Abstract
Renal ischemia/reperfusion injury (IRI) is a leading cause of acute kidney injury (AKI), a potentially fatal syndrome characterized by a rapid decline in kidney function. Excess production of superoxide contributes to the injury. We hypothesized that oral administration of a high dose of vitamin B12 (B12 - cyanocobalamin), which possesses a superoxide scavenging function, would protect kidneys against IRI and provide a safe means of treatment. Following unilateral renal IR surgery, C57BL/6J wild type (WT) mice were administered B12 via drinking water at a dose of 50 mg/L. After 5 days of the treatment, plasma B12 levels increased by 1.2-1.5x, and kidney B12 levels increased by 7-8x. IRI mice treated with B12 showed near normal renal function and morphology. Further, IRI-induced changes in RNA and protein markers of inflammation, fibrosis, apoptosis, and DNA damage response (DDR) were significantly attenuated by at least 50% compared to those in untreated mice. Moreover, the presence of B12 at 0.3 μM in the culture medium of mouse proximal tubular cells subjected to 3 hr of hypoxia followed by 1 hr of reperfusion in vitro showed similar protective effects, including increased cell viability and decreased reactive oxygen species (ROS) level. We conclude that a high dose of B12 protects against perfusion injury both in vivo and in vitro without observable adverse effects in mice and suggest that B12 merits evaluation as a treatment for I/R-mediated AKI in humans.
Keywords: Cyanocobalamin, Ischemia/reperfusion injury, Superoxide dismutase mimetic, Mouse proximal tubular cells, Inflammation, Fibrosis
Graphical abstract
Highlights
-
•
B12 decreases the level of ROS induced by ischemia/reperfusion in vivo and in vitro.
-
•
B12 suppresses inflammation and fibrosis induced by ischemia/reperfusion injury.
-
•
B12 suppresses DNA damage response and apoptosis induced by ischemia/reperfusion.
-
•
B12 has potential for the prevention/treatment of acute kidney injury.
1. Introduction
Vitamin B12 (B12 – cyanocobalamin), the last vitamin characterized, is the largest (MW1355) of all vitamins and has several unique features. All cobalamins (vitamin B12 derivatives) contain the rare transition metal, cobalt (Co), positioned in the center of a corrin ring. While cobalamins are synthesized only in certain bacteria and archaea [1], they are co-enzymes essential for all life, except in plants. In higher vertebrates, methyl-cobalamin and 5′-deoxyadenosyl-cobalamin are essential for the function of methionine synthase and methylmalonyl CoA mutase, respectively [2]. Intestinal absorption, circulation, cellular uptake, and function of dietary vitamin B12 requires complex systems involving multiple binding proteins, cell surface receptors, lysosomal transfer, and converting enzymes [3]. B12 deficiency caused by defects in any of these systems can lead to megaloblastic anemia, homocysteinemia, methylmalonic acidemia, and other conditions associated with reduced cellular energy metabolism and DNA synthesis [4].
More recently, subclinically low B12 levels have drawn increasing attention as a risk factor for subtle age-related conditions, including neurological disorders, cognitive impairment, inflammatory conditions, and cancer [4]. Once inside the cell, the oxidation state of the cobalt atom is reduced from Co(III) to Co(II) and to Co(I). Importantly, one of us reported that Co(II)balamin is a highly effective intracellular superoxide (O2• −) scavenger with a reaction rate close to that of superoxide dismutases (SOD) [5,6]. As superoxide is a major ROS in biological systems [7], the role of B12 as an antioxidant may account for the association between subclinical B12 deficiency and increased risk for several age-related diseases, for many of which oxidative stress has been implicated as playing a significant role [7].
The daily requirement of B12 intake in humans is less than 5 μg [8]. The absorption of dietary B12 requires intrinsic factor (IF), a B12-binding protein secreted by the gastric mucosa, which acts as an efficient ligand for the B12 uptake receptor located in the intestine. Consequently, the bioavailability of oral B12 may be limited by the availability of IF. However, Kuzuminski et al. have reported that when high amounts of B12 (2 mg/day) are given orally to patients lacking IF, approximately 1% of B12 was absorbed via an IF-independent mechanism [9]. Given its antioxidant property, a protective effect of B12 in ROS-induced cell injuries seems promising. However, although no adverse effects have been noted with high doses of B12 and B12 is generally considered safe by the FDA, studies exploring its therapeutic use against cellular injury have been surprisingly scarce.
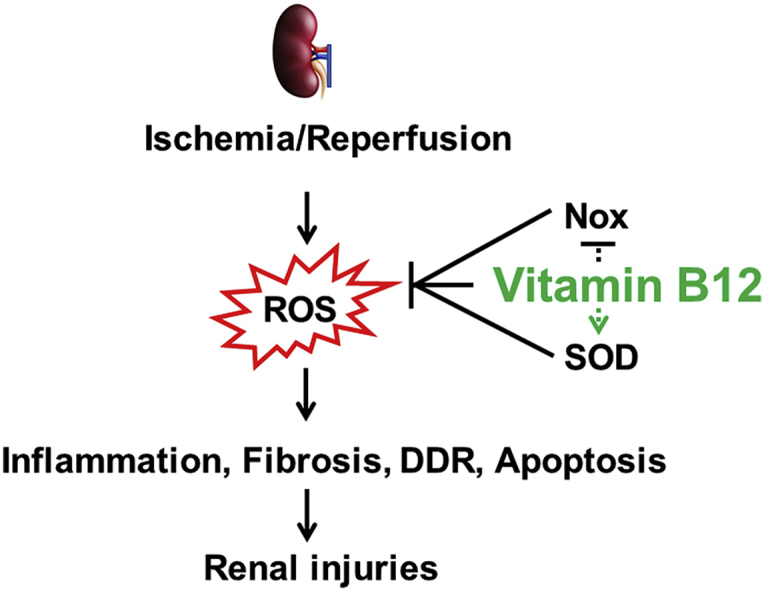
In the current study, we investigated the therapeutic effects of high-dose dietary B12 in mice on kidney IRI, in which critical involvement of ROS has been well established [10]. During the ischemic phase, the absence of oxygen leads to the accumulation of metabolic intermediates. During reperfusion, these metabolic intermediates react with oxygen to produce a sudden increase in oxygen radicals, resulting in the uncontrolled oxidation of cellular components [10]. Furthermore, impaired mitochondria electron transfer chain system and NADPH oxidases (Nox) significantly contribute to ROS production during the processes of IRI and repair [10]. Our results demonstrate that a high-dose oral B12 administration significantly blunts the kidney damage caused by I/R via marked suppression of ROS and associated inflammation, fibrosis, and apoptotic cell death.
2. Materials and methods
Mice: Wild type male mice (C57BL/6J) at age of 12–16 weeks were housed in standard cages on a 12 h light/dark cycle and were allowed free access to food and water. Rodent chow [(Cat# 3002909–203, PicoLab, Fort Worth, Texas) contained B12 at 79 μg/kg. All experiments were carried out in accordance with the National Institutes of Health guideline for use and care of experimental animals, as approved by the IACUC of the University of North Carolina at Chapel Hill.
Renal ischemia/reperfusion (I/R) procedure: Mice were anesthetized with isoflurane (1.5%) inhalation, and core body temperature was maintained at 37 °C by using a homeothermic blanket. After a midline laparotomy, in I/R groups, the left renal pedicle was occluded by using microaneurysm clamps for 30 min, then the incision was double-sutured after the clamps were removed. In sham-operated groups, renal pedicle was not occluded, but the abdomen was kept open for 30 min. After waking from the surgery, mice with I/R or sham surgery were randomly enrolled into either vehicle (water)-treatment groups or B12 treatment groups at dose of 50 mg/L via drinking water. Body fluid and tissues were collected for analyses 5 days after surgery.
Biochemical analyses: Plasma creatinine and BUN were measured by Vet Axcel (Diagnostic Technologies, LLC, West Coldwell, New Jersey). B12 and cystatin C were measured by ELISA kits (# MBS762328, MyBioSource INC, San Diego, CA; #NR-E10516, Novateinbio, Woburn, MA).
Immunohistochemistry (IHC): Rabbit antibodies against phospho-histone H2A.X (#9718, Cell Signaling), cleaved Caspase-3 (#9664, 5A1E, Cell Signaling) and F4/80 (#70076, Cell Signaling) were used. IHC was performed in the Bond fully-automated slide staining system (Leica Biosystems Inc. Vista, CA). Antigen retrieval was performed at 100 °C in Bond-epitope retrieval solution 1 pH6.0 (AR9961) for 20 min. Positive and negative controls (no primary antibody) were included for each antibody. IHC stained sections were digitally imaged (20x objective) in the Aperio ScanScope XT using line-scan camera technology (Leica Biosystems) [11].
