Abstract
Otostegia fruticosa, a plant belonging to the family Lamiaceae, is endemic to Ethiopia. In Ethiopian traditional medicine, O. fruticosa has been used for the treatment of several respiratory-related disorders. The present study was designed to evaluate the bronchodilatory and antimicrobial activities of O. fruticosa leaves crude extract (Of.Cr). Ex-vivo experiments were conducted on guinea-pig trachea provided with physiological oxygenated buffer solution using emkaBath setup. The crude extract was analyzed by gas chromatography-mass spectrometry. Of.Cr, showed the presence of terpenes, fragrance components, saponins, and higher fatty acids. Of.Cr when tested on contracted tracheal chains with carbamylcholine (CCh, 1 µM) and high K+ (80 mM) produced relaxation by showing higher potency against CCh with incomplete inhibition of high K+. Dicyclomine, used as a positive control, also showed selectively higher potency to inhibit CCh when compared with its effect against K+. In the anticholinergic curves, Of.Cr at 1 mg/mL deflected CCh-induced concentration–response curves (CRCs) competitively to the right like dicyclomine (0.03 µM) and atropine whereas a higher dose of Of.Cr (3 mg/mL) produced a non-parallel shift in the CCh curves like a higher dose of dicyclomine (0.1 µM). In the calcium channel inhibitory assay, Of.Cr at 3 & 5 mg/mL, deflected CRCs of Ca++ to the right like verapamil, used as positive control. Of.Cr, at concentrations (1–3 mg/mL) increases cAMP levels in isolated tracheal homogenates, similar to positive control phosphodiesterase inhibitor (papaverine). When tested for antibacterial activity against standard and clinical strains, Of.Cr was found more active (MIC 475 µg/ml) against S. aureus (NCTC 6571), while the maximum inhibition (MIC 625 µg/ml) was observed by the extract when tested against MRSA. These results determine the mechanistic pathways of the observed bronchodilatory effect of Otostegia fruticosa with a combination of anticholinergic and dual inhibition of phosphodiesterase and voltage-gated Ca++ channels.
Keywords: Antimicrobial, Antimuscarinic, Asthma, Bronchodilatation, Ca++ channel blocker, Otostegia fruticosa, Phosphodiesterase inhibitor
1. Introduction
Bacteria cause respiratory tract infections such as laryngitis, pharyngitis/tonsillitis, acute rhinosinusitis, acute otitis media, bronchitis, pneumonia, and tracheitis. The infections caused by antibiotic-resistant germs such as methicillin-resistant S. aureus (MRSA) are difficult to treat. It has been reported that medicinal plants exert an antimicrobial effect (Costa et al., 2015). One of the most promising approaches for combating multidrug-resistant bacteria is the combination between antibiotics and natural antimicrobial substances such as plant extracts (Cheesman et al., 2017).
Otostegia fruticosa (Forssk.) Schweinf. ex Penzig is a shrub widely distributed in West and Eastern Africa, Ethiopia, and Middle Eastern countries including Saudi Arabia. In Northern Ethiopia, it is known as “sasa” in Tigrigna (Kidane et al., 2013), “Tinjut” (D’avigdor et al., 2014) or “geram tungut” (Getaneh and Girma, 2014) in Amharic, “Fesi hadima” in Eritrea (Andemariam, 2010) and Sharm in Arabic (Adgaba et al., 2017). The leaves of Otostegia fruticosa are taken orally for the treatment of asthma, tonsillitis and febrile illness (Enyew et al., 2013, Getaneh and Girma, 2014). In Fiche, Ethiopia, it is used for unexplained stomach ache (“megagna”) by inhaling the smoke of burned leaves, as an insecticide, disinfectant, fumigant, and the branch is used for cleaning teeth (D’avigdor et al., 2014). In Kolla Temben District, Tigray, Northern Ethiopia the leaf part is claimed for its mosquito repellent activity (Kidane et al., 2013). In Eretria, the leaf and stem part of this plant is used for arthritis, tonsillitis and gynecological problems (Andemariam, 2010). In Yemen, it is reported as antiparalytic and for treatment of eye diseases (Mothana et al., 2011). Furthermore, In Saudi Arabia infusion of the flowering branches of this plant is used as a remedy for sun-stroke (Rahman et al., 2004).
Al-Musayeib and his coworkers have isolated labdane diterpenes; otostegin A, otostegin B and 15-epi-otostegin B from the aerial parts of Otostegia fruticosa (Al-Musayeib et al., 2000). The anti-bacterial activity of Otostegia fruticosa has been pharmacologically documented (Aboutabl et al., 1995). Although there is an ethnobotanical claim of this plant in asthma and respiratory distress, no scientific evidence can be found on the leaf of the plant concerning bronchodilatory activity. Therefore, the current study was designed to validate the traditional claim and to further explore the possible mechanism(s) of the observed bronchodilatory effect of the leaf extract of Otostegia fruticosa.
2. Material and methods
2.1. Plant material and extraction
The leaves of Otostegia fruticosa were collected in January 2017 from Wukro Kilteawlaelo 42 Km east of Mekelle, Northern Ethiopia. The collected plant specimen was identified and authenticated by Mr. Shamble Alemu and a voucher specimen of the plant (0 0 1) was deposited at the National Herbarium of College of Natural and Computational Science, Addis Ababa University. The leaves were air-dried under the shade and then ground into a coarse powder using mortar and pestle. The powdered plant material (800 g) was soaked with 6.4L (in a ratio of 1:8, wt./vl) of 70% ethanol; mixed and kept on occasional shaking by orbital shaker at 130 rotations per minute (rpm). After 72 h, the extract was filtered by ordinary cloth and Whitman filter paper No 1. The residue was re-macerated twice to exhaustively extract the plant material. The filtrates were combined and the solvent was removed from the hydro-alcoholic extract using drying oven at 40 °C. Finally, the dried extract was stored in a closed container at −4 °C.
