Abstract
Dioscorealide B is an important secondary metabolite isolated from Dioscorea membranacea Pierre ex Prain & Burkill. The effect on secondary metabolite content of different concentrations of two elicitors [jasmonic acid (JA) and salicylic acid (SA)], and of medium status and JA exposure period were investigated. In the JA and SA concentration experiment, 6-week-old shoots were cultured on MS medium supplemented with 8.87 µM BA (6-benzyladenine) in combination with 100–500 µM JA or 50–200 µM SA for 3 weeks. MS medium supplemented only with 8.87 µM BA was used as a control. The highest dioscorealide B content was recorded in the 100 µM JA shoots. To determine the optimal medium status and JA exposure period, shoots were cultured on solid and in liquid MS media supplemented with 8.87 µM BA and 100 µM JA for 2, 3, 4 and 5 weeks. No interaction was found between the medium status and the elicitor exposure period in the dioscorealide B production. Shoots cultured on the solid MS medium supplemented with 100 µM JA had a higher dioscorealide B content (0.57 ± 0.35% w/w) than those cultured in liquid medium (0.36 ± 0.40% w/w) and 5-week JA exposure produced the highest dioscorealide B content of 1.05 ± 0.15% (w/w).
Keywords: Medicinal plant, Elicitor, Jasmonic acid, Salicylic acid, Secondary metabolites
Introduction
Dioscorea membranacea Pierre ex Prain & Burkill is a rhizomatous perennial (Huber 1998) which has a distribution ranging from mixed deciduous forests to lower mountain evergreen forests (Wilkin and Thapyai 2009). The rhizomes of this medicinal plant have been used to treat dermopathy, lymphopathy, leprosy, venereal disease as well as inflammation, cancer and AIDS (Itharat et al. 2003, 2004; Saetung et al. 2005; Tewtrakul et al. 2006). The important secondary metabolite isolated from the rhizomes is dioscorealide B (Sukkarn and Itharat 2009). This compound exhibits high cytotoxic activity against lung cancer cells (CORL-23) and breast cancer cells (MCF-7) but is less toxic to normal cells (Itharat et al. 2004). The rhizomes of D. membranacea have been extensively used by Thai traditional doctors as ingredients in many medicinal preparations (Tewtrakul and Itharat 2006). As the demand for D. membranacea has increased, the plant populations in the forests have dramatically decreased (Rithichai et al. 2013). This plant species typically propagates vegetatively by rhizome, but with poor multiplication rates, resulting in insufficient supply to the pharmaceutical industry. Moreover, the secondary metabolite content from natural propagation varies according to the growing conditions and is usually slow to accumulate. As reported by Rithichai et al. (2013), cultivated rhizomes 15 months after planting (MAP) showed a lower dioscorealide B content of 2.7% (w/w), while the mother rhizomes collected from the forest had a higher content of 4.0% (w/w).
Plant tissue culture is an alternative technique used to produce secondary metabolites in many medicinal plants (Sauerwein et al. 1991; Amoo et al. 2012; Tan et al. 2010; Jirakiattikul et al. 2016). The advantages of this technique over conventional production are independence from environmental factors, continuous sourcing of products of consistent quality and quantity, and production of novel compounds (Ramachandra Rao and Ravishankar 2002). In vitro shoots of D. membranacea have been successfully regenerated but microrhizomes could not be induced under aseptic conditions (unpublished data). Therefore, in vitro shoots of this plant species were used to determine the secondary metabolite content. Boonyuen et al. (2014) reported that the 4–12-week-old regenerated shoots of D. membranacea contain 0.03 ± 0.02–0.97 ± 0.00% (w/w) of dioscorealide B, lower than those of the 15 MAP rhizomes or the mother rhizomes reported by Rithichai et al. (2013). Zhao et al. (2005) revealed that low yield of secondary metabolites is a major limitation of cell or organ cultures. This problem can be overcome by stimulation with elicitors, which induces the expression of defense-related genes and stimulates defense-related secondary metabolic pathways (Qian et al. 2006). Jasmonic acid (JA) and salicylic acid (SA) are biotic elicitors that have been applied to enhance secondary metabolite production under aseptic culture in medicinal species including Hypericum perforatum (Walker et al. 2002), H. hirsutum and H. maculatum (Coste et al. 2011), Morinda elliptica (Chong et al. 2005), Panax ginseng (Yu et al. 2002) and Rubia cordifolia (Bulgakov et al. 2002). However, there have been no reports of the use of JA and SA as elicitors in D. membranacea under aseptic conditions. Thus, the objectives of the present study were to investigate the effects of the JA and SA concentration, medium status and JA exposure period on the dioscorealide B content, total phenolic content, and DPPH radical scavenging activity in shoot cultures of D. membranacea.