Morphological examination: Tissues were fixed with 4% paraformaldehyde, paraffin sectioned (5 μm), and stained with hematoxylin and eosin, or with Masson's Trichrome. Semi-quantification of tubular injury was scored using a scale of 0–4: 0, no injury; 1, <25%; 2, 25–50%; 3, 50–75%; 4, >75%. Scoring was done by one nephrologist and one renal-pathologist who were blinded to the experimental groups [12].
Cell culture, In vitro hypoxia/reperfusion (H/R) experiment: BU.MPT cells, a conditionally immortalized mouse kidney proximal tubular epithelial cell (PTEC) line, were used [13]. For H/R experiments, briefly, cells were grown to ≥90% confluence at 39 °C, in the absence of IFN-γ, as described elsewhere [13]. Under these conditions, BU.MPT cells behave like primary cultures of PTEC [13]. At confluence, cells were starved for 24 h without FBS. Cells were then randomly divided into 4 groups: (1) normal culture conditions (21% oxygen, 10% FBS, and 4.5 g/L glucose in medium); (2) normal culture condition with 0.3 μM cyanocobalamin (B12); (3) hypoxia/reperfusion (H/R); (4) H/R with 0.3 μM B12. Hypoxia was induced by placing cells in an anaerobic chamber equilibrated to approximately 1% O2, 5%CO2, and 94% N2 using culture medium lacking glucose and FBS with or without B12. After 3 h of hypoxia, cells were returned to normoxic culture conditions with addition of glucose (4.5 g/L) and FBS (10%, v/v) for a period of 1 hr, which was regarded as reperfusion [14]. Medium and cells were then collected for analysis.
Quantitative RT-PCR: Total RNA from tissues or cells was extracted using Trizol (Life Technologies, St. Paul, MN) following the manufacturer's instruction. mRNA was quantified with TaqMan real-time quantitative RT-PCR (7500 real time PCR system, Applied Biosystems, Foster City, CA) by using the one-step RT-PCR Kit (Bio Rad, Hercules, CA) with 18s as the reference gene in each reaction [11]. The primer and probe sequences are listed in Table S2.
Microarray analyses: After H/R treatment, cells were collected and total RNA was isolated using Trizol as described above. RNA was purified using RNeasy Mini Kit (QIAGEN), and RNA samples were submitted to the microarray core at UNC-CH to assess RNA quality and analyze the transcriptome using Clariom™ S Mouse WT (Affymetrix). The data has been deposited in GEO (GSE142674).
Superoxide and ROS measurement: Dihydroethidium (DHE) (10 μM) was added to the culture medium of four groups of cells in glass-bottom 96-well plates (#655891, Fisher) treated according to the H/R protocol described above. Fluorescence was detected after 1 hr of reperfusion using a Cytation 5 imaging reader (BioTeck, Vermont) using a filter cube and LED with λex = 405 nm and λem = 560–580 nm to minimize ethidium fluorescence interference [15]. Another batch of four groups of cells, and kidneys collected at 5 days after surgery, was subjected to high performance liquid chromatography (HPLC) analysis of 2-hydroxyethidium (2-OH-E) as described previously [16]. Detailed methods are described in Supplementary material.
Western blot (WB): The antibodies used in the study including: p-H2A.X (#9718, Cell Signaling), p-Chk1 (#2348; Cell Signaling), caspase 3 (#9665; Cell Signaling), cleaved caspase 3 (#9664; Cell Signaling), β-actin (#5125; Cell Signaling), catalase (#12980S; Cell Signaling). Detailed methods are described in Supplementary material.
Cell viability assay: Cells were cultured in 96-well plates under four different conditions as described above. After 1 hr of reperfusion, CCK-8 solution (#96992, Sigma) was added to each well (final 1/10 dilution) and incubated for 1hr. The absorbance was measured at 450 nm using a microplate reader (SpectraMax M5 Microplate Reader, Molecular Devices). The percent cell viability in individual treatment groups was determined from the optical density (OD) relative to the optical density of control cells, whose mean was set as one.
Statistical analysis: Data are presented as mean ± SEM. Multifactorial ANOVA test was used to assess statistical significance with the program JMP 13.0 (SAS Institute Inc. Cary, NC). Post hoc analyses were done using the Tukey–Kramer Honest Significant Difference test.
3. Results
3.1. Oral B12 supplementation at a high dose increases kidney B12 content
We first evaluated how different amounts of vitamin B12 given in the drinking water would influence the plasma and tissue levels in adult C57BL/6J WT male mice for five days. The murine diet contains ~80 μg/kg of B12. Since food and water consumption did not differ among the groups (data not shown), daily B12 intake was estimated using 4 g of feed and 4 ml of water. Neither plasma nor kidney B12 increased with up to 67 μg/day of dietary B12, but significant increases were observed at 200 μg/day (Fig. 1). Plasma B12 levels in mice on 200 μg/day were ~2x higher than those of mice with no supplement. Kidney B12 levels increased by ~6x at 200 μg/day intake and by ~11x in mice fed 600 μg/day compared to those without supplement (Fig. 1). Based on these data, we selected a dose of 200 μg/day (50 mg/L in drinking water) in our experiments.
Fig. 1.
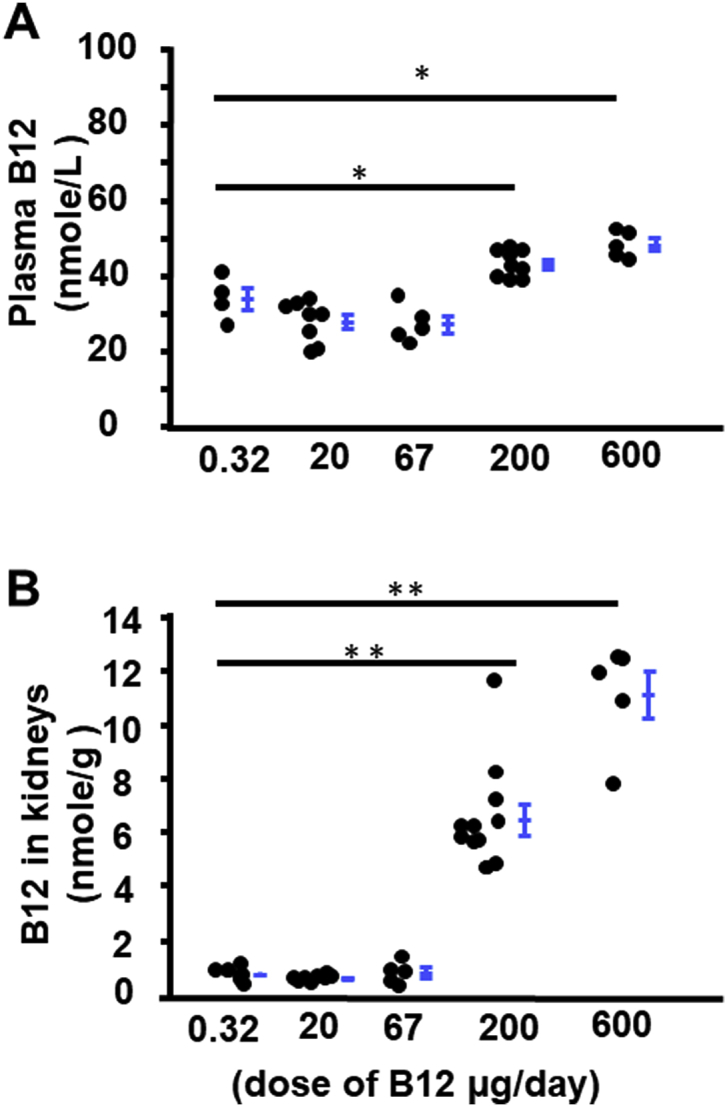
Dose effect of oral B12 intake on plasma and kidney B12 content. B12 was administered to WT male mice via drinking water for 5 days and dose per day was calculated as 4 ml of water intake. A) plasma concentration of B12. B) B12 level in kidneys. n ≥ 5. *p < 0.05, **p < 0.01 by Tukey–Kramer Honest Significant Difference test. Error bars indicate group mean ± SEM.