2.2. Reagents and laboratory animals
Salts of atropine, carbamylcholine, papaverine, dicyclomine, isoprenaline, and verapamil were procured from Sigma Chemicals Company, St. Louis, MO, USA. Different salts to prepare physiological buffer (Kreb’s solution) were: potassium chloride (Sigma Co), calcium chloride, ethylenediamine tetra-acetic acid (EDTA), glucose, magnesium sulfate, potassium dihydrogen phosphate, sodium bicarbonate and sodium chloride (E.Merck, Darmstadt, Germany). cAMP enzyme immunoassay kit (Sigma-Aldrich Co., USA). Guinea-pigs (510–560 g) of both genders were kept at the Animal Care Unit, College of Pharmacy, PSAU, KSA, maintained at 23–25 °C. Animals were given tap water ad libitum commercial standard pellet diet. Experiments were performed in compliance with the rulings of the Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council (NRC, 1996).
2.3. GC–MS analysis
The hydro-alcoholic extract of O. fruticosa was analyzed by a single quadrupole mass detector attached with gas chromatograph (Clarus 600). The sample injection volume was 1 µL and the capillary column used was Elite 5-MS (30 m × 250 µm I.D, 0.25 µm film thickness) in the splitless mode of 1:20. The starting temperature of the column oven was 40 °C stable at 2 min and the increase in temperature to 150 °C at the rate of 10 °C per min and then again stabilize for 2 min and in another ramp the temperature increases to 300 °C and hold for 2 min and the total run time completed was 32 min. The injector temperature was at 280 °C and inlet line temperature at 220 °C and the source temperature was 220 °C. Helium was used as a carrier gas at a flow rate of 1.0 ml per min. The electron ionizing mode was used at 70 eV at a multiplier voltage of 380 and the range was from 40 to 600 m/z.
The phytoconstituents were identified based on their retention time and their masses were confirmed by matching with a mass spectral library of NIST (National Institute of Standards and Technology) library and Wiley library. As a result, a total of 20 compounds were identified.
2.4. Guinea-pig tracheal tissues
Guinea-pig was procured and sacrificed following neck dislocation. Their rib cage was opened and tracheal tube excised and immediately stored in Kreb’s solution bubbled with carbogen (pH 7.4). The final concentration (mM) of the Kreb’s solution was: NaCl 118.2, NaHCO3 25.0, CaCl2 2.5, KCl 4.7, KH2PO4 1.3, MgSO4 1.2 and glucose 11.7. After cleaning from additional tissues, the tracheal was cut in a way that its cartilage is opened opposite to smooth muscle in 2–3 mm length. The cartilage was tied from both the cartilaginous sides with a cotton thread to mount it in the hook of an isolated organ bath. A forced tension equal to 1 g was applied to the mounted tracheal tissue and maintained constant throughout the assay. The mounted tissues were stabilized for approximately 1 h with changing the Kreb’s solution after every 15 min. After 1 h incubation, the tissues were exposed repeatedly to 1 µM CCh and 80 mM of K+ final bath concentration till the same level of contractions were achieved. The Of.Cr from low to high final bath concentrations was tested for possible tracheal relaxation against the spasmogens such as CCh and K+.
2.4.1. Anticholinergic activity
To study the possible interaction of Of.Cr with the muscarinic type of cholinergic receptors, cumulative concentration-response curves (CRCs) of CCh were induced in the absence and presence of the pre-incubated (1 h) tracheal tissues with increasing bolus doses of Of.Cr (Shah and Gilani, 2010).
2.4.2. Voltage-dependent Ca++ ion inhibitory assay
After the pilot CCB assay results obtained from the inhibitory effects of Of.Cr high K+-mediated contractions, further conformational detailed experiments were conducted on the tracheal tissues with the replacement of the normal Kreb’s with Ca++-free Kreb’s solution with added EDTA (0.1 mM) for the chelation of Ca++ from the tissues for half-hour. Afterward, K+-rich without Ca++ Kreb’s solution filled in the organ bath to further release and then chelate the endoplasmic reticulum stored Ca++. The concentration of K+-rich and Ca++ free Kreb’s solution (mM) was: KCl 50, NaCl 50.58, MgSO4 3.10., NaHCO3 23.8, KH2PO4 1.26, glucose 11.1 and EDTA 0.1. After an hour of incubation in the K+-rich and Ca++ free Kreb’s solution (containing EDTA) where tracheal tissues were assumed completely Ca++ free, the solution was replaced by K+-rich and Ca++ free Kreb’s without EDTA and the CRCs of Ca++ were induced by cumulative addition of exogenous Ca++ into organ bath in the absence and presence of pre-incubation (1 h) of the increasing doses of Of.Cr.
2.4.3. cAMP estimation
Freshly isolated trachea from guinea-pig was snap-frozen in liquid nitrogen and ground to fine powder followed by homogenization in 10 volumes of 0.1 M HCl under liquid nitrogen. The homogenate was centrifuged at 600g for 10 min at room temperature. The sample was further diluted with 0.1 M HCl and stored at − 80 °C until the time of assay. The influence of Of.Cr on cAMP content in the guinea-pig trachea was measured by direct cAMP enzyme immunoassay kit as per manufacturer instruction (Sigma-Aldrich, Saint Lewis, USA). The cAMP content was expressed as picomole per mL.
2.5. Antimicrobial activity
2.5.1. Bacterial strains
The antibacterial assays of Of.Cr were carried out against reference strains from American Type Culture Collection (ATCC) and National Collection of Type Culture (NCTC) available in the Microbiology Laboratory of College of Pharmacy, Prince Sattam Bin Abdulaziz University (Al-Kharj-Arabia Saudi). The strains used for the test were Klebsiella pneumoniae (NCTC 9633), Pseudomonas aeruginosa (ATCC 10145), and Staphylococcus aureus (NCTC 6571). The Of.Cr was also tested against clinical pathogens (K. pneumoniae and methicillin-resistant Staphylococcus aureus (MRSA) which were isolated and identified by Dr. Khalil in cooperation with King Khalid Hospital (research study IRB: 18-477E). The teste strains were routinely cultivated in MacConkey agar for gram-negative bacteria and Mannitol Salt Agar for S. aureus
2.5.2. Determination of minimum inhibitory concentration (MIC)
MIC was determined by the broth dilution method described by Clinical and Laboratory Standards Institute guidelines (Patel et al., 2015). Eight dilutions were made ranging from 400 to 750 µg/mL and a volume (10 µL) of target bacteria was inoculated to each concentration. Mueller-Hinton broth (MHB, Scharlau) alone was tested as sterility control, and untreated bacteria inoculated on MHB alone and with 1% DMSO were labeled as growth control. Minimal Bacterial Concentration (MBC) was determined by sub culturing 10 µL from the broth dilution in Mueller-Hinton agar (MHA, Scharlau) that kills 100% of microbes after 24 h incubation. All assays were carried out in duplicate to confirm the results.
