Abstract
Background
Carotid patch angioplasty (with either a venous or a synthetic patch) may reduce the risk of carotid artery restenosis and subsequent ischaemic stroke. This is an update of a Cochrane Review originally published in 1995 and previously updated in 2004.
Objectives
To assess the safety and efficacy of routine or selective carotid patch angioplasty compared to carotid endarterectomy with primary closure.
Search methods
We searched the Cochrane Stroke Group Trials Register (last searched 5 May 2009), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, Issue 1, 2009), MEDLINE (1966 to November 2008), EMBASE (1980 to November 2008) and Index to Scientific and Technical Proceedings (1980 to November 2008). We handsearched journals and conference proceedings, checked reference lists, and contacted experts in the field.
Selection criteria
Randomised and quasi‐randomised trials comparing carotid patch angioplasty with primary closure in any patients undergoing carotid endarterectomy.
Data collection and analysis
Two review authors independently assessed eligibility, trial quality and extracted data.
Main results
We included 10 trials involving 1967 patients undergoing 2157 operations. The quality of trials was generally poor. Follow up varied from hospital discharge to five years. Carotid patch angioplasty was associated with a reduction in the risk of ipsilateral stroke during the perioperative period (odds ratio (OR) 0.31, 95% confidence interval (CI) 0.15 to 0.63, P = 0.001) and long‐term follow up (OR 0.32, 95%CI 0.16 to 0.63, P = 0.001). It was also associated with a reduced risk of perioperative arterial occlusion (OR 0.18, 95% CI 0.08 to 0.41, P < 0.0001), and decreased restenosis during long‐term follow up in eight trials (OR 0.24, 95% CI 0.17 to 0.34, P < 0.00001). These results are more certain than those of the previous review since the number of operations and events have increased. However, the sample sizes are still relatively small, data were not available from all trials, and there was significant loss to follow up. Very few arterial complications, including haemorrhage, infection, cranial nerve palsies and pseudo‐aneurysm formation were recorded with either patch or primary closure. No significant correlation was found between use of patch angioplasty and the risk of either perioperative or long‐term all‐cause death rates.
Authors' conclusions
Limited evidence suggests that carotid patch angioplasty may reduce the risk of perioperative arterial occlusion and restenosis. It would appear to reduce the risk of ipsilateral stroke and there is a non significant trend towards a reduction in perioperative any stroke rate and all‐cause case fatality.
Plain language summary
Patch angioplasty versus primary closure for carotid endarterectomy
Evidence from this review of 10 trials involving 1967 patients undergoing 2157 operations now suggests a benefit from using routine patch angioplasty during carotid endarterectomy. About 20% of strokes result from narrowing of the carotid artery (the main artery supplying blood to the brain). Carotid endarterectomy is an operation that involves opening the carotid artery to remove this narrowing and, therefore, reduce the risk of stroke. However, there is a 2% to 10% risk of the operation itself causing a stroke. Some surgeons advocate the incorporation of a patch made out of either synthetic material or the patient's own vein, into the arterial closure. This may help to reduce the risk of the artery being narrowed during suture placement and may, therefore, reduce the risk of recurrent blockage and consequent stroke or death or both. However, use of a patch may increase surgical difficulty and operation length. Furthermore, thin‐walled vein patches may rupture with potentially fatal consequences and synthetic materials are vulnerable to infection.
Background
Carotid endarterectomy has been shown in large, well‐conducted randomised controlled trials to reduce the risk of stroke in patients with recently symptomatic, severe (greater than 70%) internal carotid artery stenosis (Rothwell 2003). There is also some evidence that it is beneficial for asymptomatic patients (ACAS 1995; Halliday 2004). What is less clear at present is whether different surgical techniques affect the outcome. One such technique is carotid patch angioplasty, with either a venous patch or a synthetic patch. Might this be as safe as primary closure, reduce the risk of restenosis and, more importantly, improve the long‐term clinical outcome?
There are relatively few good prospective studies of restenosis following carotid endarterectomy and studies are difficult to compare because of differences in the definitions of stenosis and lengths of follow up. However, it appears that carotid restenosis of greater than 50% diameter reduction (as detected by Doppler ultrasound) occurs in 6% to 36% of patients during long‐term follow up (Bernstein 1990; Knudsen 1990; Ouriel 1987; Volteas 1994; Zierler 1982). The majority of stenoses occur in the first two years (Frericks 1998). Carotid patch angioplasty may reduce the risk of restenosis, and so reduce the long‐term risk of recurrent ipsilateral ischaemic stroke (Awad 1989; Ouriel 1987). However, the risk of symptomatic restenosis appears to be much lower ‐ about 2% to 4% (Das 1985; Frericks 1998), and patch angioplasty may also be associated with certain perioperative risks: routine patching involves a longer carotid occlusion time, two suture lines instead of one and the use of a patch material, all of which may increase the risk of early re‐occlusion, arterial rupture, infection or pseudoaneurysm formation (Awad 1989; Bernstein 1992). In addition, if a venous patch is used, there may be morbidity associated with vein harvesting, such as neuralgia, haemorrhage, and infection.
A survey from the United Kingdom in a trial showed considerable variations among vascular surgeons in the use of carotid patching, which may reflect uncertainty in its benefits: 76% of surgeons always used patching, 19.4% sometimes and 4.6% never (Girn 2008). Analysis of the ECST trial data showed significant heterogeneity in frequency of use of patch angioplasty at an individual surgeon, national and international level (ECST 1991). Given the uncertainty implied by such variation in practice, it is clearly important to establish whether routine or selective patching is more effective than, and as safe as, primary closure. Randomised controlled trials provide the most reliable evidence on which to base these assessments. We, therefore, performed a systematic review of all such trials that compared routine or selective patching with primary closure.
NB: The first version of this review included trials comparing one type of patch with another. These trials have now been included in a separate Cochrane review (Bond 2003).
This is an update of a Cochrane Review originally published in 1995 and previously updated in 2004.
Objectives
To assess the safety and efficacy of routine or selective carotid patch angioplasty with either a venous patch or a synthetic patch compared to primary closure. We wished to test the primary hypothesis that carotid patch angioplasty resulted in a lower rate of significant arterial restenosis and therefore fewer recurrent strokes and stroke‐related deaths without a significant increase in perioperative complications.
Methods
Criteria for considering studies for this review
Types of studies
We sought to identify all unconfounded randomised trials of carotid patching. We included quasi‐randomised trials in which allocation to different treatment regimens was not adequately concealed (e.g. allocation by alternation, date of birth, hospital number, day of the week, or by using an open random number list), but foreknowledge of treatment allocation might lead to biased treatment allocation and exaggerated treatment effects (Schulz 1995).
Types of participants
We considered trials that included any type of patient undergoing carotid endarterectomy as eligible, whether the initial indication for endarterectomy was symptomatic or asymptomatic carotid disease.
Types of interventions
We sought to identify all trials comparing routine carotid patch angioplasty (i.e. patching attempted in all patients) with primary closure. Any type of patch material was eligible e.g. venous, Dacron, or polytetrafluoroethylene (PTFE). We also intended to include trials comparing selective patch angioplasty (i.e. patching attempted only in patients thought likely to benefit) with primary closure, but we failed to identify any such trials. Trials which compared one type of patch with another are included in a separate Cochrane review (Bond 2003).
Types of outcome measures
We aimed to extract from each trial the number of patients originally allocated to each treatment group to allow an intention‐to‐treat analysis. Within each treatment group we then extracted the number of patients:
who died within 30 days of the operation and during subsequent follow up. We tried to classify each death as stroke‐related or not;
who had any stroke within 30 days of the operation and during subsequent follow up. A separate analysis of strokes ipsilateral to the endarterectomy was also performed;
who had known occlusion of the artery that was operated on within 30 days of the operation;
who had a significant complication related to surgery, such as haemorrhage from or rupture of the artery, infection of the endarterectomy site, cranial nerve palsy or pseudoaneurysm formation;
who developed restenosis greater than 50% or occlusion of the artery that was operated on during follow up.
Search methods for identification of studies
See the 'Specialized register' section in the Cochrane Stroke Group module.
We searched the Cochrane Stroke Group trials register, which was last searched by the Managing Editor in May 2009. We also updated the electronic searches and handsearched additional issues of relevant journals as follows.
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, Issue 1, 2009); MEDLINE (1966 to November 2008) (Appendix 1), EMBASE (1980 to November 2008) (Appendix 2), and Index to Scientific and Technical Proceedings (1980 to November 2008), which was searched using the terms "carotid" and ("trial* or random*").
-
We handsearched the following journals, including conference supplements:
Annals of Surgery (1981 to September 2008);
Annals of Vascular Surgery (1994 to September 2008);
Cardiovascular Surgery (now Vascular) (1994 to September 2008);
European Journal of Vascular Surgery (now European Journal of Vascular and Endovascular Surgery) (1987 to September 2008);
Journal of Vascular Surgery (1994 to September 2008);
Stroke (1994 to September 2008).
We reviewed the reference lists of all relevant studies.
We contacted experts in the field to identify further published and unpublished studies
-
For the previous version of the review:
-
we hand‐searched the following journals, including conference supplements:
American Journal of Surgery (1994 to 2001);
British Journal of Surgery (1985 to 2001);
World Journal of Surgery (1978 to 2001).
-
we handsearched abstracts of the following meetings for the years 1995 to 2001:
AGM of the Vascular Surgical Society (UK);
AGM of the Association of Surgeons of Great Britain and Ireland;
AHA Stroke Conference;
Annual Meeting of the Society for Vascular Surgery (USA);
European Stroke Conference.
-
We did not apply any language restriction in the searches and arranged translation of all possibly relevant non‐English language publications.
