Abstract
Earlier we reported that seed pre-treatment with PHF promoted early seedling growth and salinity tolerance in wheat. As a way forward, experiments were conducted to investigate whether and to what extent foliar spray of fullerol could influence growth and physio-biochemical responses in salt stressed wheat. In a control experiment, seeds were sown in sand filled pots (500 g) under control and 150 mM NaCl stress. After 15 days, foliar spray of fullerol at 0, 10, 40, 80 and 120 nM concentration was applied and the data for various morpho-biochemical attributes recorded after 2 weeks. Fullerol caused improvements in shoot growth attributes while had least effect on root growth traits. Increase in total chlorophyll while reduction in Car/Chl ratio was evident under salinity in response to fullerol spray. Only 40 and 80 nM spray treatments improved antioxidant activities and reduced H2O2 contents while MDA contents which increased due to salt stress, remained unaffected by foliar spray. Fullerol spray also improved sugars, proline and free amino acids under salinity. During second experiment under natural conditions, 60 day old plants grown in sand filled pots (10 kg) under 0 and 150 mM NaCl were foliar sprayed with selected concentrations (0, 40 and 80 nM) of fullerol. Salinity inhibited gas exchange and grain yield attributes while fullerol-sprayed plants exhibited recovery. Fullerol spray resulted in high root and shoot K+ and shoot Ca2+ contents. Also, increase in shoot and root P, while lesser shoot Na+ was recorded due to 80 nM spray under salt stress. Overall, 40 and 80 nM fullerol spray improved photosynthetic activity, osmolytes accumulation and altered tissue ion compartmentalization which contributed to improvement in grain yield attributes under salinity.
Keywords: Antioxidants, Abiotic stress, Nanoparticles, Fullerol, Salinity, Wheat, Yield
Introduction
Nanoparticles are solid state materials with at least one dimension between 1 and 100 nm and the smaller diameter contribute to their unique properties. To an estimate, over 8000 nano-scale products are commercially available (StatNano 2018). There is considerable interest in nanomaterials based agriculture to modulate plant growth responses and different type of metal oxides and carbon based NPs are of prime importance. Among various carbon nanoparticles, fullerene (C60) molecule is made up of 60 carbon atoms joined in the form of soccer ball and contains 30 carbon double bonds, poor electron delocalization which makes it behave as electron deficient alkenes (Ali et al. 2004; Acquah et al. 2017). Fullerols [C60(OH)x] or polyhydroxy fullerenes (PHF) are derivatives of C60 that exhibit stronger electron affinities and are radical sponges (Andrievsky et al. 2005; Sachkova et al. 2017).
Unfortunately, the C60 solutions prepared in toxic organic solvents cannot be applied directly to plants. Therefore for biological applications, the use of fullerol is preferred over pristine fullerene due to its water solubility and extraordinary antioxidant properties. A single fullerol molecule can accept up to six electrons and can scavenge all ROS and NOS (Gust et al. 2001; Yanagi et al. 2007). The mechanism of fullerol mediated scavenging of hydroxyl radical (OH−) generated via Fenton’s reaction is already reported (Grebowski et al. 2014). In addition, fullerol neutralized superoxide anion (Ali et al. 2004), singlet oxygen (Yanagi et al. 2007), hydrogen peroxide and reactive nitrogen species (Yin et al. 2009). Hence fullerol can potentially neutralize all forms of ROS and RNS and its role in protection of cell integrity is substantial (Zhou et al. 2017). Studies conducted with animal and human models reported positive effects of fullerols referring them as non-toxic electron scavenging nano-antioxidants with health beneficial effects (Liu et al. 2013; Darabi and Mohammadi 2017; Grebowski et al. 2018).
In case of plants, fullerol improved agronomic traits and phytochemical contents in Momordica charantia under control conditions (Kole et al. 2013). Exogenous fullerol conferred water stress tolerance in Beta vulgaris L. (Borišev et al. 2016) and Brassica napus L. through mitigation of oxidative stress and modulation of ABA levels (Xiong et al. 2018). In our previous study, seed pre-treatment with PHF at nano-molar concentrations promoted salt tolerance in 30-day old wheat seedlings through improvements in chlorophyll contents, free amino acids, total sugars and enhancing the activities of H2O2 scavenging enzymes viz. CAT, POD and APX (Shafiq et al. 2019). Also, the seedlings exhibited better osmotic ion adjustments (particularly K+ and P ions) and less content of MDA and H2O2 (Shafiq et al. 2019). On the basis of these short-term effects of PHF as seed priming agent, we further hypothesized that its foliar spray might also promote salt tolerance in wheat. Accordingly, both short and long term effects of foliar applied fullerol on salinity stressed wheat plants were studied in two separate experiments. From an agricultural viewpoint, salinity is a global problem (Tadesse et al. 2017; Lagacherie et al. 2018) and the accumulation of soluble salts in soils interferes with plant physio-biochemical functioning of glycophytes (Iqbal and Ashraf 2013; Geilfus 2018). In turn, ROS over-production impairs cellular metabolism and hampers crop growth (Feki et al. 2017; Shafiq et al. 2018). Carefully summarizing this information, the modulation of oxidative stress is a crucial aspect in functional plant biology and could be targeted to induce crop stress tolerance. Thus, the principal objective of the present study was to assess that whether foliar-applied fullerol [C60(OH)20] nanoparticles could alter antioxidant capacity, ion homeostasis and photosynthetic activity to enhance grain yield attributes in wheat exposed to salinity.
Materials and methods
Influence of fullerol foliar spray on salinity stressed wheat was performed in separate experiments.
Experiment-1: Effect of fullerol spray on wheat seedling growth and biochemical traits under control conditions
Seedling growth and fullerol spray application
Seeds of wheat (cv. Ujala-2016) were germinated in pots under two salinity levels (0 and 150 mM NaCl) given through Hoaglands’ nutrient solution. The seedlings were grown in growth incubator (Sanyo, Model MLR-351H) set at 10 h photoperiod, 18/23 °C day/night temperature, and 370 µmol m−2 s−1 light intensity. Fullerol [C60(OH)20] nanoparticles were purchased from BuckyUSA, Houston Texas, USA. Different fullerol concentrations were prepared in MiliQ water with continuous sonication. The foliar application of fullerol nanoparticles to control and salinity stressed seedlings was performed at 0, 10, 40, 80 and 120 nM concentrations after 15 days of germination. Growth and biochemical attributes were studied after 15 days of fullerol spray treatments (30 days old plants).
Determination of growth attributes and photosynthetic pigments
Growth parameters such as shoot and root lengths and fresh and dry weights were recorded. For pigments determination, fresh material (100 mg) was extracted with acetone and the optical density (OD) was recorded spectrophotometrically at three wavelengths viz. 663, 645 and 480 nm. The pigments were calculated as described earlier (Arnon 1949; Kirk and Allen 1965).
Determination of oxidative stress, antioxidants and osmolytes
Oxidative stress markers
Changes in MDA and H2O2 contents were determined from plant-trichloroacetic acid (TCA) extracts. Plant-TCA extracts were prepared by homogenization of fresh material (500 mg) in 8% TCA solution (10 mL) and centrifuged at 7000 rpm for 10 min, and the supernatant referred as plant-TCA extract. For MDA determination, 1 mL TBA solution (0.1% TBA in 10% TCA) and 1 mL TCA extract were mixed, and placed in boiling water for 30 min (Dhindsa et al. 1981). The OD was recorded at three different wavelengths viz. 600, 532 and 450 nm. For H2O2 determination, 0.5 mL of plant-TCA extract and 2 mL KI (1 M) were mixed and placed in dark at 25 °C for 30 min. The OD was recorded at 390 nm and H2O2 contents calculated from a standard curve (Velikova et al. 2000).
Enzymatic antioxidant activities
For antioxidant enzyme activities determination, fresh leaf (200 mg) was grinded in 100 mM potassium phosphate (K-P) buffer (pH 7.8). After centrifugation at 12,000 rcf, the supernatant was separated and referred as crude protein extract. During SOD activity determination, 1 mL assay mixture contained 50 mM K-P buffer (pH 7.4), 50 µM NBT, 0.1% Triton X-100, 13 mM methionine, 1.3 µM riboflavin, 75 mM EDTA and 50 µL crude protein extract in the 1 mL final volume. After irradiation of cuvettes at 75 µmol m−2 s−1 for 5 min, OD was recorded at 560 nm (Beauchamp and Fridovich 1971).
