Abstract
BACKGROUND: Chemotherapy-resistant cancer stem cells (CSC) may lead to tumor recurrence in glioblastoma (GBM). The poor prognosis of this disease emphasizes the critical need for developing a treatment stratification system to improve outcomes through personalized medicine. METHODS: We present a case series of 12 GBM and 2 progressive anaplastic glioma cases from a single Institution prospectively treated utilizing a CSC chemotherapeutics assay (ChemoID) guided report. All patients were eligible to receive a stereotactic biopsy and thus undergo ChemoID testing. We selected one of the most effective treatments based on the ChemoID assay report from a panel of FDA approved chemotherapy as monotherapy or their combinations for our patients. Patients were evaluated by MRI scans and response was assessed according to RANO 1.1 criteria. RESULTS: Of the 14 cases reviewed, the median age of our patient cohort was 49 years (21–63). We observed 6 complete responses (CR) 43%, 6 partial responses (PR) 43%, and 2 progressive diseases (PD) 14%. Patients treated with ChemoID assay-directed therapy, in combination with other modality of treatment (RT, LITT), had a longer median overall survival (OS) of 13.3 months (5.4-NA), compared to the historical median OS of 9.0 months (8.0–10.8 months) previously reported. Notably, patients with recurrent GBM or progressive high-grade glioma treated with assay-guided therapy had a 57% probability to survive at 12 months, compared to the 27% historical probability of survival observed in previous studies. CONCLUSIONS: The results presented here suggest that the ChemoID Assay has the potential to stratify individualized chemotherapy choices to improve recurrent and progressive high-grade glioma patient survival.
Importance of the Study
Glioblastoma (GBM) and progressive anaplastic glioma are the most aggressive brain tumor in adults and their prognosis is very poor even if treated with the standard of care chemoradiation Stupp's protocol. Recent knowledge pointed out that current treatments often fail to successfully target cancer stem cells (CSCs) that are responsible for therapy resistance and recurrence of these malignant tumors. ChemoID is the first and only CLIA (clinical laboratory improvements amendment) -certified and CAP (College of American Pathologists) -accredited chemotherapeutic assay currently available in oncology clinics that examines patient's derived CSCs susceptibility to conventional FDA (Food and Drugs Administration) -approved drugs. In this study we observed that although the majority of our patients (71.5%) presented with unfavorable prognostic predictors (wild type IDH-1/2 and unmethylated MGMT promoter), patients treated with ChemoID assay-directed therapy had an overall response rate of 86% and increased median OS of 13.3 months compared to the historical median OS of 9.1 months (8.1–10.1 months) previously reported [1] suggesting that the ChemoID assay may be beneficial in personalizing treatment strategies.
Introduction
Glioblastoma (GBM) and progressive anaplastic glioma are the most aggressive brain tumor in adults, exhibiting a very poor prognosis with a median time to recurrence for GBM of approximately 7 months, and a median survival of 15–18 months even if treated with standard of care consisting of surgical resection/biopsy, and concurrent radiation with Temozolomide followed by adjuvant Temozolomide (TMZ) [2]. Despite this treatment, recurrence is almost inevitable, [3] and the prognosis of recurrent GBM remains poor with a median PFS of 5.5 months, and a median OS of 8–9 months [4]. Unfortunately, no universally held standard of care is available for recurrent GBM and progressive anaplastic glioma. Treatment options depend on specific aspects of its presentation, including secondary cytoreductive surgery when possible, focused re-irradiation [5], and numerous second-line chemotherapy treatment options [6]. While most patients eventually succumb to the progression of recurrent disease, second-line chemotherapy treatments have provided variable remission and symptom-free survival in a percentage of patients [6]. As such, the selection of effective chemotherapy is extremely important for these patients. Additionally, with emerging value-based payment models where outcomes-based contracts link to payment for indications of specific cancer drug prices, there are further concerns about the accessibility and affordability for treatment of recurrent GBM patients; therefore, there is a need for effective treatments that limit overall cost while also increasing treatment value for these patients.
Herein we describe our experience in using ChemoID, a clinical laboratory improvements amendment (CLIA)-certified and College of American Pathologists (CAP)-accredited clinical diagnostic test that is performed in a clinical pathology laboratory, which identifies chemotherapeutic agent(s) that kill both the cancer stem cells (CSCs) and the bulk tumor cells, to guide treatment of recurrent GBM and progressive anaplastic glioma.
Methods
Patients
Fourteen patients (11 males and 3 females), 18 years and older, that were clinically diagnosed with recurrent GBM (12 patients) or progressive anaplastic glioma (2 patients), received a concurrent stereotactic biopsy for histological diagnosis and for prospective ChemoID chemotherapeutic testing between October 2016 and October 2017. This study was approved by the Institutional Review Board (IRB) and informed consent was obtained. Patients were treated with ChemoID-guided chemotherapy according to their overall functional status and ability to tolerate the recommended treatment. Radiological data was collected before surgery, immediately post-surgery and following chemo/radiation therapy with an MRI follow-up every 2 months. Supportive care was also allowed at the discretion of the treating physician. Disease status was measured by radiologic examination (MR scan as the primary imaging method), physical examination, and measurements using the Response Assessment in Neuro-Oncology (RANO 1.1) criteria [7], which takes into account parameters and evaluations to identify pseudo-progression as well as pseudo-response. The RANO criteria, similarly to other systems, divide response into 4 types of response based on imaging (MRI) and clinical features [8,9]: complete response, partial response, stable disease, and progression.