2.6. Statistical analysis
Data expressed are mean ± standard error of the mean (SEM, n = number of the experiment) and the median effective concentrations (EC50) with 95% confidence intervals (CI). Student’s t-test was applied to compare the curves and results with p < 0.05 were considered statistically significant. CRCs were analyzed by non-linear regression using the Graph Pad program (Graph Pad, San Diego, CA, USA).
3. Results
3.1. Hydro-alcoholic extract yield (%)
The leaves of O. fruticosa (Fig. 1) yielded 17.5% (v/w) of crude extract (Of.Cr).
Fig. 1.
Picture of Otostegia fruticosa plant leaves (Available on http://botany.cz/cs/otostegia-fruticosa).
3.2. GC–MS analysis
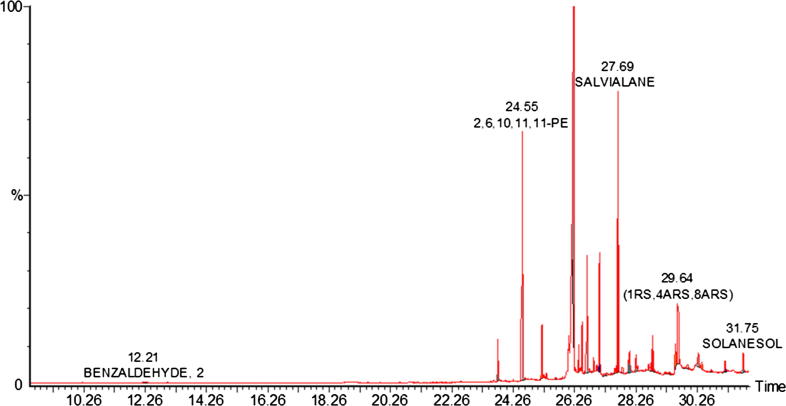
Of.Cr was found to contain terpenes, terpene alcohols, aliphatic hydrocarbons, higher fatty acids, and sterols. Santalene is a sesquiterpene and dimethyl bornane is a derivative of camphor moiety. Salvialene is an octa-hydro azulene derivative having potent biological activity. Lavandulyl acetate is a terpene present in 52% of the extract and having potent skin applications. Solanesol is a derivative of non-cyclic terpene alcohol having isoprene units. (Table 1 and Fig. 2).
Table 1.
GC–MS analysis of hydro-alcoholic extract of Otostegia fruticosa (Of.Cr).
| # | Name | RT | Area % | Area |
|---|---|---|---|---|
| 1 | 2-METHYL-BENZALDEHYDE | 12.22 | 0.260 | 150,480 |
| 2 | 3,7,11,15-TETRAMETHYL-2-HEXADECEN-1-OL | 23.76 | 2.840 | 1,632,143 |
| 3 | E AND Z ISOMERS OF 1-(2,6,6-TRIMETHYL-1-CYCLOHEXEN-1-YL)-3,4,4-TRIMETHYL-2-PENT-ENE | 24.55 | 6.680 | 3,839,953 |
| 4 | SANTALANE | 25.19 | 2.740 | 1,574,217 |
| 5 | (+)-2-ENDO,3-ENDO-DIMETHYLBORNANE | 25.35 | 0.150 | 85,324 |
| 7 | LAVANDULYL ACETATE | 26.23 | 52.230 | 30,022,698 |
| 10 | BOTRYOCOCCANONE | 26.51 | 3.000 | 1,724,785 |
| 11 | 2,8-DIMETHYL-4-(1-METHYLPROPYL)-4,6-DECADIENE | 26.66 | 3.520 | 2,022,087 |
| 12 | OLEAN-12-EN-28-AL | 27.08 | 5.580 | 3,205,149 |
| 13 | TRANS-TETRAHYDROIONONE | 27.31 | 0.540 | 310,335 |
| 15 | LAVANDULYL ACETATE | 27.57 | 0.280 | 162,610 |
| 16 | SALVIALANE | 27.69 | 12.200 | 7,015,119 |
| 17 | DIHYDROTORULOSOL | 28.60 | 0.110 | 61,480 |
| 18 | PEROXYERGOSTEROL | 28.80 | 1.180 | 676,631 |
| 19 | PALUSTROL | 29.95 | 1.290 | 738,770 |
| 20 | 2,5-DIHYDROXY-4′-METHOXY-FLAVANONE | 30.72 | 1.710 | 982,168 |
| 21 | (ALL-E)-2,6,10,14-TETRAMETHYL-16-(PHENYLTHIO)HEXADECA-2,6,10,14-TETRAEN-1-OL | 31.15 | 0.830 | 479,122 |
| 22 | SOLANESOL | 31.75 | 1.640 | 941,182 |
| 23 | 9-OCTADECENOIC ACID | 31.95 | 0.570 | 325,331 |
Fig. 2.
GC–MS chromatogram of hydro-ethanolic extract of Otostegia fruticosa (Of.Cr).