For determination of CAT activity, assay mixture (3 mL) contained 100 µL crude protein extract, 50 mM K-P buffer (pH 7.4) and 5.9 mM H2O2 (Aebi 1984). Change in absorbance was recorded at 240 nm for 180 s with UV–Vis spectrophotometer. In addition, for POD activity determination, 3 mL assay mixture contained 50 µL crude protein extract, 50 mM K-P buffer (pH 7.0), 40 mM H2O2 and 20 mM guaiacol. The change in OD was recorded at 470 nm for 180 s as described earlier (Chance and Maehly 1955). Similarly for APX activity determination, 3 mL assay mixture comprised of 100 µL plant sample, 50 mM K-P buffer (pH 7.0), 10 mM H2O2 and 5 mM L-ascorbate. Change in absorbance was recorded at 290 nm for 180 s as described earlier (Nakano and Asada 1981). Finally, 0.01 absorbance change was used as one unit of POD, APX and CAT enzyme activity and the activities presented in U mg−1 protein.
Non-enzymatic antioxidants and osmolytes
Plant ethanol (EtOH) extracts were prepared as described earlier (Shafiq et al. 2019). Determination of amino acids was performed using ninhydrin (2%) and pyridine (10%) and the OD was recorded at 570 nm (Hamilton and Van Slyke 1943). The phenolic contents were quantified after reaction with 7.5% Na2CO3 and Folin–ciocalteau reagent. The absorbance was recorded at 765 nm and total phenolics were calculated (Bray and Thorpe 1954). For quantification of total soluble sugars, EtOH extracts were mixed with cold anthrone reagent (2% anthrone in 65% H2SO4). The absorbance of this assay mixture was recorded at 620 nm (Dubois et al. 1956). Flavonoids were quantified from the assay mixture containing 0.3 mL AlCl3 (10%), 0.3 mL NaNO2 (5%) and 1 mL EtOH extracts. After that, 2 mL NaOH was added to assay mixture and test tubes were placed at 30 °C for 20 min. Finally, absorbance was recorded at at 510 nm (Pękal and Pyrzynska 2014).
Leaf free proline was determined from fresh leaf material (200 mg) after its homogenization in 5-sulfosalicylic acid. After centrifugation (10,000 rpm for 15 min), the assay mixture for proline comprised glacial acetic acid (2 mL) mixed with ninhydrin reagent (2 mL) and placed in boiling water for 1 h. Finally, this assay mixture was extracted with toluene and the OD of the supernatant was taken at 520 nm (Bates et al. 1973).
Ascorbic acid was determined from plant-TCA extracts. For this purpose, 2 mL extract was mixed with 0.3 mL of DTC reagent (3 g 2–4 Dinitrophenyl hydrazine, 0.4 g Thiourea and 0.05 g CuSO4 dissolved in 9 N H2SO4). The assay mixture was incubated at 30 °C for 3 h and the osazone formed were dissolved in 65% H2SO4 and the OD was taken at 520 nm (Mukherjee and Choudhuri 1983).
Experiment-2: Effect of fullerol spray on wheat physiological functioning and yield attributes under natural conditions
The influence of selected fullerol spray concentrations on the photosynthetic activity, ion compartmentalization and wheat productivity was studied in the second experiment.
Fullerol spray application
Seeds of wheat cv. Ujala 2016 were sown in plastic pots containing sand (10 kg) under two different levels of salinity (0 and 150 mM NaCl). After germination and establishment of seedlings, thinning was executed to retain 6 plants per pot. Foliar application of selected fullerol concentrations (0, 40 and 80 nM) was performed on 30 days old control and salinity stressed plants and the data for physiological attributes was recorded after 30 days (60 days old plants).
Changes in photosynthetic activity
The gas exchange attributes of wheat plants after 30 days of foliar treatments (60 days old plants) were recorded by using steady state porometer (model LI-1600, LI-COR, Inc) during 10–11 am morning time. Different parameters like diffusive resistance (DR), relative humidity (RH), leaf temperature (LT), quantum (Q) and transpiration rate (TR) were recorded. Leaf average temperature ranged from 25 ± 2 °C. Leaf transpiration rates (E) were calculated using Dalton’s law of partial pressures and the values presented as mol m−2 s−1 (Pearcy et al. 2000). Stomatal conductance (gs) was calculated using diffusive resistivity while photosynthesis rate (Pn) was calculated by using E and gs values. In addition, the intrinsic water use efficiency (WUE) was calculated as Pn/E.
Ion analyses
Root and shoot dry plant material was subjected to wet-acid digestion using H2SO4 and H2O2 mixture (Wolf 1982). The determination of sodium, potassium and calcium ions was carried out flame photometerically (Jenway PFP-7, U.K) while the phosphorus was determined spectrophotometerically (U-4000, Hitachi, Japan) after its reaction with Barton’s reagent.
Determination of yield attributes
At physiological maturity, various yield attributes were recorded. Further, after 2 months of harvest, seeds from control and fullerol sprayed plants were tested for germination so as to observe any beneficial or toxic effects of fullerols accumulated in seed.
Statistical analyses
Both experiments were performed using completely randomized layout with four replications per treatment. There were five and three spray treatments in the initial control experiment and the second experiment. The total number of experimental units for first and second experiment was 40 and 24, respectively. Analyses of variance were performed to compute statistical significance among various treatments using GLM module of CoStat (CoHort Software, Monterey, CA, USA). Also, the Duncan’s Multiple Range (DMR) test at 5% probability level was used to compute differences among mean values.
Results
Influence of fullerol spray on salt stressed wheat seedlings: Experiment-I
Changes in growth parameters
Salinity caused prominent reduction (P < 0.001) in the wheat shoot growth attributes, which manifest in the decrease of length and weight of shoots (Table 1). Fullerol foliar application at 40 and 80 nM concentration positively improved (P < 0.005) shoot growth traits of seedlings particularly under salt stress treatments (See ANOVA analyses presented in Table 6). Root growth attributes of wheat seedlings were also significantly decreased by salinity (Table 1). While under salinity, seedlings treated with fullerol foliar spray at 80 nM exhibited significantly improvement (P < 0.005) in root growth traits. Moreover, 40 nM spray improved the root dry weight of salinity stressed seedlings (P < 0.005).
Table 1.
Influence of fullerol foliar application on growth attributes of salinity stressed wheat seedlings (means; n = 4 ± SE)
| Source of variation | Root | Shoot | ||||
|---|---|---|---|---|---|---|
| Length (cm) | Fresh weight (g) | Dry weight (g) | Length (cm) | Fresh weight (g) | Dry weight (g) | |
| Control | ||||||
| Control | 13.2c ± 0.51 | 3.29bc ± 0.08 | 0.69b ± 0.05 | 34.3bc ± 0.32 | 3.92c ± 0.20 | 0.97b ± 0.02 |
| 10 nM PHF | 16.2b ± 0.90 | 3.75a ± 0.11 | 0.72b ± 0.04 | 35.3ab ± 0.42 | 4.58ab ± 0.21 | 1.08a ± 0.05 |
| 40 nM PHF | 15.7b ± 1.01 | 3.69a ± 0.12 | 0.85a ± 0.05 | 36.2ab ± 0.44 | 4.71ab ± 0.24 | 1.15a ± 0.04 |
| 80 nM PHF | 17.4b ± 0.93 | 3.54ab ± 0.04 | 0.87a ± 0.06 | 36.6a ± 0.46 | 4.84a ± 0.09 | 1.12a ± 0.06 |
| 120 nM PHF | 19.5a ± 1.02 | 3.07c ± 0.16 | 0.64b ± 0.03 | 32.4c ± 0.43 | 4.33bc ± 0.05 | 0.94b ± 0.02 |
| 150 mM NaCl | ||||||
| Control | 10.3d ± 0.27 | 1.31f ± 0.05 | 0.35d ± 0.02 | 24.8e ± 0.69 | 2.15fg ± 0.07 | 0.38d ± 0.01 |
| 10 nM PHF | 11.9 cd ± 0.25 | 1.72de ± 0.12 | 0.45 cd ± 0.01 | 30.0d ± 0.74 | 2.54ef ± 0.10 | 0.49c ± 0.02 |
| 40 nM PHF | 11.0d ± 0.17 | 1.58ef ± 0.12 | 0.50c ± 0.01 | 29.6d ± 0.84 | 2.92de ± 0.17 | 0.58c ± 0.04 |
| 80 nM PHF | 13.1c ± 0.61 | 1.93d ± 0.12 | 0.52c ± 0.02 | 33.3c ± 0.80 | 3.13d ± 0.03 | 0.52c ± 0.01 |
| 120 nM PHF | 10.2d ± 0.45 | 1.38f ± 0.03 | 0.35d ± 0.01 | 26.3e ± 0.49 | 1.91g ± 0.11 | 0.34d ± 0.03 |
Means with different lowercase letter(s) within a column differ significantly at 5% probability level
Table 6.
