Abstract
With a development of radiotherapeutic techniques, availability of radiotherapy data on cardiotoxicity, and slowly improving esophageal cancer outcomes, an increasing emphasis is placed on the heart protection in radiation treated esophageal cancer patients. Radiation induced heart complications encompass mainly pericardial disease, cardiomyopathy, coronary artery atherosclerosis, valvular heart disease, and arrhythmias. The most frequent toxicity is pericardial effusion which is usually asymptomatic in the majority of patients. The use of modern radiotherapy techniques is expected to reduce the risk of cardiotoxicity, although this expectation has to be confirmed by clinical data.
Keywords: Esophageal cancer, Radiation therapy, Cardiac toxicity
1. Introduction
The incidence rate of esophageal cancer varies considerably according to geographic location. The highest rates occur in Central and Eastern Asia, and Eastern and Southern Africa, while the lowest rates are in Western Africa.1, 2 The main histological types of esophageal cancer are squamous cell carcinoma (SCC) and adenocarcinoma (AC). Smoking and alcohol consumption is considered to be the most significant risk factors for SCC, while obesity and gastroesophageal reflux disease are strong risk factors of AC.3 Adenocarcinomas usually occur in the lower third of the esophagus and there is a high increase of this histological type in the last decades in Europe, the United States, and Australia.4, 5
According to the guidelines, in esophageal cancer generally, radiotherapy plays a role mainly in locoregional or locally advanced disease (clinical stage IIA - IVA). It is provided as preoperative chemoradiation with subsequent surgery or even chemoradiation alone as a definitive treatment. Chemoradiation alone as a definitive therapy is preferred mainly in patients unfit for surgery or patients refusing surgery, in unresectable disease, and in cervical esophageal cancer.6, 7
The common regimen of concurrent chemoradiotherapy is radiotherapy in a dose of 50–50.4 Gy in 5–5.5 weeks and concurrent chemotherapy, 5-fluorouracil and cisplatin. This scheme was defined as an effective definitive treatment by two randomized trials. The first one, RTOG 8501, demonstrated that concurrent chemoradiotherapy is more effective than radiotherapy alone, although with lower dose of radiation.8 The second one, Intergroup study 0123, did not confirm a survival benefit associated with a higher dose of radiation than 50.4 Gy in concurrent chemoradiotherapy.9 Currently, other regimens of chemotherapy are accepted as similarly effective, e.g. FOLFOX, paclitaxel-carboplatin, etc.10, 11
The preoperative chemoradiation is based on results of clinical trials and meta-analyses that confirmed a benefit of preoperative chemoradiation and surgery compared to surgery alone in esophageal cancer in terms of overall survival.12, 13 In the preoperative setting, until recently, the regimen of chemoradiation was not different compared to definitive treatment in most cases. The CROSS trial, one of the largest trial published recently, that confirmed the survival benefit of preoperative chemoradation compared to surgery alone used radiation with a lower dose (41.4 Gy in 23 fractions in 4.5 weeks) and concurrent chemotherapy consisting of paclitaxel and carboplatin in a weekly regimen.14 Therefore, this regimen is accepted in the preoperative setting, as well.
According to the International Commission on Radiation Units and Measurements (ICRU) reports 50 and ICRU Supplement 62, the gross tumor volume (GTV) is defined as a macroscopic primary tumor and involved lymph nodes.15, 16 It is advantageous to use FDG-positron emission tomography (PET) for precise definition of GTV.17 The clinical target volume (CTV) includes a primary tumor, lymph nodes (GTV) and areas at risk of a microscopic spread of the disease. In general, cranial and caudal margin is recommended of at least 3 cm because of the submucosal spread and 1 cm radially. The lymph nodes at risk are included in the CTV according to the location of the primary tumour.17, 18 Planning target volume (PTV) includes CTV plus a margin for internal movements, mainly respiratory movements, (internal margin) and for set-up uncertainties (setup margin). The margin of 1 cm beyond the CTV is commonly used.7, 19
It follows from the above that delineated PTV usually has an elongated cylindrical shape in the posterior part of the mediastinum and, depending on primary tumor location, it is often located beyond the heart. Although modern radiation therapy techniques have been introduced to clinical practice, the dose distribution in many cases of esophageal cancer continues to be a compromise between sparing of the lungs, heart and spinal cord.20 Therefore, the heart is usually considered a significant organ at risk for the radiation of esophageal cancer.
