Abstract
Background
Delivering Stereotactic Body Radiotherapy (SBRT) for Hepatocellular Carcinoma (HCC) is challenging mainly for two reasons: first, motion of the liver occurs in six degrees of freedom and, second, delineation of the tumor is difficult owing to a similar density of HCC to that of the adjoining healthy liver tissue in a non-contrast CT scan. To overcome both these challenges simultaneously, we performed a feasibility study to synchronize intravenous contrast to obtain an arterial and a delayed phase 4D CT.
Materials and Methods
We included seven HCC patients of planned for SBRT. 4D CT simulation was performed with synchronized intravenous contrast based on the formula TSCAN DELAY = Tpeak – (L0/Detector Coverage × Cine Duration in Seconds). This was followed by a delayed 4D CT scan.
Results
We found that, with our protocol, it is feasible to obtain a 4DCT with an arterial and a delayed phase making it comparable to a diagnostic multi-phase CT. The peak HU of the 4D scan and diagnostic CT were similar (mean peak HU 134.2 vs 143.1, p value = 0.58 N.S). Whereas in comparison with a non-contrast CT a significant rise in the peak HU was seen (mean peak 134.2 vs 61.4 p value = .00003).
Conclusion
A synchronized contrast 4D CT simulation for HCC is safe and feasible. It results in good contrast enhancement comparable to a diagnostic 3D contrast CT scan.
Keywords: Index terms – hepatocellular carcinoma, 4DCT simulation, Synchronized intravenous contrast scans, Target delineation
1. Introduction
Hepatocellular carcinoma (HCC) is the sixth most commonly occurring cancer, and the fourth most common cause of death worldwide.1 At present, liver transplant and resection are the only curative options available.2 Unfortunately, only 10–20 % of the patients are surgical candidates, as the majority have impaired liver function, portal hypertension, or advanced tumor stage.3,4 Stereotactic Body Radiotherapy (SBRT) has demonstrated high rates of local control, (defined as no progression of the disease as per RECIST criteria) ranging from 87% to 100% at 1–3 years in unresectable and non-surgical HCC patients.5, 6, 7 Delivering SBRT for HCC is challenging, mainly for two reasons. First, due to the motion of the liver in six degrees of freedom.8 This has been resolved by taking 4D CT scans, which capture the position of the liver in different phases of respiration, or by taking breath-hold scans. Second, due to HCC’s CT density, which is similar to the surrounding normal liver tissue in non-contrast CT scans, making delineation of the tumor difficult. HCC has a characteristic appearance on contrast imaging with arterial phase enhancement and washout in Porto-venous and delayed phases which results in a positive predictive value of nearly 100%.9, 10, 11 To take advantage of this characteristic appearance, oncologists have used breath-hold 3D scans with contrast,12 but the breath-hold 3D scan is not feasible in all HCC patients.13 Some have also tried to fuse diagnostic scans with non-contrast 4D scans, but there is an inherent fusion error.14 Oncologists popularly take a triphasic planning 3DCT and use the 4DCT only to gauge the motion, but a breathing fast spiral CT may not accurately represent the mean target position since each slice localizes the target positions at a different respiratory phase away from the actual mean position.15 Multislice scanners could take a snapshot of the entire tumor at a position that may not represent the mean and, in fact, could be at an extreme position away from the mean. To tackle all the above mentioned challenges simultaneously; we need a synchronized contrast-enhancing 4D CT. The time of acquisition of a 4D CT is 1−4 min (depending on the type of scanner) which results in a mismatch of contrast timing if we follow 3D CT protocols. Very few studies have been published to address these issues. In one study, no HCC patients were taken,16 and in the other, only a delayed phase was taken.17 We aim to solve these issues by conducting a feasibility study in HCC patients where we would acquire a contrast-enhanced and a delayed phase 4D CT for SBRT planning and compare its quality with the diagnostic counterparts.