Materials and methods
Plant materials, medium and culture conditions
Rhizomes of D. membranacea (Fig. 1a) were collected from their natural forest environment in Chumphon province, Thailand and planted in a greenhouse at Thammasat University, Pathum-thani, Thailand. Young aerial shoots (Fig. 1b) were collected, cut into pieces with a single lateral bud and surface sterilized in sodium hypochlorite (Clorox®) using a two-step procedure: 10% and 5% (v/v) sodium hypochlorite with a few drops of Tween-20 for 15 min and 10 min, respectively. Next, they were rinsed twice in sterile distilled water and inoculated vertically onto solid MS medium supplemented with 8.87 µM BA (6-benzyladenine) and 3% sucrose for shoot induction. The pH of the medium was adjusted to 5.8 with 1N NaOH and gelled with 0.8% agar before autoclaving at 121 °C for 15 min. The cultures were maintained at 25 ± 2 °C under a 16/8 h (light/dark) photoperiod using cool white fluorescent tubes. After shoots developed, single nodes were cut and cultured on the same medium. Subculturing was performed every 6 weeks to obtain the explants for the experiments.
Fig. 1.
Dioscorea membranacea: a rhizome; b aerial shoots growing in the greenhouse; c six-week-old shoots on MS medium supplemented with 8.87 µM BA were used as the explants for elicitation
Preparation of elicitors
Jasmonic acid (Sigma) was prepared following the method of Wiktorowska et al. (2010) and salicylic acid (BDH Prolabo) following Wang et al. (2004). Both chemicals were dissolved in 70% (v/v) ethanol and further diluted in distilled water to give final stock concentrations of 4.75 mM JA and 0.1 M SA. Filter sterilized stock solutions of each elicitor were made using a 0.22 µm syringe filter and added to the culture medium.
Experiment 1: JA and SA concentration
Six-week-old in vitro shoots of D. membranacea cultured on MS medium supplemented with 8.87 µM BA (Fig. 1c) were taken to determine the shoot fresh weight (FW). They were then transferred to culture on solid MS medium supplemented with 8.87 µM BA in combination with 100, 250 and 500 µM JA or 50, 100 and 200 µM SA for 3 weeks. MS medium supplemented only with 8.87 µM BA was used as a control. The experiment was arranged in a completely randomized design (CRD) with seven treatments and three replicates. The shoot FW of each treatment was recorded and the increase rate (mg/shoot/day) was calculated as follows (Marcelis 1993 cited in Wien 2002):
Shoots were dried at 50 °C for 48 h and powdered to determine the dioscorealide B and total phenolic contents. The antioxidant activity was also determined using DPPH radical scavenging assay after elicitation with JA and SA.
Experiment 2: Medium status and JA exposure
Six-week-old in vitro shoots of D. membranacea were cultured on solid and in liquid media supplemented with 8.87 µM BA and 100 µM JA (the results from experiment 1) for 2, 3, 4 and 5 weeks. The shoots were dried at 50 °C for 48 h and the dioscorealide B content was analyzed. The experiment used a 2 × 4 factorial in CRD with three replications.