Mean square values from analyses of variance of the data for growth and biochemical attributes of wheat plants treated with fullerol foliar spray
| Source of variation | df | Shoot length | Shoot FW | Shoot DW | Root length |
|---|---|---|---|---|---|
| Salt stress (SS) | 1 | 383.3*** | 37.8*** | 3.497*** | 258.7*** |
| Fullerol spray (FS) | 4 | 43.9*** | 1.38*** | 0.073*** | 15.1*** |
| SS × FS | 4 | 11.6*** | 0.158NS | 0.0004NS | 12*** |
| Error | 30 | 1.39 | 0.082 | 0.005 | 1.89 |
| Source of variation | df | Root FW | Root DW | Chl a | Chl b |
|---|---|---|---|---|---|
| Salt stress (SS) | 1 | 35.4*** | 1.023*** | 102,093.6*** | 8613.6*** |
| Fullerol spray (FS) | 4 | 0.483*** | 0.065*** | 10,455.7*** | 3019.1*** |
| SS × FS | 4 | 0.096NS | 0.002NS | 449.8NS | 467.5** |
| Error | 30 | 0.041 | 0.004 | 658.2 | 108.3 |
| Source of variation | df | Chl (a + b) | Car | Chl a/b ratio | Car/Chl ratio |
|---|---|---|---|---|---|
| Salt stress (SS) | 1 | 170,016.3*** | 1489.0*** | 0.083NS | 0.0033*** |
| Fullerol spray (FS) | 4 | 11,131.1*** | 1363.4*** | 9.84*** | 0.0019*** |
| SS × FS | 4 | 999.5NS | 448.8** | 2.10*** | 0.00075** |
| Error | 30 | 772.3 | 78.9 | 0.288 | 0.00013 |
| Source of variation | df | SOD | CAT | POD | APX |
|---|---|---|---|---|---|
| Salt stress (SS) | 1 | 1.834*** | 0.903*** | 23.8*** | 0.189*** |
| Fullerol spray (FS) | 4 | 0.025NS | 0.091** | 1.90* | 0.046** |
| SS × FS | 4 | 0.047* | 0.312*** | 2.95** | 0.217*** |
| Error | 30 | 0.011 | 0.018 | 0.602 | 0.010 |
| Source of variation | df | MDA | H2O2 | Ascorbic acid | Amino acid |
|---|---|---|---|---|---|
| Salt stress (SS) | 1 | 126.2*** | 4.51*** | 0.0951*** | 5879*** |
| Fullerol spray (FS) | 4 | 10.5** | 0.080* | 0.219*** | 372.1* |
| SS × FS | 4 | 19.9*** | 0.212*** | 0.444*** | 1208.8*** |
| Error | 30 | 2.47 | 0.021 | 0.013 | 94.7 |
| Source of variation | df | Phenolics | Sugars | Flavonoids | Proline |
|---|---|---|---|---|---|
| Salt stress (SS) | 1 | 6.47** | 916.7* | 47.7* | 9583.3*** |
| Fullerol spray (FS) | 4 | 4.77*** | 1868.6*** | 79.2*** | 243.7* |
| SS × FS | 4 | 1.93* | 3727.3*** | 37.2** | 289.8* |
| Error | 30 | 0.499 | 193.1 | 8.24 | 79.9 |
ns non-significant
*,**,***Significant at 0.05, 0.01 and 0.001 levels, respectively
Changes in photosynthetic pigments
Substantial reduction (P < 0.001) in chlorophyll was recorded in wheat under salinity (Table 2). On the other hand, salinity mediated considerable increase in the carotenoids was evident (P < 0.05). The foliar application of fullerol at 10, 40 and 80 nM concentration improved total Chl contents of salt stressed seedlings (P < 0.05; Table 2). Furthermore, reduction in carotenoids was evident in response to 40 nM fullerol spray (P < 0.05). We also recorded prominent increase (P < 0.01) in the Chl ab−1 relative ratio while decrease (P < 0.05) in the Car Chl−1 ratio in response to 40 and 80 nM fullerol spray under salinity was recorded.
Table 2.
Influence of fullerol foliar application on photosynthetic pigments of salinity stressed wheat seedlings (means; n = 4 ± SE)
| Source of variation | Photosynthetic pigments | |||||
|---|---|---|---|---|---|---|
| Chl a (µg/g FW) | Chl b (µg/g FW) | Chl (a + b) (µg/g FW) | Car (µg/g FW) | Chl a/b ratio | Car/Chl ratio | |
| Control | ||||||
| Control | 303.9ab ± 10.3 | 110.0ab ± 2.31 | 413.9bc ± 10.9 | 79.9b ± 3.95 | 2.76c ± 0.1 | 0.19e ± 0.006 |
| 10 nM PHF | 346.7a ± 16.7 | 129.6a ± 9.28 | 476.4a ± 14.8 | 82.5ab ± 3.81 | 2.75c ± 0.3 | 0.17f ± 0.002 |
| 40 nM PHF | 359.3a ± 6.84 | 99.0abc ± 3.66 | 458.3a ± 10.3 | 79.8b ± 5.81 | 3.63b ± 0.07 | 0.17f ± 0.010 |
| 80 nM PHF | 360.1a ± 11.0 | 84.2abc ± 3.44 | 444.3ab ± 12.3 | 84.6ab ± 2.33 | 4.29b ± 0.2 | 0.19e ± 0.007 |
| 120 nM PHF | 266.8ab ± 17.9 | 106.5ab ± 4.33 | 373.3 cd ± 17.1 | 49.4c ± 5.50 | 2.53c ± 0.23 | 0.13 g ± 0.009 |
| 150 mM NaCl | ||||||
| Control | 195.3b ± 5.64 | 74.3abc ± 2.93 | 269.5f ± 4.37 | 105.9a ± 6.15 | 2.65c ± 0.17 | 0.39a ± 0.02 |
| 10 nM PHF | 233.8ab ± 12.2 | 115.6ab ± 6.28 | 349.4d ± 18.4 | 102.2a ± 2.66 | 2.02c ± 0.01 | 0.29b ± 0.007 |
| 40 nM PHF | 249.0ab ± 17.4 | 48.2c ± 4.52 | 297.3ef ± 21.7 | 75.9b ± 5.14 | 5.21a ± 0.2 | 0.25d ± 0.010 |
| 80 nM PHF | 263.4ab ± 16.2 | 68.9bc ± 4.11 | 332.4de ± 12.2 | 80.4ab ± 4.79 | 3.92b ± 0.4 | 0.24d ± 0.011 |
| 120 nM PHF | 190.1b ± 4.35 | 75.5abc ± 7.05 | 265.6f ± 7.40 | 72.7b ± 1.81 | 2.60c ± 0.2 | 0.27c ± 0.001 |
Means with different lowercase letter(s) within a column significantly at 5% probability level
Chl chlorophyll, Car carotenoids
Changes in oxidative stress, antioxidants and osmolyte accumulation
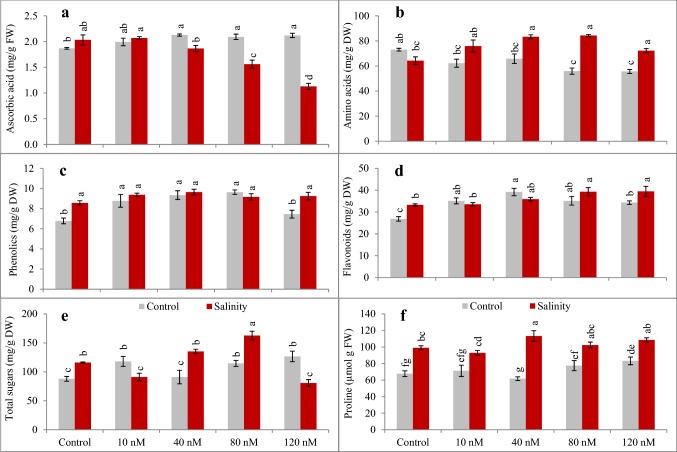
Under salinity, increase in the MDA and H2O2 contents was significant (P < 0.05) among seedlings (Table 3). Furthermore, considerable increase in the SOD and CAT activities for un-sprayed plants under salt stress (P < 0.05) was observed. Foliar application of fullerol to salt stressed wheat seedlings at 40 nM, significantly enhanced activities of enzymatic antioxidants (P < 0.05). Moreover, 80 nM and 120 nM fullerol spray also prominently improved SOD and CAT activities (P < 0.001). In addition, fullerol spray treatment resulted in lesser (P < 0.05) oxidative stress indicated by lower MDA and H2O2 fraction under salinity (Table 3). Fullerol spray caused decrease in ascorbic acid contents under salinity (P < 0.05; Fig. 1a) while increased (P < 0.05) amino acids (Fig. 1b), phenolics and flavonoids (Table 6; Fig. 1c–e). Fullerol also markedly increased total soluble sugars (P < 0.05; Fig. 1d) and proline accumulation (Fig. 1f) with or without salinity.