3.2. B12 lessens kidney IRI
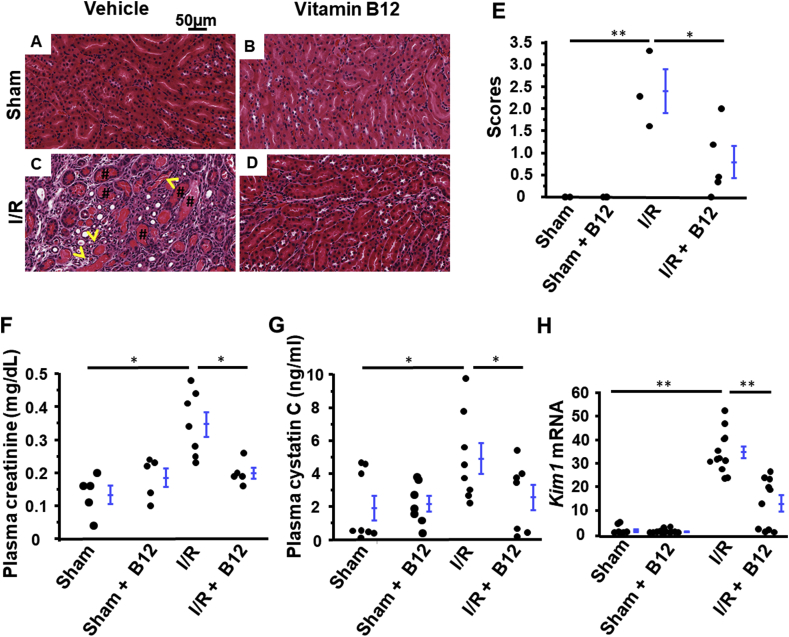
A burst of superoxide production in the early reperfusion stage has been considered as the source of the variety of ROS, but excess ROS production continues during the cellular damage and repair processes. While in some I/R events, such as kidney transplantation, are predictable, most acute renal injuries are unpredictable. To examine whether B12 can prevent post I/R kidney damage, we provided mice with B12(cyanocobalamin) in drinking water after mice recovered from surgery at a dose of 50 mg/L. Plasma B12 levels on day 5 were increased by ~1.5x, and kidney levels were increased by ~6x in both sham and experimental mice (Fig. S1). Renal tubular necrosis and cast formation subsequent to I/R (Fig. 2C, E & S2) were significantly mitigated by B12 (Fig. 2D and E). Moreover, while I/R surgery reduced renal function, as indicated by elevated plasma creatinine and cystatin C levels (Fig. 2F and G), mice receiving B12 had significantly lower plasma creatinine and cystatin C levels (Fig. 2F and G) than untreated I/R mice. B12 had no effect on renal morphology and function of sham mice (Fig. 2A, B, E, F, & G).
Fig. 2.
Functional and morphological protection from kidney ischemia/reperfusion (I/R) injury with B12. A-D) Histological assessment of the tubular structures of the 4 groups of mice; Sham operated with or without B12 and I/R operated with or without B12. Hematoxylin and eosin staining. Yellow arrows: necrotic tubules. #: protein casts. E) Semi-quantitative injury score of the kidneys of the four groups of mice. n ≥ 3. F) Plasma creatinine concentration in the four groups of mice. n ≥ 57. G) Plasma cystatin C concentration in the four groups of mice. n ≥ 7. H) Kim1 mRNA levels in kidneys of the four groups of mice. n ≥ 9. longer line: I/R vs. sham; shorter line: I/R + B12 vs I/R. *p < 0.05, **p < 0.01. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Kidney injury molecule-1 (KIM-1), a type 1 transmembrane protein with an immunoglobulin and mucin domain, is abundantly expressed in post-ischemic kidneys, and sustained upregulation of Kim1 is an excellent marker of kidney damage including peritubular inflammation and interstitial fibrosis [17,18]. mRNA levels of Kim1 in the kidneys subjected to I/R were increased ~30x compared to sham mice. B12 halved the induction of Kim1 dramatically in the kidneys of I/R mice, but had no effect on Kim1 expression in sham kidneys (Fig. 2H).
3.3. B12 reduces kidney inflammation and fibrosis
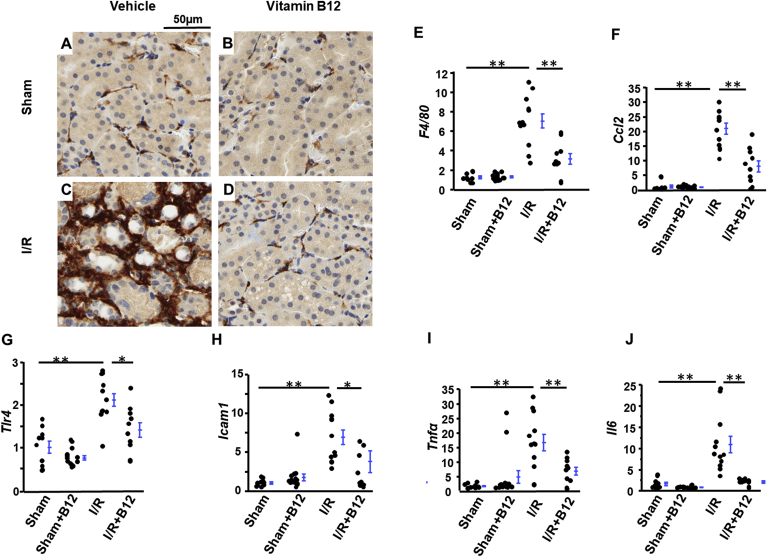
Inflammation response plays pivotal roles in the I/R injury process and transition from AKI to chronic kidney disease (CKD) [19]. At post I/R day 5, the kidneys of I/R mice contained abundant infiltrating macrophages, as demonstrated by prominent immunostaining of F4/80, a pan-macrophage marker, around injured tubules (Fig. 3C). B12 significantly reduced the F4/80 staining in I/R kidneys (Fig. 3D). The staining in kidneys from sham mice, both treated and untreated with B12, was very low (Fig. 3A and B). Quantitative RT-PCR confirmed these results. I/R increased the level of F4/80 mRNA about 7x in I/R kidneys relative to sham, and B12 reduced this induction to ~2.5x (Fig. 3E). Together, these data indicate that B12 strongly limited the number of infiltrating macrophages. Consistent with this observation, expression of Ccl2, the gene encoding monocyte chemoattractant protein-1 (MCP1), which attracts monocytes to the site of damaged tissues, was induced ~20x in I/R kidneys compared to sham, with B12 decreasing the induction of Ccl2 by more than 50% (Fig. 3F). Similar patterns of enhanced expression were seen in I/R kidneys for several other genes involved in inflammation and innate immunity. Suppression by B12 was approximately 50% for Tlr4, Icam1, and Tnfα (Fig. 3G–I), while suppression was almost complete in the case of Il6 (Fig. 3J).
Fig. 3.
Supression of ischemia/reperfusion (I/R)-induced inflammation with B12. A-D) Immunostaining for F4/80 (pan-macrophage marker) in kidneys of the four groups of mice. E-J) mRNA levels of inflammatory genes in kidneys of the four groups of mice. n ≥ 9. longer line: I/R vs. sham; shorter line: I/R + B12 vs I/R. *p < 0.05, **p < 0.01.
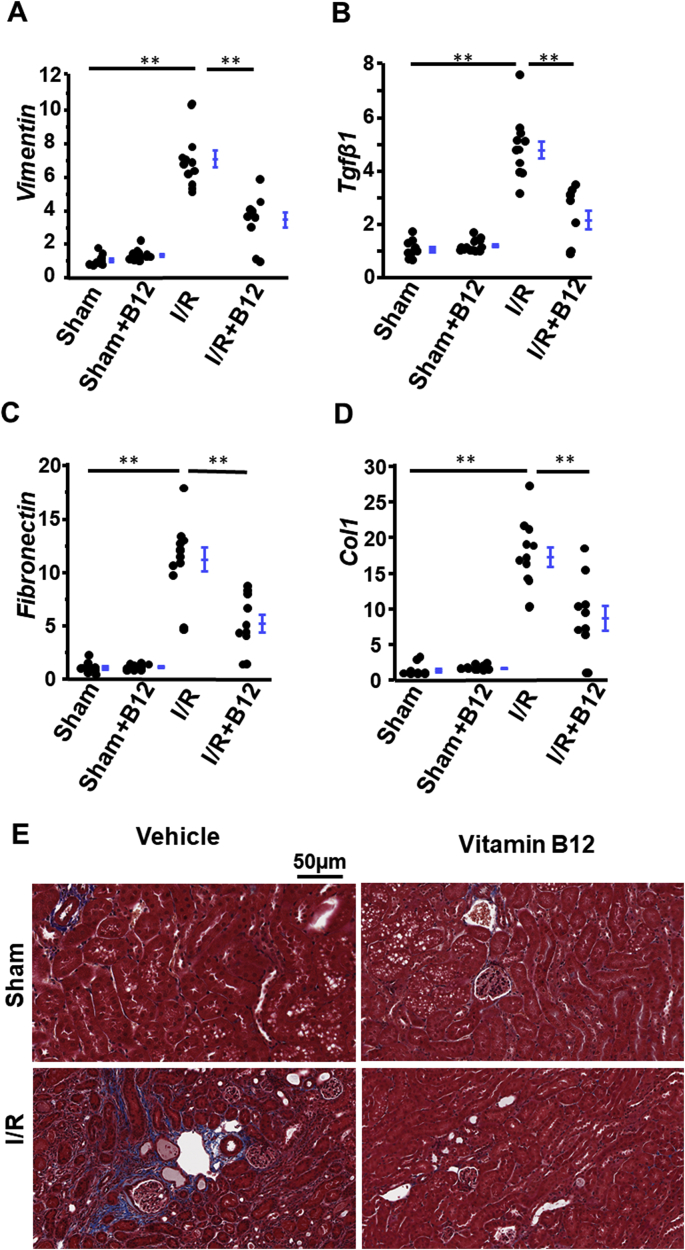
Proximal tubular cells injured by I/R undergo epithelial-to-mesenchymal transition (EMT) and express vimentin, the major cytoskeletal component of mesenchymal cells [20]. Kidneys subjected to I/R expressed ~7x increased levels of Vimentin mRNA as compared to sham kidneys. B12 reduced this increase to ~2x in I/R kidneys, and had no effect on Vimentin expression in sham kidneys (Fig. 4A). Tgfβ1, a well-known inducer of EMT, showed the same pattern of expression as Vimentin (Fig. 4B).