2. Radiation-induced cardiotoxicity
Radiotherapy-induced cardiotoxicity has been extensively studied mainly in breast cancer21, 22 and Hodgkin’s lymphoma patients.23, 24 The reason of scientific concentration on cardiotoxicity in these diagnoses is the high number of patients with breast cancer, very young age at the time of treatment of Hodgkin’s lymphoma and a high number of long term survivals in both diagnoses.
On the other hand, esophageal cancer is a rare diagnosis in young people. The highest incidence is at an older age (>65 years of age).25, 26 And, despite modern treatment approaches, the prognosis of esophageal cancer is not good regardless of a curative therapeutic approach. The 5-year overall survival is often less than 30% in unselected locally advanced esophageal cancer patients,27, 28 although there are studies with trimodality therapy (preoperative chemoradiation and surgery) with 5-year overall survival ≥45%.29, 30 Furthermore, some heart diseases can be caused by similar risk factors as esophageal cancer, like smoking and alcohol in SCC, and obesity in AC. Therefore, the cardiotoxicity of radiation in esophageal cancer is described in literature much less than in diagnoses mentioned above.
Radiation induced heart complications encompass mainly: pericardial disease, cardiomyopathy, coronary artery atherosclerosis, valvular heart disease, and arrhythmias. Pericardial disease can present itself as acute pericarditis or chronic pericardial effusion, often asymptomatic. Cardiomyopathy is usually characterized pathologically as diffuse fibrosis of the myocardium that can result in decreased ejection fraction and sometimes in arrhythmias. Radiation enhances the atherosclerotic process in coronary arteries and may lead to myocardial infarction. Radiation induced valvular disease means focal leaflet fibrosis, valve calcifications and stenosis, and these changes cause reduced ejection fraction and valve regurgitation. Arrhythmias are usually supraventricular.31, 32
3. Radiation-induced heart toxicity in esophageal cancer
The higher risk of cardiac death in survivors after radiation for esophageal cancer was noted in an analysis of the Surveillance, Epidemiology, and End Results (SEER). Garzai et al. analyzed 5630 patients treated from 1973 to 2012 for esophageal cancer. There was higher probability of cardiac death among patients after radiotherapy compared to no radiation group (p < 0.0001) and in lower esophageal carcinomas compared to upper and middle esophageal carcinomas (p = 0.01 and p = 0.04, resp.).33
Data from SEER were earlier analyzed also by Frandsen et al. with the aim of evaluating the risk of heart disease related death following radiation therapy for esophageal cancer. They found a total number of 40,677 esophageal cancer patients with 26,377 of them having received radiation as a part of their initial therapy. While the overall survival for all patients at 5-, 10- and 20-year was 19.7%, 11.8% and 4.5%, resp., the absolute risk of heart disease related death was 2.8%, 5.3% and 9.4%, resp. According to primary tumor location, the highest risk of heart disease related death was for mid-esophageal tumors.34
The review of Beukema et al. analyzed published data on cardiotoxicity and radiation dose parameters between 1970 and 2013. They identified 13 relevant papers with total number of 781 patients treated with radiotherapy in dose of 30−70 Gy. Based on an analysis of these papers they concluded that the overall incidence of clinically relevant cardiotoxicity was 10.8% (data available in six papers). Most events occurred within 2 years after the treatment. Pericardial effusions were the most common cardiac toxicity with an actuarial rate of 48%, although in most cases these effusions were asymptomatic and were found using imaging methods.35
Retrospective study of Ogino et al. found a probability of symptomatic radiation-induced cardiac complications 13.8% at 5 years, based on data from follow-up in 58 long term survivors after chemoradiotherapy for esophageal cancer. The most frequent complications were pericardial effusion and atrial tachyarrhythmias.36
The clinical and dosimetric factors influencing the risk of pericardial effusion after definitive radiochemotherapy for esophageal cancer were evaluated by Wei et al. They reported in 101 patients a development of pericardial effusion in 27.7% at a median of 5.3 months. The effusion was revealed at follow-up CT as asymptomatic complication. The risk of pericardial effusion increased significantly with a mean pericardial dose of 26.1 Gy and V30 of the pericardium greater than 46 % (13% vs. 73%, p = 0,001.37