2. Material and methods
Our department review board cleared the study, and we took informed consent from all the patients. We performed a 4DCT simulation for seven patients who were not suitable for breath hold SBRT using our protocol. After ensuring normal renal function, an 18 G three-way IV cannula was inserted in the antecubital vein to ensure an adequate flow of contrast. All patients underwent respiratory coaching to ensure good quality 4DCT. We used the Anzai respiratory gating system AZ-733 V (Anzai Medical Co. Ltd., Tokyo, Japan), composed of a respiratory sensor, sensor port, wave deck, and a laptop for obtaining 4DCT scans. The patients were made to lie in a supine position and positioned with BlueBAG™ on our GE Discovery PET/CT 710 with time-of-flight (TOF PET-CT) technology having 128 slices GE optima CT equipment (GE Medical Systems, USA) and we took a 4D CT from carina to 6 cm below the inferior most point of the liver. An automatic contrast injector Ulrich CT motion contrast injector (Ulrich medical® interface, Ulrich GmbH & Co. KG, Germany) was connected to the 18 or 20 G IV line, and 125 ml of VisipaqueTM (iodixanol) containing 270 mg of iodine per ml was injected at a rate of 3 ml/s. The CT scan parameters used for the synchronized intravenous contrast 4D CT scan were: 140 kVp, 140–160 mA depending on patient body weight, the rotation speed varied from 0.5 to 1 s/rotation with a constant detector coverage of 2 cm (detector coverage is the length scanned by the CT scanner for a duration equal to the patients’ cine time). The CT scans were acquired with 2.5 mm slice thickness and eight images/rotation.
We calculated the scan delay for all our patients using the formula,
| TSCAN DELAY = Tpeak – (L0/Detector Coverage × Cine Duration in Seconds) |
Where,
Time of Scan Delay (TSCAN DELAY) is the time between the start of the contrast injection and the start of the CT acquisition from its initial level. With an appropriately calculated delay, the 4D CT acquisition and contrast enhancement synchronize in a way that generates a peak contrast enhancement at the level of interest (see Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5).
Fig. 6.
Patient 7 Infiltrative HCC (a) Synchronized 4D CT contrast showing no enhancement in arterial phase(hypoenhancing HCC) (b) Diagnostic 3D CT contrast of same slide showing no contrast enhancement. (c) Synchronized 4D CT delayed showing washout but n washout in the THAD component. (d) Diagnostic non contrast scan- difficult to appreciate the tumor.
Fig. 1.
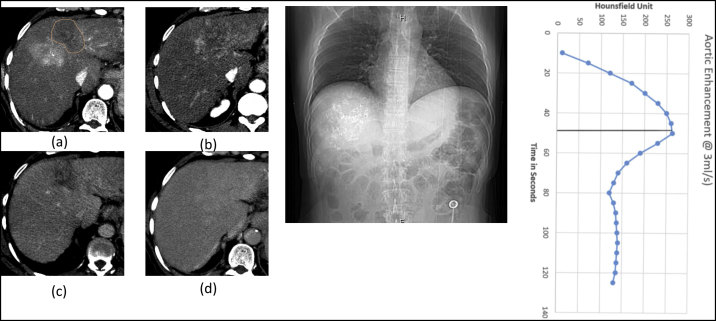
Patient 1 HCC post TACE (a) Synchronized 4D CT contrast showing peak HU of 136 in average intensity projection (AIP). (b) Diagnostic 3D CT contrast of same slice showing peak HU of 154. (c) Synchronized 4D CT delayed showing washout. (d) Diagnostic non contrast scan showing lipiodol and poor demarcation between liver parenchyma and HCC. (e) DRR showing Synchronized 4D CT scan length. L0, Ls(distance between scan start position and the start of the region of interest which is the liver in this case), TSCANDELAY and corresponding aortic enhancement at 4D CT scan length and LOI.
Fig. 2.
