Abstract
Esophageal squamous cell carcinoma (ESCC) is a common cancer occurring in males and females worldwide. Accumulating evidence continues to highlight the crucial roles of long non-coding RNAs (lncRNAs) in the process of tumorigenesis. However, the regulatory mechanism of lncRNAs in ESCC remains unclear. The aim of this study is to elucidate the role of lncRNA Krüppel-like factor 3 antisense RNA 1 (KLF3-AS1) in ESCC by regulating miR-185-5p and KLF3. Initially, ESCC cell spheres with stem cell-like properties were prepared by suspension culture, and subsequently characterized by assessing colony formation ability and stem cell markers. LncRNA KLF3-AS1 was found to be poorly expressed in ESCC and could upregulate the expression of KLF3 by binding to miR-185-5p. lncRNA KLF3-AS1 upregulation was observed to inhibit miR-185-5p, thereby contributing to decreased expression of SOX2 and Oct4 (octamer-binding transcription factor 4). Furthermore, enhancement of lncRNA KLF3-AS1 resulted in reduced colony formation ability, cell invasion and migration, and tumor volume in vivo while promoting cell apoptosis in ESCC through downregulation of miR-185-5p. Collectively, this study indicated that lncRNA KLF3-AS1 inhibited ESCC cell invasion and migration by impairing miR-185-5p-mediated inhibition of KLF3, highlighting a promising novel potential target for ESCC treatment.
Keywords: long non-coding RNA Krüppel-like factor 3 antisense RNA 1, competing endogenous RNA, miR-185-5p, Krüppel-like factor 3, esophageal squamous cell carcinoma stem cells, biological characteristics, nude mice, migration, apoptosis
Introduction
Esophageal squamous cell carcinoma (ESCC) continues to be one of the deadliest malignant tumors with high mortality and morbidity worldwide, and it is also the main esophageal carcinoma histologic type in Asian countries. Additionally, ESCC ranks sixth in the predominant cancer-related death cause, depriving more than 400,000 patients of their lives each year.1 The occurrence and progression of ESCC have been chiefly attributed to a diet deficient of minerals and vitamins, in addition to the overconsumption of alcohol and tobacco.2 The enormous mortality of patients diagnosed with ESCC is mainly caused by a poor prognosis and high recurrence rate after definitive chemotherapy and radiation treatment or curative surgery.3 Recently, radiation therapy with more precise intensity modulation accompanied by adjuvant chemotherapy increased the cure rate of ESCC, but the 5-year survival rate of patients with ESCC remains below 30%, with chemoresistance and radioresistance often occurring during and after the therapy.4 Long non-coding RNAs (lncRNAs) have been demonstrated to be associated with the pathogenesis of various tumors.5
lncRNAs are as non-coding RNA sequences with the length exceeding 200 nt, yet without any capacity for encoding protein expression.6 Previous data have highlighted the ability of lncRNAs to perform as crucial regulators of cancer, including ESCC.7 Additionally, lncRNAs play a key role in the tumorigenesis process by functioning as competing endogenous RNAs (ceRNAs).8 ceRNAs, the newly discovered members of RNAs, function in gene regulation mediated by microRNA (miRNA) and share miRNA recognition elements, thus showing mutual regulation.9 During the current study, the differential (gene expression) analysis on ESCC chip GEO: GSE17351 and GSE45670 revealed that the level of Krüppel-like factor 3 antisense RNA 1 (KLF3-AS1) was low in ESCC, and the analysis through the RNA22 website revealed the existence of binding sites between miR-185-5p and the KLF3 3′ untranslated region (UTR) and lncRNA KLF3-AS1, respectively. Aberrantly expressed miRNAs have been demonstrated to exert a role in tumor cell invasion, apoptosis, motility, and proliferation in ESCC.10 miR-185-5p is often overexpressed in various cancers, including renal cancer, breast cancer, and hepatocellular carcinoma, and studies have highlighted its ability to act as an oncogenic miRNA (oncomiRNA) capable of enhancing cancer cell proliferation.11 KLF3, a transcriptional inhibitor bound with the cofactor C-terminal-binding protein, possesses a wide range of biological impacts on regulating apoptosis, differentiation, and proliferation in various tissues during the entire progression process.12 Furthermore, KLF3 has been proposed as a tumor suppressor in colorectal cancer.13 Based on the aforementioned exploration of the literature, we hypothesized that lncRNA KLF3-AS1 is involved in ESCC cell invasion and migration through mediating miR-185-5p and KLF3. Therefore, the central objective of the current study was to elucidate the underlying molecular mechanisms by which lncRNA KLF3-AS1 interacts with miR-185-5p and KLF3 in ESCC, with the hope of identifying a fresh therapeutic strategy for ESCC treatment.
Results
Eca109 Cell Spheres Present Markedly Enhanced Colony Formation Ability
A clonogenic assay was primarily performed to evaluate cell colony formation ability. The results showed that after Eca109 cell suspension culture, compared with the Eca109 cells, the Eca109 cell spheres exhibited significantly elevated colony formation ability (p < 0.05) (Figures 1A and 1B). The western blot analysis results illustrated that the expression of the stem cell marker octamer-binding transcription factor 4 (Oct4) in Eca109 cell spheres was significantly increased (p < 0.05) (Figure 1C). Collectively, the results suggested that Eca109 cell spheres had stem cell characteristics, and they were therefore used in subsequent experiments.
Figure 1.
Characterization of ESCC Stem Cells Isolated from Eca109 Cells
(A) Representative images of spheres formed in Eca109 cells after suspension culture (original magnification, ×100). (B) Images and statistical analysis of colony formation ability of Eca109 cells and spheres. (C) Gray value of Oct4 protein band in Eca109 cells and spheres measured by western blot assay. *p < 0.05 versus the Eca109 spheres; the values are measurement data and are presented as the mean ± standard deviation. Data were analyzed using an independent sample t test, and the experiment was repeated three times.
lncRNA KLF3-AS1 Is Poorly Expressed in ESCC, and KLF3-AS1 May Regulate KLF3 by Competitively Binding to miR-185-5p
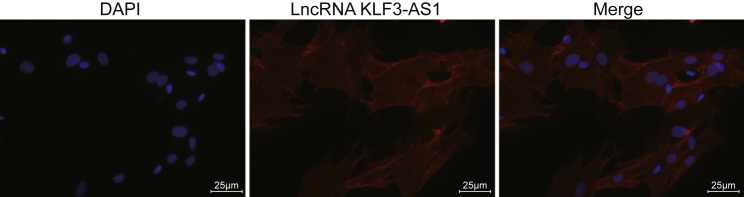
A fluorescence in situ hybridization (FISH) assay was conducted in order to identify the location of KLF3-AS1 in the Eca109 cells. The results signified that KLF3-AS1 could potentially function in the cytoplasm of the Eca109 cells (Figure 2).
Figure 2.
lncRNA KLF3-AS1 Localized in the Cytoplasm of Eca109 Cells
Original magnification, ×400. DAPI shows nuclei with a blue signal.
