Abstract
Background
Lower respiratory tract infection (LRTI) is the third leading cause of death worldwide and the first leading cause of death in low‐income countries. Community‐acquired pneumonia (CAP) is a common condition that causes a significant disease burden for the community, particularly in children younger than five years, the elderly and immunocompromised people. Antibiotics are the standard treatment for CAP. However, increasing antibiotic use is associated with the development of bacterial resistance and side effects for the patient. Several studies have been published regarding optimal antibiotic treatment for CAP but many of these data address treatments in hospitalised patients. This is an update of our 2009 Cochrane Review and addresses antibiotic therapies for CAP in outpatient settings.
Objectives
To compare the efficacy and safety of different antibiotic treatments for CAP in participants older than 12 years treated in outpatient settings with respect to clinical, radiological and bacteriological outcomes.
Search methods
We searched CENTRAL (2014, Issue 1), MEDLINE (January 1966 to March week 3, 2014), EMBASE (January 1974 to March 2014), CINAHL (2009 to March 2014), Web of Science (2009 to March 2014) and LILACS (2009 to March 2014).
Selection criteria
We looked for randomised controlled trials (RCTs), fully published in peer‐reviewed journals, of antibiotics versus placebo as well as antibiotics versus another antibiotic for the treatment of CAP in outpatient settings in participants older than 12 years of age. However, we did not find any studies of antibiotics versus placebo. Therefore, this review includes RCTs of one or more antibiotics, which report the diagnostic criteria and describe the clinical outcomes considered for inclusion in this review.
Data collection and analysis
Two review authors (LMB, TJMV) independently assessed study reports in the first publication. In the 2009 update, LMB performed study selection, which was checked by TJMV and MMK. In this 2014 update, two review authors (SP, SM) independently performed and checked study selection. We contacted trial authors to resolve any ambiguities in the study reports. We compiled and analysed the data. We resolved differences between review authors by discussion and consensus.
Main results
We included 11 RCTs in this review update (3352 participants older than 12 years with a diagnosis of CAP); 10 RCTs assessed nine antibiotic pairs (3321 participants) and one RCT assessed four antibiotics (31 participants) in people with CAP. The study quality was generally good, with some differences in the extent of the reporting. A variety of clinical, bacteriological and adverse events were reported. Overall, there was no significant difference in the efficacy of the various antibiotics. Studies evaluating clarithromycin and amoxicillin provided only descriptive data regarding the primary outcome. Though the majority of adverse events were similar between all antibiotics, nemonoxacin demonstrated higher gastrointestinal and nervous system adverse events when compared to levofloxacin, while cethromycin demonstrated significantly more nervous system side effects, especially dysgeusia, when compared to clarithromycin. Similarly, high‐dose amoxicillin (1 g three times a day) was associated with higher incidence of gastritis and diarrhoea compared to clarithromycin, azithromycin and levofloxacin.
Authors' conclusions
Available evidence from recent RCTs is insufficient to make new evidence‐based recommendations for the choice of antibiotic to be used for the treatment of CAP in outpatient settings. Pooling of study data was limited by the very low number of studies assessing the same antibiotic pairs. Individual study results do not reveal significant differences in efficacy between various antibiotics and antibiotic groups. However, two studies did find significantly more adverse events with use of cethromycin as compared to clarithromycin and nemonoxacin when compared to levofloxacin. Multi‐drug comparisons using similar administration schedules are needed to provide the evidence necessary for practice recommendations. Further studies focusing on diagnosis, management, cost‐effectiveness and misuse of antibiotics in CAP and LRTI are warranted in high‐, middle‐ and low‐income countries.
Plain language summary
Antibiotics for community‐acquired pneumonia in adolescent and adult outpatients
Review question
This review studied the effects of antibiotics on adolescents and adults with pneumonia acquired and treated in the community (as opposed to acquiring pneumonia in hospital and/or being treated for pneumonia in hospital). The evidence is current to March 2014.
Background
Lower respiratory tract infection is the third leading cause of death worldwide and the first leading cause of death in low‐income countries. Pneumonia, or infection of the lungs, is a common condition representing a significant disease burden for the community. Pneumonia is especially life‐threatening in children younger than five years, in older people and in people with other illnesses that may affect their immune system (such as diabetes or HIV/AIDS, or solid organ transplant recipients). Antibiotics are the most common treatment for pneumonia and these can vary in their effectiveness and adverse effects.
Study characteristics
We identified 11 trials (with 3352 participants older than 12 years with a diagnosis of community‐acquired pneumonia), fully published in peer‐reviewed journals, focused on treatment of pneumonia in adolescents and adults treated in the community in outpatient settings. This included five new trials included since our last review published in 2009. None of the trials included antibiotics versus placebo; all trials included one or more antibiotics. All participants were diagnosed with pneumonia based upon clinical diagnosis by the physician and chest X‐ray.
Study funding sources
All included trials were well conducted; nine of the 11 trials were sponsored by bio‐pharmaceutical companies manufacturing the antibiotics used in the study, or their authors were closely linked with the company.
Key results
Nine of the included trials compared different antibiotics and, hence, we could not combine the results of the individual trials to present our overall conclusion. There were some notable adverse events in seven studies: 1) erythromycin demonstrated significant gastrointestinal side effects compared to clarithromycin in two studies; 2) nemonoxacin demonstrated higher gastrointestinal (nausea, diarrhoea) and nervous system (dizziness, headache) adverse events compared to levofloxacin; 3) cethromycin demonstrated more side effects, especially a distortion of the sense of taste, than clarithromycin; 4) gastritis and diarrhoea were more common in the high‐dose amoxicillin group (1 g three times a day) compared to the other three antibiotic groups (clarithromycin, azithromycin and levofloxacin).
Conclusion
Unfortunately, there were not enough trials to compare the effects of different antibiotics for pneumonia acquired and treated in the community.
Background
Description of the condition
Community‐acquired pneumonia (CAP), which excludes cases acquired in hospital, nursing homes and long‐term care facilities, is a common condition that carries a high burden of mortality and morbidity. This burden is carried particularly in children younger than five years, the elderly and immunocompromised people. Prospective studies conducted in the United Kingdom, Finland and the United States have estimated the annual incidence of CAP in community‐dwelling adults at 5 to 11 cases per 1000 adult population; the incidence is known to vary markedly with age, being higher in the very young and the elderly (BTS 2009; Foy 1979; Jokinen 1993; Torres 1991; Woodhead 1987).
CAP is the most important cause of death from infectious causes in high‐income countries and the seventh most important cause of death overall (IDSA/ATS 2007; Mandell 2007). CAP can be caused by a broad range of pathogens, including bacteria, atypical bacteria Chlamydophila pneumoniae (C. pneumoniae),Mycoplasma pneumoniae (M. pneumoniae), Legionella pneumophila (L. pneumophila) and viruses (IDSA/ATS 2007; Mandell 2007; Welte 2012). In fact, more than 100 different micro‐organisms have been associated with CAP (Loeb 2002). Furthermore, a patient with CAP can be infected with more than one microbe, as in the case of a bacterial superinfection of an underlying influenza infection. The most common pathogens in normal hosts include Streptococcus pneumoniae (S. pneumoniae) (usually by far the most common),C. pneumoniae,Haemophilus influenzae (H. influenzae),M. pneumoniae and influenza viruses (BTS 2009; IDSA/ATS 2007; Loeb 2002; Mandell 2007; Welte 2012).
Significant costs are associated with the diagnosis and management of CAP. In the UK, 22% to 42% of adults with CAP are admitted to hospital (BTS 2009) and of those 1.2% to 10% need to be admitted to an intensive care unit (BTS 2009). In a recent European review, pneumonia accounted for 10.1 billion Euros annually (Welte 2012).
Description of the intervention
Antibiotics are the mainstay of treatment for CAP and the causative organisms usually respond well. Consequently, CAP treatment is associated with the development of bacterial resistance. In treating patients with CAP, the choice of antibiotic is a difficult one. Factors that must be considered are the possible aetiologic pathogen, the efficacy of the substance, potential side effects, the treatment schedule, patient adherence to treatment, the particular regional resistance profile of the causative organism, common pathogens in particular age groups (children versus elderly), co‐morbidities that might influence the range of potential pathogens (such as in cystic fibrosis or solid organ transplant recipients), the dosage of antibiotics (as in the case of renal insufficiency) and the clinical severity of CAP.
How the intervention might work
Bacterial pathogens remain the most common causative agents of CAP. Use of appropriate antibiotics is essential, otherwise the chosen antibiotic may not be effective and could pose a danger to the patient due to side effects. If a specific pathogen has been identified, antibiotic therapy should be directed at this pathogen, so that it is more effective and less harmful.
Why it is important to do this review
Many clinical trials have been performed to evaluate and compare the efficacy of antibiotics for CAP. However, the vast majority of them were conducted in hospitalised patients. These patients usually suffer from more severe manifestations of the disease and often have other co‐morbid conditions that affect their response to treatment and their time to recovery. Consequently, it is unclear if results of therapy in hospitalised patients can be extrapolated to outpatients. Numerous guidelines exist to aid clinicians with the treatment of CAP: in recent years, guidelines have been published by the American Thoracic Society (ATS 2001), the Infectious Diseases Society of America (IDSA 2000, updated December 2003 and later in 2007; IDSA 2003; IDSA/ATS 2007; Mandell 2007), the British Thoracic Society (BTS 2004; update: BTS 2009), the Canadian Community‐Acquired Pneumonia Working Group (CCAPWG 2000), the European Respiratory Society (Woodhead 2011), a German Guidelines Group (Höffken 2010), a Dutch CAP Guidelines group (Wiersinga 2011), the Gulf Cooperation Council (Memish 2007), the Japanese Respiratory Society (JRS 2006), the Latin American Thoracic Association (ALAT 2001, update: ALAT 2004), the South African Thoracic Society (SATS 2007) and the Swedish Society of Infectious Diseases (Hedlund 2005; update: Strålin 2007; Spindler 2012). All these guidelines include recommendations for the choice of antibiotic treatment for CAP in outpatient settings. However, the evidence on which these recommendations are based is derived mainly from studies carried out almost exclusively in hospitalised patients. Although many studies have been published concerning CAP and its treatment, there is no concise summary of the available evidence concerning its treatment in unselected ambulatory outpatients.
This review is an update of our review (Bjerre 2004; Bjerre 2009), and addresses the comparative efficacy of new antibiotic treatments for CAP in outpatients above 12 years of age.
Objectives
To compare the efficacy and safety of different antibiotic treatments for CAP in participants older than 12 years treated in outpatient settings with respect to clinical, radiological and bacteriological outcomes.
Methods
Criteria for considering studies for this review
Types of studies
We looked for studies comparing antibiotics versus placebo, as well as antibiotics versus another antibiotic. However, we did not find studies comparing antibiotics versus placebo. Therefore, we included RCTs of one or more antibiotics in adolescent and adult outpatients with CAP, which reported on clinical outcome parameters, clinical cure rates or bacteriologic response, or both, and adverse events.
Types of participants
Participants older than 12 years of age in outpatient settings with the following.
Symptoms and signs consistent with an acute lower respiratory tract infection associated with new radiographic shadowing for which there is no other explanation (for example, not pulmonary oedema or infarction).
The illness is the primary clinical problem and is managed as pneumonia.
(Modified from the criteria for CAP as defined by the British Thoracic Society (BTS 2009)).
Types of interventions
We considered all double‐blind RCTs comparing one antibiotic and a placebo or at least two antibiotics used to treat CAP. We did not include trials comparing two doses, two treatment durations or two different application methods (example: intravenous versus oral) of the same drug. However, we included trials comparing two different pharmacological formulations of the same substance (for example, microspheres versus pure substance), as they are likely to differ in their pharmacodynamic and pharmacokinetic properties and thus may differ in their efficacy.
Comparisons involving intravenous drugs are usually carried out in a hospital setting. However, as this might occasionally be performed in an ambulatory setting, we did not exclude studies dealing with intravenous drug applications a priori.
We included trials allowing concurrent use of other medications, such as antitussives, antipyretics, bronchodilators or mucolytics, if they allowed equal access to such medications for participants in both arms of the trial.
Types of outcome measures
Primary outcomes
Test‐of‐clinical‐cure: clinical response: improvement of signs and symptoms, usually at a pre‐defined test‐of‐cure (TOC) visit. Where possible, we used duration of clinical signs and symptoms as outcome measures. We used a clinical definition of cure as the primary outcome since radiographic resolution lags behind clinical improvement (Macfarlane 1984).
Secondary outcomes
Radiologic response: resolution or improvement of a new finding on chest X‐ray after antibiotic therapy.
Bacteriologic response: negative sputum culture in patients previously found to have had pathogens in their sputum.
Adverse events: adverse events related to the intervention were reported.
Hospitalisation.
Mortality.
Search methods for identification of studies
Electronic searches
For this 2014 update, to improve our comprehensiveness and coverage of the literature, we edited our search strategy and extended our searches of the electronic databases to include CINAHL, Web of Science and LILACS. Details of the previous search are in Appendix 1.
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (2014, Issue 1) (accessed 28 March 2014), which contains the Cochrane Acute Respiratory Infections Group's Specialised Register, MEDLINE (January 2009 to March week 3, 2014), EMBASE (February 2009 to March 2014), CINAHL (2009 to March 2014), Web of Science (2009 to March 2014) and LILACS (2009 to March 2014).
We searched MEDLINE and CENTRAL using the search strategy shown below. We combined the MEDLINE search strategy with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐and precision‐maximising version (2008 revision) (Lefebvre 2011). We adapted the search strategy for EMBASE (Appendix 2), CINAHL (Appendix 3), Web of Science (Appendix 4) and LILACS (Appendix 5).
MEDLINE (Ovid)
1 exp Pneumonia/ 2 pneumonia.tw. 3 1 or 2 4 Community‐Acquired Infections/ 5 community‐acquired.tw. 6 Outpatients/ (7479) 7 (outpatient* or out‐patient*).tw. 8 exp Ambulatory Care/ 9 (ambulat* adj2 (care or patient*)).tw. 10 or/4‐9 11 3 and 10 12 (community acquired pneumon* or cap).tw. 13 11 or 12 14 exp Anti‐Bacterial Agents/ 15 antibiotic*.tw. 16 exp Macrolides/ 17 exp beta‐Lactams/ 18 exp Quinolones/ 19 exp Tetracyclines/ 20 (beta‐lactam* or macrolide* or makrolide* or quinolone* or tetracycline* or aciclovir or amikacin or amoxicillin or amoxycillin or ampicillin or azithromycin or cefepim or cefotaxim* or ceftarolin or ceftazidim* or ceftibuten or ceftriaxon* or cefuroxim* or cethromycin or ciprofloxacin or clarithromycin or clavulanic acid or clindamycin or co‐amoxiclav or co‐trimoxacol or doxycyclin* or ertapenem or erythromycin or fluoroquinolon* or fluorchinolon* or gemifloxacin or gentamicin or imipenem or levofloxacin or linezolide or meropenem or moxifloxacin or penicillin* or piperacillin or roxithromycin or sultamicillin or tazobactam or telithromycin or tetracyclin* or ticarcillin or tobramycin).tw,nm. 21 or/14‐20 22 13 and 21
Searching other resources
We also identified studies by checking the bibliographies of studies and review articles retrieved and, if necessary, by contacting the first or corresponding authors of relevant studies. In our first review of this topic, published in 2004 (Bjerre 2004), we had contacted the following antibiotics manufacturers to identify any additional published or unpublished studies: Abbott, AstraZeneca, Aventis, Boehringer‐Ingelheim, Bristol‐Myers‐Squibb, GlaxoSmithKline, Hoffmann‐LaRoche, Lilly, Merck, Merck Sharp & Dohme, Novartis, Pfizer, Pharmacia, Sanofi and Yamanouchi. This search yielded no new studies.
We decided not to contact pharmaceutical companies for future review updates. We made this decision for two reasons: first, because of the very low yield of this search strategy, compared to the significant amount of time it requires; and second, because this search strategy provides an unfair advantage to unpublished studies carried out by industry, as opposed to government or academia, where an equivalent search strategy is not readily available. For the same reason, we decided only to include studies that have been fully published in peer‐reviewed journals. We applied no language restrictions to the search and selection process.
Data collection and analysis
Selection of studies
Two or one review authors (SP and SM for this 2014 update; and LMB and TJMV for the 2004 update; and LMB for the 2009 update) used the titles and abstracts of the identified citations to exclude trials that clearly did not meet our inclusion criteria in the previous two publications of this review (Bjerre 2004; Bjerre 2009). If the review authors felt that a study might possibly fulfil the inclusion criteria, we obtained the full paper for further study.
Two review authors (LMB, TJMV in the first review, LMB in the second review and SP, SM in this updated review) independently reviewed articles having passed this initial screen to determine whether they met the inclusion criteria of the review.
Studies could be excluded for any one of the following reasons: if they were not randomised; if they were conducted exclusively in hospitalised patients; if they only compared two doses or two application forms of the same substance, if the indication for treatment consisted of a mix of diagnoses (most commonly: acute bronchitis, exacerbation of chronic bronchitis and pneumonia) and if the results were not reported separately for each diagnostic group.
Another reason for exclusion was that some studies included a mix of in‐ and outpatients without reporting the data separately for these two subgroups. Whenever this was the case, we contacted the trial authors to obtain separate data for outpatients only.
We also excluded studies including only bacteriologically evaluable patients, because these studies typically included only patients with positive cultures of pathogens susceptible to study antibiotics or excluded patients with serologic confirmation of infection with atypical agents (such as M. pneumoniae or C. pneumoniae). A priori exclusion of patients with resistant strains, as well as of patients with non‐bacterial or atypical causes of CAP, would falsely increase the treatment success rate to levels that would be unrealistic in real practice. We chose to exclude these 'narrow‐focus' studies because we are interested in the efficacy of treatment in patients as they present to their general practitioner (GP), that is, unselected and unfiltered. We consider this essential to the generalisability of our results.
We also excluded studies if the diagnosis of pneumonia was not confirmed by chest X‐ray. This exclusion criterion was necessary to ensure that only participants with a very high likelihood of having pneumonia be included in the review, since this was the patient population in which the efficacy of various treatment alternatives was to be assessed.
Furthermore, we excluded studies if the total number of patients was fewer than 30, because below this limit the estimate of a binomial parameter (in this case, the proportion of patients cured or improved) becomes too unstable (Armitage 1994).
Similar to the previous versions of this review (Bjerre 2004; Bjerre 2009), we excluded studies of antibiotics that have been withdrawn from the market or are no longer licensed for the treatment of outpatients with CAP, due to severe adverse effects. For example, we excluded studies assessing the following fluoroquinolones: gatifloxacin, grepafloxacin, sparfloxacin, temafloxacin and trovafloxacin (Black Box Warning: not recommended for CAP because of serious side effects). Consequently, we excluded a study that had been included in the first publication of this review from the present review and the previous update (Ramirez 1999).
Data extraction and management
Two review authors independently extracted the following data from each study, whenever possible:
description of participants, in particular: age range and gender of participants, smoking status, co‐morbidities;
description of potential pathogens identified and their antimicrobial resistance profiles;
description of intervention;
description of control therapy;
total number of participants in each arm of the trial;
study setting;
mean duration of symptoms in each arm of the trial;
clinical, radiographic and bacteriologic cure rates in each arm of the trial;
number of patients lost to follow‐up;
types of adverse effects experienced and number of patients experiencing adverse effects;
number of drop‐outs due to adverse effects;
proportion of patients admitted to hospital in each arm of the trial;
mortality rates in each arm of the trial;
study sponsor/s and role of sponsor/s in study conception, design, implementation, analysis, manuscript writing and publication.
There were no irreconcilable disagreements. Review authors were not blinded to the identity and affiliation of the study authors.
Assessment of risk of bias in included studies
We used the Cochrane 'Risk of bias' tool to systematically assess the risk of bias in included studies (Higgins 2011). (See Characteristics of included studies table).
Measures of treatment effect
We used the Mantel‐Haenszel approach to estimate the common odds ratios (ORs) with approximate 95% confidence intervals (CIs). This was done using RevMan 2014.
Unit of analysis issues
The unit of analysis was the individual patient. All included studies were RCTs without any design particularities, such as cross‐over design or multiple interventions, which would warrant special attention to the units of analysis.