Secondary metabolite determination
The method for preparation of plant extract was modified from Rithichai et al. (2013). Two grams of ground dried shoot sample were extracted over 3 days using chloroform at a ratio of 1:3. This procedure was repeated two more times on the residues. The extracts were filtered and evaporated at 50 °C. All extracts from each treatment were combined and stored at − 20 °C until analysis.
HPLC, as described by Sirikatitham et al. (2007) and Rithichai et al. (2013), was used to determine the dioscorealide B content. Plant extract samples were prepared and the analysis was performed in triplicate.
Total phenolic content was measured using the Folin–Ciocalteu method as described in Jirakiattikul et al. (2016). Gallic acid was used as the standard. Total phenolic content was expressed as milligrams of gallic acid equivalent per gram of dry extract (mg GAE/g dry extract). All tests were performed in triplicate.
The DPPH radical scavenging activity of the extracts was determined based on the methods of Yamasaki et al. (1994), as described by Jirakiattikul et al. (2016). BHT (butylated hydroxyltuluene) was used as the standard. Each sample was determined in triplicate and the EC50 value (effective concentration of sample required to scavenge DPPH radicals by 50%) was calculated using a regression equation.
Data analysis
Data obtained were subjected to analysis of variance using SAS version 9.0. Differences between means were assessed by Duncan’s new multiple range test at a 0.05 probability level.
Results and discussion
Experiment 1: JA and SA concentration
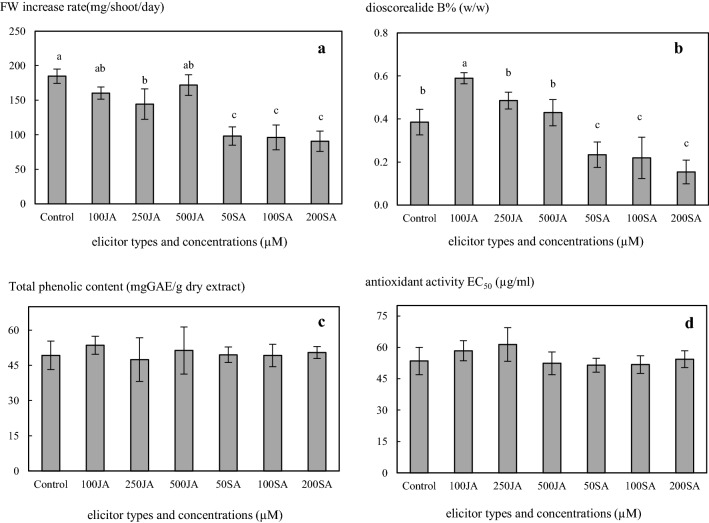
After the in vitro shoots had been cultured on media containing JA and SA at different concentrations for 3 weeks, significant differences were found in the FW increase rates under the different treatments. The control treatment had the highest FW increase rate of 8.80 ± 0.50 mg/shoot/day, which was not significantly different from the 100 or 500 µM JA cultures (7.64 ± 0.42 and 8.19 ± 0.71 mg/shoot/day, respectively). The 50, 100 and 200 µM SA shoots showed the lowest FW increase rates of 4.68 ± 0.63, 4.57 ± 0.86 and 4.32 ± 0.69 mg/shoot/day, respectively. (Figure 2a). This indicated that JA had weak effect on shoot growth of D. membranacea whereas SA had a negative effect. The results were inconsistent with a report on H. hirsutum and H. Maculatum, in which JA significantly inhibited shoot biomass but the effect of SA was insignificant (Coste et al. 2011). This suggests that plant growth response to elicitors may vary among plant species. Elicitor retardation of plant cell or shoot growth has been observed in other species such as R. cordifolia (Bulgakov et al. 2002), P. ginseng (Yu et al. 2002) and Salvia miltiorrhiza (Chen and Chen 2000).
