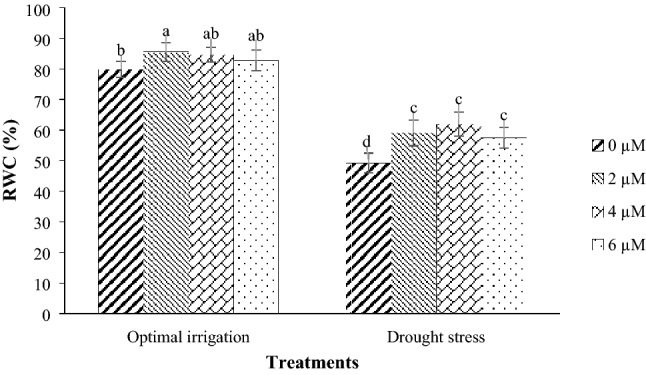
Mean comparison for interaction effects of irrigation levels × hormone spraying on RWC. Mean values sharing similar letter(s) are not significant at p ≤ 0.05, according to Duncan’s multiple range tests
Proline content
Drought stress in the 2016 and the 2017 periods increased the proline content by 126.06% and 31.06% respectively in contrast with the optimal irrigation (Fig. 3). Moreover, the application of different concentrations of EBL increased the proline content compared with the control (Table 3). The application of 4 µM EBL resulted in the highest increase in proline content by 49.01% in contrast with the control, but it was not significant with the concentration of 2 µM EBL (Table 3).
Fig. 3.
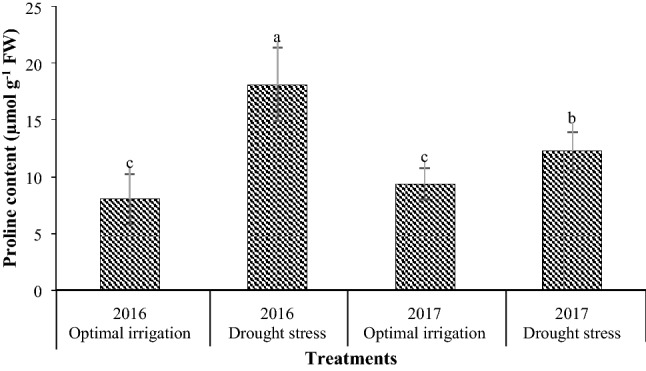
Mean comparison for interaction effects of years × irrigation levels on proline content. Mean values sharing similar letter(s) are not significant at p ≤ 0.05, according to Duncan’s multiple range tests
Table 3.
Mean comparison for different traits of two common bean genotypes evaluated at two levels of irrigation and four concentrations of exogenous EBL in 2 years
| Traits | Years | Irrigation levels | Genotypes | Exogenous EBL (µM) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2016 | 2017 | Optimal irrigation | Drought stress | COS16 | Kusha | Control | 2 | 4 | 6 | |
| RWC (%) | 70.19 ± 17.32 a | 70.03 ± 12.83 a | 83.25 ± 6.04 a | 56.96 ± 8.66 a | 68.87 ± 13.57 b | 71.35 ± 16.65 a | 64.57 ± 16.67 b | 72.37 ± 15.29 a | 73.32 ± 13.29 a | 70.17 ± 14.58 a |
| Proline content (µmol g−1 FW) | 13.08 ± 7.47 a | 10.82 ± 3.36 b | 8.69 ± 3.70 a | 15.21 ± 5.86 a | 11.86 ± 5.43 a | 12.04 ± 6.33 a | 9.63 ± 5.59 c | 12.56 ± 5.44 ab | 14.35 ± 6.72 a | 11.27 ± 4.88 bc |
| MDA content (µmol g−1 FW) | 2.68 ± 1.34 b | 4.52 ± 1.00 a | 3.02 ± 1.41 a | 4.18 ± 1.37 a | 3.70 ± 1.34 a | 3.50 ± 1.65 a | 4.12 ± 1.37 a | 3.54 ± 1.39 ab | 3.49 ± 1.43 b | 3.25 ± 1.73 b |
| CAT activity (µmol mg−1 Pr min−1) | 0.022 ± 0.008 a | 0.021 ± 0.007 a | 0.018 ± 0.005 b | 0.025 ± 0.008 a | 0.022 ± 0.008 a | 0.021 ± 0.007 a | 0.016 ± 0.005 b | 0.021 ± 0.006 a | 0.025 ± 0.008 a | 0.024 ± 0.007 a |
| POD activity (µmol mg−1 Pr min−1) | 1.25 ± 0.57 a | 1.13 ± 0.43 a | 0.90 ± 0.30 a | 1.48 ± 0.50 a | 1.35 ± 0.56 a | 1.03 ± 0.39 b | 0.89 ± 0.27 b | 1.28 ± 0.50 a | 1.33 ± 0.57 a | 1.26 ± 0.53 a |
| APX activity (µmol mg−1 Pr min−1) | 1.58 ± 0.55 a | 2.04 ± 0.85 a | 1.54 ± 0.63 b | 2.07 ± 0.77 a | 1.85 ± 0.70 a | 1.77 ± 0.80 a | 1.26 ± 0.44 c | 1.62 ± 0.62 b | 2.25 ± 0.78 a | 2.10 ± 0.70 a |
| SOD activity (U mg−1 Pr) | 1306.01 ± 436.01 a | 1021.38 ± 160.15 b | 1063.29 ± 307.57 a | 1264.11 ± 376.74 a | 1162.61 ± 330.82 a | 1164.79 ± 384.29 a | 891.16 ± 292.80 b | 1192.78 ± 252.89 a | 1266.40 ± 323.01 a | 1304.45 ± 401.67 a |
| NR activity (µmol NO2 mg−1 Pr h−1) | 7.00 ± 2.03 a | 2.20 ± 0.70 b | 5.01 ± 2.99 a | 4.19 ± 2.66 a | 4.39 ± 2.97 a | 4.82 ± 2.73 a | 3.54 ± 2.61 b | 4.58 ± 2.66 a | 5.20 ± 2.94 a | 5.09 ± 3.02 a |
Mean values sharing similar letter(s) in a parameter are not significant at p ≤ 0.05, according to Duncan’s multiple range tests
Values in the table are mean ± SD
RWC relative water content, MDA malondialdehyde, CAT catalase, POD guaiacol peroxidase, APX ascorbate peroxidase, SOD superoxide dismutase, NR nitrate reductase
MDA content
Drought stress in the 2016 and the 2017 periods increased the MDA content by 93.96% and 14.22% respectively in contrast with the optimal irrigation (Fig. 4). Moreover, the application of 2, 4, and 6 µM EBL in the optimal irrigation decreased the MDA content by 16.08, 15.20, and 15.20% compared with the control respectively, but in the drought stress, it decreased the MDA content by 12.27, 15.18, and 25.16% respectively compared with the control (Fig. 5).
Fig. 4.
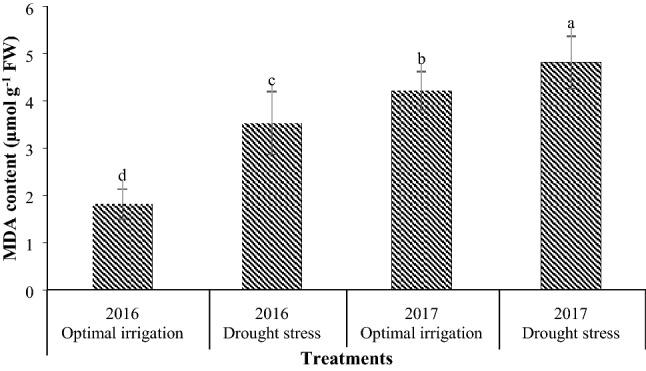
Mean comparison for interaction effects of years × irrigation levels on MDA content. Mean values sharing similar letter(s) are not significant at p ≤ 0.05, according to Duncan’s multiple range tests
Fig. 5.