Dealing with missing data
We were to deal with missing data arising, for example, from failure to report on outcomes such as radiological cure rates in some studies, by excluding the specific outcome from the 'Data and analysis' section for the study in question. As for data missing from individual studies (more specifically, patients lost to follow‐up), whenever possible, we used data from the clinical per protocol population, because this excluded patients who had not been sufficiently exposed to the study drug to be able potentially to benefit from the drug.
Assessment of heterogeneity
We carried out the assessment of heterogeneity by means of the Chi2 test for heterogeneity, available in RevMan 2014.
Assessment of reporting biases
If applicable, we assessed reporting/publication bias using funnel plots.
Data synthesis
Whenever possible, we synthesised data using a fixed‐effect meta‐analysis model (Mantel‐Haenszel odds ratio, available in RevMan 2014).
Subgroup analysis and investigation of heterogeneity
As all included studies compared different antibiotic pairs, a subgroup analysis was not possible.
Sensitivity analysis
We did not include studies with fewer than 30 participants in the new review, so no sensitivity analysis was done.
Results
Description of studies
Results of the search
This search yielded a total of 1828 references in our first review (1966 to 2003) (Bjerre 2004), the second updated search (2003 to 2009) yielded an additional 1298 records and this current updated search (2009 to 2014) yielded 730 records, for a grand total of 3856 records. After independently reading all the selected full articles, we selected 11 studies for this review. Some records were double entries, due to the overlapping content of databases.
Included studies
We included 11 RCTs involving a total of 3352 patients aged 12 years and older diagnosed with CAP (Anderson 1991; Chien 1993; Drehobl 2005; D'Ignazio 2005; English 2012; Kohno 2003; Mathers Dunbar 2004; Oldach 2013; Udupa 2011; Vacarezza 2010; van Rensburg 2010a; van Rensburg 2010b). None of the studies included antibiotics versus placebo; all studies included one or more antibiotics. The van Rensburg 2010 article is presented here as van Rensburg 2010a; van Rensburg 2010b for simplicity as in this article two doses of nemonoxacin were tested against levofloxacin (in 1:1:1 ratio randomisation). The trials included varying numbers of patients, the largest having 1025 participants (English 2012), which is comprised of two studies, the smallest 31 (Udupa 2011). The mean size of studies included in the analysis was 310 participants and the median size was 131 (Figure 1). Figure 2 is reproduced from the previous review depicting antibiotic pairs included in that review (Bjerre 2009).
1.
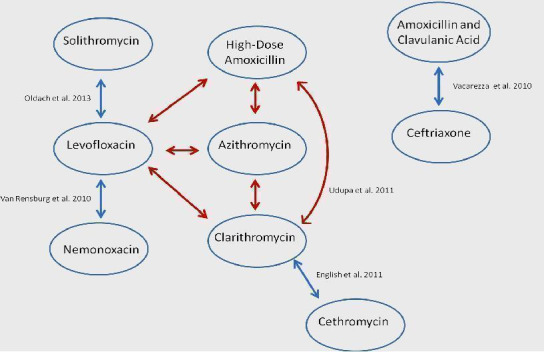
Figure1: Antibiotic comparisons in new studies included in this review. The red arrow indicates antibiotic comparisons studied in Udupa 2011.
2.
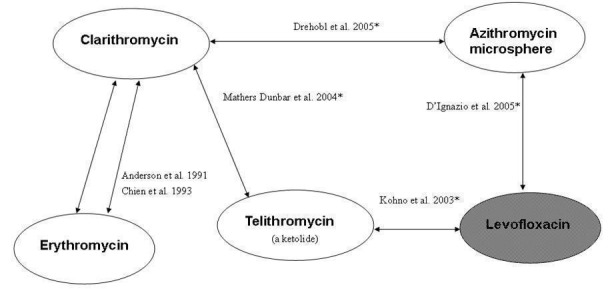
Figure 2. Overview of included studies and antibiotic pairs studied in 2009 review. *Indicates studies new to this review; shaded ovals indicate quinolones (gyrase inhibitors), white ovals indicate macrolides
All trials enrolled outpatients with CAP and the diagnosis was based on clinical signs and symptoms as well as radiographic findings in all participants. The signs and symptoms included fever, chills, recent onset of productive cough, pleuritic chest pain, shortness of breath, tachypnoea, dullness to percussion, egophony, rales, localised reduced breath sounds and bronchial breath sounds. In all included trials, participants were treated exclusively as outpatients.
Participant inclusion and exclusion criteria
Eight trials included adult participants only, aged 18 years and older. Three trials included adolescents: Chien 1993 included adolescents 12 years of age and older. Drehobl 2005 and Kohno 2003 included adolescents 16 years of age and older. Three trials included participants aged 75 years and older (24% to 30% greater than 65 years age) (English 2012; Oldach 2013), and two trials excluded older participants (Udupa 2011 excluded participants aged 55 years and older and in Kohno 2003 participants up to 80 years were included). Inpatients and outpatients were studied in Vacarezza 2010, however, results were presented separately for both groups. Overall, the trials excluded patients with conditions that could have affected the treatment or interfered with follow‐up. Exclusion criteria were reported in sufficient detail in all study reports. The most common criteria reported were: pregnancy and lactation, women not using adequate contraception (usually oral contraceptives or a barrier method), history of allergic reaction to the study drugs, recent treatment with or concomitant use of an antimicrobial agent, concurrent medication with ergotamine, cyclosporin, antacids (except H2‐antagonists) or digitalis, conditions affecting gastrointestinal (GI) absorption, severe renal or hepatic impairment, terminal illness or conditions precluding study completion, infectious mononucleosis, HIV/AIDS and prior participation in the study.
Antibiotics
The trials varied with respect to the antibiotics studied (Figure 1; Figure 2). Four trials studied the same antibiotic pair: Udupa 2011 and Vacarezza 2010 studied clarithromycin versus amoxicillin and Anderson 1991 and Chien 1993 studied erythromycin versus clarithromycin. However, Udupa 2011 also studied two additional antibiotics including azithromycin and levofloxacin. All other trials studied different antibiotic pairs, namely clarithromycin versus azithromycin microspheres (Drehobl 2005), clarithromycin versus telithromycin (Mathers Dunbar 2004), azithromycin microspheres versus levofloxacin (D'Ignazio 2005), telithromycin versus levofloxacin (Kohno 2003), cethromycin versus clarithromycin (English 2012), solithromycin versus levofloxacin (Oldach 2013), and nemonoxacin versus levofloxacin (van Rensburg 2010a; van Rensburg 2010b).
Excluded studies
We excluded 109 studies. A large number of studies were excluded because they were conducted exclusively in hospitalised participants. Furthermore, a number of studies reported including a mix of in‐ and outpatients without reporting data separately for these two subgroups. For these studies, we contacted the trial authors to try to obtain separate data on outpatients. Out of seven trial authors, only two responded and both were unable to provide us with the necessary data.
Risk of bias in included studies
The extent of reporting was variable between studies but was generally good to very good. However, in Vacarezza 2010 and Udupa 2011, overall frequency of signs and symptoms was presented rather than presentation and resolution of signs and symptoms in each treatment arm. Treatment adherence relied on self reporting in all except three studies where treatment adherence was explicitly assessed by pill count (Anderson 1991; Chien 1993; Mathers Dunbar 2004). None reported any difference in the number of pills remaining between the two groups. However, in the Chien 1993 study, 40 participants were excluded because they received "less than the minimum therapy" (seven days) and these patients were distributed unevenly across the two groups (10 in the clarithromycin group and 30 in the erythromycin group). In the two studies using azithromycin microspheres (D'Ignazio 2005; Drehobl 2005), the treatment adherence in the azithromycin group was 100% in both studies, because the drug was administered in a single dose under directly observed therapy (DOT) at the initial treatment visit.
Regarding co‐interventions with other medications, most studies excluded patients whose co‐medication included certain drugs such as other antibiotics, chemotherapeutics or anti‐retrovirals. Only one study reported how many patients were excluded because of forbidden co‐medication (Chien 1993).
The risk of bias in included studies was systematically assessed using 'Risk of bias' tables (RevMan 2014). See Characteristics of included studies table, Figure 3 and Figure 4.
3.
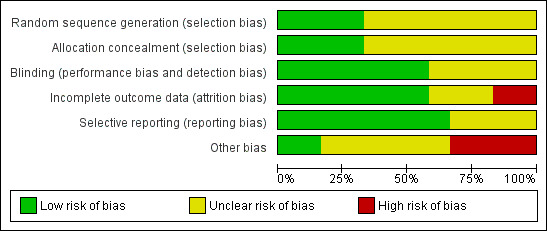
'Risk of bias' graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
4.
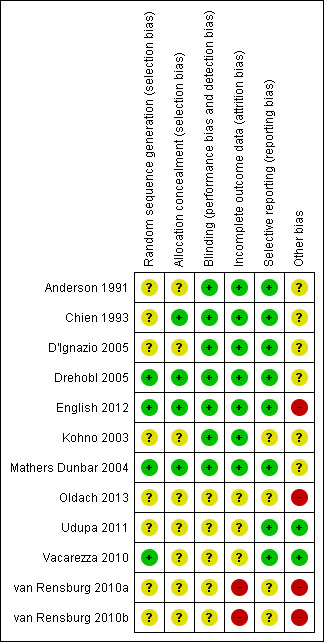
'Risk of bias' summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Only three of the included studies clearly stated the randomisation method used (using interactive voice response systems) and allocation using coded blister cards (Drehobl 2005; English 2012; Mathers Dunbar 2004). More female participants and more participants with chronic obstructive pulmonary disease (COPD) or asthma (or both) received solithromycin as compared to levofloxacin in Oldach 2013.
Blinding
All trials were randomised, double‐blind evaluations comparing two or more antibiotics. None of the trials reported any test of effectiveness of the blinding procedures used.
Incomplete outcome data
Withdrawals were generally reported in sufficient detail. However, three studies did not report a CONSORT diagram (Oldach 2013; Udupa 2011; Vacarezza 2010). The number of patients lost to follow‐up was reported in all studies. Losses to follow‐up appeared to be minor, amounting to a maximum of 10% of the initially randomised participants. One study did not present intention‐to‐treat (ITT) analysis results (Chien 1993).
Since only two studies addressed the efficacy of the same antibiotic pair and both studies provided the same information about outcomes (Anderson 1991; Chien 1993), there were no missing data issues in the combined analysis of the data arising from these two studies (Figure 1; Figure 2).
Selective reporting
There were no obvious concerns about the selective availability of data, or selective reporting of outcomes.
Other potential sources of bias
The main concern about other potential sources of bias was that nine included studies were sponsored by bio‐pharmaceutical companies manufacturing the antibiotics used in the study, or authors were closely linked with the company (Anderson 1991; Chien 1993; D'Ignazio 2005; Drehobl 2005; English 2012; Kohno 2003; Mathers Dunbar 2004; Oldach 2013, van Rensburg 2010a; van Rensburg 2010b). Funding sources were not described in two trials (Udupa 2011; Vacarezza 2010).
Effects of interventions
Primary outcome
1. Test‐of‐clinical‐cure
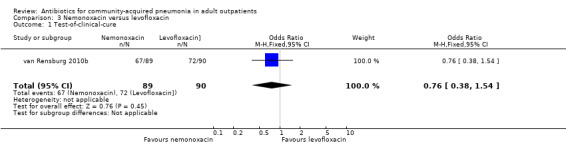
The success rates for each of the treatment arms of the 11 trials are shown in the Data and analyses section of this review. 'Success' was defined as cure or improvement, be it clinical or bacteriological, as assessed at a predefined follow‐up visit ('test‐of‐cure' (TOC) visit). Overall, success rates were very high, usually ranging from 76% to 89% though they were similar in treatment and comparator arms in individual studies. Efficacy in the studies by Udupa 2011 and Vacarezza 2010 is difficult to determine because the data are not presented clearly for treatment groups, rather the frequency of signs and symptoms is presented. Neither clinical nor statistical significance was achieved when the results of the two studies of clarithromycin versus erythromycin were pooled together (Anderson 1991; Chien 1993) (Analysis 7.1; Analysis 7.2; Analysis 7.3; Analysis 7.4).
7.1. Analysis.
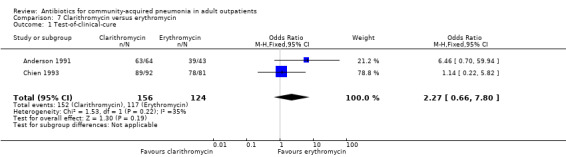
Comparison 7 Clarithromycin versus erythromycin, Outcome 1 Test‐of‐clinical‐cure.
7.2. Analysis.
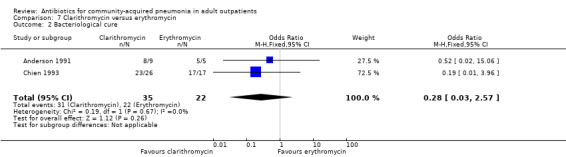
Comparison 7 Clarithromycin versus erythromycin, Outcome 2 Bacteriological cure.
7.3. Analysis.
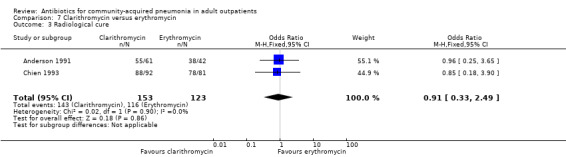
Comparison 7 Clarithromycin versus erythromycin, Outcome 3 Radiological cure.
7.4. Analysis.
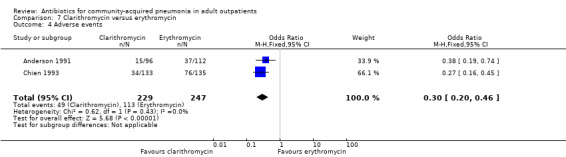
Comparison 7 Clarithromycin versus erythromycin, Outcome 4 Adverse events.
Secondary outcomes
1. Radiological response
Radiological response was reported only in two studies (Anderson 1991; Chien 1993), where it was not significant despite pooling the data (Analysis 7.1; Analysis 7.2; Analysis 7.3; Analysis 7.4).
2. Bacteriologic response
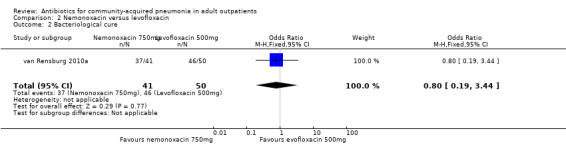
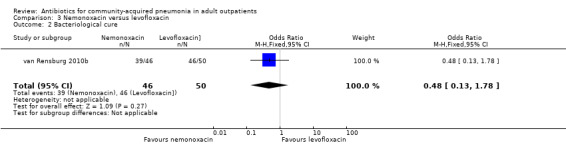
In the study by Oldach 2013, bacterial success rate was higher in the solithromycin group when compared to the levofloxacin group. In the Kohno 2003 study, bacteriological success favoured levofloxacin over telithromycin, while overall efficacy was not different in both these studies. In the studies by van Rensburg 2010a, van Rensburg 2010b and English 2012, bacteriological cure ranged from 48% to 89%.
Detailed bacteriological data are not presented in Udupa 2011 and Vacarezza 2010, and only Gram stain identification was performed on sputum samples in Udupa 2011 at the baseline.
3. Adverse events
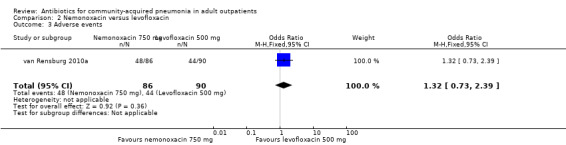
Detailed descriptions of adverse events were presented in all included studies. In all studies, the most common side effects attributable to the study drugs were gastrointestinal side effects. Most side effects were minor and comparable in all treatment arms, except for seven studies (Anderson 1991; Chien 1993; D'Ignazio 2005; English 2012; Mathers Dunbar 2004, van Rensburg 2010a; van Rensburg 2010b; Udupa 2011). In the two studies comparing clarithromycin with erythromycin (Anderson 1991; Chien 1993), there were significantly more side effects in the erythromycin group, the majority being gastrointestinal side effects. However, this was not reflected in the rate of side effects leading to withdrawal from the study, which was not significantly different across treatment arms. However, as noted above, in the Chien 1993 study, 40 patients were excluded because they received "less than the minimum therapy" (seven days) and these patients were distributed unevenly across the two groups (10 in the clarithromycin group and 30 in the erythromycin group). Although not listed as drop‐outs due to side effects, it is quite plausible that these differences in pre‐study drop‐out rates were due to the unfavourable gastrointestinal side effects of erythromycin. In van Rensburg 2010a; van Rensburg 2010b, nemonoxacin demonstrated higher gastrointestinal (nausea, diarrhoea) and nervous system (dizziness, headache) adverse events compared to levofloxacin. In English 2012, cethromycin demonstrated more side effects, especially dysgeusia, than clarithromycin. In Udupa 2011, gastritis and diarrhoea were higher in the high‐dose amoxicillin group (1 g three times a day) compared to the other three antibiotic groups (clarithromycin, azithromycin and levofloxacin).
4. Hospitalisation
Hospitalisation was not explicitly reported as an outcome in any study.
5. Mortality
Mortality was not explicitly reported as an outcome in any trial.
Comparisons across antibiotic groups
Different antibiotic pairs were studied in nine studies (Figure 1; Figure 2). Notably, though, Udupa 2011 and Vacarezza 2010 studied clarithromycin and amoxicillin (high‐dose amoxicillin was studied in Udupa 2011 in addition to azithromycin and levofloxacin). There were no significant differences in clinical or bacteriological success, except in Oldach 2013 and Kohno 2003. Radiological outcomes were not reported separately for the treatment arms.
Bacteriological pathogens
Various pathogens were identified with varying frequency across studies. The proportion of samples yielding an identifiable pathogen ranged from 19% (Anderson 1991) to 65% in Udupa 2011 (only Gram stain on sputum samples at the baseline). H. influenzae was the most common pathogen identified by Anderson 1991 (62% of positive cultures) and Kohno 2003 (43% of positive cultures), whereas S. pneumoniae was the main causative organism in Chien 1993 (56% of positive cultures) and Mathers Dunbar 2004 (52% of positive cultures). D'Ignazio 2005 and Drehobl 2005 reported C. pneumoniae as being the most common pathogen (20% and 23% of positive cultures, respectively). In Kohno 2003, the bacteriological success rate (i.e. eradication of previously identified pathogen) significantly favoured levofloxacin (Analysis 11.1; Analysis 11.2; Analysis 11.3; Analysis 11.4). Most failures were due to failure to eradicate H. influenzae, so the authors looked at the clinical success rate of patients with H. influenzae at baseline, which turned out to not be significantly different between levofloxacin and telithromycin.
11.1. Analysis.
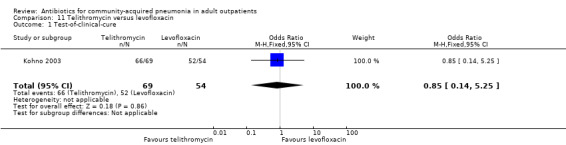
Comparison 11 Telithromycin versus levofloxacin, Outcome 1 Test‐of‐clinical‐cure.
11.2. Analysis.
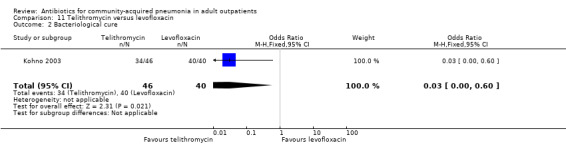
Comparison 11 Telithromycin versus levofloxacin, Outcome 2 Bacteriological cure.
11.3. Analysis.
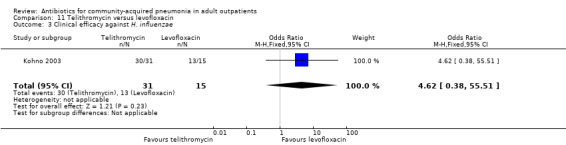
Comparison 11 Telithromycin versus levofloxacin, Outcome 3 Clinical efficacy against H. influenzae.