ChemoID Assay
Multiple fresh GBM tissue samples for ChemoID functional testing were collected in the operating room from each patient at the time of the stereotactic biopsy procedure. Details regarding cell culture conditions, the CSCs enrichment procedure and the assay have been previously described [[10], [11], [12]]. The bulk of tumor cells and CSCs were counted using trypan blue exclusion to determine cellular viability and cell number prior to chemosensitivity testing. Cells were also incubated with florescent antibodies for phenotypic characterization by flow cytometry and also this procedure has been previously described [10,11]. Percent of cell survival was assessed using an MTT assay on 1 × 10∧3 cells plated in 5 replicas into 96-well plates. An equal number of the bulk of tumor cells and CSCs were seeded in 96-well dishes and incubated at 37 °C for 24-hours. Three concentrations of each chemotherapy treatment were prepared by serial dilution. Each concentration was added to five replicate wells on the microtiter plate. Additionally, three replicates wells (control 1 = no treatment) and three replicates wells (control 2 = equal amount of solvent) were associated with each treatment. The cells were challenged for a 1-hour pulse with the panel of anticancer drugs (Table 1). MTT assay was performed 24-hours following chemotherapy treatment to assess cell survival as previously described [[10], [11], [12]].
Table 1.
List of single chemotherapeutic agents and combinations tested on the GBM and progressive high-grade glioma cohort and clinical dose
| Single drug | Dose | |
|---|---|---|
| 1 | Carboplatin | 350 mg/m2 |
| 2 | Irinotecan | 125 mg/m2 |
| 3 | Etoposide | 50 mg/m2 |
| 4 | BCNU | 100 mg/m2 |
| 5 | CCNU | 100 mg/m2 |
| 6 | Temozolomide | 200 mg/m2 |
| 7 | Procarbazine | 60 mg/m2 |
| 8 | Vincristine | 1.4 mg/m2 |
| 9 | Imatinib | 400 mg |
| Drug Combinations | Dose | |
| 1 | Procarbazine | 60 mg/m2 |
| CCNU | 100 mg/m2 | |
| Vincristine | 1.4 mg/m2 | |
| 2 | Carboplatin | 350 mg/m2 |
| Irinotecan | 125 mg/m2 | |
| 3 | Carboplatin | 350 mg/m2 |
| Etoposide | 50 mg/m2 | |
| 4 | Temozolomide | 50 mg/m2 |
| Etoposide | 50 mg/m2 | |
| 5 | Temozolomide | 50 mg/m2 |
| Imatinib | 200 mg |
Inhibition of bulk of tumor cells and CSCs survival was measured for each concentration (average counts in five replicates ± SE) of a given treatment (15–18 per patient). Survival of tumor cells at each concentration was calculated as compared to control-2 and overall percent of the bulk of tumor cells and CSCs killed were calculated for each treatment as the primary measures of potential therapy efficacy.
Statistical Analysis
Descriptive statistics were constructed as medians with ranges for continuous variables and counts and percentages for categorical variables. Due to the small sample size, no survival models were constructed. A graph displaying censoring/death times for each participant with annotations was constructed along with a Kaplan–Meier plot showing survival probabilities. All statistics and graphics were compiled using Stata v15.1 (StataCorp, College Station, TX).
Results
Patients' Survival Analysis
Fresh tissue samples were collected for drug sensitivity testing from 12 GBM and 2 progressive anaplastic glioma patients, with a median age of 49 years (range 21–61), 79% of which were male, all eligible for stereotactic biopsy. Patients were treated with chemotherapies predicted to be most efficacious by the ChemoID chemotherapeutics assay taking into consideration the patient's tolerability of that particular treatment based on their health status.
IDH-1 mutation and methylation status of the promoter region of the O6-Methyl-guanyl-methyl-transferase (MGMT) gene were studied for our entire patient cohort. About 29% of our patients had methylation of the MGMT gene promoter, and 86% a wild-type IDH-1/2 status from their pathology report (Table 2).
Table 2.
Patient characteristics
| Median age | 49 / (21–63) |
| Female | 3 (21%) |
| Male | 11 (79%) |
| IDH-1/2 wild-type | 12/14 (86%) |
| Unmethylated MGMT promoter | 10/14 (71%) |
| Response | |
| Complete response (CR) | 6 (43%) |
| Partial response (PR) | 6 (43%) |
| Stable disease (SD) | 0 (0%) |
| Progressive disease (PD) | 2 (14%) |
Table 3 reports the clinical characteristics we have analyzed in our patient cohort. In particular, 10 patients (72%) presented with IDH-1/2 wild-type and unmethylated MGMT promoter, 2 patients (14%) with IDH-1/2 wild-type and methylated MGMT promoter, 1 patient (7%) with IDH-1 mutated and unmethylated MGMT promoter, and 1 patient (7%) with IDH-1/2 mutated and a methylated MGMT promoter, indicating that the vast majority of the patients in our cohort had negative prognostic predictors for their disease (i.e. presence of wild-type IDH-1/2 protein and unmethylated MGMT gene promoter) [[13], [14], [15], [16]]. The median KPS status of our patients was 70 (KPS range 60–90). 57% of our patients were re-irradiated following ChemoID guided therapy. Only one patient in our cohort underwent surgical resection as part of the treatment for their recurrent GBM, and another one was operated on as a treatment for an infection.