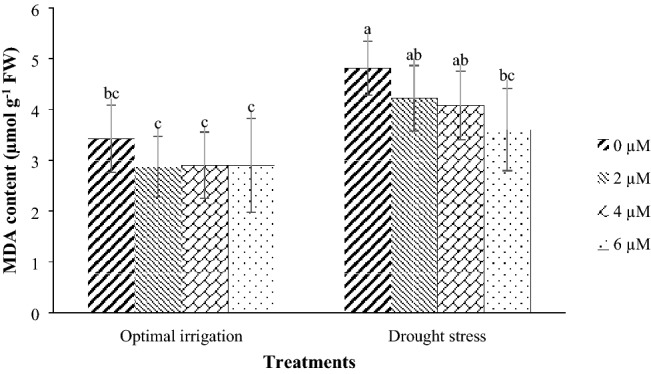
Mean comparison for interaction effects of irrigation levels × hormone spraying on MDA content. Mean values sharing similar letter(s) are not significant at p ≤ 0.05, according to Duncan’s multiple range tests
Antioxidant enzymes activity
Drought stress increased the CAT and APX activities by 38.89% and 34.42% respectively in contrast with the optimal irrigation (Table 3). Drought stress in the 2016 and the 2017 periods increased the POD activity by 84.09% and 47.25% respectively in contrast with the optimal irrigation (Fig. 6). Also, the POD activity in the COS16 genotype was higher than that in the Kusha cultivar (Table 3). Drought stress in the 2016 and the 2017 periods increased the SOD activity by 27.37% and 8.92% respectively in contrast with the optimal irrigation (Fig. 7). Moreover, the application of different concentrations of EBL increased the CAT, POD, APX, and SOD activities compared with the control (Table 3). The application of 2, 4, and 6 µM EBL in the optimal irrigation increased the CAT activity by 38.46, 46.15, and 46.15% compared with the control respectively, but in the drought stress, it increased the CAT activity by 38.89, 66.67, and 55.56% respectively compared with the control (Fig. 8). Also, the application of 2, 4, and 6 µM EBL in the optimal irrigation increased the POD activity by 32.39, 38.03, and 33.80% compared with the control respectively, but in the drought stress, it increased the POD activity by 50.47, 57.01, and 45.79% respectively compared with the control (Fig. 9). The application of 4 µM EBL resulted in the highest increase in the APX activity by 78.57% in contrast with the control, but it was not significant with the concentration of 6 µM EBL (Table 3). Also, the application of 6 µM EBL resulted in the highest increase in the SOD activity by 46.38% in contrast with the control, but it was not significant with the other concentrations of EBL (Table 3).
Fig. 6.
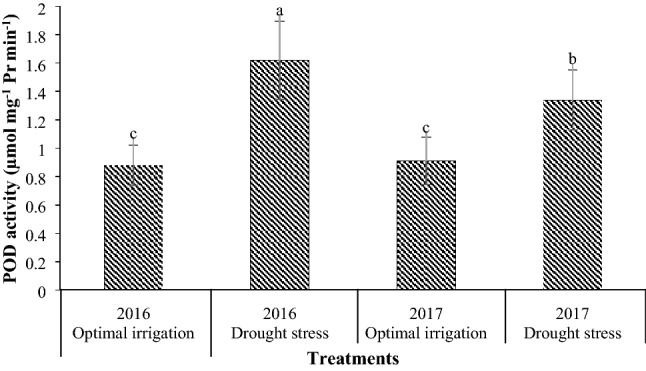
Mean comparison for interaction effects of years × irrigation levels on POD activity. Mean values sharing similar letter(s) are not significant at p ≤ 0.05, according to Duncan’s multiple range tests
Fig. 7.
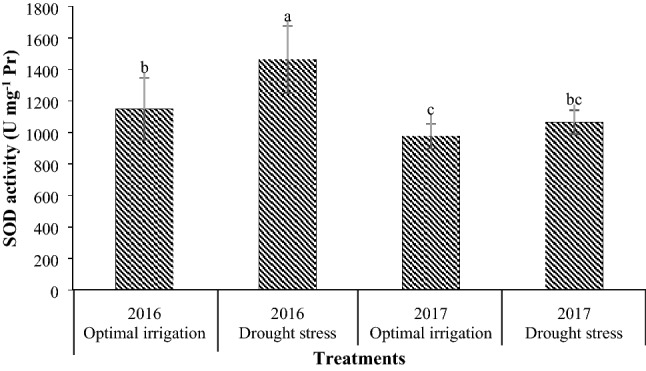
Mean comparison for interaction effects of years × irrigation levels on SOD activity. Mean values sharing similar letter(s) are not significant at p ≤ 0.05, according to Duncan’s multiple range tests
Fig. 8.
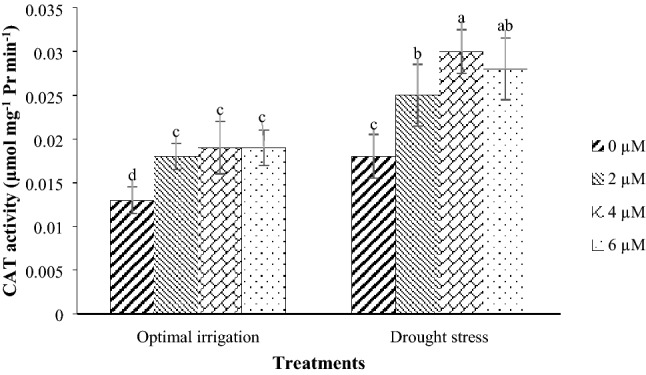
Mean comparison for interaction effects of irrigation levels × hormone spraying on CAT activity. Mean values sharing similar letter(s) are not significant at p ≤ 0.05, according to Duncan’s multiple range tests
Fig. 9.
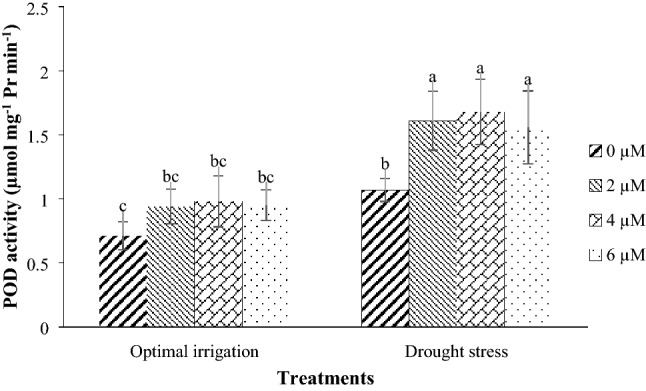
Mean comparison for interaction effects of irrigation levels × hormone spraying on POD activity. Mean values sharing similar letter(s) are not significant at p ≤ 0.05, according to Duncan’s multiple range tests
NR activity
The NR activity in 2016 was higher than that in 2017 (Table 3). Moreover, the application of 2, 4, and 6 µM EBL in the optimal irrigation increased the NR activity by 15.13, 26.95, and 32.15% compared with the control respectively, but in the drought stress, it increased the NR activity by 50.53, 76.14, and 61.05% respectively compared with the control (Fig. 10).