Fig. 2.
a fresh weight increase rate, b dioscorealide B content, c total phenolic content and d DPPH radical scavenging activity of D. membranacea shoots after elicitation with JA and SA at different concentrations for 3 weeks
The effect of JA and SA on the dioscorealide B content of D. membranacea shoots is shown in Fig. 2b. It was found that the addition of 100 µM JA induced an increase in dioscorealide B content of 0.59 ± 0.08% (w/w), or 1.51 times that of the control (0.39 ± 0.17% w/w). At all SA concentrations (50–200 µM), the production of dioscorealide B (0.15 ± 0.06–0.23 ± 0.06% w/w) was significantly lower than that of the control. The effect of these two elicitors on total phenolic content and DPPH radical scavenging activity was also investigated. Phenolic compounds are secondary metabolites and play important roles in antioxidant activity (Matkowski 2008; Saxena et al. 2012) However, the total phenolic contents and DPPH radical scavenging activities in the D. membranacea shoots did not vary significantly with the elicitor type or concentration. The levels of total phenolic content were 47.44 ± 9.25–53.54 ± 3.85 mg GAE/g dry extract (Fig. 2c) and the DPPH radical scavenging activities with EC50 ranged from 51.45 ± 3.32 to 61.35 ± 7.99 µg/ml (Fig. 2d). The results revealed that JA and SA had no effect on the total phenolic content or DPPH radical scavenging activity in the D. membranacea shoots. However, SA had a deleterious effect on dioscorealide B production, while JA as an elicitor enhanced the production of dioscorealide B under aseptic conditions, especially at a concentration of 100 µM JA. This suggested that the effectiveness of elicitation depends on the elicitor used and its concentration. This is in agreement with Vasconsuelo and Boland (2007) and Coste et al. (2011) who reported that elicitation of secondary metabolite is a very complex process which depends on several factors: elicitor specificity, concentration of elicitors, stage of plant growth, contact time with the elicitor and culture conditions. JA and its derivatives, such as methyl jasmonate (MeJA), have been recommended as key signal compounds and are involved in the part of the signal transduction pathway that leads to accumulation of secondary metabolites (Gundlach et al. 1992; Mizukami et al. 1993; Yu et al. 2002). It has also been reported that JA is an effective elicitor of secondary metabolite production of numerous medicinal plants under aseptic conditions. However, the optimal concentration of JA depends on the plant species. In a study of M. elliptica, the highest anthraquinone level was obtained by supplementation of 50 µM JA on day 12 and harvesting on day 15 (Chong et al. 2005). Wiktorowska et al. (2010) reported that elicitation with 100 µM JA for 72 h produced the greatest accumulation of oleanolic acid in cell suspension cultures of Calendula officinalis. Yu et al. (2002) reported that the addition of 2 mg/l JA to the culture medium increased the ginsenoside yield of P. Ginseng, and Blando et al. (2005) showed that supplementation with 50 µM JA enhanced cyanidin 3-glucoside synthesis in callus culture of Prunus cerasus. In the present study, the highest dioscorealide B [0.59 ± 0.08% (w/w)] content occurred when the 6-week-old D. membranacea shoots were cultured on solid MS medium supplemented with 8.87 µM BA and 100 µM JA for 3 weeks. However, the most appropriate medium status or optimum JA exposure period for elicitation of dioscorealide B in shoot cultures of D. membranacea had not yet been determined. Therefore, the effects of these two factors were investigated in a second experiment.