Data collection and analysis
One review author (KR) selected those trials that met the inclusion criteria, and the other review author (PMR) independently reviewed these decisions. We resolved all disagreements through discussion.The same two review authors also assessed the methodological quality of each trial. We decided not to use a scoring system to assess quality but simply to record the following details: the randomisation method, the blinding of the clinical and Doppler assessments, whether outcomes were reported for all patients originally randomised in each group irrespective of whether they received the operation they were allocated to or whether the patient was excluded after randomisation, and the number of patients lost to follow up. We sought data on the number of outcome events in all patients originally randomised to allow an intention‐to‐treat analysis. We extracted and cross‐checked all data. In addition, we also extracted details about the patients included in the trial, the inclusion and exclusion criteria, the comparability of the treatment and control groups for important prognostic factors, the type of patch, the type of anaesthetic, the use of shunts, and the use of antiplatelet therapy during follow up. If any of the above data were not available from the publication, we sought further information by correspondence with the trialists.
All of the trials included both patients who had unilateral carotid endaterectomies and patients who had bilateral carotid endarterectomies, and in most the artery was randomised to a particular procedure rather than the patient (Al‐Rawi 2006; Lord 1989; Mannheim 2005; Myers 1994; Pratesi 1986; Ranaboldo 1993; Vleeschauwer 1987). In these trials, it was therefore possible for one patient to have primary closure on one side and carotid patching on the other side. Indeed, in one trial if a patient required bilateral endarterectomies, each artery had to have a different procedure (Myers 1994). In the reporting of these trials, the results were given for each artery that was randomised rather than for each individual patient. This makes sense for arterial complications such as haemorrhage or occlusion, for ipsilateral events, and for complication within 30 days of surgery (since most patients waited at least this period between the first and second operation), but it is not ideal for patient‐related long‐term clinical outcome events such as death or any stroke. In patients with bilateral endarterectomies who had both patching and primary closure, it would not be possible to relate death or stroke to one particular procedure. Therefore, In trials where it was possible for a patient to have both procedures, we analysed death and any stroke only in those who had unilateral procedures or the same procedure to both arteries. These data were available from the authors in all except two trials (Lord 1989; Myers 1994). In one trial, the investigators no longer had the original data on patients with unilateral operations and so this trial was excluded from the analyses of these outcomes (Lord 1989). In the other trial (Myers 1994), the number of patients undergoing unilateral endarterectomies was reported and we were able to estimate the number of clinical events per patient in each group using the number of events per artery and the total number of deaths that were reported.
A separate analysis of only strokes ipsilateral to the operated artery was also performed for each artery. However, this may be less useful as the more important outcome is the total number of strokes and not just the ipsilateral strokes. We analysed arterial complications, such as occlusion, haemorrhage from the endarterectomy site, restenosis, infection at the operation site, or pseudoaneurysm formation for all arteries rather than patients. The analyses based on arteries assumed that, in patients who had bilateral endarterectomies, outcome events in each carotid artery were independent. This is unlikely to be true but given that relatively few patients had bilateral procedures (10% overall) we felt it reasonable to perform such analyses. However, their results should be interpreted with caution.
About 40 patients were lost to follow up. For the intention‐to‐treat analyses, we assumed that patients who were lost to follow up did not have an outcome event. For the main analyses, we assumed that patients who were lost did not have an outcome event. In the previous version of this review, where statistically significant results were found, worst‐case sensitivity analyses were performed to determine whether the results were robust. These analyses assumed that all patients lost from the patching arm had an adverse outcome, whereas none of those lost from the control arm did. However, for this review these analyses have not been included since the current authors consider them to be unreasonably critical.
We calculated proportional risk reductions based on a weighted estimate of the odds ratio using the Peto method (APT 1994). Since all the outcome events assessed were rare, the odds ratios quoted will be similar to the relative risks. We calculated absolute risk reductions from the crude risks of each outcome in all trials combined (APT 1994). We assessed heterogeneity between study results using the I2 statistic (Higgins 2003). This examined the percentage of total variation across studies due to heterogeneity rather than to chance. Values of I2 over 75% indicate a high level of heterogeneity.
Results
Description of studies
Seven trials that fulfilled the eligibility criteria were identified by the 2004 version of this review, but only two new randomised controlled trials have been reported since then (Al‐Rawi 2006; Mannheim 2005). We also found one trial published in 1986 that was not identified in the previous verison of the review and it is included in this version (Pratesi 1986). We have not identified any additional ongoing trials, and there are no trials presently awaiting assessment. We excluded two trials: in one unpublished trial, an intention‐to‐treat analysis was not possible because one‐third of the 300 patients randomised did not receive their allocated operation and results for these patients were not available (Gale 1985); in the discussion section of one of the published papers (Eikelboom 1988), another unpublished trial was quoted but after discussion with the principal investigator, it became apparent that this trial was not in fact random or quasi‐random (Hertzer 1987).
All of the trials we included compared routine patching with primary closure. Three of the trials used only saphenous vein patches (Eikelboom 1988; Myers 1994; Vleeschauwer 1987), and three used synthetic patches (Al‐Rawi 2006; Katz 1994; Mannheim 2005). Four trials used both vein and synthetic ‐ PTFE or Dacron ‐ patches (AbuRahma 1996; Lord 1989; Pratesi 1986; Ranaboldo 1993), but in two of these (Pratesi 1986; Ranaboldo 1993), results were not recorded by the type of patch that the patient received. In the previous review, the results of one of these trials (Lord 1989) were presented by splitting them into vein patching versus control and synthetic patching versus control. However, on hindsight, this was incorrect since it allowed the number of operations with primary closure to be counted twice in the overall analysis. Therefore, for the updated review we have analysed these three trials as any patch versus no patch.
One of the trials included a group that was allocated to obligate patching without randomisation (Myers 1994). This group of patients was not included in the analyses. All operations were performed under general anaesthetic, and most were also performed with shunting. Most of the patients in all the trials received antiplatelet or anticoagulant drugs long term after the operation. All the trials with follow up beyond hospital discharge included Doppler ultrasound of the arteries during follow up, and one also included intravenous digital subtraction angiography (Eikelboom 1988).
The average age of patients involved in these trials was about 67 years and there were approximately twice as many men as women. All of the trials included patients with asymptomatic carotid disease with the proportion varying from 8% (Ranaboldo 1993) to 51% (Mannheim 2005). All trials compared routine patching in all patients in the treatment group with primary closure. In four trials, narrow carotid arteries were excluded before randomisation on the basis that it was not safe to close these with primary closure: in one trial, 38 out of a total of 163 arteries were excluded because the internal diameter (assessed at operation) was less than 5 mm (Myers 1994); in one trial, one patient out of a possible 110 patients was excluded because the arterial diameter was less than 3.5 mm (assessed from the preoperative angiogram) (Katz 1994); in one trial, 12 out of 399 carotid endarterectomies were excluded if internal carotid artery diameter was less than 4 mm (AbuRahma 1996); and in one trial, 24 out of 422 were excluded if small diameter internal carotid artery or the need for an interposition graft. In the remaining trials, only one patient randomised to primary closure required a patch because the artery was felt to be too narrow (Eikelboom 1988). Seven other patients randomised to primary closure required patching either because the stenosis was very high (two patients) or because the artery became occluded postoperatively (five patients). Seven patients from the patch group did not receive a patch either because no vein was available (two patients), because rapid closure was required due to possible ischaemic changes on an EEG during the operation (one patient), or for no apparent reason (four patients). The average follow up varied from hospital discharge (Lord 1989) to five years (Eikelboom 1988; Myers 1994). In all trials in which the data were available, the treatment groups were comparable for important prognostic factors.
Risk of bias in included studies
There were several significant flaws in most of the trials. Firstly, allocation concealment was only adequate in six trials which used numbered, sealed, opaque, envelopes as the method of randomisation (AbuRahma 1996; Al‐Rawi 2006; Lord 1989; Mannheim 2005; Myers 1994; Ranaboldo 1993). One study used envelope randomisation but they were not numbered or opaque (Vleeschauwer 1987). Two trials used quasi‐random allocation based on the patient's hospital number (Eikelboom 1988) or social security number (Katz 1994). Secondly, adequate blinding is important in order to reduce bias in the detection of certain outcome events. For instance, ultrasound assessment of restenosis should probably be assessed blind, although experienced practitioners may be able to detect the slight dilatation associated with a carotid patch even when blinded. Correspondence with the authors confirmed that clinical assessment was definitely blinded in only three trials (AbuRahma 1996; Ranaboldo 1993; Vleeschauwer 1987), but that restenosis was assessed blind in all except two trials (Katz 1994; Lord 1989).
As mentioned previously, one of the main flaws in eight of the trials was that a patient undergoing bilateral carotid endarterectomy could be randomised twice and have their two carotid arteries randomised to different treatment groups (AbuRahma 1996; Al‐Rawi 2006; Lord 1989; Mannheim 2005; Myers 1994; Pratesi 1986; Ranaboldo 1993; Vleeschauwer 1987). In these trials, it was unclear from the published reports exactly how many patients (as opposed to arteries) were randomised to each group and how many patients with bilateral endarterectomies had different procedures to each artery (Table 3). We were able to obtain these data from all except one trial (Lord 1989). Demographic features, such as age and sex, as well as results were usually reported for each randomised artery rather than per patient. True intention‐to‐treat analysis was only possible for three trials after we obtained additional data from the authors (AbuRahma 1996; Al‐Rawi 2006; Ranaboldo 1993). In the other trials, data on patients lost to follow up were not available, and in one trial four patients who did not have the procedure that they were randomised to receive were excluded from the analysis (Lord 1989).