Fukada et al. retrospectively analyzed 167 patients treated between 2001 and 2010 with concurrent chemoradiation. At the median follow-up of 29 months, pericardial effusion of all grades developed in 35.9% patients with an onset in median of 6 months. Symptomatic pericardial effusion (grade ≥ 3) developed in 8.4% patients with a median of 22 months (4–108 months). The average mean pericardial dose was 43.2 Gy in patients with symptomatic pericardial effusion, and it was significantly higher compared to 36.2 Gy in those with asymptomatic pericardial effusion (p < 0.05) and 26.7 Gy in those without pericardial effusion (p < 0.001). Pericardial V30 to V45 was significantly greater in patients with symptomatic effusion than in those with asymptomatic pericardial effusion (p < 0.05).38
Pericardial disease as the most frequent cardiac toxicity of chemoradiation for esophageal cancer was also discussed in the study of Ishikura et al. The symptomatic pericarditis was revealed in 10% of 78 patients in complete remission. Other severe cardiac complications were myocardial infarction (2 cases) and heart failure (2 cases).39
Ogino et al. evaluated 86 patients with a minimum 24 months of follow-up after concurrent chemoradiotherapy for esophageal cancer. Pericardial effusion was revealed during follow-up at CT in 49 pts. (57%), but symptomatic effusion was only in 5 patients (6%). The pericardial V50 in patients with symptomatic effusion ranged between 17.1 and 21.7%; therefore, the authors recommend the threshold for the pericardial V50 ≤ 17%.40
Konski et al. evaluated 102 pts. treated with preoperative or definitive chemoradiotherapy for esophageal cancer and found a 12-month actuarial incidence of any cardiotoxicity of 20.4%, and 8.5% when symptomatic toxicities were considered. There was significantly higher mean heart V20, V30 and V40 in patients with symptomatic cardiotoxicity and the authors recommended that these parameters should be kept below 70%, 65%, and 60%, resp. In this study women had higher probability to develop symptomatic cardiotoxicity (p = 0.0013) or any cardiotoxicity (p = 0.0046).41
The higher risk of cardiotoxicity for women was noted in retrospective study of Tait et al. This study evaluated 100 males and 27 females after preoperative or definitive chemoradiation and found heart complication of grade ≥ 3 in 16 males (16%), but in 12 females (44%). The risk of cardiotoxicity in females was 4.15 times higher (95% CI 1.63–10.50, p = 0.0017) than in males. The most frequent complication was pericardial effusion (23 cases).42
Lester et al. evaluated the risk of cardiopulmonary toxicity depending on age in 571 patients treated with preoperative chemoradiation and surgery at three US centers. The rate of cardiotoxicity including atrial fibrillation and other arrhythmias, myocardial infarction, and congestive heart failure, was significantly higher in patients older than 65 years than in younger (25% vs. 13%, p < 0.001).43
Recent paper of Hayashi et al. evaluated cardiotoxicity in 80 patients treated with chemoradiotherapy for invasive squamous cell superficial esophageal cancer. With a median follow-up of 73 months, thirteen patients developed severe cardiac events: ischemic heart diseases (7 pts.), pericardial effusion (3 pts.), atrial fibrillation (1 pt.), and sudden death. The 5-year rate of severe cardiac events was 16.3%. The ratio of irradiated to the total heart volume and the absolute heart’s exposure to radiation was significantly higher in patients who developed a cardiac event. The authors found volume cut-off of 280 ml of the heart irradiated with a dose higher than 50 Gy (V50) with a risk ratio of 16.80 (95% CI, 4.94–53.07).44
4. Modern techniques in radiation therapy
The dose that the heart receives is greatly dependent on the location of the target volume, but also on the irradiation technique. Historically, conventional radiotherapy has almost not evaluated dose-volume parameters for organs at risk. Three-dimensional conformal radiotherapy (3D-CRT) introduced treatment planning based on three-dimensional computer tomography. Modern software made it possible to calculate dose in target volumes and organs at risk, as well as include dose-volume histograms and, based on these data, we obtained the possibility to find an association between dose-volume parameters and clinical data of radiation toxicity.