Fig. 4.
Suppression of ischemia/reperfusion (I/R)-induced fibrosis with B12. A-D) mRNA levels of fibrotic genes in kidneys of the four groups of mice. n ≥ 9. longer line: I/R vs. sham; shorter line: I/R + B12 vs I/R. *p < 0.05, **p < 0.01. E) Representative Masson's Trichrome stain of kidneys in the four groups of mice.
Activated myofibroblasts secrete extracellular matrix proteins including collagen and fibronectin during IRI [18]. The mRNA levels of Fibronetin and Col1 (coding collagen1) were markedly increased to 10x and 20x, respectively, in I/R kidneys. B12 reduced the induction of these two genes by ~50% (Fig. 4C&D). Collagen deposition detected by Masson's Trichrome staining was increased in the kidneys of untreated I/R mice (Fig. 4E) but was markedly less in the kidneys of I/R mice treated with B12 (Fig. 4E). Collagen staining did not differ between sham groups with or without B12 (Fig. 4E).
3.4. B12 suppresses DNA damage response (DDR) and apoptosis
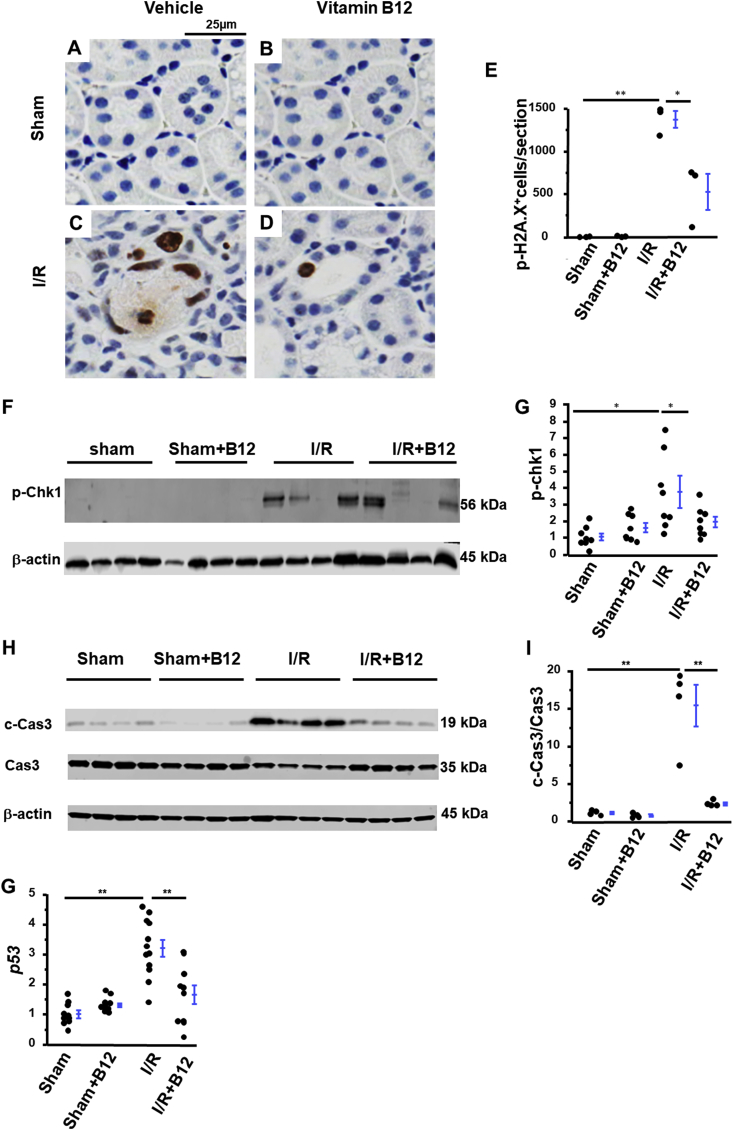
Several molecules, known to be involved in the DDR, were upregulated in the kidneys of mice subjected to I/R [14]. These included p-H2A.X, as detected by Immunohistochemistry (IHC) (Fig. 5C and E), and phosphorylated checkpoint kinase 1 (p-Chk1), as detected by western blotting (WB) (Fig. 5F and G). Treatment with B12 significantly decreased expression of both p-H2A.X (Fig. 5D and E) and p-Chk1 (Fig. 5F and G) in the kidneys of I/R mice. B12 did not alter the renal expression of p-H2A.X or p-Chk1 in sham mice (Fig. 5A, B, E, F, & G).
Fig. 5.
Suppression of ischemia/reperfusion (I/R)-induced DNA damage response (DDR) and apoptosis with B12. A-D) Representatives of immunostaining of p-H2A.X (a hallmark of DDR) in kidneys of the four groups of mice. E) semi-quantification of positive staining of p-H2A.X. n = 3. F) Western blot and G) densitometric quantitation of p-Chk1. n = 8. H) Western blot of cleaved caspase 3 (c-Cas3, effector of apoptosis) and caspase 3 (Cas3) and I) the ratios of their densitometrically quantitated amounts in kidneys of four groups of mice. n = 4. G) mRNA level of p53 in the kidneys of the four groups of mice. n ≥ 9. longer line: I/R vs. sham; shorter line: I/R + B12 vs. I/R. *p < 0.05, **p < 0.01.
Activation of the DDR contributes to renal tubular cell apoptosis [14]. The kidneys of I/R mice showed markedly increased staining for cleaved caspase 3 (c-Cas3), the main effector of apoptosis. B12 significantly decreased cleaved caspase 3 in I/R kidneys (Fig. S3 C&D). Cleaved caspase 3 could not be detected in sham mice, regardless of treatment with B12 (Fig. S3 A&B). As assessed by densitometry, treatment with B12 reduced the ratio of cleaved/full length caspase 3 in I/R kidneys from ~15x to levels not significantly different from those seen in sham kidneys (Fig. 5H and I). p53, a key component of apoptosis, contributes to activation of the mitochondrial pathway of apoptosis, resulting in cytochrome C release from mitochondria and subsequent activation of caspases [21]. The mRNA levels of p53 were increased more than 3x in the kidneys of I/R mice, as compared to the sham kidneys, and B12 reduced the extent of induction (Fig. 5G). Finally, neither I/R nor B12 altered mRNA levels of renal Bcl2 (data not shown).