1. Number of cases lost to follow up.
| Study | Total patients | Total operations | Patch lost at 30 days | Patch lost at end | Primary lost at 30 days | Primary lost at end | Number of exclusions | Crossover patch ‐ non | Crossover non ‐ patch |
| AbuRahma 1996 | 357 | 399 | 0 | 0 | 0 | 0 | 4 | 0 | 0 |
| Al‐Rawi 2006l | 315 | 338 | 0 | 7 | 0 | 8 | 10 | Data not available | Data not available |
| Eikelboom 1988 | 126 | 129 | 0 | 10 patients lost to doppler FU but not clinical FU | 0 | 7 to doppler FU but not clinical FU | 0 | 3 | 3 |
| Katz 1994 | 87 | 100 | 0 | 5 | 0 | 7 | 0 | 0 | 0 |
| Lord 1989 | 123 | 140 | 0 | 0 | 0 | 0 | 4 | Between 0 and 4 | Between 0 and 4 |
| Mannheim 2005 | 404 | 422 | 0 | Data not available | 0 | Data not available | Data not available | 0 | 0 |
| Myers 1994 | 136 (109 after exclusion of 27 patients undergoing obligatory vein patching) | 152 (122 analysed as 30 operations got obligatory vein patches) | 0 | 6 | 0 | 8 | 30 operations underwent obligatory vein patch closure and 16 patients had both sides done (total 46) | 0 | 0 |
| Pratesi 1986 | 100 | 100 | Data not available | Data not available | Data not available | Data not available | Data not available | Data not available | Data not available |
| Ranaboldo 1993 | 199 | 213 | 0 | 5 | 0 | 12 | 0 | 0 | 0 at 30‐day FU but 4 at 1‐year FU |
| Vleeschauwer 1987 | 126 | 174 | 0 | Data not available | 0 | Data not available | 0 | 0 | 0 |
FU: follow up
Effects of interventions
We included data from 10 trials (1967 patients, 2157 operations) in this review. The results presented may differ from those in the published reports where additional information has been obtained from the authors. There was no statistical heterogeneity in any of the analyses.
Outcomes within 30 days of operation
Stroke
Any stroke (fatal, non‐fatal, contralateral, ipsilateral, brainstem, haemorrhage, or infarct)
The overall perioperative risk of any stroke was 2.5% (45/1769). Patching was associated with a non‐significant reduction in the odds of any stroke (odds ratio (OR) 0.57, 95% confidence interval (CI) 0.31 to 1.03, P = 0.06) (Analysis 1.1). Within each closure type there was no heterogeneity, but there was significant between‐group heterogeneity (I2 = 60.3). None of the trials recorded the severity of stroke in terms of residual disability, but only three of these strokes were fatal (one in the patch group, two in the primary closure group).
1.1. Analysis.
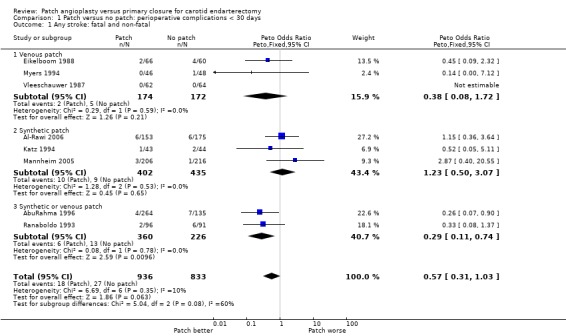
Comparison 1 Patch versus no patch: perioperative complications < 30 days, Outcome 1 Any stroke: fatal and non‐fatal.
Ipsilateral stroke (haemorrhage or infarct)
If effective, patching would be expected to reduce mainly stroke ipsilateral to the operated artery. The number of ipsilateral strokes per artery randomised was available from seven trials, although in several instances we required additional data from the authors. No data were available from the three new trials. In total, 2.8% (33/1201) of operations were associated with an ipsilateral stroke. Carotid patching was associated with a statistically significant reduction in the relative odds of perioperative ipsilateral stroke (OR 0.31, 95% CI 0.15 to 0.63, P = 0.001) (Analysis 1.3).
1.3. Analysis.
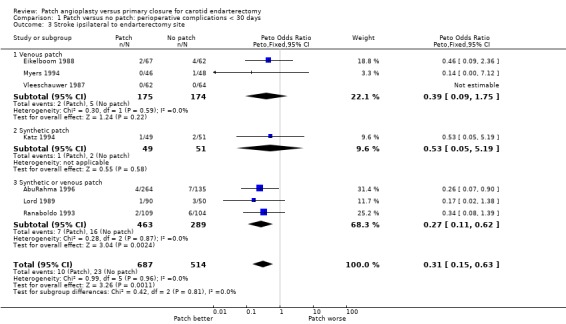
Comparison 1 Patch versus no patch: perioperative complications < 30 days, Outcome 3 Stroke ipsilateral to endarterectomy site.
Death
There were only 11 deaths in the nine trials with available data (overall risk = 0.6%, 11/1869), and so it remains unclear whether patching is associated with a higher or lower perioperative mortality than primary closure (OR 0.62, 95% CI 0.18 to 2.09, P = 0.4) (Analysis 1.4).
1.4. Analysis.
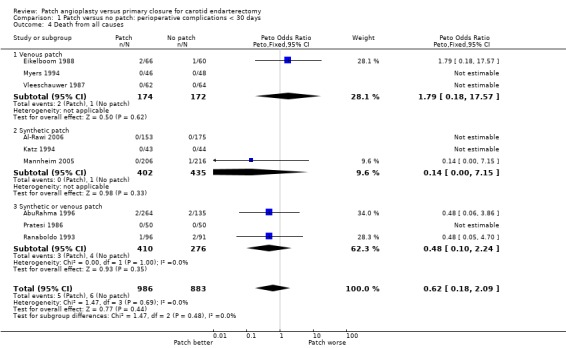
Comparison 1 Patch versus no patch: perioperative complications < 30 days, Outcome 4 Death from all causes.
Stroke or death
Combined stroke/death rate was non‐significantly lower in the patching group (OR 0.58, 95% CI 0.33 to 1.01, P = 0.06) (Analysis 1.5).
1.5. Analysis.
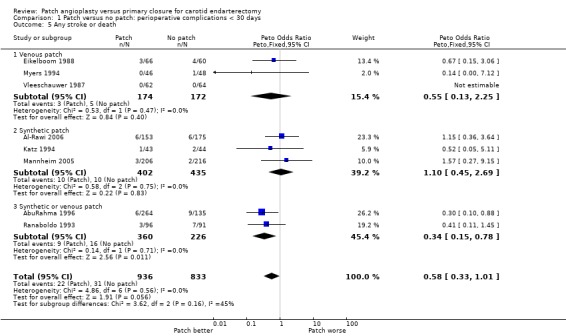
Comparison 1 Patch versus no patch: perioperative complications < 30 days, Outcome 5 Any stroke or death.
Arterial complications
As noted in the Methods section, these results should be interpreted with caution since, in patients who underwent bilateral endarterectomies, outcomes in each artery were probably not independent. We were unable to identify how many patients with bilateral endarterectomies had outcomes events in both arteries.
Arterial occlusion
Three trials did not provide data on perioperative arterial occlusion (Mannheim 2005; Pratesi 1986; Vleeschauwer 1987). Of the other trials, four used ultrasound (Duplex) scanning (AbuRahma 1996; Al‐Rawi 2006; Katz 1994; Ranaboldo 1993), two used intravenous digital subtraction angiography (Eikelboom 1988; Lord 1989) and one used ocular pneumoplethysmography (Myers 1994). At least 26 of the randomised arteries were not assessed within 30 days of operation (14 patch, 12 primary closure) and these arteries were assumed to be not occluded for the purpose of this analysis. Patching was associated with a highly statistically significant 82% reduction (OR 0.18 P < 0.0001) in the odds of perioperative arterial occlusion. However, this result was based on small numbers (4/794 (0.5%) patching versus 20/641 (3.1%) primary closure) and so the confidence interval was wide (OR 0.18, 95% CI 0.08 to 0.41 P < 0.0001) (Analysis 1.6). As documented above, however, the consequences to the patients (in terms of stroke‐related death and non‐fatal stroke) resulting from this reduction in arterial occlusion were unclear.
1.6. Analysis.
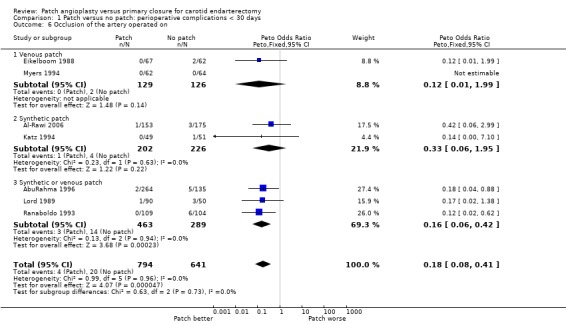
Comparison 1 Patch versus no patch: perioperative complications < 30 days, Outcome 6 Occlusion of the artery operated on.
Arterial rupture/haemorrhage
The overall risk of rupture and haemorrhage in all patients combined was low (1.5%). There was no significant difference between patching and primary closure but the confidence interval was wide (OR 1.24, 95% CI 0.61 to 2.54) (Analysis 1.7). None of the arterial haemorrhages was associated with a fatal or major stroke.
