Graphical abstract
Keywords: Hansen solubility parameter, Lipid nanoparticles and nanocarriers, Solubility, Clarithromycin, Efavirenz, Minocycline hydrochloride, Mometasone furoate, Didanosine
Abstract
The aim of these studies was to determine the miscibility of different API with lipid excipients to predict drug loading and encapsulation properties for the production of solid lipid nanoparticles and nanostructured lipid carriers. Five API exhibiting different physicochemical characteristics, viz., clarithromycin, efavirenz, minocycline hydrochloride, mometasone furoate, and didanosine were used and six solid lipids in addition to four liquid lipids were investigated. Determination of solid and liquid lipids with the best solubilization potential for each API were performed using a traditional shake-flask method and/or a modification thereof. Hansen solubility parameters of the API and different solid and liquid lipids were estimated from their chemical structure using Hiroshi Yamamoto’s molecular breaking method of Hansen Solubility Parameters in Practice software. Experimental results were in close agreement with solubility parameter predictions for systems with ΔδT < 4.0 MPa1/2. A combination of Hansen solubility parameters with experimental drug-lipid miscibility tests can be successfully applied to predict lipids with the best solubilizing potential for different API prior to manufacture of solid lipid nanoparticles and nanostructured lipid carriers.
1. Introduction
Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) are two major types of lipid-based nanocarriers developed to overcome the limitations of other colloidal carriers, such as emulsions, liposomes and polymeric nanoparticles (Naseri et al., 2015). Some of these limitations include limited stability when stored over extended periods, poor batch-to-batch reproducibility, low drug loading capacity (LC) and failure to manipulate biological membrane barriers to achieve sufficient API delivery for therapeutic activity (Beija et al., 2012, Riehemann et al., 2009). The encapsulation of drug molecules in nanoparticles shields them from the effect of efflux transporters and the small particle size facilitates uptake of drugs across biological membranes (Ahmad et al., 2018b). SLN and NLC are nanovectors manufactured using solid lipids or a combination of solid and liquid lipids, respectively (Mehnert and Mader, 2002, Riehemann et al., 2009). SLN are usually used as aqueous dispersions and are produced using a solid lipid, an API and surfactant(s) which impart stability to the system (Mehnert and Mader, 2002). NLC differ from SLN only from an excipients point of view, in that binary mixtures of solid and liquid lipids are used for formulation (Uner, 2006). In addition to excellent physical stability, SLN and NLC have an ability to exhibit complex functions, such as controlled delivery of API across different biological membrane barriers and consequently targeting organs leading to adhesion and improved cellular uptake (Ahmad et al., 2018b, Naseri et al., 2015, Riehemann et al., 2009).
The manufacture of SLN or NLC formulations involves melting a solid lipid or a binary mixture of solid and liquid lipid, followed by re-dispersion of the molten lipids as submicron-size droplets in an aqueous medium containing surfactant(s) (Uner, 2006). Acute and/or chronic toxicity during in vivo use has been associated with carriers of traditional colloidal systems, including polymeric-based systems (Müller et al., 2002). Thus, a prerequisite for manufacture of SLN and NLC is that only pharmaceutical grade excipients that are generally regarded as safe (GRAS) are used for production (Muchow et al., 2008, Müller et al., 2002, Wissing et al., 2004). Mehnert and Mader (2002), in addition to Souto and Müller (2007), have reported broad lists of lipids and surfactants that can and have been used for the manufacture of SLN and NLC.
An important aspect to be considered prior to the development and optimization of SLN/NLC is the solubility of the API in the lipid(s) to be used. The usefulness of SLN and/or NLC as API carrier systems is usually dependent on LC and encapsulation efficiency (EE) of the nanocarriers for that particular API (Souto and Muller, 2011). Consequently a major factor affecting the LC and EE of SLN and/or NLC for an API is the solubility of that API in molten lipid (Hou et al., 2003, Müller et al., 2000, Wissing et al., 2004). Thus an adequate LC and EE can only be achieved if the solubility of an API in the molten lipid is relatively high (Hou et al., 2003, Müller et al., 2000, Wissing et al., 2004). Consequently it is imperative to evaluate the solubility of the API in different solid and liquid lipids, in order to select a solid and/or liquid lipid combination with the best solubilizing potential for that API.
Screening of solubility of API in lipids for production of SLN and NLC has been performed using the traditional shake flask method in order to determine equilibrium solubility (Baka et al., 2008, Cirri et al., 2018, Kasongo et al., 2011, Son et al., 2019). Modification of the shake flask method have also been used in the case of some poorly lipid soluble API (Joshi and Patravale, 2008, Joshi and Patravale, 2006). In addition, the selection of oil excipients for the production of nanoemulsions has been done two-fold through investigating API solubilty in a variety of oils using the shake flask method followed by comparative stability studies of produced nanoemulsions using four oils which demonstrated the highest API solubility (Ahmad et al., 2018a). Determination of the solubilization potential of different lipids is established using high performance liquid chromatography (HPLC) analysis, UV spectrophotometry and/or visual inspection (Ahmad et al., 2018a, Joshi and Patravale, 2008, Joshi and Patravale, 2006, Kasongo et al., 2011, Parveen et al., 2011). The shake-flask technique is a simple procedure but is time-consuming, costly and requires performing a number of laboratory experiments. Moreover, there is no accepted or standard approach when using this method (Box et al., 2006) and published solubility study data reveal large differences in the experimental conditions used, in particular stirring/shake times, sample preparation and separation techniques prior to analysis. Furthermore the solubility of ten compounds with different physicochemical profiles in ten lipid excipients was unsuccessful in elucidating a clear link between the physicochemical properties of API investigated and solubility in the excipients (Thi et al., 2009). Solubilization of API in lipids is complex and is comprised of different kinetic and thermodynamic factors that include parameters such as interfacial tension, molecular volume, crystal structure, hydrophilicity, surface charge and/or charge density as well as the physical and chemical environment of the reaction media (Shah and Agrawal, 2013, Steven Abbott, 2015). Recent advances in computational technologies have facilitated the development of more powerful in silico simulation and modelling approaches in which molecular structure, physiochemical properties and specific solute-solvent interactions may be taken into account (Kasimova et al., 2012, Persson et al., 2013, Rane et al., 2008).