3.5. B12 dampens kidney oxidative stress induced by IRI
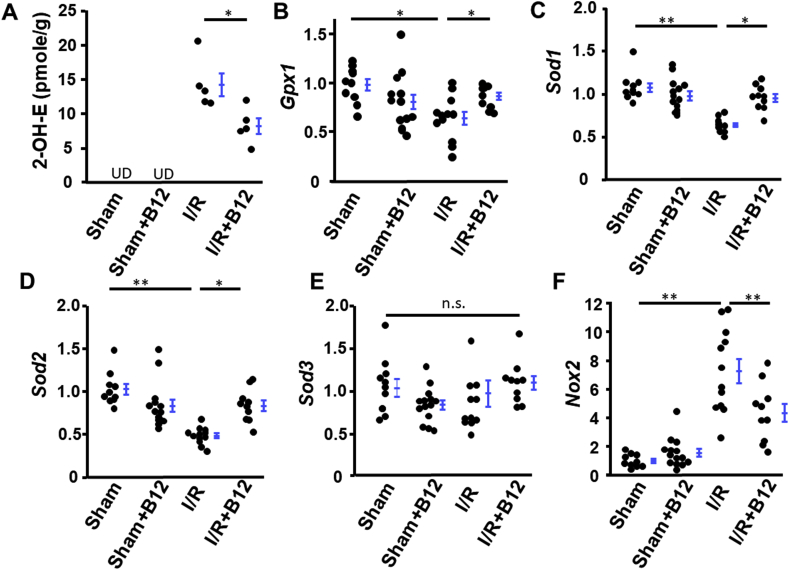
Excess ROS production in post I/R tissues aggravates the kidney injury, and oxidative stress is directly connected to Inflammation, fibrosis, DNA damage and apoptosis. Cellular amounts of antioxidant enzymes thus play important roles in preventing the damage due to oxidative stress. We assessed the level of superoxide by its reaction with dihydroethidium (DHE) to produce the fluorescent product, 2-hydroxyethidium (2-OH-E). B12 decreased the production of 2-OH-E in kidneys subjected to I/R (Fig. 6A). In addition, mRNA levels of Gpx1 (encoding glutathione peroxidase 1), Sod1 (encoding superoxide dismutase 1), and Sod2 (encoding superoxide dismutase 2), were all reduced significantly by I/R to ~0.4-0.6x those of sham. They were normalized by B12 (Fig. 6B–D). No significant changes in the expression of Sod3 (encoding superoxide dismutase 3, extracellular SOD) were altered by either I/R or B12 (Fig. 6E). Rac1-GTP dependent NADPH oxidase (Nox2) mRNA level was increased ~8x in I/R kidneys consistent with previous reporting [10,22], and B12 halved the induction of this gene (Fig. 6F). Finally, protein levels of catalase were not different among the four groups (data not shown).
Fig. 6.
Normalization of antioxidant capacities in ischemia/reperfusion (I/R) kidneys with B12. A) The levels of 2-OH-E in kidneys of the four groups of mice. UD: undetectable. n = 5. B-D) B12 normalized the mRNA levels of Gpx1, Sod1, and Sod2 that were down-regulated in kidneys by I/R. E) Neither I/R nor B12 had effects on Sod3 mRNA level in kidneys. F) B12 decreased the mRNA levels of Nox2 that were up-regulated in the kidneys by I/R. n ≥ 9. longer line: I/R vs. sham; shorter line: I/R + B12 vs. I/R. *p < 0.05, **p < 0.01. n.s.: non-significantly different.
3.6. B12 mitigates hypoxia/reperfusion (H/R) injury in vitro
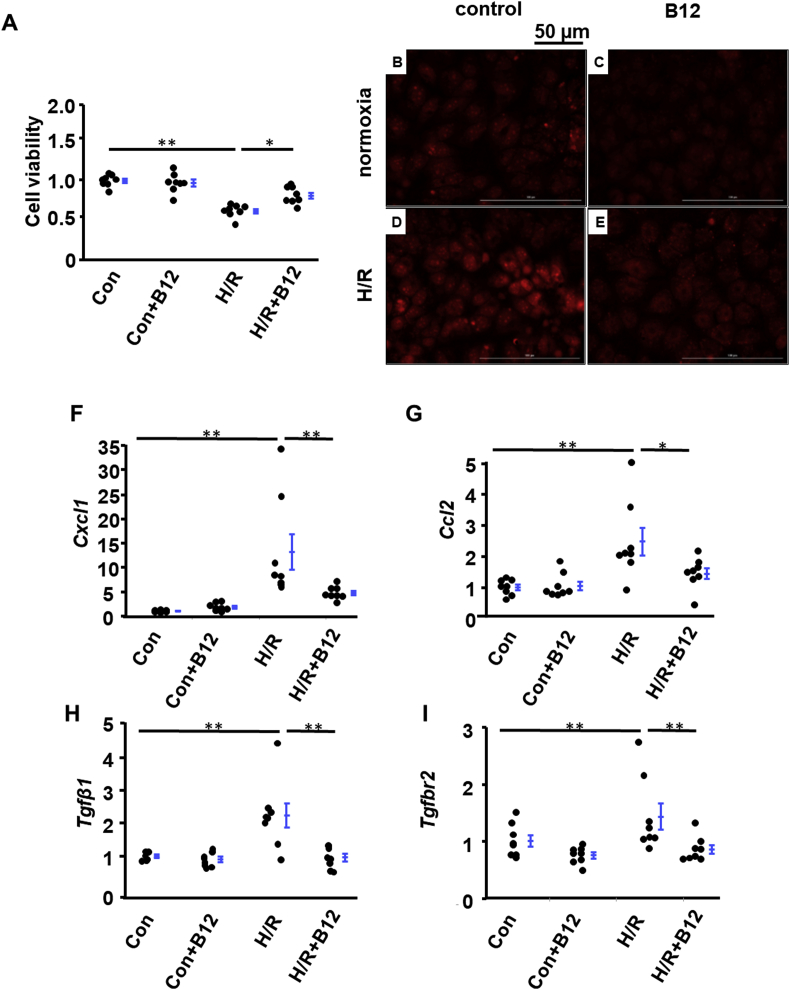
While our observations are consistent with the SOD mimetic action of B12, characterization of ROS in tissues in vivo at post day 5 is complex due to kidneys being composed of multiple cell types that may differ in pathological features. Proximal tubular cells are more sensitive to IRI than other types of cells in the kidney [23,24]. We therefore used mouse proximal tubular cells (BU.MPT), a conditionally immortalized proximal tubular cell line, to test the effects of B12 on the acute phase of reperfusion. At a B12 concentration of 0.3 μM, which is close to the maximal plasma B12 level seen in mice with saturation of the B12 transport system [25], B12 prevented cell death induced by H/R (Fig. 7A). 2-OH-E was below the detection limit by HPLC in cells. However, we observed a decrease in DHE-probed-fluorescence of B12-treated cells, which was induced by H/R, using a filter that maximizes the specificity for 2-OH-E by minimizing interference from ethidium (λex = 405 nm, λem = 560–580 nm) [15](Fig. 7D&E). Control cells, regardless of B12, had very low signals (Fig. 7B&C).
Fig. 7.
Effects of B12 on early stage of reperfusion injury in vitro. Mouse proximal tubular cells (BU.MPT) were treated with hypoxia/reperfusion (H/R) as described in the materials and methods section. A) B12 increased the survival rate of cells treated with H/R. n = 8. B-E) Representative images of fluorescence of ROS probed by DHE. The signal of fluorescence was markedly increased in cells subjected to H/R (D) but was blunted by B12 treatment (0.3 μM) present throughout the entire H/R procedure (E). F–I) mRNA levels of markers of inflammation and fibrosis. n = 8. longer line: H/R vs. control; shorter line: H/R + B12 vs. H/R. *p < 0.05, **p < 0.01.
3.7. B12 modulates H/R induced chemokine gene expression in cultured proximal tubular cells
To gain insight on how B12 influences early H/R events, we globally examined genes that are differentially expressed in the four groups of BU.MPT cells. Microarray analysis identified 6641 genes whose expression significantly differed in at least one group versus the others by ANOVA (F-test p < 0.05). Among these differentially expressed genes, H/R increased the transcripts of 595 genes more than 2x compared to control, while it reduced the transcripts of another 592 genes to less than 0.5x. The genes induced by H/R were enriched with those that function in transcriptional regulation (9E-15), kinase activities (1.2E-11), and oxidoreductase activities (3.3E-7). Although genes suppressed by H/R were overall less related to each other, they were enriched with mitochondrial proteins involved in metabolic pathways (2E-7), such as electron transport NADH dehydrogenases (1.5E-7). This pattern of gene expression is generally consistent with that observed by others [26]. The heatmap of the 33 most up- and down-regulated genes are illustrated in Fig. S4.
As compared to the H/R response, the number of genes responding to treatment with B12 was extremely limited. The transcripts of only 20 genes were increased more than 2x in cells treated with B12 as compared to control cells, while the transcripts of 13 were reduced to less than 0.50x. However, none of these B12 effects was significant by the Tukey-Kramer HSD test.