1.7. Analysis.
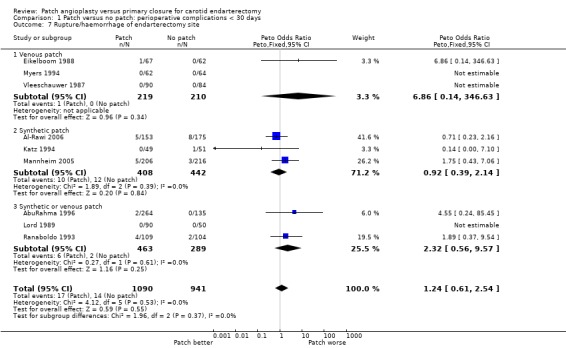
Comparison 1 Patch versus no patch: perioperative complications < 30 days, Outcome 7 Rupture/haemorrhage of endarterectomy site.
Local infection
There was only a two reported case of infection at the endarterectomy site (Katz 1994; Mannheim 2005). These occured in two patients in the synthetic patching group and six patients in the primary closure group. There was no significant difference between patching and primary closure but the confidence interval was wide (OR 0.38, 95% CI 0.09 to 1.54) (Analysis 1.8). Within each closure type there was significant heterogeneity (I2 = 61).
1.8. Analysis.
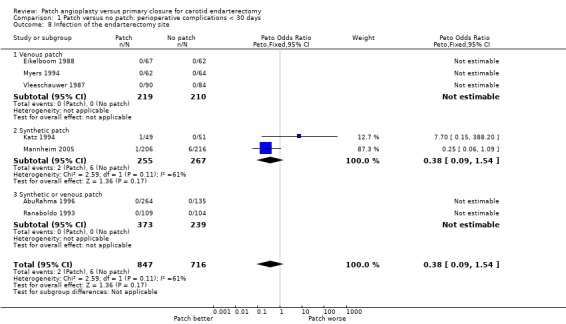
Comparison 1 Patch versus no patch: perioperative complications < 30 days, Outcome 8 Infection of the endarterectomy site.
Cranial nerve palsy
Only four trials supplied data on this outcome (AbuRahma 1996; Katz 1994; Mannheim 2005; Myers 1994), and in one of these no outcomes occurred. The risk of nerve palsy was low (2.6%) with no significant difference between patching and primary closure (OR 0.78, 95% CI 0.36 to 1.69) (Analysis 1.9).
1.9. Analysis.
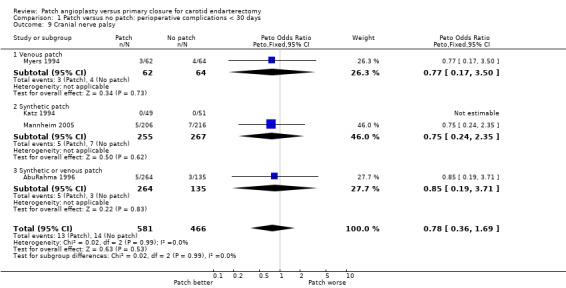
Comparison 1 Patch versus no patch: perioperative complications < 30 days, Outcome 9 Cranial nerve palsy.
Complications requiring return to theatre
When the number of complications (occlusion, haemorrhage, infection) that required return to theatre for re‐operation within 30 days of the first operation were considered, there was a significant trend in favour of carotid patching, that is carotid patching was associated with fewer returns to theatre (OR 0.35, 95% CI 0.16 to 0.79) (Analysis 1.10).
1.10. Analysis.
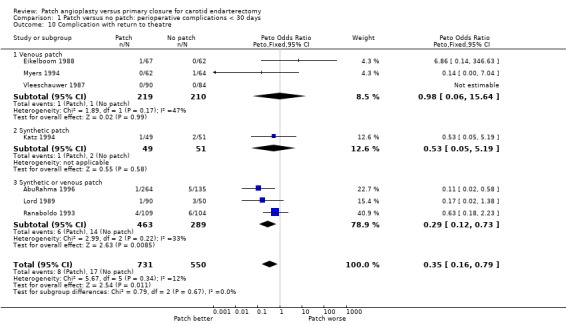
Comparison 1 Patch versus no patch: perioperative complications < 30 days, Outcome 10 Complication with return to theatre.
Outcomes during long‐term follow up (at least one year) including events during the first 30 days
One trial followed up patients for 30 days only (Lord 1989) and this trial has been excluded from these analyses. In the remaining trials, at least 56 patients (28 patch, 28 primary closure) were lost to follow up. These patients were assumed to be event free for the main analyses.
Stroke
Any stroke (fatal, non‐fatal, ipsilateral, contralateral, brainstem, infarct or haemorrhage)
There was a significant reduction in the risk of any stroke during follow up with patching (OR 0.49, 95% CI 0.27 to 0.90, P = 0.02) (Analysis 2.1). A similar reduction was seen in fatal strokes (OR 0.27 0.05 to 1.6 P = 0.15) (Analysis 2.2) but this was based on only five events. Within each closure type there was no heterogeneity, but there was significant between‐group heterogeneity (I2 = 72.5).
2.1. Analysis.
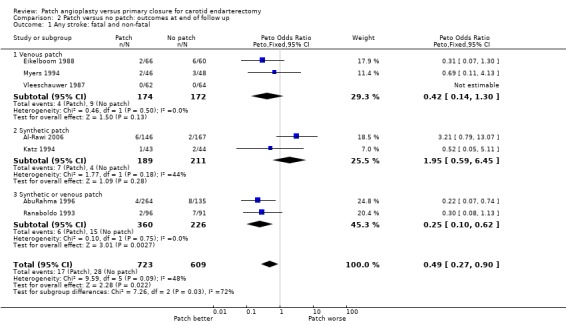
Comparison 2 Patch versus no patch: outcomes at end of follow up, Outcome 1 Any stroke: fatal and non‐fatal.
2.2. Analysis.
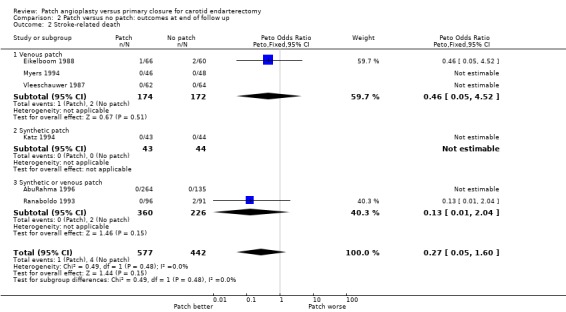
Comparison 2 Patch versus no patch: outcomes at end of follow up, Outcome 2 Stroke‐related death.
Ipsilateral stroke
Thirty‐three strokes were definitely ipsilateral and one other stroke was assumed to be ipsilateral although it was unclear whether it actually was (Eikelboom 1988). The reduction in risk of ipsilateral stroke with patching was similar to that for all strokes (OR 0.32, 95% CI 0.16 to 0.63, P = 0.001) (Analysis 2.3).
2.3. Analysis.
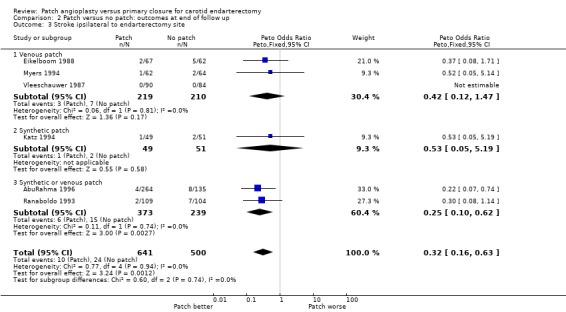
Comparison 2 Patch versus no patch: outcomes at end of follow up, Outcome 3 Stroke ipsilateral to endarterectomy site.
Death
One‐hundred‐and‐forty‐three patients died during follow up (10.7%). Even if all patients lost to follow up were assumed to be alive, patching was associated with a non‐significant reduction in the risk of death (OR 0.78, 95% CI 0.54 to 1.12, P = 0.18)(Analysis 2.4). Again, as outlined above, few of these deaths were directly attributable to stroke.
2.4. Analysis.
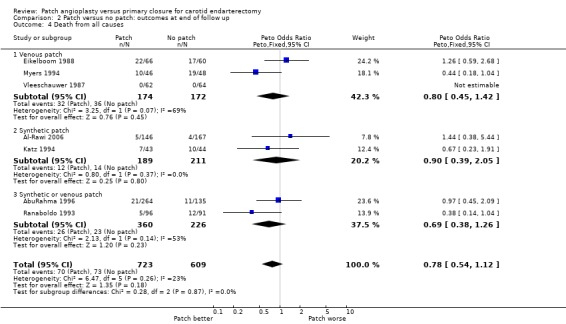
Comparison 2 Patch versus no patch: outcomes at end of follow up, Outcome 4 Death from all causes.
Any stroke or death
Patching was associated with a significant reduction in the risk of stroke or death (OR 0.59, 95% CI 0.42 to 0.84, P = 0.004) (13% patch versus 20.6% primary closure) (Analysis 2.5).
2.5. Analysis.
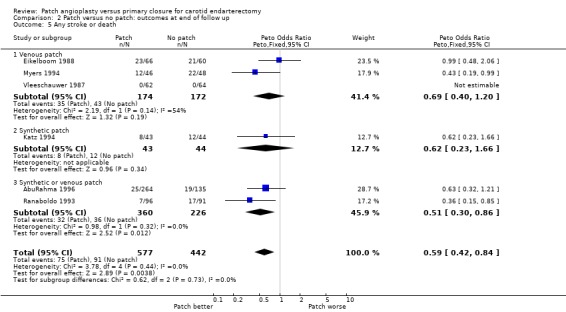
Comparison 2 Patch versus no patch: outcomes at end of follow up, Outcome 5 Any stroke or death.
Arterial complications
As noted in the Methods section, these results should be interpreted with caution since, in patients who underwent bilateral endarterectomies, outcomes in each artery were probably not independent. We were unable to identify how many patients with bilateral endarterectomies had outcomes events in both arteries.