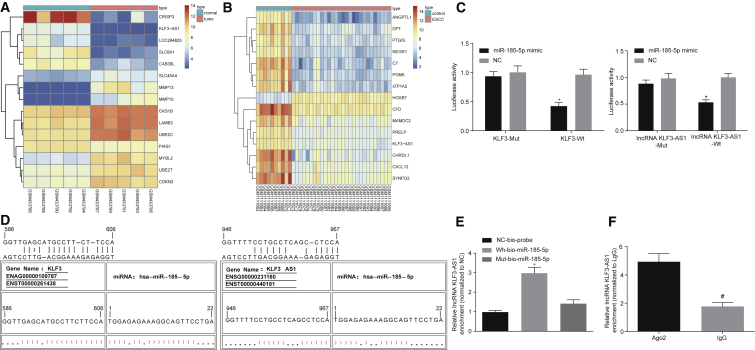
Following application of the FISH assay, a dual-luciferase reporter gene assay, RNA pull-down, and RNA immunoprecipitation (RIP) were applied to analyze the relationships between lncRNA KLF3-AS1 and KLF3, and between lncRNA KLF3-AS1 and miR-185-5p. The differential analysis of the ESCC microarray data GEO: GSE17351 and GSE45670 demonstrated that KLF3-AS1 was poorly expressed in ESCC (Figures 3A and 3B). Through analysis using the RNA22 website (https://cm.jefferson.edu/rna22/), we found that binding sites existed between miR-185-5p and KLF3 3′ UTR and between miR-185-5p and lncRNA KLF3-AS1, respectively, indicating that KLF3 and lncRNA KLF3-AS1 were the target genes of miR-185-5p. According to the dual-luciferase reporter gene assay, the luciferase activity of KLF3 wild-type (WT) 3′ UTR was restrained by miR-185-5p mimic (p < 0.05), while the luciferase activity of KLF3 mutant (MUT) 3′ UTR was unaffected (p > 0.05), indicating that miR-185-5p could specifically bind to KLF3 3′ UTR and subsequently negatively regulate the KLF3 gene on a post-transcriptional level (Figure 3C). The luciferase activity of the binding domain between lncRNA KLF3-AS1 WT and miR-185-5p was suppressed by miR-185-5p mimic, while that between lncRNA KLF3-AS1 MUT and miR-185-5p remained unchanged, signifying that miR-185-5p specifically bound to lncRNA KLF3-AS1 (Figure 3D). RNA pull-down revealed that in contrast to MUT-miR-185-5p, the binding of lncRNA KLF3-AS1 to WT-miR-185-5p was significantly enhanced (p < 0.05), suggesting that miR-185-5p could directly bind to lncRNA KLF3-AS1 (Figure 3E). A RIP assay revealed that the enrichment of lncRNA KLF3-AS1 on Ago2 was remarkably elevated when compared with immunoglobulin G (IgG) (p < 0.05), indicating that lncRNA KLF3-AS1 could bind to Ago2 protein (Figure 3F). The aforementioned findings led us to conclude that lncRNA KLF3-AS1 might participate in KLF3 regulation by competitively binding to miR-185-5p.
Figure 3.
ESCC Cells Exhibit Poor KLF3-AS1 Expression, and lncRNA KLF3-AS1 Is Likely to Mediate KLF3 by Binding to miR-185-5p
(A) Heatmap of ESCC-related expression profile GEO: GSE17351. (B) Heatmap of ESCC-related expression profile GEO: GSE45670. In (A) and (B), the abscissa represents sample number, and the ordinate represents names of the DEGs; the upper right histogram indicates color gradation, with color change from top to bottom representing the expression of microarray data in descending order; each rectangle corresponds to the expression of each sample, and each column represents all gene expression in each sample; the dendrogram on the left represents cluster analysis results of different genes in different samples; the uppermost crossband represents the sample type, while the upper right pane represents the sample color reference, with the sample in blue representing the normal control sample while the sample in red represents the tumor sample. (C) Target interaction verification between miR-185-5p and KLF3 by dual-luciferase reporter gene assay. ∗p < 0.05 versus the NC group. (D) Target interaction verification between miR-185-5p and KLF3-AS1 by dual-luciferase reporter assay. (E) RNA pull-down revealed that miR-185-5p could directly bind to lncRNA KLF3-AS1. ∗p < 0.05 versus the NC-bio-probe group. (F) RIP indicated that lncRNA KLF3-AS1 could bind to Ago2 protein. ∗p < 0.05 versus the NC group; #p < 0.05 versus the Ago2 group. Resulting values of the dual-luciferase reporter gene assay are measurement data, which are presented as the mean ± standard deviation and verified using two-factor ANOVA. Resulting values of RNA pull-down were analyzed using one-way ANOVA with Tukey’s post hoc test. The resulting values of the RIP assay were analyzed using an independent sample t test. All experiments were repeated three times.
lncRNA KLF3-AS1 Overexpression or miR-185-5p Inhibition Leads to Elevated KLF3 Expression yet Decreased Expression of SOX2 and Oct4
In order to further investigate the effects associated with lncRNA KLF3-AS1 and miR-185-5p on the expression of KLF3, as well as the ESCC stem cell markers (SOX2 and Oct4), Eca109 cell spheres were transfected with si-KLF3-AS1, miR-185-5p inhibitor, and miR-185-5p mimic alone or in combination. Quantitative reverse transcription polymerase chain reaction (qRT-PCR) (Figure 4) and western blot analyses (Figures 5A and 5B) showed that compared with the control group, the empty vector group exhibited no significant difference in expression of lncRNA KLF3-AS1, miR-185-5p, and mRNA and protein levels of KLF3, SOX2, and Oct4 (p > 0.05), while the miR-185-5p inhibitor group showed increased lncRNA KLF3-AS1 expression and mRNA and protein levels of KLF3, but decreased miR-185-5p expression and mRNA and protein levels of SOX2 and Oct4. Compared with the control group, the miR-185-5p mimic, si-KLF3-AS1, and si-KLF3-AS1 + miR-185-5p mimic groups displayed decreased lncRNA KLF3-AS1 expression and mRNA and protein levels of KLF3, but increased miR-185-5p expression and mRNA and protein levels of SOX2 and Oct4. Compared with the si-KLF3-AS1 and miR-185-5p mimic groups, the si-KLF3-AS1 + miR-185-5p mimic group demonstrated reduced lncRNA KLF3-AS1 expression and mRNA and protein levels of KLF3, but enhanced miR-185-5p expression and mRNA and protein levels of SOX2 and Oct4 (all p < 0.05). However, no significant difference concerning the aforementioned factors was found between the si-KLF3-AS1 and miR-185-5p mimic groups (p > 0.05).