11.4. Analysis.
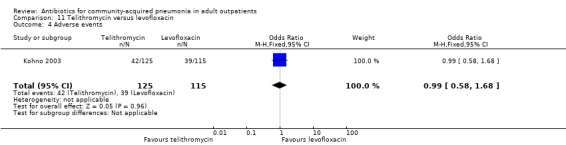
Comparison 11 Telithromycin versus levofloxacin, Outcome 4 Adverse events.
Serologically identified pathogens
The most frequently identified pathogen was M. pneumoniae, which represented 22% (English 2012), 39% (D'Ignazio 2005), 52% (Kohno 2003), 67% (van Rensburg 2010a; van Rensburg 2010b), 69% (Anderson 1991) and 74% (Chien 1993) of positive serology results. In addition, C. pneumoniae was identified in 17% of cases in the van Rensburg 2010a; van Rensburg 2010b study and 7% of cases in the English 2012 study. Legionella pneumoniae (L. pneumoniae) was identified in 4% and 6% in the microbiological evaluable population in the English 2012; van Rensburg 2010a; van Rensburg 2010b studies respectively. In the study by D'Ignazio 2005, C. pneumoniae was predominant, representing 61% of atypical pathogens. Likewise in the studies by Drehobl 2005 and Mathers Dunbar 2004, C. pneumoniae represented just over half of atypical pathogens (53% and 52%, respectively). Only one patient tested positive for L. pneumoniae in the Mathers Dunbar 2004 study and no samples were positive for Chlamydia psittaci (C. psittaci) in any of the studies.
Discussion
Summary of main results
The overwhelming feature of this review update remains the paucity of relevant evidence that could be identified and included in the review. Nonetheless, in this update, five new studies were included (English 2012; Oldach 2013; Udupa 2011; Vacarezza 2010; van Rensburg 2010a; van Rensburg 2010b). Inclusion of these studies did not alter the conclusions of our previous reviews conducted in 2004 and 2009 (Bjerre 2004; Bjerre 2009).
Unfortunately, only two of the 11 studies focused on the same antibiotic pairs (Anderson 1991; Chien 1993), so that, once again, no formal meta‐analysis of the data could be carried out. At most, it can be stated that individual study results did not reveal significant differences in efficacy between various antibiotics and antibiotic groups, but that there were some significant differences with respect to the frequency of side effects. Given this current state of affairs, it is not possible to make strong evidence‐based recommendations regarding the choice of antibiotic to be used for the treatment of community‐acquired pneumonia in ambulatory outpatients. Under such circumstances, other factors such as tolerability, duration and frequency of treatment and cost will take on more importance in determining the choice of treatment.
Importantly, the majority of the studies were conducted in high‐income countries and, hence, the available evidence does not adequately address the issue of community‐acquired pneumonia (CAP) or lower respiratory tract infection (LRTI) in low‐ and middle‐income countries. Lastly, antibiotic misuse is a global threat, although the antibiotic stewardship programmes are gaining some momentum in a few academic centres (Nussenblatt 2013).
Overall completeness and applicability of evidence
One important reason for this lack of evidence is that a large number of the trials originally identified were conducted in hospitalised patients and therefore are not necessarily relevant to the treatment of ambulatory patients. It could be argued that the inclusion/exclusion criteria for this review were too strict and that this is the reason why so few studies were retained. However, we do believe that the criteria we applied are necessary in order to address validly the question of the efficacy of treatment of CAP in ambulatory patients. In particular, it could be argued that the decision to exclude studies based on size is not desirable, since one aim of the review is to pool results and that each study therefore would contribute some information. However, we felt that this criterion was necessary to exclude studies where the number of patients with pneumonia was so small that randomisation could no longer be expected to achieve a balanced distribution of confounders, both known and unknown, across study groups.
As for the requirement that the diagnosis of CAP be confirmed by a chest radiograph, we felt that this was necessary to avoid diagnostic misclassification, which could, for example, have led to the inclusion of patients with bronchitis in this review. This could have biased the estimation of the efficacy of various antibiotic treatments, either differentially or non‐differentially, depending on the distribution of non‐CAP cases across treatment groups. Indeed, most recent clinical guidelines recommend the routine use of chest X‐rays to confirm a suspected pneumonia (BTS 2009; IDSA/ATS 2007; Woodhead 2011; Wunderink 2011). However, we are aware that this diagnostic test is often not used in practice and that patients are therefore treated empirically according to the clinical findings and the severity of the clinical picture. In such a situation, patients with an empirical diagnosis of pneumonia (i.e. diagnosis without chest X‐ray) are probably, on average, less severely ill than the participants in the trials we reviewed.
The diversity of pathogens identified as the most common causative organisms in our present review underscores the need for conducting studies of CAP treatment in a variety of different geographical locations. This also points to a possible limitation of such studies, namely their questionable generalisability to different clinical and geographical situations than the ones included in the present studies. For example, studies conducted in low‐ and middle‐income countries might have a different bacteriological profile than those conducted in high‐income countries.
Finally, a lot of potentially useful information is lost because investigators often included a mix of in‐ and outpatients in their studies without reporting results separately for each of these subgroups. Investigators and journal editors should be strongly encouraged to report such data separately, as these patients have different co‐morbidity profiles and, potentially, respond to treatment differently. Putting heterogeneous patient groups into the same analytic basket may reduce the generalisability and thus the usefulness of such study results. Contacting trial authors after publication to get additional data, sometimes years after a study was published, is a time‐consuming process with a very low yield, as was our experience and that of other Cochrane review authors (Robenshtok 2008).
Quality of the evidence
Overall, the quality of the included studies was relatively good, although there were some differences in the completeness of reporting. The fact that we chose to include only double‐blind, controlled, prospective randomised controlled trials (RCTs) led to a priori exclusion of studies of lesser quality.
Potential biases in the review process
By choosing to include only studies published in peer‐reviewed journals and by choosing no longer to contact pharmaceutical companies for information on unpublished studies, we believe that we have contributed to increasing study quality and reducing bias in our review to a minimum. Furthermore, by excluding data from studies focusing on selected subgroups of ambulatory participants (such as participants with suspected bacterial pneumonia), we believe we have maximised the generalisability of our review results to unselected patients presenting to their physician.
It is noteworthy that, once again, nine studies meeting the inclusion criteria for our review were sponsored by bio‐pharmaceutical companies. This could potentially introduce a publication bias, as it would be in the interest of manufacturers not to publish studies yielding unfavourable results about their products. We are of the opinion that there is an urgent need for industry‐independent research into the treatment of CAP in ambulatory patients.
Agreements and disagreements with other studies or reviews
As mentioned above, we feel that our decision to exclude studies focusing only on a subset of outpatients (for example, "only bacteriologically evaluable patients" or "excluding patients with atypical pneumonia") is necessary to maintain the generalisability of our results to patients presenting to first‐line physicians. In contrast, a recent meta‐analysis we encountered in the process of searching the literature was more inclusive (Maimon 2008). The object of this meta‐analysis was different from that of our review. However, the target population (outpatients with CAP, treated as outpatients) was the same. Despite the authors' stated focus on "the inclusion of only randomised prospective double‐blind studies using only oral therapy exclusively in outpatients" (p.1974), this work included a number of open‐label (non‐blinded) studies, studies including a mix of in‐ and outpatients (for which subgroup data were reportedly obtained from study authors) as well as narrow‐focus studies (i.e. studies focusing exclusively on bacterial pneumonia, for example). Of the 13 studies included in this meta‐analysis, none met the inclusion criteria for our review, which leads us to question the generalisability of the results of this meta‐analysis to unselected patients presenting in general practice.
Finally, a recent RCT confirmed that patients with CAP of moderate severity (Fine Score II or III) can be treated as outpatients, just as safely and effectively as inpatients (Carratalà 2005), at only a fraction of the cost. This further underscores the need for solid, evidence‐based data on the treatment of patients with CAP in the ambulatory care setting.
Authors' conclusions
Implications for practice.
Currently available evidence from randomised controlled trials (RCTs) is insufficient to make evidence‐based recommendations for the choice of antibiotic to be used in the treatment of community‐acquired pneumonia (CAP) in ambulatory patients. At most, it can be stated that individual study results do not reveal significant differences in efficacy between various antibiotics and antibiotic groups.
Implications for research.
Multi‐drug, multi‐drug‐group, double‐blind comparisons conducted in various geographical settings are needed to provide the evidence necessary for practice recommendations if these are to be applicable in the ambulatory setting. Study conditions should ensure that diagnosis and management of patients with CAP is as similar as possible to real practice, while still ensuring that the study question is addressed in a valid way. In studies recruiting a mix of in‐ and outpatients, it is imperative that data be reported separately for these two subgroups. Finally, good quality studies are needed in high as well as low‐ and middle‐income countries to address CAP and lower respiratory tract infection diagnosis, management, cost‐effectiveness and misuse of antibiotics.
What's new
| Date | Event | Description |
|---|---|---|
| 28 March 2014 | New citation required but conclusions have not changed | Three new review authors joined the original team to update this review. |
| 28 March 2014 | New search has been performed | Searches updated. We included five new trials (English 2012; Oldach 2013; Udupa 2011; Vacarezza 2010; van Rensburg 2010a; van Rensburg 2010b) and excluded 43 new trials (Alcacer 1993; Arifin 2013; Bai 2014; Blasi 2013; Block 2006; Bothra 2012; Brittain‐Long 2011; Casapao 2012; Critchley 2010; Daniel 1999a; Daniel 1999b; Dartois 2013; Esposito 2012; File 2012a; File 2012b; Fogarty 2004; Ghebremedhin 2012; Kohno 2013; Lagler 2012; Lee 2012; Little 2012; Long 2011; Lopez‐Vejar 2013; Matzneller 2013; Montassier 2013; Naderer 2013; Navarta 2010; Nussenblatt 2013; Polverino 2013; Rank 2011; Seki 2009; Shorr 2013; Skalsky 2012; Smith 2013; Snyman 2009; Stille 2000; Sun 2012; Viasusa 2013; Worrall 2010; Wunderink 2011; Yamamoto 2013; Yuan 2012; Zhang 2012). |
History
Protocol first published: Issue 2, 2000 Review first published: Issue 2, 2004
| Date | Event | Description |
|---|---|---|
| 20 February 2009 | New search has been performed | Searches conducted. |
| 20 February 2009 | New citation required but conclusions have not changed | Four new studies included. |
| 26 February 2004 | New search has been performed | Review first published, Issue 2, 2004. |
Acknowledgements
We thank the authors we contacted during the second revision of this review (Bjerre 2009), who kindly replied to our requests for additional information: Dr. Lorenzo Aguilar, Dr. Claude Carbon, Dr. Lars Hagberg, Dr. Karen Higgins, Dr. Shigeru Kohno, Dr. Hartmut Lode, Dr. Lala Mathers Dunbar and Dr. Antoni Torres Martí. Many thanks to Dr. Frederike Behn for precious help with parts of the data extraction process in the first version of this review (Bjerre 2004). We also wish to thank the following people for commenting on drafts of this review: Clare Jeffrey, Anne Lyddiatt, Mary Baldwin, Deviprasad Mohapatra, Tina Tan, Balwinder Singh, William Cayley, Mark Jones, Robert Ware and Roger Damoiseaux. Last, but not least, we thank the Acute Respiratory Infections Group editorial team for enduring support and guidance, in particular Liz Dooley (Managing Editor), as well as Ruth Foxlee and Sarah Thorning (respectively, past and present Trials Search Co‐ordinators).
In this current 2014 update, we wish to thank Dr. Rosendo Rodriguez, Dr. Gonzalo Alvarez, Dr. Mathieu Saint Pierre, Dr. Jacqueline Sandoz and Dr. Keiko Asakawa for their assistance with translation of Spanish, French and Japanese articles. We also acknowledge and thank Ms. Lara Bajar, administrative assistant for Dr. Smita Pakhale, for administrative assistance with management of the articles.
Appendices
Appendix 1. Details of previous search
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, 2009, issue 1), which contains the Cochrane Acute Respiratory Infections Group's Specialised Register; MEDLINE (January 1966 to February week 2, 2009) and EMBASE (January 1974 to February 2009).
MEDLINE and CENTRAL were searched using the search strategy shown below. We combined the MEDLINE search string with the Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐maximising version (2008 revision) (Lefebvre 2011). The search string was adapted for EMBASE, as shown in Appendix 2.
MEDLINE (Ovid)
1 exp Anti‐Bacterial Agents/ 2 antibiotic$.mp. 3 or/1‐2 4 exp Pneumonia/ 5 exp Community‐Acquired Infections/ 6 and/4‐5 (3356) 7 community acquired pneumonia.mp. 8 or/6‐7 9 3 and 8
EMBASE (Elsevier)
#1. 'antibiotic agent'/exp AND [embase]/lim #2. antibiotic*:ti,ab AND [embase]/lim #3. #1 OR #2 #4. 'pneumonia'/exp AND [embase]/lim #5. 'communicable disease'/exp AND [embase]/lim #7. 'community acquired pneumonia'/exp AND [embase]/lim #8. #4 AND #5 #9. 'community acquired pneumonia':ti,ab AND [embase]/lim #10. #7 OR #8 OR #9 #11. #3 AND #10 #12. 'randomized controlled trial'/exp OR 'double blind procedure'/exp OR 'single blind procedure'/exp OR 'crossover procedure'/exp AND [embase]/lim #13. random*:ti,ab OR factorial*:ti,ab OR crossover*:ti,ab OR 'cross over':ti,ab OR assign*:ti,ab OR allocat*:ti,ab OR volunteer*:ti,ab OR 'single blind':ti,ab OR 'single blinding':ti,ab OR 'single blinded':ti,ab OR 'double blind':ti,ab OR 'double blinded':ti,ab OR 'double blinding':ti,ab AND [embase]/lim #14. #12 OR #13 #15. #11 AND #14
Appendix 2. Embase.com search strategy
#32 #23 AND #31 #31 #26 NOT #30 #30 #27 NOT #29 #29 #27 AND #28 #28 'human'/de #27 'animal'/de OR 'nonhuman'/de OR 'animal experiment'/de #26 #24 OR #25 #25 random*:ab,ti OR placebo*:ab,ti OR trial:ti OR allocat*:ab,ti OR crossover*:ab,ti OR 'cross over':ab,ti OR (doubl* NEXT/1 blind*):ab,ti #24 'randomized controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp #23 #14 AND #229264 #22 #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 #21 macrolide*:ab,ti OR makrolide*:ab,ti OR quinolone*:ab,ti OR tetracycline*:ab,ti OR aciclovir:ab,ti OR amikacin:ab,ti OR amoxicillin:ab,ti OR amoxycillin:ab,ti OR ampicillin:ab,ti OR azithromycin:ab,ti OR cefepim:ab,ti OR cefotaxim*:ab,ti OR ceftarolin:ab,ti OR ceftazidim*:ab,ti OR ceftibuten:ab,ti OR ceftriaxon*:ab,ti OR cefuroxim*:ab,ti OR cethromycin:ab,ti OR ciprofloxacin:ab,ti OR clarithromycin:ab,ti OR 'clavulanic acid':ab,ti OR clindamycin:ab,ti OR doxycyclin*:ab,ti OR ertapenem:ab,ti OR erythromycin:ab,ti OR fluoroquinolon*:ab,ti OR fluorchinolon*:ab,ti OR gemifloxacin:ab,ti OR gentamicin:ab,ti OR imipenem:ab,ti OR levofloxacin:ab,ti OR linezolide:ab,ti OR meropenem:ab,ti OR moxifloxacin:ab,ti OR penicillin*:ab,ti OR piperacillin:ab,ti OR roxithromycin:ab,ti OR sultamicillin:ab,ti OR tazobactam:ab,ti OR tobramycin:ab,ti OR 'beta‐lactam':ab,ti OR 'beta‐lactams':ab,ti OR 'co‐amoxiclav':ab,ti OR 'co‐trimoxacol':ab,ti #20 'tetracycline derivative'/exp #19 'quinolone derivative'/de #18 'beta lactam'/de #17 'macrolide'/exp #16 antibiotic*:ab,ti #15 'antibiotic agent'/exp #14 #1 OR #2 OR #13 #13 #5 AND #12 #12 #6 OR #7 OR #8 OR #9 OR #10 OR #11 #11 (ambulat* NEAR/2 (care OR patient*)):ab,ti #10 'ambulatory care'/exp #9 outpatient*:ab,ti OR 'out‐patient':ab,ti OR 'out‐patients':ab,ti #8 'outpatient'/de #7 'community acquired':ab,ti #6 'communicable disease'/de #5 #3 OR #4 #4 pneumon*:ab,ti #3 'pneumonia'/exp #2 'community acquired pneumonia':ab,ti OR cap:ab,ti #1 'community acquired pneumonia'/de
Appendix 3. CINAHL (EBSCO) search strategy
S34 S23 and S33 S33 S24 or S25 or S26 or S27 or S28 or S29 or S30 or S31 or S32 S32 (MH "Random Assignment") S31 (MH "Quantitative Studies") S30 TI placebo* OR AB placebo* S29 (MH "Placebos") S28 TI random* OR AB random* S27 TI ( (singl* or doubl* or tripl* or trebl*) W1 (blind* or mask*)) OR AB ((singl* or doubl* or tripl* or trebl*) W1 (blind* or mask*)) S26 TI clinical* trial* OR AB clinical* trial* S25 PT clinical trial S24 (MH "Clinical Trials+") S23 S14 and S22 S22 S15 or S16 or S17 or S18 or S19 or S20 or S21 S21 TI (beta‐lactam* or macrolide* or makrolide* or quinolone* or tetracycline* or aciclovir or amikacin or amoxicillin or amoxycillin or ampicillin or azithromycin or cefepim or cefotaxim* or ceftarolin or ceftazidim* or ceftibuten or ceftriaxon* or cefuroxim* or cethromycin or ciprofloxacin or clarithromycin or clavulanic acid or clindamycin or co‐amoxiclav or co‐trimoxacol or doxycyclin* or ertapenem or erythromycin or fluoroquinolon* or fluorchinolon* or gemifloxacin or gentamicin or imipenem or levofloxacin or linezolide or meropenem or moxifloxacin or penicillin* or piperacillin or roxithromycin or sultamicillin or tazobactam or telithromycin or tetracyclin* or ticarcillin or tobramycin) OR AB (beta‐lactam* or macrolide* or makrolide* or quinolone* or tetracycline* or aciclovir or amikacin or amoxicillin or amoxycillin or ampicillin or azithromycin or cefepim or cefotaxim* or ceftarolin or ceftazidim* or ceftibuten or ceftriaxon* or cefuroxim* or cethromycin or ciprofloxacin or clarithromycin or clavulanic acid or clindamycin or co‐amoxiclav or co‐trimoxacol or doxycyclin* or ertapenem or erythromycin or fluoroquinolon* or fluorchinolon* or gemifloxacin or gentamicin or imipenem or levofloxacin or linezolide or meropenem or moxifloxacin or penicillin* or piperacillin or roxithromycin or sultamicillin or tazobactam or telithromycin or tetracyclin* or ticarcillin or tobramycin) S20 (MH "Antibiotics, Lactam+") S19 (MH "Tetracyclines+") S S18 (MH "Antiinfective Agents, Quinolone+") S17 (MH "Antibiotics, Macrolide+") S16 TI antibiotic* OR AB antibiotic* S15 (MH "Antibiotics+") S14 S11 or S12 or S13 S13 TI (community acquired pneumon* or cap) OR AB (community acquired pneumon* or cap) S12 (MH "Community‐Acquired Pneumonia") S11 S3 and S10 S10 S4 or S5 or S6 or S7 or S8 or S9 S S9 TI (ambulat* N1 (care or patient*)) OR AB (ambulat* N1 (care or patient*)) S8 (MH "Ambulatory Care") S7 TI (outpatient* or out patient*) OR AB (outpatient* or out patient*) S6 (MH "Outpatients") S5 TI community acquired OR AB community acquired S4 (MH "Community‐Acquired Infections+") S3 S1 or S2 S2 TI pneumon* OR AB pneumon* S1 (MH "Pneumonia+")
Appendix 4. Web of Science (Thomson Reuters) search strategy
| # 9 | 204 | #7 AND #6 Refined by: Publication Years=( 2011 OR 2009 OR 2010 OR 2012 ) Databases=SCI‐EXPANDED, CPCI‐S, CCR‐EXPANDED, IC Timespan=1985‐2012 Lemmatization=On |
|
| # 8 | 755 | #7 AND #6 Databases=SCI‐EXPANDED, CPCI‐S, CCR‐EXPANDED, IC Timespan=1985‐2012 Lemmatization=On |
|
| # 7 | 1,077,429 | Title=(trial) OR Topic=(random* or placebo* or ((singl* or doubl*) NEAR/1 blind*)) Databases=SCI‐EXPANDED, CPCI‐S, CCR‐EXPANDED, IC Timespan=1985‐2012 Lemmatization=On |
|
| # 6 | 4,075 | #5 AND #4 Databases=SCI‐EXPANDED, CPCI‐S, CCR‐EXPANDED, IC Timespan=1985‐2012 Lemmatization=On |
|
| # 5 | 266,150 | Topic=(antibiotic* or beta‐lactam* or macrolide* or makrolide* or quinolone* or tetracycline* or aciclovir or amikacin or amoxicillin or amoxycillin or ampicillin or azithromycin or cefepim or cefotaxim* or ceftarolin or ceftazidim* or ceftibuten or ceftriaxon* or cefuroxim* or cethromycin or ciprofloxacin or clarithromycin or clavulanic acid or clindamycin or co‐amoxiclav or co‐trimoxacol or doxycyclin* or ertapenem or erythromycin or fluoroquinolon* or fluorchinolon* or gemifloxacin or gentamicin or imipenem or levofloxacin or linezolide or meropenem or moxifloxacin or penicillin* or piperacillin or roxithromycin or sultamicillin or tazobactam or telithromycin or tetracyclin* or ticarcillin or tobramycin) Databases=SCI‐EXPANDED, CPCI‐S, CCR‐EXPANDED, IC Timespan=1985‐2012 Lemmatization=On |
|
| # 4 | 9,196 | #3 OR #2 OR #1 Databases=SCI‐EXPANDED, CPCI‐S, CCR‐EXPANDED, IC Timespan=1985‐2012 Lemmatization=On |
|
| # 3 | 44 | Topic=((ambulat* NEAR/2 (care or patient* or setting)) NEAR/3 pneumon*) Databases=SCI‐EXPANDED, CPCI‐S, CCR‐EXPANDED, IC Timespan=1985‐2012 Lemmatization=On |
|
| # 2 | 194 | Topic=((outpatient* or "out patient" or "out patients") NEAR/3 pneumon*) Databases=SCI‐EXPANDED, CPCI‐S, CCR‐EXPANDED, IC Timespan=1985‐2012 Lemmatization=On |
|
| # 1 | 9,089 | Topic=(community acquired*) AND Topic=(pneumonia) Databases=SCI‐EXPANDED, CPCI‐S, CCR‐EXPANDED, IC Timespan=1985‐2012 Lemmatization=On |
Appendix 5. LILACS (BIREME) search strategy
Search > ("community‐acquired pneumonia" OR ((MH:pneumonia OR pneumon$ OR Neumonía OR MH:C08.381.677$ OR MH:C08.730.610$) AND (MH:"Community‐Acquired Infections" OR "Community‐Acquired Infections" OR "Infecciones Comunitarias Adquiridas" OR "Infecções Comunitárias Adquiridas" OR MH:Outpatients OR outpatient$ OR "out patient" OR "out patients" OR "Pacientes Ambulatorios" OR "Pacientes Ambulatoriais" OR MH:"Ambulatory Care" OR "ambulatory care" OR "Atención Ambulatoria" OR "Assistência Ambulatorial" OR "Cuidados Ambulatorios" OR "Cuidados de Pacientes Externos" OR "Cuidados ambulatoriais"))) AND (MH:"Anti‐Bacterial Agents" OR antibiot$ OR antibacteria$ OR MH:macrolides OR Macrólidos OR Macrolídeos OR MH:D02.540.505$ OR MH:"beta‐Lactams" OR "beta‐Lactamas" OR MH:D02.065.589.099$ OR MH:D02.886.108$ OR MH:D04.075.080.875.099.221$ OR MH:Quinolones OR Quinolonas OR Quinolinones OR Ketoquinolines OR Oxoquinolines OR MH:D03.438.810.835$ OR MH:Tetracyclines OR tetraciclinas OR tetraciclinas OR MH:D02.455.426.559.847.562.900$ OR MH:D04.615.562.900$ OR "beta‐lactam" OR "beta‐lactams" OR macrolide$ OR makrolide$ OR quinolone$ OR tetracycline$ OR aciclovir OR amikacin OR amoxicillin OR amoxycillin OR ampicillin OR azithromycin OR cefepim OR cefotaxim$ OR ceftarolin OR ceftazidim$ OR ceftibuten OR ceftriaxon$ OR cefuroxim$ OR cethromycin OR ciprofloxacin OR clarithromycin OR "clavulanic acid" OR clindamycin OR "co‐amoxiclav" OR "co‐trimoxacol" OR doxycyclin$ OR ertapenem OR erythromycin OR fluoroquinolon$ OR fluorchinolon$ OR gemifloxacin OR gentamicin OR imipenem OR levofloxacin OR linezolide OR meropenem OR moxifloxacin OR penicillin$ OR piperacillin OR roxithromycin OR sultamicillin OR tazobactam OR telithromycin OR tetracyclin$ OR ticarcillin OR tobramycin) > clinical_trials
Data and analyses
Comparison 1. Solithromycin versus levofloxacin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Test‐of‐clinical‐cure | 1 | 132 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.32, 2.26] |
| 2 Bacteriological cure | 1 | 32 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.4 [0.28, 6.98] |
| 3 Adverse events | 1 | 132 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.19, 1.40] |