Occlusion or restenosis greater than 50%
Patching was associated with a highly significant reduction in the risk of arterial occlusion or restenosis (OR 0.24, 95% CI 0.17 to 0.34, P < 0.00001) (Analysis 2.6). Within each closure type there was significant heterogeneity (I2 = 65) and there was significant between‐group heterogeneity (I2 = 72.4). Lack of data meant that it was not possible to correct for those patients who had died during follow up. However, the result appears to be particularly robust and is likely to remain significant even if corrected for the small numbers who died. Another problem is that the clinical significance of a reduction in occlusion or restenosis is unknown: the important outcome from the patient's point of view is a reduction in the risk of stroke. The trial by Eikelboom et al suggested that the reduction in restenosis or occlusion was confined to women, but this may be a chance subgroup effect or because women had an increased absolute risk of restenosis and so the numbers who developed restenosis were greater (Eikelboom 1988).
2.6. Analysis.
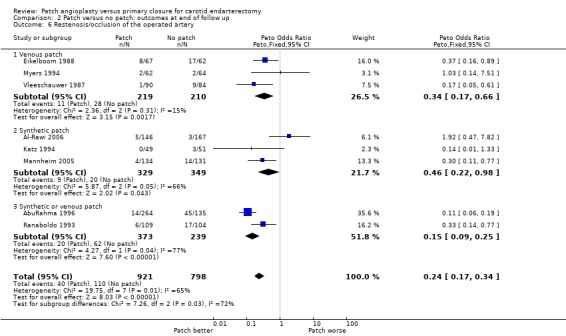
Comparison 2 Patch versus no patch: outcomes at end of follow up, Outcome 6 Restenosis/occlusion of the operated artery.
Pseudoaneurysm formation
No pseudoaneurysms were documented during follow up of at least one year in 1141 arteries.
Discussion
The results of this systematic review when first published in 1995 were considered to be inconclusive, although there appeared to be promising and potentially clinically important trends in favour of routine patching in terms of both short and long‐term reductions in risks of ipsilateral stroke. The results were felt to be unreliable because they were based on small number of outcome events (33 ipsilateral strokes in total), there were a number of losses to follow up and because methodological quality of the trials was on the whole poor (Table 3). However, in the 2004 update a good quality trial including 399 operations and 45 perioperative and long term events was added (AbuRahma 1996) and contributed additional weight to the conclusions drawn in the previous analyses. In the current update, three new small trials have been added, which has resulted in loss of statistical significance of the apparently reduced risk of any perioperative stroke in the patching group.
The significant reductions in the risk of acute occlusion or long‐term restenosis with patching may be less useful than data on clinically important outcomes such as stroke. Acute occlusion, though feared, is not always associated with stroke. Similarly, restenosis detected by routine Duplex scanning may not be clinically important. In some cases, remodelling of the arterial wall after endarterectomy can be mistaken for stenosis and in other cases spontaneous regression of Duplex defined stenosis has occurred (Bernstein 1990; Ranaboldo 1993). Moreover, in one study there was no significant association between restenosis and recurrent neurological symptoms (Knudsen 1990), whilst in another, patients with restenosis greater than 50% had a better long‐term prognosis in terms of death or stroke than patients with no significant restenosis (Bernstein 1990)!
Most surgeons agree that carotid patching does play a role in carotid endarterectomy since they are faced with situations when this type of closure is either unavoidable or positively desirable, for example an artery with a very narrow internal diameter or a very long plaque (Eikelboom 1988). However, it is unclear how frequently such situations arise and how narrow an artery should be before it has to be patched. For example, only two trials in this review excluded narrow arteries on the grounds that they must be patched. One trial excluded 23% of arteries because they were less than 5 mm diameter (Myers 1994), whilst another trial excluded only 1% of arteries because they were less than 3.5 mm diameter (Katz 1994). In the other trials, very few patients had to cross over from primary closure to patching because the artery was deemed too narrow for primary closure. A British survey also demonstrated that there is divided opinion on how often patching is required: some surgeons use it all of the time, others rarely or never (Girn 2008). The trials of patch versus no patch included in this review tested the policy of routinely patching all arteries against a policy of never patching in those patients in whom there was no definite indication for a patch. A policy of selective patching of only those arteries thought to require a patch at the time of operation compared to no patching has not been tested in randomised controlled trials.
It is possible that if patching is effective its benefit may be restricted to narrow arteries (Golledge 1996). This would be analogous to carotid endarterectomy for symptomatic carotid stenosis where the benefit is restricted to those with severe artery stenosis (ECST 1991). We were unable to test this hypothesis because the results of the trials were not reported according to the degree of narrowing of the artery. One trial did exclude a significant number of arteries because they were less than 5 mm diameter (Myers 1994). The results of this trial were no worse than those of the other trials, which might suggest that there is little difference in the effect of patching between arteries greater than or less than 5 mm diameter. However, such indirect comparisons between trials are unreliable.
There were significant methodological flaws in these trials which should be addressed in future trials. Inadequate methods of randomisation and blinding were frequently used, which can seriously bias the results of trials (Schulz 1995). In most trials, the blinding of outcome assessment was unclear. Three trials mentioned blinding, but no trial assessed outcome by neurologists or stroke physicians. It is well known that studies that have neurologists as assessors are associated with higher stroke and death rates (Rothwell 1995). The trials were generally too small to achieve adequate statistical power and none were analysed on a true intention‐to‐treat basis, partly because there were significant losses to follow up. Problems arose with the randomisation of arteries rather than patients, and there was poor reporting of the numbers of ipsilateral strokes and disabling strokes in each treatment group.
Authors' conclusions
Implications for practice.
At present, most vascular surgeons in some countries such as the United Kingdom do routinely use patching in all patients undergoing carotid endarterectomy (Girn 2008). The results of this review provide some further support for routine patching, although more conclusive evidence is required as numbers are still small. Individual surgeons (and patients) may still interpret the evidence differently and, therefore, it is up to each surgeon to decide whether to patch routinely or not. The use of selective patching (such as for very narrow arteries) has not been studied in randomised controlled trials and so, although it is likely to be required on occasions, no clear indications for selective patching can be given.
Implications for research.
The potential benefit of routine patching could be clinically important but in order to have reliable evidence on the risks and benefits of patching compared with primary closure, a large multicentre randomised controlled trial will be required. This trial should concentrate on clinical outcomes (deaths, all strokes, particularly fatal or disabling strokes and ipsilateral strokes) as opposed to restenosis, and have long‐term follow up (perhaps five years). Assuming a 30‐day risk of stroke or death of 5%, the trial would need to recruit about 3000 patients to have a 90% chance of detecting a reduction in the absolute risk of death or stroke to 2.5% (this number would also give a greater‐than‐90% chance of detecting a reduction in the risk of stroke or death at five years from 25% to 20%). Such a trial should use a secure method of randomisation and be performed on a true intention‐to‐treat basis with complete follow up of all patients. Patients rather than arteries should be randomised so that the number of deaths and strokes are reported on a patient basis rather than an artery basis. Clinical follow up should be blinded with independent assessment of strokes, preferably by neurologists (Rerkasem 2009; Rothwell 1995). The results should be analysed according to the degree of narrowing of the artery and whether the patient has had a previous stroke or transient ischaemic attack or not. It would be possible to use a factorial design for such a trial so that some other procedure could be tested simultaneously, such as routine shunting. Until the benefit of carotid patching in terms of clinical outcomes for the patient is established, any future trials should include a control group of primary closure.
What's new
| Date | Event | Description |
|---|---|---|
| 5 May 2009 | New search has been performed | The searches have been completed to November 2008. In the four years since the previous version of this Cochrane Review was published, there have been two new randomised trials and we also added a trial published in 1986 which was not identified in the previous review. The new trials included data on 750 operations and 17 stroke/death outcomes. The conclusion of this updated review is more conservative than that in the previous review. |
| 5 May 2009 | New citation required and conclusions have changed | The authorship of the review has changed. The conclusion of the review has changed. |
History
Protocol first published: Issue 3, 1996 Review first published: Issue 3, 1996
| Date | Event | Description |
|---|---|---|
| 9 September 2008 | Amended | Converted to new review format. |
| 28 February 2003 | New search has been performed | Differences between this review and the previous version: one new trial (357 patients and 399 operations), comparing primary closure with venous patching (saphenous or jugular vein) and with synthetic patching, has published its early and late results (AbuRahma 1996 and 1998) and has been included in the review. |
Acknowledgements
We would like to thank Dr Rick Bond, Professor Ali AbuRahma and Professor Ross Naylor for their contribution to previous versions of this review; and Hazel Fraser for providing us with references to relevant trials from the Cochrane Stroke Group Trials Register.
Ongoing trials
If anyone is aware of any randomised trials that we have omitted, please contact Professor Peter Rothwell.