3.3. Effect of Of.Cr on different spasmogen-mediated contractions
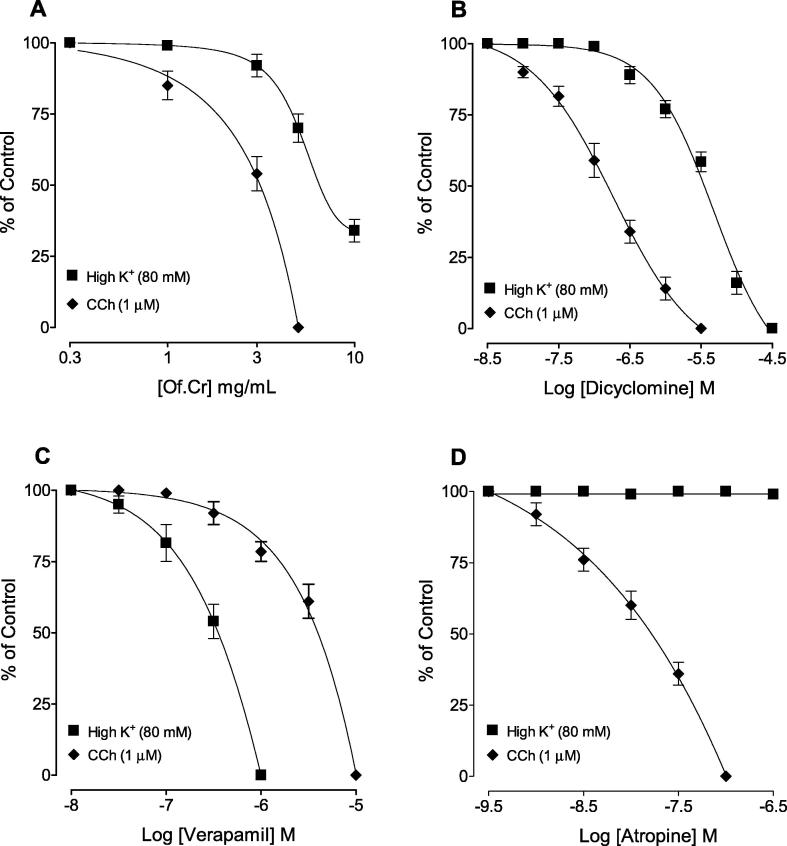
The inhibitory CRCs of the crude extract (Of.Cr) against CCh was obtained at lower concentration with a resultant EC50 value of 4.32 mg/mL (3.98–5.14, 95% CI, n = 4), compared to its effect against K+ (80 mM) with EC50 value of 8.44 mg/mL (7.44–8.92, n = 5). The relaxation against high K+ was incomplete with maximum efficacy of 70%, as shown in Fig. 3A. Dicyclomine, similar to Of.Cr, showed inhibitory effect against CCh and high K+ with EC50 values of 0.46 µM (0.33–0.69, n = 4) and 6.11 µM (5.22–7.86, n = 6), respectively (Fig. 3B) whereas the relaxant effects observed with verapamil was more potent against K+ with EC50 value of 0.44 µM (0.38–0.49, n = 5) compared to its inhibitory effects against CCh [5.22 µM (4.22–6.92, n = 5)] as shown in Fig. 3C. Atropine only relaxed the CCh (1 µM)-induced contraction, with EC50 value of 0.08 µM (0.07–0.09, n = 6), without any effect on K+ (80 mM)-induced contractions (Fig. 3D), as expected.
Fig. 3.
Concentration-response curves showing comparison of (A) hydro-ethanolic extract of Otostegia fruticosa (Of.Cr), (B) dicylomine, (C) verapamil and (D) atropine for the inhibitory effect against carbachol (CCh) and high K+-induced contractions in isolated guinea-pig tracheal preparations. Values shown are mean ± SEM, n = 5–6.
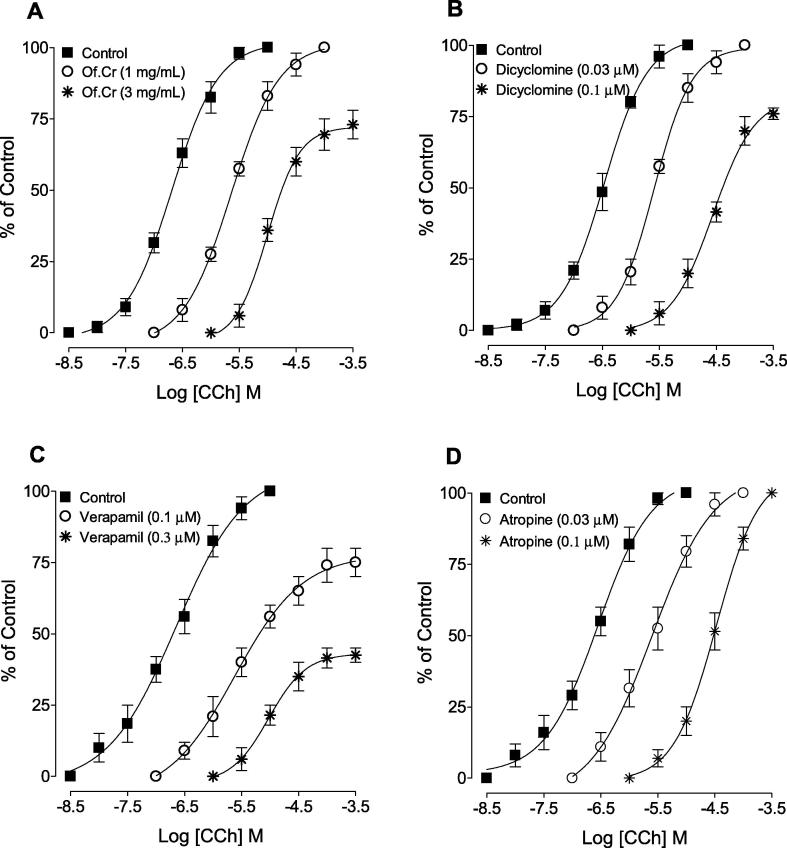
3.4. Anticholinergic effect of Of.Cr
Of.Cr at 1.0 mg/mL, deflected CCh-mediated CRCs towards the right side in a parallel way without depressing the maximum peak while the next higher dose of 3.0 mg/mL (Of.Cr) showed suppression and maximum efficacy was decreased (Fig. 4A). In similar experiments conducted, dicyclomine shifted to the right CCh-induced CRCs without suppressing the maximum response at lower doses of 0.03 µM while its higher dose of 0.1 µM affected the maximum efficacy of CCh (Fig. 4B). Verapamil at both tested concentrations (0.1–0.3 µM) produced a non-parallel rightward shift with dose-dependent suppression of the maximum of contractions of CCh-induced CRCs (Fig. 4C). Atropine, dose-dependently (0.03–0.1 µM) deflected the CCh curves to the right in a parallel way without effect on maximum efficacy (Fig. 4D).
Fig. 4.
Concentration-response curves of carbachol (CCh) in the absence and presence of different concentrations of (A) hydro-ethanolic extract of Otostegia fruticosa (Of.Cr), (B) dicyclomine, (C) verapamil and (D) atropine in isolated guinea-pig tracheal preparations. Values shown are mean ± SEM, n = 3–4.