The development of computer technology, as well as availability of modern imaging techniques and tools during radiotherapy planning and radiotherapy application, enabled the improvement of organ at risk sparing. For a definition of GTV, it is appropriate to use hybrid PET/CT. Similarly, the dose distribution is improved using modern irradiation techniques, mainly intensity-modulated radiation therapy (IMRT) and its variants, including volumetric modulated arc therapy (VMAT) or tomotherapy (Fig. 1). Image-guided radiotherapy means acquisition of two orthogonal images or cone-beam CT before individual fraction of radiation, matching the reference images and individual setup corrections. IGRT enables reduction of set-up margins and, subsequently, a dose reduction in organs at risk during radiotherapy planning. The motion during the respiratory cycle can be significant, mainly in lower esophageal tumors.45 It is possible to verify the movement of target volumes and organs at risk during respiratory cycle using 4D-computer tomography, and in the case of an advantage of irradiation in specific phase of respiratory cycle it is possible to use respiratory control techniques for irradiation (respiratory gating, tracking etc.). Recently, proton therapy has been increasingly discussed because of advantageous dose distribution.19
Fig. 1.
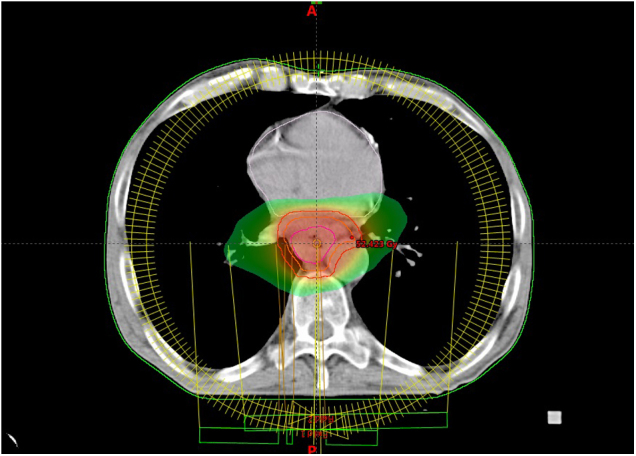
Heart-sparing radiotherapy using volumetric modulated arc therapy (VMAT) in esophageal cancer. The volume irradiated with a dose greater than 25 Gy is displayed.
There are several papers comparing dosimetric isodose plans using modern irradiation techniques. Chen et al. compared IMRT, tomotherapy and 3D-CRT techniques and found better sparing of the heart (V30 and V45) in IMRT and tomotherapy although lung V10 was more acceptable in 3D-CRT.46 Similarly, Choi et al. noted better heart protection using IMRT and VMAT compared to 3D-CRT in V20, V30, and V40 parameters.47 Kataria et al. found dosimetric advantages of the VMAT technique compared to IMRT, although the data for the heart were not statistically significant.48 On the other hand, Wu et al. compared isodose plans of VMAT, IMRT and 3D-CRT and, although they found a better protection of the heart, they recommended 3D-CRT as a feasible and cost-effective option for middle thoracic esophageal cancer radiotherapy.49
Xu et al. retrospectively analyzed 65 patients with thoracic esophageal cancer treated with the VMAT technique using 4D-CT and PET based planning and compared plans and results with 130 pts. treated with the IMRT step and shoot technique (ssIMRT). With a median follow-up of 14.3 months in the VMAT group and 31.8 months in the ssIMRT group, there was no difference in overall survival or recurrence-free survival in statistical analysis. Dosimetric analysis did not reveal a significant difference for heart parameters V30 and V40, V5 was higher, while V50 was lower in the VMAT group compared to ssIMRT. The differences in cardiac toxicity were not statistically significant between the groups.50 Hirano et al. compared proton therapy, IMRT and 3D-CRT plans in CT data of 27 esophageal cancer patients treated with proton therapy. Proton therapy reached a better dose distribution compared to IMRT and 3D-CRT in the heart and lung sparing. Clinically, four patients (15%) developed late pericardial effusion of grade 2 after proton therapy.51
Similarly, Ling et al. compared proton therapy, IMRT, and 3D-CRT in 10 esophageal cancer patient CT data. That study did not find a benefit of IMRT compared to 3D-CRT, but proton therapy was beneficial in lower heart doses compared to both, IMRT and 3D-CRT.52 Similarly, dosimetric study of Shiraishi et al. reported that proton therapy is associated with significantly lower radiation exposure to the whole heart and cardiac substructures than IMRT.53