The Hildebrand solubility parameter (δ) is a numerical value that indicates the relative solvency behavior of a specific solvent and is represented by Eq. (1) (Martin et al., 1980). The parameter expresses the square root of the cohesive energy density (CED) of the components holding the substances together. It is derived from the CED of the solvent, which in turn is derived from the heat of vaporization (Martin et al., 1980, Shah and Agrawal, 2013).
| (1) |
where ΔEv is the molar energy of vaporization and Vm is the molar volume of the solvent.
The Hildebrand approach works well for low molecular weight non polar solvents but fails to adequately describe the solubility behavior when polar and hydrogen bonding solvents are introduced. Consequently, Steven Abbott (2015) developed an approach to solubility parameters that takes into account the two latter mentioned forces. Hansen solubility parameters (HSP) have been applied to select API carriers using a combination of the theoretical solubility parameters and experimentally determined partition coefficients (Hossin et al., 2016).
The HSP divides the total solubility parameter (δT) into individual parts arising from dispersion forces (δD), permanent dipole–permanent dipole forces (δP), and hydrogen bonding (δH) (Steven Abbott, 2015) and can be estimated using Equation (2).
| (2) |
The use of theoretical solubility parameter prediction based on the molecular structure of compounds provides an early, rapid screening approach for the selection of lipid candidates without the need for lengthy experimental procedures to generate data (Hansen and Smith, 2004, Stefanis and Panayiotou, 2008, Steven Abbott, 2015). According to the HSP, the best miscibility of an API and an excipient is predictable when intermolecular forces., viz dispersion, polar, and hydrogen bonding forces between the molecules of the solute and solvent are of similar strength (Medarević et al., 2019, Shah and Agrawal, 2013, Steven Abbott, 2015). The difference in the solubility parameters between an API and an excipient can be used to estimate their compatibility and thus miscibility (Long et al., 2006). The HSP has therefore been used to describe numerous physical properties of materials in addition to predicting the miscibility and compatibility of API and excipients (Forster et al., 2001, Long et al., 2006, Rowe, 1988).
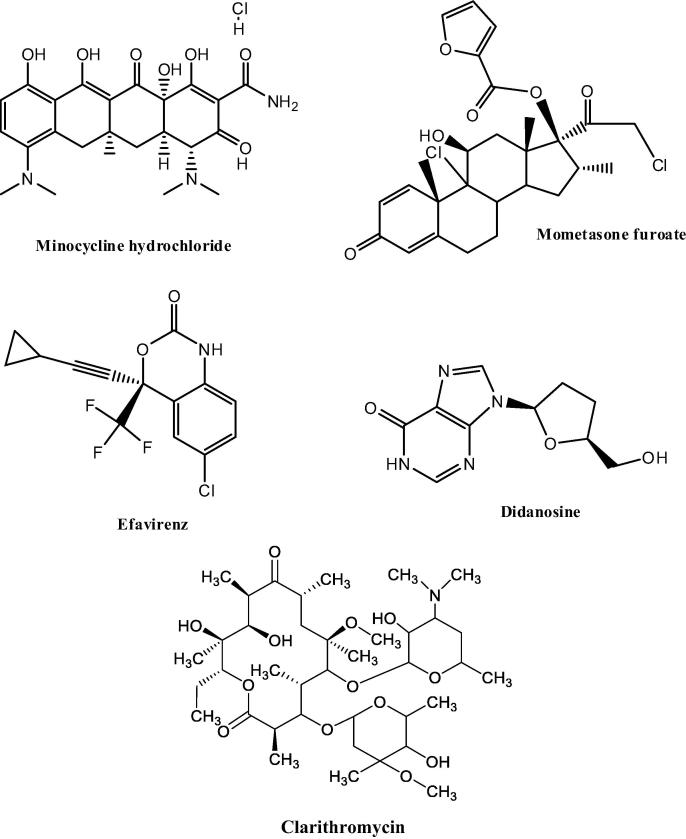
This study reports the validity of the HSP approach in screening lipid excipients for use in SLN and NLC production. Apparent solubility studies were performed with five (5) API with different physicochemical properties and the data generated by different researchers in our research group was used to evaluate and augment theoretical calculations based on the HSP. Minocycline hydrochloride (MNH), mometasone furoate (MF), efavirenz (EFV), didanosine (DDN) and clarithromycin (CLA) were evaluated to establish their miscibility and compatibility with different lipids in an attempt to identify and select the lipids with the best solubilization potential for the different API. The selection of lipids used in the studies was based on the similarity of application and availability. EFV, MF and CLA have low aqueous solubility and high intestinal permeability and are classified as a Biopharmaceutical Classification System (BCS) Class II compounds (Kristin et al., 2017, Madgulkar et al., 2019, Taneja et al., 2016). MNH and DDN exhibit high aqueous solubility and low intestinal permeability and are classified as BCS Class III compounds (Papich and Martinez, 2015, Pretorius and Bouic, 2009). The molecular structure of each API are depicted in Fig. 1.