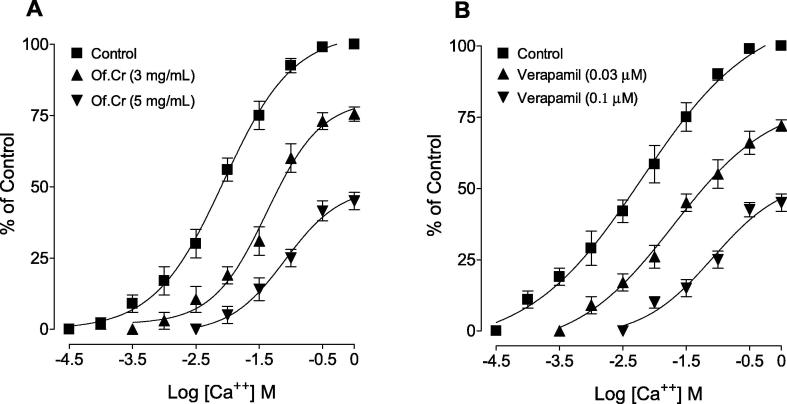
3.5. Effect on Ca++-curves
When tested for the possible interaction with Ca++ channels, Of.Cr (3 & 5 mg/mL) produced a rightward shift in the Ca++ curves (Fig. 5A), similar to that caused by verapamil (Fig. 5B).
Fig. 5.
Concentration-response curves of Ca++ in the absence and presence of the increasing concentrations of (A) hydro-ethanolic extract of Otostegia fruticosa (Of.Cr) and (B) verapamil in isolated guinea-pig tracheal preparations. Values shown are mean ± SEM, n = 3–4.
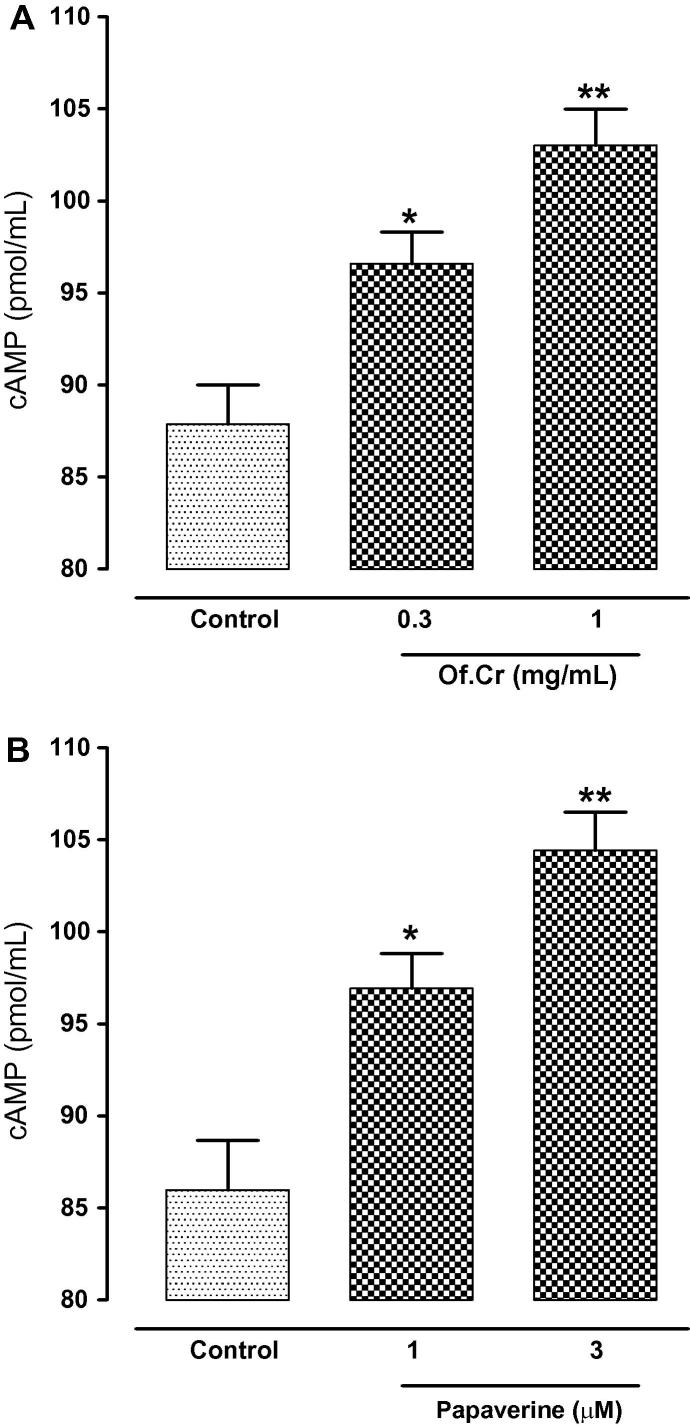
3.6. Effect on cAMP levels
The cAMP levels were estimated in the control CCh treated tissues and compared with Of.Cr and papaverine pre-treated tracheal tissues. Untreated tissues measured 87.87 ± 2.134 pmol of cAMP/mL of tissue homogenate. Compared to this, tissues after treatment with 0.3 and 1 mg/mL of Of.Cr against CCh-induced contraction measured the cAMP levels up to 96.58 ± 1.744 (p = <0.05) and 103.01 ± 1.969 (p < 0.01) pmol/mL respectively as can evident in Fig. 6A. The papaverine control showed the similar pattern of rise in the cAMP levels with control CCh treated tissues showing cAMP levels of 85.98 ± 2.680 pmol/mL whereas treatment with 1 µM and 3 µM of papaverine in CCh-contracted tissues, the cAMP levels increased to 96.93 ± 1.892 pmol/mL (p < 0.05) and 104.43 ± 2.078 (p < 0.01) pmol/mL respectively, as evident in Fig. 6B.
Fig. 6.
Effect of (A) hydro-ethanolic extract of Otostegia fruticosa (Of.Cr) and (B) papaverine on the cyclic nucleotide content of guinea-pig trachea. *p < 0.05, **p < 0.01, showed comparison with control (Unpaired t-test). Values shown are mean ± SEM, n = 4.
3.7. Antibiotic susceptibility pattern of clinical pathogens
In the present investigation, clinical isolates (K. pneumoniae and MRSA) were used. Antibiotic Susceptibility test was done in Microbiology lab/ King Khalid hospital which uses Phoenix 100/BD company machine. The results were interpreted according to Clinical and laboratory standard institute (CLSI) guidelines. Table 2 shows the antibiotic susceptibility pattern for both bacteria. K. pneumoniae was resistant to Ampicillin, Amoxicillin/clavulanate only. While, MRSA was found to be resistant against Imipenem, Cefoxitin, Cefotaxime, Ampicillin, PencillinG, Oxacillin, and Amoxicillin/clavulanate.