Fig. 10.
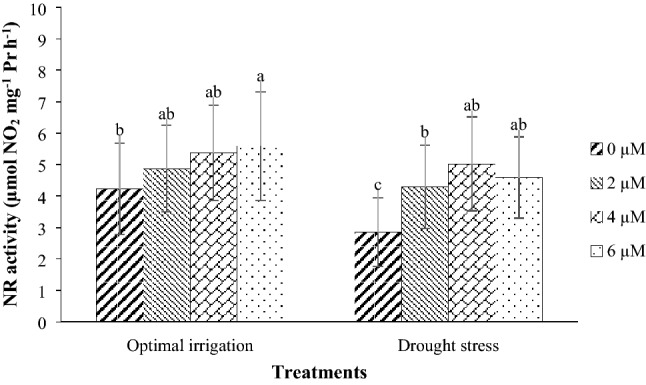
Mean comparison for interaction effects of irrigation levels × hormone spraying on NR activity. Mean values sharing similar letter(s) are not significant at p ≤ 0.05, according to Duncan’s multiple range tests
Cu/Zn-SOD expression
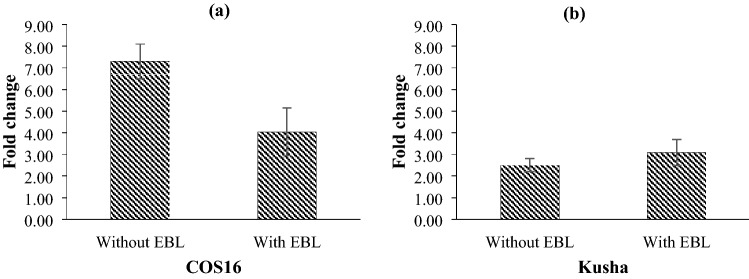
In response to the drought stress, Cu/Zn-SOD was up-regulated, but two common bean genotypes showed different expression at two levels of exogenous EBL. So that, Cu/Zn-SOD was up-regulated 7.29 and 4.05 fold-changes in the COS16 genotype at the conditions of non-application of hormone and application of hormone, respectively, and the Kusha cultivar at the conditions of non-application of hormone and application of hormone showed up-regulation 2.51 and 3.08 fold-changes, respectively (Fig. 11).
Fig. 11.
Effect of drought stress on the relative expression of Cu/Zn-SOD of a COS16 genotype and b Kusha cultivar, evaluated at two concentrations of exogenous EBL
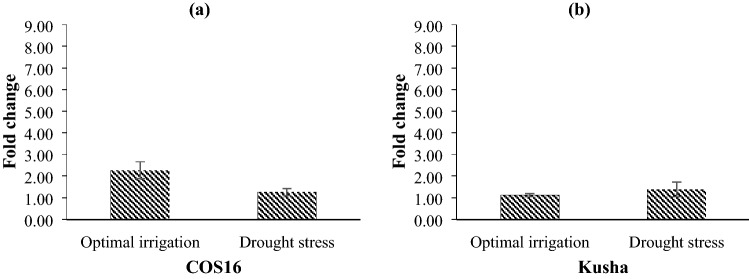
Also, Cu/Zn-SOD was up-regulated in response to the exogenous EBL, but this up-regulation of Cu/Zn-SOD was lower than the up-regulation of this gene in response to the drought stress (Fig. 12). In response to the exogenous EBL, two common bean genotypes showed different expression at two levels of irrigation. So that, Cu/Zn-SOD was up-regulated 2.27 and 1.26 fold-changes in the COS16 genotype under optimal irrigation and drought stress conditions, respectively, and the Kusha cultivar under optimal irrigation and drought stress conditions showed up-regulation 1.12 and 1.38 fold-changes, respectively (Fig. 12).
Fig. 12.
Effect of exogenous EBL on the relative expression of Cu/Zn-SOD of a COS16 genotype and b Kusha cultivar, evaluated at two levels of irrigation
In general, Cu/Zn-SOD was up-regulated in response to the drought stress and exogenous EBL. The COS16 genotype (9.19 fold change) showed a higher increase in the relative expression of Cu/Zn-SOD in response to the drought stress and exogenous EBL compared with the Kusha cultivar (3.46 fold change) (Fig. 13).
Fig. 13.
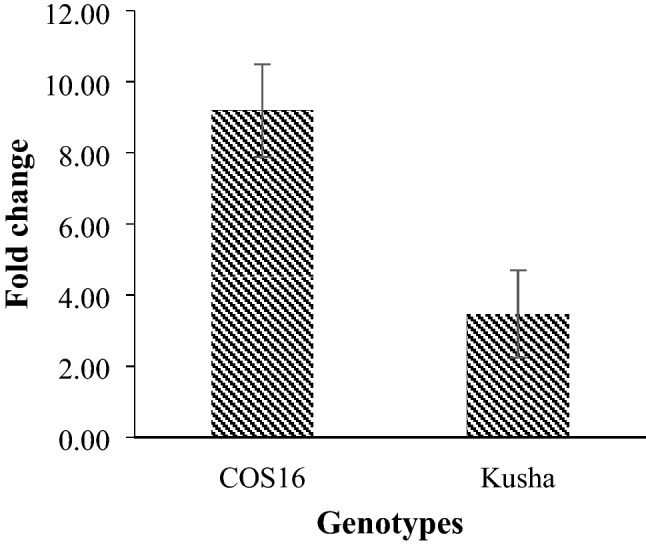
Effect of drought stress and exogenous EBL on the relative expression of Cu/Zn-SOD of two common bean genotypes
Phylogenetic analysis
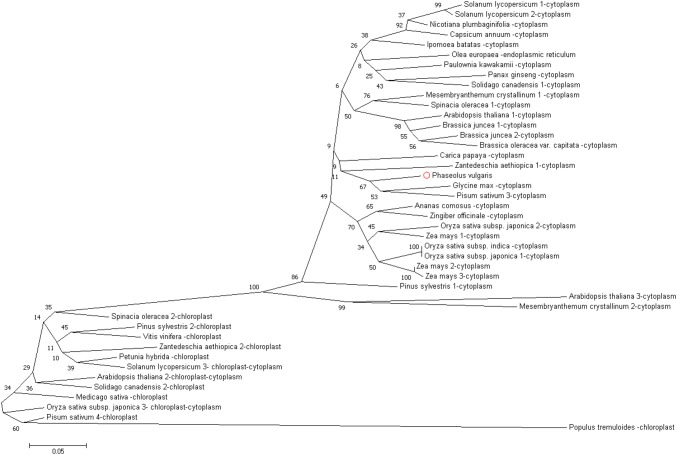
The activity site of the Cu/Zn-SOD enzymes can be chloroplasts or cytoplasm. In some plants, the activity site of this enzyme has been identified, but in the common bean, no attempt has been made to identify the likely site of its activity. In this study, the protein sequences of all known Cu/Zn-SODs of the plants where their activity sites were determined was compared with common bean Cu/Zn-SOD. The results showed that all chloroplastic Cu/Zn-SODs were placed in one group and also cytoplasmic Cu/Zn-SODs were placed together in another group. Considering that, the studied common bean Cu/Zn-SOD is localized in the cytoplasmic Cu/Zn-SODs group, it can be said that the Cu/Zn-SOD activity site is in the cytoplasm (Fig. 14). The results also showed that the Cu/Zn-SOD in common bean is most similar to the Cu/Zn-SODs in pea and soybean (Fig. 14). The results are also confirmed using the Softberry online tool to predict the potential site of the Cu/Zn-SOD activity.