Fig. 1.
Chemical structures of MNH, MF, EFV, DDN and CLA.
2. Materials and methods
2.1. Materials
MNH and DDN were donated by Aspen Pharmacare (Port Elizabeth, Eastern Cape, South Africa). MF was purchased from Symbiotec Pharmalab Limited (Rau, Indore, India) and EFV was donated by Adcock Ingram® Limited (Johannesburg, Gauteng, South Africa). CLA was purchased from Skyrun Industrial Co. Limited (Taizhou, China). Gelucire® 48/16 (polyethylene glycol monostearate), Compritol® 888 (glyceryl behenate), Precirol® ATO 5 (glyceryl distearate), Geleol™ (glyceryl monostearate) and cetyl palmitate were donated by Gattefossé SAS (Gattefossé SAS, Saint-Priest Cedex, France). Stearic acid was purchased from Sigma Aldrich Chemical Co. (Milwaukee, WI, USA). Transcutol® HP (diethylene glycol monoethyl ether), Labrafac® PG (propylene glycol dicaprylate), Lauroglycol® FCC (propylene glycol monolaurate) and Capryol™ 90 (propylene glycol caprylate) were donated by Gattefossé SAS (Gattefossé SAS, Saint-Priest Cedex, France). MeOH and ACN (Romil®) was purchased from Microsep® (Port Elizabeth, Eastern Cape, South Africa). Potassium dihydrogen phosphate and sodium hydroxide pellets were purchased from Merck® Chemicals (Midrand, Gauteng, South Africa). HPLC-grade water was prepared using a Milli-RO® 15 water purification system (Millipore Co., Bedford, MA, USA) that consisted of a Super-C® carbon cartridge, two Ion-X® ion exchange cartridges and an Organex-Q® cartridge. The water was filtered through a 0.22 μm Millipak® 40 stack filter (Millipore Co., Bedford, MA, USA) prior to use. HPLC-water was also prepared using a Milli Q Plus (Millipore Co, Schwalbach, Germany). All reagents and solvents were of analytical grade and used without further purification.
2.2. Solubility studies
2.2.1. Selection of solid lipids
The solubility of API in different solid lipids was determined by either dissolving increasing amounts of individual API in a fixed amount of molten lipid or dissolving fixed amounts of API by addition of increasing amounts of solid lipid whilst heating and shaking the mixtures. Evaluation of the melt was performed visually (Joshi and Patravale, 2006, Kasongo et al., 2011). Required amounts of API and/or solid lipid were accurately weighed using a Model PA 2102 Ohaus® top-loading analytical balance (Ohaus® Corp. Pine Brook, NJ USA) and transferred into individual test tubes (Pyrex® Laboratory Glassware, England). The samples heated at 85 °C for an hour using a LABOTEC® shaking water bath (Laboratory Thermal Equipment, Greenfield NR. Oldham) set at 100 rpm.
An excess amount of API (MNH and EFV) was added to molten solid lipid individually and the melt evaluated visually. Following solution, additional aliquots of API were added until saturation was observed and no additional API dissolved in the molten lipid after shaking for 24 h at 85 °C. In order to study the solubility of DDN, MF and CLA in solid lipids, 50 mg of the individual API was weighed and placed into a test tube and solid lipid was added in 1.0 g aliquots after which the test tube was exposed to a temperature of 85 °C at 100 rpm using a LABOTEC® shaking water bath (Laboratory Thermal Equipment, Greenfield NR. Oldham). The amount of lipid required to solubilize the API in the molten state was estimated at the point that no further solid API could be solubilized by the molten lipid after shaking at 100 rpm at 85 °C for 24 h.
2.2.2. Selection of liquid lipids
The solubility of EFV, CLA and MF in different liquid lipids was determined by dissolving increasing amounts of the API in a fixed amount of molten lipid and evaluation of the melt visually as described in Section 2.2.1.
The saturation solubility of DDN in different liquid lipids was determined after shaking a liquid lipid containing an excess of DDN at 200 rpm for 24 h at 85 °C using a Model 4230 Innova refrigerated incubator shaker (New Brunswick Scientific). The oil–DDI mixtures were centrifuged using a Model 22 R Heraeus Biofuge centrifuge (Thermo Electron LED GmbH, Langenselbold, Germany) at 17 000 rpm for 30 min in order to separate DDI from the oil. The supernatant was filtered through a 0.45 µm hydrophilic Sartorius® membrane filter (Sartorius AG, Goettingen, Germany). The filtrate was diluted with MeOH and analyzed using a validated reversed-phase (RP)-HPLC method (Kasongo et al., 2011). The saturation solubility of MNH was determined by dispersing 10 mg MNH in 2.0 g of molten lipid to which 2 mL of hot distilled water was added. The mixture was shaken for 30 min at 85 °C using a LABOTEC® shaking water bath (Laboratory Thermal Equipment, Greenfield NR. Oldham) set at a speed of 100 rpm. The oil-MNH mixture was separated by centrifugation using a Model HN-SII IEC centrifuge (Damon, Needham HTS, MA, USA) at 1500 rpm for 10 min prior to analysis using a validated HPLC method (Ranchhod, 2017).