Table 2.
Antibiotic susceptibility pattern of clinical pathogens.
| Bacteria | Antibiotics |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GN | IMI | FOX | CTX | AMP | PG | OX | AUG | TS | VAN | NI | CIP | CXM | CAZ | CPM | ATM | |
| MRSA | S | R | R | R | R | R | R | R | S | S | S | S | – | – | – | – |
| K. pneumoniae | S | S | S | S | R | – | – | R | – | – | S | S | S | S | S | S |
Susceptible (S), Resistant (R), No Result (-),
GN: Gentamicin, IMI: Imipenem, FOX: Cefoxitin, CTX: Cefotaxime, AMP:Ampicillin, PG: PencillinG, OX: Oxacillin, AUG:Amox/Calv, TS:Trimethoprim/Sulfa, VAN: Vancomycin, NI: Nitrofurantoin, CIP: Ciprofloxacin, CXM: Cefuroxime, CAZ: Ceftazidime, CPM: Cefepime, ATM: Aztreonam.
3.8. MIC and MBC determination
Table 3 illustrates the MIC and MBC of Of.Cr against the tested bacterial strains. The Of.Cr (MIC 475 µg/mL) observed highly active against S. aureus (NCTC 6571) compared to other tested bacteria. On another hand, Of.Cr was less active (MIC 625 µg/mL) when tested with MRSA which is multi-resistant bacteria. It was also noted that there was no difference in efficacy of Of.Cr (MIC 550 µg/mL) against both strains of tested standard and clinical K. pneumoniae.
Table 3.
MIC and MBC of hydro-alcoholic extract of Otostegia fruticosa (Of.Cr) versus Tested Bacteria.
| Tested Microbes | MIC | MBC |
|---|---|---|
| K. pneumoniae (NCTC 9633) | 550 µg/mL | 575 µg/mL |
| P. aeruginosa (ATCC 10145) | 525 µg/mL | 550 µg/mL |
| S. aureus (NCTC 6571) | 475 µg/mL | 500 µg/mL |
| *MRSA | 625 µg/mL | 650 µg/mL |
| *K. pneumoniae | 550 µg/mL | 575 µg/mL |
Clinical pathogens.
Regarding gram-negative bacteria, P. aeruginosa appeared more sensitive to Of.Cr (MIC 525 µg/mL) among other types in the same group.
4. Discussion
The traditional importance of the use of Otostegia fruticosa in respiratory diseases has been documented by multiple sources (Enyew et al., 2013, Getaneh and Girma, 2014), but no scientific study has been conducted to rationalize its use in such disorders. This study was therefore conducted to analyze the chemical composition of the hydro-alcoholic extract of this plant (Of.Cr) by GC–MS while ex-vivo studies were conducted on guinea-pig trachea to evaluate the possible bronchodilatory effect. Contractions were produced in isolated tracheal tissues with muscarinic agonist (CCh) and high concentration of K+ (80 mM) and then Of.Cr were added on the sustained contractions in a cumulative way. Interestingly, Of.Cr completely reversed CCh-mediated contractions at lower concentrations while the incomplete inhibitory effect was observed against high K+ at the maximum tested concentration of 10 mg/mL. Similar to Of.Cr, a positive control drug; dicyclomine, also showed more potency against CCh as compared to high K+. Dicyclomine a dual blocker of the cholinergic receptor of muscarinic types and Ca++ channels inhibitor (Downie et al., 1977). Hence hypothesis was developed from these preliminary experiments that the bronchodilatory effect of Of.Cr possesses dual inhibitory mechanisms; inhibition of muscarinic receptors and Ca++ channels. Carbachol, a muscarinic receptors agonist (Jude et al., 2019), produces sustained contractions in isolated trachea by receptor-operated Ca++ entry, hence well documented for the evaluation of antimuscarinic activity of any test material (Arunlakhshana and Schild, 1959) whereas a substance that inhibits high K+-mediated contractions is considered indirectly as calcium channel blocker as K+ (>30 mM) specifically opens voltage-gated L-type Ca++ channels that results sustained contractions of smooth muscles (Godfraind et al., 1986, Hussain et al., 2008). To further strengthen the antimuscarinic effect of Of.Cr, CCh-mediated cumulative CRCs were made in the absence and presence of the preincubated tracheal tissues with a low and high dose of Of.Cr. The plant extract at a lower dose displaced CCh curves to the right toward higher concentrations without effecting the maximum response in a competitive manner (Arunlakhshana and Schild, 1959), similar to atropine, a known anticholinergic drug (Delmendo et al., 1989). Whereas, the higher dose of plant extract caused further rightward shift but suppressed the maximum response of CCh, a typical characteristic of the non-specific type of antagonists (Gilani and Khan, 2008). Similar nonspecific inhibition of CCh curves was found with a high dose of dicyclomine and verapamil, a known Ca++ inhibitor (Fleckenstein, 1977). As our plant extract inhibited high K+ with 70% efficacy and also a non-specific shift in CCh CRCs was observed, hence we further expanded our experiments to confirm the Of.Cr effect on voltage-gated Ca++ channels. For this purpose, tracheal tissues were first made Ca++ free by replacing normal Krebs solution with that of potassium normal and Ca++ free Krebs solution with EDTA to chelate the intracellular Ca++. Cumulative CRCs of Ca++ was constructed by exogenous calcium chloride addition in the absence and presence of Of.Cr extract. Parallel experiments were run by using verapamil at different concentrations to compare our results. Of.Cr shifted Ca++ CRCs to right similar to verapamil, a known voltage-gated L-type calcium inhibitor (Fleckenstein, 1977).