Table 3.
Influence of fullerol foliar application on oxidative stress markers and enzymatic antioxidant activities of salinity stressed wheat seedlings (means; n = 4 ± SE)
| Source of variation | Oxidative stress and enzymatic antioxidant activities | |||||
|---|---|---|---|---|---|---|
| MDA contents (nmol g−1 FW) | H2O2 contents (mmol g−1 FW) | SOD activity (U mg−1 protein) | CAT activity (U mg−1 protein) | POD activity (U mg−1 protein) | APX activity (U mg−1 protein) | |
| Control | ||||||
| Control | 7.75ab ± 0.72 | 0.65d ± 0.03 | 0.43f ± 0.04 | 0.85efg ± 0.06 | 4.70bc ± 0.09 | 0.59 cd ± 0.05 |
| 10 nM PHF | 7.41b ± 0.14 | 0.77d ± 0.06 | 0.54e ± 0.04 | 0.91def ± 0.10 | 3.65c ± 0.41 | 0.67bc ± 0.08 |
| 40 nM PHF | 8.70ab ± 0.24 | 0.72d ± 0.06 | 0.43f ± 0.05 | 0.69 g ± 0.04 | 3.68c ± 0.55 | 0.49d ± 0.01 |
| 80 nM PHF | 8.90ab ± 0.38 | 0.82d ± 0.08 | 0.43f ± 0.05 | 1.00cdef ± 0.05 | 3.50c ± 0.19 | 0.47d ± 0.03 |
| 120 nM PHF | 7.73ab ± 0.57 | 0.86 cd ± 0.03 | 0.46f ± 0.04 | 1.04cde ± 0.06 | 4.91b ± 0.37 | 0.68bc ± 0.05 |
| 150 mM NaCl | ||||||
| Control | 15.9a ± 1.50 | 1.85a ± 0.06 | 0.73d ± 0.04 | 1.09 cd ± 0.01 | 5.70b ± 0.25 | 0.72bc ± 0.04 |
| 10 nM PHF | 11.3ab ± 1.13 | 1.37b ± 0.10 | 0.79c ± 0.08 | 1.14c ± 0.09 | 4.58bc ± 0.53 | 0.49d ± 0.03 |
| 40 nM PHF | 9.08ab ± 0.37 | 1.42b ± 0.11 | 1.01a ± 0.04 | 1.57a ± 0.10 | 6.94a ± 0.46 | 1.06a ± 0.08 |
| 80 nM PHF | 9.65ab ± 0.73 | 1.13bc ± 0.06 | 1.01a ± 0.08 | 1.37b ± 0.06 | 5.78b ± 0.51 | 0.82b ± 0.04 |
| 120 nM PHF | 12.3ab ± 0.95 | 1.41b ± 0.10 | 0.89b ± 0.07 | 0.81 fg ± 0.04 | 5.15b ± 0.22 | 0.50d ± 0.07 |
Means with different lowercase letter(s) within a column differ significantly at 5% probability level. MDA, malondialdehyde; H2O2, hydrogen peroxide
SOD superoxide dismutase, CAT catalase, POD peroxidise, APX ascorbate peroxidase
Fig. 1.
a–f Influence of fullerol foliar spray on non-enzymatic antioxidants and osmolytes in wheat under control (grey filled square box) and 150 mM NaCl salinity (red filled square box) (n = 4; mean ± SE). Different lowercase letters in each attribute are significantly different at P < 0.05 (DMR). DW, dry weight; FW, fresh weight
Influence of fullerol spray on wheat productivity under salinity: Experiment-II
Changes in photosynthetic activity
We recorded considerable reduction in stomatal conductance (gs), transpiration rate (E) and net photosynthetic rate (Pn) among plants under salinity (P < 0.05; Table 4). On the other hand, fullerol sprayed salinity stressed plants exhibited significant recovery (P < 0.01) in the Pn rate, E and gs (Table 4). Furthermore, considerable recovery in WUE calculated as Pn/E, was evident under salinity due to fullerol spray at both 40 and 80 nM spray treatments (P < 0.05).
Table 4.
Influence of fullerol foliar application on photosynthetic activity of salinity stressed wheat plants (means; n = 4 ± SE)
| Source of variation | Pn (µmol m−2 s−1) | gs (mol(H2O) m−2 s−1) | E (mol(H2O) m−2 s−1) | WUE |
|---|---|---|---|---|
| Control | ||||
| Control | 24.9a ± 0.44 | 1.11ab ± 0.013 | 4.59a ± 0.19 | 5.37bc ± 0.06 |
| 40 nM PHF | 25.9a ± 0.79 | 0.815bc ± 0.043 | 3.20bc ± 0.31 | 6.46a ± 0.49 |
| 80 nM PHF | 25.8a ± 0.32 | 1.186a ± 0.122 | 4.56a ± 0.48 | 5.71ab ± 0.28 |
| Salinity | ||||
| Control | 18.2b ± 1.30 | 0.695c ± 0.016 | 2.30d ± 0.18 | 4.79c ± 0.18 |
| 40 nM PHF | 23.8a ± 1.19 | 0.768c ± 0.021 | 2.73 cd ± 0.09 | 5.85ab ± 0.22 |
| 80 nM PHF | 24.2a ± 0.22 | 0.994ab ± 0.041 | 3.90ab ± 0.14 | 6.07ab ± 0.27 |
Means with different lowercase letter(s) in a column differ significantly at 5% probability level. Pn, photosynthesis rate; gs, stomatal conductance
E transpiration rate, WUE water use efficiency
Changes in inorganic ions
Under salinity, increase (P < 0.001) in Na+ fraction of shoot and root was recorded (Table 5). Similar trend was recorded for root and shoot Ca2+ which increased under salinity (P < 0.05). Also, P and K+ contents increased in the root while these contents reduced in the leaf fraction for salinity stressed plants (P < 0.05). Wheat plants sprayed with 40 and 80 nM fullerol concentration had less reduction (P < 0.05) in root K+, Ca2+ and P (Table 5). The 40 nM fullerol treated plants exhibited an increase in root Na+ fraction (P < 0.05). Furthermore, increase (P < 0.01) in shoot K+, Ca2+ and P was recorded under salinity in response to fullerol spray treatments. In addition, marked reduction was recorded in the shoot Na+ concentration due to 40 and 80 nM fullerol spray treatments respectively (P < 0.05; Table 5).
Table 5.