Clinical data of intensity-modulated proton therapy in 19 pts. with esophageal cancer was recently published by Prayongrat et al. With a median follow-up of 17 months, they noted only two grade 2 toxicities: pericardial effusion and atrial fibrillation.54
Despite modern radiation techniques in use, there can be a higher risk of heart complications in the case of dose escalation. Venkat et al. compared treatment results after preoperative chemoradiation with prescribed dose of 56 Gy a 50.4 Gy. Radiation was applied as IMRT and planned using 4D-CT. Although the higher dose was associated with better treatment results in terms of better pathologic complete response rate (56.2% vs. 30.0%), 3-year locoregional control (93.8% vs. 78.5%; p = 0.022), 3-year freedom from failure (73.7% vs. 52.2%; p = 0.051), there was a statistically significant increase in postoperative atrial fibrillation (30.1% vs. 12.5%; p = 0.036).55
5. Conclusion
With a development of radiotherapeutic techniques, availability of radiotherapy data on cardiotoxicity, and slowly improving esophageal cancer outcomes, an increasing emphasis is placed on the heart sparing in radiation treated esophageal cancer patients. Modern techniques and tools improve the dose distribution with organ at risk sparing, including heart protection, and, therefore, the risk of cardiotoxicity is expected to be reduced when using these techniques. Naturally, expectations must be confirmed by clinical data. However, it is equally important, even at this time, to be aware of the cardiotoxicity risk in patients irradiated for esophageal cancer.
Financial disclosure
None declared.
Conflict of interest
None declared.
Acknowledgments
The present study was supported by the MH CZ-DRO (UHHK, grant number 00179906) and by the program PROGRESQ40/06. Thanks to Dr. Daniel Díaz for his assistance in English language proofreading.
References
- 1.Gupta B., Kumar N. Worldwide incidence, mortality and time trends for cancer of the oesophagus. Eur J Cancer Prev. 2017;26:107–118. doi: 10.1097/CEJ.0000000000000249. [DOI] [PubMed] [Google Scholar]
- 2.Wong M.C.S., Hamilton W., Whiteman D.C. Global Incidence and mortality of oesophageal cancer and their correlation with socioeconomic indicators temporal patterns and trends in 41 countries. Sci Rep. 2018;8:4522. doi: 10.1038/s41598-018-19819-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xie S.H., Lagergren J. Risk factors for oesophageal cancer. Best Pract Res Clin Gastroenterol. 2018;36–37:3–8. doi: 10.1016/j.bpg.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 4.Thrift A.P., Whiteman D.C. The incidence of esophageal adenocarcinoma continues to rise: Analysis of period and birth cohort effects on recent trends. Ann Oncol. 2012;23:3155–3162. doi: 10.1093/annonc/mds181. [DOI] [PubMed] [Google Scholar]
- 5.Malhotra G.K., Yanala U., Ravipati A., Follet M., Vijayakumar M., Are C. Global trends in esophageal cancer. J Surg Oncol. 2017;115:564–579. doi: 10.1002/jso.24592. [DOI] [PubMed] [Google Scholar]
- 6.Lordick F., Mariette C., Haustermans K., Obermannová R., Arnold D., ESMO Guidelines Committee Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl 5):v50–v57. doi: 10.1093/annonc/mdw329. [DOI] [PubMed] [Google Scholar]
- 7.The National Comprehensive Cancer Network (NCCN). Esophageal and Esophagogastric Junction Cancer. Version 1.2019. https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf [accessed 23 April 2019].