Based on our previously documented studies that herbal extracts having traditional famous to treat airways related disorders often contain enzyme inhibitory potential, specifically phosphodiesterase enzyme (PDE), thus causing bronchodilation by increasing tissue cAMP levels (Barnes, 2006, Gilani et al., 2005, Gilani et al., 2009, Khan et al., 2012). Of.Cr was therefore incubated in tracheal tissues at increasing concentrations and their homogenates were further analyzed for an increase in the cAMP levels by enzyme immunoassay kit with parallel experiments conducted with papaverine, a standard PDE-inhibitor (Choo and Mitchelson, 1978, Sato et al., 2006). Interestingly, Of.Cr produced an elevation in the levels of cAMP in CCh pre-contracted tissues compared to control tissues, similar to papaverine. The cAMP is synthesized from ATP by the action of adenylate cyclase, which stimulates cAMP-dependent protein kinase A (PKA) (Brown and Lee, 2012). Since cAMP levels are increased when PDE enzyme is inhibited in the tissues (Harris and Connell, 1989), therefore, we conclude that PDE-inhibitory-like components co-exist in the bronchodilatory effect of Of.Cr in combination with anticholinergic and CCB.
The significance of muscarinic receptor antagonists and PDE inhibitors are well-established facts in asthma (Barnes, 2006), despite their major limitation as that both produce cardiac stimulation (Nawarth, 1981, Nicholas, 2006). Interestingly, studies show the possible potential clinical implications of CCBs in bronchoconstriction (Twiss et al., 2002) parallel to its cardiac-inhibitory actions (Billman, 1992). The co-existence of voltage-gated CCB-constituent(s) with antimuscarinic and PDE inhibitors in Of.Cr perhaps meant by nature to oppose the cardiac stimulant effect seen with anticholinergics or PDE inhibitors if administered alone. The current observation with Of.Cr further supports the philosophical approach of an herbalist that natural products express synergism and side-effect neutralizing capabilities (Ernst, 2005).
The antibacterial effectiveness of any plant depends on its chemical constituents as well as the type of target bacteria and their structure (Gram-positive and Gram-negative bacteria). The results obtained in our study are in accordance with that of previous studies in that Gram-negative bacteria are more resistant to growth inhibition than Gram-positive bacteria (El abed and Guesmi, 2014, Guesmi et al., 2017). Scientists attribute these differences in part to the complexity of the cell envelope in Gram-negative bacteria (double-membrane) compared to the single membrane structure of the Gram-positive (El abed et al., 2014). On the other hand, these differences could be due to the presence of the outer membrane which is composed of lipopolysaccharides in the Gram-negative bacteria, which provides a hydrophilic surface which prevents permeability of any plant extracts, antibiotics, detergents, and lipophilic compounds (Burt, 2004, El abed and Guesmi, 2014).
The observed biological activities may be due to the presence of terpenes lavandulyl acetate, santalene and solanesol. The lavandulyl acetate is present as a major portion in the extract and might be responsible for bronchodilatory effects and though the contribution of additional constituents cannot be ruled out.
5. Conclusions
In conclusion, these results show that the hydro-alcoholic extract of Otostegia fruticosa possesses bronchodilatory potential mediated by multiple mechanisms i.e. receptor-operated (antimuscarinic), voltage-gated Ca++ channels blockade in addition to cAMP elevations, hence provide a sound rationale to the traditional utilization of this plant in airways disorders.
Acknowledgments
Acknowledgments
This work was funded by the Deanship of Scientific Research, Prince Sattam Bin Abdulaziz University, Saudi Arabia (Research project number: 2019/03/10464).
The authors are thankful to Dr. Maged Saad AbdelKader for providing some of the required chemicals to perform experiments.
Declaration of Competing Interest
The authors declare no conflict of interest.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Mohd Nazam Ansari, Email: nazam.ansari@gmail.com.
Najeeb Ur Rehman, Email: n_rehman5@hotmail.com.
References
- Aboutabl E.A., Sokkar N.M. Composition and antimicrobial activity of Otostegia fruticosa Forssk. J. Essential Oil Res. 1995;7(3):299–303. [Google Scholar]
- Adgaba N., Al-Ghamdi A. Nectar secretion dynamics and honey production potentials of some major honey plants in Saudi Arabia. Saudi J. Biol. Sci. 2017;24(1):180–191. doi: 10.1016/j.sjbs.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Musayeib N.M., Abbas F.A. Labdane diterpenes from Otostegia fruticosa. Phytochemistry. 2000;54(8):771–775. doi: 10.1016/s0031-9422(00)00185-0. [DOI] [PubMed] [Google Scholar]
- Andemariam W.S. Legislative regulation of traditional medicinal knowledge in Eritrea vis-à-vis Eritrea’s commitments under the convention on biological diversity: issues and alternatives. Law Environ. Dev. J. 2010;6(2):130–162. [Google Scholar]
- Arunlakhshana O., Schild H.O. Some quantitative uses of drug antagonists. Br. J. Pharmacol. 1959;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes P.J. Drugs for asthma. Br. J. Pharmacol. 2006;147(Suppl 1):S297–S303. doi: 10.1038/sj.bjp.0706437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billman G.E. The antiarrythmic effects of the calcium antagonists. In: Epstein M., editor. Calcium Antagonists in Clinical Medicine. Hanley and Belfus Inc.; Philadelphia, USA: 1992. pp. 183–212. [Google Scholar]
- Brown K.M., Lee L.C. Cyclic AMP-specific phosphodiesterase, PDE8A1, is activated by protein kinase A-mediated phosphorylation. FEBS Letter. 2012;586:1631–1637. doi: 10.1016/j.febslet.2012.04.033. [DOI] [PubMed] [Google Scholar]
- Burt S. Essential oils: their antibacterial properties and potential applications in foods—a review. Int. J. Food Microbiol. 2004;94(3):223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Cheesman M.J., Ilanko A. Developing new antimicrobial therapies: are synergistic combinations of plant extracts/compounds with conventional antibiotics the solution? Pharmacognosy Rev. 2017;11(22):57–72. doi: 10.4103/phrev.phrev_21_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo L.K., Mitchelson F. Antagonism of cholinomimetics by troxy pyrrolidinium in guinea-pig atria and longitudinal ileal muscle: comparison with hemicholinium-3. Eu. J. Pharmacol. 1978;52(3):313–322. doi: 10.1016/0014-2999(78)90284-4. [DOI] [PubMed] [Google Scholar]