Influence of fullerol foliar application on inorganic ions of salinity stressed wheat plants (means; n = 4 ± SE)
| Source of variation | Na+ (mg g−1 DW) | K+ (mg g−1 DW) | Ca2+ (mg g−1 DW) | P (mg g−1 DW) | ||||
|---|---|---|---|---|---|---|---|---|
| Root | Shoot | Root | Shoot | Root | Shoot | Root | Shoot | |
| Control | ||||||||
| Control | 9.00d ± 0.90 | 4.38c ± 0.22 | 12.4bc ± 0.4 | 20.4c ± 0.4 | 4.81d ± 0.23 | 5.56c ± 0.24 | 6.27b ± 0.41 | 8.23c ± 0.36 |
| 40 nM PHF | 9.59cd ± 0.36 | 3.70d ± 0.22 | 12.7bc ± 0.4 | 16.4d ± 0.5 | 6.69b ± 0.39 | 5.82c ± 0.37 | 4.93c ± 0.49 | 9.57b ± 0.50 |
| 80 nM PHF | 10.9c ± 0.18 | 3.31d ± 0.19 | 16.1a ± 0.8 | 19.4c ± 1.2 | 7.72a ± 0.39 | 6.21c ± 0.24 | 4.70c ± 0.52 | 6.78d ± 0.35 |
| Salinity | ||||||||
| Control | 17.1b ± 0.41 | 7.23a ± 0.07 | 16.3a ± 0.9 | 16.0d ± 0.6 | 6.29bc ± 0.24 | 8.08b ± 0.31 | 7.80a ± 0.25 | 6.86d ± 0.39 |
| 40 nM PHF | 19.1a ± 0.71 | 5.80b ± 0.19 | 11.1c ± 0.3 | 22.9b ± 0.2 | 4.76d ± 0.20 | 9.14ab ± 0.39 | 6.33b ± 0.45 | 10.0ab ± 0.25 |
| 80 nM PHF | 17.4b ± 0.64 | 6.11b ± 0.14 | 14.0b ± 0.4 | 25.4a ± 0.4 | 5.42 cd ± 0.35 | 9.92a ± 0.42 | 4.80c ± 0.41 | 11.2a ± 0.56 |
Means with different lowercase letter(s) in a column differ significantly at 5% probability level
Na+, sodium; K+, potassium; Ca2+, calcium; P, phosphorus
Changes in yield attributes
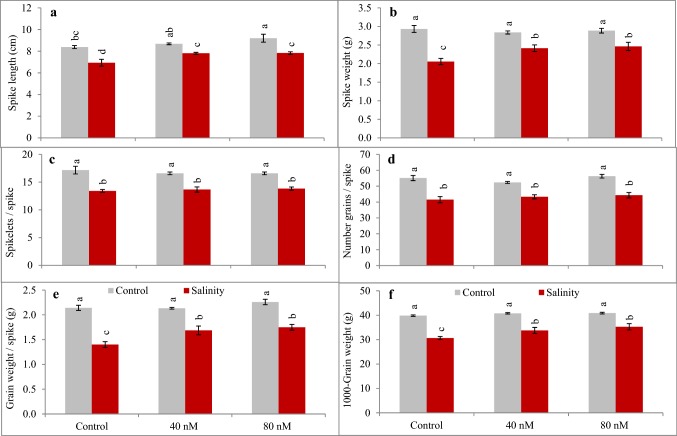
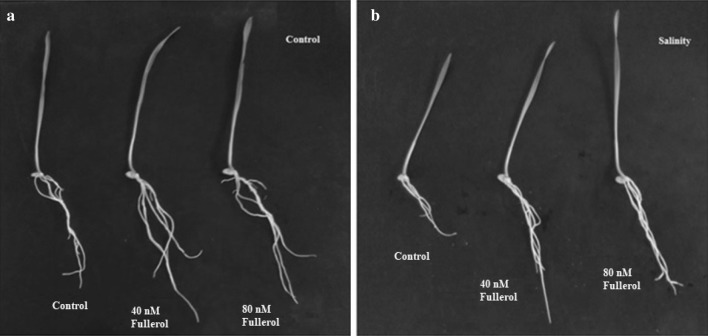
Salinity caused reduction (P < 0.01) in various yield attributes including spike length (Fig. 2a), spike weight (Fig. 2b), numbers of spikelet (Fig. 2c) and grains spike−1 (Fig. 2d), grain weight spike−1 (Fig. 2e) and 100-grain weight (Fig. 2f). Fullerol foliar spray caused prominent recovery in spike weight (P < 0.05). Furthermore, fullerol spray also significantly improved (P < 0.05) grain weight spike−1 and 100-grain weight. In contrast, numbers of spikelet spike−1 and grains spike−1 remained unaffected by spray treatments. Above all, the harvested seeds from 40 and 80 nM fullerol sprayed plants exhibited better germination when grown under salinity (Fig. 3). The ANOVA analyses related to interactive effects of fullerol spray and salinity treatments for different physiological, ion and yield attributes are given in the Table 7.
Fig. 2.
a–f Influence of fullerol foliar spray on yield attributes of wheat under control (grey filled square box) and 150 mM NaCl salinity (red filled square box) (n = 4; mean ± SE). Different lowercase letters in each attribute differ significantly at P < 0.05 (DMR)
Fig. 3.
Photograph showing the growth of seedlings raised from seeds harvested from plants treated with 40 and 80 nM fullerol foliar spray. Harvested seeds were grown in Petri plates under a control (0 mM NaCl) and b salinity (150 mM NaCl)
Table 7.
Mean square values from analyses of variance of the data for gas exchange, ions and yield attributes of plants treated with fullerol foliar spray
| Source of variation | df | Pn | E | gs | WUE |
|---|---|---|---|---|---|
| Salt stress (SS) | 1 | 73.2*** | 7.82*** | 2.91*** | 0.465NS |
| Fullerol spray (FS) | 2 | 29.5*** | 3.23*** | 1.81*** | 2.48** |
| SS × FS | 2 | 15.7* | 2.02** | 0.715* | 0.603NS |
| Error | 18 | 2.72 | 0.280 | 0.129 | 0.319 |
| Source of variation | df | Na+ root | Na+ shoot | K+ root | K+ shoot |
|---|---|---|---|---|---|
| Salt stress (SS) | 1 | 393.9*** | 40*** | 0.053ns | 42.2*** |
| Fullerol spray (FS) | 2 | 3.94ns | 3.08*** | 21.6*** | 35.9*** |
| SS × FS | 2 | 4.67ns | 0.349ns | 21.6*** | 76.5*** |
| Error | 18 | 1.37 | 0.131 | 1.27 | 1.64 |
| Source of variation | df | Ca2+ root | Ca2+ shoot | P root | P shoot |
|---|---|---|---|---|---|
| Salt stress (SS) | 1 | 5.06** | 75*** | 6.13* | 8.63** |
| Fullerol spray (FS) | 2 | 2.40** | 0.878 ns | 10.6*** | 10.6*** |
| SS × FS | 2 | 8.69*** | 3.86** | 1.25ns | 17.8*** |
| Error | 18 | 0.383 | 0.561 | 0.746 | 0.691 |
| Source of variation | df | Spike length | Spike weight | Spikelet/spike | Grains/spike |
|---|---|---|---|---|---|
| Salt stress (SS) | 1 | 9.07*** | 1.989*** | 59.1*** | 793.5*** |
| Fullerol spray (FS) | 2 | 1.53** | 0.069ns | 0.055ns | 13.6ns |
| SS × FS | 2 | 0.192ns | 0.084ns | 0.574ns | 10.7ns |
| Error | 18 | 0.184 | 0.04 | 0.643 | 8.36 |
| Source of variation | df | Grain weight/spike | 100-Grain weight |
|---|---|---|---|
| Salt stress (SS) | 1 | 1.94*** | 317.4*** |
| Fullerol spray (FS) | 2 | 0.109** | 17.2** |
| SS × FS | 2 | 0.047ns | 6.56ns |
| Error | 18 | 0.013 | 2.61 |
ns non-significant
*, **, ***Significant at 0.05, 0.01 and 0.001 levels, respectively
Discussion
Influence of fullerol spray on early growth and biochemical traits
In general, when wheat is exposed to the salinity, the growth and photosynthetic processes are inhibited (Roy et al. 2014; Geilfus 2018; Shafiq et al. 2018). Further, salt stress results in chlorophyll degradation and ROS overproduction (Santos 2004; Iqbal and Ashraf 2013; Stefanov et al. 2016). In agreement with this, we recorded salt stress induced reduction in growth parameters and chlorophyll contents in the present studies. The fullerol spray at 40 and 80 nM concentrations improved shoot length, shoot fresh and dry weights parallel with recovery in total chlorophyll contents under salinity stress. The foliar spray (80 nM) significantly improved root growth parameters such as root length, fresh and dry weights under salt stress. The concentration dependent effect of fullerol was more evident in case of its foliar spray while as priming agent, it was effective at all concentrations used viz. 10, 40, 80 and 120 nM (Shafiq et al. 2019). Nonetheless, positive influence of exogenous fullerol is reported on Arabidopsis (Gao et al. 2011), bitter gourd (Kole et al. 2013), sugar beet (Borišev et al. 2016) and canola (Xiong et al. 2018). Similarly, improvement in chlorophyll contents of control grown wheat plants in response to a radio-labeled fullerol have been reported earlier (Wang et al. 2016).
Our results of increase in MDA and H2O2 concentration among salt stressed plants indicated oxidative stress. The fullerol spray did not reduce leaf MDA contents although it substantially decreased leaf H2O2 concentrations. Also, only fullerol spray at 40 nM dose increased SOD, CAT, POD and APX activities under salt stress while 80 nM dose only caused improvements in SOD and CAT activities. If we compare present findings with our previous study (Shafiq et al. 2019), seed pre-treatment substantially reduced MDA and H2O2 contents at all concentrations applied which clearly is not the case here due to foliar spray. Yet, increased enzymatic antioxidants particularly in response to 40 and 80 nM fullerol spray suggested detoxification of O2− radical and H2O2 and could be linked with antioxidant properties of fullerol to quench free radicals (Foley et al. 2002; Sachkova et al. 2017). Under salt stress, increase in the contents of phenolics, flavonoids, soluble sugars and free proline was recorded. In addition, fullerol spray at 80 nM concentration further improved flavonoids, soluble sugars, free amino acids and proline concentration while decreased ascorbic acid under salt stress. In contrast, foliar spray of fullerol (40 nM) only improved free amino acids and proline concentrations under salt stress. This is again in contrast to our previous results, where seed pre-treatment with fullerol caused increase in amino acids and soluble sugars under salinity stress at all concentrations while decreased phenolics (Shafiq et al. 2019). However, consistent with our present findings, fullerol spray increased proline contents of sugar beet plants under drought stress (Borišev et al. 2016). Proline is a vital osmolyte that accumulates under abiotic stresses and plays a significant role in cellular osmo-regulation (Szabados and Savoure 2009; Arias-Baldrich et al. 2015). Further, its exogenous application as seed pre-treatment has been reported to improve wheat salt tolerance (Shafiq et al. 2018). Accumulation and retention of amino acids and sugars is effective for osmotic adjustments (Flowers and Colmer 2008; Per et al. 2017). By contrast, prominent reduction in the ascorbic acid was evident under salinity which further decreased in response to increasing fullerol doses. Both flavonoids and ascorbic acid protect vital biomolecules from ROS (Kelly et al. 2002; Akram et al. 2017). Ascorbic acid rapidly participates in free radical scavenging primarily H2O2 contents (Akram et al. 2017) and its oxidation in our study was consistent with increase in APX activity which uses ascorbic acid as a substrate for H2O2 neutralization (Mittler et al. 2004; van Doorn and Ketsa 2014). Above all, increase in the flavonoids indicated better non-enzymatic antioxidant capacity. Overall, 40 and 80 nM fullerol concentrations as foliar spray were much more effective in inducing salt tolerance in wheat.