Figure 4.
lncRNA KLF3-AS1 Inhibits miR-185-5p to Upregulate KLF3 Expression and to Decrease mRNA Levels of SOX2 and Oct4
Eca109 cell spheres used in this experiment were non-transfected or transfected with empty vector, si-KLF3-AS1, miR-185-5p mimic, or miR-185-5p inhibitor, or co-transfected with si-KLF3-AS1 and miR-185-5p mimic. *p < 0.05 versus the control group; #p < 0.05 versus the si-KLF3-AS1 and miR-185-5p mimic groups. The results of qRT-PCR are measurement data, which are presented as the mean ± standard deviation. Analyses include a one-way ANOVA with Tukey’s post hoc test. The experiment was repeated three times.
Figure 5.
lncRNA KLF3-AS1 Inhibits miR-185-5p to Upregulate the Protein Level of KLF3 and to Suppress the Protein Levels of SOX2 and Oct4
Eca109 cell spheres used in this experiment were non-transfected or transfected with empty vector, si-KLF3-AS1, miR-185-5p mimic, or miR-185-5p inhibitor, or co-transfected with si-KLF3-AS1 and miR-185-5p mimic. (A) Gray values of the SOX2, Oct4, and KLF3 protein bands were assessed by western blot analysis in Eca109 cells. (B) Quantitative analysis of protein levels of SOX2, Oct4, and KLF3 in Eca109 cells. *p < 0.05 versus the control group; #p < 0.05 versus the si-KLF3-AS1 and miR-185-5p mimic groups. The results of western blot analysis are measurement data, which are presented as the mean ± standard deviation. Analyses include a one-way ANOVA with Tukey’s post hoc test. The experiment was repeated three times.
Based on the above results, we concluded that both lncRNA KLF3-AS1 upregulation and miR-185-5p inhibition increased expression of KLF3 but decreased that of SOX2 and Oct4 in Eca109 cell spheres.
lncRNA KLF3-AS1 Reduces Migration and Invasion Abilities while Inducing the Apoptosis of ESCC Cell Spheres by Inhibiting miR-185-5p
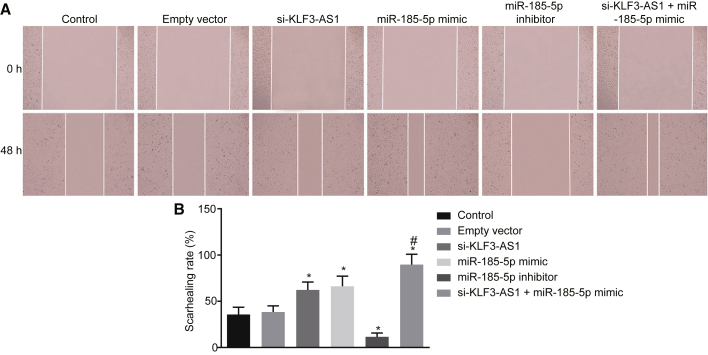
Next, a scratch test, Transwell assay, and flow cytometry were performed to detect migration (Figures 6A and 6B), invasion (Figures 7A and 7B), and apoptosis (Figures 8A and 8B) of ESCC cell spheres with stem cell-like properties, respectively. The results showed that compared with the control group, the si-KLF3-AS1, miR-185-5p mimic, and si-KLF3-AS1 + miR-185-5p mimic groups demonstrated significantly elevated cell migration and invasion abilities but a reduced cell apoptotic rate (p < 0.05), while the empty vector group displayed no significant difference in relationship to cell migration, invasion, and apoptosis (p > 0.05). In contrast, reductions in cell migration and invasion properties but increases in the cell apoptotic rate were observed in the miR-185-5p inhibitor group (p < 0.05). Compared with the si-KLF3-AS1 and miR-185-5p mimic groups, the si-KLF3-AS1 + miR-185-5p mimic group exhibited significantly enhanced cell migration and invasion properties and reduced cell apoptotic rate (p < 0.05), while no significant difference was found in those aforementioned cellular behaviors between the si-KLF3-AS1 group and the miR-185-5p mimic group (p > 0.05). The results revealed that cell migration and cell invasiveness were reduced whereas apoptosis was enhanced by lncRNA KLF3-AS1 upregulation through downregulation of miR-185-5p in ESCC stem cells.
Figure 6.
Overexpression of lncRNA KLF3-AS1 Leads to Suppression of miR-185-5p to Prominently Reduce ESCC Cell Migration
Eca109 cell spheres used in this experiment were non-transfected or transfected with empty vector, si-KLF3-AS1, miR-185-5p mimic, or miR-185-5p inhibitor, or co-transfected with si-KLF3-AS1 and miR-185-5p mimic. (A) Eca109 cell migration detected by a scratch test. (B) Quantitative analysis of the scratch healing rate of Eca109 cells. *p < 0.05 versus the control group; #p < 0.05 versus the si-KLF3-AS1 and miR-185-5p mimic groups. The results of scratch tests are measurement data, which are presented as the mean ± standard deviation. Analyses include a one-way ANOVA with Tukey’s post hoc test. The experiment was repeated three times.
Figure 7.
lncRNA KLF3-AS1 Inhibits miR-185-5p to Decrease Cell Invasion in ESCC
Eca109 cell spheres used in this experiment were non-transfected or transfected with empty vector, si-KLF3-AS1, miR-185-5p mimic, or miR-185-5p inhibitor, or co-transfected with si-KLF3-AS1 and miR-185-5p mimic. (A) Eca109 cell invasion as evaluated by a Transwell assay (original magnification, ×200). (B) Quantitative analysis of the number of Eca109 cells invaded through the Transwell chamber. *p < 0.05 versus the control group; #p < 0.05 versus the si-KLF3-AS1 and miR-185-5p mimic groups. The results of Transwell assays are measurement data, which are presented as the mean ± standard deviation. Analyses include a one-way ANOVA with Tukey’s post hoc test. The experiment was repeated three times.
Figure 8.
lncRNA KLF3-AS1 Overexpression or miR-185-5p Suppression Contributes to Enhanced Cell Apoptosis in ESCC
Eca109 cell spheres used in this experiment were non-transfected or transfected with empty vector, si-KLF3-AS1, miR-185-5p mimic, or miR-185-5p inhibitor, or co-transfected with si-KLF3-AS1 and miR-185-5p mimic. (A) Eca109 cell apoptosis detected by flow cytometry. (B) Quantitative analysis of the apoptosis rate of Eca109 cells. *p < 0.05 versus the control group; #p < 0.05 versus the si-KLF3-AS1 and miR-185-5p mimic groups. The results of flow cytometry are measurement data, which are presented as the mean ± standard deviation. Analyses include a one-way ANOVA with Tukey’s post hoc test. The experiment was repeated three times.