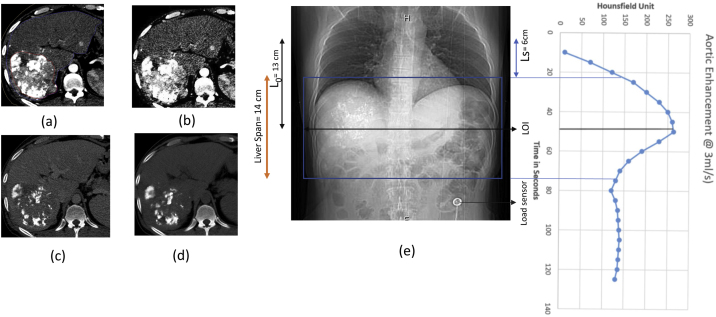
Patient 2(a) Synchronized 4D CT contrast showing peak HU of 161 in average intensity projection (AIP). (b) Diagnostic 3D CT contrast of same slide showing peak HU 0f 168. (c) Diagnostic non contrast scan.(d) DRR showing Synchronized 4D CT scan length. L0, Ls, TSCANDELAY and corresponding aortic enhancement at 4D CT scan length and LOI.
Fig. 3.
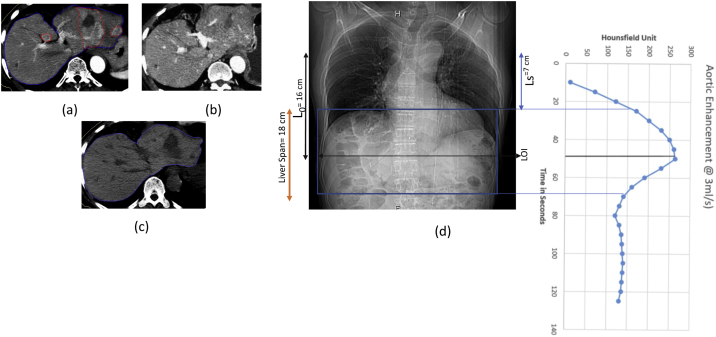
Patient 4 (a) Synchronized 4D CT contrast showing peak HU of 138 in average intensity projection (AIP). (b) Diagnostic 3D CT contrast of same slide showing peak HU 0f 141. (c) Synchronized 4D CT delayed showing washout. (d) Diagnostic non contrast scan. (e) DRR showing Synchronized 4D CT scan length. L0, Ls, TSCANDELAY and corresponding aortic enhancement at 4D CT scan length and LOI.
Fig. 4.
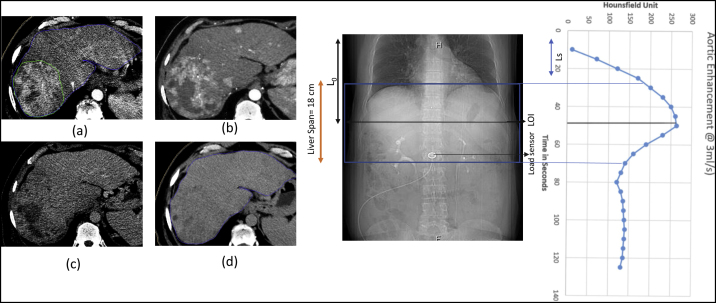
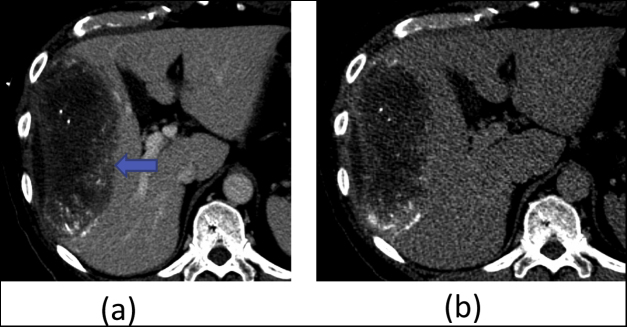
Patient 5. HCC post TACE (a) Synchronized 4D CT contrast showing peak HU of 122 in average intensity projection (AIP). (b) Non Contrast 3DCT of the same patient. Arrow points towards the peripheral contrast enhancement and a better edge contrast which helps in target delineation.
Fig. 5.
