Abstract
Propose
To report two cases of severe acute multi-systemic failure with bilateral ocular toxoplasmosis in immunocompetent patients from urban settings in Colombia.
Observations
We report two immunocompetent male patients aged 44- and 67-years-old who, despite not having visited the Amazonian region in Colombia, had severe bilateral posterior uveitis and extensive-bilateral macular lesions and multiple organ failure that required admission to an intensive care unit. Toxoplasma gondii was positive by PCR assay in vitreous humor samples. Patients were treated with intravitreal clindamycin and dexamethasone in addition to systemic treatment with trimethoprim-sulfamethoxazole. In both patients, infection by atypical strains was confirmed; in one case by serotyping and in another one by genotyping (ROP 18 virulent allele). After 2 and 4 months of treatment respectively, the patients showed improvement of the posterior uveitis and its systemic manifestations. However, there was no significant visual acuity improvement due to bilateral extensive macular involvement.
Conclusions and importance
Clinicians should be aware that toxoplasmosis originating from South America could be associated with severe acute multisystemic and intraocular bilateral involvement, even in patients with no history of exposure to jungle environments.
Keywords: Toxoplasma gondii, Atypical strains, ROP18, Ocular toxoplasmosis, Multiple organ failure
1. Introduction
Clinical toxoplasmosis in immunocompetent patients is usually auto limited. However, it has been described that cases originated in the Amazonian regions in South America can be more severe.1 In particular, outbreaks in Colombia2 and French Guiana3,4 have been described in soldiers that participated in jungle campaigns as well as in communities living near to the jungle, who presented with respiratory and cardiac involvement.5 Until now, no severe cases have been described in people living in South American urban settlements. We report two cases of severe toxoplasmosis with multisystem and ocular involvement, without previous exposure to the Amazonian environment.
2. Findings
2.1. Case 1
A 44-year old male from Bogota, without prior history of travel to the Amazonian region or relevant medical history, was hospitalized for a period of 32 days due to fever of unknown origin, profuse sweating, headache, general malaise, diarrhea, and weight loss. During hospitalization, he developed pneumonia, ventilatory failure, and required treatment at the intensive care unit. He was diagnosed with myocarditis, pericarditis, nephritis and distal polyneuropathy, with unidentified etiology.
10 days after hospitalization, without complete improvement of the systemic condition, but conscious and without dependence on mechanical ventilation, the patient complained of floaters and decreased visual acuity in his left eye. At the initial ophthalmological examination, best-corrected visual acuity (BCVA) was hand motion in the right eye and 20/400 in the left eye. Anterior segment examination revealed mild conjunctival hyperemia, fine pigmented keratic precipitates, and 1+ anterior chamber cells in both eyes. Intraocular pressures were 10 mmHg in both eyes. There were 1+ vitreous cells in both eyes. The fundus examination disclosed multiple white creamy yellow subretinal lesions with irregular borders associated with hemorrhage in the macular area and in the mid periphery and a posterior serous retinal detachment in both eyes (Fig. 1).
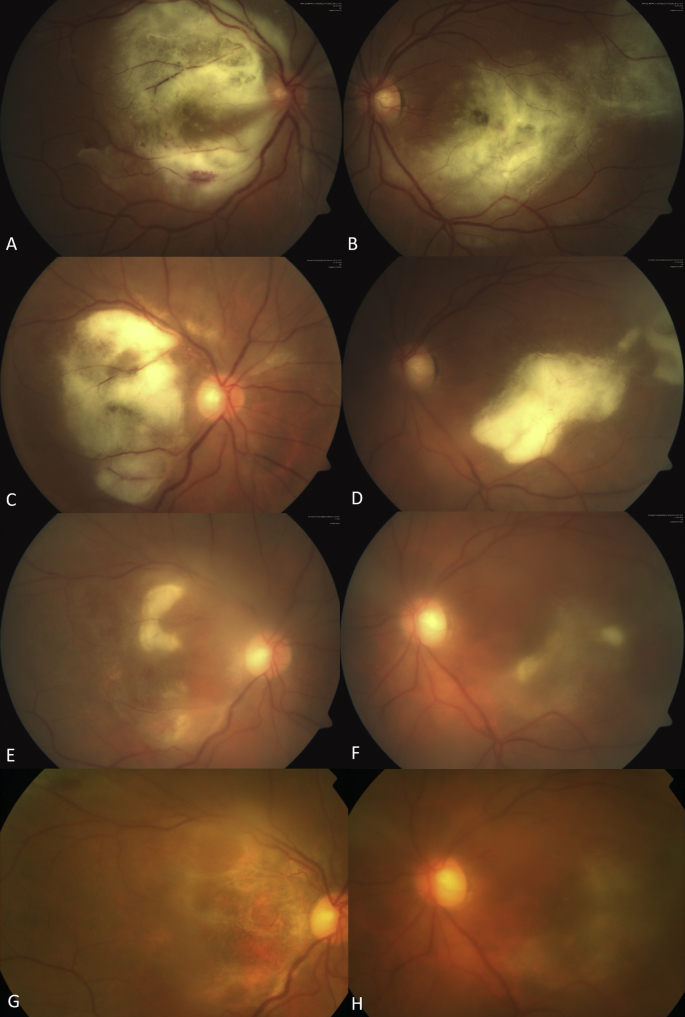
Fig. 1.