Fig. 14.
Investigation of the phylogenetic relationship of the Cu/Zn-SOD in common bean
Discussion
RWC is one of the important physiological parameters that shows a good correlation with drought tolerance (Colom and Vazzana 2003). RWC of both genotypes was reduced by drought stress, but reduction was higher in cultivar Kusha (Fig. 1). In drought stress, because of decreasing soil moisture content, the roots are not able to provide water lost through transpiration. As a result, leaf water potential and leaf water content decreased. The researchers reported that water scarcity reduced the RWC of bean (Korir et al. 2006; Lizana et al. 2006), which is in line with the findings of this study. Under optimal irrigation, the Kusha cultivar is superior than the COS16 genotype in RWC. While, the situation is changed under drought stress, so that the COS16 genotype is superior than the Kusha cultivar in terms of this trait. In fact, the COS16 genotype is more tolerant to drought stress than the Kusha cultivar (Fig. 1). The high RWC in drought tolerant genotypes can be due to mechanisms that reduce water loss through stomata or increase water absorption through the development of a root system (Rosales-Serna et al. 2004). Researchers investigated the reaction of two susceptible and tolerant soybean cultivars to drought stress and showed that drought stress reduced the RWC, which was more severe in drought sensitive cultivar than drought tolerant cultivar (Angra et al. 2010). In this study, different concentrations of exogenous EBL caused a slight increase in RWC under optimal irrigation condition but significantly increased the RWC under drought stress compared with the control, indicating the more effective use of EBL in drought stress relative to optimal irrigation (Fig. 2). The more effective use of BRs in adverse conditions relative to favorable environmental conditions has also been reported in other studies (Talaat and Shawky 2016; Vardhini and Anjum 2015). The increase in the RWC may be due to the effect of BRs as a result of osmotic regulation and increased water absorption from the soil. Drought stress has been reported to reduce the RWC of maize leaves, but the application of 0.1 mg l−1 of brassinolide improved the RWC of leaves in both drought stress and optimal irrigation conditions (Anjum et al. 2011). Also, under severe drought stress, RWC of leaves and water potential of Robinia pseudoacacia seedlings treated with 0.2 mg l−1 of BRs was significantly higher than control treatment, and the researchers considered that increasing in the RWC was associated with a reduction in water loss of seedlings due to transpiration (Li et al. 2008). Researchers demonstrated that the ability of exogenous application of BRs to maintain high RWC in stressed plants might be attributed to their contribution to osmotic adjustment by increasing the internal total free amino acid concentration (Talaat and Shawky 2016). The application of BRs in various plants, including bean (Rady 2011), wheat (Talaat and Shawky 2012), tomato (Hayat et al. 2012; Yuan et al. 2010), and rice (Thussagunpanit et al. 2015) also increases the RWC of leaves.
Drought stress increased the amount of proline compared to optimal irrigation in both years, and this increase was higher in the 2016 period than the 2017 period (Fig. 3). Probably because of the difference in weather conditions during the measurement of this trait, drought stress has shown a greater increment in proline content in the 2016 period compared to the 2017 period. Increasing proline content under stress conditions protects cell membranes, proteins, cytoplasmic enzymes, and reduces the amount of reactive oxygen species (Liang et al. 2013). Drought stress significantly increased the amount of proline in chickpea (Najaphy et al. 2010) and faba bean (Siddiqui et al. 2015). Proline accumulation in drought stress can be due to stimulation of its synthesis or prevention of its decomposition or protein degradation (Gomes et al. 2010). Although in some plants, it has been proven that changes in proline content are associated with their ability to tolerate stress conditions and can be used as an indicator for selecting tolerant plants (Niknam et al. 2006), but it has been seen that in drought-sensitive plants such as cassava (Sundaresan and Sudhakaran 1995) and bean (Andrade et al. 1995), proline accumulation is just a sign of stress. Considering that, in this experiment there was no significant difference between the two common bean genotypes in terms of proline changes, it seems that in this study proline accumulation is just a sign of stress. The application of 4 µM EBL significantly increased the proline content compared with the control (Table 3). It has been reported that BRs increase proline content by affecting the expression of proline biosynthesis genes (Talaat and Shawky 2013). On the other hand, BRs increase biosynthesis of nucleic acids in plant cells (Bajguz 2000). Therefore, the increase in proline content with the application of EBL is probably due to the increment in synthesis of nucleic acids in plant cells and their conversion to the amino acids needed, such as proline. It has been reported that BRs increased the proline content in stress conditions (Agami 2013; Rady 2011).
Peroxidation of membrane lipids is considered as a sign of oxidative damage and is often used as an indicator for determining the extent of damage to the membrane under stress (Khan and Panda 2008). The levels of peroxidation of membrane lipids increase in drought stress, and as a result, MDA levels increase in cells under stress (Eraslan et al. 2007; Gunes et al. 2006). Low MDA levels indicate a low level of damage caused by stress to the cell membrane, which itself means that the plant is more tolerant to stress. In this study, drought stress increased the MDA content compared to optimal irrigation in both years, and this increase was higher in the 2016 period than the 2017 period (Fig. 4). Researchers have been reported that MDA content increased in stress conditions (Svetleva et al. 2012; Yuan et al. 2010). Since peroxidase activity is related to the amount of peroxidation of membrane lipids, a significant increase in the level of MDA of plants under stress indicates an inadequate activity of the peroxidase in collecting reactive oxygen species to prevent membrane damage and MDA production. In this study, different concentrations of exogenous EBL caused a slight decrease in MDA content under optimal irrigation condition but the application of 6 µM EBL decreased the MDA content under drought stress, significantly compared with the control, indicating the more effective use of EBL in drought stress relative to optimal irrigation (Fig. 5). Reducing MDA content due to the use of BRs demonstrates the role of these compounds in maintaining the structure and stability of plasma membranes in the presence of stress. Therefore, reduction of MDA accumulation in EBL treatment under drought stress indicates a decrease in lipid peroxidation and maintaining membrane health under drought stress conditions. Reduction in the accumulation of MDA by the application of BRs has also been reported by other researchers (Hayat et al. 2010; Yuan et al. 2010; Rady 2011; Thussagunpanit et al. 2015; Talaat and Shawky 2013).