Table 3.
Patients' detailed characteristics
| Patient number | Age at diagnosis | Gender | Alive | Response by RANO 1.1 | IDH-1/2 | MGMT Promoter Methylation Status |
Tumor Location |
KPS Status | Surgery before ChemoID | Repeat Surgical Resection after ChemoID/Biopsy |
LITT before ChemoID | LITT after ChemoID | Irradiation before ChemoID | Irradiation after ChemoID |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 32 | M | No | PR | Wild-type | Methylated | Left Frontal, Multifocal | 80 | Yes | No | Yes | No | Yes | No |
| 2 | 60 | F | No | PD | Wild-type | Unmethylated | Right frontal, Multifocal | 70 | No | Yes (for infection) |
No | No | No | No |
| 3 | 49 | F | No | CR | Wild-type | Unmethylated | Right Temporal | 80 | Yes | No | No | No | No | Yes |
| 4 | 60 | M | No | PD | Wild-type | Unmethylated | Left Temporal | 70 | Yes | No | No | No | No | No |
| 5 | 48 | M | Yes | CR | Wild-type | Unmethylated | Right Frontal | 60 | Yes | No | No | No | Yes | No |
| 6 |
49 |
F |
No |
CR |
Mutant |
Unmethylated |
Diffuse, Right Frontal, Parietal, Temporal | 60 | Yes | No | No | No | No | Yes |
| 7 | 56 | M | No | CR | Wild-type | Methylated | Left Temporal | 90 | Yes | No | No | No | Yes | No |
| 8 | 61 | M | No | PR | Wild-type | Unmethylated | Left Temporal | 90 | No | Yes | No | No | No | No |
| 9 | 21 | M | Yes | CR | Wild-type | Unmethylated | Left Thalamus and Midbrain | 90 | No | No | No | No | No | Yes |
| 10 | 52 | M | Yes | PR | Wild-type | Unmethylated | Right Frontal/Insula | 70 | Yes | No | No | No | No | Yes |
| 11 | 35 | M | Yes | CR | Wild-type | Unmethylated | Left Occipital | 70 | No | No | No | No | No | Yes |
| 12 | 63 | M | Yes | PR | Mutant | Methylated | Left Frontal-Temporal | 80 | Yes | No | No | No | No | Yes |
| 13 | 36 | M | Yes | PR | Wild-type | Unmethylated | Right Temporal | 70 | No | No | No | No | No | Yes |
| 14 | 33 | M | Yes | PR | Wild-type | Unmethylated | Diffuse, Right Frontal, Temporal | 70 | Yes | No | No | No | No | Yes |
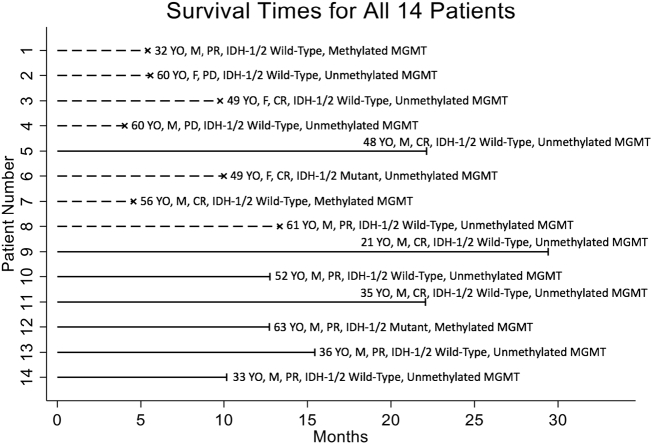
Patients were prospectively monitored by response assessment in neuro-oncology (RANO 1.1) for tumor response, time to progression, progression-free survival (PFS), and overall survival (OS). Table 2, Table 3 describe the characteristics of our patient series and their response to therapy observed as per RANO 1.1. We observed 6 complete responses (CR) 43% (6 out of 14 patients), 6 partial response (PR) 43% (6 out of 14 patients), and 2 progressive diseases (PD) 14% (2 out of 14 patients). However, the two PD (patients #2 and 4, Figure 1) were observed because patients could not be treated with ChemoID recommended therapy for their health status.
Figure 1.
Diagram illustrating patient's characteristics, their survival times in months, and their response to treatment according to RANO 1.1 criteria. Abscissae axis shows survival time in months, ordinate axis shows patient number. Dashed lines indicate deaths, while solid lines indicate right censoring. Treatment response according to RANO 1.1 criteria is indicated by Complete Response (CR), Partial Response (PR) and Progressive Disease (PD).
All patients had follow-up MRI every 2 months, and the median follow-up from pre-ChemoID tumor biopsy was 11.4 months (range 4.0–29.4 months). At the end of our follow-up, 7 patients (50%) were still alive. Figure 1 shows survival times of the recurrent GBM patients prospectively treated using the ChemoID test results and chemotherapies predicted by the CLIA-certified and CAP-accredited CSC drug assay.