- 8.Cooper J.S., Guo M.D., Herskovic A. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA. 1999;281:1623–1627. doi: 10.1001/jama.281.17.1623. [DOI] [PubMed] [Google Scholar]
- 9.Minsky B.D., Pajak T.F., Ginsberg R.J. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20:1167–1174. doi: 10.1200/JCO.2002.20.5.1167. [DOI] [PubMed] [Google Scholar]
- 10.Ruppert B.N., Watkins J.M., Shirai K. Cisplatin/Irinotecan versus carboplatin/paclitaxel as definitive chemoradiotherapy for locoregionally advanced esophageal cancer. Am J Clin Oncol. 2010;33:346–352. doi: 10.1097/COC.0b013e3181aaca26. [DOI] [PubMed] [Google Scholar]
- 11.Conroy T., Galais M.P., Raoul J.L. Fédération Francophone de Cancérologie Digestive and UNICANCER-GI Group. Definitive chemoradiotherapy with FOLFOX versus fluorouracil and cisplatin in patients with oesophageal cancer (PRODIGE5/ACCORD17): final results of a randomised, phase 2/3 trial. Lancet Oncol. 2014;15:305–314. doi: 10.1016/S1470-2045(14)70028-2. [DOI] [PubMed] [Google Scholar]
- 12.Urschel J.D., Vasan H. A meta-analysis of randomized controlled trials that compared neoadjuvant chemoradiation and surgery to surgery alone for resectable esophageal cancer. Am J Surg. 2003;185:538–543. doi: 10.1016/s0002-9610(03)00066-7. [DOI] [PubMed] [Google Scholar]
- 13.Fiorica F., Di Bona D., Schepis F. Preoperative chemoradiotherapy for oesophageal cancer: a systematic review and meta-analysis. Gut. 2004;53:925–930. doi: 10.1136/gut.2003.025080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shapiro J., van Lanschot J.J., Hulshof M.C. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090–1098. doi: 10.1016/S1470-2045(15)00040-6. [DOI] [PubMed] [Google Scholar]
- 15.ICRU; Bethesda, MD: 1993. International commission on radiation units and measurements (ICRU). prescribing, recording, and reporting photon beam therapy. ICRU report 50. [Google Scholar]
- 16.ICRU; Bethesda, MD: 1999. International Commission on Radiation Units and Measurements. ICRU Report 62: Prescribing, recording, and reporting photon beam therapy (supplement to ICRU Report 50) [Google Scholar]
- 17.Han D., Yuan Y., Song X., Yu Y., Yu J. What is the appropriate clinical target volume for esophageal squamous cell carcinoma? Debate and consensus based on pathological and clinical outcomes. J Cancer. 2016;7:200–206. doi: 10.7150/jca.13873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong Y., Guan H., Huang W. Precise delineation of clinical target volume for crossing-segments thoracic esophageal squamous cell carcinoma based on the pattern of lymph node metastases. J Thorac Dis. 2015;7(Dec (12)):2313–2320. doi: 10.3978/j.issn.2072-1439.2015.12.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vosmik M., Petera J., Sirak I. Technological advances in radiotherapy for esophageal cancer. World J Gastroenterol. 2010;16:5555–5564. doi: 10.3748/wjg.v16.i44.5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vieillevigne L., Vidal M., Izar F., Rives M. Is dose escalation achievable for esophageal carcinoma? Rep Pract Oncol Radiother. 2015;20(Jan 5(2)):135–140. doi: 10.1016/j.rpor.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Darby S.C., Ewertz M., McGale P. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 22.van den Bogaard V.A., Ta B.D., van der Schaaf A. Validation and modification of a prediction model for acute cardiac events in patients with breast cancer treated with radiotherapy based on three-dimensional dose distributions to cardiac substructures. J Clin Oncol. 2017;35:1171–1178. doi: 10.1200/JCO.2016.69.8480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hahn E., Jiang H., Ng A. Late cardiac toxicity after mediastinal radiation therapy for Hodgkin lymphoma: contributions of coronary artery and whole heart dose-volume variables to risk prediction. Int J Radiat Oncol Biol Phys. 2017;98:1116–1123. doi: 10.1016/j.ijrobp.2017.03.026. [DOI] [PubMed] [Google Scholar]
- 24.van Nimwegen F.A., Schaapveld M., Cutter D.J. Radiation dose-response relationship for risk of coronary heart disease in survivors of Hodgkin Lymphoma. J Clin Oncol. 2016;34:235–243. doi: 10.1200/JCO.2015.63.4444. [DOI] [PubMed] [Google Scholar]
