Abstract
Ultraviolet radiation induces biochemical and genetic changes in plants. The aim of this study was to investigate the effects of UV-B radiation on genetic stability, phenolic compounds and antioxidant activity of Pelargonium graveolens L′Her. Plant cuttings were exposed to 0, 0.12. 0.26 and 0.38 W/m2 of UV-B radiation. Results indicated that by increasing the UV-B radiation intensity, total phenols, flavonoids and anthocyanin contents, Phenylalanine ammonia lyase activity and antioxidant capacity were increased. Analysis of four flavonols (quercetin, myricetin, kaempferol and rutin) contents of leaves extract by HPLC indicated that these four flavonols were enhanced in all treated plants and also the ratio of quercetin to kaempferol (Q/K) showed a significant increase (P ≤ 0.05) in UV-B treated plants in compare to control. To evaluate the genetic variation in treated plants, 10 ISSR primers were used. The highest level of percentage of polymorphism (P%), Shannon index (I), number of effective allele (Ne) and Nei’ genetic diversity (He), were observed at the highest UV-B radiation (0.38 W/m2). The AMOVA analysis also showed a significant genetic differentiation (P ≥ 0.001) among the studied groups, and confirmed the differentiation of groups obtained by the cluster analysis of molecular data. Overall, these results showed that biochemical changes in different intensities of UV-B were in line with genetic variations, so that the highest biochemical and genetic variations were observed in 0.38 W/m2 treatment.
Keywords: Pelargonium graveolens, UV-B radiation, Flavonol, HPLC, ISSR
Introduction
Pelargonium graveolens, belonging to the Geraniaceae family, is a high value medicinal plant (Sharopov et al. 2014), which is planted for its valuable oil that is used in cosmetic and food industries (Pandey and Patra 2015). In adittion, this plant has a very high antioxidant activity against diseases such as cancer, heart disease and diabetes. Also, the leaf of this plant is used as a kind of herbal tea to solve mental problems such as stress and anxiety (Saraswathi et al. 2011; Fayed 2009). Compared to the total rays of the sun reaching the ground, UV-B rays, with a wavelength of 290–315 nm includes a small part (Neugart and Schreiner 2018). Ozone (O3) is the important component absorbing UV-B radiation in atmosphere. Enhanced use of environmental pollutants such as chlorofluorocarbon gas (CFCs) have destroyed ozone layer. Therefore, more UV-B radiation reaches the surface of the earth (Inostroza-Blancheteau et al. 2016). Studies show that this ray can cause harmful effects on the plant. These changes include changes in genomics, lipid destruction, oxidative damage, changes in plant biochemistry and reduced growth (Neugart and Schreiner 2018). In addition to numerous UV-B damages on plants, sometimes, this radiation can induce the plants to synthesize some compounds that are useful to humans (Jansen et al. 2008). Plants have a many strategies to cope with UV-B stress that allow them to offset the negative effects of this radiation. As an avoidance strategy, when plants are exposed to UV-B radiation, the production of secondary metabolites is one of the best known solutions to protect the plant against UV-B. For example the activity of phenylpropanoids pathway including phenols, flavonoids and anthocyanin increases in response to UV-B rays. Many of the enzymes in phenylpropanoid pathway are activated by UV-B. One of them is Phenylalanine ammonia-lyase (PAL), the important and a key enzyme in this pathway (Matsuura et al. 2013; Escobar et al. 2017). These compounds accumulate in the epidermal cell vacuoles and prevent the penetration of UV-B rays into sensitive leaf areas, such as photosynthetic tissues (Zlatev et al. 2012). Also, because of redox properties, these compounds might function as single oxygen scavengers (antioxidants) and can effectively prevent the macromolecular oxidation, such as lipids, proteins and nucleic acids, to reduce plant damage (Karaman et al. 2010; Tawaha et al. 2007; Zheng and Wang 2001).
Antioxidants are divided into chemical and natural antioxidants. Natural antioxidants are made in plants. Phenols and terpenoids are an example of natural antioxidants. Flavonoids are probably the largest group of low molecular weight polyphenolic secondary metabolites that are widespread throughout the plant kingdom and an important group of natural antioxidants. They include several sub classes, such as flavonols, flavones, and so on (Matsuura et al. 2013; Gupta et al. 2014). UV-B induces accumulation of flavonols (especially quercetin and kaempferol) in plants. Flavonols are not as efficient as other secondary metabolites in absorbing UV-B radiation, but show the greatest ability to control stress-induced changes in cellular reactive oxygen species homeostasis, and to manage the development of separate organs and the whole plant (Pollastri and tattini 2011). Today, the use of herbal extracts or metabolites extracted from plants as antioxidants is widely used in the medical, pharmaceutical and food industries, and, sometimes, the amount of these compounds increases in the presence of stress factors such as UV-B radiation, drought, salinity, and so forth (Matsuura et al. 2013; Joo et al. 2010).
At the molecular level, UV-B radiation damages lipids, proteins and nucleic acid.
DNA under UV-B radiation produces multiple DNA photoproducts which may change DNA sequence and cause some mutations during replication. The most widespread DNA damages are the dimerization which produces cyclobutane-type pyrimidine dimers (CPDs) and the pyrimidine (6, 4) pyrimidon. In addition, deletion, insertion of base pairs, DNA protein cross link and DNA strand breaks may be generated under UV-B radiation. These DNA alterations can induce phenotype changes, but sometimes these changes are silent mutations (Cuadra et al. 2004). Due to this, molecular biology has provided a useful tool for analysis of DNA changes in living organisms in recent years. Molecular markers such as ISSRs, which are PCR based markers, are designed based on target microsatellite sites for genetic and molecular studies (Shiran et al. 2007; Irshad et al. 2014; Chaves et al. 2009).
In previous work (Azarafshan et al. 2019), we analyzed the effect of UV-B radiation on the essential oils composition and antioxidant enzymes activity. In order to find out more about the effect of UV-B exposure on P. graveolens, this study was set up to investigate the effect of UV-B radiation on the alteration in Pelargonium’s DNA, PAL activity, total phenols, flavonoids and anthocyanin contents as well as antioxidant properties. In addition, Rutin, Quercetin, Myricetin, and Kaempferol as some important flavonoid components in this plant were analyzed with high-performance liquid chromatography (HPLC).
Materials and methods
The aromatic geranium plant was prepared from a greenhouse belonging to the Botanical Garden of Iran. More than 60 planting cuttings (without leaves) were prepared from the mother plant and each were planted in plastic pots (12 × 10 cm) containing sand and kept in a greenhouse under the following conditions, 16 h of light and 8 h (day/night) at 23 ± 2 °C and relative humidity of 56%. The PAR for these cuttings was 194 μmol/m2 s, which was generated by two 40 W lamps (Pars khazar, Iran). The cuttings were rooted after about 4 weeks and transferred to pots containing mix sterilized loam soil and kept in a greenhouse till they produced 5 leaves, then they were treated with UV-B radiation.