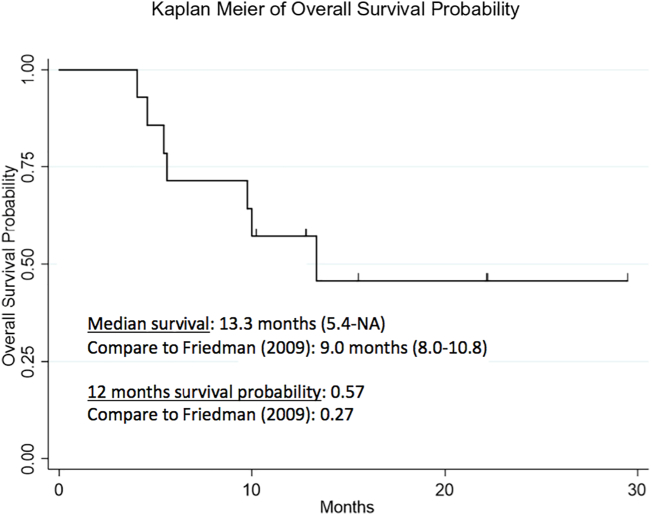
We observed that the median OS of patients treated with ChemoID assay-directed therapy was 13.3 months (5.4-NA) compared to the historical median OS of 9.1 months (8.1–10.1 months) previously reported (Figure 2) [1]. Notably, our recurrent and progressive high-grade glioma patient cohort treated with ChemoID assay-guided therapy had a 57% probability to survive at 12 months, compared to the 27% historical probability of survival at 12 months observed in previous studies [17]. Of the surviving patients, all have exceeded the expected survivals of previously reported studies [1,4,17]. One-year overall survival was 57% (95% CI: 28% - 78%), based upon Kaplan–Meier estimates.
Figure 2.
Kaplan-Meier plot of overall survival probability. Overall survival (OS) for recurrent GBM patients treated with ChemoID-guided responsive drugs. Patients receiving ChemoID responsive drugs had a median survival of 13.3 months. Twelve months survival probability was 57% (95% CI: 28% to 78%), based upon Kaplan–Meier estimates.
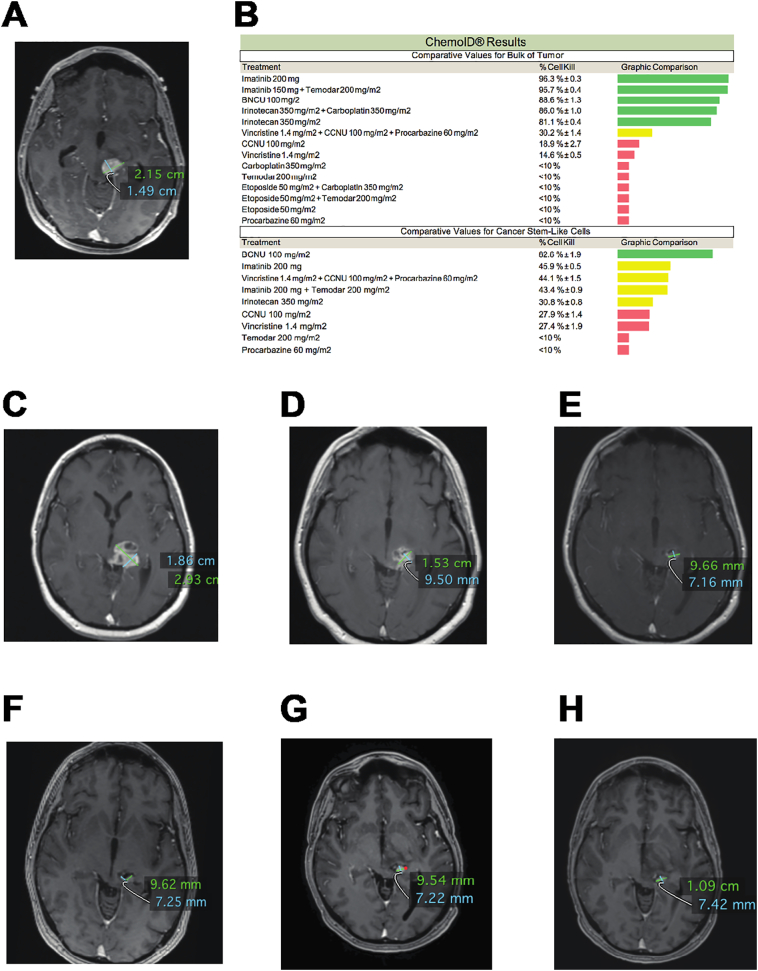
Figure 3 shows the MRI image of a wild type IDH-1, unmethylated MGMT, non-operable left midbrain/thalamus GBM patient (Figure 3A), the ChemoID drug response assay results (Figure 3B), and the subsequent MRI images (Figure 3, C–H) following treatment with ChemoID-guided therapy and re-irradiation. This patient received a stereotactic biopsy for an inoperable left brainstem mass to confirm the presence of a grade IV glioblastoma in December of 2016. A concomitant fresh biopsy was sent to the ChemoID lab for testing. The patient was treated with a full course of irradiation to 60 Gy and Temozolomide (TMZ) as per standard of care; however, the tumor showed radiologic progression in April 2017 (Figure 3C). At this point, after multidisciplinary discussion, treatment was switched to BCNU, which had demonstrated bulk tumor effectiveness (88.6% cell kill) as well as CSC (82.6% cell kill) by the ChemoID assay. MRI images in November of 2017 following the completion of treatment with 6 cycles of BCNU (150 mg/m2 every 6 weeks) showed a dramatic reduction of the tumor burden (Figure 3D). Patient requested maintenance therapy and was then reirradiated and started on Imatinib (400 mg daily), which also demonstrated effectiveness on the bulk of tumor (96.3% cell kill) and on CSC (45.9% cell kill) by the ChemoID assay. MRI images following treatment with Imatinib (February–November 2018) showed an even greater reduction of the tumor burden for 24 months with no new lesions (Figure 3, E–H).