Among the 595 genes up-regulated by H/R, two were significantly modulated by B12: Lrig1 (Leucine rich repeat and Ig like domain, p = 0.0061), a negative regulator of epidermal growth factor receptor signaling [27], and Tob1 (Transducer of ErbB2-1, p < 0.008), which negatively regulates cell proliferation [28]. When the analysis was broadened to include genes up-regulated by H/R more than 1.62x as compared to control, we found significant modulation by B12 in an additional 5 genes (p < 0.05) (Fig. S5). Among the 592 genes down-regulated by H/R, 11 were significantly modulated by B12. These included Romo1 (ROS modulator 1, p = 0.005), a positive regulator of ROS metabolism required for mitochondrial fusion and for normal integrity of cristae; Vamp7 (Vesicle associated membrane protein 7, p = 0.005), which plays a role in ER to Golgi protein transport and vesicle fusion as well as in the regulation of autophagy and mitochondrial homeostasis [29]; and Tram1 (Translocation associated membrane protein 1, p = 0.003), an ER component that facilitates the secretion of proteins across the ER membrane and the alleviation of ER stress [30]. The number of B12-modulated genes expanded to 28 when the threshold for down-regulation by H/R was relaxed to less than 0.62x that of control cells (Fig. S5). Notably, while B12 modulated expression of these genes in the setting of H/R, B12 did not significantly affect their expression in control cells.
Although those with highly significant p-values are worth further consideration, dampening of the expression of only a small number of genes that responded to H/R suggests possibilities that either early effects of B12 on gene transcription are subtle or the major effects are at the level of protein modifications. We therefore performed qRT-PCR analyses for some genes of interest, such as those of the chemokine family and TGFβ1 signaling, to determine whether B12 influences pathways known to play a role in the early H/R injury process. Confirming the microarray data, Cxcl1 (encoding chemokine [C-X-C motif] ligand 1] and Ccl2, were significantly up-regulated by H/R. As opposed to our microarray analysis, qRT-PCR detected a significant dampening effect of B12 for Cxcl1 induction (p < 0.003), and a trend towards reduction for Ccl2 induction (p = 0.044, one-tail) (Fig. 7F&G). Since CXCL1 is a strong chemoattractant for neutrophils, limiting local production of CXCL1 with consequent mitigation of inflammatory cascades may be an important early contribution of B12. TGFβ1-dependent signaling induced EMT is another early process in H/R. In contrast to microarray analysis, which showed induction of Tgfb2 and Tgfb3, but not Tgfb1, by H/R, qRT-PCR revealed a significant induction of Tgfb1 by H/R that was further modulated by B12 (Fig. 7H). In addition, while microarray analysis detected B12-induced upregulation of Tgfbr1 (encoding TGFβ receptor 1) but not of Tgfbr2 (p = 0.055, Tukey Kramer HSD). qRT-PCR detected a significant modulation of Tgfbr2 by B12 (Fig. 7I). Although these expression data are not fully consistent, they do suggest in aggregate a role of these pathways in early reperfusion injury.
4. Discussion
Our in vivo studies demonstrate dietary loading can increase kidney B12 content more than 6x. Because B12 was provided after I/R, our results show that B12 has reno-protective effects even when given after IRI, making our findings translationally relevant. The beneficial effects of B12 were evidenced by multiple criteria, including decreased tubular injury and decreased plasma creatinine and cystatin C levels, as well as inhibition of markers of inflammation, fibrosis, apoptosis, and DDR at 5 days following IRI. In addition, our in vitro data using cultured proximal tubular cells revealed that B12 decreased ROS level induced by H/R, resulting in increased cell survival. Taken together, our in vitro and in vivo data suggest that B12 may be directly protective in the early stages of I/R injury, at least in part through suppression of ROS.
IRI is a leading cause of AKI, a common clinical condition associated with an increased risk for developing chronic kidney disease (CKD) and end stage renal disease (ESRD) [31]. Agents considered as an optimal treatment for AKI must not only minimize the acute injury, but also promote adaptive repair, with inhibition of inflammation and fibrosis, thereby preventing/halting progression to CKD and ESRD. Currently, no effective therapeutic strategies are available for AKI [32]. This is especially true for agents administered after the initial injury has occurred. Notably, in our studies, B12 administered after surgical IRI not only ameliorated the course of AKI, but also reduced markers of inflammation and fibrosis, suggesting that B12 has tremendous potential as a treatment for AKI.
Many agents with antioxidant capacity have been tested in animal models of I/R and have shown similar degrees of benefit to those of B12 as observed by us [[33], [34], [35]]. In the majority of these reports, treatment was started at the same time or prior to I/R, in part because of the widely accepted belief that ROS are produced during the reperfusion stage. However, IRI and repair are both ongoing processes, with ongoing production of ROS. Our experiments suggest that redox modulation even at later stages following IRI can be strongly beneficial. Synthetic mimetics of SOD, including those specifically targeted to mitochondria, have been examined for their protective effects in animals, with some currently under preclinical and clinical development for diseases including cancers [36], central nervous system diseases [37], diabetes, and radiation injury [38]. The efficacy and safety of these agents will take significant time to be established. B12, a naturally occurring SOD mimetic that distributes in both cytoplasm and mitochondria, is an excellent alternative. Indeed, recent publications have demonstrated the benefits of B12 in animal models of DiGeorge syndrome [39], Parkinson's disease [40], optical neuropathy [41], and methylcobalamin prevents mutant human SOD1 (G93A) induced motor neuron death in vitro [42].
Our demonstration that orally administered B12 can achieve blood and tissue levels sufficient to exert protection post I/R has significant implications. Daily intake of approximately 0.32 μg of B12 is enough for WT mice to have normal physiological function. However, production of ROS during IRI overwhelms and/or impairs the normal antioxidant defense system. Since absorption of dietary B12 is limited by a small amount of intrinsic factor available, we hypothesized that pharmacological/supraphysiological doses of B12 will be needed to overcome this limitation and have protective effects on IRI. A pro-oxidant environment under I/R might also reduce the rapid conversion of cob(III)alamin to cob(II)alamin, requiring more cobalamin substrate is needed to see a significant antioxidant effect. Importantly, we selected dose of 200 μg/day in our study, since the B12 levels in plasma and kidneys after administration of B12 via drinking water in WT mice was substantially increased at this dose, and further increased at a dose of 600 μg/day. Further evaluations are required to determine whether there is a threshold of B12 availability to initiate its superoxide scavenging function and/or a lower dose exerts protection effects in I/R.
The effects of high-dose B12 supplementation has been tested in diabetic patients [43]. These trials, which generally used 1 mg/day of B12 in combination with B6 and B9, failed to establish clinical efficacy, despite a reduction in plasma homocysteine levels. The dose used in our study with mice translates to approximately 50 mg/day in humans [44]. In previous work by Kira et al., B12 was given to Japanese patients with multiple sclerosis at 60 mg/day [45]. Although the reduction in clinical symptom was not satisfactory, the authors reported no adverse effects of B12 at this high level. Therapeutic benefits seen in animals do not always translate to patients. Multiple reasons for this have been suggested, including species differences [46]. In this regard, we note that humans have two circulating B12 binding proteins, transcobalamin 1 and 2 (TCN1 and TCN2) of which only TCN2 can bind to cellular receptors for B12. Unlike in humans, Tcn1 is a pseudogene in rodents and therefore only TCN2 carries circulating B12 in rodents [47]. Although TCN1-deficient humans show no symptoms [48], the efficacy of cellular delivery of high-dose B12 into cells in humans also requires detailed evaluation.
Previously, one of us showed that reduced B12 reacts with superoxide at a rate approaching that of SOD [5], and proposed that B12 acts as a second line of defense in conditions of oxidative stress [6]. For cobalamin to directly react with superoxide, it needs to be reduced to cob(II)alamin first [5]. Intracellular processing of cobalamin includes removal of the β-axial ligand; for alkylcobalamins and cyanocobalamin it is accomplished by the methylmalonic aciduria and homocystinuria type C (MMHAC) protein, concomitantly with reduction of the metal center of cobalamin [[49], [50], [51]]. While many of the intracellular forms of cobalamin will not directly scavenge superoxide, up to 80% of mitochondrial cobalamin could be in its cob(II)alamin reduced form [52]. In endothelial cells the mitochondrial coenzyme, adenosylcobalamin, was shown to be higher than the cytosolic methylcobalamin [53].
Importantly, in the current work, we showed evidence of decreased superoxide levels in the I/R kidneys of B12-treated mice, supporting the idea that enough cob(II)alamin could be acting as a second line of defense when ROS levels increase due to I/R. However, we also observed increasing effects of B12 treatment on antioxidant genes, including Sod1 and Sod2, as well as the opposite effect on the superoxide producing Nox2 gene. An increase in superoxide dismutases and/or a decrease in generation of superoxide could also account for the deceased levels of superoxide. Although we did not specifically assess the protein levels of genes we analyzed, the literature suggests a good correlation between mRNA and protein levels. For example, Yi et al. has reported a correlation between mRNA and protein levels for Sod1, Sod2 and Gpx [54]. Wan et al. has similarly reported that the mRNA levels of Nox2 correlate with its protein levels [55]. Additionally, one of us has also reported that a correlation among mRNA, protein level and enzyme activity for SOD1 in a model of arterial injury [56].