UV-B treatment
The experiment was conducted in a completely randomized design. Each treatment was performed with 15 replicates (pots) and one cutting plant was planted in each pot.
In each replicate, five-leaf cuttings were treated. The cuttings received UV-B irradiation under control: T0: 0.00, T1: 0.12, T2: 0.26, and T3: 0.38 W/m2. Treatments were applied to cuttings for 7 days and daily for 10 min. UV-B radiation source was artificially supplied with the Sankyo Denki lamps (G15T8E/Japan) located 70 cm above the cutting plants.
After 2 weeks of UV treatments, fresh leaves of cuttings were harvested for biochemical and genetically analysis.
Sample preparation
The dried leaves (0.1 g) samples (for flavonoids and DPPH assay) and 0.1 g of fresh leaves samples (for total phenols) hydrolysed with 5 ml of methanol 80%, and shaken for 24 h. The resulted solutions were then centrifuged at 13,500×g for 15 min, and the supernatants were used for the determination of total phenols, flavonoids and antioxidant activity.
Total phenols assay
The total phenols in the sample extracts were analyzed spectrophotometrically using a modification of folin-ciocalteu colorimetric by the method of Marinova et al. (2005). Gallic acid was used as standard.
Total flavonoids content
The total flavonoids content was measured by the aluminum chloride colorimetric assay by Chang et al. (2002) method. Quercetin was used as a standard.
Antioxidant activity
The DPPH assay was determined according to Akowuah et al. (2005) method. 2,2-diphenyl-1-picrylhydrazyl (DPPH, 0.004%) was prepared freshly before analysis and ascorbic acid was used as positive control. Five volumes (50–250 μl) of the test extract were added to 1 ml of DPPH and incubated for 30 min. The absorbance was read at 517 nm and the inhibition percentage was calculated with I % = ((Ab − As)/Ab) × 100) where Ab is the absorbance of negative control reaction and As is the absorbance of samples. Percentage of inhibition after 30 min was plotted against concentration, and the equation for the line was used to obtain the IC50 (half maximal inhibitory concentration) value.
Anthocyanin assay
The 0.1 g of fresh leaves of each sample was homogenized with 10 ml methanol-HCl (99:1) according to Nogues and Baker (2000) method. The absorbance was scanned spectrophotometrically (530 nm) to calculate the content of anthocyanin.
Determination of PAL activity
Approximately 0.5 g of the fresh leaves of each sample was homogenized with 5 ml tris-glycine buffer. The extract was used to determine the PAL activity by Wang et al. (2006) method. Cinnamic acid was used as a standard.
HPLC analysis
0.5 g of dried leaves were hydrolysed with 5 ml of methanol 80%, and shaken for 24 h, then centrifuged at 13,500×g for 15 min, and the supernatants were made up to 5 ml by methanol 80%. The extracts were filtered via 0.45 μm nylon filters and stored at 4 °C for further analysis.
Quercetin, Kaempferol, Myricetin, and Rutin standards were provided by Sigma (USA), and prepared in 80% methanol. For each individual compounds, the calibration curves were drawn using different concentrations of standards.
The analysis of Quercetin, Kaempferol, Myricetin, and Rutin was done using Agilent 1260 infinity ii HPLC system (Agilent, USA), covering Binary Pump, Vial sampler with integrated column compartment, 6.0 µl heater and sample cooler, Diode Array Detector WR with a standard 10-mm flow cell, 1100 Autosampler, 1100 Thermostatted Column Compartment, 1100 Diode Array Detector with a standard 10-mm flow cell, and Agilent OpenLAB CDS Version 2.1. The separation of compounds was carried out using Agilent ZORBAX Eclipse Plus C18, 4.6 × 150 mm, 5 µm. The used mobile phase was methanol: 200, acetonitrile: 100, acetic acid: 10, phosphoric acid: 10, and 200 ml of water. The injection volume was 20 μl by a flow rate of 0.6 ml/min. The integrated peak areas of the sample against the standard curves were used for quantification of compounds, in terms of mg/ml.
Molecular analyses
DNA extraction
Five leaves as a pooled sample was used for each treatment. The genomic DNA was extracted from each sample by CTAB-based method according to Doyle and Doyle (1990). The quality of extracted DNA was examined by its separation on 0.8% (w/v) agaros gel. In addition, DNA concentration was measured by NanoDrop. The A260/A280 ratio was greater than 1.8.
ISSR-amplification
Ten ISSR primers; UCB810, UCB811, UCB834, (GA)9 A, (GA)9 T, (GA)9 C, (AGC)5 GC, (AGC)5 GG, (AGC)5 GT and (AGC)5 GA, commercialized by UBC (the university of British Colombia) were used. ISSR-amplifications were performed in Bio-Rad (T-100, USA) thermal cycler. PCRs were run in 25 µl volumes with following program: 5 min initial denaturation step 94 °C, 40 cycles of 1 min at 94 °C, 1 min at 55 °C and 1:30 min at 72 °C. The reaction was completed by final extension step of 10 min at 72 °C. The amplification products were visualized by electrophoresis on 2% (w/v) agaros gel.
Statistical analysis
Genetic analysis
The fragment size was estimated by using 100 bp molecular size ladder (fermentase, Germany). ISSR bands obtained were treated as binary characters and coded accordingly (present: 1, absence: 0).
Parameter like Nei’s gene diversity (He), Shannon information index (I), number of effective alleles (Ne), and percentage of polymorphism (P%) were determined (Weising et al. 2005; Freeland et al. 2011). Nei’s genetic distance was determined among the studied groups and used for clustering (Freeland et al. 2011; Huson and Bryant 2006). For grouping of the treated plants, Neighbor joining (NJ) clustering was performed after 1000 bootstraping (Huson and Bryant 2006; Freeland et al. 2011). Molecular variance (AMOVA) test (with 1000 per mutations) and PCoA analyzed with the use of GenAlex 6.4 (Peakall and Smouse 2006). Gene Alex ver. 6.4 (2012) and DARwin ver. 5 (2012) programs were used for these analysis.
Biochemical analysis
One Way ANOVA and Duncan test at P ≤ 0/05 level of significance, were used to compare the mean differences among samples by using SPSS Ver. 16. All experiments were conducted by three replications and data were expressed as the mean ± SE.