Upregulation of lncRNA KLF3-AS1 Reduces Tumor Growth In Vivo through Inhibition of miR-185-5p
A tumor xenograft in nude mice was conducted to assess the effect of lncRNA KLF3-AS1 on tumor progression. The results showed that tumor size was gradually increased over time. Compared with the control group, the si-KLF3-AS1, miR-185-5p mimic, and si-KLF3-AS1 + miR-185-5p mimic groups exhibited a significant increase in tumor volume (p < 0.05). Interestingly, the miR-185-5p inhibitor group displayed a marked reduction in tumor volume (p < 0.05), while the empty vector group displayed no significant difference in tumor volume (p > 0.05). Compared with the si-KLF3-AS1 and miR-185-5p mimic groups, the si-KLF3-AS1 + miR-185-5p mimic group showed significantly elevated tumor volume (p < 0.05). No significant difference was detected between the si-KLF3-AS1 group and the miR-185-5p mimic group (p > 0.05) (Figures 9A and 9B). These results suggested that tumorigenesis was restrained by upregulation of lncRNA KLF3-AS1 in ESCC, which could be achieved through downregulation of miR-185-5p.
Figure 9.
lncRNA KLF3-AS1 Inhibits miR-185-5p expression, Thereby Resulting in Decline in Tumor Volume in ESCC
The nude mice used for tumor xenograft experiment were injected with non-transfected Eca109 cell spheres or Eca109 cell spheres stably transfected with empty vector, si-KLF3-AS1, miR-185-5p mimic, or miR-185-5p inhibitor, or co-transfected with si-KLF3-AS1 and miR-185-5p mimic. (A) Representative images of tumors formed in nude mice after different treatments. (B) Tumor growth curve of nude mice after different treatments. *p < 0.05 versus the control group; #p < 0.05 versus the si-KLF3-AS1 and miR-185-5p mimic groups. The results of the tumor xenograft experiment are measurement data, which are presented as the mean ± standard deviation. Values at different points were analyzed using a repeated ANOVA with Tukey’s post hoc test (n = 5). The experiment was repeated three times.
Discussion
The frequently unsuccessful therapy and high mortality related to ESCC are thought to be mainly due to delayed diagnosis.2 With optimized medical therapies applied for treating ESCC, the 5-year survival rate of ESCC patients remains below 30% often due to metastasis, drug resistance, and tumor recurrence.1 Recently, the discovery of lncRNA has shed new light on enhanced treatment targets for ESCC, serving as a prognostic marker and therapeutic target.14 Therefore, the current study was performed in order to investigate the role of lncRNA KLF3-AS1 in the migration and invasion of ESCC stem cells, and the experimental data suggested that lncRNA KLF3-AS1 can act as ceRNA of miR-185-5p and upregulated KLF3, which ultimately leads to suppression of migration and invasion of ESCC stem cells.
Initially, our study showed that the lncRNA KLF3-AS1 was lowly expressed in ESCC. Similarly, previous results indicated that the lncRNA GAS5 level is frequently reduced in 86 paired ESCC tissues. Furthermore, lncRNA GAS5 suppresses the growth of ESCC by miR-196a involvement in the RNA-induced silencing complex.15 Furthermore, based on the information provided by the target prediction websites, dual-luciferase reporter assay, RNA pull-down, and RIP, we found that lncRNA KLF3-AS1 may serve as a ceRNA against miR-185-5p. This pathway ultimately regulates KLF3 expression, thus inhibiting cell migration and invasion in ESCC. lncRNAs possess the ability to interact with miRNAs, thus exerting their modulatory effects.16 For example, lncRNA HOTAIR participated in the regulation of cell cycle and enhancement of cell proliferation by functioning as a ceRNA to elevate the cyclin D1 level via competitively binding to miR-1 in ESCC cells, highlighting a promising new insight for the therapy and diagnosis of ESCC.17 More specifically, lncRNA KLF3-AS1 serves as a ceRNA to induce elevated GIT1 expression in osteoarthritis by competitively binding to miR-206.18 In the current study, we uncovered the regulatory network showing that lncRNA KLF3-AS1 could competitively bind to miR-185-5p to upregulate KLF3.
Another important finding of our study was that lncRNA KLF3-AS1 overexpression induced miR-185-5p inhibition, which resulted in upregulation in mRNA and protein levels of KLF3 and reduction of mRNA and protein levels of ESCC stem cell markers SOX2 and Oct. KLF3, concentrated at multiple muscle gene promoters, plays a role in synergizing the serum response factor at KLF binding sites and regulating gene expression in muscle, with its two isoforms found to increase when muscle is differentiating.19 SOX2 is a main transcription factor that contributes to the maintenance of pluripotency, self-renewal, and cell fate decision in embryonic stem cells.20 Similarly, as a member of the POU domain family, Oct4 plays a key role in embryonic development and contributes to stem cell self-renewal and differentiation as well as carcinogenesis.21 The levels of SOX2 and Oct4 have been linked to the histological grade of tongue squamous cell carcinoma, and their increased expressions are possibly responsible for a poorer overall survival rate in tongue squamous cell carcinoma patients.22
Furthermore, we identified that inhibition of miR-185-5p by lncRNA KLF3-AS1 triggered a significant reduction in colony formation ability, invasion ability, migration ability, and tumor volume while enhancing cell apoptosis in ESCC. At present, an ESCC-specific ceRNA network has been constructed on the basis of the expression profiles of lncRNA, miRNA, and mRNA, which offer novel pathways for controlling ceRNA networks for the treatment of cancers.23 A previous study demonstrated that lncRNA TP73-AS1 influenced cell apoptosis and cell proliferation in ESCC,24 and lncRNA AFAP1-AS1 also involves in cell colony-forming and proliferation ability and cell apoptosis in ESCC.25 A similar regulatory network has been proposed by Zhang et al.26 that lncRNA RNCR3 acts as a ceRNA of miR-185-5p to increase KLF16, which restrains the progression of glioblastoma.
In conclusion, this study suggests that lncRNA KLF3-AS1 can suppress cell migration and invasion via binding to miR-185-5p and impairing miR-185-5p-targeted inhibition of KLF3 in ESCC (Figure 10). These findings shed light on a novel chapter for the development of future ESCC treatments. Further studies concerning the mechanism by which lncRNA KLF3-AS1, miR-185-5p, and KLF3 influence ESCC are still required for further investigation in order to optimize this experimental approach for targeted ESCC therapy.
Figure 10.
Schematic Diagram Illustrating the Molecular Mechanism by Which the lncRNA KLF3-AS1/miR-185-5p/KLF3 Axis Regulates ESCC Progression
lncRNA KLF3-AS1 acted as ceRNA of miR-185-5p to elevate KLF3, and consequently reduced the expression of SOX2 and Oct4, ultimately inhibiting the migration and invasion of ESCC cells.
Materials and Methods
Ethics Statement
The protocols of this study were approved by the Animal Research Ethics Committee of The First Affiliated Hospital of Zhengzhou University (201806002). All animal experiments were performed using the Guide for the Care and Use of Laboratory Animals as approved by International Committees.
Bioinformatics Screening and Prediction
ESCC-related microarray data GEO: GSE17351 and GSE45670 and gene probe annotation files were initially downloaded from GEO (https://www.ncbi.nlm.nih.gov/geo) in order to systematically analyze the differentially expressed genes (DEGs) in ESCC samples, with the normal samples as control. The limma package of R language was used to conduct differential analysis between the ESCC samples and control samples. |Log fold change (FC)| >2 and p value <0.05 were set as thresholds to screen out the DEGs in ESCC. The pheatmap package of R language was applied to draw a heatmap of DEGs.