1.1. Analysis.
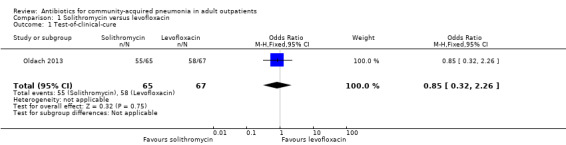
Comparison 1 Solithromycin versus levofloxacin, Outcome 1 Test‐of‐clinical‐cure.
1.2. Analysis.
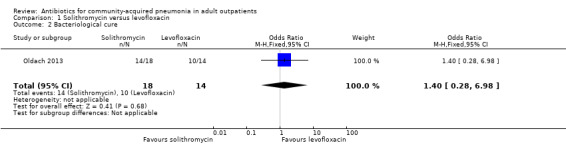
Comparison 1 Solithromycin versus levofloxacin, Outcome 2 Bacteriological cure.
1.3. Analysis.
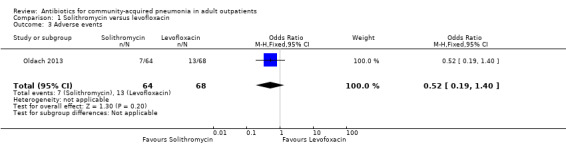
Comparison 1 Solithromycin versus levofloxacin, Outcome 3 Adverse events.
Comparison 2. Nemonoxacin versus levofloxacin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Test‐of‐clinical‐cure | 1 | 176 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.55, 2.53] |
| 2 Bacteriological cure | 1 | 91 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.19, 3.44] |
| 3 Adverse events | 1 | 176 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.73, 2.39] |
2.1. Analysis.
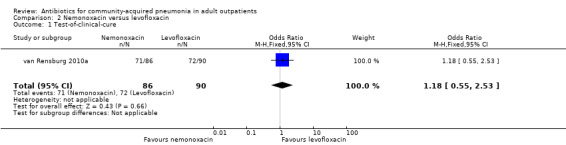
Comparison 2 Nemonoxacin versus levofloxacin, Outcome 1 Test‐of‐clinical‐cure.
2.2. Analysis.
Comparison 2 Nemonoxacin versus levofloxacin, Outcome 2 Bacteriological cure.
2.3. Analysis.
Comparison 2 Nemonoxacin versus levofloxacin, Outcome 3 Adverse events.
Comparison 3. Nemonoxacin versus levofloxacin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Test‐of‐clinical‐cure | 1 | 179 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.38, 1.54] |
| 2 Bacteriological cure | 1 | 96 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.13, 1.78] |
| 3 Adverse events | 1 | 179 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.47, 1.54] |
3.1. Analysis.
Comparison 3 Nemonoxacin versus levofloxacin, Outcome 1 Test‐of‐clinical‐cure.
3.2. Analysis.
Comparison 3 Nemonoxacin versus levofloxacin, Outcome 2 Bacteriological cure.
3.3. Analysis.
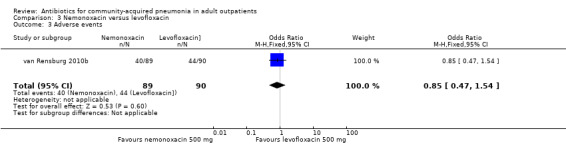
Comparison 3 Nemonoxacin versus levofloxacin, Outcome 3 Adverse events.
Comparison 4. Clarithromycin versus amoxicillin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Test‐of‐clinical‐cure | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
4.1. Analysis.
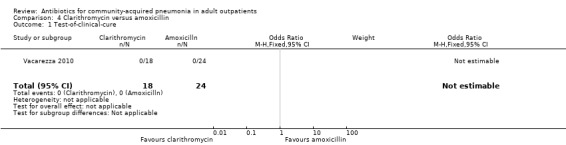
Comparison 4 Clarithromycin versus amoxicillin, Outcome 1 Test‐of‐clinical‐cure.
Comparison 5. Clarithromycin versus azithromycin versus levofloxacin versus amoxicillin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Test‐of‐clinical‐cure | Other data | No numeric data | ||
| 2 Adverse events | Other data | No numeric data |
5.1. Analysis.
Comparison 5 Clarithromycin versus azithromycin versus levofloxacin versus amoxicillin, Outcome 1 Test‐of‐clinical‐cure.
| Test‐of‐clinical‐cure | |||
|---|---|---|---|
| Study | Antibiotics | Events | Total |
| Udupa 2011 | Clarithromycin | 8 | |
| Udupa 2011 | Azithromycin | 7 | |
| Udupa 2011 | Levofloxacin | 7 | |
| Udupa 2011 | High‐dose amoxicillin | 9 | |
5.2. Analysis.
Comparison 5 Clarithromycin versus azithromycin versus levofloxacin versus amoxicillin, Outcome 2 Adverse events.
| Adverse events | |||
|---|---|---|---|
| Study | Antibiotic | Events | Total |
| Udupa 2011 | Clarithromycin | 4 | 8 |
| Udupa 2011 | Azithromycin | 5 | 7 |
| Udupa 2011 | Levofloxacin | 5 | 7 |
| Udupa 2011 | Amoxicillin | 7 | 9 |
Comparison 6. Cethromycin versus clarithromycin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Test‐of‐clinical‐cure | 1 | 1025 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.63, 1.22] |
| 2 Bacteriological cure | 1 | 363 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.50, 1.58] |
| 3 Adverse events | 1 | 1096 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.68 [1.32, 2.15] |
6.1. Analysis.
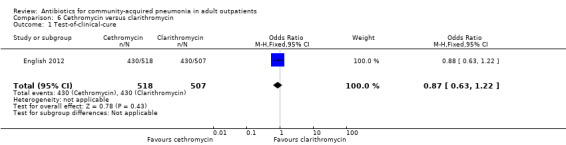
Comparison 6 Cethromycin versus clarithromycin, Outcome 1 Test‐of‐clinical‐cure.
6.2. Analysis.
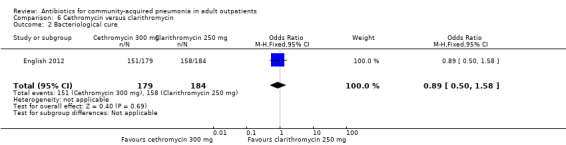
Comparison 6 Cethromycin versus clarithromycin, Outcome 2 Bacteriological cure.
6.3. Analysis.
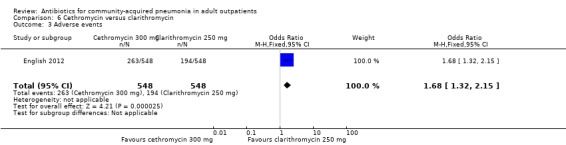
Comparison 6 Cethromycin versus clarithromycin, Outcome 3 Adverse events.
Comparison 7. Clarithromycin versus erythromycin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Test‐of‐clinical‐cure | 2 | 280 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.27 [0.66, 7.80] |
| 2 Bacteriological cure | 2 | 57 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.03, 2.57] |
| 3 Radiological cure | 2 | 276 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.33, 2.49] |
| 4 Adverse events | 2 | 476 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.20, 0.46] |
Comparison 8. Azithromycin microspheres versus levofloxacin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Test‐of‐clinical‐cure | 1 | 363 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.27, 1.26] |
| 2 Bacteriological cure | 1 | 237 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.32, 2.02] |
| 3 Adverse events | 1 | 423 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.78 [1.04, 3.03] |
8.1. Analysis.
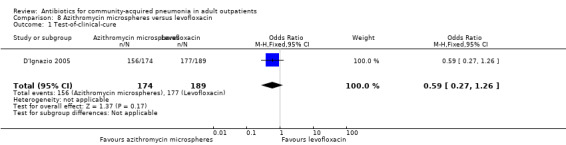
Comparison 8 Azithromycin microspheres versus levofloxacin, Outcome 1 Test‐of‐clinical‐cure.
8.2. Analysis.
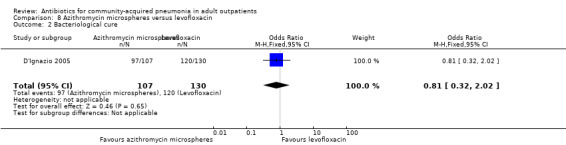
Comparison 8 Azithromycin microspheres versus levofloxacin, Outcome 2 Bacteriological cure.
8.3. Analysis.
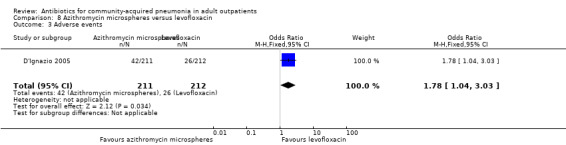
Comparison 8 Azithromycin microspheres versus levofloxacin, Outcome 3 Adverse events.
Comparison 9. Azithromycin microspheres versus clarithromycin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Test‐of‐clinical‐cure | 1 | 411 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.31, 1.55] |
| 2 Bacteriological cure | 1 | 303 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.52, 2.61] |
| 3 Adverse events | 1 | 499 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.73, 1.64] |
9.1. Analysis.
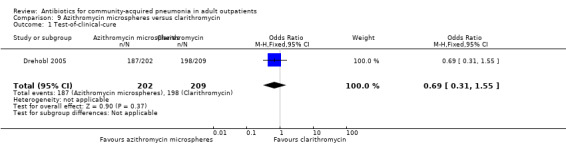
Comparison 9 Azithromycin microspheres versus clarithromycin, Outcome 1 Test‐of‐clinical‐cure.
9.2. Analysis.
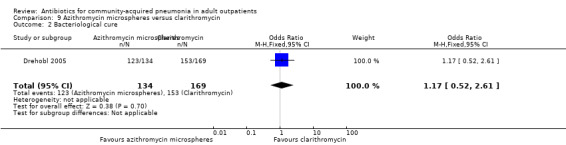
Comparison 9 Azithromycin microspheres versus clarithromycin, Outcome 2 Bacteriological cure.
9.3. Analysis.
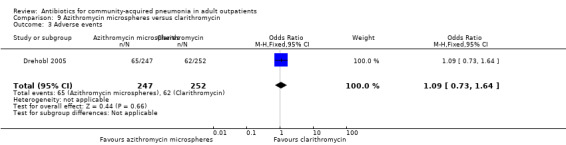
Comparison 9 Azithromycin microspheres versus clarithromycin, Outcome 3 Adverse events.
Comparison 10. Telithromycin versus clarithromycin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Test‐of‐clinical‐cure | 1 | 318 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.49, 1.95] |
| 2 Bacteriological cure | 1 | 62 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.03, 2.29] |
| 3 Adverse events | 1 | 443 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.61 [1.08, 2.40] |
10.1. Analysis.
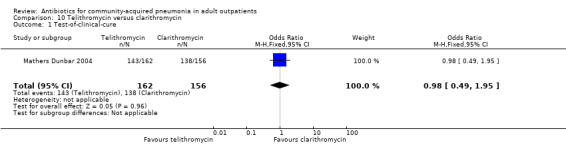
Comparison 10 Telithromycin versus clarithromycin, Outcome 1 Test‐of‐clinical‐cure.
10.2. Analysis.
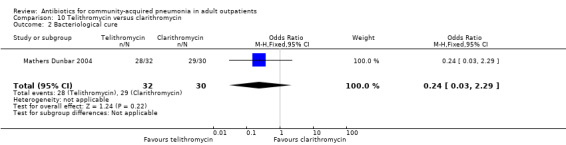
Comparison 10 Telithromycin versus clarithromycin, Outcome 2 Bacteriological cure.
10.3. Analysis.
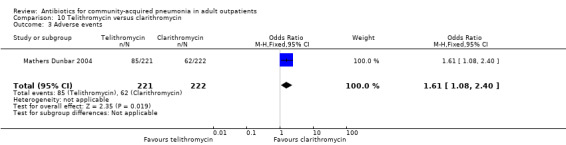
Comparison 10 Telithromycin versus clarithromycin, Outcome 3 Adverse events.