Influence of fullerol spray on physiological attributes and productivity of salt-stressed wheat plants
During the second experiment, the influence of two comparatively most effective fullerol spray concentrations (40 and 80 nM), selected from first experiment was investigated on physiological functioning, tissue ion contents and wheat grain yield attributes under salinity. We recorded prominent reduction in the gas exchange attributes such as Pn, gs, E and WUE of wheat plants under salt stress. It is reported that salinity inhibits gas exchange attributes and interferes with stomatal conductance and transpiration rate thereby affecting transpiration pull, nutrient uptake and evaporative cooling (Ruffino et al. 2010; Tardieu et al. 2011). Here, exogenous fullerol spray at 80 nM conc. improved all the gas exchange attributes while 40 nM spray only improved Pn rate and WUE under salinity. In contrast to our findings, Borišev et al. (2016) did not record any beneficial effect of foliar applied fullerol (70 and 700 μmol concentration) on the photosynthesis of drought stressed sugar beet. The fullerol-mediated improvements in gas exchange attributes in the present study could be linked with higher chlorophyll contents in fullerol treated plants which may have contributed to better photosynthetic rate. Further, improvement in E and gs can be due to better cellular osmotic adjustments induced by fullerol spray. In this context, the experiment-1 results revealed that salinity stressed wheat seedlings treated with 80 nM fullerol spray exhibited higher free amino acids, sugars and proline contents. The accumulation of such compatible solutes lowers water potential under osmotic stress (Adolf et al. 2013; Per et al. 2017; Shafiq et al. 2018), and thus regulate growth under stress conditions. However, further investigations are required to prove the direct involvement of fullerol in the regulation of photosynthesis.
Our results indicated increase in root and shoot Na+ concentration under salt stress while reduction in shoot K+ and P contents. The effect of fullerol spray was differential and concentration dependent i.e., 80 nM fullerol decreased shoot Na+, increased root and shoot K+, increased shoot Ca2+ and P contents while the 40 nM fullerol spray did not affect shoot Na+ and P contents. Again this is in contrast to our previous findings, where seed treatment at all concentrations improved root K+, root P and shoot P concentrations (Shafiq et al. 2019). Here, only 80 nM spray improved these traits. Salinity disturbs root and shoot ion homeostasis (Hasegawa 2013; Lee et al. 2016) and through the mechanism of Na+ xylem loading, plant roots tend to transport Na+ to shoot for better osmotic adjustments (Shabala et al. 2010). In response, increase in the root K+ contents can improve Na+/K+ ratio and it is an effective strategy to counter the effects of salinity (Cuin et al. 2009; Wang et al. 2017). Therefore, salt tolerant plants mainly use K+ ions as cheap osmolyte to balance water relations (Iannucci et al. 2002). Generally, salt stress inhibits the K+ uptake in root. Usually, the plants which can keep higher K+ and low Na+ contents in the root show better salt tolerance. In contrast, in the present study, fullerol spray increased the accumulation of K+ in the shoots rather than in roots at both concentrations under salt stress. Similarly, Ca2+ is an important ion involved in the regulation of various cellular and biochemical responses under abiotic stresses (Marshner 1995; Shabala 2017). In this study, fullerol sprayed plants had better K+, Ca2+ and P contents in the shoot than root. In agreement with our results, salt tolerant barley genotypes exhibited higher K+ and Ca2+ retention (Hammami et al. 2017). In addition, the exogenous Ca2+ and K+ conferred salinity tolerance in Oryza sativa (Rahman et al. 2016), Canavalia ensiformis, Capsicum annum, Glycine max and Solanum melongena (Baba et al. 2017). Fullerol as priming agent also promoted P uptake in the root and shoot of wheat seedlings under salinity (Shafiq et al. 2019). Cell viability under salinity has also been linked with Na+, Ca2+ and ROS homeostasis (Nath et al. 2016).
Above all, salinity negatively affected grain yield attributes of wheat. Decrease in grain weight under salinity has been linked with vulnerability of fertilization process and grain-filling stage of wheat (Munns et al. 2016; Munns and Gilliham 2015) as well as decrease in fertile tillers (El-Hendawy et al. 2012). We recorded fullerol-mediated increase in spike length, spike weight, grain weight/spike and 1000-grain weight of salinity stressed wheat plants. The better germination ability of seed collected from fullerol treated plants under both control and salt stress showed its beneficial and non-toxic effects at selected 40 and 80 nM concentrations as foliar application.
Conclusions and prospects
Salinity negatively affected wheat growth, biochemical and ion attributes while fullerol as foliar spray resulted in certain improvements to promote salinity tolerance. Of different concentrations, 40 and 80 nM fullerol spray was much more effective in inducing salinity tolerance in wheat. Further experimentation confirmed the beneficial effects of 80 nM fullerol on photosynthetic activity and ion compartmentalization which contributed to improvement in yield attributes. Interestingly, seed collected from fullerol-treated wheat exhibited better germination under both normal and saline conditions. Based on our previously published data and the present findings, it can be concluded that fullerol applied as seed pre-treatment and foliar spray differentially affect plant growth and biochemical attributes under salinity. This differential response might be due to the timing and mode of fullerol application. For instance, seed pre-treatment with fullerol may allow early salinity tolerance response while in case of the fullerol as foliar spray; plants are already exposed to salt stress for some duration that affects their biochemistry. This is what we observed here that fullerol spray decreased H2O2 but MDA concentrations remained unaffected possibly due to lipid peroxidation before foliar spray treatments. In future, it will be interesting to investigate that how genetically diverse wheat cultivars respond to fullerol under different abiotic stresses.
Acknowledgements
The study was partially funded by Higher Education Commission (HEC), Islamabad, Pakistan through Project Grants No. 20-1522/R&D/09 and 20-1523/R&D/10 to MI.