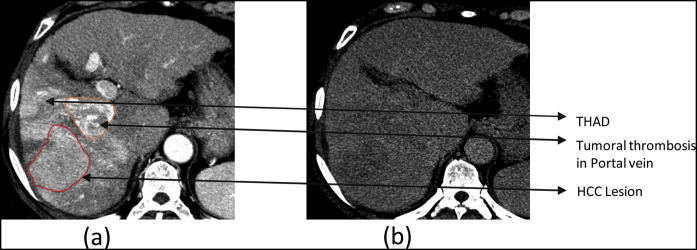
Patient 6. HCC with Transient hepatic attenuation difference (THAD) (a) Synchronized 4D CT contrast showing primary lesion with tumoral thrombosis in portal vein along with Transient hepatic attenuation difference (THAD) in average intensity projection (AIP). (b) Synchronized 4D CT delayed showing washout.
Level of Interest(LOI) is the desired level of the Tpeak. We kept it in the middle of the liver span so that we can have an enhancement of at least 150 HU for 50 s across 20 cm (assuming a max liver span of 20 cm with the contrast flow rate of 3 ml/s).18
For diagnostic triple phase, scans the scan delay to obtain a late arterial phase is around 25–30 seconds with a contrast flow rate of 4–5 ml/s.
Time to Peak (Tpeak) is the time elapsed from the start of the contrast injection to the peak enhancement of the aorta [quantified by Hounsfield Units (HU)]. TID is the injection duration and CTT is the transit time for the contrast from the antecubital vein to the aorta. In our study, we followed a fixed contrast volume method and gave 125 ml of contrast at 3 ml/sec giving us a TID of 41.6 s. We assumed a transit time of 10 s and, hence, the general Tpeak for all our cases was calculated to be 50 s. Multiple studies have also reported a similar Tpeak for aortic enhancement.18,19
| Tpeak = TID + CTT2018 |
Length above the level of interest (L0); is the length between the scan start position and the level at which we desire to have the Tpeak. i.e. the LOI.
Cine Duration is the time required to image one respiratory cycle. It was calculated based on the formula given by the vendor that provides the hardware for the 4DCT acquisition. In our case, it was 1 respiratory cycle + 1. Cine duration for our cases varied from 4 to 6 s.
2.1. 4D CT Delayed
After the completion of the arterial enhancing phase of the 4D CT, we took another 4D CT after a delay of 5 min.
3. Results
We obtained diagnostic quality contrast-enhanced 4DCTs in all patients safely, except one (patient 3). Patient three had a hypoenhancing HCC on diagnostic as well as 4DCT and was visualized well on a delayed scan. No major complications were encountered using our protocol. except that patient two experienced multiple episodes of vomiting after the scan which we managed with routine antiemetics.
The mean peak HU for diagnostic 3DCT, 4DCT, and Non-Contrast 3DCT was 143.1, 134.2 and 61.4HU, respectively. No statistically significant difference was found between the mean peak HUs of diagnostic contrast 3DCT and contrast 4DCT scans (143.1 HU vs. 134.2 HU p-value = 0.58). There was a significant statistical difference in the mean peak HU between 4DCT and non-contrast 3D CT scans (134.2 vs. 61.4 HU p-value = .0003)
We also noted the presence of Transient Hepatic Attenuation Difference (THAD) for two patients, (patient 6 and 7) which is a common occurrence in patients with portal vein thrombosis. The delayed scans enabled us to differentiate the tumor from THAD.
Following are the parameters which we obtained from the scans of the seven patients along with the calculated T scan delay (Table 1).
Table 1.
Scan Parameters and delay.