Comparison 11. Telithromycin versus levofloxacin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Test‐of‐clinical‐cure | 1 | 123 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.14, 5.25] |
| 2 Bacteriological cure | 1 | 86 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.03 [0.00, 0.60] |
| 3 Clinical efficacy against H. influenzae | 1 | 46 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.62 [0.38, 55.51] |
| 4 Adverse events | 1 | 240 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.58, 1.68] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Anderson 1991.
| Methods | Date and duration of study: not specified. Follow‐up: 6 to 8 weeks. Patients were included from 57 general practitioners in the UK. Double‐blind, double‐dummy technique, intention‐to‐treat results provided | |
| Participants | Patients with CAP older than 18 years. CAP diagnosis confirmed by 3 of the following features: pyrexia, dyspnoea, tachypnoea, rales, localised reduced breath sounds and cough. Diagnosis of CAP was later confirmed radiographically. Total: n = 208. Evaluable for efficacy: n = 108 (exclusion usually due to failure to confirm initial diagnosis on CXR), n = 64 (clarithromycin), n = 44 (erythromycin). Exclusion criteria clear | |
| Interventions | Clarithromycin 250 mg twice daily for 14 days or erythromycin 500 mg 4 times daily for 14 days, each given at least 1 hour before or 2 hours after meals, mean treatment duration: 13 days (clarithromycin), or 10 days (erythromycin). Compliance assessment: tablet count | |
| Outcomes | Primary outcome: clinical response at 2 weeks (test‐of‐cure visit): 98% (clarithromycin), 91% (erythromycin). Treatment‐related adverse events: 16% (clarithromycin) versus 33% (erythromycin), P value = 0.004, mainly gastrointestinal side effects | |
| Notes | 3 of 5 authors from Abbott Laboratories, source of funding not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not specified |
| Allocation concealment (selection bias) | Unclear risk | Randomisation method not specified |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, double‐dummy technique |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Detailed list of reasons for exclusion from efficacy analyses, with number of patients affected |
| Selective reporting (reporting bias) | Low risk | Pre‐specified outcomes appear to be fully reported |
| Other bias | Unclear risk | 3 of 5 authors from Abbott Laboratories, source of funding not specified |
Chien 1993.
| Methods | Date and duration of study: January 1989 to June 1990. Follow‐up: 4 to 6 weeks. Multicentre study (15 centres of the Canada‐Sweden Clarithromycin‐Pneumonia Study Group, 11 in Canada, 4 in Sweden). Double‐blind, double‐dummy technique, no intention‐to‐treat results provided | |
| Participants | Ambulatory patients older than 12 years with CAP. N = 268 all patients, after exclusions 173 "evaluable patients": n = 92 (clarithromycin), n = 81 (erythromycin). Patients with mild or moderate infection. Drop‐outs: 35% (due to less than minimum therapy, premature discontinuation, unavailable for follow‐up, misdiagnosis, inadequate data collection, concomitant medication, underlying condition). Exclusion criteria clear | |
| Interventions | Clarithromycin: 250 mg every 12 hours, or erythromycin stearate: 500 mg every 6 hours. Mean treatment duration not specified (minimum duration: 7 days, intended duration: 7 to 14 days). Compliance assessment: tablet count | |
| Outcomes | Primary outcome: clinical success (cure and improvement) 97% clarithromycin, 96% erythromycin. Treatment‐related adverse events: 31% clarithromycin, 59% erythromycin (P value < 0.001) | |
| Notes | Research supported by Abbott Laboratories, Chicago | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not specified |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomisation was blinded to both the investigators and the patients." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, double‐dummy technique |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Detailed list of reasons for exclusion from efficacy analyses, with number of patients affected |
| Selective reporting (reporting bias) | Low risk | Pre‐specified outcomes appear to be fully reported |
| Other bias | Unclear risk | Research supported by Abbott Laboratories, Chicago |
D'Ignazio 2005.
| Methods | Date and duration of study: April 2003 to April 2004. Double‐blind, double‐dummy, non‐inferiority trial, patients were recruited from 56 centres worldwide (Canada, Chile, India, Lithuania, Mexico, Peru, Russia, United States) | |
| Participants | 427 outpatients, 18 years or older, 423 patients received the study medication. Patients were eligible for enrolment if they had a clinical diagnosis of mild to moderate CAP (signs and symptoms) and radiographic evidence of new pulmonary infiltrate. Patients also had to have a Fine Mortality risk class of I, II or III (< 90 points). Exclusion criteria are clearly listed | |
| Interventions | Single, 2 g dose of azithromycin microspheres versus levofloxacin 500 mg once daily for 7 days | |
| Outcomes | Primary endpoint: clinical response in the clinical per protocol population, on the basis of signs and symptoms of CAP, assessed at test‐of‐cure visit (TOC; day 13 to 21): azithromycin 89.7%, levofloxacin 93.7%, non‐significant difference. Treatment‐related adverse effects: azithromycin 19.9%, levofloxacin 12.3%, P value = 0.0063, mainly gastrointestinal side effects | |
| Notes | Study funded by Pfizer Inc., 3 of 5 authors are employees of Pfizer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not specified |
| Allocation concealment (selection bias) | Unclear risk | Randomisation method not specified |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, double‐dummy technique |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Detailed list of reasons for exclusion from efficacy analyses, with number of patients affected |
| Selective reporting (reporting bias) | Low risk | Pre‐specified outcomes appear to be fully reported |
| Other bias | Unclear risk | Study funded by Pfizer Inc., 3 of 5 authors are employees of Pfizer |
Drehobl 2005.
| Methods | Date and duration of study: not specified. Double‐blind, double‐dummy, phase III trial, conducted in 58 outpatient centres worldwide (United States, Canada, Argentina, Russia, India, Estonia, Lithuania) | |
| Participants | 501 outpatients, 16 years or older. Patients were eligible for enrolment if they had a clinical diagnosis of mild to moderate CAP (signs and symptoms) and radiographic evidence of new pulmonary infiltrate. Patients also had to have a modified Fine risk score of I or II (< 70 points) | |
| Interventions | Single 2 g dose of azithromycin microspheres administered as an oral suspension versus extended‐release clarithromycin administered orally as 2 x 500 mg capsules once daily for 7 days | |
| Outcomes | Primary endpoint: clinical response in the clinical per protocol population, on the basis of signs and symptoms of CAP, assessed at test‐of‐cure visit (TOC; day 14 to 21): azithromycin 91.8% versus clarithromycin 94.7% (non‐significant difference). Treatment‐related adverse effects: azithromycin 26.3% versus clarithromycin 24.6% (non‐significant difference), mainly gastrointestinal side effects | |
| Notes | Study funded by Pfizer Inc., 2 of 4 authors are employees of Pfizer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were randomised according to computer‐generated pseudo random code using the method of permutated blocks, balanced within investigational site" |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization numbers were assigned by a central Web/telephone computer‐based telerandomization system" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, double‐dummy technique |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Detailed list of reasons for exclusion from efficacy analyses, with number of patients affected |
| Selective reporting (reporting bias) | Low risk | Pre‐specified outcomes appear to be fully reported |
| Other bias | Unclear risk | Study sponsored by Pfizer |
English 2012.
| Methods | 2 phase 3 prospective, double‐blinded RCTs, parallel‐group, multicentre, multinational studies | |
| Participants | Male or female participants > 18 years from outpatient clinics | |
| Interventions | Oral cethromycin versus oral clarithromycin | |
| Outcomes | Primary: clinical and radiological response. Secondary: bacteriological response, adverse effects | |
| Notes | This trial included results from 2 separate RCTs. However, results were pooled and the interventions were identical. The results of the 2 studies are reported as 1 in the article. Study setting was South Africa, USA, Canada, South America, Europe, Israel | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation done by interactive voice response system (IVRS) |
| Allocation concealment (selection bias) | Low risk | IVRS assigned numerically coded blister cards to each participant containing either cethromycin or clarithromycin or placebo |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and assessors were blinded to treatment and assessments. Study medications and placebos were over‐encapsulated and appeared identical |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All inclusions and exclusions were explicitly stated |
| Selective reporting (reporting bias) | Low risk | All enrolled participants were accounted for. Reasons for participant exclusion from the final analysis were provided |
| Other bias | High risk | All authors were members of Advanced Life Sciences Inc, a biopharmaceutical company (USA) |
Kohno 2003.
| Methods | Date and duration of study: December 2000 to June 2001. Double‐blind, double dummy, randomised phase III trial, conducted in 117 centres in Japan | |
| Participants | 270 patients (mix of in‐ and outpatients), aged 16 to 80 years. 123 outpatients were included. Exclusion criteria are clearly stated and patient selection flow chart is provided | |
| Interventions | Telithromycin 600 mg (= 2 x 300 mg) once daily after breakfast versus levofloxacin 100 mg 3 times daily for 7 days | |
| Outcomes | Results are reported separately for in‐ and outpatients. Primary endpoint: clinical response in the clinical per protocol population, on the basis of signs and symptoms of CAP, assessed at end of treatment (EOT; 7 days after treatment initiation) telithromycin 95.7% versus clarithromycin 96.3% (non‐significant difference). Treatment‐related adverse effects: telithromycin 33.6% versus levofloxacin 33.9% (non‐significant difference), mostly gastrointestinal side effects | |
| Notes | Study report is in Japanese, but extensive figures and tables are provided in English, so that most of the necessary information could be extracted. Missing information was kindly provided by the main author, Prof. Shigeru Kohno, MD, PhD, of Nagasaki University, Japan. Funding provided by Astellas (Fujisawa) Pharmaceutical Co. Ltd | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Text in Japanese; tables and figures in English |
| Allocation concealment (selection bias) | Unclear risk | Text in Japanese; tables and figures in English |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, double‐dummy technique |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Detailed flow chart of reasons for exclusion from efficacy analyses, with number of patients affected |
| Selective reporting (reporting bias) | Unclear risk | Text in Japanese; tables and figures in English |
| Other bias | Unclear risk | Study sponsored by Fujisawa |
Mathers Dunbar 2004.
| Methods | Date and duration of study: May 1998 to September 1999. Double‐blind, double‐dummy, parallel‐group clinical trial conducted at 54 centres in 4 countries (United States, Canada, Argentina and Chile) | |
| Participants | 493 outpatients aged 18 and over. Patients were eligible for enrolment if they had a clinical diagnosis of acute CAP (signs and symptoms) and chest radiographic findings supporting the clinical diagnosis of bacterial pneumonia | |
| Interventions | Telithromycin 800 mg (= 2 x 400 mg capsules) once daily in the morning versus clarithromycin 500 mg (= 2 x 250 mg capsules) twice daily for 10 days | |
| Outcomes | Primary endpoint: clinical response in the clinically assessable per protocol population, on the basis of signs and symptoms of CAP, assessed at test‐of‐cure visit (TOC; day 17 to 24): telithromycin 88.3% versus clarithromycin 88.5% (non‐significant difference). Treatment‐related adverse effects: telithromycin 38.5% versus clarithromycin 27.9%, mostly gastrointestinal side effects | |
| Notes | Funded by an unrestricted educational grant from Aventis Pharmaceuticals | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients... were randomised according to a schedule generated by the sponsor for each centre. The schedule linked sequential treatment assignment number to treatment codes allocated at random." |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization schedules were held by the sponsor." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, double‐dummy technique. Quote: "Treatment blinding was ensured by encapsulation of both active study medication and placebo within identical opaque capsules." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Detailed list of reasons for exclusion from efficacy analyses, with number of patients affected |
| Selective reporting (reporting bias) | Low risk | Pre‐specified outcomes appear to be fully reported |
| Other bias | Unclear risk | Funded by an unrestricted educational grant from Aventis Pharmaceuticals |
Oldach 2013.
| Methods | Randomised, double‐blind, multicentre, phase 2 study | |
| Participants | Males and females > 18 years | |
| Interventions | Oral solithromycin versus oral levofloxacin | |
| Outcomes | Primary: clinical response. Secondary: bacteriological response, adverse effects | |
| Notes | Study done in USA and Canada. Did not explicitly state the setting in which patients were recruited | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation stratified by age and pneumonia severity index (PORT score), but method of randomisation not explicitly stated |
| Allocation concealment (selection bias) | Unclear risk | Not clearly stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not clearly stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | CONSORT diagram of patient inclusion and reasons for exclusion is not provided in the paper |
| Selective reporting (reporting bias) | Unclear risk | Since CONSORT diagram missing, we are unclear regarding the reasons patients were excluded from the outcome analysis |
| Other bias | High risk | Study was funded by Cempra Inc., a pharmaceutical company. All investigators are member of Cempra Inc. USA, or InClin Inc. USA. InClin is a consulting firm for biotechnology and pharmaceutical companies |
Udupa 2011.
| Methods | Randomised study | |
| Participants | Males and females 18 to 55 years of age | |
| Interventions | Oral clarithromycin/oral azithromycin or oral levofloxacin/oral high‐dose amoxicillin | |
| Outcomes | Primary: clinical response. Secondary: adverse effects | |
| Notes | Study conducted in Rural Health Training Centre in India | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No stated method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not explicitly stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not explicitly stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No CONSORT diagram presented, therefore reasons for exclusion of patients at the entry level are unclear |
| Selective reporting (reporting bias) | Low risk | The study reported on every patient that was included in the trial (n = 31) |
| Other bias | Low risk | No explicit mention of biopharmaceutical industry involvement |
Vacarezza 2010.
| Methods | Randomised, double‐blinded study (outpatients), single‐blinded (inpatients) | |
| Participants | Adult males and females > 18 years | |
| Interventions | Oral clarithromycin versus oral amoxicillin for outpatients study arm | |
| Outcomes | Primary: clinical response. Secondary: adverse effects | |
| Notes | Article in Spanish; translation done by Dr. RA Rodriguez, Dr. Gonzalo Alvarez and Dr. Jacqueline Sandoz (Department of Medicine, University of Ottawa) Hospitalised patients are included in the study but separate results for inpatients and outpatients are provided. Study conducted in Uruguay |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation by PORT score was used |
| Allocation concealment (selection bias) | Unclear risk | Not explicitly stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No explicit mention of patient and assessor blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No CONSORT diagram presented, therefore reasons for exclusion of patients at the entry level are unclear |
| Selective reporting (reporting bias) | Low risk | The study reported on every patient that was included in the trial |
| Other bias | Low risk | No financial conflicts |
van Rensburg 2010a.
| Methods | Randomised, double‐blind, multicentre, multinational study, with 3 treatment arms | |
| Participants | Adult males and females > 18 years | |
| Interventions | Oral nemonoxacin 750 mg versus levofloxacin 500 mg daily for 7 days | |
| Outcomes | Primary: clinical response. Secondary: bacteriological response | |
| Notes | Study was partially supported by grant from Government of Taiwan. Study conducted in Taiwan and South Africa | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No explicit mention of randomisation methods |
| Allocation concealment (selection bias) | Unclear risk | No explicit mention of allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No explicit mention of patient and assessor blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | CONSORT diagram presented and numbers of included and excluded patients stated. However, reasons for exclusions are not clearly stated |
| Selective reporting (reporting bias) | Unclear risk | No evidence of selective reporting; all outcomes are reported in the included study population |
| Other bias | High risk | Several authors of the study are employees of TaiGen Biotechnology Co. Ltd. |
van Rensburg 2010b.
| Methods | Randomised, double‐blind, multicentre, multinational study, with 3 treatment arms | |
| Participants | Adult males and females > 18 years | |
| Interventions | Oral nemonoxacin 500 mg versus levofloxacin 500 mg daily for 7 days | |
| Outcomes | Primary: clinical response. Secondary: bacteriological response | |
| Notes | Study was partially supported by grant from Government of Taiwan. Study conducted in Taiwan and South Africa | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No explicit mention of randomisation methods |
| Allocation concealment (selection bias) | Unclear risk | No explicit mention of allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No explicit mention of patient and assessor blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | CONSORT diagram presented and numbers of included and excluded patients stated. However, reasons for exclusions are not clearly stated |
| Selective reporting (reporting bias) | Unclear risk | No evidence of selective reporting; all outcomes are reported in the included study population |
| Other bias | High risk | Several authors of the study are employees of TaiGen Biotechnology Co. Ltd. |
CAP: community‐acquired pneumonia CXR: chest x‐ray EOT: end of treatment IVRS: interactive voice response system n: number RCT: randomised controlled trial TOC: test‐of‐cure
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alcacer 1993 | Hospitalised patients only included |
| Arifin 2013 | Conference abstract only |
| Bai 2014 | Meta‐analysis of RCTs on ertapenem and ceftriaxone: inpatients only |
| Balgos 1999 | Mix of in‐ and outpatients (unspecified proportions) Mix of diagnoses (chronic bronchitis and CAP), only about 50% with CAP; results reported separately Comparison of 2 dosage regimens of the same drug (amoxycillin/clavulanic acid 875/125 mg twice daily versus 500/125 mg 3 times daily) |
| Ball 1994 | Review of a series of unblinded trials Mix of patients from different studies |
| Balmes 1991 | Mix of diagnoses (acute bronchitis and pneumonia) Only 8/110 patients (7%) had a diagnosis of pneumonia Mix of in‐ and outpatients (unspecified proportions) |
| Bantz 1987 | Mix of diagnoses (bronchitis and pneumonia) Only 15/108 patients (14%) had a diagnosis of pneumonia Data not reported separately |
| Biermann 1988 | No chest X‐ray for every patient with suspected pneumonia |
| Blasi 2013 | Patients with chronic bronchitis were included, not CAP |