Appendices
Appendix 1. MEDLINE search strategies
MEDLINE 1966 to 1995
Term used "carotid endarterectomy"
MEDLINE (Ovid) 1994 to 2008 and Cochrane Central Register of Controlled Trials (lines 1 to 12)
1 Endarterectomy, carotid/ 2 exp carotid arteries/su (surgery) 3 exp carotid artery diseases/su 4 exp carotid arteries/ 5 exp carotid artery diseases/ 6 carotid.tw. 7 4 or 5 or 6 8 endarterectomy/ 9 (endarterectom$ or surg$).tw. 10 8 or 9 11 7 and 10 12 1 or 2 or 3 or 11 13 randomized controlled trial.pt. 14 randomized controlled trials as topic/ 15 controlled clinical trial.pt. 16 controlled clinical trials as topic/ 17 random allocation/ 18 clinical trial.pt. 19 exp clinical trials as topic/ 20 (clin$ adj25 trial$).tw. 21 random$.tw. 22 research design/ 23 intervention studies/ 24 control$.tw. 25 patch$.tw. 26 or/13‐25 27 12 and 26 28 limit 27 to humans
Appendix 2. EMBASE search strategies
EMBASE 1980 to 1995
Terms used "carotid endarterectomy" and "carotid surgery"
EMBASE (Ovid) 1994 to 2008
1 carotid endarterectomy/ 2 carotid artery surgery/ 3 exp carotid artery disease/su 4 exp carotid artery/ 5 exp carotid artery disease/ 6 4 or 5 7 artery surgery/ or endarterectomy/ or vascular surgery/ or surgery/ 8 6 and 7 9 (carotid adj5 (endarterect$ or surgery)).tw. 10 1 or 2 or 3 or 8 or 9 11 Clinical trial/ 12 randomized controlled trial/ 13 controlled study/ 14 randomization/ 15 random$.tw. 16 Prospective study/ 17 "Evaluation and follow up"/ or Follow up/ 18 versus.tw. 19 prospective.tw. 20 types of study/ 21 methodology/ 22 comparative study/ 23 ((intervention or experiment$) adj5 group$).tw. 24 Parallel design/ 25 intermethod comparison/ 26 (controls or control group$).tw. 27 (control$ adj trial$).tw. 28 patch$.tw. 29 or/11‐28 30 10 and 29 31 limit 30 to humans
Data and analyses
Comparison 1. Patch versus no patch: perioperative complications < 30 days.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any stroke: fatal and non‐fatal | 8 | 1769 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.57 [0.31, 1.03] |
| 1.1 Venous patch | 3 | 346 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.38 [0.08, 1.72] |
| 1.2 Synthetic patch | 3 | 837 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.23 [0.50, 3.07] |
| 1.3 Synthetic or venous patch | 2 | 586 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.29 [0.11, 0.74] |
| 2 Stroke‐related death | 7 | 1441 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.47 [0.05, 4.56] |
| 2.1 Venous patch | 3 | 346 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.91 [0.06, 14.73] |
| 2.2 Synthetic patch | 2 | 509 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Synthetic or venous patch | 2 | 586 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.00, 6.46] |
| 3 Stroke ipsilateral to endarterectomy site | 7 | 1201 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.31 [0.15, 0.63] |
| 3.1 Venous patch | 3 | 349 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.39 [0.09, 1.75] |
| 3.2 Synthetic patch | 1 | 100 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.53 [0.05, 5.19] |
| 3.3 Synthetic or venous patch | 3 | 752 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.27 [0.11, 0.62] |
| 4 Death from all causes | 9 | 1869 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.62 [0.18, 2.09] |
| 4.1 Venous patch | 3 | 346 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.79 [0.18, 17.57] |
| 4.2 Synthetic patch | 3 | 837 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.00, 7.15] |
| 4.3 Synthetic or venous patch | 3 | 686 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.48 [0.10, 2.24] |
| 5 Any stroke or death | 8 | 1769 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.58 [0.33, 1.01] |
| 5.1 Venous patch | 3 | 346 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.55 [0.13, 2.25] |
| 5.2 Synthetic patch | 3 | 837 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.10 [0.45, 2.69] |
| 5.3 Synthetic or venous patch | 2 | 586 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.34 [0.15, 0.78] |
| 6 Occlusion of the artery operated on | 7 | 1435 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.18 [0.08, 0.41] |
| 6.1 Venous patch | 2 | 255 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.12 [0.01, 1.99] |
| 6.2 Synthetic patch | 2 | 428 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.33 [0.06, 1.95] |
| 6.3 Synthetic or venous patch | 3 | 752 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.16 [0.06, 0.42] |
| 7 Rupture/haemorrhage of endarterectomy site | 9 | 2031 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.24 [0.61, 2.54] |
| 7.1 Venous patch | 3 | 429 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 6.86 [0.14, 346.63] |
| 7.2 Synthetic patch | 3 | 850 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.92 [0.39, 2.14] |
| 7.3 Synthetic or venous patch | 3 | 752 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.32 [0.56, 9.57] |
| 8 Infection of the endarterectomy site | 7 | 1563 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.38 [0.09, 1.54] |
| 8.1 Venous patch | 3 | 429 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Synthetic patch | 2 | 522 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.38 [0.09, 1.54] |
| 8.3 Synthetic or venous patch | 2 | 612 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Cranial nerve palsy | 4 | 1047 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.78 [0.36, 1.69] |
| 9.1 Venous patch | 1 | 126 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.77 [0.17, 3.50] |
| 9.2 Synthetic patch | 2 | 522 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.75 [0.24, 2.35] |
| 9.3 Synthetic or venous patch | 1 | 399 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.85 [0.19, 3.71] |
| 10 Complication with return to theatre | 7 | 1281 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.35 [0.16, 0.79] |
| 10.1 Venous patch | 3 | 429 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.98 [0.06, 15.64] |
| 10.2 Synthetic patch | 1 | 100 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.53 [0.05, 5.19] |
| 10.3 Synthetic or venous patch | 3 | 752 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.29 [0.12, 0.73] |
1.2. Analysis.
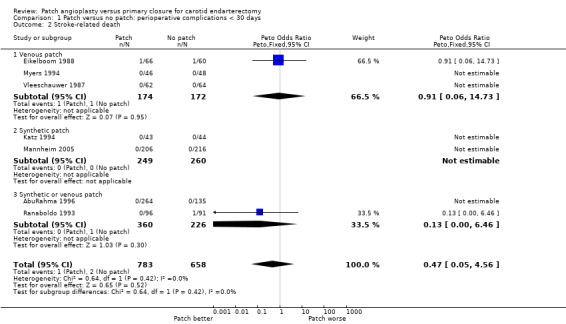
Comparison 1 Patch versus no patch: perioperative complications < 30 days, Outcome 2 Stroke‐related death.
Comparison 2. Patch versus no patch: outcomes at end of follow up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any stroke: fatal and non‐fatal | 7 | 1332 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.49 [0.27, 0.90] |
| 1.1 Venous patch | 3 | 346 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.42 [0.14, 1.30] |
| 1.2 Synthetic patch | 2 | 400 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.95 [0.59, 6.45] |
| 1.3 Synthetic or venous patch | 2 | 586 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.25 [0.10, 0.62] |
| 2 Stroke‐related death | 6 | 1019 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.27 [0.05, 1.60] |
| 2.1 Venous patch | 3 | 346 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.46 [0.05, 4.52] |
| 2.2 Synthetic patch | 1 | 87 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Synthetic or venous patch | 2 | 586 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.01, 2.04] |
| 3 Stroke ipsilateral to endarterectomy site | 6 | 1141 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.32 [0.16, 0.63] |
| 3.1 Venous patch | 3 | 429 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.42 [0.12, 1.47] |
| 3.2 Synthetic patch | 1 | 100 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.53 [0.05, 5.19] |
| 3.3 Synthetic or venous patch | 2 | 612 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.25 [0.10, 0.62] |
| 4 Death from all causes | 7 | 1332 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.78 [0.54, 1.12] |
| 4.1 Venous patch | 3 | 346 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.80 [0.45, 1.42] |
| 4.2 Synthetic patch | 2 | 400 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.90 [0.39, 2.05] |
| 4.3 Synthetic or venous patch | 2 | 586 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.69 [0.38, 1.26] |
| 5 Any stroke or death | 6 | 1019 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.59 [0.42, 0.84] |
| 5.1 Venous patch | 3 | 346 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.69 [0.40, 1.20] |
| 5.2 Synthetic patch | 1 | 87 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.62 [0.23, 1.66] |
| 5.3 Synthetic or venous patch | 2 | 586 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.51 [0.30, 0.86] |
| 6 Restenosis/occlusion of the operated artery | 8 | 1719 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.24 [0.17, 0.34] |
| 6.1 Venous patch | 3 | 429 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.34 [0.17, 0.66] |
| 6.2 Synthetic patch | 3 | 678 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.46 [0.22, 0.98] |
| 6.3 Synthetic or venous patch | 2 | 612 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.15 [0.09, 0.25] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
AbuRahma 1996.
| Methods | R = computer‐generated sealed envelopes (artery randomised) Both duplex and clinical FU blinded Crossovers: 4 patients in vein group underwent primary closure and were excluded from the trial; 3 jugular vein patients had saphenous vein and were included in trial | |
| Participants | USA 357 patients, 399 operations in three arms: 130 vein, 134 PTFE and 135 primary closure 50% male Mean age: 68 years 33% asymptomatic Comparability: age, sex, vascular risk factors, % asymptomatic disease similar in each group | |
| Interventions | Rx: polytetrafluoroethylene patch or alternating saphenous vein patch (from ankle) and jugular vein Control: primary closure Routine shunting for all and GA 325 mg daily aspirin was started within 24 hours of surgery for all patients | |
| Outcomes | Death, ipsilateral stroke, ipsilateral TIA and ipsilateral RIND at 30 days and 48 months Duplex evidence of restenosis > 50% during follow up | |
| Notes | Ex: patients with ICA < 4 mm or combined CABG or redo surgery FU: mean 30 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Al‐Rawi 2006.