Results
Total phenols, flavonoids, anthocyanin and PAL activity
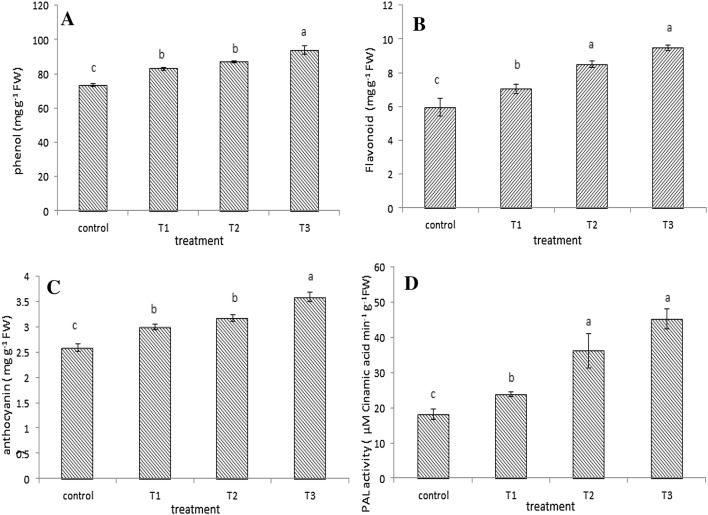
Leaves of plant treated with different intensities of UV-B showed a significant increase in the mean of phenols and PAL activity (P ≤ 0.05) at the highest intensity of UV-B radiation.
The highest total phenols content (93.99 mg/gfw) was observed in 0.38 W/m2 treatment and the lowest (73.45 mg/gfw) in the control plants (Fig. 1a).
Fig. 1.
Phenol (a), flavonoid (b), anthocyanin (c) and PAL activity (d) under different UV-B radiation (control: 0, T1: 0.12 W/m2, T2: 0.26 W/m2 and T3: 0.38 W/m2). Bars are standard errors (Mean ± SE). Different letters indicate significant differences between treatments (P ≤ 0.05)
The highest amount of flavonoid (9.50 mg/gdw) was achieved in 0.38 W/m2 treatment and the lowest (5.97 mg/gdw) in the control plants (Fig. 1b).
The highest anthocyanin content (3.58 mg/gfw) was observed in 0.38 W/m2 treatment and the lowest (2.59 mg/gfw) in control plants (Fig. 1c).
The PAL activity significantly increased by increasing the intensity of UV-B radiation. The highest activity (45.28 µM cinammic acid/min gfw) was gained in 0.38 W/m2 treatment and the lowest (18.16 µM cinammic acid/min gfw) in control plants. The pattern of PAL activity was the same as flavonoid content and it had a moderate similarity by phenol and anthocyanin amounts (Fig. 1d).
Flavonol content
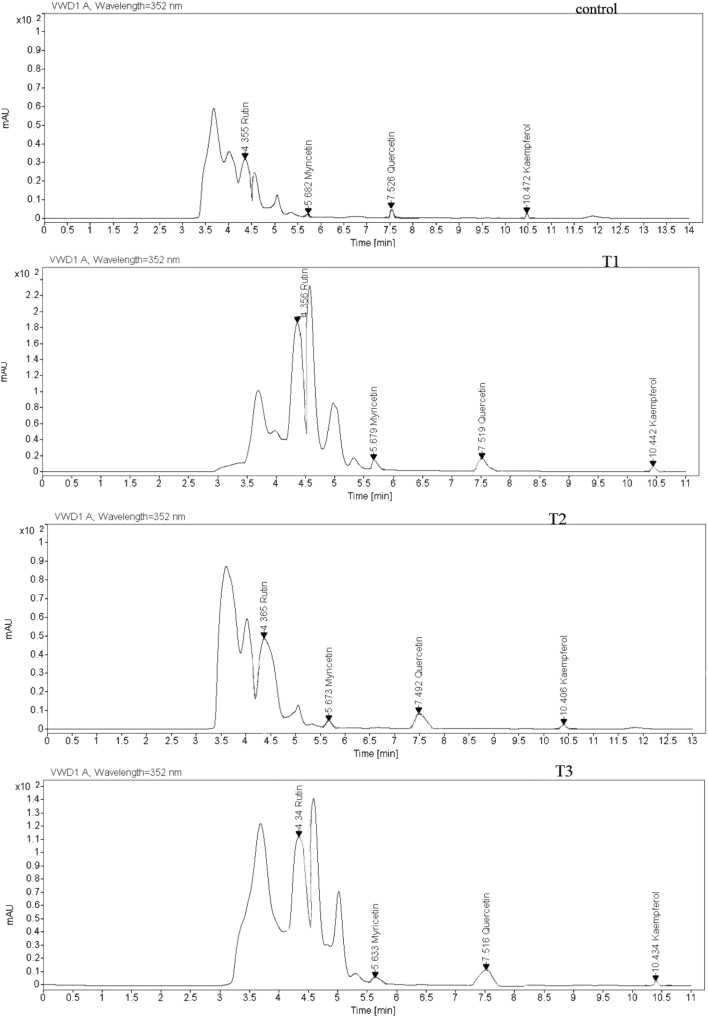
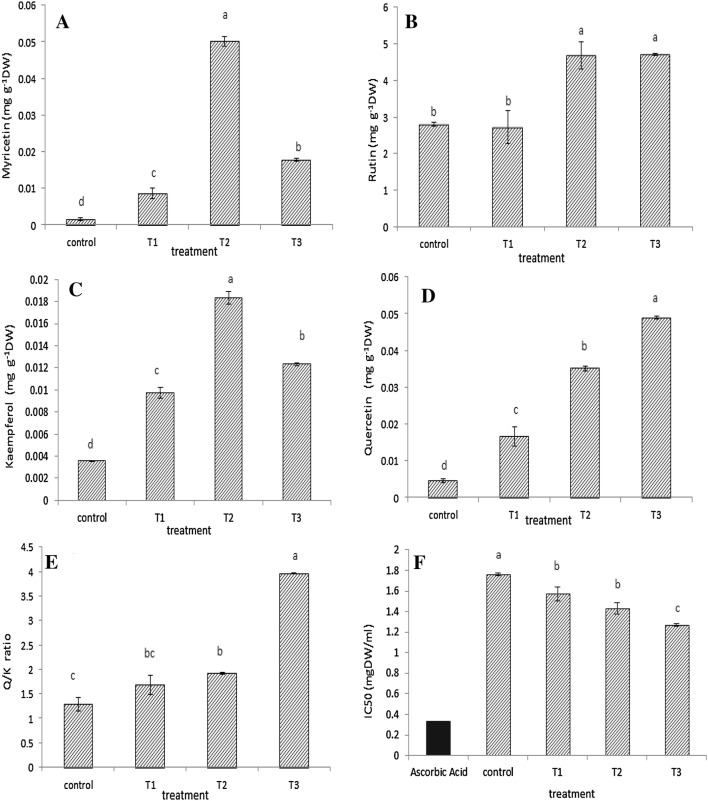
Four flavonols (quercetin, myricetin, kaempferol and rutin) in treated plants were observed by HPLC analysis (Fig. 2). Results showed that with increasing intensity of UV-B radiation, all of the above compounds increased significantly (P ≤ 0.05) compared to the control plant (Fig. 3a–e). The minimum of all of them were determined in control plants. High level of myricetin, rutin and kaempferol were observed in 0.26 W/m2 treatment while the highest amount of quercetin was gained in 0.38 W/m2 treatment.