| Block 2006 | Children with a mean age of 11; comparison of same antibiotic with different doses; indication for treatment consists of mix of diagnoses besides CAP; some diagnoses not confirmed by chest X‐ray |
| Bonvehi 2003 | Focus on pneumococcal CAP; positive sputum cultures used as a selection criterion |
| Bothra 2012 | Study not randomised, expert opinion only |
| Brittain‐Long 2011 | Study did not address antibiotic treatment for CAP and URTIs |
| Carbon 1999 | Mix of in‐ and outpatients, data not reported separately; authors did not respond, or separate data could not be provided by authors |
| Casapao 2012 | Study not randomised, expert opinion only |
| Chodosh 1991 | Only bacteriologically evaluable patients were included |
| Critchley 2010 | Hospitalised patients only included |
| Daniel 1999a | Hospitalised patients included; study uses trovafloxacin ‐ not recommended for treatment of CAP |
| Daniel 1999b | Hospitalised patients included; study uses trovafloxacin ‐ not recommended for treatment of CAP |
| Dark 1991 | Mix of diagnoses (bronchitis and pneumonia) Only 23/272 patients (8%) had a diagnosis of pneumonia Mix of in‐ and outpatients (unspecified proportions) |
| Dartois 2013 | Pooled analysis of 2 phase 3 studies on tigecycline versus levofloxacin iv ‐ inpatients only |
| Dautzenberg 1992 | Open‐label study |
| De Cock 1988 | Mix of diagnoses (acute bronchitis, acute superinfection of chronic bronchitis, pneumonia) and results reported separately Only 42/198 patients (21%) had a diagnosis of pneumonia (results not reported separately for each diagnostic group) |
| Dean 2006 | Open‐label study |
| Donowitz 1997 | Focus on bacterial pneumonia (acute onset, purulent sputum, Gram stain and chest X‐ray consistent with bacterial pneumonia were required for inclusion into the study) |
| Esposito 2012 | Review article, not a RCT |
| File 1997 | Mix of in‐ and outpatients, data not reported separately; authors did not respond, or separate data could not be provided by authors |
| File 2001 | Mix of in‐ and outpatients, data not reported separately; authors did not respond, or separate data could not be provided by authors |
| File 2004 | Focus on bacterial pneumonia |
| File 2012a | Report on safety and efficacy of ceftaroline fosamil; not a RCT; hospitalised patients included |
| File 2012b | Hospitalised patients included |
| Fogarty 1999 | Mix of in‐ and outpatients, data not reported separately; authors did not respond, or separate data could not be provided by authors |
| Fogarty 2002 | Focus on bacterial pneumonia. Serologic evidence of M. pneumoniae or C. pneumoniae was an exclusion criterion; serologic testing was systematically carried out pre‐treatment as well as twice after treatment |
| Fogarty 2004 | Hospitalised patients only included |
| Fong 1995 | Too small (n < 30) |
| Fujita 2012 | Not a RCT |
| Ghebremedhin 2012 | Study on pharmacokinetics and pharmacodynamics of sitafloxacin, not a RCT |
| Gotfried 2002 | Culture confirmation of bacterial CAP required |
| Gris 1996 | Too small (n < 30) |
| Hagberg 2002 | Mix of in and outpatients, data not reported separately; authors did not respond, or separate data could not be provided by authors |
| Higuera 1996 | Open‐label study |
| Hoeffken 2001 | Patients were recruited as outpatients. However, 30% were hospitalised in the course of the study, data for in‐ and outpatients are not reported separately, and hospitalisation was not considered treatment failure |
| Hoepelman 1993 | Mix of diagnoses (infective exacerbation of chronic obstructive pulmonary disease, purulent bronchitis and pneumonia) and results not reported separately Only 9/99 patients (9%) had pneumonia. Pneumonia not necessarily community‐acquired |
| Hoepelman 1998 | Mix of diagnoses (infective exacerbation of chronic obstructive pulmonary disease, purulent bronchitis and pneumonia) and results not reported separately; only 3 of 144 patients had CAP |
| Kammer 1991 | Only bacteriologically evaluable patients were included |
| Kiani 1990 | Mix of in and outpatients, data not reported separately Mix of diagnoses (acute bronchitis, pneumonia, acute exacerbation of chronic bronchopulmonary infection) but data reported separately Only 10/110 patients (9%) had a diagnosis of pneumonia |
| Kinasewitz 1991 | Focus on bacterial pneumonia (purulent sputum and leucocytosis were required for inclusion into study, patients without sputum pathogens were excluded from efficacy analysis, etc.) |
| Kohno 2013 | Hospitalised patients only included |
| Lacny 1972 | Mix of diagnoses (infection of soft tissue, infection of the upper respiratory tract, otitis, skin infection, conjunctivitis, pneumonia) and results not reported separately Only 2/121 patients (2.6%) had a diagnosis of pneumonia Completely institutionalised population 32.5% patients were younger than 16 years |
| Lagler 2012 | Comparison of same antibiotic with different doses (abstract only) |
| Laurent 1996 | Mix of diagnoses (acute bronchitis, acute infectious exacerbations of chronic bronchitis or pneumonia) but results reported separately Only 43/204 patients (21%) had a diagnosis of pneumonia Mix of in‐ and outpatients (unclear in which proportions) |
| Lee 2012 | Hospitalised patients only included |
| Leophonte 2004 | Focus on pneumococcal pneumonia |
| Liipo 1994 | Dirithromycin withdrawn from market |
| Little 2012 | Patients with CAP excluded, only patients with lower respiratory tract infection were included |
| Lode 1995 | Only inpatients (personal communication H. Lode), contrary to study report (patients reportedly treated as either in‐ or outpatients) |
| Lode 1998 | Combines patients from 4 other RCTs, including only those patients with S. pneumoniae pneumonia confirmed by blood culture, i.e. highly select subgroup, generalisability questionable. The data from the 4 studies can be obtained from the original reports (one of which is Örtqvist 1996, already excluded from this review because open‐label) |
| Lode 2004a | Gatifloxacin withdrawn from market |
| Lode 2004b | Gatifloxacin withdrawn from market |
| Long 2011 | Not comparing treatment looking at antibiotic reduction, not comparing antibiotic use |
| Lopez‐Vejar 2013 | Comparison levofloxacin versus ceftriaxone/clarithromycin iv ‐ inpatients only |
| MacFarlane 1996 | Single‐blind study, no chest X‐ray required |
| Matzneller 2013 | A study about tissue pharmacokinetics and pharmacodynamics, not a RCT |
| Montassier 2013 | A critical and synthetic review of the literature, not a RCT |
| Moola 1999 | Grepafloxacin withdrawn from market |
| Müller 1992 | Only bacteriologically evaluable patients were included |
| Naderer 2013 | A pharmacokinetic study on healthy volunteers, not a RCT |
| NAPSG 1997 | Fleroxacin withdrawn from market |
| Navarta 2010 | Hospitalised patients only included, not clear if diagnosis confirmed by chest X‐ray |
| Neu 1993 | Mix of diagnoses (acute bacterial exacerbation of chronic bronchitis or asthmatic bronchitis and bacterial pneumonia) and data not reported separately Unclear whether in‐ or outpatients. Only 43/213 (20%) had a diagnosis of CAP |
| Nussenblatt 2013 | A review of interventions to improve outcomes in CAP patients, not a RCT |
| O'Doherty 1997 | Grepafloxacin withdrawn from worldwide market in October 1999 due to risk of severe arrhythmias |
| O'Doherty 1998 | Open‐label study |
| Pavie 2005 | Descriptive study, not a RCT |
| Petitpretz 2001 | Focus on pneumococcal pneumonia |
| Peugeot 1991 | Open‐label study |
| Polverino 2013 | Hospitalised patients only |
| Pullman 2003 | Excluded because trovafloxacin no longer available for outpatients due to severe hepatotoxicity. Study recruitment was terminated because FDA restricted use of trovafloxacin to hospitalised patients with severe life‐ or limb‐threatening infections (1999) |
| Rahav 2004 | Open‐label study |
| Ramirez 1999 | Sparfloxacin withdrawn from market due to increased risk of severe phototoxicity and rash, as well as rhabdomyolysis |
| Rank 2011 | Cohort of RCTs, not a RCT |
| Rayman 1996 | Comparison of different dosage regimens of the same drug Included patients with either CAP or acute exacerbation of chronic bronchitis (i.e. mixed indications) Groups were not similar at baseline (more smokers and more failures of previous treatment in the "twice daily" group, for which more adverse reactions were reported) |
| Saito 2008 | Mix of in‐ and outpatients, separate data for outpatients not available Note: article in Japanese. However, tables and figures in English; additional information obtained through personal communication with one of the authors (Kohno S) |
| Salvarezza 1998 | Open‐label study |
| Schleupner 1988 | Mix of diagnoses (bronchitis, pneumonia) Only 34/61 patients (56%) had a diagnosis of pneumonia Mix of in‐ and outpatients (unspecified proportions) |
| Seki 2009 | Hospitalised patients included |
| Shorr 2013 | Hospitalised patients only |
| Siquier 2006 | Focus on pneumococcal pneumonia |
| Skalsky 2012 | A systematic review and meta‐analysis of RCTs for CAP. We searched the back references of this paper to check for any studies suitable for our review. Not a RCT |
| Smith 2013 | Cost‐effectiveness analysis, not a RCT |
| Snyman 2009 | Comparison of same antibiotic; mix of diagnoses not just CAP |
| Sokol 2002 | Excluded because trovafloxacin no longer indicated for outpatients, later removed from market due to severe hepatotoxicity. Study recruitment was terminated because FDA restricted use of trovafloxacin to hospitalised patients with severe life‐ or limb‐threatening infections (1999) |
| Sopena 2004 | Open‐label study |
| Stille 2000 | Hospitalised patients |
| Sun 2012 | Healthy participants |
| Tellier 2004 | Mix of in‐ and outpatients, data not reported separately; authors did not respond, or separate data could not be provided by authors |
| Tilyard 1992 | Too small (n < 30) |
| Torres 2003 | Mix of in‐ and outpatients, data not reported separately; authors did not respond, or separate data could not be provided by authors |
| Trémolières 1998 | Trovafloxacin withdrawn from market due to severe hepatotoxicity |
| Trémolières 2005 | Focus on bacterial pneumonia |
| van Zyl 2002 | Focus on bacterial pneumonia; purulent sputum sample required; patients with serologic evidence of Mycoplasma pneumoniae or Chlamydophila pneumoniae were excluded |
| Viasusa 2013 | A review of CAP treatment |
| Worrall 2010 | Not a RCT |
| Wunderink 2011 | Not a RCT |
| Yamamoto 2013 | Hospitalised patients only |
| Yuan 2012 | Meta‐analysis of RCTs, not a RCT |
| Zhang 2012 | Systematic review and meta‐analysis, not a RCT |
| Zuck 1990 | Open‐label, unblinded study comparing oral administration twice daily versus intramuscular administration once daily. Included only high‐risk patients; unclear whether treated as in‐ or outpatients |
| Örtqvist 1996 | Patients had CAP but were treated exclusively as inpatients |
CAP: community‐acquired pneumonia FDA: Food and Drug Administration iv: intravenous RCT: randomised controlled trial URTIs: upper respiratory tract infections
Differences between protocol and review
In our first review of this topic, published in 2004 (Bjerre 2004), as per protocol we contacted the following antibiotics manufacturers to identify any additional published or unpublished studies: Abbott, AstraZeneca, Aventis, Boehringer‐Ingelheim, Bristol‐Myers‐Squibb, GlaxoSmithKline, Hoffmann‐LaRoche, Lilly, Merck, Merck Sharp & Dohme, Novartis, Pfizer, Pharmacia, Sanofi and Yamanouchi. This search yielded no new studies. Starting with this update, we decided to no longer contact pharmaceutical companies to ask about unpublished studies. This decision was made for two reasons: first, because of the very low yield of this search strategy, compared to the significant amount of time it requires; and second, because this search strategy provides an unfair advantage to unpublished studies carried out by industry, as opposed to government or academia, where an equivalent search strategy is not readily available.
In this review and the second update of this review (Bjerre 2009), we excluded studies of antibiotics that have been withdrawn from the market or are no longer licensed for the treatment of outpatients with CAP, due to severe adverse effects. For example, studies assessing the following fluoroquinolones were excluded: gatifloxacin, grepafloxacin, sparfloxacin, temafloxacin and trovafloxacin.
Finally, in the second update (Bjerre 2009), and in this 2014 update, we applied the new 'Risk of bias' tools (tables, summary figures and graphs) newly made available in RevMan 2014. These tools were not available at the time our protocol and our first review (Bjerre 2004) were written and published.
Contributions of authors
Smita Pakhale and Sunita Mulpuru independently screened abstracts and full articles for inclusion in this review. They decided, by mutual agreement, which articles to include, extracted the data from these articles, performed the quantitative analyses, wrote the text, tables and figures of this review and made modifications to this review based on the comments of peer and consumer referees.
Lise M Bjerre was the lead author on the first two iterations of this review (2004, 2009); as such, she made substantial contributions to study design and to data acquisition, analysis and interpretation; she also wrote the text of these two earlier reviews, which form the backbone of the present review, with editorial input from MMK and TJMV. For the present review, she provided background information and guidance to the new lead author (Smita Pakhale) with respect to the search criteria and the overall process of the Cochrane review; she critically reviewed the manuscript for important intellectual content and approved the final version for submission.
Michael M Kochen (MMK) co‐wrote the protocol, defined the search strategy and did a preliminary screening of abstracts for inclusion into the 2004 and 2009 reviews. He also critically reviewed and edited the text of this review at various stages in its development.
Theo JM Verheij (TJMV) co‐wrote the protocol, screened abstracts and full articles for inclusion in the study and decided, in agreement with LMB, which articles to include for 2004 and 2009 reviews. He also critically reviewed and edited the text of the review at various stages in its development for all three reviews.
Gernot GU Rohde critically reviewed the protocol and data extraction tool and contributed to the revision and critical review of this manuscript.
All authors agreed upon the final draft of this review prior to submission.
Sources of support
Internal sources
No external funding was received for this review. Dr. Smita Pakhale being a full‐time staff respirologist and a clinician scientist receives support from the Department of Medicine, the Ottawa Hospital, and Ottawa Hospital Research Institute, Canada.
External sources
None, Other.
Declarations of interest
Smita Pakhale: no potential conflicts of interest to declare. Sunita Mulpuru: no potential conflicts of interest to declare. Theo JM Verheij: I participated in an RCT on the effects of pneumococcal vaccination, funded by Pfizer. Michael M Kochen: no potential conflicts of interest to declare. Gernot GU Rohde: Dr. Rohde received financial benefits for advisory board memberships from Pfizer and consultancy work for Novartis and Takeda as well as payments for lectures from Pfizer, Chiesi, Astra‐Zeneca, GSK, Novartis and Grünenthal. Lise M Bjerre: Dr. Bjerre currently holds operating foundation grants as principal investigator from the Canadian Institutes for Health Research (CIHR) and the Centre for Learning, Research and Innovation in Long‐term care (CLRI) of the Ministry of Health of Ontario; she is also co‐investigator on other CIHR grants. She has never accepted remuneration, gifts or research funds in any form from drug or device manufacturers, or other member of the pharmaceutical industry.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Anderson 1991 {published data only}
- Anderson G, Esmonde TS, Coles S, Macklin J, Carnegie C. A comparative safety and efficacy study of clarithromycin and erythromycin stearate in community‐acquired pneumonia. Journal of Antimicrobial Chemotherapy 1997;27(Suppl A):117‐24. [DOI] [PubMed] [Google Scholar]
Chien 1993 {published data only}
- Chien SM, Pichotta P, Siepman N, Chan CK and the Canada‐Sweden Clarithromycin‐Pneumonia Group. Treatment of community‐acquired pneumonia: a multicenter, double‐blind, randomized study comparing clarithromycin with erythromycin. Chest 1993;103:697‐701. [DOI] [PubMed] [Google Scholar]
D'Ignazio 2005 {published data only}
- D'Ignazio J, Camere MA, Lewis DE, Jorgensen D, Breen JD. Novel, single‐dose microsphere formulation of azithromycin versus 7‐day levofloxacin therapy for treatment of mild to moderate community‐acquired pneumonia in adults. Antimicrobial Agents and Chemotherapy 2005;49(10):4035‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Drehobl 2005 {published data only}
- Drehobl MA, Salvo MC, Lewis DE, Breen JD. Single‐dose azithromycin microspheres vs clarithromycin extended release for the treatment of mild‐to‐moderate community‐acquired pneumonia in adults. Chest 128;4:2230‐7. [DOI] [PubMed] [Google Scholar]
English 2012 {published data only}
- English ML, Fredericks CE, Milanesio NA, Rohowsky N, Xu Z‐Q, Jenta TRJ, et al. Cethromycin versus clarithromycin for community‐acquired pneumonia: comparative efficacy and safety outcomes from two double‐blinded, randomized, parallel‐group, multicenter, multinational noninferiority studies. Antimicrobial Agents and Chemotherapy 2011;56(4):2037‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welte T, Torres A, Nathwani D. Clinical and economic burden of community‐acquired pneumonia among adults in Europe. Thorax 2012;67:71‐9. [Welte 2012] [DOI] [PubMed] [Google Scholar]
Kohno 2003 {published data only}
- Kohno S, Watanabe A, Aoki N, Niki Y. Clinical evaluation of telithromycin for community‐acquired pneumonia ‐ phase III double‐blind comparative study of telithromycin versus levofloxacin [Japanese]. Japanese Journal of Chemotherapy 2003;51(Suppl 1):255‐78. [Google Scholar]
Mathers Dunbar 2004 {published data only}
- Mathers Dunbar L, Hassman J, Tellier G. Efficacy and tolerability of once‐daily oral telithromycin compared with clarithromycin for the treatment of community‐acquired pneumonia in adults. Clinical Therapeutics 2004;26(1):48‐62. [DOI] [PubMed] [Google Scholar]
Oldach 2013 {published data only}
- Oldach D, Clark K, Schranz J, Das A, Craft JC, Scott D, et al. Randomized, double‐blind, multicentre phase 2 study comparing the efficacy and safety of oral solithromycin (CEM‐101) to those of oral levofloxacin in the treatment of patients with community‐acquired bacterial pneumonia. Antimicrobial Agents and Chemotherapy 2013;57(6):2526‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Udupa 2011 {published data only}
- Udupa A, Gupta P. Antibiotic therapy in pneumonia: a comparative study of oral antibiotics in a rural healthcare centre. International Journal of Pharmacy and Pharmaceutical Sciences 2011;3:156‐8. [Google Scholar]
Vacarezza 2010 {published data only}
- Vacarezza M, Pedrouzo RV, Bartesaghi L, Sicca M, Lerena V, Perdomo J, et al. Community‐acquired acute pneumonias in the adult. Controlled therapeutic study. Uruguay [Neumona aguda del adulto adquirida en la comunidad. Ensayo terapéutico controlado. Uruguay]. Archivos de Medicina Interna 2010;XXXII(2‐3):31‐5. [Google Scholar]
van Rensburg 2010a {published data only}
- Rensburg D, Perng R‐P, Mitha IH, Bester AJ, Kasumba J, Wu R‐G, et al. Efficacy and safety of nemonoxacin versus levofloxacin for community acquired pneumonia. Antimicrobial Agents and Chemotherapy 2010;54(10):4098‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Rensburg 2010b {published data only}
- Rensburg D, Perng R‐P, Mitha IH, Bester AJ, Kasumba J, Wu R‐G, et al. Efficacy and safety of nemonoxacin versus levofloxacin for community acquired pneumonia. Antimicrobial Agents and Chemotherapy 2010;54(10):4098‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Alcacer 1993 {published data only}
- Alcacer F, Belda A, Custardoy J, Borras F, Redondo A, Borja J, et al. [Estudio comparativo entre ofloxacino y eritromicina en el tratamiento de la neumonia de la comunidad]. Revista Española de Quimioterapia 1993;6(3):213‐8. [Google Scholar]
Arifin 2013 {published data only}
- Arifin NM, Soepandi P, Burhan E, Isbaniah F, Arimah C, Kusumo D, et al. P319 Multicenter, comparative study of levofloxacin high‐dose, IV oral sequential therapy for CAP with moxifloxacin in Indonesia. International Journal of Antimicrobial Agents 2013;42(Suppl):S142. [Google Scholar]