Experiment 2: Medium status and JA exposure
As the first experiment had revealed that JA had no effect on total phenolic content or DPPH radical scavenging activity, in this experiment only dioscorealide B content was determined after 6-week-old in vitro shoots of D. membranacea were cultured on solid and in liquid media supplemented with 8.87 µM BA and 100 µM JA for 2, 3, 4 and 5 weeks. No interaction was found between the status of the medium and the elicitor exposure period (Table 1). This suggests that the effect of the medium status on the dioscorealide B content did not depend on the elicitor exposure period. Shoots cultured on the solid medium had an average dioscorealide B content of 0.57 ± 0.35%, (w/w), which was significantly higher than those cultured in liquid medium (0.36 ± 0.40% w/w), suggesting that the solid medium is appropriate for dioscorealide B elicitation by JA in shoot cultures of D. membranacea. As noted above, the culture condition is one of many factors that have an effect on secondary metabolite enhancement (Vasconsuelo and Boland 2007; Coste et al. 2011). Studies using a solid medium for elicitation have investigated a number of plant species, including R. cordifolia (Bulgakov et al. 2002), Dionaea muscipula and Drosera capensis (Krolicka et al. 2008) Bacopa monnieri (Largia et al. 2015) and Silybum marianum (Gabr et al. 2016). Medicinal plants cultured in liquid medium have included M. elliptica (Chong et al. 2005), Artemisia annua (Putalun et al. 2007), C. officinalis (Wiktorowska et al. 2010) and H. hirsutum and H. maculatum (Coste et al. 2011).
Table 1.
Dioscorealide B content of D. membranacea shoots cultured on solid or in liquid MS medium supplemented with 8.87 µM BA and 100 µM JA for 2–5 weeks
| Treatments | Dioscorealide B (% w/w) |
|---|---|
| Media status (S) | |
| Solid | 0.57 ± 0.351/a |
| Liquid | 0.36 ± 0.40b |
| Elicitor exposure periods (T: weeks) | |
| 2 | 0.15 ± 0.14c |
| 3 | 0.24 ± 0.15c |
| 4 | 0.43 ± 0.13b |
| 5 | 1.05 ± 0.15a |
| Media status (S) | ** |
| Elicitor exposure periods (T) | ** |
| S × T | ns |
ns not significantly different
**Significantly different at P < 0.01
1/Mean values (mean ± SD) within a column followed by different letters are significantly different at P < 0.05 according to Duncan’s New Multiple Range Test (DMRT)
The exposure period was also demonstrated to play a role in dioscorealide B production. Shoots treated with 100 µM JA for 5 weeks exhibited the highest content of 1.05 ± 0.15% (w/w) while those elicited for 2 and 3 weeks showed the lowest contents at 0.15 ± 0.14 and 0.24 ± 0.15% (w/w), respectively. The results indicated that the elicitor exposure period or contact time with the elicitor had an effect on the dioscorealide B content of D. membranacea shoots. Vasconsuelo and Boland (2007) reported that the time required for high secondary metabolite accumulation is distinctive in each plant species. Many studies have investigated the different periods of elicitor exposure needed to enhance the production of secondary metabolites. Yu et al. (2002) reported that total ginsenoside and Rb group ginsenoside was maximized when adventitious roots of P. ginseng were cultured on medium supplemented with 2 mg/l of JA for 7 days. In A. annua, hairy roots cultured in ½ MS liquid medium containing 150 mg/l of chitosan for over 6 days resulted in the highest artemisinin production (Putalun et al. 2007). The production of hypercin and pseudohypericin in H. hirsutum increased when shoots were cultured in liquid medium supplemented with 50 µM SA for 28 days (Coste et al. 2011). In addition, anthraquinone production of Robia tinctorum was markedly enhanced after 24 h of treatment with 200 mg/l chitosan, but no further enhancement was noted when exposure was extended to 48 h (Vasconsuelo et al. 2003). The optimal elicitor exposure period therefore depends on the plant species and must be independently determined for each system. In conclusion, production of dioscorealide B in D. membranacea shoots is enhanced by culturing the shoots on a solid MS medium supplemented with 100 µM of JA, with a culture period of at least 5 weeks.
Acknowledgements
This work was supported by The Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission. Thanks are also given to Mr. Norman R. Mangnall from Faculty of medicine, Thammasat University and Mr. John Winward from Thammasat University for their kind assistance in the proof reading of this manuscript.