Fundus photography. Evolution of the retinochoroidal lesions Case 1.
A and B, fundus images from both eyes at first visit, a central whitish confluent deep retinal lesion is present in both eyes, associated serous retinal detachment is observed in OD. C and D, decrease and consolidation of whitish lesion after 2 weeks of intravitreal clindamycin injections, with resolution of serous retinal detachment. E and F, continuing improvement after the second intravitreal injection of clindamycin is noted in both eyes. G and H, significant improvement after third intravitreal injection of clindamycin with a mild sub-macular scarring involving the fovea in both eyes.
A vitreous sample was taken and 0.1 cc of intravitreal vancomycin was administrated due to the initial rational clinical diagnosis was probable bacterial endogenous endophthalmitis. Laboratory tests were performed. Results showed positive serum antibodies: IgG anti-CMV (23.6 mg/dL), IgM anti-Epstein Barr Virus (21.9 mg/dL), and IgG (2000 mg/dL, cutoff 1.0) and IgM (38.3 mg/dL, cutoff 0.55) anti Toxoplasma gondii. Globular sedimentation velocity was elevated (45 mm/h) and real-time-PCR for Toxoplasma gondii was positive in the vitreous sample (positive DNA in the 17th cycle). Consequently, intravitreal clindamycin and dexamethasone were injected and therapy with trimethoprim-sulfamethoxazole 160/800 mg BID was initiated. From that moment the systemic condition improved.
2 weeks after the start of treatment, BCVA improved to 20/300 in the right eye and 20/300 in the left eye, another vitreous sample was performed. Toxoplasma gondii was still positive in vitreous (positive DNA intr the 24th cycle on real-time-PCR test) and a new intravitreal injection of clindamycin and dexamethasone was performed in both eyes. After 2 months under systemic treatment with trimethoprim-sulfamethoxazole, BCVA was finally stable in both eyes, however, based on the persistence of vitritis, a third vitreous sample was performed and a new injection of clindamycin and dexamethasone was performed in both eyes. The DNA amplified in vitreous humor was sequenced at the ROP18 gene locus as described previously6 and comparisons with Genebank sequences from reference strains were used for molecular phylogenetic analysis by maximum likelihood method.6 The parasite sequence was grouped with virulent ROP 18 allele.