Figure 3.
MRI Images and comparative analysis of ChemoID test results on Bulk of Tumor and Cancer Stem Cells of a patient affected by left midbrain/thalamus, WHO grade 4, IDH wild-type, MGMT unmethylated gene, not operable, recurrent GBM.
A) Preoperative (stereotactic biopsy) MRI shows an intra-axial enhancing mass centered in the left midbrain/thalamus measuring 2.51 × 1.49 cm (12/22/2016).
B) Comparative ChemoID analysis on Bulk of Tumor and Cancer Stem Cells obtained from fresh stereotactic biopsy.
C) Control MRI (04/06/2017) following standard of care treatment with Temodar chemotherapy (75 mg/m2 daily) and radiation therapy (with radiation boost – 7 fractions) shows increased size of the left midbrain/thalamus lesion measuring 2.93 × 1.86 cm.
D) Failure of standard of care treatment prompted treatment with BCNU (150 mg/m2 every 6 weeks). Control MRI (11/08/2017) after 6 cycles of BCNU shows initial regression of the lesion measuring 1.53 × 0.95 cm.
E) Control MRI (02/20/2018) following Imatinib (400 mg daily) treatment shows regression of the lesion measuring 9.66 × 7.16 mm.
F) Control MRI (05/14/2018) following continued Imatinib (400 mg daily) treatment shows stable lesion measuring 9.62 × 7.25 mm.
G) Control MRI (07/19/2018) following continued Imatinib (400 mg daily) treatment shows stable lesion measuring 9.54 × 7.22 mm.
H) Control MRI (11/21/2018) following continued Imatinib (400 mg daily) treatment shows stable lesion measuring 9.66 × 7.16 mm.
Discussion
Medical management of recurrent GBM and progressive anaplastic glioma is typically a multimodality treatment plan consisting of maximal safe surgical resection (when possible), followed by radiotherapy with concomitant and adjuvant Temozolomide and/or other secondary chemotherapies [4,18,19] which continually increases treatment morbidity leading to further cost with diminishing returns on the outcome. Moreover, despite aggressive therapy and emerging chemotherapeutic treatment options, because current therapies are not curative and improving overall survivability has proven to be problematic, the management of these patients remains difficult.
TMZ is a key component of standard therapy for both newly diagnosed and recurrent GBM patients; however, the major challenge with recurrent GBM treatment is the numerous clinically-acceptable and oftentimes equivalent treatment options identified in treatment guidelines [20] . Currently, there is insufficient evidence to indicate a superior agent or treatment strategy for GBM patients as a whole as well as for the individual patient.
The presence of CSCs appears to be responsible, at least in part, for resistance to standard treatments, the variable responses seen to treatment, and thus has important implications for the development of a diagnostic assay to guide personalized treatment regimens [21]. The current study evaluated the clinical advantage of using the ChemoID chemotherapeutics assay to measure CSC response against a panel of FDA approved chemotherapies to treat recurrent GBM. Patients were treated with chemotherapies (Table 1) chosen from those drugs showing the highest cell kill as determined by the CSCs and bulk of tumor chemotherapeutic test response, and by taking into consideration the patient's tolerability of the treatment.
This study is the first example demonstrating that ChemoID, a CSC chemotherapeutics assay, can prospectively identify and stratify more effective chemotherapy agents versus other possible choices on an individual patient level in poor prognosis recurrent GBM and progressive anaplastic glioma. In particular, although 10/14 of our patient cohort (71.5%) presented with unfavorable prognostic predictors (IDH-1/2 wild type and unmethylated MGMT promoter) [[13], [14], [15]], we observed that patients treated with ChemoID assay-directed therapy had an overall response rate of 86%. Notably, PD was observed in those patients that could not be treated with assay recommended therapy due to their health status and co-morbidities.
Interestingly, systematic reviews and meta-analysis of re-resection and re-irradiation for recurrent GBM showed that both practices were associated with better overall survival and post-progression survival, providing encouraging disease control and survival rates [22,23]. 57% of the GBM patients in our cohort, received irradiation following ChemoID guided therapy, but only two recurrent patients underwent surgical resection as part of their treatment, with one of the two patients that were operated to treat an infection and not the neoplastic lesion.