2.3. Solubility parameter calculations
The Hansen solubility parameter of the API and different solid and liquid lipids were calculated using the chemical structure and applying Hiroshi Yamamoto’s molecular breaking method (Y-MB) using version 5.2.02 Hansen Solubility Parameters in Practice (HSPiP) software (Hansen Solubility, Hørsholm, Denmark). The chemical structures of the API and lipids were transformed by ChemDraw Ultra version 10.0 (CambridgeSoft corporation, Cambridge, MA, USA) to their simplified molecular input line entry syntax (SMILES) notation which was then used to calculate the solubility parameters in situ using Equation (2). The units of these solubility parameters are reported as (Joules/cm3)½ or, equivalently, MPa½ (Steven Abbott, 2015).
3. Results and discussion
3.1. Selection of lipid excipients
The results of the solubility studies of the API in different solid lipids are depicted in Table 1, Table 2.
Table 1.
Solubility of EFV and MNH in solid lipid excipients.
| EFV |
MNH |
||
|---|---|---|---|
| Lipid (1.0 g) | Solubility (g) | Lipid (2.0 g) | Solubility (g) |
| Compritol® 888 ATO | 0.25 | Compritol® 888 ATO | 0.0125 |
| Precirol® ATO 5 | 0.30 | Precirol® ATO 5 | 0.0075 |
| Gelucire® 48/16 | 0.40 | Gelucire® 48/16 | 0.0175 |
| Cetyl palmitate | 0.05 | Cetyl palmitate | <0.0025 |
| Stearic acid | 0.10 | Stearic acid | – |
| Geleol™ | 5.50 | Geleol™ | 0.0300 |
-Solubility studies not performed.
Table 2.
Solubility of MF, DDN and CLA in solid lipid excipients.
| MF (0.005 g) |
DDN (0.01 g) |
CLA (0.01 g) |
|||
|---|---|---|---|---|---|
| Lipid | Amount(g) | Lipid | Amount(g) | Lipid | Amount(g) |
| Compritol® 888 ATO | – | Compritol® 888 ATO | 3.0 | Compritol® 888 ATO | – |
| Precirol® ATO 5 | – | Precirol® ATO 5 | 4.0 | Precirol® ATO 5 | – |
| Gelucire® 48/16 | 7.0 | Gelucire® 48/16 | – | Gelucire® 48/16 | – |
| Cetyl palmitate | – | Cetyl palmitate | – | Cetyl palmitate | – |
| Stearic acid | – | Stearic acid | – | Stearic acid | 3.0 |
| Geleol™ | 6.0 | Geleol™ | – | Geleol™ | – |
-Complete solubilization of API not achieved as lipid(s) with best solubilization potential for the API had been identified.
The results of the solubility studies of the API in liquid lipids are depicted in Table 3, Table 4.
Table 3.
Solubility of EFV, MF and CLA in liquid lipid excipients.
| EFV |
MF |
CLA |
|||
|---|---|---|---|---|---|
| Liquid Lipid | Amount (g) | Solid Lipid | Amount (g) | Solid Lipid | Amount (g) |
| Labrafac® PG | 1.50 | Labrafac® PG | <0.05 | Labrafac® PG | <0.10 |
| Transcutol® HP | 4.50 | Transcutol® HP | 0.10 | Transcutol® HP | 0.20 |
| Capryol™ 90 | 2.10 | Capryol™ 90 | <0.05 | Capryol™ 90 | <0.10 |
| Lauroglycol® FCC | 1.50 | Lauroglycol® FCC | <0.05 | Lauroglycol ® FCC |
<0.10 |
Table 4.
Solubility of MNH and DDN in liquid lipid excipients.
| MNH |
DDN |
||
|---|---|---|---|
| Liquid Lipid | Solubility (g) | Liquid Lipid | Solubility (g) |
| Labrafac® PG | 0.0117 ± 0.001 | Labrafac® PG | 0.014 ± 0.00035 |
| Transcutol® HP | 0.3624 ± 0.017 | Transcutol® HP | 0.267 ± 0.0160 |
| Capryol™ 90 | 0.0097 ± 0.003 | Capryol™ 90 | 0.079 ± 0.00038 |
| Lauroglycol® FCC | 0.0043 ± 0.001 | Lauroglycol® FCC | 0.022 ± 0.00029 |
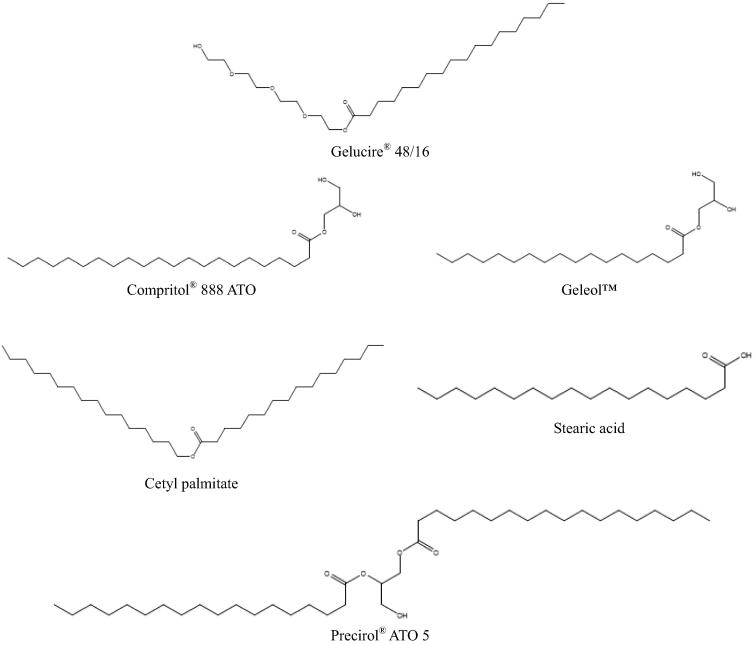
The data listed in Table 1, Table 2 reveal that Geleol™ was the solid lipid with the best solubilizing potential for EFV, MNH and MF. Geleol™ has the highest δP (4.2 MPa1/2) value of the solid lipids investigated (Table 5) due to the presence of two hydroxyl groups of the glycerin and the shortest alkyl chain of solid lipids investigated (Fig. 2). The presence of oxygen and hydroxyl functional groups increases the polarity and hydrogen bonding possibilities of the compound thus possibly contributing to the increased solubility of EFV, MNH and MF in Geleol™. In addition, the presence of mono- and diglycerides in lipid matrices has been shown to promote solubilization of API (Müller et al., 2000). DDN showed greatest solubility in Compritol® 888 while CLA showed greatest solubility in stearic acid. Compritol® 888 and stearic acid also possess oxygen and hydroxyl functional groups thus aiding molecular interactions leading to API solubilization in these lipids. However, there is no clear link between the physicochemical properties of the each API, the BCS classification and their ability to be solubilized in lipid excipients tested.