ESCC Cell Preparation
The ESCC cell line Eca109 (iCell-h056) was purchased from iCell Bioscience (Shanghai, China). The purchased cells were cultured with Dulbecco’s modified Eagle’s medium (DMEM) solution containing 10% fetal bovine serum (FBS) at 37°C with 5% CO2. Following cell adherence, the cells were subcultured and detached with 0.25% trypsin. Cells at the logarithmic growth phase were collected and diluted into a 1 × 108 cells/L single-cell suspension with serum-free DMEM/F-12 (Gibco, Grand Island, NY, USA) medium containing B27 (Invitrogen, Carlsbad, CA, USA). Next, 10 μL of suspension was transferred into a culture bottle coated with poly(2-hydroxyethyl methacrylate) (Poly-HEMA; ZSGB-Bio, Beijing, China) and incubated at 37°C with 5% CO2. The process of the Eca109 cells growing into spheres in the serum-free culture medium was monitored and recorded every day. The newly formed, tightly interconnected, and suspended Eca109 cell spheres with larger volume were collected and subcultured for subsequent experiments.27,28
Clonogenic Assay
The prepared Eca109 cell spheres and cells were separated by mechanical triturating and then counted. Subsequently, the spheres and cells were separately resuspended in complete medium and then seeded into two six-well plates at a density of 500 cells/well for 7–14 days of culture. After the cell colony was visible to the naked eye, the culture medium was removed gently and the spheres and cells were washed twice with phosphate-buffered saline (PBS) solution and fixed with methanol at room temperature for 30 min. The spheres and cells were stained with 0.1% crystal violet for 30 min and finally washed again with PBS. After staining, the formed colonies in spheres and cells were counted and compared.29
RNA FISH Assay
A FISH kit (C10910, Guangzhou RiboBio, Guangzhou, Guangdong, China) was used to perform an in situ test on the expression of lncRNA KLF3-AS1 in the Eca109 cells. The Eca109 cells at the logarithmic growth phase were transferred onto the cell slide (about 6 × 104 cells/well), which was placed at the bottom of a 24-well plate. When cell confluence reached 60%–70%, the cells were washed with 1× PBS for 5 min, fixed with 4% paraformaldehyde at room temperature for 10 min, and then washed three times with 1× PBS (5 min each). Cells in each well were added with 1 mL of pre-cooled permeabilization solution (0.1% Triton X-100) and allowed to stand at 4°C for 5 min. After discarding the transparent solution, the cells were washed three times with 1× PBS (5 min each). The cells in each well were subsequently blocked with 200 μL of pre-hybridization solution at 37°C for 30 min. Meanwhile, the hybridization solution was preheated at 37°C followed by the addition of 2.5 μL of FISH probe mix (20 μM) stock solution under conditions devoid of light. After the pre-hybridization solution in each well was discarded, an appropriate amount of probe hybridization solution containing probe was added into each well for reaction overnight at 37°C under conditions devoid of light. After hybridization, the cells were rinsed at 42°C in dark conditions: the procedure consisted of three initial washes in lotion 1 (5 min each) to lower the background signal, followed by one wash in lotion 2, and a wash in lotion 3. Subsequently, the cells were then stained by the addition of DAPI solution for 10 min. Under conditions devoid of light the cell slide was cautiously taken out from each well and fixed on a glass slide with mounting medium for fluorescence determination. The specific probe of lncRNA KLF3-AS1 was synthesized by RiboBio (Guangzhou, Guangdong, China).
Dual-Luciferase Reporter Gene Assay
The biological prediction website RNA22 (https://cm.jefferson.edu/rna22/) was explored to analyze and obtain the sequence of the binding site of miR-185-5p, lncRNA KLF3-AS1, and KLF3. The full lengths of KLF3-AS1 and the 3′ UTR of KLF3 were amplified by cloning to the luciferase carrier pmirGLO (E1330, Promega, Madison, WI, USA), which were then respectively designated as pKLF3-AS1-WT and pKLF3-WT. The bioinformatics software was utilized to predict the binding site and site-specific mutagenesis between miR-185-5p and lncRNA KLF3-AS1, and between miR-185-5p and KLF3. Next, pKLF3-AS1-MUT and pKLF3-MUT vectors were constructed. The PRL-TK vector (E2241, Promega, Madison, WI, USA) with Renilla luciferase was employed as an internal reference, while miR-185-5p mimic and miR-185-5p empty vector were co-transfected with the luciferase reporter vectors into the Eca109 cells (CRL-1415, ATCC, Rockville, MD, USA). A dual-luciferase reporter gene assay kit (GM-040502A, Qcbio Science and Technologies, Shanghai, China) was employed to determine the fluorescence signal at a wavelength of 560 nm (firefly relative light unit [RLU]) and 465 nm (Renilla RLU). The ratio of firefly RLU to Renilla RLU was used to determine the binding intensity.
RNA Pull-Down
Eca109 cells were transfected with WT-bio-miR-185-5p or MUT-bio-miR-185-5p labeled with 50 nM biotin. After 48 h of transfection, the cells were collected, washed with PBS, and incubated in specific lysis buffer (Ambion, Austin, TX, USA) for 10 min. The lysate was centrifuged at 14,000 × g and the supernatant was collected, which was subsequently incubated with a M-280 Streptavidin MagneSphere (S3762, Sigma-Aldrich, St. Louis, MO, USA) pre-coated with RNase-free bovine serum albumin (BSA) and yeast tRNA (TRNABAK-RO, Sigma-Aldrich, St. Louis, MO, USA). The magnetic beads were incubated at 4°C for 3 h and washed twice with pre-cooled lysis buffer, twice with low-salt buffer, and once with high-salt buffer. The combined RNA in the complex was purified by TRIzol and the expression of lncRNA KLF3-AS1 was assessed by qRT-PCR.
RIP
The Eca109 cells were lysed by the lysis buffer (25 mM Tris-HCl [pH 7.4], 150 mM NaCl, 0.5% nonylphenol [NP]-40, 2 mM ethylenediaminetetraacetic acid [EDTA], 1 mM NaF, and 0.5 mM dithiothreitol) containing RNasin (Takara, Kyoto, Japan) and protease inhibitor (B14001a, Roche, USA). The lysate was centrifuged at 12,000 × g for 30 min followed by collection of the supernatant, with magnetic beads coated with anti-human Ago-2 (BMFA-1, Biomarker Technologies, Beijing, China) or anti-IgG added. The lysate supernatant was incubated with beads at 4°C for 4 h and washed three times with washing buffer containing 50 mM Tris-HCl, 300 mM NaCl (pH 7.4), 1 mM MgCl2, and 0.1% NP-40. RNA was extracted from the magnetic beads using the TRIzol method, and the expression of lncRNA KLF3-AS1 was subsequently determined by qRT-PCR.