Abbreviations
- BA
6-Benzyladenine
- FW
Fresh weight
- JA
Jasmonic acid
- MAP
Months after planting
- MS medium
Murashige and Skoog medium
Author contributions
YJ, PR and AI conceived and designed the experiments. YJ, TB and SR performed the experiments. YJ and PR prepared the manuscript. All authors discussed the results and implications and commented on the manuscript at all stages.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Amoo SO, Aremu AO, Van Staden J. In vitro plant regeneration, secondary metabolite production and antioxidant activity of micropropagated Aloe arborescens Mill. Plant Cell Tissue Organ Cult. 2012;11:345–358. doi: 10.1007/s11240-012-0200-3. [DOI] [Google Scholar]
- Blando F, Scardino AP, De Bellis L, Nicoletti I, Giovinazzo G. Characterization of in vitro anthocyanin-producing sour cherry (Prunus cerasus L.) callus cultures. Food Res Int. 2005;38:937–942. doi: 10.1016/j.foodres.2005.02.014. [DOI] [Google Scholar]
- Boonyuen T, Jirakiattikul Y, Rithichai P, Ruangnoo S, Itharat A. Dioscorealide B content of in vitro Khao–Yen–Tai (Dioscorea membranacea Pierre ex Prain & Burkill) shoots at different culture periods. Khon Kaen Agric J. 2014;3:306–310. [Google Scholar]
- Bulgakov VP, Tchernoded GK, Mischenko NP, Khodakovskaya MV, Glazunov VP, Radchenko SV, Zvereva EV, Fedoreyev SA, Zhuravlev YN. Effect of salicylic acid, methyl jasmonate, ethephon and cantharidin on anthraquinone production by Rubia cordifolia callus cultures transformed with the rolB and rolC genes. J Biotechnol. 2002;97:213–221. doi: 10.1016/S0168-1656(02)00067-6. [DOI] [PubMed] [Google Scholar]
- Chen H, Chen F. Effect of yeast elicitor on the secondary metabolism of Ti-transformed Salvia miltiorrhiza cell suspension culture. Plant Cell Rep. 2000;19:710–717. doi: 10.1007/s002999900166. [DOI] [PubMed] [Google Scholar]
- Chong TM, Abdullah MA, Lai OM, Nor’Aini FM, Lajis NH. Effective elicitation factors in Morinda elliptica cell suspension culture. Process Biochem. 2005;40:3397–3405. doi: 10.1016/j.procbio.2004.12.028. [DOI] [Google Scholar]
- Coste A, Vlase L, Halmagyi A, Deliu C, Coldea G. Effects of plant growth regulators and elicitors on production of secondary metabolites in shoot cultures of Hypericum hirsutum and Hypericum maculatum. Plant Cell Tissue Organ. 2011;106:279–288. doi: 10.1007/s11240-011-9919-5. [DOI] [Google Scholar]
- Gabr AMM, Ghareeb H, El Shabrawi HM, Smetanska I, Bekheet SA. Enhancement of silymarin and phenolic compound accumulation in tissue culture of Milk thistle using elicitor feeding and hairy root cultures. J Genet Eng Biotechnol. 2016;14:327–333. doi: 10.1016/j.jgeb.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundlach H, Müller MJ, Kutchan TM, Zenk MH. Jasmonic acid is a signal transducer in elicitor-induced plant cell cultures. Plant Biol. 1992;89:2389–2393. doi: 10.1073/pnas.89.6.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber H. Dioscoreaceae. In: Kubitzki K, editor. The families and genera of vascular plants. Flowering plant monocotyledons, Lilianae (except Orchidaceae) Germany: Springer; 1998. pp. 216–235. [Google Scholar]
- Itharat A, Plubrukarn A, Kongsaeree P, Bui T, Keawpradub N, Houghton PJ. Dioscorealides and dioscoreanone, novel cytotoxi naphthofuranoxepins and 1,4-phenanthraquinone from Dioscorea membranacea Pierre. Org Lett. 2003;5:2879–2882. doi: 10.1021/ol034926y. [DOI] [PubMed] [Google Scholar]