Table 5.
Solubility parameters of solid and liquid lipids.
| Solid Lipid | δD | δP | δH | δT |
|---|---|---|---|---|
| Compritol® 888 ATO | 16.5 | 1 | 1.2 | 16.6 |
| Precirol® ATO 5 | 16.2 | 2.4 | 7.6 | 18 |
| Gelucire® 48/16 | 15.9 | 4 | 8.3 | 18.4 |
| Cetyl palmitate | 16 | 1.4 | 1.8 | 16.1 |
| Geleol™ | 16.2 | 4.2 | 10.3 | 19.7 |
| Stearic acid | 16.2 | 2.8 | 5.2 | 17.2 |
| Liquid Lipid | δD | δP | δH | δT |
| Lauroglycol ®FCC | 16.3 | 4.2 | 8.7 | 18.9 |
| Labrafac® PG | 16.2 | 3.2 | 4.2 | 17.1 |
| Transcutol® HP | 16.3 | 7.4 | 12 | 21.6 |
| Capryol™ 90 | 16.4 | 5.1 | 8.7 | 19.3 |
Fig. 2.
Chemical structures of solid lipids tested.
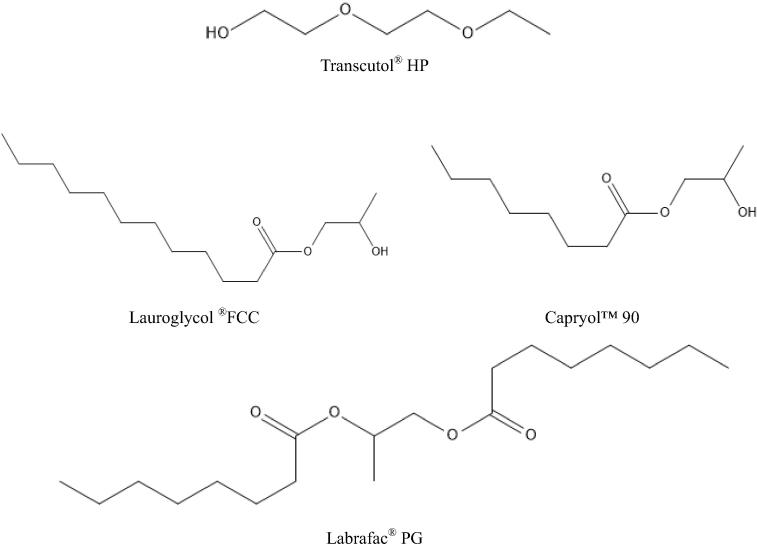
The data reported in Table 3, Table 4 reveal that all API were highly soluble in Transcutol® HP, which is a combination of diethylene glycol monoethyl ethers (Gattefosse, n.d.). Transcutol® HP has the highest δP and δH values (Table 5) due to the presence of two ester and a hydroxyl functional group in addition to possessing the shortest alkyl chain of all liquid lipids examined (Fig. 3).
Fig. 3.
Chemical structures of liquid lipids.
3.2. Solubility parameter calculations
The results of the solubility parameter estimation for the API and lipids tested and exhibiting the best solubilizing potential using HSPiP software are listed in Table 6. Although the difference between solubility parameters of API and polymer should be small, if components are miscible, it is difficult to establish a threshold for the ΔδT value below which components are considered to be miscible. Greenhalgh et al. (1999) proposed limits for ΔδT which indicate components are likely be miscible if ΔδT < 7.0 MPa1/2, while ΔδT > 10.0 MPa1/2, suggests the likelihood of components being immiscible. However Forster et al. (2001) suggested more stringent limits which predict materialization of a solid solution if ΔδT < 2.0 MPa1/2, while immiscibility is anticipated for systems with ΔδT > 10.0 MPa1/2. API-polymer systems with a ΔδT between 5.0 and 10.0 MPa1/2 are likely to ensure an unreliable conclusion as to whether the system would be miscible or immiscible. Our studies confirm alignment of API-lipid miscibility studies of systems having ΔδT < 4.0 MPa1/2 (Group 1) as summarized in Table 6. For API-lipid systems with a ΔδT > 4.0 MPa1/2 (Group 2), HSPiP was unable to predict the lipid with the best solubilization potential for the API and results for these are based solely on experimental studies. This conclusion is based on a ΔδT of 4.9 MPa1/2 for the DDN-Geleol™ system prediction which failed to align with experimental findings that suggested Compritol® 888 ATO was the solid lipid with best solubilization potential for DDN with a ΔδT of 8.0 MPa1/2 for this API-lipid system. In addition, Y-MB fails to provide HSP values for molecules with more than 120 atoms, other than H atoms, such as CLA. Consequently, the recommendation is to split the molecule (into two parts) while finding an appropriate functionality at the splitting point. The Y-MB of each structure is then calculated and a conclusion as to how the combined molecule is likely to behave then elucidated (Steven Abbott, 2015). This procedure resulted in inconsistent findings between HSP and experimental procedures for the solubility predictions for CLA in the lipids investigated thus preventing grouping of the system.