Bai 2014 {published data only}
- Bai N, Sun C, Wang J, Cai Y, Liang B, Zhang L, et al. Ertapenem versus ceftriaxone for the treatment of complicated infections: a meta‐analysis of randomized controlled trials. Chinese Medical Journal 2014;127(6):1118‐25. [PUBMED: 24622445] [PubMed] [Google Scholar]
Balgos 1999 {published data only}
- Balgos AA, Rodriguez‐Gomez G, Nasnas R, Mahasur AA, Margono BP, Tinoco‐Favila JC. Efficacy of twice‐daily amoxycillin/clavulanate in lower respiratory tract infections. International Journal of Clinical Practice 1999;53(5):325‐30. [PubMed] [Google Scholar]
Ball 1994 {published data only}
- Ball P. Efficacy and safety of cefprozil versus other beta‐lactam antibiotics in the treatment of lower respiratory tract infections. European Journal of Clinical Microbiology and Infectious Diseases 1994;13(10):851‐6. [DOI] [PubMed] [Google Scholar]
Balmes 1991 {published data only}
- Balmes P, Clerc G, Dupont B, Labram C, Pariente P, Poirier R. Comparative study of azithromycin and amoxicillin/clavulanic acid in the treatment of lower respiratory tract infections. European Journal of Clinical Microbiology and Infectious Diseases 1991;10:437‐9. [DOI] [PubMed] [Google Scholar]
Bantz 1987 {published data only}
- Bantz PM, Grote J, Peters‐Haertel W, Stahmann J, Timm J, Kasten R, et al. Low‐dose ciprofloxacin in respiratory tract infections: a randomized comparison with doxycycline in general practice. American Journal of Medicine 1987;82(Suppl 4A):208‐10. [Google Scholar]
Biermann 1988 {published data only}
- Biermann C, Loken A, Riise R. Comparison of spiramycin and doxycycline in the treatment of lower respiratory infections in general practice. Journal of Antimicrobial Chemotherapy 1988;22(Suppl B):155‐8. [DOI] [PubMed] [Google Scholar]
Blasi 2013 {published data only}
- Blasi F, Tarsia P, Mantero M, Morlacchi LC, Piffer F. Cefditoren versus levofloxacin in patients with exacerbations of chronic bronchitis: serum inflammatory biomarkers, clinical efficacy, and microbiological eradication. Journal of Therapeutics and Clinical Risk Management 2013;9:55‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Block 2006 {published data only}
- Block SL. Comparative tolerability, safety and efficacy of tablet formulations of twice‐daily clarithromycin 250 mg versus once‐daily extended‐release clarithromycin 500 mg in pediatric and adolescent patients. Clinical Pediatrics 2006;45(7):641‐8. [DOI] [PubMed] [Google Scholar]
Bonvehi 2003 {published data only}
- Bonvehi P, Weber K, Busman T, Shortridge D, Notario G. Comparison of clarithromycin and amoxicillin/clavulanic acid for community‐acquired pneumonia in an era of drug‐resistant streptococcus pneumoniae. Clinical Drug Investigation 2003;23(8):491‐501. [DOI] [PubMed] [Google Scholar]
Bothra 2012 {published data only}
- Bothra M, Lodha R, Kabra SK. Tobramycin for the treatment of bacterial pneumonia in children. Expert Opinion on Pharmacotherapy 2012;13(4):565‐71. [DOI] [PubMed] [Google Scholar]
Brittain‐Long 2011 {published data only}
- Brittain‐Long R, Westin J, Olofsson S, Lindh M, Andersson LM. Access to a polymerase chain reaction assay method targeting 13 respiratory viruses can reduce antibiotics: a randomised, controlled trial. BMC Medicine 2011;9:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carbon 1999 {published data only}
- Carbon C, Ariza H, Rabie WJ, Salvarezza CR, Elkharrat D, Rangaraj M, et al. Comparative study of levofloxacin and amoxycillin/clavulanic acid in adults with mild‐to‐moderate community‐acquired pneumonia. Clinical Microbiology and Infection 1999;5:724‐32. [Google Scholar]
Casapao 2012 {published data only}
- Casapao AM, Steed ME, Levine DP, Rybak MJ. Ceftaroline fosamil for community‐acquired bacterial pneumonia and acute bacterial skin and skin structure infection. Expert Opinion on Pharmacotherapy 2012;13(8):1177‐86. [DOI] [PubMed] [Google Scholar]
Chodosh 1991 {published data only}
- Chodosh S. Temafloxacin compared with ciprofloxacin in mild to moderate lower respiratory tract infections in ambulatory patients. Chest 1991;100:1497‐502. [DOI] [PubMed] [Google Scholar]
Critchley 2010 {published data only}
- Critchley I, Friedland D, Eckburg P, Jandourek A, Han S, Thye D. Microbiological outcomes of 2 multicenter phase 3 clinical trials of ceftaroline in community‐acquired bacterial pneumonia. American Journal of Respiratory and Critical Care Medicine 2010;181:A5481. [Google Scholar]
Daniel 1999a {published data only}
- Daniel R. Oral trovafloxacin compared with amoxicillin (plus optional erythromycin) for the treatment of mild to moderate community‐acquired pneumonia. Drugs 1999;58(Suppl 2):320‐2. [Google Scholar]
Daniel 1999b {published data only}
- Daniel R. Trovafloxacin vs high dose amoxicillin in the treatment of community‐acquired bacterial pneumonia. Drugs 1999;58(Suppl 2):304‐5. [DOI] [PubMed] [Google Scholar]
Dark 1991 {published data only}
- Dark D. Multicenter evaluation of azithromycin and cefaclor in acute lower respiratory tract infections. American Journal of Medicine 1991;91(Suppl 3A):31‐5. [DOI] [PubMed] [Google Scholar]
Dartois 2013 {published data only}
- Dartois N, Cooper CA, Castaing N, Gandjini H, Sarkozy D. Tigecycline versus levofloxacin in hospitalized patients with community‐acquired pneumonia: an analysis of risk factors. Open Respiratory Medicine Journal 2013;7:13‐20. [PUBMED: 23526572] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dautzenberg 1992 {published data only}
- Dautzenberg B, Scheimberg A, Brambilla C, Camus P, Godard P, Guerin JC. Comparison of two oral antibiotics, roxithromycin and amoxicillin plus clavulanic acid, in lower respiratory tract infections. Diagnostic Microbiology and Infectious Disease 1992;15(Suppl):85‐9. [DOI] [PubMed] [Google Scholar]
Dean 2006 {published data only}
- Dean NC, Sperry P, Wikler M, Suchyta MS, Hadlock C. Comparing gatifloxacin and clarithromycin in pneumonia symptom resolution and process of care. Antimicrobial Agents and Chemotherapy 2006;50(4):1164‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
De Cock 1988 {published data only}
- Cock L, Poels R. Comparison of spiramycin with erythromycin for lower respiratory tract infections. Journal of Antimicrobial Chemotherapy 1988;22(Suppl B):159‐63. [DOI] [PubMed] [Google Scholar]
Donowitz 1997 {published data only}
- Donowitz GR, Brandon ML, Salisbury JP, Harman CP, Tipping DM, Urick AE, et al. Sparfloxacin versus cefaclor in the treatment of patients with community‐acquired pneumonia: a randomized, double‐masked, comparative, multicenter study. Clinical Therapeutics 1997;19(5):936‐53. [DOI] [PubMed] [Google Scholar]
Esposito 2012 {published data only}
- Esposito S, Esposito I, Leone S. Considerations of antibiotic therapy duration in community and hospital‐acquired bacterial infections. Journal of Antimicrobial Chemotherapy 2012;67(11):2570‐5. [DOI] [PubMed] [Google Scholar]
File 1997 {published data only}
- File TM Jr, Segreti J, Dunbar L, Player R, Kohler R, Williams RR, et al. A multicenter, randomized study comparing the efficacy and safety of intravenous and/or oral levofloxacin versus ceftriaxone and/or cefuroxime axetil in treatment of adults with community‐acquired pneumonia. Antimicrobial Agents and Chemotherapy 1997;41(9):1965‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
File 2001 {published data only}
- File TM Jr, Schlemmer B, Garau J, Cupo M, Young C, 049 Clinical Study Group. Efficacy and safety of gemifloxacin in the treatment of community‐acquired pneumonia: a randomized, double‐blind comparison with trovafloxacin. Journal of Antimicrobial Chemotherapy 2001;48(1):67‐74. [DOI] [PubMed] [Google Scholar]
File 2004 {published data only}
- File TM Jr, Lode H, Kurz H, Kozak R, Xie H, Berkowitz E, et al. Double‐blind, randomized study of the efficacy and safety of oral pharmacokinetically enhanced‐amoxicillin clavulanate (2,000/125 milligrams) versus those of amoxicillin‐clavulanate (875/125 milligrams), both given twice daily for 7 days, in treatment of bacterial community‐acquired pneumonia in adults. Antimicrobial Agents and Chemotherapy 2004;48(9):3323‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
File 2012a {published data only}
- File Jr T, Eckburg P, Low D, Talbot G, llorens L, Friedland HD. Assessment of outcomes at an entry time point may identify a differential effect of macrolide therapy on community‐acquired pneumonia due to atypical pathogens. Clinical Microbiology and Infection 2012;18(55):133‐4. [Google Scholar]
File 2012b {published data only}
- File Jr TM, Wilcox MH, Stein GE. Summary of ceftaroline fosamil clinical trial studies and clinical safety. Clinical Infectious Diseases 2012;55(Suppl 3):173‐80. [DOI] [PubMed] [Google Scholar]
Fogarty 1999 {published data only}
- Fogarty C, Grossman C, Williams J, Haverstock D, Church D for the Community‐Acquired Pneumonia Study Group. Efficacy and safety of moxifloxacin vs clarithromycin for community‐acquired pneumonia. Infections in Medicine 1999;16:748‐63. [Google Scholar]
Fogarty 2002 {published data only}
- Fogarty CM, Cyganowski M, Palo WA, Hom RC, Craig WA. A comparison of cefditoren pivoxil and amoxicillin/clavulanate in the treatment of community‐acquired pneumonia: a multicenter, prospective, randomized, investigator‐blinded, parallel‐group study. Clinical Therapeutics 2002;24(11):1854‐70. [DOI] [PubMed] [Google Scholar]
Fogarty 2004 {published data only}
- Fogarty C, Siami G, Kohler R, File Jr TM, Tennenberg AM, Olson WH, et al. Multicenter, open‐label, randomized study to compare the safety and efficacy of levofloxacin versus ceftriaxone sodium and erythromycin followed by clarithromycin and amoxicillin‐clavulanate in the treatment of serious community‐acquired pneumonia in adults. Clinical Infectious Diseases 2004;38(Suppl 1):16‐23. [Google Scholar]
Fong 1995 {published data only}
- Fong IW, Laforge J, Dubois J, Small D, Grossman R, Zakhari R, et al. Clarithromycin versus cefaclor in lower respiratory tract infections. Clinical and Investigative Medicine 1995;18:131‐8. [PubMed] [Google Scholar]
Fujita 2012 {published data only}
- Fujita J, Niki Y, Kadota J, Yanagihara K, Kaku M, Watanabe A, et al. Clinical and bacteriological efficacies of sitafloxacin against community‐acquired pneumonia caused by Streptococcus pneumoniae: nested cohort within a multicenter clinical trial. Journal of Infection and Chemotherapy (Official Journal of the Japan Society of Chemotherapy) 2013;19(3):472‐9. [DOI] [PubMed] [Google Scholar]
Ghebremedhin 2012 {published data only}
- Ghebremedhin B. Bacterial infections in the elderly patient: focus on sitafloxacin. Clinical Medicine Insights: Therapeutics 2012;4:185‐200. [Google Scholar]
Gotfried 2002 {published data only}
- Gotfried MH, Dattani D, Riffer E, Devcich KJ, Busman TA, Notario GF, et al. A controlled, double‐blind, multicenter study comparing clarithromycin extended‐release tablets and levofloxacin tablets in the treatment of community‐acquired pneumonia. Clinical Therapeutics 2002;24(5):736‐51. [DOI] [PubMed] [Google Scholar]
Gris 1996 {published data only}
- Gris P. Once‐daily, 3‐day azithromycin versus a three‐times‐daily, 10‐day course of co‐amoxiclav in the treatment of adults with lower respiratory tract infections: results of a randomized, double‐blind comparative study. Journal of Antimicrobial Chemotherapy 1996;37(Suppl C):93‐101. [DOI] [PubMed] [Google Scholar]
Hagberg 2002 {published data only}
- Hagberg L, Torres A, Rensburg D, Leroy B, Rangaraju M, Ruuth E. Efficacy and tolerability of once‐daily telithromycin compared with high‐dose amoxicillin for treatment of community‐acquired pneumonia. Infection 2002;30(6):378‐86. [DOI] [PubMed] [Google Scholar]
Higuera 1996 {published data only}
- Higuera F, Hidalgo H, Feris J, Giguere G, Collins JJ. Comparison of oral cefuroxime axetil and oral amoxycillin/clavulanate in the treatment of community‐acquired pneumonia. Journal of Antimicrobial Chemotherapy 1996;37:555‐64. [DOI] [PubMed] [Google Scholar]
Hoeffken 2001 {published data only}
- Hoeffken G, Meyer HP, Winter J, Verhoef L, CAP1 Study Group. The efficacy and safety of two oral moxifloxacin regimens compared to oral clarithromycin in the treatment of community‐acquired pneumonia. Respiratory Medicine 2001;95(7):553‐64. [DOI] [PubMed] [Google Scholar]
Hoepelman 1993 {published data only}
- Hoepelman AIM, Sips AP, Helmond JLM, Barneveld PWC, Neve AJ, et al. A single‐blind comparison of three‐day azithromycin and ten‐day co‐amoxiclav treatment of acute lower respiratory tract infections. Journal of Antimicrobial Chemotherapy 1993;31(Suppl E):147‐52. [DOI] [PubMed] [Google Scholar]
Hoepelman 1998 {published data only}
- Hoepelman IM, Möllers MJ, Schie MH, Greefhorst APM, Schlösser NJJ, Sinninghe Damsté EJ, et al. A short (3‐day) course of azithromycin tablets versus a 10‐day course of amoxycillin‐clavulanic acid (co‐amoxiclav) in the treatment of adults with lower respiratory tract infections and effects on long‐term outcome. International Journal of Antimicrobial Agents 1998;9:141‐6. [DOI] [PubMed] [Google Scholar]
Kammer 1991 {published data only}
- Kammer RB, Ress R. Randomized comparative study of ceftibuten versus cefaclor in the treatment of acute lower respiratory tract infections. Diagnostic Microbiology and Infectious Disease 1991;14:101‐5. [DOI] [PubMed] [Google Scholar]
Kiani 1990 {published data only}
- Kiani R, Coulson L, Johnson D, Hammershaimb L. Comparison of once‐daily and twice‐daily cefixime regimens with amoxicillin in the treatment of acute lower respiratory tract infections. Current Therapeutic Research, Clinical and Experimental 1990;48(5):841‐52. [Google Scholar]
Kinasewitz 1991 {published data only}
- Kinasewitz G, Wood RG. Azithromycin versus cefaclor in the treatment of acute bacterial pneumonia. European Journal of Clinical Microbiology and Infectious Diseases 1991;10(10):872‐7. [DOI] [PubMed] [Google Scholar]
Kohno 2013 {published data only}
- Kohno S, Yanagihara K, Yamamoto Y, Tokimatsu I, Hiramatsu K, Higa F, et al. Early switch therapy from intravenous sulbactam/ampicillin to oral garenoxacin in patients with community‐acquired pneumonia: a multicenter, randomized study in Japan. Journal of Infection and Chemotherapy (Official Journal of the Japan Society of Chemotherapy) 2013;19(6):1035‐41. [DOI] [PubMed] [Google Scholar]
Lacny 1972 {published data only}
- Lacny J. A comparison of oral therapy with clindamycin and penicillin G in common gram‐positive infections. Current Therapeutic Research 1972;14(1):26‐30. [PubMed] [Google Scholar]
Lagler 2012 {published data only}
- Lagler H, Gattringer R, Derler V, Wlazny D, Graninger W, Burgmann H. Intravenous azithromycin ‐ single dose 1.5 g vs. 500 mg once daily for 3 days in patients with community‐acquired pneumonia: a prospective and randomized study. Clinical Microbiology and Infection 2012;18(65):137‐8. [Google Scholar]
Laurent 1996 {published data only}
- Laurent K. Efficacy, safety and tolerability of azithromycin versus roxithromycin in the treatment of acute lower respiratory tract infections. Journal of Antimicrobial Chemotherapy 1996;37(Suppl C):115‐24. [DOI] [PubMed] [Google Scholar]
Lee 2012 {published data only}
- Lee JH, Kim SW, Kim JH, Ryu YJ, Chang JH. High‐dose levofloxacin in community‐acquired pneumonia: a randomized, open‐label study. Clinical Drug Investigation 2012;32(9):569‐76. [DOI] [PubMed] [Google Scholar]
Leophonte 2004 {published data only}
- Léophonte P, File T, Feldman C. Gemifloxacin once daily for 7 days compared to amoxicillin/clavulanic acid thrice daily for 10 days for the treatment of community‐acquired pneumonia of suspected pneumococcal origin. Respiratory Medicine 2004;98(8):708‐20. [DOI] [PubMed] [Google Scholar]
Liipo 1994 {published data only}
- Liippo K, Tala E, Puolijoki H, Brückner OJ, Rodrig J, Smits JPH. A comparative study of dirithromycin and erythromycin in bacterial pneumonia. Journal of Infectious Diseases 1994;28:131‐9. [DOI] [PubMed] [Google Scholar]
Little 2012 {published data only}
- Little P, Stuart B, Moore M, Coenen S, Butler CC, Godycki‐Cwirko M, et al. Amoxicillin for acute lower‐respiratory‐tract infection in primary care when pneumonia is not suspected: a 12‐country, randomised, placebo‐controlled trial. Lancet Infectious Diseases 2013;13(2):123‐9. [DOI] [PubMed] [Google Scholar]
Lode 1995 {published data only}
- Lode H, Garau J, Grassi C, Hosie J, Huchon G, Legakis N, et al. Treatment of community‐acquired pneumonia: a randomized comparison of sparfloxacin, amoxycillin‐clavulanic acid and erythromycin. European Respiratory Journal 1995;8:1999‐2007. [DOI] [PubMed] [Google Scholar]
Lode 1998 {published data only}
- Lode H, Aubier M, Portier H, Ortqvist A and the Sparfloxacin Study Group. Sparfloxacin as alternative treatment to standard therapy for community‐acquired bacteremic pneumococcal pneumonia. Clinical Microbiology and Infection 1998;4(3):135‐43. [DOI] [PubMed] [Google Scholar]
Lode 2004a {published data only}
- Lode H, Magyar P, Muir JF, Loos U, Kleutgens K, International Gatifloxacin Study Group. Once‐daily oral gatifloxacin vs three‐times‐daily co‐amoxiclav in the treatment of patients with community‐acquired pneumonia. Clinical Microbiology and Infection 2004;10(6):512‐20. [DOI] [PubMed] [Google Scholar]
Lode 2004b {published data only}
- Lode H, Aronkyto T, Chuchalin AG, Jaaskevi M, Kahnovskii I, Kleutgens K, et al. A randomised, double‐blind, double‐dummy comparative study of gatifloxacin with clarithromycin in the treatment of community‐acquired pneumonia. Clinical Microbiology and Infection 2004;10(5):403‐8. [DOI] [PubMed] [Google Scholar]
Long 2011 {published data only}
- Long W, Deng X, Zhang Y, Lu G, Xie J, Tang J. Procalcitonin guidance for reduction of antibiotic use in low‐risk outpatients with community‐acquired pneumonia. Respirology 2011;16(5):819‐24. [DOI] [PubMed] [Google Scholar]
Lopez‐Vejar 2013 {published data only}
- López‐Véjar CE, Castellanos de la Cruz L, Meraz‐Ortega R, Román‐ Flores A, Geuguer‐Chávez L, Pedro‐González A, et al. [Eficacia del levofloxacino en el tratamiento de neumonía adquirida en la comunidad]. Medicina Interna de México 2013;29(587):594. [Google Scholar]
MacFarlane 1996 {published data only}
- MacFarlane JT, Prewitt J, Gard P, Guion A. Comparison of amoxycillin and clarithromycin as initial treatment of community‐acquired lower respiratory tract infections. British Journal of General Practice 1996;46(407):357‐60. [PMC free article] [PubMed] [Google Scholar]
Matzneller 2013 {published data only}
- Matzneller P, Krasniqi S, Kinzig M, Sörgel F, Hüttner S, Lackner E, et al. Blood, tissue, and intracellular concentrations of azithromycin during and after end of therapy. Antimicrobial Agents and Chemotherapy 2013;57(4):1736‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Montassier 2013 {published data only}
- Montassier E, Goffinet N, Potel G, Batard E. How to reduce antibiotic consumption for community‐acquired pneumonia. Medicines et Maladies Infectieuses 2013;43(2):52‐9. [DOI] [PubMed] [Google Scholar]
Moola 1999 {published data only}
- Moola S, Hagberg L, Churchyard GA, Dylewski JS, Sedani S, Staley H. A multicenter study of grepafloxacin and clarithromycin in the treatment of patients with community‐acquired pneumonia. Chest 1999;116(4):974‐83. [DOI] [PubMed] [Google Scholar]
Müller 1992 {published data only}
- Müller O, Wettich K. Comparison of loracarbef (LY 163892) versus amoxicillin in the treatment of bronchopneumonia and lobar pneumonia. Infection 1992;20(3):176‐82. [DOI] [PubMed] [Google Scholar]
Naderer 2013 {published data only}
- Naderer OJ, Dumont E, Zhu J, Kurtinecz M, Jones LS. Single‐dose safety, tolerability, and pharmacokinetics of the antibiotic GSK1322322, a novel peptide deformylase inhibitor. Antimicrobial Agents and Chemotherapy 2013;57(5):2005‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
NAPSG 1997 {published data only}
- The Nordic Atypical Pneumonia Study Group. Atypical pneumonia in the Nordic countries: aetiology and clinical results of a trial comparing fleroxacin and doxycycline. Journal of Antimicrobial Chemotherapy 1997;39:499‐508. [DOI] [PubMed] [Google Scholar]
Navarta 2010 {published data only}
- Navarta AC, Bocklet ML, Anzorena A, Cuello H, Carena JA. Not available [Eficacia clínica de los macrólidos como parte del tratamiento empírico en neumonía de la comunidad que se interna]. Revista Americana de Medicina Respiratoria 2010;3:97‐104. [Google Scholar]
Neu 1993 {published data only}
- Neu HC, Chick TW. Efficacy and safety of clarithromycin compared to cefixime as outpatient treatment of lower respiratory tract infections. Chest 1993;104:1393‐9. [DOI] [PubMed] [Google Scholar]
Nussenblatt 2013 {published data only}