An increase in superoxide dismutase activity and/or a decrease in generation of superoxide could also account for the decreased levels of superoxide. Although reduced tissue injuries and macrophage infiltration account for at least parts of these results, B12 could also be contributing to the reno-protective effects through other functions than direct superoxide scavenging. In this regard, our attempts to analyze early changes in gene expression by proximal tubular epithelial cells, although limited in scope, suggest that chemokines, ER stress, and EMT are among the early processes for which B12 may exert a protective role. These processes are likely ongoing and continue to contribute to IRI long after reperfusion has occurred. Importantly, transcription of chemokine genes such as CXCL1 and CCL2 are under the regulation of the transcription factor, nuclear factor κB (NFκB). The early phases of oxidative stress have been shown to induce NFκB activation in multiple ways. For example, H2O2 activates NFκB mainly through the classical IKK-dependent pathway. Moreover, phosphorylation of Ser-276 on the p65 subunit of NFκB has been shown to be ROS-dependent and is required for transcriptional expression of some NFκB target genes, including CXCL1 and CCL2 [57]. Although exposure to sustained oxidative stress may inhibit NFκB activation as Wu et al. has shown [58], superoxide scavenging by B12 could effectively suppress early chemokine expression and recruitment of inflammatory cells to the site of IRI.
5. Conclusion
We have demonstrated that high-dose B12 improves the function and morphology of kidneys in mice injured by I/R. B12 effectively suppressed inflammation, fibrosis, and apoptosis, all of which are associated with ROS generation. Because B12 is generally regarded as safe in humans, it merits further evaluation for the prevention and/or treatment of human AKI mediated by IRI.
Author contribution
F.L. and N.M-S designed the study; F.L., J. W., E.M. B., J. H., S. H., A.A., R.S. and N.T. carried out experiments; F.L., N. M-S., E.M.B., V.N., and N.T. analyzed and interpreted the data; L.F. and J.S.L. directed the BU.MPT cell culture; F.L., N. M − S drafted and F.L. N.M-S., E.M.B., and J.S.L revised the paper.
Declaration of competing interest
None.
Acknowledgments
We thank Phillip K Huynh for assisting with Western blot analysis. We thank Dr. Oliver Smithies for his inspiring thoughts to allow us to complete this project.
This work was supported by a grant from the National Institutes of Health (R01HL049277 to N.M-S and K01HL145354 to E.M.B.) The histology core facility at UNC is supported by NIH Grant DK 034987.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2020.101504.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Roth J.R., Lawrence J.G., Bobik T.A. Cobalamin (coenzyme B12): synthesis and biological significance. Annu. Rev. Microbiol. 1996;50:137–181. doi: 10.1146/annurev.micro.50.1.137. [DOI] [PubMed] [Google Scholar]
- 2.Kapadia C.R. Vitamin B12 in health and disease: part I--inherited disorders of function, absorption, and transport. Gastroenterol. 1995;3:329–344. [PubMed] [Google Scholar]
- 3.Obeid R., Heil S.G., Verhoeven M.M.A. Vitamin B12 intake from animal foods, biomarkers, and health aspects. Front. Nutr. 2019;6:93. doi: 10.3389/fnut.2019.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Green R., Allen L.H., Bjorke-Monsen A.L. Vitamin B12 deficiency. Nat. Rev. Dis. Prim. 2017;3:17040. doi: 10.1038/nrdp.2017.40. [DOI] [PubMed] [Google Scholar]
- 5.Suarez-Moreira E., Yun J., Birch C.S. Vitamin B(12) and redox homeostasis: cob(II)alamin reacts with superoxide at rates approaching superoxide dismutase (SOD) J. Am. Chem. Soc. 2009;131:15078–15079. doi: 10.1021/ja904670x. [DOI] [PubMed] [Google Scholar]
- 6.Moreira E.S., Brasch N.E., Yun J. Vitamin B12 protects against superoxide-induced cell injury in human aortic endothelial cells. Free Radic. Biol. Med. 2011;51:876–883. doi: 10.1016/j.freeradbiomed.2011.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lambert A.J., Brand M.D. Reactive oxygen species production by mitochondria. Methods Mol. Biol. 2009;554:165–181. doi: 10.1007/978-1-59745-521-3_11. [DOI] [PubMed] [Google Scholar]
- 8.Doets E.L., van Wijngaarden J.P., Szczecinska A. Vitamin B12 intake and status and cognitive function in elderly people. Epidemiol. Rev. 2013;35:2–21. doi: 10.1093/epirev/mxs003. [DOI] [PubMed] [Google Scholar]
- 9.Kuzminski A.M., Del Giacco E.J., Allen R.H. Effective treatment of cobalamin deficiency with oral cobalamin. Blood. 1998;92:1191–1198. [PubMed] [Google Scholar]
- 10.Granger D.N., Kvietys P.R. Reperfusion injury and reactive oxygen species: the evolution of a concept. Redox Biol. 2015;6:524–551. doi: 10.1016/j.redox.2015.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li F., Kakoki M., Smid M. Causative effects of genetically determined high maternal/fetal endothelin-1 on preeclampsia-like conditions in mice. Hypertension. 2018;71:894–903. doi: 10.1161/HYPERTENSIONAHA.117.10849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li F., Wang C.H., Wang J.G. Elevated tissue factor expression contributes to exacerbated diabetic nephropathy in mice lacking eNOS fed a high fat diet. J. Thromb. Haemostasis. 2010;8:2122–2132. doi: 10.1111/j.1538-7836.2010.03976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vujicic S., Feng L., Antoni A. Identification of intracellular signaling events induced in viable cells by interaction with neighboring cells undergoing apoptotic cell death. J. Vis. Exp. 2016;118 doi: 10.3791/54980. 1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen H., Wang L., Wang W. ELABELA and an ELABELA fragment protect against AKI. J. Am. Soc. Nephrol. 2017;28:2694–2707. doi: 10.1681/ASN.2016111210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nazarewicz R.R., Bikineyeva A., Dikalov S.I. Rapid and specific measurements of superoxide using fluorescence spectroscopy. J. Biomol. Screen. 2013;18:498–503. doi: 10.1177/1087057112468765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zielonka J., Vasquez-Vivar J., Kalyanaraman B. Detection of 2-hydroxyethidium in cellular systems: a unique marker product of superoxide and hydroethidine. Nat. Protoc. 2008;3:8–21. doi: 10.1038/nprot.2007.473. [DOI] [PubMed] [Google Scholar]
- 17.Ichimura T., Bonventre J.V., Bailly V. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J. Biol. Chem. 1998;273:4135–4142. doi: 10.1074/jbc.273.7.4135. [DOI] [PubMed] [Google Scholar]
- 18.Oh E., Humphreys B.D. Fibrotic changes mediating acute kidney injury to chronic kidney disease transition. Nephron. 2017;137:264–267. doi: 10.1159/000474960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu C.Y., Winterberg P.D., Chen J. Acute kidney injury: a conspiracy of Toll-like receptor 4 on endothelia, leukocytes, and tubules. Pediatr. Nephrol. 2012;27:1847–1854. doi: 10.1007/s00467-011-2029-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kusaba T., Lalli M., Kramann R. Differentiated kidney epithelial cells repair injured proximal tubule. Proc. Natl. Acad. Sci. U. S. A. 2014;111:1527–1532. doi: 10.1073/pnas.1310653110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao C.F., Ren S., Zhang L. Caspase-dependent cytosolic release of cytochrome c and membrane translocation of Bax in p53-induced apoptosis. Exp. Cell Res. 2001;265:145–151. doi: 10.1006/excr.2001.5171. [DOI] [PubMed] [Google Scholar]
- 22.Barrera-Chimal J., Andre-Gregoire G., Nguyen Dinh Cat A. Benefit of mineralocorticoid receptor antagonism in AKI: role of vascular smooth muscle Rac1. J. Am. Soc. Nephrol. 2017;28:1216–1226. doi: 10.1681/ASN.2016040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Basile D.P., Anderson M.D., Sutton T.A. Pathophysiology of acute kidney injury. Comp. Physiol. 2012;2:1303–1353. doi: 10.1002/cphy.c110041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chevalier R.L. The proximal tubule is the primary target of injury and progression of kidney disease: role of the glomerulotubular junction. Am. J. Physiol. Ren. Physiol. 2016;311:F145–F161. doi: 10.1152/ajprenal.00164.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lildballe D.L., Mutti E., Birn H. Maximal load of the vitamin B12 transport system: a study on mice treated for four weeks with high-dose vitamin B12 or cobinamide. PloS One. 2012;7 doi: 10.1371/journal.pone.0046657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Supavekin S., Zhang W., Kucherlapati R. Differential gene expression following early renal ischemia/reperfusion. Kidney Int. 2003;63:1714–1724. doi: 10.1046/j.1523-1755.2003.00928.x. [DOI] [PubMed] [Google Scholar]