| Patient no. | Tpeak (s) | Liver Span (cm) | L0 (cm) |
Cine Duration (s) | TSCAN DELAY (s) |
Peak HU Tumor Non Contrast |
Peak HU in Tumor- Contrast (in AIP) | Peak HU in diagnostic CT |
|---|---|---|---|---|---|---|---|---|
| Patient 1 | 50 | 14 | 13 | 5 | 17.5 | 64 | 136 | 154 |
| Patient 2 | 50 | 18 | 16 | 4 | 18 | 68 | 161 | 178 |
| Patient 3 | 50 | 20 | 14 | 5 | 15 | 62 | 89 | 91 |
| Patient 4 | 50 | 18 | 13.5 | 6 | 9 | 48 | 138 | 141 |
| Patient 5 | 50 | 17 | 13.5 | 5 | 16 | 62 | 142 | 148 |
| Patient 6 | 50 | 15 | 11.5 | 5 | 21 | 54 | 169 | 172 |
| Patient 7 | 50 | 16 | 13 | 4 | 18 | 72 | 105 | 118 |
| Mean | 61.4 | 134.2 | 143.1 | |||||
| S.D | 7.5 | 26.4 | 28.1 | |||||
| pvalue unpaired t test |
61.4 vs 134.2 P = .00003 |
134.2 vs 143.1 p = 0.58 |
4. Discussion
HCC is one of the very few tumors where imaging alone is sufficient for diagnosis. This is due to its characteristic appearance on contrast imaging with arterial phase enhancement and washout in porto-venous and delayed phases resulting in a positive predictive value of 100%. Its arterial phase enhancement is due to neo-arterialization which is a feature of hepatocarcinogenesis and is complemented by the fact that it derives its blood supply from the hepatic artery. Arterial enhancement alone is not sufficient to make the diagnosis as it can also be seen in early stages of hepatic carcinogenesis (which includes dysplastic and cirrhotic nodules), as well as in other benign lesions, such as small hemangiomas, benign perfusion alterations, etc. Venous washout is defined as the temporal reduction of HCC enhancement relative to the background of liver parenchyma. The mechanism is likely to be multifactorial, including reduced intratumoral portal venous blood supply, early venous drainage, progressive enhancement of background parenchyma, and intrinsic hypoattenuation.11,21 It is imperative for a radiation oncologist to take advantage of this distinctive feature of HCC and reproduce it on a CT simulation scan so that precise target delineation is done for delivering SBRT. A good contrast-enhanced 4DCT is essential to reduce systematic errors due to erroneous target delineations for motion encompassing, gated and compression treatments. Another advantage of a diagnostic quality contrast-enhanced 4D CT scan is the ability to view any new lesions which would have developed during the time lag between the diagnostic CT and the planning CT as seen in our second patient (Fig. 2), thus, making the patient unsuitable for SBRT. Some work has been done for synchronized intravenous contrast in HCC (Beddar et al.),17 but that was done only in the delayed phase. Some researchers have taken smaller scan regions of the arterial phase, containing only the tumor region (Helou J et al.),16 but this is not ideal for treatment planning.22 To address all these problems, we propose a protocol for simultaneous synchronized intravenous contrast scans with arterial and delayed phase 4D CT. In this feasibility study, we were able to obtain 4DCT scans which were quite similar to diagnostic quality 3D scans.
We performed two scans, one for the arterial and one for the delayed phase. To successfully perform a synchronized contrast 4DCT, it is essential to understand the concept of Tpeak, contrast arrival time, contrast transit time (CTT), injection duration (TID), level of interest (LOI) and scan duration which is explained in detail by Kyongtae T Bae.18 In short, in our study, we kept the level of interest in the middle of the liver so that we achieve the Tpeak at the LOI and a good enhancement of the HCC in all the segments of the liver.23 With this approach, we obtained arterially enhancing 4D scans as seen in Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5. Even after tailoring most of the parameters to achieve a good contrast enhancement, our study has some limitations. We have assumed the same Tpeak, contrast arrival and CTT for all patients. To be scientifically precise and individualize the synchronized intravenous contrast 4D CT, patient specific timings for the above parameters need to be calculated as done by Choi et al.20 for pancreatic 4DCT scans.