- Costa D.C., Costa H.S. Advances in phenolic compounds analysis of aromatic plants and their potential applications. Trends Food Sci. Technol. 2015;45(2):336–354. [Google Scholar]
- D’avigdor E., Wohlmuth H. The current status of knowledge of herbal medicine and medicinal plants in Fiche, Ethiopia. J. Ethnobiol. Ethnomed. 2014;10 doi: 10.1186/1746-4269-10-38. Article Number 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmendo R.E., Michel A.D. Affinity of muscarinic receptor antagonists for three putative muscarinic receptor binding sites. Br. J. Pharmacol. 1989;96(2):457–464. doi: 10.1111/j.1476-5381.1989.tb11838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downie J.W., Twiddy D.A. Antimuscarinic and non-competitive antagonist properties of dicyclomine hydrochloride in isolated human and rabbit bladder muscle. J. Pharmacol. Exp. Ther. 1977;201(3):662–668. [PubMed] [Google Scholar]
- El abed N., Guesmi F. Phytochemical screening and assessment of antioxidant, antibacterial and cytotoxicity activities of five Tunisian medicinal plants. Int. J. Pharm. Res. Biosci. 2014;3(4):770–789. [Google Scholar]
- Enyew A., Asfaw Z. Status of medico-cultural commercial plants at Fiche town market Ethiopia. Int. J. Pharm. Health Care Res. 2013;1(4):227–236. [Google Scholar]
- Ernst E. The efficacy of herbal medicine-an overview. Fundam. Clin. Pharmacol. 2005;19(4):405–409. doi: 10.1111/j.1472-8206.2005.00335.x. [DOI] [PubMed] [Google Scholar]
- Fleckenstein A. Specific pharmacology of Ca++ in myocardium, cardiac pacemakers and vascular smooth muscle. Rev. Pharmacol. Toxicol. 1977;17:149–166. doi: 10.1146/annurev.pa.17.040177.001053. [DOI] [PubMed] [Google Scholar]
- Getaneh S., Girma Z. An ethnobotanical study of medicinal plants in Debre Libanos Wereda, Central Ethiopia. Afr. J. Plant Sci. 2014;8(7):366–379. [Google Scholar]
- Gilani A.H., Khan A.U. Mechanisms underlying the antispasmodic and bronchodilatory properties of Terminalia bellerica fruit. J. Ethnopharmacol. 2008;116:528–538. doi: 10.1016/j.jep.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Gilani A.H., Shah A.J. Chemical composition and mechanisms underlying the spasmolytic and bronchodilatory properties of the essential oil of Nepeta cataria L. J. Ethnopharmacol. 2009;121(3):405–411. doi: 10.1016/j.jep.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Gilani A.H., Khan A. Antispasmodic and bronchodilator activities of St. John’s wort are putatively mediated through dual inhibition of calcium influx and phosphodiesterase. Fundam. Clin. Pharmacol. 2005;19(6):695–705. doi: 10.1111/j.1472-8206.2005.00378.x. [DOI] [PubMed] [Google Scholar]
- Godfraind T., Miller R. Calcium antagonism and calcium entry blockade. Pharmacol. Rev. 1986;38(4):321–416. [PubMed] [Google Scholar]
- Guesmi F., Ben Hadj A.S. Investigation of extracts from Tunisian Ethnomedicinal plants as antioxidants, cytotoxins, and antimicrobials. Biomed. Environ. Sci. 2017;30(11):811–824. doi: 10.3967/bes2017.109. [DOI] [PubMed] [Google Scholar]
- Harris A.L., Connell M.J. Role of low Km cyclic AMP phosphodiesterase inhibition in tracheal relaxation and bronchodilation in the guinea pig. J. Pharmacol. Exp. Ther. 1989;251(1):199–206. [PubMed] [Google Scholar]
- Hussain A.I., Anwar F. Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem. 2008;108(3):986–995. doi: 10.1016/j.foodchem.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Jude J., Botelho D. Salicylic acid amplifies Carbachol-induced bronchoconstriction in human precision-cut lung slices. Respir. Res. 2019;20(1) doi: 10.1186/s12931-019-1034-x. Article Number 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M., Khan A. Pharmacological explanation for the use of Juniperus excelsa in hyperactive gastrointestinal and respiratory disorders. J. Natural Med. 2012;66(2):292–301. doi: 10.1007/s11418-011-0605-z. [DOI] [PubMed] [Google Scholar]
- Kidane D., Tomass Z. Community knowledge of traditional mosquito repellent plants in Kolla Temben District, Tigray, Northern Ethiopia. Sci. Res. Essays. 2013;8(24):1139–1144. [Google Scholar]
- Mothana R., Kriegisch S. Assessment of selected Yemeni medicinal plants for their in vitro antimicrobial, anticancer, and antioxidant activities. Pharm. Biol. 2011;49(2):200–210. doi: 10.3109/13880209.2010.512295. [DOI] [PubMed] [Google Scholar]
- National Research Council . National Academy Press; Washington: 1996. Guide for the Care and Use of Laboratory Animals; pp. 1–7. [Google Scholar]
- Nawarth H. Action potential, membrane currents and force of contraction in cat ventricular heart muscle treated with papaverine. J. Pharmacol. Exp. Ther. 1981;218(2):544–549. [PubMed] [Google Scholar]
- Nicholas J.G. Anticholinergic agents in asthma and COPD. Eur. J. Pharmacol. 2006;533(1–3):36–39. doi: 10.1016/j.ejphar.2005.12.072. [DOI] [PubMed] [Google Scholar]
- Patel J.B., Cockerill F.R. 35(3) Clinical and Laboratory Standards Institute; 2015. Performance standards for antimicrobial susceptibility testing; pp. 1–15. (Twenty Fourth Informational Supplement M100–S24). [Google Scholar]
- Rahman A., Mossa S. Medicinal plant diversity in the flora of Saudi Arabia: a report on seven plant families. Fitoterapia. 2004;75(2):149–161. doi: 10.1016/j.fitote.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Sato Y., Akao T. Glycycoumarin from Glycyrrhizae Radix acts as a potent antispasmodic through inhibition of phosphodiesterase 3. J. Ethnopharmacol. 2006;105(3):409–414. doi: 10.1016/j.jep.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Shah A.J., Gilani A.H. Bronchodilatory effect of Acorus calamus is mediated through multiple pathways. J. Ethnopharmacol. 2010;131(2):471–477. doi: 10.1016/j.jep.2010.07.024. [DOI] [PubMed] [Google Scholar]
- Twiss M.A., Harman E. Efficacy of calcium channel blockers as maintenance therapy for asthma. Br. J. Clin. Pharmacol. 2002;53(3):243–249. doi: 10.1046/j.0306-5251.2001.01560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]