Table 6.
Solubility parameters of API and lipidic excipients.
| API- Lipid System | δD (MPa½) | δP (MPa½) | δH (MPa½) | δT (MPa½) | ΔδT (MPa1/2) | Group* |
|---|---|---|---|---|---|---|
| EFV | 18.4 | 8.9 | 5.6 | 21.1 | ||
| Geleol™ | 16.2 | 4.2 | 10.3 | 19.7 | 1.4 | 1 |
| Transcutol® HP | 16.3 | 7.4 | 12 | 21.6 | 0.5 | 1 |
| MN** | 20.1 | 15.1 | 13.6 | 28.6 | ||
| HCL | 20 | 0.1 | 19.8 | 28.1 | ||
| MNH Average | 28.35 | |||||
| Geleol™ | 16.2 | 4.2 | 10.3 | 19.7 | 8.65 | 2 |
| Transcutol® HP | 16.3 | 7.4 | 12 | 21.6 | 6.75 | 2 |
| MF | 19.6 | 9.4 | 3.6 | 22.1 | ||
| Geleol™ | 16.2 | 4.2 | 10.3 | 19.7 | 2.4 | 1 |
| Transcutol® HP | 16.3 | 7.4 | 12 | 21.6 | 0.5 | 1 |
| DDN | 19 | 12.4 | 9.4 | 24.6 | ||
| Compritol® 888 ATO | 16.5 | 1 | 1.2 | 16.6 | 8 | 2 |
| Transcutol® HP | 16.3 | 7.4 | 12 | 21.6 | 3 | 1 |
| CLA | 17.7 | 4.1 | 4.4 | 18.6 | ||
| 16.2 | 4.5 | 7.6 | 18.5 | |||
| CLA Average | 18.55 | |||||
| Stearic acid | 16.2 | 2.8 | 5.2 | 17.2 | 1.35 | Ng*** |
| Transcutol® HP | 16.3 | 7.4 | 12 | 21.6 | 3.05 | Ng*** |
Group 1 lipids with best solubilization potential based on ΔδT. Group 2 lipids likely to be miscible with API and require experimental confirmation.
MN = Minocycline.
Not grouped.
4. Conclusions
Evaluation of API-lipid miscibility and solubility is integral to the rational design, formulation and manufacture of lipid nanocarrier technologies. The selection of lipids for production with a specific API can be based on differences between calculated total solubility parameters of API and lipid without having to conduct lengthy and tedious laboratory experiments. When the difference between API and lipid total solubility parameters is <4.0 MPa1/2, the best solubilization of API is likely to result from that specific lipid, when compared to other lipids. When the difference between API and lipid total solubility parameters is >4.0 MPa1/2 HSP predictions alone cannot be used to identify the lipid with the best solubilizing potential for that API. Confirmation with experimental studies is required in addition to an understanding of the physicochemical properties of the API and lipid. Furthermore the molecular weight of the API and/or lipid was found to have limitations when predicting miscibility using HSP alone, as molecules with more than 120 atoms other than H atoms require splitting prior to using Y-MB, resulting in inaccurate predictions. Further studies using a larger sample size of model API are required to determine if the proposed model of solubility parameter use for lipid screening in SLN and NLC manufacture is applicable to different BCS class API and other lipids.