Abbreviations
- Na+
Sodium
- K+
Potassium
- Ca2+
Calcium
- P
Phosphorus
- Chl
Chlorophyll
- Car
Carotenoids
- ROS
Reactive oxygen species
- MDA
Malondialdehyde
- H2O2
Hydrogen peroxide
- SOD
Superoxide dismutase
- CAT
Catalase
- POD
Peroxidase
- APX
Ascorbate peroxidase
- AsA
Ascorbate
- Pn
Photosynthetic rate
- E
Transpiration rate
- gs
Stomatal conductance
- WUE
Water use efficiency
- DW
Dry weight
- FW
Fresh weight
- EtOH
Ethyl alcohol
- PHF
Polyhydroxy fullerene
Authors’ contribution
MI, FS and MA conceived and designed the research. FS carried out the experimentation and analyses. FS and MI analyzed data and wrote the manuscript. All authors commented on the manuscript, discussed the results and approved the final manuscript for submission.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Acquah SF, Penkova AV, Markelov DA, Semisalova AS, Leonhardt BE, Magi JM. The beautiful molecule: 30 years of C60 and its derivatives. ECS J Solid State Sci Technol. 2017;6(6):3155–3162. doi: 10.1149/2.0271706jss. [DOI] [Google Scholar]
- Adolf VI, Jacobsen SE, Shabala S. Salt tolerance mechanisms in quinoa (Chenopodium quinoa Willd.) Environ Exp Bot. 2013;92:43–54. doi: 10.1016/j.envexpbot.2012.07.004. [DOI] [Google Scholar]
- Aebi H (1984) Catalase in vitro. In: Pac L (ed) Orlando: Academic Press
- Akram NA, Shafiq F, Ashraf M. Ascorbic Acid: a potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front Plant Sci. 2017;8:613. doi: 10.3389/fpls.2017.00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali SS, Hardt JI, Quick KL, Sook Kim-Han J, Erlanger BF, Huang T, Epstien CJ, Dugan LL. A biologically effective fullerene (C60) derivative with superoxide dismutase mimetic properties. Free Radical Biol Med. 2004;37(8):1191–1202. doi: 10.1016/j.freeradbiomed.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Andrievsky G, Klochkov V, Derevyanchenko L. Is the C60 fullerene molecule toxic? Fuller Nanotub Carbon N. 2005;13(4):363–376. doi: 10.1080/15363830500237267. [DOI] [Google Scholar]
- Arias-Baldrich C, Bosch N, Begines D, Feria AB, Monreal JA, García-Mauriño S. Proline synthesis in barley under iron deficiency and salinity. J Plant Physiol. 2015;183:121–129. doi: 10.1016/j.jplph.2015.05.016. [DOI] [PubMed] [Google Scholar]
- Arnon DI. Copper enzymes in isolated chlorlasts: polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba T, Sato Y, Katsube Y, Kuroda C, Fujiyama H. Relationship between plant responses to high Na and ameliorative effects of supplemental K and Ca. J Plant Nutr. 2017;40(1):33–39. doi: 10.1080/01904167.2016.1193606. [DOI] [Google Scholar]
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39(1):205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Borišev M, Borišev I, Župunski M, Arsenov D, Pajević S, Ćurčić Ž, Vasin J, Djordjevic A. Drought impact is alleviated in sugar beets (Beta vulgaris L.) by foliar application of fullerenol nanoparticles. PLoS ONE. 2016;11(11):1–20. doi: 10.1371/journal.pone.0166248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray HG, Thorpe WV. Analysis of phenolic compounds of interest in metabolism. New York: Wiley; 1954. pp. 27–52. [DOI] [PubMed] [Google Scholar]
- Chance B, Maehly AC. Assay of catalases and peroxidases. Methods Enzymol. 1955;2:764–775. doi: 10.1016/S0076-6879(55)02300-8. [DOI] [PubMed] [Google Scholar]
- Cuin TA, Tian Y, Betts SA, Chalmandrier R, Shabala S. Ionic relations and osmotic adjustment in durum and bread wheat under saline conditions. Funct Plant Biol. 2009;36(12):1110–1119. doi: 10.1071/FP09051. [DOI] [PubMed] [Google Scholar]
- Darabi S, Mohammadi MT. Fullerenol nanoparticles decrease ischaemia-induced brain injury and oedema through inhibition of oxidative damage and aquaporin-1 expression in ischaemic stroke. Brain Inj. 2017;31(8):1142–1150. doi: 10.1080/02699052.2017.1300835. [DOI] [PubMed] [Google Scholar]
- Dhindsa RS, Plumb-Dhindsa P, Thorpe TA. Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot. 1981;32(1):93–101. doi: 10.1093/jxb/32.1.93. [DOI] [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers PT, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28(3):350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- El-Hendawy SE, Hu Y, Sakagami JI, Schmidhalter U. Screening egyptian wheat genotypes for salt tolerance at early growth stages. Intl J Plant Prod. 2012;5(3):283–298. [Google Scholar]
- Feki K, Tounsi S, Masmoudi K, Brini F. The durum wheat plasma membrane Na+/H+ antiporter SOS1 is involved in oxidative stress response. Protoplasma. 2017;254(4):1725–1734. doi: 10.1007/s00709-016-1066-8. [DOI] [PubMed] [Google Scholar]
- Flowers TJ, Colmer TD. Salinity tolerance in halophytes. New Phytol. 2008;2008:945–963. doi: 10.1111/j.1469-8137.2008.02531.x. [DOI] [PubMed] [Google Scholar]
- Foley S, Crowley C, Smaihi M, Bonfils C, Erlanger BF, Seta P, Larroque C. Cellular localisation of a water-soluble fullerene derivative. Biochem Biophys Res Commun. 2002;294(1):116–119. doi: 10.1016/S0006-291X(02)00445-X. [DOI] [PubMed] [Google Scholar]
- Gao J, Wang Y, Folta KM, Krishna V, Bai W, Indeglia P, Georgieva A, Nakamura H, Koopman B, Moudgil B. Polyhydroxy fullerenes (Fullerols or fullerenols): beneficial effects on growth and lifespan in diverse biological models. PLoS ONE. 2011;6(5):e19976. doi: 10.1371/journal.pone.0019976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geilfus CM. Chloride: from nutrient to toxicant. Plant Cell Physiol. 2018;59(5):877–886. doi: 10.1093/pcp/pcy071. [DOI] [PubMed] [Google Scholar]
- Grebowski J, Krokosz A, Konarska A, Wolszczak M, Puchala M. Rate constants of highly hydroxylated fullerene C60 interacting with hydroxyl radicals and hydrated electrons. Pulse radiolysis study. Radiat Phys Chem. 2014;103:146–152. doi: 10.1016/j.radphyschem.2014.05.057. [DOI] [Google Scholar]
- Grebowski J, Kazmierska P, Litwinienko G, Lankoff A, Wolszczak M, Krokosz A. Fullerenol C60(OH)36 protects human erythrocyte membrane against high-energy electrons. Biochim Biophys Acta Biomembr. 2018;1860(8):1528–1536. doi: 10.1016/j.bbamem.2018.05.005. [DOI] [PubMed] [Google Scholar]
- Gust D, Moore TA, Moore AL. Mimicking photosynthetic solar energy transduction. Acc Chem Res. 2001;34(1):40–48. doi: 10.1021/ar9801301. [DOI] [PubMed] [Google Scholar]
- Hamilton PB, Van Slyke DD. The gasometric determination of free amino acids in blood filtrates by the ninhydrin-carbon dioxide method. J Biol Chem. 1943;150(1):231–250. [Google Scholar]
- Hammami Z, Gauffreteau A, BelhajFraj M, Sahli A, Jeuffroy MH, Rezgui S, Bergaoui K, McDonnell R, Trifaa Y. Predicting yield reduction in improved barley (Hordeum vulgare L.) varieties and landraces under salinity using selected tolerance traits. Field Crops Res. 2017;211:10–18. doi: 10.1016/j.fcr.2017.05.018. [DOI] [Google Scholar]
- Hasegawa PM. Sodium (Na+) homeostasis and salt tolerance of plants. Environ Exp Bot. 2013;92:19–31. doi: 10.1016/j.envexpbot.2013.03.001. [DOI] [Google Scholar]
- Iannucci A, Russo M, Arena L, Di Fonzo N, Martiniello P. Water deficit effects on osmotic adjustment and solute accumulation in leaves of annual clovers. Eur J Agron. 2002;16(2):111–122. doi: 10.1016/S1161-0301(01)00121-6. [DOI] [Google Scholar]
- Iqbal M, Ashraf M. Gibberellic acid mediated induction of salt tolerance in wheat plants: growth, ionic partitioning, photosynthesis, yield and hormonal homeostasis. Environ Exp Bot. 2013;86:76–85. doi: 10.1016/j.envexpbot.2010.06.002. [DOI] [Google Scholar]
- Kelly EH, Anthony RT, Dennis JB. Flavonoid antioxidants: chemistry, metabolism, and structure-activity relationships. J Nutr Biochem. 2002;13(10):572–584. doi: 10.1016/S0955-2863(02)00208-5. [DOI] [PubMed] [Google Scholar]
- Kirk JT, Allen RL. Dependence of chloroplast pigment synthesis on protein synthesis: effect of actidione. Biochem Biophys Res Commun. 1965;21(6):523–530. doi: 10.1016/0006-291X(65)90516-4. [DOI] [PubMed] [Google Scholar]