- 25.Mathieu L.N., Kanarek N.F., Tsai H.L., Rudin C.M., Brock M.V. Age and sex differences in the incidence of esophageal adenocarcinoma: results from the Surveillance, Epidemiology, and End Results (SEER) Registry (1973–2008) Dis Esophagus. 2014;27:757–763. doi: 10.1111/dote.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Q.L., Xie S.H., Wahlin K., Lagergren J. Global time trends in the incidence of esophageal squamous cell carcinoma. Clin Epidemiol. 2018;10:717–728. doi: 10.2147/CLEP.S166078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Worni M., Castleberry A.W., Gloor B. Trends and outcomes in the use of surgery and radiation for the treatment of locally advanced esophageal cancer: a propensity score adjusted analysis of the surveillance, epidemiology, and end results registry from 1998 to 2008. Dis Esophagus. 2014;27:662–669. doi: 10.1111/dote.12123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sio T.T., Wilson Z.C., Stauder M.C. Long-term treatment outcomes for locally advanced esophageal cancer: a single-institution experience. Am J Clin Oncol. 2016;39:448–452. doi: 10.1097/COC.0000000000000089. [DOI] [PubMed] [Google Scholar]
- 29.Fakhrian K., Ordu A.D., Lordick F. Long-term outcomes of trimodality treatment for squamous cell carcinoma of the esophagus with cisplatin and/or 5-FU: more than 20 years’ experience at a single institution. Strahlenther Onkol. 2014;190:1133–1140. doi: 10.1007/s00066-014-0711-4. [DOI] [PubMed] [Google Scholar]
- 30.van Hagen P., Hulshof M.C., van Lanschot J.J. CROSS Group. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 31.Yusuf S.W., Venkatesulu B.P., Mahadevan L.S., Krishnan S. Radiation-induced cardiovascular disease: a clinical perspective. Front Cardiovasc Med. 2017;4:66. doi: 10.3389/fcvm.2017.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spetz J., Moslehi J., Sarosiek K. Radiation-induced cardiovascular toxicity: mechanisms, prevention, and treatment. Curr Treat Options Cardiovasc Med. 2018;20:31. doi: 10.1007/s11936-018-0627-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gharzai L., Verma V., Denniston K.A., Bhirud A.R., Bennion N.R., Lin C. Radiation therapy and cardiac death in long-term survivors of esophageal cancer: an analysis of the surveillance, epidemiology, and end result database. PLoS One. 2016;11 doi: 10.1371/journal.pone.0158916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frandsen J., Boothe D., Gaffney D.K., Wilson B.D., Lloyd S. Increased risk of death due to heart disease after radiotherapy for esophageal cancer. J Gastrointest Oncol. 2015;6:516–523. doi: 10.3978/j.issn.2078-6891.2015.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beukema J.C., van Luijk P., Widder J., Langendijk J.A., Muijs C.T. Is cardiac toxicity a relevant issue in the radiation treatment of esophageal cancer. Radiother Oncol. 2015;114:85–90. doi: 10.1016/j.radonc.2014.11.037. [DOI] [PubMed] [Google Scholar]
- 36.Ogino I., Watanabe S., Iwahashi N. Symptomatic radiation-induced cardiac disease in long-term survivors of esophageal cancer. Strahlenther Onkol. 2016;192:359–367. doi: 10.1007/s00066-016-0956-1. [DOI] [PubMed] [Google Scholar]
- 37.Wei X., Liu H.H., Tucker S.L. Risk factors for pericardial effusion in inoperable esophageal cancer patients treated with definitive chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2008;70:707–714. doi: 10.1016/j.ijrobp.2007.10.056. [DOI] [PubMed] [Google Scholar]
- 38.Fukada J., Shigematsu N., Takeuchi H. Symptomatic pericardial effusion after chemoradiation therapy in esophageal cancer patients. Int J Radiat Oncol Biol Phys. 2013;87:487–493. doi: 10.1016/j.ijrobp.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 39.Ishikura S., Nihei K., Ohtsu A. Long-term toxicity after definitive chemoradiotherapy for squamous cell carcinoma of the thoracic esophagus. J Clin Oncol. 2003;21:2697–2702. doi: 10.1200/JCO.2003.03.055. [DOI] [PubMed] [Google Scholar]
- 40.Ogino I., Watanabe S., Sakamaki K., Ogino Y., Kunisaki C., Kimura K. Dosimetric predictors of radiation-induced pericardial effusion in esophageal cancer. Strahlenther Onkol. 2017;193:552–560. doi: 10.1007/s00066-017-1127-8. [DOI] [PubMed] [Google Scholar]