Cell Grouping and Transfection
The prepared ESCC stem cells were assigned into six groups: (1) control group (cells without any treatment), (2) empty vector group (cells transfected with empty vector), (3) si-KLF3-AS1 group (cells transfected with si-lncRNA KLF3-AS1), (4) miR-185-5p mimic group (cells transfected with miR-185-5p mimic), (5) miR-185-5p inhibitor group (cells transfected with miR-185-5p inhibitor), and (6) si-KLF3-AS1 + miR-185-5p mimic group (cells co-transfected with si-lncRNA KLF3-AS1 and miR-185-5p mimic). The si-lncRNA KLF3-AS1, miR-185-5p mimic, and miR-185-5p inhibitor were all purchased from RiboBio (Guangzhou, Guangdong, China). For the cell preparation, the cells were seeded into a 24-well plate and cultured until cell density reached 50%–60% confluence. Next, the Eca109 cells were transfected according to the instructions for Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). Lipofectamine 2000 and RNA ready for transfection were prepared independently in two sterile Eppendorf (EP) tubes. Next, 1 μL of Lipofectamine 2000 was added with 50 μL of serum-free medium and allowed to stand at room temperature for 5 min. RNA ready for transfection (20 pmol) was added with 50 μL of serum-free medium (the final concentration was 40 μM after transfection). Solutions in these two tubes were mixed and allowed to stand at room temperature for 20 min, permitting the RNA and liposome to form into complexes. The final mixture was added to the cells ready for transfection in culture dish and then further cultured at 37°C with 5% CO2. After a 6- to 8-h period of incubation, the culture medium was replaced with complete medium.
qRT-PCR
The Eca109 stem cells at the logarithmic growth phase were transfected for 48 h, after which the cells were collected to extract total RNA by using the miRNeasy mini kit (QIAGEN, Hilden, Germany). Next, RNA samples were subjected to cDNA synthesis through a 20-μL reverse transcription system on a PCR Mastercycler according to the instructions of the reverse transcription kit (1.7-2.1, Beijing TransGen Biotech, Beijing, China). The primers employed for cDNA synthesis (primers of lncRNA KLF3-AS1, miR-185-5p, KLF3, SOX2, and Oct4) were designed and synthesized by Shanghai Sangon Biotechnology (Shanghai, China) (Table 1). The cDNA synthesis was performed based on the instructions for the EasyScript First-Strand cDNA Synthesis SuperMix (AE301-02, Beijing TransGen Biotech, Beijing, China), with a PCR instrument (9700, Beijing Dingguo Changsheng Biotechnology, Beijing, China) used to conduct reverse transcription. The obtained cDNA was kept at 20°C followed by quantitative real-time PCR based on the instructions of the SYBR Premix Ex Taq II kit (Takara Biotechnology, Dalian, Liaoning, China). Real-time qRT-PCR was performed using fluorescent quantitative PCR 7500 (ABI, Oyster Bay, NY, USA), with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) regarded as the internal reference. The expression of lncRNA KLF3-AS1, miR-185-5p, KLF3, SOX2, and Oct4 was determined, and 2−ΔΔCt represented the fold changes of target genes between the experimental group and the control group. The threshold cycle was defined as the cycle at which the fluorescence intensity crossed over a level where the amplification entered a logarithmic growth phase. The experiment was conducted three times.
Table 1.
qRT-PCR Primer Sequences
| Items | Sequence |
|---|---|
| U6 | F: 5′-AAAGCAAATCATCGGACGACC-3′ |
| R: 5′- GTACAACACATTGTTTCCTCGGA-3′ | |
| GAPDH | F: 5′-TGTGGGCATCAATGGATTTGG-3′ |
| R: 5′-ACACCATGTATTCCGGGTCAAT-3′ | |
| KLF3 | F: 5′-TGTCTCAGTGTCATACCCATCT-3′ |
| R: 5′-CCTTCTGGGGTCTGAAAGAACTT-3′ | |
| SOX2 | F: 5′-CTCGTGCAGTTCTACTCGTCG-3′ |
| R: 5′-AGCTCTCGGTCAGGTCCTTT-3′ | |
| Oct4 | F: 5′-GTGGAGAGCAACTCCGATG-3′ |
| R: 5′-TGCTCCAGCTTCTCCTTCTC-3′ | |
| miR-185-5p | F: 5′-UGGAGAGAAAGGCAGUUCCUGA-3′ |
| R: 5′-AGGAACTGCCTTTCTCTCCATT-3′ | |
| lncRNA KLF3-AS1 | F: 5′-GGCTGGCGGGAGAATAACT-3′ |
| R: 5′-CGTACGTGTGTGTGCTCATC-3′ |
F, forward; R, reverse; qRT-PCR, reverse transcription quantitative polymerase chain reaction; U6, small nuclear ribonucleic acid 6; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; KLF3, Krüppel-like factor 3; SOX2, sex determining region Y (SRY)-box 2; Oct4, octamer-binding transcription factor 4; miR, microRNA; lncRNA, long non-coding RNA.
Western Blot Analysis
The transfected Eca109 stem cells at exponential phase were transfected. The cells were then lysed by the addition of radioimmunoprecipitation assay (RIPA) lysis buffer on ice for 30 min and centrifuged in order to harvest the supernatant. Next, the protein was extracted with the protein concentration and then assessed by the bicinchoninic acid (BCA) method. After separation by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), the protein was wet-transferred onto the nitrocellulose (NC) membrane and sealed with 5% BSA at room temperature for 1 h. Subsequently, the membranes were incubated with diluted primary antibodies, including rabbit polyclonal antibodies to KLF3 (ab154531, 1:1,000), sex determining region Y (SRY)-box 2 (SOX2, ab97959, 1 μg/mL), Oct4 (ab18976, 1 μg/mL), and GAPDH (ab37168, 1 μg/mL), which were added into the membrane for incubation overnight at 4°C. The next day, the membrane was washed three times with PBS with Tween 20 (PBST) (10 min each). After washing, the membranes were incubated with secondary antibody, that is, goat anti-rabbit polyclone antibody (ab205718, 1:5,000) diluted in 5% skimmed milk. The aforementioned antibodies were all purchased from Abcam (Cambridge, UK). Later, the membrane was visualized using a Bio-Rad gel imaging system (MG8600, Beijing Thmorgan Biotechnology, Beijing, China) and quantitatively analyzed with IPP7.0 software (Media Cybernetics, Bethesda, MD, USA). The gray value ratio of KLF3, SOX2, and Oct4 bands to that of the internal reference GAPDH represented their respective concentration levels.
Scratch Test
Eca109 stem cells were seeded in a six-well plate at a density of 5 × 105 cells/well. After adherence, the cells were subjected to transfection. After 24 h of transfection, a 200-μL sterile spearhead was used to scratch a straight line on the cell surface with the help of a straightedge. The plate was washed three times with PBS and imaged under an inverted microscope (CX23, Olympus, Tokyo, Japan; original magnification, ×100). After 48 h of incubation, the plate was removed from the incubator, and additional images were taken in order to determine the healing rate of cells after being scratched.