| Methods | R = envelope Duplex blind, clinical FU not blind Crossovers: unknown Indication for shunt in any sign of ischaemia from transcranial Doppler, cerebral function monitor, near infrared spectrscopy, Doppler flowmetry Exclusion during trial: none Patients lost to FU: 7 patch, 8 no patch | |
| Participants | England 321 patients 338 operations 68% male Mean age: 69 years 10% asymptomatic carotid disease % stenosis unknown Comparability: age, sex, vascular risk factors, % asymptomatic disease were similar in each group | |
| Interventions | Rx: collagen‐coated polyester vascular patch Control: primary closure % shunted: unknown After surgery, all patients were given rectal aspirin (600 mg) | |
| Outcomes | Deaths < 30 days and end of FU Strokes < 30 days and end of FU Perioperative occlusion Bleeding or evacuation clot Cardiac event Restenosis > 50% or occlusion at end of FU (ultrasound) | |
| Notes | Ex: 10 patients due to: poor cerebral blood flow (3 patients), ST depression (1 patient), high tortuosity (6 patients) FU: 12 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | C ‐ Inadequate |
Eikelboom 1988.
| Methods | R = odd/even hospital number (patients randomised) Duplex blind, clinical FU not blind Crossovers: 3 primary closure to patch, 3 patch to primary closure (all analysed in original group) Exclusion during trial: none Patients lost to FU: 10 patch, 7 no patch | |
| Participants | The Netherlands 126 patients 129 operations 73% male Mean age: 63 years 18% asymptomatic carotid disease All arteries > 60% stenosis Comparability: age, sex, vascular risk factors, % asymptomatic disease were similar in each group | |
| Interventions | Rx: saphenous vein patch Control: primary closure 20% shunted Postoperative warfarin +/‐ antiplatelet for all | |
| Outcomes | Deaths < 30 days and end of FU Strokes < 30 days and end of FU Perioperative occlusion (intravenous DSA) Wound haemorrhage Restenosis > 50% or occlusion at end of FU (Duplex and intravenous DSA) | |
| Notes | Ex: simultaneous cardiac surgery FU: mean 5 years | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Katz 1994.
| Methods | R = odd/even social security number (patients randomised) Neither duplex nor clinical FU blind Crossovers: none Exclusions during trial: none Patients lost to FU: 12 in total | |
| Participants | USA 87 patients 100 operations 56% male Mean age: 67 years 40% asymptomatic carotid disease Comparability: age, sex, vascular risk factors, % asymptomatic disease were similar in each group | |
| Interventions | Rx: polytetrafluoroethylene patch Control: primary closure Routine shunting for all Postoperative aspirin (325 mg) for all | |
| Outcomes | Death < 30 days and end of FU Stroke < 30 days and end of FU Perioperative occlusion (Duplex) Wound haemorrhage, infection, cranial nerve palsy Restenosis > 50% or occlusion at end of FU (Duplex) | |
| Notes | Ex: previous endarterectomy, simultaneous cardiac surgery, internal carotid artery diameter < 3.5 mm FU: mean 29 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Lord 1989.
| Methods | R = sealed envelopes (artery randomised) Probably neither duplex nor clinical FU blind Crossovers: 4 (unclear which group these were in) Exclusions during trial: 4 crossovers Patients lost to FU: none | |
| Participants | Australia 123 patients 140 operations. 62% male Mean age: 63 years % asymptomatic carotid disease unknown Comparability: age, sex, vascular risk factors, % symptomatic disease similar in each group | |
| Interventions | Rx: saphenous vein patch or polytetrafluoroethylene patch (random allocation) Control: primary closure 17% shunted Postoperative aspirin for all | |
| Outcomes | Ipsilateral stroke < 30 days Perioperative occlusion (intravenous DSA) Wound haemorrhage | |
| Notes | Ex: unknown FU: until hospital discharge | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Mannheim 2005.
| Methods | R = sealed envelopes Duplex and clinical FU unknown for blinding Crossovers: none Exclusions during trial: none Patients lost to FU: unknown | |
| Participants | Israel 404 patients 422 operations 65.6% male Mean age: 69.5 years 53.7% asymptomatic carotid disease Comparability: sex, vascular risk factors, % asymptomatic disease similar in each group | |
| Interventions | Rx: polyester urethane patch angioplasty Control: primary closure Indication shunt for change in neurological status during carotid clamping or in patients in general anesthesia with stump pressure < 40 mmHg Peri and postoperative aspirin: unknown | |
| Outcomes | Death < 30 days and end of FU Stroke < 30 days and end of FU Coronary event Wound haemorrhage, infection, cranial nerve palsy Restenosis > 50% or occlusion at end of FU (Duplex) | |
| Notes | Ex: small ICA or need for interposition graft FU: 5 years | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | C ‐ Inadequate |
Myers 1994.
| Methods | R = opaque, sequentially numbered sealed envelopes (artery randomised) Duplex blind, clinical FU not blind Crossovers: none Exclusions during trial: none Patients lost to FU: 6 patch, 8 no patch | |
| Participants | USA 136 (109) patients 152 (126) operations 99% male Mean age: 62 years 23% asymptomatic carotid disease Comparability: age, sex, vascular risk factors, % asymptomatic disease similar in each group | |
| Interventions | Rx: saphenous vein patch Control: primary closure Routine shunt for all Peri and postoperative aspirin (325 mg) for all | |
| Outcomes | Death < 30 days and end of FU Stroke < 30 days and end of FU Perioperative occlusion (ocular pneumoplethysmography) Wound haemorrhage, infection, cranial nerve palsy Restenosis > 50% or occlusion at end of FU (Duplex) | |
| Notes | Ex: ICA diameter < 5mm, arteriotomy > 3 cm, looped or kinked ICA FU: 4 to 5 years | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Pratesi 1986.
| Methods | R = unknown Duplex and clinical FU: unknown Crossovers: unknown Exclusions during trial: unknown Patients lost to FU: unknown | |
| Participants | Italy 90 patients 100 operations Ratio: male:female 6:5 Mean age: unknown 10% asymptomatic carotid disease % stenosis: unknown Comparability: age, sex, vascular risk factors not reported by treatment group | |
| Interventions | Rx: % autologous vein or synthetic (unknown) Control: primary closure Shunt: unknown indication Aspirin before surgery, unknown after surgery | |
| Outcomes | Death < 30 days and FU period Stroke < 30 days and FU period Restenosis FU period (by DSA) | |
| Notes | Ex: unknown FU: 2 years | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | C ‐ Inadequate |
Ranaboldo 1993.
| Methods | R = opaque, sequentially‐numbered, sealed envelopes (artery randomised) Duplex and clinical FU blind Crossovers: 4 primary closure to patch (all analysed in original group) Exclusions during trial: none Patients lost to FU: none | |
| Participants | United Kingdom 199 patients 213 operations 69% male Mean age: 66 years 8% asymptomatic carotid disease 60% arteries > 75% stenosis Comparability: age, sex, vascular risk factors not reported by treatment group | |
| Interventions | Rx: autologous vein (N = 53) or Dacron (N = 56) patches (non‐random allocation) Control: primary closure Shunt 'when technically possible' Aspirin before surgery, unknown after surgery | |
| Outcomes | Death < 30 days and end of FU Stroke < 30 days and end of FU Perioperative occlusion (Duplex) Wound haemorrhage, infection Restenosis > 50% or occlusion at end of FU (Duplex) | |
| Notes | Ex: unknown FU: 12 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Vleeschauwer 1987.
| Methods | R ‐ sealed envelopes (not opaque or numbered) Clinical and Duplex FU blind Crossovers: none Exclusions during trial: patients with residual stenosis/occlusion (number known) Patients lost to FU: none | |
| Participants | Germany 126 patients 174 operations 60% male Mean age: 64 years 30% asymptomatic carotid disease Comparability: age, sex, vascular risk factors, % asymptomatic disease not reported by treatment group | |
| Interventions | Rx: autologous vein patch Control: primary closure Routine shunting for all Postoperative aspirin (1 g) for all | |
| Outcomes | Death < 30 days and end of FU Stroke < 30 days and end of FU Wound haemorrhage, infection Restensosis > 50% or occlusion at end of FU (Duplex) | |
| Notes | Ex: Recurrent stenosis, kinked ICA FU: 1 year | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
CABG: coronary artery bypass graft DSA: digital subtraction angiography Ex: exclusion criteria FU: follow up GA: general anaesthesia ICA: internal carotid artery R: concealment of allocation RIND: reversible ischaemic neurological deficit Rx: treatment TIA: transient ischaemic attack
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Gale 1985 | Randomised by flipping a coin Not intention‐to‐treat About 300 patients randomised to either vein patch or primary closure: 194 patients had the operation to which they were randomised, the remaining patients were randomised to 1 procedure but actually had the other procedure for some reason Results only available for the 194 patients who remained in the group to which they were originally allocated |
| Hertzer 1987 | Non‐random comparison of patching performed by one surgeon and primary closure performed by other surgeons in the same institute (personal communication with Dr Hertzer) |
Contributions of authors
Kittipan Rerkasem: designed the protocol, performed searches, selected studies for inclusion or exclusion, extracted data and updated the review. Peter Rothwell: selected studies for inclusion or exclusion, advised on the design of the protocol, updated the review, extracted data and locally co‐ordinated the update.
Sources of support
Internal sources
Oxford University Medical Research Fund (MRF), UK.
External sources
Medical Research Council, UK.
Chiang Mai University, Thailand.
NHS Executive Research and Development Directorate, UK.