Fig. 2.
HPLC chromatogram of P. graveolens methanolic extract under different UV-B radiation (control: 0, T1: 0.12 W/m2, T2: 0.26 W/m2 and T3: 0.38 W/m2)
Fig. 3.
Myricetin (a), Rutin (b), Kaempferol (c), Quercetin (d), Q/K ratio (e) and IC50 (f) under different UV-B radiation (control: 0, T1; 0.12 W/m2, T2: 0.26 W/m2 and T3: 0.38 W/m2). Bars are standard errors (Mean ± SE). Different letters indicate significant differences between treatments (P ≤ 0.05)
The ratio of quercetin to kaempferol (Q/K) also showed a significant increase (P ≤ 0.05) in all treatments, whereas the highest Q/K ratio (3.97) was recorded in 0.38 W/m2 treatment (Fig. 3e).
Antioxidant activity
Analysis of data showed that the mean IC50 decreased significantly (P ≤ 0.05) with increasing the intensity of the UV-B treatment. The lowest IC50 (1.27 mgdw/ml), was observed in the 0.38 W/m2 treatment and the highest IC50 (1.76 mgdw/ml) in the control plants. All of the studied plants had an IC50 higher than that of ascorbic acid, indicating that the antioxidant capacity of all the samples was approximately half the antioxidant capacity of ascorbic acid (Fig. 3f).
Genetic analyses
P. graveolens plants which were subjected to different UV-B radiation, showed the highest content of percentage of polymorphism (P%), Shannon index (I), number of effective allele (Ne) and Nei’ genetic diversity (He) (23.87%, 0.192, 1.231 and 0.131 respectively) in the 0.38 W/m2 treatment with the highest radiation intensity (Table 1).
Table 1.
Genetic parameters under UV-B radiation
| Treatment | Parameters | ||||
|---|---|---|---|---|---|
| Ne | I | He | UHe | P% | |
| Control | 1.143 ± 0.016 | 0.117 ± 0.013 | 0.080 ± 0.009 | 0.096 ± 0.011 | 19.89 |
| T1 (0.12 W/m2) | 1.127 ± 0.015 | 0.107 ± 0.012 | 0.073 ± 0.008 | 0.087 ± 0.010 | 18.5 |
| T2 (0.26 W/m2) | 1.163 ± 0.017 | 0.140 ± 0.013 | 0.094 ± 0.009 | 0.113 ± 0.011 | 24.59 |
| T3 (0.38 W/m2) | 1.123 ± 0.019 | 0.192 ± 0.015 | 0.131 ± 0.010 | 0.157 ± 0.012 | 32.87 |
Ne number of effective alleles, I Shannon’s information index, He gene diversity, UHe unbiased gene diversity, P% percentage of polymorphism
The highest genetic distance (0.301) was obtained between control plants and those exposed to the highest radiation intensity i.e. 0.38 W/m2 treatment, and the lowest genetic distance (0.188) was between 0.12 W/m2 and 0.26 W/m2 treatments (Table 2).
Table 2.
Genetic distance (Nei) between treatments (control: 0, T1: 0.12 W/m2, T2: 0.26 W/m2 and T3: 0.38 W/m2)
| Control | T1 (0.12 W/m2) | T2(0.28 W/m2) | T3(0.38 W/m2) | |
|---|---|---|---|---|
| 0.00 | Control | |||
| 0.220 | 0.00 | T1 (0.12 W/m2) | ||
| 0.252 | 0.188* | 0.00 | T2 (0.28 W/m2) | |
| 0.301** | 0.288 | 0.253 | 0.00 | T3 (0.38 W/m2) |
*Lowest genetic distance, **highest genetic distance
According to NJ tree, all groups and all individuals were completely separated and located in three clusters, and the plants of control and 0.12 W/m2 treatments were the sub clusters of one (the same) cluster (Fig. 4). The study of plants under greenhouse conditions with PCoA showed that all treatments were almost separated in this environment (Fig. 5). This result is in agreement with the NJ tree result.
Fig. 4.
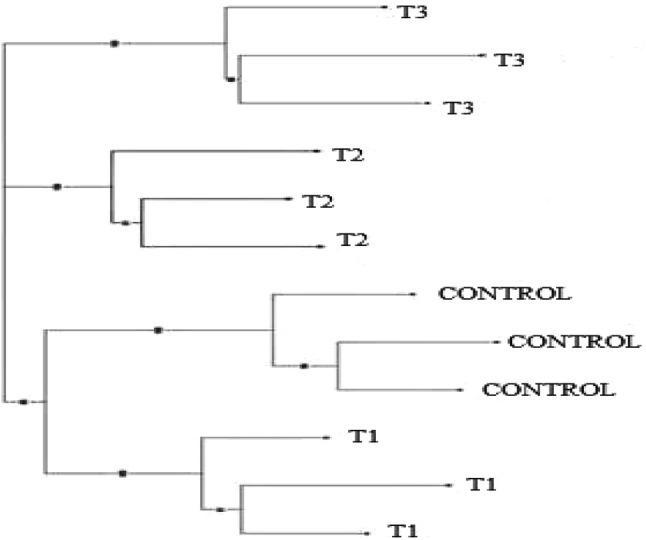
Neigbor joining diagram of P. graveolens under UV-B radiation (control: 0, T1: 0.12 W/m2, T2: 0.26 W/m2 and T3: 0.38 W/m2)
Fig. 5.
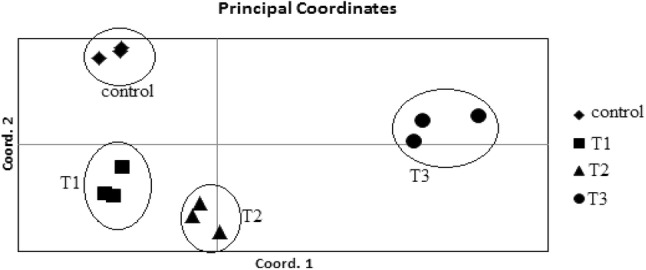
PCoA of P. graveolens under UV-B radiation (control: 0, T1: 0.12 W/m2, T2: 0.26 W/m2 and T3: 0.38 W/m2)
Analysis of AMOVA showed 53% variance between-group with significant difference (P ≥ 0.001) and 47% variance within-group studied.
Discussion
In present study, the content of total phenols, flavonoids and anthocyanin increased with increasing UV-B radiation.