- 27.Chen X., Cai G., Liu C. IL-17R-EGFR axis links wound healing to tumorigenesis in Lrig1(+) stem cells. J. Exp. Med. 2019;216:195–214. doi: 10.1084/jem.20171849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ho K.J., Do N.L., Otu H.H. Tob1 is a constitutively expressed repressor of liver regeneration. J. Exp. Med. 2010;207:1197–1208. doi: 10.1084/jem.20092434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aoyagi K., Ohara-Imaizumi M., Itakura M. VAMP7 regulates autophagy to maintain mitochondrial homeostasis and to control insulin secretion in pancreatic beta-cells. Diabetes. 2016;65:1648–1659. doi: 10.2337/db15-1207. [DOI] [PubMed] [Google Scholar]
- 30.Tang Z., Zhang W., Wan C. TRAM1 protect HepG2 cells from palmitate induced insulin resistance through ER stress-JNK pathway. Biochem. Biophys. Res. Commun. 2015;457:578–584. doi: 10.1016/j.bbrc.2015.01.027. [DOI] [PubMed] [Google Scholar]
- 31.Nath K.A., Croatt A.J., Haggard J.J. Renal response to repetitive exposure to heme proteins: chronic injury induced by an acute insult. Kidney Int. 2000;57:2423–2433. doi: 10.1046/j.1523-1755.2000.00101.x. [DOI] [PubMed] [Google Scholar]
- 32.Kaushal G.P., Shah S.V. Challenges and advances in the treatment of AKI. J. Am. Soc. Nephrol. 2014;25:877–883. doi: 10.1681/ASN.2013070780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Long C., Yang J., Yang H. Attenuation of renal ischemia/reperfusion injury by oleanolic acid preconditioning via its antioxidant, antiinflammatory, and antiapoptotic activities. Mol. Med. Rep. 2016;13:4697–4704. doi: 10.3892/mmr.2016.5128. [DOI] [PubMed] [Google Scholar]
- 34.Lima-Posada I., Fontana F., Perez-Villalva R. Pirfenidone prevents acute kidney injury in the rat. BMC Nephrol. 2019;20:158. doi: 10.1186/s12882-019-1364-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yin M., Wheeler M.D., Connor H.D. Cu/Zn-superoxide dismutase gene attenuates ischemia-reperfusion injury in the rat kidney. J. Am. Soc. Nephrol. 2001;12:2691–2700. doi: 10.1681/ASN.V12122691. [DOI] [PubMed] [Google Scholar]
- 36.Tovmasyan A., Sampaio R.S., Boss M.K. Anticancer therapeutic potential of Mn porphyrin/ascorbate system. Free Radic. Biol. Med. 2015;89:1231–1247. doi: 10.1016/j.freeradbiomed.2015.10.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheng H., Chaparro R.E., Sasaki T. Metalloporphyrins as therapeutic catalytic oxidoreductants in central nervous system disorders. Antioxidants Redox Signal. 2014;20:2437–2464. doi: 10.1089/ars.2013.5413. [DOI] [PubMed] [Google Scholar]
- 38.Reboucas J.S., Spasojevic I., Batinic-Haberle I. Quality of potent Mn porphyrin-based SOD mimics and peroxynitrite scavengers for pre-clinical mechanistic/therapeutic purposes. J. Pharmaceut. Biomed. Anal. 2008;48:1046–1049. doi: 10.1016/j.jpba.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lania G., Bresciani A., Bisbocci M. Vitamin B12 ameliorates the phenotype of a mouse model of DiGeorge syndrome. Hum. Mol. Genet. 2016;25:4369–4375. doi: 10.1093/hmg/ddw267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schaffner A., Li X., Gomez-Llorente Y. Vitamin B12 modulates Parkinson's disease LRRK2 kinase activity through allosteric regulation and confers neuroprotection. Cell Res. 2019;29:313–329. doi: 10.1038/s41422-019-0153-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chan W., Almasieh M., Catrinescu M.M. Cobalamin-associated superoxide scavenging in neuronal cells is a potential mechanism for vitamin B12-deprivation optic neuropathy. Am. J. Pathol. 2018;188:160–172. doi: 10.1016/j.ajpath.2017.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ito S., Izumi Y., Niidome T. Methylcobalamin prevents mutant superoxide dismutase-1-induced motor neuron death in vitro. Neuroreport. 2017;28:101–107. doi: 10.1097/WNR.0000000000000716. [DOI] [PubMed] [Google Scholar]
- 43.Elbarbary N.S., Ismail E.A.R., Zaki M.A. Vitamin B complex supplementation as a homocysteine-lowering therapy for early stage diabetic nephropathy in pediatric patients with type 1 diabetes: a randomized controlled trial. Clin. Nutr. 2019;39:49–56. doi: 10.1016/j.clnu.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 44.Nair A.B., Jacob S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016;7:27–31. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kira J., Tobimatsu S., Goto I. Vitamin B12 metabolism and massive-dose methyl vitamin B12 therapy in Japanese patients with multiple sclerosis. Intern. Med. 1994;33:82–86. doi: 10.2169/internalmedicine.33.82. [DOI] [PubMed] [Google Scholar]
- 46.de Jong M., Maina T. Of mice and humans: are they the same?--Implications in cancer translational research. J. Nucl. Med. 2010;51:501–504. doi: 10.2967/jnumed.109.065706. [DOI] [PubMed] [Google Scholar]
- 47.Frater-Schroder M., Haller O., Gmur R. Allelic forms of mouse transcobalamin 2. Biochem. Genet. 1982;20:1001–1014. doi: 10.1007/BF00484073. [DOI] [PubMed] [Google Scholar]
- 48.van Asselt D.Z., Thomas C.M., Segers M.F. Cobalamin-binding proteins in normal and cobalamin-deficient older subjects. Ann. Clin. Biochem. 2003;40:65–69. doi: 10.1258/000456303321016187. [DOI] [PubMed] [Google Scholar]
- 49.Kim J., Gherasim C., Banerjee R. Decyanation of vitamin B12 by a trafficking chaperone. Proc. Natl. Acad. Sci. U. S. A. 2008;105:14551–14554. doi: 10.1073/pnas.0805989105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koutmos M., Gherasim C., Smith J.L. Structural basis of multifunctionality in a vitamin B12-processing enzyme. J. Biol. Chem. 2011;286:29780–29787. doi: 10.1074/jbc.M111.261370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hannibal L., Kim J., Brasch N.E. Processing of alkylcobalamins in mammalian cells: a role for the MMACHC (cblC) gene product. Mol. Genet. Metabol. 2009;97:260–266. doi: 10.1016/j.ymgme.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Padovani D., Banerjee R. Assembly and protection of the radical enzyme, methylmalonyl-CoA mutase, by its chaperone. Biochemistry. 2006;45:9300–9306. doi: 10.1021/bi0604532. [DOI] [PubMed] [Google Scholar]
- 53.Hannibal L., Axhemi A., Glushchenko A.V. Accurate assessment and identification of naturally occurring cellular cobalamins. Clin. Chem. Lab. Med. 2008;46:1739–1746. doi: 10.1515/CCLM.2008.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yi R., Zhang J., Sun P. Protective effects of kuding tea (Ilex kudingcha C. J. Tseng) polyphenols on UVB-induced skin aging in SKH1 hairless mice. Molecules. 2019;24 doi: 10.3390/molecules24061016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wan L.L., Xia J., Ye D. Effects of quercetin on gene and protein expression of NOX and NOS after myocardial ischemia and reperfusion in rabbit. Cardiovasc. Ther. 2009;27:28–33. doi: 10.1111/j.1755-5922.2009.00071.x. [DOI] [PubMed] [Google Scholar]
- 56.Bahnson E.S., Koo N., Cantu-Medellin N. Nitric oxide inhibits neointimal hyperplasia following vascular injury via differential, cell-specific modulation of SOD-1 in the arterial wall. Nitric Oxide. 2015;44:8–17. doi: 10.1016/j.niox.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morgan M.J., Liu Z.G. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 2011;21:103–115. doi: 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu M., Bian Q., Liu Y. Sustained oxidative stress inhibits NF-kappaB activation partially via inactivating the proteasome. Free Radic. Biol. Med. 2009;46:62–69. doi: 10.1016/j.freeradbiomed.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.