Drought stress increased the activities of CAT, POD, APX, and SOD, which indicates the inhibition of reactive oxygen species in drought stress conditions (Figs. 6, 7, Table 3). There is a strong correlation between tolerance to oxidative stress caused by environmental stresses and an increase in the activity of antioxidant enzymes in plants (Mittler 2002). Increasing the activity of antioxidant enzymes in drought stress has also been reported by other researchers (Habibi 2013; Hameed et al. 2011; Khanna-Chopra and Selote 2007; Zeid and Shedeed 2006). This suggests that plants have been shown to increase the activity of their antioxidant enzymes to cope with drought stress and eliminate reactive oxygen species. The activity of POD in the COS16 genotype was higher than that in the Kusha cultivar, which indicates higher capacity for trapping the reactive oxygen species in this genotype, and thus damage to plasma membrane lipids in this genotype is lower (Table 3). The results of study in tolerant and susceptible cultivars of rice to salinity stress showed that under salinity stress, the level of POD activity in salinity resistant cultivar was more than salinity sensitive cultivar (Khan and Panda 2008). The CAT, APX, and SOD enzymes had the same activities in the two genotypes, and there was no significant difference in the activities of these enzymes between the two genotypes (Table 3). In other words, due to the type of activities of these enzymes, genotypes can’t be differentiated and none of the genotypes was superior to the other in terms of the activity levels of these enzymes. The application of EBL increased the activities of APX and SOD compared with the control (Table 3), and also caused a slight increase in CAT and POD activities under optimal irrigation condition and increased the CAT and POD activities under drought stress, significantly compared with the control (Figs. 8, 9). In general, different concentrations of exogenous EBL increased the seed yield of both common bean genotypes under both conditions of drought stress and optimal irrigation and decreased the seed yield loss and induced tolerance to drought stress by decreasing the MDA content and increasing the RWC, proline content and CAT, POD, APX, and SOD activities (Table 4). The use of BRs significantly improved the plant drought tolerance and reduced the accumulation of reactive oxygen species by increasing the activity of antioxidant enzymes (Talaat et al. 2015). The effect of BRs on antioxidant enzymes may be due to its effect on transcription or translation of genes (Bajguz 2000; Bajguz and Hayat 2009; Cao et al. 2005; Choe 2006). Increase in the activity of antioxidant enzymes after application of BRs has also been reported in bean (Rady 2011), maize (Bhardwaj et al. 2007), tomato (Yuan et al. 2010), mustard (Arora et al. 2010; Fariduddin et al. 2009; Sirhindi et al. 2009), soybean (Zhang et al. 2008), and barley (Kartal et al. 2009).
Table 4.
Seed yield of two common bean genotypes evaluated at two levels of irrigation and four concentrations of exogenous EBL in 2 years and seed yield loss in drought stress condition
| Years | Genotypes | Exogenous EBL (µM) | Seed yield (kg ha−1) | Seed yield loss (%) | |
|---|---|---|---|---|---|
| Optimal irrigation | Drought stress | ||||
| 2016 | COS16 | 0 | 1921.79 | 659.93 | 65.66 |
| 2 | 2537.76 | 880.57 | 65.30 | ||
| 4 | 2320.53 | 1159.84 | 50.02 | ||
| 6 | 2318.15 | 1223.23 | 47.23 | ||
| Kusha | 0 | 2329.23 | 752.73 | 67.68 | |
| 2 | 3426.31 | 1428.35 | 58.31 | ||
| 4 | 3229.47 | 1296.80 | 59.84 | ||
| 6 | 3116.79 | 1313.99 | 57.84 | ||
| 2017 | COS16 | 0 | 1911.66 | 800.99 | 58.10 |
| 2 | 2336.77 | 1179.65 | 49.52 | ||
| 4 | 2659.33 | 1192.53 | 55.16 | ||
| 6 | 2515.24 | 1028.75 | 59.10 | ||
| Kusha | 0 | 3393.57 | 1187.48 | 65.01 | |
| 2 | 3496.45 | 2076.01 | 40.63 | ||
| 4 | 3709.55 | 2052.56 | 44.67 | ||
| 6 | 3048.17 | 1675.23 | 45.04 | ||
Several studies have been reported different responses of NR activity in different environmental conditions. It has been reported that drought stress reduced NR activity and decreased nitrate absorption (Casadebaig et al. 2008; Fresneau et al. 2007; Talaat and Shawky 2016). In this study, drought stress decreased the NR activity by 16.53% in contrast with the optimal irrigation, which was not statistically significant (Table 3). In this study, different concentrations of exogenous EBL caused a slight increase in NR activity under optimal irrigation condition but increased the NR activity under drought stress, significantly compared with the control, indicating the more effective use of EBL in drought stress relative to optimal irrigation (Fig. 10). Improvement in the activity of NR can be related to the effect of BRs to transcription or translation of NR (Bajguz 2000) or nitrate absorption at the membrane surface (Mai et al. 1989). BRs have been reported to maintain the fluidity of the plasma membrane that has been altered by water stress and improve nitrate absorption, which induces the activity of NR (Talaat and Shawky 2016). In a study, the use of BRs led to an increase in the activity of NR, and the researchers considered this increase to be due to improved leaf water balance and increased leaf water potential under water deficit conditions (Sairam 1994). The use of BRs has led to an increase in the activity of NR in other studies (Fariduddin et al. 2009; Zheng et al. 2017; Talaat and Shawky 2016).
Drought stress increased the relative expression of Cu/Zn-SOD (Fig. 11). The induction of Cu/Zn-SOD expression in drought stress has also been reported in Caragana (Zhang et al. 2014), maize (Malan et al. 1990), and tomato (Perl-Treves and Galun 1991). Also, the use of EBL increased the relative expression of Cu/Zn-SOD (Fig. 12). BRs increase the tolerance of plants in a wide range of environmental stresses, and this increase is generally due to the increase in transcription of genes in response to stress (Clouse et al. 1992). Resistance to stress due to BRs is associated with the increased activity of SOD, CAT, APOX, DHAR, MDHAR, and GR as well as their expressions (Vardhini and Anjum 2015). It has also been reported that BRs increase the expression of APOX, CAT, and SOD in Brassica juncea under salinity stress (Kaur et al. 2015). Lower decrease in RWC in the COS16 genotype under drought stress (Fig. 1), lower negative effects of drought stress on photosynthesis and gas-exchange parameters and chlorophyll and carotenoids contents in the COS16 genotype as we reported in our previous study (Mohammadi et al. 2019), and also a higher increase in the relative expression of Cu/Zn-SOD in response to the drought stress and exogenous EBL in the COS16 genotype compared to the Kusha cultivar (Fig. 13) may indicate that the COS16 genotype is more tolerant to drought stress than the Kusha cultivar. In an experiment, the SOD expression pattern in maize was investigated under drought stress and the effect of water stress on SOD isozymes of two resistant and sensitive genotypes was studied. The results showed that transcription of Mn-SOD and Fe-SOD in the resistant plant was more pronounced than the sensitive genotype (Shiriga et al. 2014). The Tortula ruralis (drought tolerant) showed lower lipid peroxidation and higher Cu/Zn-SOD activity than Cratoneuron filicinum (drought sensitive) (Dhindsa and Matowe 1981).
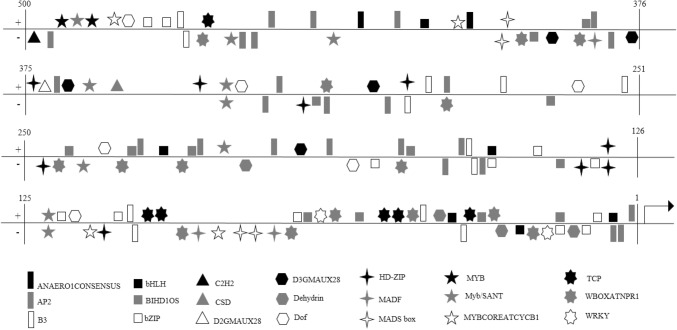
The density of the detected elements was very high at the upstream of the studied CSD, and there were 67 types of regulatory elements in the promoter of this gene. AP2 transcription factor binding site was observed to be the most frequent regulator sequence at the upstream of this gene (25 binding sites). There are several reports in relation to the role of AP2 transcription factor in activating genes responsive to abiotic stresses (Mizoi et al. 2012). Other extant elements in the upstream of this gene are the bZIP transcription factor binding site. These transcription factors play an essential role in adjusting the plant’s response to different environmental conditions (Wang et al. 2017). Other important elements involved in plant response to environmental stresses are shown in the following figure (Fig. 15). The presence of high levels of this regulatory element on the upstream side of this gene can indicate the role and importance of regulation of this gene in response to stresses. The results of this study showed that there are high numbers of specific regulatory elements in the upstream of this gene, which provide the interface of important regulatory proteins in the stress. Further studies in this field will provide an understanding of how this gene will respond in different situations.