Declarations of interest
None known
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
AbuRahma 1996 {published data only}
- AbuRahma AF, Khan JH, Robinson PA, Saiedy S, Short YS, Boland JP, et al. Prospective randomized trial of carotid endarterectomy with primary closure and patch angioplasty with saphenous vein, jugular vein, and polytetrafluoroethylene: perioperative (30 day) results. Journal of Vascular Surgery 1996;24:998‐1007. [DOI] [PubMed] [Google Scholar]
- AbuRahma AF, Robinson PA, Saiedy S, Kahn JH, Boland JP. Prospective randomized trial of carotid endarterectomy with primary closure and patch angioplasty with saphenous vein, jugular vein, and polytetrafluoroethylene: long‐term follow‐up. Journal of Vascular Surgery 1998;27(2):222‐32. [DOI] [PubMed] [Google Scholar]
Al‐Rawi 2006 {published data only}
- Al‐Rawi PG, Turner CL, Waran V, Ng I, Kirkpatrick PJ. A randomized trial of synthetic patch versus direct primary closure in carotid endarterectomy. Neurosurgery 2006;59:822‐29. [DOI] [PubMed] [Google Scholar]
Eikelboom 1988 {published and unpublished data}
- Eikelboom BC, Ackerstaff RGA, Hoeneveld H, Ludwig JW, Teeuwen C, Vermeulen FEE, et al. Benefits of carotid patching: a randomized study. Journal of Vascular Surgery 1988;7:240‐7. [DOI] [PubMed] [Google Scholar]
- Letter JAM, Moll FL, Welten RJT, Eikelboom BC, Ackerstaff RGA, Vermeulen FEE, et al. Benefits of carotid patching: a prospective randomized study with long‐term follow‐up. Annals of Vascular Surgery 1994;8:54‐8. [DOI] [PubMed] [Google Scholar]
Katz 1994 {published and unpublished data}
- Katz D, Snyder SO, Gandhi RH, Wheeler JR, Gregory RT, Gayle RG, et al. Long‐term follow up for recurrent stenosis: a prospective randomized study of expanded polytetrafluoroethylene patch angioplasty versus primary closure after carotid endarterectomy. Journal of Vascular Surgery 1994;19:198‐205. [DOI] [PubMed] [Google Scholar]
Lord 1989 {published and unpublished data}
- Lord RSA, Raj TB, Stary DL, Nash PA, Graham AR, Goh KH. Comparison of saphenous vein patch, polytetrafluoroethylene patch, and direct arteriotomy closure after carotid endarterectomy. Part I: Perioperative results. Journal of Vascular Surgery 1989;9:521‐9. [PubMed] [Google Scholar]
Mannheim 2005 {published data only}
- Mannheim D, Weller B, Vahadim E, Karmeli R. Carotid endarterectomy with a polyurethane patch versus primary closure: a prospective randomized study. Journal of Vascular Surgery 2005;41:403‐8. [DOI] [PubMed] [Google Scholar]
Myers 1994 {published and unpublished data}
- Clagett GP, Patterson CB, Fisher Jr DF, Fry RE, Eidt JF, Humble TH, et al. Vein patch versus primary closure for carotid endarterectomy. A randomized prospective study in a selected group of patients. Journal of Vascular Surgery 1989;9:213‐23. [DOI] [PubMed] [Google Scholar]
- Myers SI, Valentine RJ, Chervu A, Bowers BL, Clagett GP. Saphenous vein patch versus primary closure for carotid endarterectomy: long term assessment of a randomized prospective study. Journal of Vascular Surgery 1994;19:15‐22. [DOI] [PubMed] [Google Scholar]
Pratesi 1986 {published data only}
- Pratesi C, Frullini A, Rega L, Fonda C, Matticari S, Alessi Innocenta A, et al. The follow‐up after carotid TEA. In: Maurer PC, Becker HM, Heidrich H, Hoffmann G, Kriessmann A, Muller‐Wiefel H, Pratorius C editor(s). Trends and Controversies. Vol. 1, Munich: W Zuckschwerdt, 1986:313‐5. [Google Scholar]
Ranaboldo 1993 {published and unpublished data}
- Ranaboldo CJ, Barros D'Sa ABB, Bell PRF, Chant ADB, Perry PM, the Joint Vascular Research Group. Randomized controlled trial of patch angioplasty for carotid endarterectomy. British Journal of Surgery 1993;80:1528‐30. [DOI] [PubMed] [Google Scholar]
Vleeschauwer 1987 {published and unpublished data}
- Vleeschauwer P, Wirthle W, Holler L, Krause E, Horsch S. Is venous patch grafting after carotid endarterectomy able to reduce the rate of restenosis? Prospective randomized pilot study with stratification. Acta Chirurgica Belgica 1987;87:242‐6. [PubMed] [Google Scholar]
References to studies excluded from this review
Gale 1985 {unpublished data only}
- Gale S. Carotid endarterectomy with and without vein patch: a prospective randomized study. Journal of Vascular Surgery 1988;7:240‐7. [Google Scholar]
Hertzer 1987 {published and unpublished data}
- Hertzer NR, Beven EG, O'Hara PJ, Krajewski LP. A prospective study of vein patch angioplasty during carotid endarterectomy. Three‐year results for 801 patients and 917 operations. Annals of Surgery 1987;206:628‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
ACAS 1995
- Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA 1995;273(18):1421‐8. [PubMed] [Google Scholar]
APT 1994
- Antiplatelet Trialists' Collaboration. Collaborative overview of trials of antiplatelet therapy ‐ I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ 1994;308:81‐106. [PMC free article] [PubMed] [Google Scholar]
Awad 1989
- Awad IA, Little JR. Patch angioplasty in carotid endarterectomy. Advantages, concerns, and controversies. Stroke 1989;20:417‐22. [DOI] [PubMed] [Google Scholar]
Bernstein 1990
- Bernstein EF, Torem S, Dilley RB. Does carotid restenosis predict an increased risk of late symptoms, stroke or death?. Annals of Surgery 1990;212:629‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bernstein 1992
- Bernstein EF. Routine versus selective carotid patching. Journal of Vascular Surgery 1992;15:868‐9. [DOI] [PubMed] [Google Scholar]
Bond 2003
- Bond R, Rerkasem K, Naylor AR, Rothwell PM. Patches of different types for carotid patch angioplasty. Cochrane Database of Systematic Reviews 2004, Issue 2. [Art. No.: CD000071. DOI: 10.1002/14651858.CD000071] [DOI] [PubMed] [Google Scholar]
Das 1985
- Das MB, Hertzer NR, Ratliff NB, O'Hara PJ, Beven EG. Recurrent carotid stenosis. A five‐year series of 65 reports. Annals of Surgery 1985;202:28‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
ECST 1991
- European Carotid Surgery Trialists' Collaborative Group. MRC European Carotid Surgery Trial: Interim results for symptomatic patients with severe (70‐99%) or with mild (0‐29%) carotid stenosis. Lancet 1991;337:1235‐43. [PubMed] [Google Scholar]
Frericks 1998
- Frericks H, Kievit J, Baalen JM, Bockel JH. Carotid recurrent stenosis and risk of ipsilateral stroke: a systematic review of the literature. Stroke 1998;29:244‐50. [DOI] [PubMed] [Google Scholar]
Girn 2008
- Girn HRS, Dellagrammaticas D, Laughlan K, Gough MJ, on behalf of the GALA Trial Collaborators. Carotid endarterectomy: technical practices of surgeons participating in the GALA trial. European Journal of Vascular and Endovascular Surgery 2008;36:385‐9. [DOI] [PubMed] [Google Scholar]
Golledge 1996
- Golledge J, Cuming R, Davies AH, Greenhalgh RM. Outcome of selective patching following carotid endarterectomy. European Journal of Vascular and Endovascular Surgery 1996;11:458‐63. [DOI] [PubMed] [Google Scholar]
Halliday 2004
- Halliday A, Mansfield A, Marro J, Peto C, Peto R, Potter J, et al. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet 2004;363:1491‐502. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Knudsen 1990
- Knudsen L, Sillesen H, Schroeder T, Hansen HJB. Eight to ten years follow‐up after carotid endarterectomy: clinical evaluation and doppler examination of patients operated on between 1978‐1980. European Journal of Vascular Surgery 1990;4:259‐64. [DOI] [PubMed] [Google Scholar]
Ouriel 1987
- Ouriel K, Green RM. Clinical and technical factors influencing recurrent carotid stenosis and occlusion after endarterectomy. Journal of Vascular Surgery 1987;5:702‐6. [PubMed] [Google Scholar]
Rerkasem 2009
- Rerkasem K, Rothwell PM. Temporal trends in the risks of stroke and death due to endarterectomy for symptomatic carotid stenosis: an updated systematic review. European Journal of Vascular and Endovascular Surgery 2009;37:504‐11. [DOI] [PubMed] [Google Scholar]
Rothwell 1995
- Rothwell P, Warlow C. Is self‐audit reliable?. Lancet 1995;346:1623. [DOI] [PubMed] [Google Scholar]
Rothwell 2003
- Rothwell PM, Eliasziw M, Gutnikov SA, Fox AJ, Taylor DW, Mayberg MR, et al. Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet 2003;361:107‐16. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Volteas 1994
- Volteas N, Labropoulos N, Leon M, Kalodiki E, Chan P, Nicolaides AN. Risk factors associated with recurrent carotid stenosis. International Angiology 1994;13:143‐7. [PubMed] [Google Scholar]
Zierler 1982
- Zierler RE, Bandyk DF, Thiele BL, Strandness Jr DE. Carotid artery stenosis following endarterectomy. Archives of Surgery 1982;117:1408‐15. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Bond 2004
- Bond R, Rerkasem K, AbuRahma AF, Naylor AR, Rothwell PM. Patch angioplasty versus primary closure for carotid endarterectomy. Cochrane Database of Systematic Reviews 2004, Issue 2. [Art. No.: CD000160. DOI: 10.1002/14651858.CD000160.pub2] [DOI] [PubMed] [Google Scholar]
Counsell 1996
- Counsell C, Salinas R, Warlow C, Naylor R. Patch angioplasty versus primary closure for carotid endarterectomy. Cochrane Database of Systematic Reviews 1996, Issue 1. [Art. No.: CD000160. DOI: 10.1002/14651858.CD000160] [DOI] [PubMed] [Google Scholar]