2.2. Case 2
A 67-year-old male cardiologist from Bogota and also without previous travel to the Amazonian region, with a prior medical history of arterial hypertension, venous mesenteric ischemia, ethmoidal meningioma, and prostatitis. Was hospitalized with a diagnosis of pneumonia that required admission to the intensive care unit due to severe respiratory insufficiency pressing mechanical ventilation. Two months later, he was admitted with an initial presumptive diagnosis of viral inflammatory myopathy vs. polymyositis. During this episode, he presented floaters and decreased visual acuity in both eyes. Tests showed positive IgM Epstein Barr serology and thrombocytopenia (platelets 80.000/ml). Imaging study showed abdominal and mediastinal adenopathy. Ocular examination showed BCVA of count fingers in the right eye and 20/200 in the left eye. Biomicroscopy revealed 2+ of inflammatory cells, flare, fine pigmented keratic precipitates in both eyes. Fundus examination showed severe vitreous opacity, extensive area of retinitis in the macula and also in the superior temporal arcade in the right eye and in the inferior-temporal arcade in the left eye (Fig. 2). Macular optical coherence tomography (OCT) showed significant edema and disorganization of the retinal architecture in both eyes. The patient underwent oral and topical valacyclovir. The vitreous sample was performed and PCR was positive for Toxoplasma gondii. We performed a serotype Toxoplasma ELISA assay as in the previous case.7 Serotyping demonstrated that the Toxoplasma gondii strain was non-type II. Antiviral treatment was tapered off and trimethoprim-sulfamethoxazole was initiated. Also, intravitreal clindamycin and dexamethasone were injected in both eyes. One month later the patient reported mild improvement in the right eye and worsening in the left eye. Ocular examination revealed the improvement of posterior uveitis in the right eye and a retinal detachment with macular involvement in the left eye. Treatment with trimethoprim-sulfamethoxazole was continued for three months. The patient underwent successful vitreoretinal surgery in the left eye. At the last follow-up examination, visual acuity improved to 20/400 in the right eye and to 20/160 in the left eye.
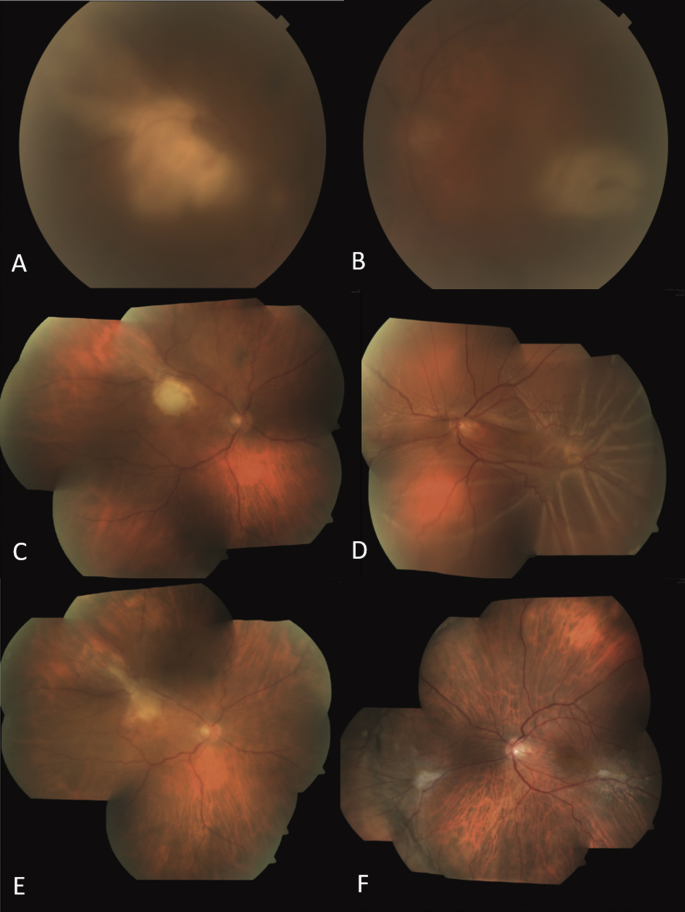
Fig. 2.
Fundus photography. Evolution of the retinochoroidal lesions Case 2.
A and B, fundus images from both eyes at first visit, media opacity due to severe vitritis is appreciate. In OD (A), a whitish confluent deep retinal lesion is present over de superotemporal arcade with foveal involving. In OS (B), a whitish deep retinal lesion is present over the inferotemporal arcade. C and D, mosaic image of both eyes after the first dose of intravitreal clindamycin in both eyes, an improvement of the vitreitis is observed, but OS (D) developed a retinal detachment. E and F, OD (E) with presence of mild subretinal scar and OD (F) with retina reattached.
Both patients provided written informed consent for the publication of this report and all accompanying medical images.