Transwell Assay
Matrigel (Becton Dickinson, Franklin Lakes, NJ, USA) was diluted at 1:10 with pre-cooled serum-free DMEM and uniformly mixed before use. Each apical chamber was added with 100 μL of diluted Matrigel, which was allowed to stand at room temperature for 2 h and later washed with 200 μL of serum-free RPMI 1640 medium before use. After 24 h of transfection, the cells were detached, resuspended with serum-free DMEM, counted, and diluted into a 3 × 105 cells/mL cell suspension. Next, 100 μL of cell suspension was added into the Transwell apical chamber (Corning Life Sciences, Corning, NY, USA), and 600 μL of DMEM containing 10% serum (serum served as a chemotactic factor) was added into the basolateral chamber. The detection of cell invasion was carried out based on the instructions of the Transwell chamber, with the cells stained with crystal violet. Three visual fields were randomly selected for counting the cells that invaded across the membrane.
Flow Cytometry
An annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) reagent kit (556547, Shanghai SOLJIA Biotechnology, Shanghai, China) was utilized to detect cell apoptosis of the Eca109 stem cells after 48 h of transfection. The binding buffer (10×) was diluted into 1× binding buffer with deionized water. The Eca109 cells in each group were centrifuged at 2,000 rpm at room temperature for 5 min and resuspended with pre-cooled 1× PBS. After that, the cells were centrifuged at 200 rpm for 5–10 min, washed, and suspended with 300 μL of 1× binding buffer. Then, the cells were uniformly mixed with 5 μL of annexin V-FITC and incubated at room temperature in darkness for 15 min. At the end, the cells were treated with 5 μL of PI in an ice bath for 5 min under conditions devoid of light. FITC was determined using a flow cytometer (Cube 6, Partec, Germany) at an excitation wavelength of 480 and 530 nm, with PI determined at an excitation wavelength of 575 nm.
Tumor Xenograft in Nude Mice
Thirty BALB/c nude mice (sex, random; age, 4 weeks; weight, 18–25 g) were purchased from Hunan Slack Jingda Animal Experimental Company (Changsha, Hunan, China) and raised in a specific pathogen-free (SPF) environment. The nude mice were randomly grouped into six groups (five mice per group), after which 1 × 106 Eca109 stem cells stably transfected with different plasmids were subcutaneously inoculated into the nude mice. The nude mice were assigned into the following groups: (1) control group (nude mice injected with untransfected Eca109 stem cells), (2) empty vector group (nude mice injected with empty vector-transfected Eca109 stem cells), (3) si-KLF3-AS1 group (nude mice injected with si-lncRNA KLF3-AS1-transfected Eca109 stem cells), (4) miR-185-5p mimic group (nude mice injected with miR-185-5p mimic-transfected Eca109 stem cells), (5) miR-185-5p inhibitor group (nude mice injected with miR-185-5p inhibitor-transfected Eca109 stem cells), and (6) si-KLF3-AS1 + miR-185-5p mimic group (nude mice injected with the Eca109 stem cells co-transfected with si-lncRNA KLF3-AS1 and miR-185-5p mimic). Tumor volume (V) was monitored and recorded once a week and calculated using V = π/6(height × length × width). The nude mice were euthanized at the end of the third week with their respective tumors were collected and analyzed.
Statistical Analysis
All data analysis was conducted using SPSS 21.0 software (IBM, Armonk, NY, USA). Measurement data were expressed as the mean ± standard deviation. Comparisons between two groups were analyzed by a t test, with correction by Welch’s method. After analyzing data normality by the Kolmogorov-Smirnov method, the skewed data were compared using the non-parametric Mann-Whitney test. When the data conformed to normal distribution, comparisons among multiple groups were conducted using one-way analysis of variance (ANOVA), followed by Tukey’s post hoc test. In the event that the data failed to conform to normal distribution, analysis was conducted using the non-parametric Kruskal-Wallis H test. Data at different time points were determined using repeated-measures ANOVA. p < 0.05 was indicative of statistical significance.
Author Contributions
R.T.F. and Y.-X.G. designed the study. L.G., D.Z., and J.Q.L. collated the data, carried out data analyses, and drafted the manuscript. J.-Q.L. and Y.-X.G. edited the manuscript. M.D., N.-N.X., and T.-X.L. prepared figures and revised the manuscript. All authors have read and approved the final submitted manuscript.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
We would like to thank all participants enrolled in the present study.
Contributor Information
Di Zhao, Email: zhaodi0110@126.com.
Rui-Tai Fan, Email: fanruitai@126.com.
References
- 1.Cui Y., Wu W., Lv P., Zhang J., Bai B., Cao W. Down-regulation of long non-coding RNA ESCCAL_1 inhibits tumor growth of esophageal squamous cell carcinoma in a xenograft mouse model. Oncotarget. 2017;9:783–790. doi: 10.18632/oncotarget.23153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palumbo A., Jr., Da Costa N.M., Esposito F., De Martino M., D’Angelo D., de Sousa V.P., Martins I., Nasciutti L.E., Fusco A., Ribeiro Pinto L.F. HMGA2 overexpression plays a critical role in the progression of esophageal squamous carcinoma. Oncotarget. 2016;7:25872–25884. doi: 10.18632/oncotarget.8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang W.L., Chang W.L., Yang H.B., Wang Y.C., Chang I.W., Lee C.T., Chang C.Y., Lin J.T., Sheu B.S. Low disabled-2 expression promotes tumor progression and determines poor survival and high recurrence of esophageal squamous cell carcinoma. Oncotarget. 2016;7:71169–71181. doi: 10.18632/oncotarget.8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zang C., Liu X., Li B., He Y., Jing S., He Y., Wu W., Zhang B., Ma S., Dai W. IL-6/STAT3/TWIST inhibition reverses ionizing radiation-induced EMT and radioresistance in esophageal squamous carcinoma. Oncotarget. 2017;8:11228–11238. doi: 10.18632/oncotarget.14495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y., Zhou J., Xu Y.J., Hu H.B. Long non-coding RNA LINC00968 acts as oncogene in NSCLC by activating the Wnt signaling pathway. J. Cell. Physiol. 2018;233:3397–3406. doi: 10.1002/jcp.26186. [DOI] [PubMed] [Google Scholar]