Investigation on their secondary metabolites pattern also shows that the plant has produced a greater amount of secondary compounds (total phenols, flavonoids and anthocyanin) to counteract the effects of radiation, especially at high intensities (0.26 and 0.38 W/m2). However, the content of phenols and anthocyanin showed no significant differences between the treatments of low and medium radiation intensities. It seems that flavonoids are the main defenders among the secondary compounds synthesized by the two treatments.
Phenols, as non-enzymatic antioxidants, protect plants against stress. It is also proven that high concentrations of phenols accumulation can give the plant greater resistance to UV-B radiation, indicating a major role for these metabolites (Katerova et al. 2012; Chang et al. 2008). The increase in total phenols content is also reported in the fruit of strawberries and in Chrysanthemum at the four stages of floral development, under the influence of ultraviolet radiation (Erkan et al. 2008; Ma et al. 2016).
Flavonoids are major compounds in plant tissues, whose protective action, that is, the defense against oxidative damage caused by UV-B is proved (Alvero-Bascos and Ungson 2012).
Santos et al. (2004) studied the changes in potato leaves influenced by UV-B radiation and reported that UV-B radiation increased the content of flavonoids. The increased content of flavonoids with increasing UV radiation is also reported for two species of Vigna species (Dwivedi et al. 2015).
UV-B is considered as the inducer of expression of anthocyanin biosynthesis pathway genes. Anthocyanin, located in the epidermis, are a UV receptor and protect the photosynthesis system against oxidative damage caused by ultraviolet radiation (Takshak and Agrawal 2015).
Basahi et al. (2014) studied the effect of ultraviolet radiation on Lactuca sativa and reported that all UV absorption components such as carotenoids, phenols, and anthocyanins increased. The increased content of anthocyanins is also reported in the seedlings of Vigna mungo from the Fabacea family, treated with UV-B (Shaukat et al. 2013).
Results showed that PAL enzyme activity increased with increasing irradiation. Since this enzyme is a key enzyme in the phenylpropanoids synthesis pathway, it seems that with increased activity of this enzyme, the pathway is activated under stress and thus synthesizes more secondary compounds. Moreover, the highest activity was observed at the highest radiation intensity, which corresponds to the pattern of increase of secondary compounds studied.
PAL converts phenylalanine into the cinnamic acid, and by successive reactions, this compound is converted into other compounds such as flavonoids, phenols, lignins, and tannins. Flavonoids and compounds derived from phenylpropanoid, as UV absorbing compounds in the epidermis, protect other plant tissues against the damage of UV radiation (Chang et al. 2008; Piri et al. 2011; Di Ferdinando et al. 2012). Escobar et al. (2017) investigated the levels of UV-B resistance in Vaccinium corymbosum cultivars and synthesis of phenylpropanoid metabolites and stated that the amount of PAL and expression of its related genes were higher in resistant cultivars than that of susceptible cultivars. Ma et al. (2016) studied the effect of increased UV-B radiation on Chrysanthemum and reported that the amount of PAL enzyme in this study increased with increasing UV.
The four flavonols examined showed an increase in this experiment. Although the incremental pattern of these compounds varied, the results showed that all the compounds were increased compared to the control plant. In comparison, the increasing pattern of quercetin is quite similar to the increasing pattern of flavonoids. It seems that under UV-B stress, the plant can reduce synthesis of some compounds in favor of the other one to make more useful compounds and protect the plant from adverse effects.
The increased content of quercetin under UV is reported by Gotz et al. (2010), Agati et al. (2011) and Morales et al. (2010).
As well, the findings reported by Demkura and Ballaré (2012), Neugart et al. (2012) and Mewis et al. (2012) show an increased content of kaempferol under UV.
The usual response to UV light is a marked increase in quercetin content, while the content of Kaempferol will increase slightly or remain unchanged. This indicates that quercetin and ortho-dihydroxyls are better protectors for plants against UV radiation. Also, the ratio of Q/K in plants under sunlight (receiving UV radiation) is more than shaded plants. Quercetin is said to be a better antioxidant to scavenge singlet oxygen as compared with Kaempferol. Therefore, in the making of mono-hydroxylated compounds such as Kaempferol and its derivatives, a change occurs towards dihydroxylated compounds such as quercetin. For this reason, a high Q/K ratio is presented in many reports (Majer et al. 2014; Agati et al. 2011; Kotilainen et al. 2008).
Rutin is a polyphenolic bioflavonoid with a high antioxidant activity (Gupta et al. 2014). Rutin can act directly by entering into the Redox reaction or indirectly by iron chelating (Namazian et al. 2008). The reason for the high antioxidant properties of this plant and its medicinal value may be due to the high content of rutin. There are several findings showing that UV increases rutin production in plants. Suzuki et al. (2005) reported that the amount of rutin increased under UV-B radiation in buckwheat plant. They suggested that the rutin plays a role in enhancing the plant’s defense against UV stress.
According to Mahdavian et al. (2008), the content of rutin in the Capsicum annuum L. was higher in UV-B and UV-C treated plants as compared with control plants.
Tegelberg et al. (2001) studied the long-term effects of UV-B on Betula pendula and stated that the content of quercetin increased. They concluded that the changes in myricetin were unpredictable under UV-B. Also, in another study on the content of myricetin in Betula Pendula leaves affected by ultraviolet radiation, it was found that the content of myricetin decreased (Morales et al. 2010).
Investigation on antioxidant activity of P. graveolens leaves methanolic extract showed that with increasing UV-B radiation, phenols, flavonoids and anthocyanin increased, whereas IC50 decreased. This reason could be due to the high phenolic compounds produced under UV-B stress. These compounds, especially flavonoids, have high antioxidant properties. The pattern of highest antioxidant activity of the cuttings plant at the highest irradiation intensity is completely consistent with the pattern of secondary compounds studied because of their antioxidant nature. Our finding indicated that all of three compounds above can have a role in decrease of IC50. Most natural antioxidants usually work together to create a wide range of antioxidant properties with a strong defense system against free radicals (Inostroza-Blancheteau et al. 2016).
Comparision between antioxidant activity with flavonols patterns showed that the decreasing template of IC50 was similar with increase in quercetin content and Q/K ratio. Li et al. (2017) found that under the stress of UV-C, the content of phenolic compounds and antioxidant activity of several types of tropical fruit increased.
In our study, with increasing intensity of UV-B, the amount of genetic changes increased and genetic stability was lost.
The results show that the highest genetic variation occurred at the highest intensity (0.38 W/m2) of UV-B irradiation. As well, the treatment T1 (0.12 W/m2) is the closest treatment to the control and the difference between T1 (0.12 W/m2) and T2 (0.26 W/m2) is less than other treatments.
Apart from being able to show the extent of damage and changes in DNA under different irradiation intensities, this pattern closely corresponds to the biochemical pattern section studied.