3. Discussion
These patients share important features. Both of them had similar systemic findings and were otherwise immunocompetent who presented a severe systemic infectious condition requiring admission to an intensive care unit for treatment. Both cases presented severe bilateral ophthalmological involvement with macular lesions. Other systemic findings were the presence of lymphadenopathies and splenomegaly. In Case 1, retroperitoneal and abdominal adenopathies were present, and in Case 2 mediastinal adenopathies were present. In Case 2, there was an inflammatory myopathy with CPK elevation and eosinophilic enteritis. These clinical findings are very similar to those found in 11 immunocompetent patients who presented systemic infection due to Toxoplasma gondii in the French Guiana between 2002 and 2008. Seven of the patients developed ventilatory failure and four had eye involvement, but the ocular specific findings were not completely as described here. The microsatellite DNA sequencing revealed atypical Toxoplasma strains.3,4
Clinical severe forms by atypical strains of Toxoplasma can be explained by the presence of virulence factors. The strain of Case 1 corresponded to the virulent ROP 18 allele group, related to severe disease.6 In Case 2, the initial serotyping demonstrated that the Toxoplasma gondii strain was non-type-II.8 In a comparative clinical study that examined 19 cases of active ocular toxoplasmosis of French origin and 23 cases of Colombian origin, significant differences were found in the size of the active lesion, macular involvement and visual acuity deterioration between the Colombian strains and French strains. These differences were attributable to the greater aggressiveness of the Colombian strain.8 The expression of IFN-γ and IL-17 was lower in the aqueous humor samples of Colombian patients which indicated that the strains of South America can cause more severe ocular toxoplasmosis due to the inhibition of the protective factor of IFN- γ.8 Clinical studies have given evidence of the participation of ROP18 virulent factor in human toxoplasmosis.6 ROP alleles codify for proteins secreted by structures called rhoptries and this organelle secretes proteins that determine the virulence of T. gondii.9 The gene of ROP18 from virulent strains codes for a protein kinase that lack an insertion sequence in the promoter of gene expression, resulting in an increase in the expression of protein kinase and, consequently, the proliferation of the parasite is enhanced.10,11 In human ocular toxoplasmosis, the absence of the insertion sequence in Toxoplasma ROP18 promoter was correlated with severe ocular inflammatory response.6 The Toxoplasma sequence of ROP18 in Case 1 precisely corresponded to this virulent group of strains. Analysis of aqueous humor cytokines showed that in Colombian patients infected by non-type II strains, IFN-γ is reduced, and there is a predominance of Th2 response that has been related to more severe clinical characteristics.7,12
On the other hand, it is notable for the finding of viral serological markers of acute infections in both cases. Case 1 was positive for IgG anti-CMV and both cases were positive for IgM anti-EBV. This raises the question if viral coinfections would potentiate the immune-paralysis mechanisms previously described in Toxoplasma. To the best of our knowledge, in severe cases of Toxoplasma in immunocompetent patients previously described, no viral coinfections were found or analyzed. The inhibition of the protective effect of IFN-γ and the Th2 response in patients infected by virulent T. gondii strains might have also a deleterious role in the defense mechanisms against viruses or vice versa. It is well known that T-helper response induction may be affected by the coinfection of a single host by multiple intracellular pathogens.13 Due to the usually adaptive feedback loops that result in the polarization of T-helper responses, it becomes complicated for the immune system to respond with effective mechanisms.14 Theoretical models also indicate that reciprocal facilitation generates a greater risk of severe disease.15 However, it should be noted that although serological tests for IgM against EBV were found, viral load by PCR was negative in the aqueous and vitreous humor, leaving no doubts that T. gondii was the responsible etiologic agent.
4. Conclusion
Virulent Toxoplasma gondii strains can cause severe multi-systemic compromise associated with severe intraocular bilateral involvement in otherwise immunocompetent individuals, living in an urban setting in Colombia. The unusual pathogenesis of the disease highlights the importance of clinical awareness about this kind of manifestation through the inclusion of serological tests and PCR amplification of Toxoplasma in diagnostic flowcharts for similar cases, as well as a renewed epidemiological vigilance against the disease in South American urban environments.
Funding
No funding or grant support.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Patient consent
Patient consent for case report publication in journal has been signed.
Declaration of competing interest
The following authors have no financial disclosures: DAC, MCA, HAR, FJR, KBM, JGE, ADLT.