- Kole C, Kole P, Randunu KM, Choudhary P, Podila R, Ke PC, Rao AM, Marcus RK. Nanobiotechnology can boost crop production and quality: first evidence from increased plant biomass, fruit yield and phytomedicine content in bitter melon (Momordica charantia) BMC Biotechnol. 2013;13(1):37. doi: 10.1186/1472-6750-13-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagacherie P, Álvaro-Fuentes J, Annabi M, Bernoux M, Bouarfa S, Douaoui A, Grünberger O, Hammani A, Montanarella L, Mrabet R, Sabir M. Managing mediterranean soil resources under global change: expected trends and mitigation strategies. Reg Environ Change. 2018;18(3):663–675. doi: 10.1007/s10113-017-1239-9. [DOI] [Google Scholar]
- Lee SJ, Jeong EM, Ki AY, Oh KS, Kwon J, Jeong JH, Chung NJ. Oxidative defense metabolites induced by salinity stress in roots of Salicornia herbacea. J Plant Physiol. 2016;206:133–142. doi: 10.1016/j.jplph.2016.08.015. [DOI] [PubMed] [Google Scholar]
- Liu, et al. A novel treatment of neuroinflammation against low back pain by soluble fullerol nanoparticles. Spine. 2013;38(17):1443. doi: 10.1097/BRS.0b013e31828fc6b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshner H. Mineral nutrition of higher plants. 2. New York: Academic Press; 1995. [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004;9(10):490–498. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Mukherjee SP, Choudhuri MA. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in vigna seedlings. Physiol Plant. 1983;58(2):166–170. doi: 10.1111/j.1399-3054.1983.tb04162.x. [DOI] [Google Scholar]
- Munns R, Gilliham M. Salinity tolerance of crops—What is the cost? New Phytol. 2015;208(3):668–673. doi: 10.1111/nph.13519. [DOI] [PubMed] [Google Scholar]
- Munns R, James RA, Gilliham M, Flowers TJ, Colmer TD. Tissue tolerance: an essential but elusive trait for salt-tolerant crops. Funct Plant Biol. 2016;43(12):1103–1113. doi: 10.1071/FP16187. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22(5):867–880. [Google Scholar]
- Nath M, Yadav S, Sahoo RK, Passricha N, Tuteja R, Tuteja N. PDH45 transgenic rice maintain cell viability through lower accumulation of Na+, ROS and calcium homeostasis in roots under salinity stress. J Plant Physiol. 2016;191:1–11. doi: 10.1016/j.jplph.2015.11.008. [DOI] [PubMed] [Google Scholar]
- Pearcy RW, Schulze ED, Zimmermann R (2000) Measurement of transpiration and leaf conductance. In: Plant physiological ecology, Springer, Dordrecht, pp 137–160
- Pękal A, Pyrzynska K. Evaluation of aluminium complexation reaction for flavonoid content assay. Food Anal Methods. 2014;7(9):1776–1782. doi: 10.1007/s12161-014-9814-x. [DOI] [Google Scholar]
- Per TS, Khan NA, Reddy PS, Masood A, Hasanuzzaman M, Khan MIR, Anjum NA. Approaches in modulating proline metabolism in plants for salt and drought stress tolerance: phytohormones, mineral nutrients and transgenics. Plant Physiol Biochem. 2017;115:126–140. doi: 10.1016/j.plaphy.2017.03.018. [DOI] [PubMed] [Google Scholar]
- Rahman A, Mostofa MG, Nahar K, Hasanuzzaman M, Fujita M. Exogenous calcium alleviates cadmium-induced oxidative stress in rice (Oryza sativa L.) seedlings by regulating the antioxidant defense and glyoxalase systems. Braz J Bot. 2016;39(2):393–407. doi: 10.1007/s40415-015-0240-0. [DOI] [Google Scholar]
- Roy SJ, Negrão S, Tester M. Salt resistant crop plants. Curr Opin Biotechnol. 2014;26:115–124. doi: 10.1016/j.copbio.2013.12.004. [DOI] [PubMed] [Google Scholar]
- Ruffino AMC, Rosa M, Hilal M, González JA, Prado FE. The role of cotyledon metabolism in the establishment of quinoa (Chenopodium quinoa) seedlings growing under salinity. Plant Soil. 2010;326:213–224. doi: 10.1007/s11104-009-9999-8. [DOI] [Google Scholar]
- Sachkova AS, Kovel ES, Vorobeva AA, Kudryasheva NS. Antioxidant activity of fullerenols. bioluminescent monitoring in vitro. Proc Tech. 2017;27:230–231. doi: 10.1016/j.protcy.2017.04.097. [DOI] [Google Scholar]
- Santos CV. Regulation of chlorophyll biosynthesis and degradation by salt stress in sunflower leaves. Sci Hort. 2004;103:93–99. doi: 10.1016/j.scienta.2004.04.009. [DOI] [Google Scholar]
- Shabala S. Signalling by potassium: another second messenger to add to the list? J Exp Bot. 2017;68(15):4003–4007. doi: 10.1093/jxb/erx238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabala S, Shabala S, Cuin TA, Pang J, Percey W, Chen Z, Conn S, Eing C, Wegner LH. Xylem ionic relations and salinity tolerance in barley. Plant J. 2010;61:839–853. doi: 10.1111/j.1365-313X.2009.04110.x. [DOI] [PubMed] [Google Scholar]
- Shafiq F, Raza SH, Bibi A, Khan I, Iqbal M. Inluence of proline priming on antioxidative potential and ionic distribution and its relationship with salt tolerance of wheat. Cereal Res Commun. 2018;46(2):286–299. doi: 10.1556/0806.46.2018.10. [DOI] [Google Scholar]
- Shafiq F, Iqbal M, Ali M, Ashraf MA. Seed pre-treatment with polyhydroxy fullerene nanoparticles confer salt tolerance in wheat through up-regulation of H2O2 neutralizing enzymes and phosphorus uptake. J Soil Sci Plant Nutr. 2019;19(4):734–742. doi: 10.1007/s42729-019-00073-4. [DOI] [Google Scholar]
- StatNano (2018) StatNano annual report 2017, StatNano Publications
- Stefanov M, Yotsova E, Rashkov G, Ivanova K, Markovska Y, Apostolova EL. Effects of salinity on the photosynthetic apparatus of two Paulownia lines. Plant Physiol Biochem. 2016;101:54–59. doi: 10.1016/j.plaphy.2016.01.017. [DOI] [PubMed] [Google Scholar]
- Szabados LS, Savoure A. Proline: a multifunctional amino acid. Trends Plant Sci. 2009;15(2):89–97. doi: 10.1016/j.tplants.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Tadesse W, Halila H, Jamal M, El-Hanafi S, Assefa S, Oweis T, Baum M. Role of sustainable wheat production to ensure food security in the Cwana region. J Exp Biol Agric Sci. 2017;5:15–32. [Google Scholar]
- Tardieu F, Granier C, Muller B. Water deficit and growth. Co-ordinating processes without an orchestrator? Curr Opin Plant Biol. 2011;14(3):283–289. doi: 10.1016/j.pbi.2011.02.002. [DOI] [PubMed] [Google Scholar]
- van Doorn WG, Ketsa S. Cross reactivity between ascorbate peroxidase and phenol (guaiacol) peroxidase. Postharvest Biol Technol. 2014;95:64–69. doi: 10.1016/j.postharvbio.2014.04.002. [DOI] [Google Scholar]
- Velikova V, Yordanov I, Edreva A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci. 2000;151(1):59–66. doi: 10.1016/S0168-9452(99)00197-1. [DOI] [Google Scholar]
- Wang C, Zhang H, Ruan L, Chen L, Li H, Chang XL, Zhang X, Yang ST. Bioaccumulation of 13C-fullerenol nanomaterials in wheat. Environ Sci Nano. 2016;3(4):799–805. doi: 10.1039/C5EN00276A. [DOI] [Google Scholar]
- Wang N, Qi H, Qiao W, Shi J, Xu Q, Zhou H, Yan G, Huang Q. Cotton (Gossypium hirsutum L.) genotypes with contrasting K+/Na+ ion homeostasis: implications for salinity tolerance. Acta Physiol Plant. 2017;39(3):77–87. doi: 10.1007/s11738-017-2381-1. [DOI] [Google Scholar]
- Wolf B. A comprehensive system of leaf analyses and its use for diagnosing crop nutrient status. Commun Soil Sci Plant Anal. 1982;13(12):1035–1059. doi: 10.1080/00103628209367332. [DOI] [Google Scholar]
- Xiong JL, Li J, Wang HC, Zhang CL, Naeem MS. Fullerol improves seed germination, biomass accumulation, photosynthesis and antioxidant system in Brassica napus L. under water stress. Plant Physiol Biochem. 2018;129:130–140. doi: 10.1016/j.plaphy.2018.05.026. [DOI] [PubMed] [Google Scholar]
- Yanagi K, Okubo S, Okazaki T, Kataura H. Endohedral metallofullerenes as strong singlet oxygen quenchers. Chem Phy Lett. 2007;435(4–6):306–310. doi: 10.1016/j.cplett.2006.12.092. [DOI] [Google Scholar]
- Yin JJ, Lao F, Fu PP, Wamer WG, Zhao Y, Wang PC, Qiu Y, Sun B, Xing G, Dong J, Chen C. The scavenging of reactive oxygen species and the potential for cell protection by functionalized fullerene materials. Biomaterials. 2009;30(4):611–621. doi: 10.1016/j.biomaterials.2008.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Li J, Ma H, Zhen M, Guo J, Wang L, Jiang L, Shu C, Wang C. Biocompatible [60]/[70] fullerenols: potent defense against oxidative injury induced by reduplicative chemotherapy. ACS Appl Mat Interfaces. 2017;9(41):35539–35547. doi: 10.1021/acsami.7b08348. [DOI] [PubMed] [Google Scholar]