Abstract
Background
Heroin smokers have high rates of COPD, respiratory morbidity, hospital admission, and mortality. We assessed the natural history of symptoms and lung function in this population over time.
Methods
A cohort of heroin smokers with COPD was followed for 18 to 24 months. At baseline and follow-up, respiratory symptoms were measured by the Medical Research Council Dyspnea Scale (MRC) and the COPD Assessment Tool (CAT), and postbronchodilator spirometry was performed. Frequency of health-care-seeking episodes was extracted from routine health records. Parametric, nonparametric, and linear regression models were used to analyze the change in symptoms and lung function over time.
Results
Of 372 participants originally recruited, 161 were assessed at follow-up (mean age, 51.0 ± 5.3 years; 74 women [46%]) and 106 participants completed postbronchodilator spirometry. All participants were current or previous heroin smokers, and 122 (75.8%) had smoked crack. Symptoms increased over time (MRC score increased by 0.48 points per year, P < .001; CAT score increased by 1.60 points per year, P < .001). FEV1 declined annually by 90 ± 190 mL (P < .001). This deterioration was not associated with change in tobacco or heroin smoking status or use of inhaled medications.
Conclusions
Heroin smokers experience a high and increasing burden of chronic respiratory symptoms and a decline in FEV1 that exceeds the normal age-related decline observed among tobacco smokers with COPD and healthy nonsmokers. Targeted COPD diagnostic and treatment services hosted within opiate substitution services could benefit this vulnerable, relatively inaccessible, and underserved group of people.
Key Words: COPD, heroin, opiate, spirometry
Abbreviations: ACO, asthma-COPD overlap; ATS, American Thoracic Society; CAT, COPD Assessment Tool; GOLD, Global Initiative for Chronic Obstructive Lung Disease; MRC, Medical Research Council Dyspnea Scale; OST, opiate substitution therapy
FOR EDITORIAL COMMENT, SEE PAGE 484
Illicit drug use is common, with 8.5% of adults in England and Wales having reported taking an illicit drug in 2016 and 2017.1 Over the last 30 years, smoking rather than injecting heroin has become more common.2, 3, 4, 5, 6 In recent years, smoking heroin rather than injecting has been used as a possible method of harm reduction.7,8
Although the effects of illicit drug use are well documented, there is limited evidence about the chronic effects of inhaled illicit drug use on the respiratory system. Multiple case reports highlight acute asthma attacks in heroin users, and observational studies report a high prevalence of respiratory disease in heroin users admitted to acute hospitals.9,10 Severe early onset emphysema associated with premature mortality has been reported among heroin users.11, 12, 13 However, large-scale diagnostic studies in this hard-to-reach population are lacking. Chronic respiratory symptoms are common in people inhaling heroin; however, access to formal diagnosis including lung function measurement is limited.14, 15, 16
We recently reported postbronchodilator spirometry in 703 heroin smokers attending for opiate substitution therapy (OST) at community drug service clinics in Liverpool, England; 50% of heroin smokers had either COPD or asthma-COPD overlap (ACO) despite a mean age of 47 years.17 This was associated with extensive respiratory symptoms, which given the known high rates of COPD hospitalization and a continuing trend toward inhalation as the mode of drug use, is likely to put increased burden on health systems.4,6,18 In light of this, screening and treatment programs for heroin smokers could be a viable method for identifying and treating disease in this relatively inaccessible patient group.19
We performed a longitudinal cohort study of heroin smokers attending community drug services and who were recruited as an original cohort of 703 heroin smokers described in terms of baseline characteristics in our previous paper.17 The aim was to ascertain their change in health status, respiratory symptoms, and lung function over an 18- to 24-month period.
Methods
Setting
The study was performed in 31 community drug service clinics in Liverpool. Clinics are run by Addaction, a large independent charity commissioned by the local city council public health department. A keyworker who knew the client and who coordinated their OST worked with the study team in each clinic.
Participants
Participants were invited to take part if they had previously completed spirometry in the baseline screening project that took place between December 2015 and June 2016,17 were > 18 years of age, and were still fully enrolled in Addaction’s service. All participants were current or previous smokers of heroin and were currently or recently treated with methadone or buprenorphine. Participants were given the study information prior to being booked for their regular appointment and were offered a study visit at their usual clinic. People missing their usual appointment were offered another at a central venue. Written informed consent was obtained from all participants.
Variables and Data Source
Baseline data collection has been previously described.17 In brief, participants completed a questionnaire detailing demographic data, and self-reported tobacco and illicit drug use. Oxygen saturations were measured, and pre- and postbronchodilator spirometry was completed.
At follow-up, participants completed a questionnaire which evaluated self-reporting medication prescriptions, health-care access, and ongoing tobacco and illicit drug use. The index of multiple deprivation, which is an official geographic measure of relative deprivation in England, was used a proxy of social-economic status.20 Participants also completed the COPD Assessment Tool (CAT)21 and the Medical Research Council Dyspnea Scale (MRC),22 and consented to allow review of 2 years of medical records for respiratory-related diagnosis and prescriptions from primary care pharmacy records (EMIS), and hospital records where applicable.