In all the sections studied, the pattern of increase in the content of examined compounds showed that the highest increase was at the highest intensity.
Furthermore, no significant difference was observed between T1 (0.12 W/m2) and T2 (0.26 W/m2) treatments for anthocyanin, antioxidant, phenol, and the ratio of quercetin to kaempferol as one of the most important ratios in UV-B radiation. Also, the highest proximity in terms of the content of compounds with control plants was observed in the low (0.12 W/m2) treatment. It seems that the changes observed at the DNA level can either be due to a change in gene sequence due to UV radiation or to a change in part of the DNA sequence, which may ultimately alter the synthesis of the compounds studied.
UV radiation is one of the most hazardous factors for biomolecules and DNA is an important target for it. Plants produce UV-absorbing compounds for coping by UV radiation as a first line for defense (Rastogi et al. 2010; Gill et al. 2015). It seems that these changes are induced by UV-B radiation as a mechanism of the signal that triggers the expression of genes involved in plant defense against UV-B radiation. Plants under UV stress try to reduce the risk of radiation by increasing secondary metabolites, neutralizing ROS species and monomerize the pyrimidine dimers. These changes are very suitable at the genetic level, especially those involved in the production of secondary metabolites, because they are very useful in coping with the stresses in the plant (Cuadra et al. 2010), but they are incapable to avoid UV-B radiation from DNA in all tissue. So when UV-B radiation reach to DNA, it can prouduces many damages such as: (1) oxidative damage (pyrimidin hydrates) and cross link (both DNA–DNA and DNA protein). (2) produce cyclobutane pyrimidine dimers (CPDs) and other photoproducts, pyrimidine (6–4) pyrimidine dimers in nuclear, chloroplast and mitochondrial DNA. Plants have variable strategies to cope with DNA damages, if the DNA repair does not work well, the accumulation of DNA damage may alter genetic stability due to the mutations occurring (Rastogi et al. 2010; Gill et al. 2015).
Increased polymorphism and genetic instability with ISSR primer is reported in the study of Gnaphalium luteo-albicans under ultraviolet radiation (Cuadra et al. 2010).
Genetic instability in tobacco plants in response to UV-B radiation is also reported by Ries et al. (2000). Genetic variation had been already proven in plants under ultraviolet radiation, in 19 different lines of Arabidopsis (kravets et al. 2012).
Conclusion
The results of this study showed that UV-B radiation affected P. graveolens and caused the increased content of secondary compounds in this plant. This is also the reason for the increased antioxidant power of the extract of this plant at higher radiation intensities. Therefore, the UV-B radiation in the intensities studied here, especially the highest intensity as an elicitor can increase the medicinal value of P. graveolens.
Moreover, the selection of ISSR marker for genetic studies of this plant was a good choice, and this marker was able to completely identify changes caused by radiation in plants and separate individuals and groups. In addition, in present study results indicated that the genetic stability in P. graveolens decreased under UV-B radiation.
Authors’ contributions
This article is part of the results of the Ph.D. thesis: 1. MA, Ph.D. student. 2. MP, Supervisor 1. 3. HA: Supervisor 2. 4. ZN: Advisor 1. 5. AM: Advisor 2.
Funding
We declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Agati G, Cerovic ZG, Pinelli P, Tattini M. Light-induced accumulation of ortho-dihydroxylated flavonoids as non-destructively monitored by chlorophyll fluorescence excitation techniques. Environ Exp Bot. 2011;73:3–9. doi: 10.1016/j.envexpbot.2010.10.002. [DOI] [Google Scholar]
- Akowuah GA, Ismail Z, Norhayati I, Sadikun A. The effects of different extraction solvents of varying polarities on polyphenols of Orthosiphon stamineus and evaluation of the free radical-scavenging activity. Food Chem. 2005;93(2):311–317. doi: 10.1016/j.foodchem.2004.09.028. [DOI] [Google Scholar]
- Alvero-Bascos EM, Ungson LB. Ultraviolet-B (UV-B) radiation as an elicitor of flavonoid production in callus cultures of jatropha (Jatropha curcas L.) Phillipp Agric Sci. 2012;95(4):335–343. [Google Scholar]
- Azarafshan M, Peyvandi M, Abbaspour H, Noormohammadi Z, Majd A. The impact of UV-B radiation on antioxidant activity, essential oil composition and physiological factors of Pelargonium graveolens L’Hér. Acta Agric Slov. 2019;113(2):211–219. doi: 10.14720/aas.2019.113.2.2. [DOI] [Google Scholar]
- Basahi JM, Ismail IM, Hassan IA. Effects of enhanced UV-B radiation and drought stress on photosynthetic performance of lettuce (Lactuca sativa L. Romaine) plants. Annu Res Rev Biol. 2014;4(11):1739–1756. doi: 10.9734/ARRB/2014/6638. [DOI] [Google Scholar]
- Chang CC, Yang H, Wen HM, Chern JC. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10(3):178–182. [Google Scholar]
- Chang A, Lim MH, Lee SW, Robb EJ, Nazar RN. Tomato phenylalanine ammonia-lyase gene family, highly redundant but strongly underutilized. J Biol Chem. 2008;283(48):33591–33601. doi: 10.1074/jbc.M804428200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves MM, Flexas J, Pinheiro C. Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot. 2009;103(4):551–560. doi: 10.1093/aob/mcn125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadra P, Herrera R, Fajardo V. Effects of UV-B radiation on the Patagonian Jaborosa magellanica Brisben. J Photochem Photobiol B. 2004;76(1–3):61–68. doi: 10.1016/j.jphotobiol.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Cuadra P, Vargas D, Fajardo V, Herrera R. Effects of UV-B radiation in morpho-genetic characters of Gnaphalium luteo-album. J Photochem Photobiol B. 2010;101(1):70–75. doi: 10.1016/j.jphotobiol.2010.06.013. [DOI] [PubMed] [Google Scholar]