- 41.Konski A., Li T., Christensen M. Symptomatic cardiac toxicity is predicted by dosimetric and patient factors rather than changes in 18F-FDG PET determination of myocardial activity after chemoradiotherapy for esophageal cancer. Radiother Oncol. 2012;104:72–77. doi: 10.1016/j.radonc.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tait L.M., Meyer J.E., McSpadden E. Women at increased risk for cardiac toxicity following chemoradiation therapy for esophageal carcinoma. Pract Radiat Oncol. 2013;3:e149–55. doi: 10.1016/j.prro.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 43.Lester S.C., Lin S.H., Chuong M. A multi-institutional analysis of trimodality therapy for esophageal cancer in elderly patients. Int J Radiat Oncol Biol Phys. 2017;98:820–828. doi: 10.1016/j.ijrobp.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 44.Hayashi Y., Iijima H., Isohashi F. The heart’s exposure to radiation increases the risk of cardiac toxicity after chemoradiotherapy for superficial esophageal cancer: a retrospective cohort study. BMC Cancer. 2019;19:195. doi: 10.1186/s12885-019-5421-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sekii S., Ito Y., Harada K. Intrafraction esophageal motion in patients with clinical T1N0 esophageal cancer. Rep Pract Oncol Radiother. 2018;23:398–401. doi: 10.1016/j.rpor.2018.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen Y.J., Liu A., Han C. Helical tomotherapy for radiotherapy in esophageal cancer: a preferred plan with better conformal target coverage and more homogeneous dose distribution. Med Dosim. 2007;32:166–171. doi: 10.1016/j.meddos.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 47.Choi K.H., Kim J., Lee S.W., Kang Y.N., Jang H. Dosimetric comparison between modulated arc therapy and static intensity modulated radiotherapy in thoracic esophageal cancer: a single institutional experience. Radiat Oncol J. 2018;36:63–70. doi: 10.3857/roj.2017.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kataria T., Govardhan H.B., Gupta D. Dosimetric comparison between volumetric modulated arc therapy (VMAT) vs intensity modulated radiation therapy (IMRT) for radiotherapy of mid esophageal carcinoma. J Cancer Res Ther. 2014;10:871–877. doi: 10.4103/0973-1482.138217. [DOI] [PubMed] [Google Scholar]
- 49.Wu Z., Xie C., Hu M. Dosimetric benefits of IMRT and VMAT in the treatment of middle thoracic esophageal cancer: is the conformal radiotherapy still an alternative option? J Appl Clin Med Phys. 2014;15:93–101. doi: 10.1120/jacmp.v15i3.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu C., Xi M., Komaki R. Dosimetric and clinical outcomes after volumetric modulated arc therapy for carcinoma of the thoracic esophagus. Adv Radiat Oncol. 2017;2:325–332. doi: 10.1016/j.adro.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hirano Y., Onozawa M., Hojo H. Dosimetric comparison between proton beam therapy and photon radiation therapy for locally advanced esophageal squamous cell carcinoma. Radiat Oncol. 2018;13:23. doi: 10.1186/s13014-018-0966-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ling T.C., Slater J.M., Nookala P. Analysis of intensity-modulated radiation therapy (IMRT), proton and 3D conformal radiotherapy (3D-CRT) for reducing perioperative cardiopulmonary complications in esophageal cancer patients. Cancers (Basel). 2014;6:2356–2368. doi: 10.3390/cancers6042356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shiraishi Y., Xu C., Yang J., Komaki R., Lin S.H. Dosimetric comparison to the heart and cardiac substructure in a large cohort of esophageal cancer patients treated with proton beam therapy or Intensity-modulated radiation therapy. Radiother Oncol. 2017;125:48–54. doi: 10.1016/j.radonc.2017.07.034. [DOI] [PubMed] [Google Scholar]
- 54.Prayongrat A., Xu C., Li H., Lin S.H. Clinical outcomes of intensity modulated proton therapy and concurrent chemotherapy in esophageal carcinoma: a single institutional experience. Adv Radiat Oncol. 2017;2:301–307. doi: 10.1016/j.adro.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Venkat P.S., Shridhar R., Naghavi A.O. Dose escalated neoadjuvant chemoradiotherapy with dose-painting intensity-modulated radiation therapy and improved pathologic complete response in locally advanced esophageal cancer. Dis Esophagus. 2017;30:1–9. doi: 10.1093/dote/dox036. [DOI] [PubMed] [Google Scholar]