Fig. 15.
Investigation of cis-regulatory elements in the upstream regions of CSD
Conclusion
The results of this study showed that drought stress decreased the RWC and increased the proline content, MDA content, and CAT, POD, APX, and SOD activities. Regarding the importance of antioxidant enzymes in the removal of radical superoxide and hydrogen peroxide, and the prevention of oxidative stress caused by drought stress, it seems that increasing the activity of antioxidant enzymes leads to less negative effects of oxidation stress induced by reactive oxygen species. As a result, tolerance to drought stress is achieved. Application of EBL reduced the seed yield loss and increased the tolerance to drought stress in both common bean genotypes by decreasing the level of MDA and increasing the RWC, proline content, antioxidant enzymes activity, NR activity, and seed yield. The effect of EBL on the SOD activity may be due to its effect on the relative expression of cyt Cu/Zn-SOD. With the findings of the current study, we can conclude that foliar spray of 4 µM EBL as the best concentration may enhance the RWC, proline content, antioxidant enzymes activity, and NR activity and enhance the stress tolerance ability of common bean. In addition, the achievement of comprehensive information on the positive effects of EBL requires a study of this hormone in different weather conditions and with other different bean genotypes.
Abbreviations
- APX
Ascorbate peroxidase
- BRs
Brassinosteroids
- CAT
Catalase
- EBL
24-Epibrassinolide
- MDA
Malondialdehyde
- NR
Nitrate reductase
- POD
Guaiacol peroxidase
- RWC
Relative water content
- SOD
Superoxide dismutase
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mahsa Mohammadi, Email: mohammadi.mahsa@znu.ac.ir.
Afshin Tavakoli, Email: tavakoli@znu.ac.ir.
Majid Pouryousef, Email: pouryousef@znu.ac.ir.
Ehsan Mohseni Fard, Email: mohsenifard.ehsan@znu.ac.ir.
References
- Agami RA. Alleviating the adverse effects of NaCl stress in maize seedlings by pretreating seeds with salicylic acid and 24-epibrassinolide. S Afr J Bot. 2013;88:171–177. [Google Scholar]
- Al-Ghamdi AA. Evaluation of oxidative stress tolerance in two wheat (Triticum aestivum) cultivars in response to drought. Int J Agric Biol. 2009;11(1):7–12. [Google Scholar]
- Andrade J, Larque-Saavedra A, Trejo C. Proline accumulation in leaves of four cultivars of Phaseolus vulgaris L. with different drought resistance. Phyton-Rev Int Bot Exp. 1995;57(2):149–158. [Google Scholar]
- Angra S, Kaur S, Singh K, Pathania D, Kaur N, Sharma S, Nayyar H. Water-deficit stress during seed filling in contrasting soybean genotypes: association of stress sensitivity with profiles of osmolytes and antioxidants. Int J Agric Res. 2010;5(6):328–345. [Google Scholar]
- Anjum S, Wang L, Farooq M, Hussain M, Xue L, Zou C. Brassinolide application improves the drought tolerance in maize through modulation of enzymatic antioxidants and leaf gas exchange. J Agron Crop Sci. 2011;197(3):177–185. [Google Scholar]
- Arora P, Bhardwaj R, Kanwar MK. 24-epibrassinolide induced antioxidative defense system of Brassica juncea L. under Zn metal stress. Physiol Mol Biol Plants. 2010;16(3):285–293. doi: 10.1007/s12298-010-0031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajguz A. Effect of brassinosteroids on nucleic acids and protein content in cultured cells of Chlorella vulgaris. Plant Physiol Biochem. 2000;38(3):209–215. [Google Scholar]
- Bajguz A, Hayat S. Effects of brassinosteroids on the plant responses to environmental stresses. Plant Physiol Biochem. 2009;47(1):1–8. doi: 10.1016/j.plaphy.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Bates L, Waldren R, Teare I. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39(1):205–207. [Google Scholar]
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Bhardwaj R, Arora N, Sharma P, Arora HK. Effects of 28-homobrassinolide on seedling growth, lipid peroxidation and antioxidative enzyme activities under nickel stress in seedlings of Zea mays L. Asian J Plant Sci. 2007;6(5):765–772. [Google Scholar]
- Borges A, Tsai SM, Caldas DGG. Validation of reference genes for RT-qPCR normalization in common bean during biotic and abiotic stresses. Plant Cell Rep. 2012;31(5):827–838. doi: 10.1007/s00299-011-1204-x. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1–2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cao S, Xu Q, Cao Y, Qian K, An K, Zhu Y, Binzeng H, Zhao H, Kuai B. Loss-of-function mutations in DET2 gene lead to an enhanced resistance to oxidative stress in Arabidopsis. Physiol Plant. 2005;123(1):57–66. [Google Scholar]
- Casadebaig P, Debaeke P, Lecoeur J. Thresholds for leaf expansion and transpiration response to soil water deficit in a range of sunflower genotypes. Eur J Agron. 2008;28(4):646–654. [Google Scholar]
- Chance B, Maehly A. [136] Assay of catalases and peroxidases. Methods Enzymol. 1955;2:764–775. doi: 10.1002/9780470110171.ch14. [DOI] [PubMed] [Google Scholar]
- Choe S. Brassinosteroid biosynthesis and inactivation. Physiol Plant. 2006;126(4):539–548. [Google Scholar]
- Clouse SD, Zurek DM, McMorris TC, Baker ME. Effect of brassinolide on gene expression in elongating soybean epicotyls. Plant Physiol. 1992;100(3):1377–1383. doi: 10.1104/pp.100.3.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colom M, Vazzana C. Photosynthesis and PSII functionality of drought-resistant and drought-sensitive weeping lovegrass plants. Environ Exp Bot. 2003;49(2):135–144. [Google Scholar]
- Contour-Ansel D, Torres-Franklin M, Zuily-Fodil Y, De Carvalho M. An aspartic acid protease from common bean is expressed ‘on call’ during water stress and early recovery. J Plant Physiol. 2010;167(18):1606–1612. doi: 10.1016/j.jplph.2010.06.018. [DOI] [PubMed] [Google Scholar]
- Dhindsa RS, Matowe W. Drought tolerance in two mosses: correlated with enzymatic defence against lipid peroxidation. J Exp Bot. 1981;32(1):79–91. [Google Scholar]
- Ding H-D, Zhu X-H, Zhu Z-W, Yang S-J, Zha D-S, Wu X-X. Amelioration of salt-induced oxidative stress in eggplant by application of 24-epibrassinolide. Biol Plant. 2012;56(4):767–770. [Google Scholar]
- Efron B, Gong G. A leisurely look at the bootstrap, the jackknife, and cross-validation. Am Stat. 1983;37(1):36–48. [Google Scholar]
- Eraslan F, Inal A, Savasturk O, Gunes A. Changes in antioxidative system and membrane damage of lettuce in response to salinity and boron toxicity. Sci Hortic. 2007;114(1):5–10. [Google Scholar]
- Fariduddin Q, Khanam S, Hasan S, Ali B, Hayat S, Ahmad A. Effect of 28-homobrassinolide on the drought stress induced changes in photosynthesis and antioxidant system of Brassica juncea L. Acta Physiol Plant. 2009;31(5):889–897. [Google Scholar]