- Itharat A, Houghton PJ, Amooquaye EE, Burke PJ, Sampson JH, Raman A. In vitro cytotoxic activity of Thai medicinal plants used traditionally to treat cancer. J Ethnopharmacol. 2004;90:33–38. doi: 10.1016/j.jep.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Jirakiattikul Y, Rithichai P, Songsri O, Ruangnoo S, Itharat A. In vitro propagation and bioactive compound accumulation in regenerated shoots of Dioscorea birmanica Prain & Burkill. Acta Physiol Plant. 2016;38:249. doi: 10.1007/s11738-016-2268-6. [DOI] [Google Scholar]
- Krolicka A, Szpitter A, Gilgenast E, Romanik G, Kaminski M, Lojkowska E. Stimulation of antibacterial naphthoquinones and flavonoids accumulation in carnivorous plants grown in vitro by addition of elicitors. Enzym Microb Technol. 2008;42:216–221. doi: 10.1016/j.enzmictec.2007.09.011. [DOI] [Google Scholar]
- Largia MJV, Pothiraj G, Shilpha J, Ramesh M. Methyl jasmonate and salicylic acid synergism enhances bacoside A content in shoot cultures of Bacopa monnieri (L.) Plant Cell Tissue Organ Cult. 2015;122:9–20. doi: 10.1007/s11240-015-0745-z. [DOI] [Google Scholar]
- Matkowski A. Plant in vitro culture for the production of antioxidants—a review. Biotechnol Adv. 2008;26:548–560. doi: 10.1016/j.biotechadv.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Mizukami H, Tabira Y, Ellis BE. Methyl jasmonate-induced rosmarinic acid biosynthesis in Lithospermum erythrorhizon cell suspension culture. Plant Cell Rep. 1993;12:706–709. doi: 10.1007/BF00233424. [DOI] [PubMed] [Google Scholar]
- Putalun W, Luealon W, De-Eknamkul W, Tanaka H, Shoyama Y. Improvement of artemisinin production by chitosan in hairy root cultures of Artemisia annua L. Biotechnol Lett. 2007;29:1143–1146. doi: 10.1007/s10529-007-9368-8. [DOI] [PubMed] [Google Scholar]
- Qian ZG, Zhao ZJ, Qian X, Zhong JJ. Novel chemically synthesized salicylate derivative as an effective elicitor for inducing the biosynthesis of plant secondary metabolites. Biotechnol Prog. 2006;22:331–333. doi: 10.1021/bp0502330. [DOI] [PubMed] [Google Scholar]
- Ramachandra Rao S, Ravishankar GA. Plant cell culture: chemical factories of secondary metabolites. Biotechnol Adv. 2002;20:101–153. doi: 10.1016/S0734-9750(02)00007-1. [DOI] [PubMed] [Google Scholar]
- Rithichai P, Itharat A, Jirakiattikul Y, Ruangnoo S. Growth of Dioscorea membranacea Pierre ex Prain & Burkill and dioscorealide B content at different harvest times. Pharmacologyonline. 2013;1:225–229. [Google Scholar]
- Saetung A, Itharat A, Dechsukum C, Wattanapiromsakul C, Keawpradub N, Ratanasuwan P. Cytotoxic activity of Thai medicinal plants for cancer treatment. Songklanakarin J Sci Technol. 2005;27:469–478. [Google Scholar]
- Sauerwein M, Flores HE, Yamazaki T, Shimomura K. Lippia dulcis shoot cultures as a source of the sweet sesquiterpene hernandulcin. Plant Cell Rep. 1991;9:663–666. doi: 10.1007/BF00235352. [DOI] [PubMed] [Google Scholar]
- Saxena M, Saxena J, Pradhan A. Flavonoids and phenolic acids as antioxidants in plants and human health. Int J Pharm Sci Rev Res. 2012;16:130–134. [Google Scholar]