It is known that recurrent GBM is associated with a median overall survival of less than a year and the majority of patients have profound tumor-related symptoms [24,25]. Interventions such as re-resection, systemic therapy and/or re-irradiation may benefit selected patients, but unfortunately, all are given with a palliative intent [25]. As such, treatment decisions must be individualized. Clinicians are tasked with selecting the most appropriate treatment, balancing the benefits of treatment with the risk of treatment-related toxicity and its impact on the quality of life [26,27]. The performance status of the patient, extent of recurrence (focal versus diffuse) and location of recurrence are important considerations in such instances [26,27].
Notably, our cohort of GBM and progressive high-grade glioma patients treated with assay-guided therapy had a 57% probability to survive at 12 months, compared to the 27% historical probability of survival at 12 months observed in previous studies [1,4,17], demonstrating the importance of determining CSCs response to chemotherapy to prolong patients' survival. The data further supports the belief that long-term tumor response in GBM, is in fact, more dependent on the intrinsic sensitivity or resistance of the CSCs to conventional chemotherapies. This concept is especially valuable and important with emerging value-based healthcare models where outcomes-based contracts linked to payment for an indication of specific anticancer-drug prices raise concerns about the accessibility and affordability for treatment of recurrent GBM patients. The power of precision medicine lies in its ability to guide health care decisions toward the most effective treatment for a given patient, and thus, improve care quality while reducing the need for unnecessary diagnostic testing and therapies.
ChemoID is a functional precision medicine test that uses patient's live bulk of tumor cells and cancer stem cells (CSCs) isolated by tumor biopsies to indicate and to stratify which chemotherapy agent (or “combinations”) is most effective [12]. Targeting of CSCs alongside the bulk of other cancer cells is a new paradigm in personalized anticancer treatment. This strategy and technological advancement constitute an important advantage of ChemoID approach over other diagnostic methods for personalized medicine.
We are currently conducting a multi-institutional phase-III clinical trial (NCT03632135) to determine the clinical validity of the ChemoID assay as a predictor of clinical response in recurrent GBM. The study has been designed as a parallel-group, controlled clinical trial that randomizes participants to either standard of care chemotherapy chosen by the physician or ChemoID-guided therapy. In the NCT03632135 trial, response to therapy will be measured by MRI imaging using RANO 1.1 criteria to assess overall survival (OS), OS at 6, 9, and 12 months, median progression-free survival (PFS), PFS at 4, 6, 9, and 12 months, objective tumor response, time to recurrence, and quality of life.
ChemoID is the first and only chemotherapeutics assay currently available in oncology clinics that examines CSCs susceptibility to conventional FDA approved drugs from solid tumors. Results from the current study indicate that the ChemoID assay may be a very useful tool for optimizing treatment selection when first-line therapy fails, and when there are multiple clinically acceptable and equivalent treatments available. The ChemoID assay takes 2 weeks to be completed from the date of receiving a live biopsy, which corresponds to the average time patients spend recovering from surgery prior to continuing further therapy. Therefore, the ChemoID assay is suitable for timely, individualized chemotherapy for cancer patients who received surgery. Furthermore, our results suggest that this individualized functional chemotherapeutic assay may indeed surpass the results achieved by empiric population-based treatment by providing more treatment options with improved outcomes for many more patients. This compelling data suggests that the ChemoID CSC assay may be beneficial in personalizing treatment strategies to increase survival time for recurrent GBM patients and to provide quality metrics for healthcare payers and providers to support access to care.
Declarations
Ethics Approval and Consent to Participate
This study was approved by the Institutional Review Board (IRB) and informed consent was obtained. Participants provided their written consent on an IRB approved informed consent form to participate in this study after being educated about the research protocol. Assay was performed after obtaining patients' written informed consent in accordance with the ethical standards of the Helsinki Declaration (1964, amended most recently in 2008) of the World Medical Association.
Consent for Publication
Not applicable
Availability of Data and Material
The datasets generated and/or analyzed during the current study are not publicly available due to individual privacy restriction on medical records, but are available from the corresponding author on reasonable request.
Competing Interests
The authors declare that they have no competing interests.
Funding
We gratefully acknowledge Marshall University and Cabell Huntington Hospital for their support. STL is partially supported by the Mississippi Center for Clinical and Translational Research and Mississippi Center of Excellence in Perinatal Research COBRE funded by the National Institute of General Medical Sciences of the National Institutes of Health under Award Numbers 5U54GM115428 and P20GM121334. CMH is partially supported by the Mississippi Center for Clinical and Translational Research funded by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number 5U54GM115428. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authors' Contributions
All authors contributed significantly to the present research and reviewed the entire manuscript.
TR: Participated in providing patients' treatment data; also participated substantially in conception and execution of the study and in the analysis and interpretation of data; participated substantially in the drafting and editing of the manuscript.
CMH: Analyzed and interpreted radiological imaging data; also participated substantially in the drafting and editing of the manuscript.
AY: Participated in providing patients' samples; also participated substantially in the execution of the study and participated substantially in the drafting and editing of the manuscript.
LX: Participated in providing patients' samples; also participated substantially in the execution of the study and participated substantially in the drafting and editing of the manuscript.
KA: Participated in providing patients' samples; also participated substantially in the execution of the study and participated substantially in the drafting and editing of the manuscript.