- Fresneau C, Ghashghaie J, Cornic G. Drought effect on nitrate reductase and sucrose-phosphate synthase activities in wheat (Triticum durum L.): role of leaf internal CO2. J Exp Bot. 2007;58(11):2983–2992. doi: 10.1093/jxb/erm150. [DOI] [PubMed] [Google Scholar]
- Gomes FP, Oliva MA, Mielke MS, Almeida A-AF, Aquino LA. Osmotic adjustment, proline accumulation and cell membrane stability in leaves of Cocos nucifera submitted to drought stress. Sci Hortic. 2010;126(3):379–384. [Google Scholar]
- Gunes A, Cicek N, Inal A, Alpaslan M, Eraslan F, Guneri E, Guzelordu T. Genotypic response of chickpea (Cicer arietinum L.) cultivars to drought stress implemented at pre-and post-anthesis stages and its relations with nutrient uptake and efficiency. Plant Soil Environ. 2006;52(8):368–376. [Google Scholar]
- Gupta KJ, Stoimenova M, Kaiser WM. In higher plants, only root mitochondria, but not leaf mitochondria reduce nitrite to NO, in vitro and in situ. J Exp Bot. 2005;56(420):2601–2609. doi: 10.1093/jxb/eri252. [DOI] [PubMed] [Google Scholar]
- Habibi G. Effect of drought stress and selenium spraying on photosynthesis and antioxidant activity of spring barley. Acta Agric Slov. 2013;101(1):31–39. [Google Scholar]
- Hameed A, Bibi N, Akhter J, Iqbal N. Differential changes in antioxidants, proteases, and lipid peroxidation in flag leaves of wheat genotypes under different levels of water deficit conditions. Plant Physiol Biochem. 2011;49(2):178–185. doi: 10.1016/j.plaphy.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Hayat S, Ali B, Hasan S, Ahmad A. Brassinosteroid enhanced the level of antioxidants under cadmium stress in Brassica juncea. Environ Exp Bot. 2007;60(1):33–41. [Google Scholar]
- Hayat S, Hasan S, Yusuf M, Hayat Q, Ahmad A. Effect of 28-homobrassinolide on photosynthesis, fluorescence and antioxidant system in the presence or absence of salinity and temperature in Vigna radiata. Environ Exp Bot. 2010;69(2):105–112. [Google Scholar]
- Hayat S, Alyemeni MN, Hasan SA. Foliar spray of brassinosteroid enhances yield and quality of Solanum lycopersicum under cadmium stress. Saudi J Biol Sci. 2012;19(3):325–335. doi: 10.1016/j.sjbs.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath RL, Packer L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophy. 1968;125(1):189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Higo K, Ugawa Y, Iwamoto M, Korenaga T. Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res. 1999;27(1):297–300. doi: 10.1093/nar/27.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaleel CA, Manivannan P, Wahid A, Farooq M, Al-Juburi HJ, Somasundaram R, Panneerselvam R. Drought stress in plants: a review on morphological characteristics and pigments composition. Int J Agric Biol. 2009;11(1):100–105. [Google Scholar]
- Jithesh M, Prashanth S, Sivaprakash K, Parida A. Monitoring expression profiles of antioxidant genes to salinity, iron, oxidative, light and hyperosmotic stresses in the highly salt tolerant grey mangrove, Avicennia marina (Forsk.) Vierh. by mRNA analysis. Plant Cell Rep. 2006;25(8):865–876. doi: 10.1007/s00299-006-0127-4. [DOI] [PubMed] [Google Scholar]
- Kang Y, Guo S. Role of brassinosteroids on horticultural crops. In: Hayat S, Ahmad A, editors. Brassinosteroids: a class of plant hormone. Dordrecht: Springer; 2011. pp. 269–288. [Google Scholar]
- Kartal G, Temel A, Arican E, Gozukirmizi N. Effects of brassinosteroids on barley root growth, antioxidant system and cell division. Plant Growth Regul. 2009;58(3):261–267. [Google Scholar]
- Kaur H, Sirhindi G, Bhardwaj R. Alteration of antioxidant machinery by 28-homobrassinolide in Brassica juncea L. under salt stress. Adv Appl Sci Res. 2015;6:166–172. [Google Scholar]
- Khan M, Panda S. Alterations in root lipid peroxidation and antioxidative responses in two rice cultivars under NaCl-salinity stress. Acta Physiol Plant. 2008;30(1):81–89. [Google Scholar]
- Khanna-Chopra R, Selote DS. Acclimation to drought stress generates oxidative stress tolerance in drought-resistant than-susceptible wheat cultivar under field conditions. Environ Exp Bot. 2007;60(2):276–283. [Google Scholar]
- Korir P, Nyabundi J, Kimurto P. Genotypic response of common bean (Phaseolus vulgaris L.) to moisture stress conditions in Kenya. Asian J Plant Sci. 2006;5(1):24–32. [Google Scholar]
- Li K, Wang H, Han G, Wang Q, Fan J. Effects of brassinolide on the survival, growth and drought resistance of Robinia pseudoacacia seedlings under water-stress. New For. 2008;35(3):255–266. [Google Scholar]
- Li Y, Liu Y, Xu X, Jin M, An L, Zhang H. Effect of 24-epibrassinolide on drought stress-induced changes in Chorispora bungeana. Biol Plant. 2012;56(1):192–196. [Google Scholar]
- Liang X, Zhang L, Natarajan SK, Becker DF. Proline mechanisms of stress survival. Antioxid Redox Signal. 2013;19(9):998–1011. doi: 10.1089/ars.2012.5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 − ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lizana C, Wentworth M, Martinez JP, Villegas D, Meneses R, Murchie EH, Pastenes C, Lercari B, Vernieri P, Horton P, Pinto M. Differential adaptation of two varieties of common bean to abiotic stress: I. Effects of drought on yield and photosynthesis. J Exp Bot. 2006;57(3):685–697. doi: 10.1093/jxb/erj062. [DOI] [PubMed] [Google Scholar]
- MacKintosh C, Douglas P, Lillo C. Identification of a protein that inhibits the phosphorylated form of nitrate reductase from spinach (Spinacia oleracea) leaves. Plant Physiol. 1995;107(2):451–457. doi: 10.1104/pp.107.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai Y, Lin J, Zeng X, Pan R. Effect of homobrassinolide on the activity of nitrate reductase in rice seedlings. Plant Physiol Commun. 1989;2:50–52. [Google Scholar]
- Majidi M, Farsi M, Bahrami A, Behravan J, Marashi S. Cloning, gene expression analysis, and phylogenic relationship of dbat gene from iranian endemic yew (Taxus baccata L.) J Med Plants. 2013;4(48):91–103. [Google Scholar]
- Malan C, Greyling MM, Gressel J. Correlation between CuZn superoxide dismutase and glutathione reductase, and environmental and xenobiotic stress tolerance in maize inbreds. Plant Sci. 1990;69(2):157–166. [Google Scholar]
- Malik AA, Li W-G, Lou L-N, Weng J-H, Chen J-F. Biochemical/physiological characterization and evaluation of in vitro salt tolerance in cucumber. Afr J Biotech. 2010;9(22):3284–3292. [Google Scholar]
- Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7(9):405–410. doi: 10.1016/s1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- Mizoi J, Shinozaki K. Yamaguchi-Shinozaki K AP2/ERF family transcription factors in plant abiotic stress responses. Biochim Biophy Acta (BBA)-Gene Regul Mech. 2012;1819(2):86–96. doi: 10.1016/j.bbagrm.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Mohammadi M, Pouryousef M, Tavakoli A, Mohseni Fard E. Improvement in photosynthesis, seed yield and protein content of common bean (Phaseolus vulgaris) by foliar application of 24-epibrassinolide under drought stress. Crop Pasture Sci. 2019;70(6):535–545. [Google Scholar]