Nevertheless, with our protocol we were able to achieve a significantly high peak HU when compared to the non-contrast CT scan (Mean peak 134.2 vs 61.4 p-value = .00003) and a similar peak HU when compared with a diagnostic CT scan (mean peak HU 134.2 vs 143.1, p-value N.S). With the contrast scan, we were also able to delineate the tumor thrombus accurately as seen in patient six and seven (Fig. 4, Fig. 5). Arterially enhancing contrast 4DCT scans allow us to delineate the tumor better in patients who present to us after trans arterial chemoembolization (TACE) as seen in patients one and five (Fig. 1, Fig. 3). The contrast helps us to distinguish the tumor from the necrotic component which would be difficult to do on a non-contrast or a delayed CT. The value of a delayed phase 4D CT is seen best in our second, third, sixth and seventh patients (Supplementary material, Figs. S6 and S7). Each phase of the arterial and delayed 4DCTs were fused which allowed us to delineate the tumor better and more confidently. In a couple of patients, we had a transient hepatic attenuation difference (THAD) which was seen on the 4D contrast scans, but as we had a delayed scan it enabled us to differentiate the THAD component from the adjacent tumor24 as seen in patients six and seven (Fig. 4, Fig. 5). With the delayed phase, we were also able to differentiate HCC from dysplastic nodules as seen in our second patient (Fig. 2). Mancosu et al. did similar work in pancreatic ductal adenocarcinoma and found good contrast-enhanced 4D CT images.25 A contrast 4DCT of HCC may also enable radiation oncologists to use a MIP (maximum intensity projection) image for contouring as there are challenges in using MIP, MinIP (minimum intensity projection) and AIP (average intensity projection) on a non-contrast 4D CT scan.26
With our work, we feel that we have addressed the issues raised by Beddar et al. Our mixture of clinical scenarios represents the common issues faced by the radiation oncologist in the clinic. By doing a contrast-enhanced and a delayed scan, we were able to solve most of these issues. A similar concept should be applied if a synchronized contrast 4DCT is to be performed for other sites.
5. Conclusion
A synchronized contrast 4D CT simulation for HCC is safe, feasible and results in good contrast enhancement and image quality which is quite similar to a diagnostic 3D contrast CT scan. Complementing it with a delayed phase allows a radiation oncologist to differentiate it from other lesions in the liver and also helps to identify HCC more accurately. With our protocol other sites that require a contrast 4DCT can also be targeted.
Conflict of interests
None.
Financial disclosure
No external funding source.
Acknowledgment
I would like to thank Dr Kritika Chopra for providing her valuable inputs in the editing of the article.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.rpor.2019.12.006.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Zaydfudim V.M., Vachharajani N., Klintmalm G.B. Liver resection and transplantation for patients with hepatocellular carcinoma beyond milan criteria. Ann Surg. 2016;264(4):650–658. doi: 10.1097/SLA.0000000000001866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Llovet J.M., Burroughs A., Bruix J. Hepatocellular carcinoma. Lancet. 2003;362(9399):1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 4.Liu C.-L., Fan S.-T. Nonresectional therapies for hepatocellular carcinoma. Am J Surg. 1997;173(4):358–365. doi: 10.1016/S0002-9610(96)00384-4. [DOI] [PubMed] [Google Scholar]
- 5.Bujold A., Massey C.A., Kim J.J. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol. 2013;31(13):1631–1639. doi: 10.1200/JCO.2012.44.1659. [DOI] [PubMed] [Google Scholar]
- 6.Lasley F.D., Mannina E.M., Johnson C.S. Treatment variables related to liver toxicity in patients with hepatocellular carcinoma, Child-Pugh class A and B enrolled in a phase 1-2 trial of stereotactic body radiation therapy. Pract Radiat Oncol. 2015;5(5):e443–9. doi: 10.1016/j.prro.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Cárdenes H.R., Price T.R., Perkins S.M. Phase I feasibility trial of stereotactic body radiation therapy for primary hepatocellular carcinoma. Clin Transl Oncol. 2010;12(3):218–225. doi: 10.1007/s12094-010-0492-x. [DOI] [PubMed] [Google Scholar]