DJ: Participated in providing patients' samples; also participated substantially in the execution of the study and participated substantially in the drafting and editing of the manuscript.
JL: Participated in providing patients' samples; also participated substantially in the execution of the study and participated substantially in the drafting and editing of the manuscript.
MAH: participated in the execution of the study; also participated in substantially drafting and editing the manuscript.
SMK: Participated substantially in the execution of the study and in drafting and editing the manuscript.
REW: Participated substantially in the execution of the study and in the analysis and interpretation of data; participated substantially in the drafting and editing of the manuscript.
RF: Participated substantially in the execution of the study and in the drafting and editing of the manuscript.
STL: Conducted the statistical analysis, participated in the interpretation of data and in the drafting and editing of the manuscript.
KD: Participated substantially in providing patients' ChemoID test results and in the drafting and editing of the manuscript.
JV: Participated substantially in conception and execution of the study and in the drafting and editing of the manuscript.
PPC: Participated substantially in conception and execution of the study and in the drafting and editing of the manuscript.
Footnotes
Key point: Cancer Stem Cell Chemotherapeutics Assay improves the outcome of recurrent high-grade glioma patients by guiding their chemotherapy treatment.
Funding: STL is partially supported by the Mississippi Center for Clinical and Translational Research and Mississippi Center of Excellence in Perinatal Research COBRE funded by the National Institute of General Medical Sciences of the National Institutes of Health under Award Numbers 5U54GM115428 and P20GM121334. CMH is partially supported by the Mississippi Center for Clinical and Translational Research funded by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number 5U54GM115428.
References
- 1.Wick W., Gorlia T., Bendszus M., Taphoorn M., Sahm F., Harting I., Brandes A.A., Taal W., Domont J., Idbaih A. Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med. 2017;377:1954–1963. doi: 10.1056/NEJMoa1707358. [DOI] [PubMed] [Google Scholar]
- 2.Johnson D.R., O'Neill B.P. Glioblastoma survival in the United States before and during the temozolomide era. J Neurooncol. 2012;107:359–364. doi: 10.1007/s11060-011-0749-4. [DOI] [PubMed] [Google Scholar]
- 3.Sundar S.J., Hsieh J.K., Manjila S., Lathia J.D., Sloan A. The role of cancer stem cells in glioblastoma. Neurosurg Focus. 2014;37 doi: 10.3171/2014.9.FOCUS14494. [DOI] [PubMed] [Google Scholar]
- 4.van Linde M.E., Brahm C.G., de Witt Hamer P.C., Reijneveld J.C., Bruynzeel A.M.E., Vandertop W.P., van de Ven P.M., Wagemakers M., van der Weide H.L., Enting R.H. Treatment outcome of patients with recurrent glioblastoma multiforme: a retrospective multicenter analysis. J Neurooncol. 2017;135:183–192. doi: 10.1007/s11060-017-2564-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gigliotti M.J., Hasan S., Karlovits S.M., Ranjan T., Wegner R.E. Re-irradiation with stereotactic radiosurgery/radiotherapy for recurrent high-grade gliomas: improved survival in the modern era. Stereotact Funct Neurosurg. 2018;96:289–295. doi: 10.1159/000493545. [DOI] [PubMed] [Google Scholar]
- 6.Steffens R., Semrau S., Lahmer G., Putz F., Lettmaier S., Eyupoglu I., Buchfelder M., Fietkau R. Recurrent glioblastoma: who receives tumor specific treatment and how often? J Neurooncol. 2016;128:85–92. doi: 10.1007/s11060-016-2079-z. [DOI] [PubMed] [Google Scholar]
- 7.Wen P.Y., Macdonald D.R., Reardon D.A., Cloughesy T.F., Sorensen A.G., Galanis E., Degroot J., Wick W., Gilbert M.R., Lassman A.B. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 8.Chinot O.L., Macdonald D.R., Abrey L.E., Zahlmann G., Kerloeguen Y., Cloughesy T.F. Response assessment criteria for glioblastoma: practical adaptation and implementation in clinical trials of antiangiogenic therapy. Curr Neurol Neurosci Rep. 2013;13:347. doi: 10.1007/s11910-013-0347-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van den Bent M.J., Wefel J.S., Schiff D., Taphoorn M.J., Jaeckle K., Junck L., Armstrong T., Choucair A., Waldman A.D., Gorlia T. Response assessment in neuro-oncology (a report of the RANO group): assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol. 2011;12:583–593. doi: 10.1016/S1470-2045(11)70057-2. [DOI] [PubMed] [Google Scholar]