- 6.Spizzo R., Almeida M.I., Colombatti A., Calin G.A. Long non-coding RNAs and cancer: a new frontier of translational research? Oncogene. 2012;31:4577–4587. doi: 10.1038/onc.2011.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y., Shi X., Yang W., Lu Z., Wang P., Chen Z., He J. Transcriptome profiling of lncRNA and co-expression networks in esophageal squamous cell carcinoma by RNA sequencing. Tumour Biol. 2016;37:13091–13100. doi: 10.1007/s13277-016-5227-3. [DOI] [PubMed] [Google Scholar]
- 8.Sun C.C., Zhang L., Li G., Li S.J., Chen Z.L., Fu Y.F., Gong F.Y., Bai T., Zhang D.Y., Wu Q.M., Li D.J. The lncRNA PDIA3P interacts with miR-185-5p to modulate oral squamous cell carcinoma progression by targeting cyclin D2. Mol. Ther. Nucleic Acids. 2017;9:100–110. doi: 10.1016/j.omtn.2017.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kartha R.V., Subramanian S. Competing endogenous RNAs (ceRNAs): new entrants to the intricacies of gene regulation. Front. Genet. 2014;5:8. doi: 10.3389/fgene.2014.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harada K., Baba Y., Ishimoto T., Shigaki H., Kosumi K., Yoshida N., Watanabe M., Baba H. The role of microRNA in esophageal squamous cell carcinoma. J. Gastroenterol. 2016;51:520–530. doi: 10.1007/s00535-016-1161-9. [DOI] [PubMed] [Google Scholar]
- 11.Pei K., Zhu J.J., Wang C.E., Xie Q.L., Guo J.Y. MicroRNA-185-5p modulates chemosensitivity of human non-small cell lung cancer to cisplatin via targeting ABCC1. Eur. Rev. Med. Pharmacol. Sci. 2016;20:4697–4704. [PubMed] [Google Scholar]
- 12.Pearson R.C., Funnell A.P., Crossley M. The mammalian zinc finger transcription factor Krüppel-like factor 3 (KLF3/BKLF) IUBMB Life. 2011;63:86–93. doi: 10.1002/iub.422. [DOI] [PubMed] [Google Scholar]
- 13.Wang X., Jiang Z., Zhang Y., Wang X., Liu L., Fan Z. RNA sequencing analysis reveals protective role of kruppel-like factor 3 in colorectal cancer. Oncotarget. 2017;8:21984–21993. doi: 10.18632/oncotarget.15766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lv X.B., Lian G.Y., Wang H.R., Song E., Yao H., Wang M.H. Long noncoding RNA HOTAIR is a prognostic marker for esophageal squamous cell carcinoma progression and survival. PLoS ONE. 2013;8:e63516. doi: 10.1371/journal.pone.0063516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang K., Li J., Xiong G., He G., Guan X., Yang K., Bai Y. Negative regulation of lncRNA GAS5 by miR-196a inhibits esophageal squamous cell carcinoma growth. Biochem. Biophys. Res. Commun. 2018;495:1151–1157. doi: 10.1016/j.bbrc.2017.11.119. [DOI] [PubMed] [Google Scholar]
- 16.Jalali S., Bhartiya D., Lalwani M.K., Sivasubbu S., Scaria V. Systematic transcriptome wide analysis of lncRNA-miRNA interactions. PLoS ONE. 2013;8:e53823. doi: 10.1371/journal.pone.0053823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ren K., Li Y., Lu H., Li Z., Li Z., Wu K., Li Z., Han X. Long noncoding RNA HOTAIR controls cell cycle by functioning as a competing endogenous RNA in esophageal squamous cell carcinoma. Transl. Oncol. 2016;9:489–497. doi: 10.1016/j.tranon.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y., Lin L., Zou R., Wen C., Wang Z., Lin F. MSC-derived exosomes promote proliferation and inhibit apoptosis of chondrocytes via lncRNA-KLF3-AS1/miR-206/GIT1 axis in osteoarthritis. Cell Cycle. 2018;17:2411–2422. doi: 10.1080/15384101.2018.1526603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Himeda C.L., Ranish J.A., Pearson R.C., Crossley M., Hauschka S.D. KLF3 regulates muscle-specific gene expression and synergizes with serum response factor on KLF binding sites. Mol. Cell. Biol. 2010;30:3430–3443. doi: 10.1128/MCB.00302-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forghanifard M.M., Ardalan Khales S., Javdani-Mallak A., Rad A., Farshchian M., Abbaszadegan M.R. Stemness state regulators SALL4 and SOX2 are involved in progression and invasiveness of esophageal squamous cell carcinoma. Med. Oncol. 2014;31:922. doi: 10.1007/s12032-014-0922-7. [DOI] [PubMed] [Google Scholar]
- 21.Izadpanah M.H., Abbaszadegan M.R., Fahim Y., Forghanifard M.M. Ectopic expression of TWIST1 upregulates the stemness marker OCT4 in the esophageal squamous cell carcinoma cell line KYSE30. Cell. Mol. Biol. Lett. 2017;22:33. doi: 10.1186/s11658-017-0065-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang X.D., Luo G., Wang X.H., Chen L.L., Ke X., Li Y. [Expression of Oct4 and Sox2 and their clinical significance in tongue squamous cell carcinoma] Zhonghua Kou Qiang Yi Xue Za Zhi. 2017;52:27–33. doi: 10.3760/cma.j.issn.1002-0098.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Xue W.H., Fan Z.R., Li L.F., Lu J.L., Ma B.J., Kan Q.C., Zhao J. Construction of an oesophageal cancer-specific ceRNA network based on miRNA, lncRNA, and mRNA expression data. World J. Gastroenterol. 2018;24:23–34. doi: 10.3748/wjg.v24.i1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zang W., Wang T., Wang Y., Chen X., Du Y., Sun Q., Li M., Dong Z., Zhao G. Knockdown of long non-coding RNA TP73-AS1 inhibits cell proliferation and induces apoptosis in esophageal squamous cell carcinoma. Oncotarget. 2016;7:19960–19974. doi: 10.18632/oncotarget.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo H.L., Huang M.D., Guo J.N., Fan R.H., Xia X.T., He J.D., Chen X.F. AFAP1-AS1 is upregulated and promotes esophageal squamous cell carcinoma cell proliferation and inhibits cell apoptosis. Cancer Med. 2016;5:2879–2885. doi: 10.1002/cam4.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang L., Cao Y., Wei M., Jiang X., Jia D. Long noncoding RNA-RNCR3 overexpression deleteriously affects the growth of glioblastoma cells through miR-185-5p/Krüppel-like factor 16 axis. J. Cell. Biochem. 2018;119:9081–9089. doi: 10.1002/jcb.27167. [DOI] [PubMed] [Google Scholar]
- 27.Ponti D., Costa A., Zaffaroni N., Pratesi G., Petrangolini G., Coradini D., Pilotti S., Pierotti M.A., Daidone M.G. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65:5506–5511. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- 28.Singh S.K., Clarke I.D., Terasaki M., Bonn V.E., Hawkins C., Squire J., Dirks P.B. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 29.Zhang D., Xiang J., Gu Y., Xu W., Xu H., Zu M., Pei D., Zheng J. Inhibition of mammalian target of rapamycin by rapamycin increases the radiosensitivity of esophageal carcinoma Eca109 cells. Oncol. Lett. 2014;8:575–581. doi: 10.3892/ol.2014.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]