Declaration of Competing Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahmad N., Ahmad R., Alam M.A., Ahmad F.J., Amir M. Impact of ultrasonication techniques on the preparation of novel Amiloride-nanoemulsion used for intranasal delivery in the treatment of epilepsy. Artif. Cells, Nanomed., Biotechnol. 2018;46:S192–S207. doi: 10.1080/21691401.2018.1489826. [DOI] [PubMed] [Google Scholar]
- Ahmad N., Alam M.A., Ahmad R., Umar S., Jalees Ahmad F. Improvement of oral efficacy of Irinotecan through biodegradable polymeric nanoparticles through in vitro and in vivo investigations. J. Microencapsul. 2018;35:327–343. doi: 10.1080/02652048.2018.1485755. [DOI] [PubMed] [Google Scholar]
- Baka E., Comer J.E.A., Takács-Novák K. Study of equilibrium solubility measurement by saturation shake-flask method using hydrochlorothiazide as model compound. J. Pharm. Biomed. Anal. 2008;46:335–341. doi: 10.1016/j.jpba.2007.10.030. [DOI] [PubMed] [Google Scholar]
- Beija M., Salvayre R., Lauth-de Viguerie N., Marty J.-D. Colloidal systems for drug delivery: from design to therapy. Trends Biotechnol. 2012;30:485–496. doi: 10.1016/j.tibtech.2012.04.008. [DOI] [PubMed] [Google Scholar]
- Box K.J., Völgyi G., Baka E., Stuart M., Takács-Novák K., Comer J.E.A. Equilibrium versus kinetic measurements of aqueous solubility, and the ability of compounds to supersaturate in solution—a validation study. J. Pharm. Sci. 2006;95:1298–1307. doi: 10.1002/jps.20613. [DOI] [PubMed] [Google Scholar]
- Cirri M., Maestrini L., Maestrelli F., Mennini N., Mura P., Ghelardini C., Di Cesare Mannelli L. Design, characterization and in vivo evaluation of nanostructured lipid carriers (NLC) as a new drug delivery system for hydrochlorothiazide oral administration in pediatric therapy. Drug Deliv. 2018;25:1910–1921. doi: 10.1080/10717544.2018.1529209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster A., Hempenstall J., Tucker I., Rades T. Selection of excipients for melt extrusion with two poorly water-soluble drugs by solubility parameter calculation and thermal analysis. Int. J. Pharm. 2001;226:147–161. doi: 10.1016/s0378-5173(01)00801-8. [DOI] [PubMed] [Google Scholar]
- Gattefosse, n.d. Gattefosse-Personal care ingredients and pharmaceutical excipients <https://www.gattefosse.com/> (accessed 20 January 2020)
- Greenhalgh D.J., Williams A.C., Timmins P., York P. Solubility parameters as predictors of miscibility in solid dispersions. J. Pharm. Sci. 1999;88:1182–1190. doi: 10.1021/js9900856. [DOI] [PubMed] [Google Scholar]
- Hansen C.M., Smith A.L. Using Hansen solubility parameters to correlate solubility of C60 fullerene in organic solvents and in polymers. Carbon N. Y. 2004;42:1591–1597. [Google Scholar]
- Hossin B., Rizi K., Murdan S. Application of Hansen Solubility Parameters to predict drug–nail interactions, which can assist the design of nail medicines. Eur. J. Pharm. Biopharm. 2016;102:32–40. doi: 10.1016/j.ejpb.2016.02.009. [DOI] [PubMed] [Google Scholar]
- Hou D., Xie C., Huang K., Zhu C. The production and characteristics of solid lipid nanoparticles (SLNs) Biomaterials. 2003;24:1781–1785. doi: 10.1016/s0142-9612(02)00578-1. [DOI] [PubMed] [Google Scholar]
- Joshi M., Patravale V. Nanostructured lipid carrier (NLC) based gel of celecoxib. Int. J. Pharm. 2008;346:124–132. doi: 10.1016/j.ijpharm.2007.05.060. [DOI] [PubMed] [Google Scholar]
- Joshi M., Patravale V. Formulation and Evaluation of Nanostructured Lipid Carrier (NLC)–based Gel of Valdecoxib. Drug Dev. Ind. Pharm. 2006;32:911–918. doi: 10.1080/03639040600814676. [DOI] [PubMed] [Google Scholar]
- Kasimova A.O., Pavan G.M., Danani A., Mondon K., Cristiani A., Scapozza L., Gurny R., Möller M. Validation of a novel molecular dynamics simulation approach for lipophilic drug incorporation into polymer micelles. J. Phys. Chem. B. 2012;116:4338–4345. doi: 10.1021/jp2104819. [DOI] [PubMed] [Google Scholar]
- Kasongo, K.W., Pardeike, J., Muller, R.H., Walker, R.B., 2011. Selection and Characterization of Suitable Lipid Excipients for use in the Manufacture of Didanosine-Loaded Solid Lipid Nanoparticles and Nanostructured Lipid Carriers. https://doi.org/10.1002/jps.22711. [DOI] [PubMed]
- Kristin F., René H., Boontida M., Buraphacheep J.V., Maximilian A., Johanna M., Peter L. Dissolution and dissolution/permeation experiments for predicting systemic exposure following oral administration of the BCS class II drug clarithromycin. Eur. J. Pharm. Sci. 2017;101:211–219. doi: 10.1016/j.ejps.2017.02.003. [DOI] [PubMed] [Google Scholar]
- Long C., Zhang L., Qian Y. Preparation and crystal modification of ibuprofen-loaded solid lipid microparticles. Chin. J. Chem. Eng. 2006;14:518–525. [Google Scholar]
- Madgulkar A.R., Padalkar R.R., Amale S.K. Preformulation studies of intranasal solid lipid nanoparticles of mometasone furoate. J. Drug Deliv. Ther. 2019;9:526–528. [Google Scholar]
- Martin A., Newburger J., Adjei A. Extended hildebrand solubility approach: solubility of theophylline in polar binary solvents. J. Pharm. Sci. 1980;69:487–491. doi: 10.1002/jps.2600690503. [DOI] [PubMed] [Google Scholar]