- 10.Kelly S.E., Di Benedetto A., Greco A., Howard C.M., Sollars V.E., Primerano D.A., Valluri J.V., Claudio P.P. Rapid selection and proliferation of CD133+ cells from cancer cell lines: chemotherapeutic implications. PLoS One. 2010;5 doi: 10.1371/journal.pone.0010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mathis S.E., Alberico A., Nande R., Neto W., Lawrence L., McCallister D.R., Denvir J., Kimmey G.A., Mogul M., Oakley G., 3rd Chemo-predictive assay for targeting cancer stem-like cells in patients affected by brain tumors. PLoS One. 2014;9 doi: 10.1371/journal.pone.0105710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howard C.M., Valluri J., Alberico A., Julien T., Mazagri R., Marsh R., Alastair H., Cortese A., Griswold M., Wang W. Analysis of chemopredictive assay for targeting cancer stem cells in glioblastoma patients. Transl Oncol. 2017;10:241–254. doi: 10.1016/j.tranon.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burgenske D.M., Yang J., Decker P.A., Kollmeyer T.M., Kosel M.L., Mladek A.C., Caron A.A., Vaubel R.A., Gupta S.K., Kitange G.J. Molecular profiling of long-term IDH-wildtype glioblastoma survivors. Neuro Oncol. 2019;21(11):1458–1469. doi: 10.1093/neuonc/noz129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao W.Z., Guo L.M., Xu T.Q., Yin Y.H., Jia F. Identification of a multidimensional transcriptome signature for survival prediction of postoperative glioblastoma multiforme patients. J Transl Med. 2018;16:368. doi: 10.1186/s12967-018-1744-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montano N., D'Alessandris Q.G., Izzo A., Fernandez E., Pallini R. Biomarkers for glioblastoma multiforme: status quo. J Clin Transl Res. 2016;2:3–10. [PMC free article] [PubMed] [Google Scholar]
- 16.Binabaj M.M., Bahrami A., ShahidSales S., Joodi M., Joudi Mashhad M., Hassanian S.M., Anvari K., Avan A. The prognostic value of MGMT promoter methylation in glioblastoma: a meta-analysis of clinical trials. J Cell Physiol. 2018;233:378–386. doi: 10.1002/jcp.25896. [DOI] [PubMed] [Google Scholar]
- 17.Friedman H.S., Prados M.D., Wen P.Y., Mikkelsen T., Schiff D., Abrey L.E., Yung W.K., Paleologos N., Nicholas M.K., Jensen R. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 18.Delgado-Lopez P.D., Corrales-Garcia E.M. Survival in glioblastoma: a review on the impact of treatment modalities. Clin Transl Oncol. 2016;11:1062–1071. doi: 10.1007/s12094-016-1497-x. [DOI] [PubMed] [Google Scholar]
- 19.Palmer J.D., Bhamidipati D., Song A., Eldredge-Hindy H.B., Siglin J., Dan T.D., Champ C.E., Zhang I., Bar-Ad V., Kim L. Bevacizumab and re-irradiation for recurrent high grade gliomas: does sequence matter? J Neurooncol. 2018;140:623–628. doi: 10.1007/s11060-018-2989-z. [DOI] [PubMed] [Google Scholar]
- 20.NCCN Guidelines Version 3.2019, Anaplastic Gliomas/Glioblastoma (GLIO 1–5) [https://www.nccn.org].
- 21.Parker N.R., Hudson A.L., Khong P., Parkinson J.F., Dwight T., Ikin R.J., Zhu Y., Cheng Z.J., Vafaee F., Chen J. Intratumoral heterogeneity identified at the epigenetic, genetic and transcriptional level in glioblastoma. Sci Rep. 2016;6:22477. doi: 10.1038/srep22477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao Y.H., Wang Z.F., Pan Z.Y., Peus D., Delgado-Fernandez J., Pallud J., Li Z.Q. A meta-analysis of survival outcomes following reoperation in recurrent glioblastoma: time to consider the timing of reoperation. Front Neurol. 2019;10:286. doi: 10.3389/fneur.2019.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kazmi F., Soon Y.Y., Leong Y.H., Koh W.Y., Vellayappan B. Re-irradiation for recurrent glioblastoma (GBM): a systematic review and meta-analysis. J Neurooncol. 2019;142:79–90. doi: 10.1007/s11060-018-03064-0. [DOI] [PubMed] [Google Scholar]
- 24.Wallner K.E., Galicich J.H., Krol G., Arbit E., Malkin M.G. Patterns of failure following treatment for glioblastoma multiforme and anaplastic astrocytoma. Int J Radiat Oncol Biol Phys. 1989;16:1405–1409. doi: 10.1016/0360-3016(89)90941-3. [DOI] [PubMed] [Google Scholar]
- 25.Easaw J.C., Mason W.P., Perry J., Laperriere N., Eisenstat D.D., Del Maestro R., Belanger K., Fulton D., Macdonald D., Canadian Glioblastoma Recommendations C. Canadian recommendations for the treatment of recurrent or progressive glioblastoma multiforme. Curr Oncol. 2011;18:e126–e136. doi: 10.3747/co.v18i3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krauze A.V., Attia A., Braunstein S., Chan M., Combs S.E., Fietkau R., Fiveash J., Flickinger J., Grosu A., Howard S. Expert consensus on re-irradiation for recurrent glioma. Radiat Oncol. 2017;12:194. doi: 10.1186/s13014-017-0928-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krauze A.V., Attia A., Braunstein S., Chan M., Combs S.E., Fietkau R., Fiveash J., Flickinger J., Grosu A., Howard S. Correction to expert consensus on re-irradiation for recurrent glioma. Radiat Oncol. 2018;13:8. doi: 10.1186/s13014-018-0955-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available due to individual privacy restriction on medical records, but are available from the corresponding author on reasonable request.