Acknowledgments
We would like to acknowledge Monica Rincon who performed ELISA serotype assay and to Dr. Nestor Cardona who performed the real-time PCR.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajoc.2020.100661.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Petersen E., Ajzenberg D., Mandelbrot L., Gomez-Marin J.E. International Encyclopedia of Public Health. 2017. Protozoan diseases: toxoplasmosis; pp. 114–132. [Google Scholar]
- 2.Pino L.E., Salinas J.E., López M.C. Descripción de un brote epidémico de toxoplasmosis aguda en pacientes inmunocompetentes miembros de las fuerzas militares de Colombia durante operaciones de selva. Infectio. 2009;13(2):83–91. [Google Scholar]
- 3.Carme B., Demar M., Ajzenberg D., Dardé M.L. Severe acquired toxoplasmosis caused by wild cycle of Toxoplasma gondii, French Guiana. Emerg Infect Dis. 2009;15(4):656–658. doi: 10.3201/eid1504.081306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carme B., Bissuel F., Ajzenberg D. Severe acquired toxoplasmosis in immunocompetent adult patients in French Guiana. J Clin Microbiol. 2002;40(11):4037–4044. doi: 10.1128/JCM.40.11.4037-4044.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demar M., Ajzenberg D., Maubon D. Fatal outbreak of human toxoplasmosis along the maroni river: epidemiological, clinical, and parasitological aspects. Clin Infect Dis. 2007;45(7):e88–e95. doi: 10.1086/521246. [DOI] [PubMed] [Google Scholar]
- 6.Sánchez V., De-la-Torre A., Gómez-Marín J.E. Characterization of ROP18 alleles in human toxoplasmosis. Parasitol Int. 2014;63(2):463–469. doi: 10.1016/j.parint.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 7.de-la-Torre A., Pfaff A.W., Grigg M.E., Villard O., Candolfi E., Gomez-Marin J.E. Ocular cytokinome is linked to clinical characteristics in ocular toxoplasmosis. Cytokine. 2014;68(1):23–31. doi: 10.1016/j.cyto.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de-la-Torre A., Sauer A., Pfaff A.W. Severe South American ocular toxoplasmosis is associated with decreased Ifn-γ/Il-17a and increased Il-6/Il-13 intraocular levels. PLoS Neglected Trop Dis. 2013;7(11) doi: 10.1371/journal.pntd.0002541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hunter C.a., Sibley L.D. Modulation of innate immunity by Toxoplasma gondii virulence effectors. Nat Rev Microbiol. 2012;10(11):766–778. doi: 10.1038/nrmicro2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niedelman W., Gold D.A., Rosowski E.E. The rhoptry proteins ROP18 and ROP5 mediate Toxoplasma gondii evasion of the murine, but not the human, interferon-gamma response. PLoS Pathog. 2012;8(6) doi: 10.1371/journal.ppat.1002784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jpj Saeij, Boyle J.P., Coller S. Polymorphic secreted kinases are key virulence factors in toxoplasmosis. Science. 2006;314(5806):1780–1783. doi: 10.1126/science.1133690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torres-Morales E., Taborda L., Cardona N. Th1 and Th2 immune response to P30 and ROP18 peptides in human toxoplasmosis. Med Microbiol Immunol. 2014;203(5):315–322. doi: 10.1007/s00430-014-0339-0. [DOI] [PubMed] [Google Scholar]
- 13.Moraru M., Black L.E., Muntasell A. NK cell and ig interplay in defense against herpes simplex Virus type 1: epistatic interaction of CD16A and IgG1 allotypes of variable affinities modulates antibody-dependent cellular cytotoxicity and susceptibility to clinical reactivation. J Immunol. 2015;195(4):1676–1684. doi: 10.4049/jimmunol.1500872. [DOI] [PubMed] [Google Scholar]
- 14.Singh R.A.K., Zhang J.Z. Differential activation of ERK, p38, and JNK required for Th1 and Th2 deviation in myelin-reactive T cells induced by altered peptide ligand. J Immunol. 2004;173(12):7299–7307. doi: 10.4049/jimmunol.173.12.7299. [DOI] [PubMed] [Google Scholar]
- 15.Eswarappa S.M., Estrela S., Brown S.P. Within-host dynamics of multi-species infections: facilitation, competition and virulence. Heil M., editor. PLoS One. 2012;7(6) doi: 10.1371/journal.pone.0038730. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.