- Najaphy A, Khamssi NN, Mostafaie A, Mirzaee H. Effect of progressive water deficit stress on proline accumulation and protein profiles of leaves in chickpea. Afr J Biotech. 2010;9(42):7033–7036. [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22(5):867–880. [Google Scholar]
- Nazari M, Amiri RM, Mehraban F, Khaneghah HZ. Change in antioxidant responses against oxidative damage in black chickpea following cold acclimation. Russ J Plant Physiol. 2012;59(2):183–189. [Google Scholar]
- Niknam V, Razavi N, Ebrahimzadeh H, Sharifizadeh B. Effect of NaCl on biomass, protein and proline contents, and antioxidant enzymes in seedlings and calli of two Trigonella species. Biol Plant. 2006;50(4):591–596. [Google Scholar]
- Pal A, Acharya K, Vats S, Kumar S, Ahuja PS. Over-expression of PaSOD in transgenic potato enhances photosynthetic performance under drought. Biol Plant. 2013;57(2):359–364. [Google Scholar]
- Perl-Treves R, Galun E. The tomato Cu, Zn superoxide dismutase genes are developmentally regulated and respond to light and stress. Plant Mol Biol. 1991;17(4):745–760. doi: 10.1007/BF00037058. [DOI] [PubMed] [Google Scholar]
- Rady MM. Effect of 24-epibrassinolide on growth, yield, antioxidant system and cadmium content of bean (Phaseolus vulgaris L.) plants under salinity and cadmium stress. Sci Hortic. 2011;129(2):232–237. [Google Scholar]
- Rosales-Serna R, Kohashi-Shibata J, Acosta-Gallegos JA, Trejo-López C, Jn Ortiz-Cereceres, Kelly JD. Biomass distribution, maturity acceleration and yield in drought-stressed common bean cultivars. Field Crops Res. 2004;85(2–3):203–211. [Google Scholar]
- Sairam R. Effects of homobrassinolide application on plant metabolism and grain yield under irrigated and moisture-stress conditions of two wheat varieties. Plant Growth Regul. 1994;14(2):173–181. [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3(6):1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Sharma P, Jha AB, Dubey RS, Pessarakli M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot. 2012;2012:1–26. [Google Scholar]
- Shiriga K, Sharma R, Kumar K, Yadav SK, Hossain F, Thirunavukkarasu N. Genome-wide identification and expression pattern of drought-responsive members of the NAC family in maize. Meta Gene. 2014;2:407–417. doi: 10.1016/j.mgene.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui MH, Al-Khaishany MY, Al-Qutami MA, Al-Whaibi MH, Grover A, Ali HM, Al-Wahibi MS, Bukhari NA. Response of different genotypes of faba bean plant to drought stress. Int J Mol Sci. 2015;16(5):10214–10227. doi: 10.3390/ijms160510214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirhindi G, Kumar S, Bhardwaj R, Kumar M. Effects of 24-epibrassinolide and 28-homobrassinolide on the growth and antioxidant enzyme activities in the seedlings of Brassica juncea L. Physiol Mol Biol Plants. 2009;15(4):335–341. doi: 10.1007/s12298-009-0038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresan S, Sudhakaran P. Water stress-induced alterations in the proline metabolism of drought-susceptible and-tolerant cassava (Manihot esculenta) cultivars. Physiol Plant. 1995;94(4):635–642. [Google Scholar]
- Svetleva D, Krastev V, Dimova D, Mitrovska Z, Miteva D, Parvanova P, Chankova S. Drought tolerance of Bulgarian common bean genotypes, characterised by some biochemical markers for oxidative stress. J Cent Eur Agric. 2012;13(2):349–361. [Google Scholar]
- Sytar O, Kumari P, Yadav S, Brestic M, Rastogi A. Phytohormone priming: regulator for heavy metal stress in plants. J Plant Growth Regul. 2019;38(2):739–752. [Google Scholar]
- Talaat N, Shawky B. 24-epibrassinolide ameliorates the saline stress and improves the productivity of wheat (Triticum aestivum L.) Environ Exp Bot. 2012;82:80–88. [Google Scholar]
- Talaat N, Shawky B. 24-Epibrassinolide alleviates salt-induced inhibition of productivity by increasing nutrients and compatible solutes accumulation and enhancing antioxidant system in wheat (Triticum aestivum L.) Acta Physiol Plant. 2013;35(3):729–740. [Google Scholar]
- Talaat N, Shawky B. Dual application of 24-epibrassinolide and spermine confers drought stress tolerance in maize (Zea mays L.) by modulating polyamine and protein metabolism. J Plant Growth Regul. 2016;35(2):518–533. [Google Scholar]
- Talaat NB, Shawky BT, Ibrahim AS. Alleviation of drought-induced oxidative stress in maize (Zea mays L.) plants by dual application of 24-epibrassinolide and spermine. Environ Exp Bot. 2015;113:47–58. [Google Scholar]
- Thussagunpanit J, Jutamanee K, Sonjaroon W, Kaveeta L, Chai-Arree W, Pankean P, Suksamrarn A. Effects of brassinosteroid and brassinosteroid mimic on photosynthetic efficiency and rice yield under heat stress. Photosynthetica. 2015;53(2):312–320. [Google Scholar]
- Tuba Bicer B, Narin Kalender A, Akar D. The effect of irrigation on spring-sown chickpea. J Agron. 2004;3(3):154–158. [Google Scholar]
- Vardhini BV, Anjum NA. Brassinosteroids make plant life easier under abiotic stresses mainly by modulating major components of antioxidant defense system. Front Environ Sci. 2015;2:1–16. [Google Scholar]
- Wang X-L, Chen X, Yang T-B, Cheng Q, Cheng Z-M. Genome-wide identification of bZIP family genes involved in drought and heat stresses in strawberry (Fragaria vesca) Int J Genom. 2017;2017:1–14. doi: 10.1155/2017/3981031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan G, Jia C, Li Z, Sun B, Zhang L, Liu N, Wang Q. Effect of brassinosteroids on drought resistance and abscisic acid concentration in tomato under water stress. Sci Hortic. 2010;126(2):103–108. [Google Scholar]
- Zeid I, Shedeed Z. Response of alfalfa to putrescine treatment under drought stress. Biol Plant. 2006;50(4):635–640. [Google Scholar]
- Zhang M, Zhai Z, Tian X, Duan L, Li Z. Brassinolide alleviated the adverse effect of water deficits on photosynthesis and the antioxidant of soybean (Glycine max L.) Plant Growth Regul. 2008;56(3):257–264. [Google Scholar]
- Zhang W, Li N, Qi F, Chen X, Hao J, Yu C, Lin X. Isolation and expression analysis of Cu/Zn superoxide dismutase genes from three Caragana species. Russ J Plant Physiol. 2014;61(5):656–663. [Google Scholar]
- Zheng Y, Xu B, Ren K, Zhang Y, Wu J. Impact of soil drench and foliar spray of 24-epibrassinolide on the growth, yield, and quality of field-grown Moringa oleifera in southwest china. J Plant Growth Regul. 2017;36(4):931–941. [Google Scholar]
- Zlatev Z, Lidon FC. An overview on drought induced changes in plant growth, water relationsand photosynthesis. Emir J Food Agric. 2012;24(1):57–72. [Google Scholar]