- Demkura PV, Ballaré CL. UVR8 mediates UV-B-induced Arabidopsis defense responses against Botrytis cinerea by controlling sinapate accumulation. Mol Plant. 2012;5(3):642–652. doi: 10.1093/mp/sss025. [DOI] [PubMed] [Google Scholar]
- Di Ferdinando M, Brunetti C, Fini A, Tattini M. Flavonoids as antioxidants in plants under abiotic stresses. In: Ahmad P, Prasad M, editors. Abiotic stress responses in plants. New York: Springer; 2012. pp. 159–179. [Google Scholar]
- Doyle JJ, Doyle JL. Isolation of plant DNA from fresh tissue. Focus. 1990;12(13):39–40. [Google Scholar]
- Dwivedi R, Singh VP, Kumar J, Prasad SM. Differential physiological and biochemical responses of two Vigna species under enhanced UV-B radiation. J Radiat Res Appl Sci. 2015;8(2):173–181. doi: 10.1016/j.jrras.2014.12.002. [DOI] [Google Scholar]
- Erkan M, Wang SY, Wang CY. Effect of UV treatment on antioxidant capacity, antioxidant enzyme activity and decay in strawberry fruit. Postharvest Biol Technol. 2008;48(2):163–171. doi: 10.1016/j.postharvbio.2007.09.028. [DOI] [Google Scholar]
- Escobar AL, De Oliveira Silva F M, Acevedo P, Nunes-Nesi A, Alberdi M, Reyes-Diaz M. Different levels of UV-B resistance in Vaccinium corymbosum cultivars reveal distinct backgrounds of phenylpropanoid metabolites. Plant Physiol Biochem. 2017;118:541–550. doi: 10.1016/j.plaphy.2017.07.021. [DOI] [PubMed] [Google Scholar]
- Fayed SA. Antioxidant and anticancer activities of Citrus reticulate (Petitgrain Mandarin) and Pelargonium graveolens (Geranium) essential oils. Res J Agric Biol Sci. 2009;5(5):740–747. [Google Scholar]
- Freeland JR, Kirk H, Peterson SD. Molecular ecology. 2. Hoboken: Wiley-Blackwell; 2011. [Google Scholar]
- Gill SS, Anjum NA, Gill R, Jha M, Tuteja N. DNA damage and repair in plants under ultraviolet and ionizing radiations. Sci: World J; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotz M, Albert A, Stich S, Heller W, Scherb H, Krins A, Langebartels C, Seidlitz HK, Ernst D. PAR modulation of the UV-dependent levels of flavonoid metabolites in Arabidopsis thaliana (L). Heynh. leaf rosettes: cumulative effects after a whole vegetative growth period. Protoplasma. 2010;243:95–103. doi: 10.1007/s00709-009-0064-5. [DOI] [PubMed] [Google Scholar]
- Gupta N, Chauhan RS, Pradhan JK (2014) Handbook of medicinal plants and their bioactive compounds 5. Rutin: a bioactive flavonoid, pp 51–57. ISBN 978-81-308-0548-1
- Huson DH, Bryant D. Application of phylogenetic networks in evolutionary studies. Mol Biol Evol. 2006;23:254–267. doi: 10.1093/molbev/msj030. [DOI] [PubMed] [Google Scholar]
- Inostroza-Blancheteau C, Acevedo P, Loyola R, Arce- Johnson P, Alberdi M, Reyes-Diaz M. Short-term UV-B radiation affects photosynthetic performance and antioxidant gene expression in highbush blueberry leaves. Plant Physiol Biochem. 2016;107:301–309. doi: 10.1016/j.plaphy.2016.06.019. [DOI] [PubMed] [Google Scholar]
- Irshad M, Idrees M, Saeed A, Naeem M, Naeem R. Genetic diversity among Asparagus species and cultivars of Asparagus officinalis L. using random amplified polymorphic DNA (RAPD) markers. Int J Biodivers Conserv. 2014;6(5):392–399. doi: 10.5897/IJBC2014.0708. [DOI] [Google Scholar]
- Jansen MAK, Hectors K, Obrien NM, Guisez Y, Potters G. Plant stress and human health: Do human consumers benefit from UV-B acclimated crops? Plant Sci. 2008;175(4):449–458. doi: 10.1016/j.plantsci.2008.04.010. [DOI] [Google Scholar]
- Joo SS, Kim YB, Lee DI. Antimicrobial and antioxidant properties of secondary metabolites from white rose flower. Plant Pathol J. 2010;26(1):57–62. doi: 10.5423/PPJ.2010.26.1.057. [DOI] [Google Scholar]
- Karaman S, Tütem E, Baskan KS, Apak R. Comparison of total antioxidant capacity and phenolic composition of some apple juices with combined HPLC–CUPRAC assay. Food Chem. 2010;120(4):1201–1209. doi: 10.1016/j.foodchem.2009.11.065. [DOI] [Google Scholar]
- Katerova Z, Todorova D, Tasheva K, Sergiev I. Influence of ultraviolet radiation on plant secondary metabolite production. Genet Plant Physiol. 2012;2(3–4):113–144. [Google Scholar]
- Kotilainen T, Tegelberg R, Julkunen-tiitto R, Lindfors A, Aphalo PJ. Metabolite specific effects of solar UV-A and UV-B on alder and birch leaf phenolics. Glob Change Biol. 2008;14(6):1294–1304. doi: 10.1111/j.1365-2486.2008.01569.x. [DOI] [Google Scholar]
- Kravets EA, Zelena LB, Zabara EP, Blume YB. Adaptation strategy of barley plants to UV-B radiation. Emir J Food Agric. 2012;24(6):632–645. doi: 10.9755/ejfa.v24i6.14682. [DOI] [Google Scholar]
- Li P, Yu X, Xu B. Effects of UV-C light exposure and refrigeration on phenolic and antioxidant profiles of subtropical fruits (Litchi, Longan, and Rambutan) in different fruit forms. J Food Qual. 2017;2017:1–12. doi: 10.1155/2017/8785121. [DOI] [Google Scholar]
- Ma CH, Chu JZ, Shi XF, Liu CQ, Yao XQ. Effects of enhanced UV-B radiation on the nutritional and active ingredient contents during the floral development of medicinal chrysanthemum. J Photochem Photobiol B. 2016;158:228–234. doi: 10.1016/j.jphotobiol.2016.02.019. [DOI] [PubMed] [Google Scholar]
- Mahdavian K, Ghorbanli M, Kalantari KM. The effects of ultraviolet radiation on the contents of chlorophyll, flavonoid, anthocyanin and proline in Capsicum annum L. Turk J Bot. 2008;32(1):25–33. [Google Scholar]
- Majer P, Neugart S, Krumbein A, Schreiner M, Hideg E. Singlet oxygen scavenging by leaf flavonoids contributes to sunlight acclimation in Tilia platyphyllos. Environ Exp Bot. 2014;100:1–9. doi: 10.1016/j.envexpbot.2013.12.001. [DOI] [Google Scholar]
- Marinova D, Ribarova F, Atanassova M. Total phenolics and total flavonoids in Bulgarian fruits and vegetables. J Chem Technol Metall. 2005;40(3):255–260. [Google Scholar]
- Matsuura HN, De Costa F, Yendo ACA, Fett-Neto AG. Photo-elicitation of bioactive secondary metabolites by ultraviolet radiation: mechanisms, strategies, and applications. In: Chandra S, Lata H, Varma A, editors. Biotechnology for medicinal plants. Berlin: Springer; 2013. pp. 171–190. [Google Scholar]