- Medarević D., Djuriš J., Barmpalexis P., Kachrimanis K., Ibrić S. Analytical and computational methods for the estimation of drug-polymer solubility and miscibility in solid dispersions development. Pharmaceutics. 2019;11:372. doi: 10.3390/pharmaceutics11080372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehnert W., Mader K. Solid lipid nanoparticles production, characterization and applications. Adv. Drug Deliv. Rev. 2002;47:165–196. doi: 10.1016/s0169-409x(01)00105-3. [DOI] [PubMed] [Google Scholar]
- Muchow M., Maincent P., Müller R.H. Lipid Nanoparticles with a Solid Matrix (SLN®, NLC®, LDC®) for Oral Drug Delivery. Drug Dev. Ind. Pharm. 2008;34:1394–1405. doi: 10.1080/03639040802130061. [DOI] [PubMed] [Google Scholar]
- Müller R.H., Mäder K., Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery- a review of the state of the art. Eur. J. Pharm. Biopharm. 2000;50:161–177. doi: 10.1016/s0939-6411(00)00087-4. [DOI] [PubMed] [Google Scholar]
- Müller R.H., Radtke M., Wissing S.A. Nanostructured lipid matrices for improved microencapsulation of drugs. Int. J. Pharm. 2002;242:121–128. doi: 10.1016/s0378-5173(02)00180-1. [DOI] [PubMed] [Google Scholar]
- Naseri N., Valizadeh H., Zakeri-Milani P. Solid lipid nanoparticles and nanostructured lipid carriers: structure, preparation and application. Adv. Pharm. Bull. 2015;5:305–313. doi: 10.15171/apb.2015.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papich M.G., Martinez M.N. Applying Biopharmaceutical Classification System (BCS) criteria to predict oral absorption of drugs in dogs: challenges and pitfalls. AAPS J. 2015;17:948–964. doi: 10.1208/s12248-015-9743-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parveen R., Baboota S., Ali J., Ahuja A., Vasudev S.S., Ahmad S. Oil based nanocarrier for improved oral delivery of silymarin: in vitro and in vivo studies. Int. J. Pharm. 2011;413:245–253. doi: 10.1016/j.ijpharm.2011.04.041. [DOI] [PubMed] [Google Scholar]
- Persson L.C., Porter C.J.H., Charman W.N., Bergström C.A.S. Computational prediction of drug solubility in lipid based formulation excipients. Pharm. Res. 2013;30:3225–3237. doi: 10.1007/s11095-013-1083-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pretorius E., Bouic P.J.D. Permeation of four oral drugs through human intestinal mucosa. AAPS PharmSciTech. 2009;10:270–275. doi: 10.1208/s12249-009-9207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranchhod J. Rhodes University; 2017. Formulation, Development and Evaluation of Lipid Nanocarriers for Minocycline Hydrochloride. [Google Scholar]
- Rane S.S., Cao Y., Anderson B.D. Quantitative solubility relationships and the effect of water uptake in triglyceride/monoglyceride microemulsions. Pharm. Res. 2008;25:1158–1174. doi: 10.1007/s11095-007-9500-4. [DOI] [PubMed] [Google Scholar]
- Riehemann K., Schneider S.W., Luger T.A., Godin B., Ferrari M., Fuchs H. Nanomedicine-challenge and perspectives. Angew. Chem. Int. Ed. 2009;48:872–897. doi: 10.1002/anie.200802585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe R.C. Adhesion of film coatings to tablet surfaces —a theoretical approach based on solubility parameters. Int. J. Pharm. 1988;41:219–222. [Google Scholar]
- Shah M., Agrawal Y. High throughput screening: an in silico solubility parameter approach for lipids and solvents in SLN preparations. Pharm. Dev. Technol. 2013;18:582–590. doi: 10.3109/10837450.2011.635150. [DOI] [PubMed] [Google Scholar]
- Son G.-H., Na Y.-G., Huh H.W., Wang M., Kim M.-K., Han M.-G., Byeon J.-J., Lee H.-K., Cho C.-W. Systemic design and evaluation of ticagrelor-loaded nanostructured lipid carriers for enhancing bioavailability and antiplatelet activity. Pharmaceutics. 2019;11:222. doi: 10.3390/pharmaceutics11050222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souto E.B., Müller R. Lipid nanoparticles (SLN and NLC) for drug delivery. In: Domb A., Tabata M., Kumar R., Farber S., editors. Nanoparticles for Pharmaceutical Applications. American Scientific Publishers; 2007. pp. 103–122. [Google Scholar]
- Souto E.B., Muller R.H. Solid lipid nanoparticles and nanostructured lipid carriers-lipid nanoparticles for medicals and pharmaceuticals. Encycl. Nanosci. Nanotechnol. 2011;23:313–328. [Google Scholar]
- Stefanis E., Panayiotou C. Prediction of Hansen solubility parameters with a new group-contribution method. Int. J. Thermophys. 2008;29:568–585. [Google Scholar]
- Steven Abbott, C.M.H. and H.Y., 2015. Hansen Solubility Parameters in Practice, 5th ed, Hansen-Solubility.com.
- Taneja S., Shilpi S., Khatri K. Formulation and optimization of efavirenz nanosuspensions using the precipitation-ultrasonication technique for solubility enhancement. Artif. Cells, Nanomedicine Biotechnol. 2016;44:978–984. doi: 10.3109/21691401.2015.1008505. [DOI] [PubMed] [Google Scholar]
- Thi T., Speybroeck M., Barillaro V., Martens J., Annaert P., Augustijns P., Humbeck J., Vermant J., Mooter Gv. Formulate-ability of ten compounds with different physicochemical profiles in SMEDDS. Eur. J. Pharm. Sci. 2009;38:479–488. doi: 10.1016/j.ejps.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Uner M. Preparation, characterization and physico-chemical properties of solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC): their benefits as colloidal drug carrier systems. Pharmazie. 2006;61:375–386. [PubMed] [Google Scholar]
- Wissing S.A., Kayser O., Müller R.H. Solid lipid nanoparticles for parenteral drug delivery. Adv. Drug Deliv. Rev. 2004;56:1257–1272. doi: 10.1016/j.addr.2003.12.002. [DOI] [PubMed] [Google Scholar]