- Sirikatitham A, Chuchom T, Itharat A. Development of the chromatographic fingerprint analysis of dioscorealides and dioscoreanone from Dioscorea membranacea Pierre. Songklanakarin J Sci Technol. 2007;29:101–107. [Google Scholar]
- Sukkarn B, Itharat A. Development of the HPLC quantitative analysis of dioscorealide B from Dioscorea membranacea Pierre. Thammasat Med J. 2009;9:156–163. [Google Scholar]
- Tan SH, Musa R, Afiff A, Maziah M. Effect of plant growth regulators on callus, cell suspension and cell line selection for flavonoid production from Pegaga (Centella asiatica L. urban) Am J Biochem Biotechnol. 2010;6:284–299. doi: 10.3844/ajbbsp.2010.284.299. [DOI] [Google Scholar]
- Tewtrakul S, Itharat A. Anti-allergic substances from the rhizomes of Dioscorea membranacea. Bioorgan Med Chem. 2006;14:8707–8711. doi: 10.1016/j.bmc.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Tewtrakul S, Itharat A, Rattanasuwan P. Anti-HIV-1 protease- and HIV-1 integrase activities of Thai medicinal plants known as Hua–Khao–Yen. J Ethnopharmacol. 2006;105:312–315. doi: 10.1016/j.jep.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Vasconsuelo A, Boland R. Molecular aspects of the early stages of elicitation of secondary metabolites in plants. Plant Sci. 2007;172:861–875. doi: 10.1016/j.plantsci.2007.01.006. [DOI] [Google Scholar]
- Vasconsuelo A, Giutetti AM, Picotto G, Rodriguez T, Boland R. Involvement of the PLC/PKC pathway in Chitosan-induced anthraquinone production by Rubia tinctorum L. cell cultures. Plant Sci. 2003;165:429–436. doi: 10.1016/S0168-9452(03)00208-5. [DOI] [Google Scholar]
- Walker TS, Bais HP, Vivanco JM. Jasmonic acid-induced hypericin production in cell suspension cultures of Hypericum perforatum L. (St. John’s wort) Phytochemistry. 2002;60:289–293. doi: 10.1016/S0031-9422(02)00074-2. [DOI] [PubMed] [Google Scholar]
- Wang YD, Yuan YJ, Wu JC. Induction studies of methyl jasmonate and salicylic acid on taxane production in suspension cultures of Taxus chinensis var. mairei. Biochem Eng J. 2004;19:256–259. doi: 10.1016/j.bej.2004.02.006. [DOI] [Google Scholar]
- Wien HC. Correlation growth in vegetables. In: Wien HC, editor. The physiology of vegetable crops. Wallingford: CABI Publishing; 2002. pp. 181–206. [Google Scholar]
- Wiktorowska E, Dlugosz M, Janiszowska W. Significant enhancement of oleanolic acid accumulation by biotic elicitors in cell suspension cultures of Calendula officinalis L. Enzym Microb Technol. 2010;46:14–20. doi: 10.1016/j.enzmictec.2009.09.002. [DOI] [Google Scholar]
- Wilkin P, Thapyai C. Dioscoreaceae. In: Santisuk T, Larsen K, editors. Flora of Thailand 10. Bangkok: Prachachon; 2009. pp. 1–140. [Google Scholar]
- Yamasaki K, Hashimoto A, Kokusenya Y, Miyamoto T, Sato T. Electrochemical method for estimating the antioxidative effect of methanol extracts of crude drugs. Chem Pharm Bull. 1994;42:1663–1665. doi: 10.1248/cpb.42.1663. [DOI] [PubMed] [Google Scholar]
- Yu KW, Gao W, Hahn EJ, Paek KY. Jasmonic acid improves ginsenoside accumulation in adventitious root culture of Panax ginseng C.A. Meyer. Biochem Eng J. 2002;11:211–215. doi: 10.1016/S1369-703X(02)00029-3. [DOI] [Google Scholar]
- Zhao J, Davis LC, Verpoorte R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol Adv. 2005;23:283–333. doi: 10.1016/j.biotechadv.2005.01.003. [DOI] [PubMed] [Google Scholar]