- Mewis I, Schreiner M, Nguyen CN, Krumbein A, Ulrichs C, Lohse M, Zrenner R. UV-B irradiation changes specifically the secondary metabolite profile in broccoli sprouts: induced signaling overlaps with defense response to biotic stressors. Plant Cell Physiol. 2012;53(9):1546–1560. doi: 10.1093/pcp/pcs096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales LO, Tegelberg R, Brosche M, Keinanen M, Lindfors A, Aphalo PJ. Effects of solar UV-A and UV-B radiation on gene expression and phenolic accumulation in Betula pendula leaves. Tree Physiol. 2010;30(7):923–934. doi: 10.1093/treephys/tpq051. [DOI] [PubMed] [Google Scholar]
- Namazian M, Zare HR, Coote ML. Determination of the absolute redox potential of Rutin: experimental and theoretical studies. Biophys Chem. 2008;132(1):64–68. doi: 10.1016/j.bpc.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Neugart S, Schreiner M. UVB and UVA as eustressors in horticultural and agricultural crops. Sci Hortic. 2018;234:370–381. doi: 10.1016/j.scienta.2018.02.021. [DOI] [Google Scholar]
- Neugart S, Zietz M, Schreiner M, Rohn S, Kroh LW, Krumbein A. Structurally different flavonol glycosides and hydroxycinnamic acid derivatives respond differently to moderate UV-B radiation exposure. Physiol Plant. 2012;145(4):582–593. doi: 10.1111/j.1399-3054.2012.01567.x. [DOI] [PubMed] [Google Scholar]
- Nogues S, Baker NR. Effects of drought on photosynthesis in Mediterranean plants grown under enhanced UV-B radiation. J Exp Bot. 2000;51(348):1309–1317. doi: 10.1093/jxb/51.348.1309. [DOI] [PubMed] [Google Scholar]
- Pandey V, Patra DD. Crop productivity, aroma profile and antioxidant activity in Pelargonium graveolens L’Her under integrated supply of various organic and chemical fertilizers. Ind Crops Prod. 2015;67:257–263. doi: 10.1016/j.indcrop.2015.01.042. [DOI] [Google Scholar]
- Peakall R, Smouse PE. GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol Notes. 2006;6:288–295. doi: 10.1111/j.1471-8286.2005.01155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piri E, Babaeian M, Tavassoli A, Esmaeilian Y. Effects of UV irradiation on plants. Afr J Microbiol Res. 2011;5(14):1710–1716. doi: 10.5897/AJMR11.263. [DOI] [Google Scholar]
- Pollastri S, Tattini M. Flavonols: old compounds for old roles. Ann Bot. 2011;108(7):1225–1233. doi: 10.1093/aob/mcr234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi RP, Kumar A, Tyagi MB, Sinha RP. Molecular mechanisms of ultraviolet radiation-induced DNA damage and repair. J Nucleic Acids. 2010 doi: 10.4061/2010/592980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries G, Heller W, Puchta H, Sandermann H, Seidlitz HK, Hohn B. Elevated UV-B radiation reduces genome stability in plants. Nature. 2000;406:98–101. doi: 10.1038/35017595. [DOI] [PubMed] [Google Scholar]
- Santos I, Fidalgo F, Almeida JM, Salema R. Biochemical and ultrastructural changes in leaves of potato plants grown under supplementary UV-B radiation. Plant Sci. 2004;167(4):925–935. doi: 10.1016/j.plantsci.2004.05.035. [DOI] [Google Scholar]
- Saraswathi J, Venkatesh K, Baburao N, Hilal MH, Rani AR. Phytopharmacological importance of Pelargonium species. J Med Plants Res. 2011;5(13):2587–2598. [Google Scholar]
- Sharopov FS, Zhang H, Setzer WN. Composition of geranium (Pelargonium graveolens) essential oil from Tajikistan. Am J Essent Oil. 2014;2(2):6–13. [Google Scholar]
- Shaukat SS, Farooq MA, Siddiqui MF, Zaidi S. Effect of enhanced UV-B radiation on germination, seedling growth and biochemical responses of Vigna mungo (L.) Hepper. Pak J Bot. 2013;45(3):779–785. [Google Scholar]
- Shiran B, Amir Bakhtiar N, Kiani S, Mohammadi SH, Sayed-Tabatabaei BE, Moradi H. Molecular characterization and genetic relationship among almond cultivars assessed by RAPD and SSR markers. Sci Hortic. 2007;111(3):280–292. doi: 10.1016/j.scienta.2006.10.024. [DOI] [Google Scholar]
- Suzuki T, Honda Y, Mukasa Y. Effects of UV-B radiation, cold and desiccation stress on rutin concentration and rutin glucosidase activity in tartary buckwheat (Fagopyrum tataricum) leaves. Plant Sci. 2005;168(5):1303–1307. doi: 10.1016/j.plantsci.2005.01.007. [DOI] [Google Scholar]
- Takshak S, Agrawal SB. Defence strategies adopted by the medicinal plant Coleus forskohlii against supplemental ultraviolet-B radiation: augmentation of secondary metabolites and antioxidants. Plant Physiol Biochem. 2015;97:124–138. doi: 10.1016/j.plaphy.2015.09.018. [DOI] [PubMed] [Google Scholar]
- Tawaha K, Alali FQ, Gharaibeh M, Mohammad M, El-Elimat T. Antioxidant activity and total phenolic content of selected Jordanian plant species. Food Chem. 2007;104(4):1372–1378. doi: 10.1016/j.foodchem.2007.01.064. [DOI] [Google Scholar]
- Tegelberg R, Julkunen-Tiitto R, Aphalo PJ. The effects of long-term elevated UV-B on the growth and phenolics of field-grown silver birch (Betula pendula) Glob Chang Biol. 2001;7(7):839–848. doi: 10.1046/j.1354-1013.2001.00453.x. [DOI] [Google Scholar]
- Wang JW, Zheng LP, Wu JY, Tan RX. Involvement of nitric oxide in an oxidative burst, phenylalanine ammonia-lyase activation and Taxol production induced by low-energy ultrasound in Taxus yunnanensis cell suspension cultures. Nitric Oxide. 2006;15(4):351–358. doi: 10.1016/j.niox.2006.04.261. [DOI] [PubMed] [Google Scholar]
- Weising K, Nybom H, Pfenninger M, Wolff K, Kahl G. DNA fingerprinting in plants: principles, methods, and applications. Boca Raton: CRC Press; 2005. [Google Scholar]
- Zheng W, Wang SY. Antioxidant activity and phenolic compounds in selected herbs. J Agric Food Chem. 2001;49(11):5165–5170. doi: 10.1021/jf010697n. [DOI] [PubMed] [Google Scholar]
- Zlatev ZS, Lidon FJ, Kaimakanova M. Plant physiological responses to UV-B radiation. Emir J Food Agric. 2012;24(6):481–501. doi: 10.9755/ejfa.v24i6.14669. [DOI] [Google Scholar]