- Nussenblatt V, Avdic E, Cosgrove S. What is the role of antimicrobial stewardship in improving outcomes of patients with CAP. Infectious Disease Clinics of North America 2013;27(1):211‐28. [DOI] [PubMed] [Google Scholar]
O'Doherty 1997 {published data only}
- O'Doherty B, Dutchman DA, Pettit R, Maroli A. Randomized, double‐blind, comparative study of grepafloxacin and amoxycillin in the treatment of patients with community‐acquired pneumonia. Journal of Antimicrobial Chemotherapy 1997;40(Suppl A):73‐81. [DOI] [PubMed] [Google Scholar]
O'Doherty 1998 {published data only}
- O'Doherty B, Muller O and the Azithromycin Study Group. Randomized, multicentre study of the efficacy and tolerance of azithromycin versus clarithromycin in the treatment of adults with mild to moderate community‐acquired pneumonia. European Journal of Clinical Microbiology and Infectious Diseases 1998;17:828‐33. [DOI] [PubMed] [Google Scholar]
Örtqvist 1996 {published data only}
- Örtqvist A, Valtonen M, Cars O, Wahl M, Saikku P, Jean C, et al. Oral empiric treatment of community‐acquired pneumonia: a multicenter, double‐blind, randomized study comparing sparfloxacin with roxithromycin. Chest 1996;110:1499‐506. [DOI] [PubMed] [Google Scholar]
Pavie 2005 {published data only}
- Pavie J, Prida JM, Diaz A, Saldias F. Assessment of the management of community‐acquired pneumonia in adult outpatients [Manejo ambulatorio de la neumonía comunitaria del adulto en las unidades de emergencia. Servicio de Salud Vina del Mar–Quillota de la V Región]. Revista Médica de Chile 2005;133:1322‐30. [DOI] [PubMed] [Google Scholar]
Petitpretz 2001 {published data only}
- Petitpretz P, Arvis P, Marel M, Moita J, Urueta J, CAP5 Moxifloxacin Study Group. Oral moxifloxacin vs high‐dosage amoxicillin in the treatment of mild‐to‐moderate, community‐acquired, suspected pneumococcal pneumonia in adults. Chest 2001;119(1):185‐95. [DOI] [PubMed] [Google Scholar]
Peugeot 1991 {published data only}
- Peugeot RL, Lipsky BA, Hooton TM, Pecoraro RE. Treatment of lower respiratory infections in outpatients with ofloxacin compared with erythromycin. Drugs in Experimental and Clinical Research 1991;17(5):253‐7. [PubMed] [Google Scholar]
Polverino 2013 {published data only}
- Polverino E, Cilloniz C, Dambrava P, Gabarrus A, Ferrer M, Agusti C, et al. Systemic corticosteroids for community‐acquired pneumonia: reasons for use and lack of benefit on outcome. Respirology 2013;18(2):263‐71. [DOI] [PubMed] [Google Scholar]
Pullman 2003 {published data only}
- Pullman J, Champlin J, Vrooman PS Jr. Efficacy and tolerability of once‐daily oral therapy with telithromycin compared with trovafloxacin for the treatment of community‐acquired pneumonia in adults. Internation Journal of Clinical Practice 2003;57(5):377‐84. [PubMed] [Google Scholar]
Rahav 2004 {published data only}
- Rahav G, Fidel J, Gibor Y, Shapiro M. Azithromycin versus comparative therapy for the treatment of community acquired pneumonia. International Journal of Antimicrobial Agents 2004;24(2):181‐4. [DOI] [PubMed] [Google Scholar]
Ramirez 1999 {published data only}
- Ramirez J, Unowsky J, Talbot GH, Zhang H, Townsend L. Sparfloxacin vs clarithromycin in the treatment of community acquired pneumonia. Clinical Therapeutics 1999;21(1):103‐17. [DOI] [PubMed] [Google Scholar]
Rank 2011 {published data only}
- Rank DR, Friedland HD, Eckburg PB, Smith A, Thye D. Integrated safety summary of ceftaroline fosamil (cpt): safety in patients overall and for patients with community‐acquired pneumonia (cap). American Journal of Respiratory and Critical Care Medicine 2011;183:A3357. [Google Scholar]
Rayman 1996 {published data only}
- Rayman J, Krchnavý M, Balciar P, Duchon J, Fortuník J, Faith L, et al. Ofloxacin once daily versus twice daily in community‐acquired pneumonia and acute exacerbation of chronic bronchitis. Chemotherapy 1996;42:227‐30. [DOI] [PubMed] [Google Scholar]
Saito 2008 {published data only}
- Saito A, Watanabe A, Aoki N, Niki Y, Kohno S, Kaku M, et al. Phase III double‐blind comparative study of sitafloxacin versus tosufloxacin in patients with community‐acquired pneumonia. Japanese Journal of Chemotherapy 2008;56(Suppl 1):49‐62. [Google Scholar]
Salvarezza 1998 {published data only}
- Salvarezza CR, Mingrone H, Fachinelli H, Kijanczuk S. Comparison of roxithromycin with cefixime in the treatment of adults with community‐acquired pneumonia. Journal of Antimicrobial Chemotherapy 1998;41(Suppl B):75‐80. [DOI] [PubMed] [Google Scholar]
Schleupner 1988 {published data only}
- Schleupner CJ, Anthony WC, Tan J, File TM, Lifland P, Craig W, et al. Blinded comparison of cefuroxime to cefaclor for lower respiratory tract infections. Archives of Internal Medicine 1988;148:343‐8. [PubMed] [Google Scholar]
Seki 2009 {published data only}
- Seki M, Higashiyama Y, Imamura Y, Nakamura S, Kurihara S, Izumikawa K, et al. A clinical comparative study of piperacillin and sulbactam/ampicillin in patients with community‐acquired bacterial pneumonia. Internal Medicine 2009;48(1):49‐55. [DOI] [PubMed] [Google Scholar]
Shorr 2013 {published data only}
- Shorr AF, Kollef M, Eckburg PB, Llorens L, Friedland HD. Assessment of ceftaroline fosamil in the treatment of community‐acquired bacterial pneumonia due to Streptococcus pneumoniae: insights from two randomized trials. Diagnostic Microbiology and Infectious Disease 2013;75(3):298‐303. [DOI] [PubMed] [Google Scholar]
Siquier 2006 {published data only}
- Siquier B, Sánchez‐Alvarez J, García‐Mendez E, Sabriá M, Santos J, Pallarés R, et al. Efficacy and safety of twice‐daily pharmacokinetically enhanced amoxicillin/clavulanate (2000/125 mg) in the treatment of adults with community‐acquired pneumonia in a country with a high prevalence of penicillin‐resistant Streptococcus pneumoniae. Journal of Antimicrobial Chemotherapy 2006;57(3):536‐45. [DOI] [PubMed] [Google Scholar]
Skalsky 2012 {published data only}
- Skalsky K, Yahav D, Lador A, Eliakim‐Raz N, Leibovici L, Paul M. Macrolides vs. quinolones for community‐acquired pneumonia: meta‐analysis of randomized controlled trials. Clinical Microbiology and Infection 2013;19(4):370‐8. [DOI] [PubMed] [Google Scholar]
Smith 2013 {published data only}
- Smith KJ, Wateska A, Nowalk MP, Raymund M, Lee BY, Zimmerman RK, et al. Cost‐effectiveness of procalcitonin‐guided antibiotic use in community acquired pneumonia. Journal of General Internal Medicine 2013;28(9):1157‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Snyman 2009 {published data only}
- Snyman JR, Schoeman HS, Grobusch MP, Henning M, Rabie W, Hira M, et al. Generic versus non‐generic formulation of extended‐release clarithromycin in patients with community‐acquired respiratory tract infections: a prospective, randomized, comparative, investigator‐blind, multicentre study. Clinical Drug Investigation 2009;29(4):265‐74. [DOI] [PubMed] [Google Scholar]
Sokol 2002 {published data only}
- Sokol WN Jr, Sullivan JG, Acampora MD, Busman TA, Notario GF. A prospective, double‐blind, multicenter study comparing clarithromycin extended‐release with trovafloxacin in patients with community‐acquired pneumonia. Clinical Therapeutics 2002;24(4):605‐15. [DOI] [PubMed] [Google Scholar]
Sopena 2004 {published data only}
- Sopena N, Martínez‐Vázquez C, Rodríguez‐Suárez JR, Segura F, Valencia A, Sabrià M. Comparative study of the efficacy and tolerance of azithromycin versus clarithromycin in the treatment of community‐acquired pneumonia in adults. Journal of Chemotherapy 2004;16(1):102‐3. [DOI] [PubMed] [Google Scholar]
Stille 2000 {published data only}
- Stille W, Sab R, Klinge R, Loos U, Althoff P‐H, Kullmann K‐H. Ceftriaxon i. v./ efetametpivoxil versus efuroxim i. v./ efuroximatxetil. Chemotherapie Journal 2000;9(2):87‐92. [Google Scholar]
Sun 2012 {published data only}
- Sun H, Maitetta R, Machineni J, Praestgaard J, Kuemell A, Stein D, et al. A single‐dose study to evaluate the pharmacokinetics, safety and tolerability of multiple formulations of PTK0796 in healthy subjects. Clinical Microbiology and Infection 2012;18(94):374‐5. [Google Scholar]
Tellier 2004 {published data only}
- Tellier G, Niederman MS, Nusrat R, Patel M, Lavin B. Clinical and bacteriological efficacy and safety of 5 and 7 day regimens of telithromycin once daily compared with a 10 day regimen of clarithromycin twice daily in patients with mild to moderate community‐acquired pneumonia. Journal of Antimicrobial Chemotherapy 2004;54(2):515‐23. [DOI] [PubMed] [Google Scholar]
Tilyard 1992 {published data only}
- Tilyard MW, Dovey SM. A randomized double‐blind controlled trial of roxithromycin and cefaclor in the treatment of acute lower respiratory tract infections in general practice. Diagnostic Microbiology and Infectious Disease 1992;15:97‐101. [DOI] [PubMed] [Google Scholar]
Torres 2003 {published data only}
- Torres A, Muir JF, Corris P, Kubin R, Duprat‐Lomon I, Sagnier PP, et al. Effectiveness of oral moxifloxacin in standard first‐line therapy in community‐acquired pneumonia. European Respiratory Journal 2003;21(1):135‐43. [DOI] [PubMed] [Google Scholar]
Trémolières 1998 {published data only}
- Trémolières F, Kock F, Pluck N, Daniel R. Trovafloxacin versus high‐dose amoxicillin (1 g three times daily) in the treatment of community‐acquired bacterial pneumonia. European Journal of Clinical Microbiology and Infectious Diseases 1998;17(6):447‐53. [DOI] [PubMed] [Google Scholar]
Trémolières 2005 {published data only}
- Trémolières F, Mayaud C, Mouton Y, Weber P, Dellatolas F, Caulin E. Efficacy and safety of pristinamycin vs amoxicillin in community acquired pneumonia in adults [Essai comparatif de l’efficacité et de la tolérance de la pristinamycine vs amoxicilline dans le traitement des pneumonies aigues communautaires de l’adulte]. Pathologie‐Biologie 2005;53(8‐9):503‐10. [DOI] [PubMed] [Google Scholar]
van Zyl 2002 {published data only}
- Zyl L, Roux JG, LaFata JA, Volk RS, Palo WA, Flamm R, et al. Cefditoren pivoxil versus cefpodoxime proxetil for community‐acquired pneumonia: results of a multicenter, prospective, randomized, double‐blind study. Clinical Therapeutics 2002;24(11):1840‐53. [DOI] [PubMed] [Google Scholar]
Viasusa 2013 {published data only}
- Viasusa D, Garcia‐Vidala C, Carratala J. Advances in antibiotic therapy for community acquired pneumonia. Current Opinion in Pulmonary Medicine 2013;19(3):209‐15. [DOI] [PubMed] [Google Scholar]
Worrall 2010 {published data only}
- Worrall G, Kettle A, Graham W, Hutchinson J. Postdated versus usual delayed antibiotic prescriptions in primary care: reduction in antibiotic use for acute respiratory infections. Canadian Family Physician 2010;56(10):1032‐6. [PMC free article] [PubMed] [Google Scholar]
Wunderink 2011 {published data only}
- Wunderink RG, Laterre PF, Francois B, Perrotin D, Artigas A, Vidal LO, et al. Recombinant tissue factor pathway inhibitor in severe community‐acquired pneumonia: a randomized trial. American Journal of Respiratory and Critical Care Medicine 2011;183(11):1561‐8. [DOI] [PubMed] [Google Scholar]
Yamamoto 2013 {published data only}
- Yamamoto Y, Izumikawa K, Morinaga Y, Nakamura S, Kurihara S, Imamura Y, et al. Prospective randomized comparison study of piperacillin/tazobactam and meropenem for healthcare‐associated pneumonia in Japan. Journal of Infection and Chemotherapy (Official Journal of the Japan Society of Chemotherapy) 2013;19(2):291‐8. [DOI] [PubMed] [Google Scholar]
Yuan 2012 {published data only}
- Yuan X, Liang B‐B, Wang R, Liu Y‐N, Sun C‐G, Cai Y, et al. Treatment of community‐acquired pneumonia with moxifloxacin: a meta‐analysis of randomized controlled trials. Journal of Chemotherapy 2012;24(5):257‐67. [DOI] [PubMed] [Google Scholar]
Zhang 2012 {published data only}
- Zhang L, Huang J, Xu T, Lin Y. Procalcitonin‐guided algorithms of antibiotic therapy in community‐acquired lower respiratory tract infections: a systematic review and meta‐analysis of randomized controlled trials. Chinese Journal of Tuberculosis and Respiratory Diseases 2010;35(4):275‐82. [PubMed] [Google Scholar]
Zuck 1990 {published data only}
- Zuck P, Rio Y, Ichou F. Efficacy and tolerance of cefpodoxime proxetil compared with ceftriaxone in vulnerable patients with bronchopneumonia. Journal of Antimicrobial Chemotherapy 1990;26(Suppl E):71‐7. [DOI] [PubMed] [Google Scholar]
Additional references
ALAT 2001
- Grupo de trabajo de la Asociación Latinoamericana del Tórax (ALAT). Latin American Thoracic Association (ALAT) recommendations on community acquired pneumonia [Recomendaciones ALAT sobre la neumonía adquirida en la comunidad]. Archivos de Bronconeumología 2001;37:340‐8. [PubMed] [Google Scholar]
ALAT 2004
- Grupo de trabajo de la Asociación Latinoamericana del Tórax (ALAT). Update to the Latin American Thoracic Association (ALAT) recommendations on community acquired pneumonia. Archivos de Bronconeumología 2004;40(8):364‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Armitage 1994
- Armitage P, Berry G. Statistical Methods in Medical Research. 3rd Edition. Oxford: Blackwell Scientific Publications, 1994. [Google Scholar]
ATS 2001
- American Thoracic Society. Guidelines for the management of adults with community‐acquired pneumonia. American Journal of Critical Care 2001;163:1730‐54. [DOI] [PubMed] [Google Scholar]
BTS 2004
- Macfarlane JT, Boldy D. 2004 update of BTS pneumonia guidelines: what's new?. Thorax 2004;59(5):364‐6. [Macfarlane 2004; www.brit‐thoracic.org.uk/guidelines] [DOI] [PMC free article] [PubMed] [Google Scholar]
BTS 2009
- Lim WS, Baudoui SV, George RC, Hill AT, Jamieson C, Jeune I, et al. BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax 2009;64(Suppl 3):iii1‐iii55. [DOI] [PubMed] [Google Scholar]
Carratalà 2005
- Carratalà J, Fernández‐Sabé N, Ortega L, Castellsagué X, Rosón B, Dorca J, et al. Outpatient care compared with hospitalization for community‐acquired pneumonia: a randomized trial in low‐risk patients. Annals of Internal Medicine 2005;142(3):165‐72. [DOI] [PubMed] [Google Scholar]
CCAPWG 2000
- Canadian Community‐Acquired Pneumonia Working Group. Canadian guidelines for the initial management of community‐acquired pneumonia: an evidence‐based update by the Canadian Infectious Diseases Society and the Canadian Thoracic Society. Clinical Infectious Diseases 2000;31:383‐421. [DOI] [PubMed] [Google Scholar]
Foy 1979
- Foy HM, Cooney MK, Allan I, Kenny GE. Rates of pneumonia during influenza epidemics in Seattle, 1964 to 1975. JAMA 1979;241:253‐8. [PubMed] [Google Scholar]
Hedlund 2005
- Hedlund J, Strålin K, Ortqvist A, Holmberg H, Community‐Acquired Pneumonia Working Group of the Swedish Society of Infectious Diseases. Swedish guidelines for the management of community‐acquired pneumonia in immunocompetent adults. Scandinavian Journal of Infectious Diseases 2005;37(11‐2):791‐805. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Höffken 2010
- Höffken G, Lorenz J, Kern W, Welte T, Bauer T, Dalhoff K, et al. Guidelines of the Paul‐Ehrlich‐Society of Chemotherapy, the German Respiratory Diseases Society, the German Infectious Diseases Society and of the Competence Network CAPNETZ for the Management of Lower Respiratory Tract Infections and Community Acquired Pneumonia: Summary of the Update 2009. Pneumologie 2010;64:149‐54. [German guidelines 2009] [DOI] [PubMed] [Google Scholar]
IDSA 2000
- Infectious Diseases Society of America. Practice guidelines for the management of community‐acquired pneumonia in adults. Clinical Infectious Diseases 2000;31:347‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
IDSA 2003
- Infectious Diseases Society of America. Update of practice guidelines for the management of community‐acquired pneumonia in immunocompetent adults. Clinical Infectious Diseases 2003;37:1405‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
IDSA/ATS 2007
- Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community‐acquired pneumonia in adults. Clinical Infectious Diseases 2007;44(Suppl 2):27‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jokinen 1993
- Jokinen C, Heiskanen L, Juvonen H, Kallinen S, Karkola K, Korppi M, et al. Incidence of community‐acquired pneumonia in the population of four municipalities in eastern Finland. American Journal of Epidemiology 1993;137:977‐88. [DOI] [PubMed] [Google Scholar]
JRS 2006
- Committee for The Japanese Respiratory Society guidelines for the management of respiratory infections. Guidelines for the management of community acquired pneumonia in adults, revised edition. Respirology 2006;11(Suppl 3):79‐133. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. The Cochrane Collaboration, 2011. [Google Scholar]
Loeb 2002
- Loeb M. Community‐acquired pneumonia. Clinical Evidence. Vol. 8, London: BMJ Publishing Group, 2002. [Google Scholar]
Macfarlane 1984
- Macfarlane JT, Miller AC, Roderick Smith WH, Morris AH, Rose DH. Comparative radiographic features of community‐acquired legionnaires' disease, pneumococcal pneumonia, mycoplasma pneumonia and psittacosis. Thorax 1984;39:28‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maimon 2008
- Maimon N, Nopmaneejumruslers C, Marras TK. Antibacterial class is not obviously important in outpatient pneumonia: a meta‐analysis. European Respiratory Journal 2008;31(5):1068‐76. [DOI] [PubMed] [Google Scholar]
Mandell 2007
- Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community‐acquired pneumonia in adults. Clinical Infectious Diseases 2007;44(Suppl 2):27‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Memish 2007
- Memish ZA, Arabi YM, Ahmed QA, Shibl AM, Niederman MS, GCC CAP Working Group. Executive summary of the Gulf Cooperation Council practice guidelines for the management of community‐acquired pneumonia. Journal of Chemotherapy 2007;19(Suppl 1):7‐11. [Memish 2007] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Robenshtok 2008
- Robenshtok E, Shefet D, Gafter‐Gvili A, Paul M, Vidal L, Leibovici L. Empiric antibiotic coverage of atypical pathogens for community acquired pneumonia in hospitalized adults. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD004418.pub3] [DOI] [PubMed] [Google Scholar]
SATS 2007
- Working Group of the South African Thoracic Society. Management of community‐acquired pneumonia in adults. South African Medical Journal 2007;97(12 Pt 2):1296‐306. [PubMed] [Google Scholar]
Spindler 2012
- Spindler C, Strålin K, Eriksson L, Hjerdt‐Goscinski G, Holmberg H, Lidman C, et al. Swedish guidelines on the management of community‐acquired pneumonia in immunocompetent adults‐‐Swedish Society of Infectious Diseases 2012. Scandinavian Journal of Infectious Diseases 2012;44(12):885‐902. [DOI] [PubMed] [Google Scholar]
Strålin 2007
- Strålin K, Goscinski G, Hedlund J, Lidman C, Spindler C, Ortqvist A, et al. Management of adult patients with community‐acquired pneumonia. Evidence‐based guidelines from the Swedish Infectious Diseases Association [Handläggning av samhälls‐förvärvad pneumoni hos vuxna. Evidensbaserade riktlinjer från Svenska infektionsläkarföreningen. (Abridged version of the 2007 update; http://www.infektion.net/klinik/lunga/pneumoni/Vardprogram_Pneumoni_slutvers_2007.pdf (in Swedish) (accessed 23 March 2009)]. Lakartidningen 2008;105(38):2582‐7. [PubMed] [Google Scholar]
Torres 1991
- Torres A, Serra‐Batlles J, Ferrer A, Jiménez P, Celis R, Cobo E, et al. Severe community‐acquired pneumonia. Epidemiology and prognostic factors. American Review of Respiratory Disease 1991;144:312‐8. [DOI] [PubMed] [Google Scholar]
Welte 2012
- Welte T, Torres A, Nathwani D. Clinical and economic burden of community‐acquired pneumonia among adults in Europe. Thorax 2012;67:71‐9. [DOI] [PubMed] [Google Scholar]
Wiersinga 2011
- Wiersinga WJ, Bonten MJ, Boersma WG, Jonkers RE, Aleva RM, Kullberg BJ, et al. Dutch guidelines on the management of community‐acquired pneumonia in adults. SWAB/NVALT Guidelines Community‐acquired Pneumonia 2011.
Woodhead 1987
- Woodhead MA, Macfarlane JT, McCracken JS, Rose DH, Finch RG. Prospective study of the aetiology and outcome of pneumonia in the community. Lancet 1987;i:671‐4. [DOI] [PubMed] [Google Scholar]
Woodhead 2011
- Woodhead M, Blasi F, Ewig S, Garau J, Huchon G, Ieven M, et al. Guidelines for the management of adult lower respiratory tract infections‐‐full version. Clinical Microbiology and Infection 2011;17(Suppl 6):E1‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Bjerre 2004
- Bjerre LM, Verheij TJ, Kochen MM. Antibiotics for community acquired pneumonia in adult outpatients. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD002109.pub2] [DOI] [PubMed] [Google Scholar]
Bjerre 2009
- Bjerre LM, Verheij TJ, Kochen MM. Antibiotics for community acquired pneumonia in adult outpatients. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD002109.pub3] [DOI] [PubMed] [Google Scholar]