- 8.Rohlfing T., Maurer C.R., O’Dell W.G., Zhong J. Modeling liver motion and deformation during the respiratory cycle using intensity-based nonrigid registration of gated MR images. Med Phys. 2004;31(3):427–432. doi: 10.1118/1.1644513. [DOI] [PubMed] [Google Scholar]
- 9.Méndez Romero A. Alternate Fractionation for Hepatic Tumors. In: Trombetta M., Pignol J.P., Montemaggi P., Brady L.W., editors. Alternate Fractionation in Radiotherapy. Medical Radiology. Springer, Cham; 2017. [Google Scholar]
- 10.Hatfield M.K., Beres R.A., Sane S.S., Zaleski G.X. Percutaneous imaging-guided solid organ core needle biopsy: coaxial versus noncoaxial method. Am J Roentgenol. 2008;190(2):413–417. doi: 10.2214/AJR.07.2676. [DOI] [PubMed] [Google Scholar]
- 11.Méndez Romero A., Brunner T.B., Kirichenko A.V. Springer; Cham: 2017. Alternate fractionation for hepatic tumors; pp. 173–201. [Google Scholar]
- 12.Hong T.S., Bosch W.R., Krishnan S. Interobserver variability in target definition for hepatocellular carcinoma with and without portal vein thrombus: radiation therapy oncology group consensus guidelines. Int J Radiat Oncol Biol Phys. 2014;89(4):804–813. doi: 10.1016/j.ijrobp.2014.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsang M.W.K. Stereotactic body radiotherapy: current strategies and future development. J Thorac Dis. 2016;8(Suppl 6):S517–27. doi: 10.21037/jtd.2016.03.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukumitsu N., Nitta K., Terunuma T. Registration error of the liver CT using deformable image registration of MIM Maestro and Velocity AI. BMC Med Imaging. 2017;17(1):30. doi: 10.1186/s12880-017-0202-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen G., Kung J., Beaudette K. Artifacts in computed tomography scanning of moving objects. Semin Radiat Oncol. 2004;14(1):19–26. doi: 10.1053/j.semradonc.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Helou J., Karotki A., Milot L., Chu W., Erler D., Chung H.T. 4DCT simulation with synchronized contrast injection in liver SBRT patients. Technol Cancer Res Treat n.d. 2016;15(1):55–59. doi: 10.1177/1533034615572341. [DOI] [PubMed] [Google Scholar]
- 17.Sam Beddar A., Briere T.M., Balter P. 4D-CT imaging with synchronized intravenous contrast injection to improve delineation of liver tumors for treatment planning. Radiother Oncol n.d. 2008;87(3):445–448. doi: 10.1016/j.radonc.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 18.Bae K.T. Intravenous contrast medium administration and scan timing at CT: considerations and approaches. Radiology. 2010;256(1):32–61. doi: 10.1148/radiol.10090908. [DOI] [PubMed] [Google Scholar]
- 19.Platt J.F., Reige K.A., Ellis J.H. Aortic enhancement during abdominal CT angiography: correlation with test injections, flow rates, and patient demographics. Am J Roentgenol. 1999;172(1):53–56. doi: 10.2214/ajr.172.1.9888738. [DOI] [PubMed] [Google Scholar]
- 20.Choi W., Xue M., Lane B.F. Individually optimized contrast-enhanced 4D-CT for radiotherapy simulation in pancreatic ductal adenocarcinoma. Med Phys. 2016;43(10):5659. doi: 10.1118/1.4963213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furlan A., Marin D., Vanzulli A. Hepatocellular carcinoma in cirrhotic patients at multidetector CT: hepatic venous phase versus delayed phase for the detection of tumour washout. Br J Radiol. 2011;84(1001):403–412. doi: 10.1259/bjr/18329080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stieber V., Meeks S., Tomé W.A. Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med Phys. 2010;37(8):4078–4101. doi: 10.1118/1.3438081. [DOI] [PubMed] [Google Scholar]
- 23.Kyongtae Bae T. Intravenous contrast medium administration and scan timing at CT: considerations and approaches. Radiology. 2010;256(1) doi: 10.1148/radiol.10090908. [DOI] [PubMed] [Google Scholar]
- 24.Shah S., Shukla A., Paunipagar B. Radiological features of hepatocellular carcinoma. J Clin Exp Hepatol. 2014;4(Suppl 3):S63–6. doi: 10.1016/j.jceh.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mancosu P., Bettinardi V., Passoni P. Contrast enhanced 4D-CT imaging for target volume definition in pancreatic ductal adenocarcinoma. Radiother Oncol. 2008;87(3):339–342. doi: 10.1016/j.radonc.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 26.Liu J., Wang J.-Z., Zhao J.-D., Xu Z.-Y., Jiang G.-L. Use of combined maximum and minimum intensity projections to determine internal target volume in 4-dimensional CT scans for hepatic malignancies. Radiat Oncol. 2012;7:11. doi: 10.1186/1748-717X-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.