Oxygen saturations were measured, and pre- and postbronchodilator spirometry was performed on all participants who consented and did not have medical contraindications. Spirometry was performed by trained clinical staff and completed according to American Thoracic Society (ATS) guidelines.23 All traces were double-reviewed for quality and grading by an experienced respiratory physician. As with the baseline survey, participants were asked not to take a short-acting bronchodilator within 8 h of visit or a long-acting bronchodilator within 24 h. If they had taken a short-acting inhaler, only postbronchodilator spirometry was recorded.
Subjects were categorized based on original screening. A diagnosis of asthma was given if airflow obstruction (FEV1/FVC ratio < 0.7) was fully reversible to inhaled salbutamol (ie, either FEV1/FVC normalized or FEV1 increased by ≥ 400 mL), or if spirometry was normal but the participant had a prior physician diagnosis of asthma. Participants with nonreversible airflow obstruction were characterized as COPD unless they had a prior physician diagnosis of asthma, in which case their condition was labeled as ACO. We report the lung function change of participants who had been diagnosed with COPD or ACO at baseline; participants with an asthma diagnosis were excluded.17
All spirometry data were reported using the European Coal and Steel Community24 reference ranges for consistency with prior work. Abnormal spirometry was defined using the Global Initiative for Chronic Obstructive Lung Disease (GOLD).25 Change in lung function was based on postbronchodilator FEV1.
Sample Size
We aimed to follow-up as many of the participants with COPD or ACO from baseline as possible.
Statistical Analysis
Univariate analysis was carried out using descriptive statistics to explore the characteristics of the study populations. Paired t tests and Wilcoxon sign rank tests (with bootstrapping to estimate the CI of the difference) were used to assess change between the two time points. Time was used as a continuous variable to account for variation between follow-up dates and to calculate an annualized change. A linear regression model was used to estimate the effect of potential factors (change in inhaled illicit drug use, change in tobacco smoking, and change in inhaler use) on changes in FEV1 over time. Variables were selected for the model a priori based on clinical data which might have varied over the course of follow-up within an individual, specifically in participants who described changes in drug or medication use. The whole model is presented without variable elimination. Data were analyzed using Stata version 14.2 statistical software (StataCorp LLC) and R version 3.4 (R Foundation for Statistical Computing). Statistical significance was tested at the conventional 5% level.
Ethics
Ethical approval was gained from the Health Research Authority via the Integrated Research Application System (No. 235151).
Results
A total of 372 participants had previous COPD or ACO and were eligible for inclusion. The study follow-up took place between December 2017 and April 2018. Baseline questionnaire and clinical data were collected from 161 participants; 109 were lost to follow-up, 49 did not attend the follow-up appointment, 26 declined at the appointment, 23 were medically unfit, and four did not take part for other reasons. A total of 106 participants completed postbronchodilator spirometry at both baseline and follow-up to ATS standards. Patients remaining (n = 55) did not meet ATS standards (n = 22), were medically unfit (n = 3), died (n = 1), or declined postbronchodilator spirometry (n = 29) (Fig 1). Compression of participants characteristics can be seen in e-Table 1.
Figure 1.
Flow of participants through the study. ACO = asthma-COPD overlap; ATS = American Thoracic Society; GP = general practitioner.
The characteristics of the population are given in Table 1. Participants had a mean age of 51 ± 5.3 years, and 46 (28.6%) were women. Most participants were unemployed with high levels of socioeconomic deprivation (mean index of multiple deprivation score 51.5, which is in the lowest quintile for England). All participants were taking OST, with 76 (47.2%) reporting current heroin use.
Table 1.
Characteristics of 161 People With Baseline COPD or Asthma-COPD Overlap Derived From Follow-Up Questionnaire Data
| Characteristic | Value |
|---|---|
| Sex, female | 46 (28.6) |
| Age, y | 51.0 ± 5.3 |
| IMD score | 51.5 ± 12.7 |
| Occupation | |
| Unemployed | 137 (85.1) |
| Employment | 24 (14.9) |
| Housing | |
| Own home (including rented) | 124 (77.0) |
| Homeless | 6 (3.7) |
| Other | 31 (19.3) |
| Cigarette smoking status | |
| Current | 133 (82.6) |
| Ex | 27 (16.8) |
| Never | 1 (0.6) |
| Cigarettes smoked per day | 11 ± 7.0 |
| Heroin smoking status | |
| Current | 76 (47.2) |
| Ex | 85 (52.8) |
| Bags smoked per weeka | 4.0 ± 7.0 |
| Crack smoking | |
| Current | 33 (20.5) |
| Ex | 89 (55.3) |
| Never | 39 (24.2) |
| Rocks smoked per week | 2.18 ± 1.4 |
| Cannabis smoking status | |
| Current | 38 (23.8) |
| Ex | 53 (33.1) |
| Never | 69 (43.1) |
| Cannabis joint per week | 12 ± 17.1 |
| Ever injected heroin | 30 (18.5) |
| Current methadone dosage, mL/d | 45.7 ± 21.6 |
| Current buprenorphine dosage, mg/d | 10.4 ± 8.8 |
Values are presented as mean ± SD or No. (%). IMD = index of multiple deprivation.
A bag is estimated to equate to 0.1 g.
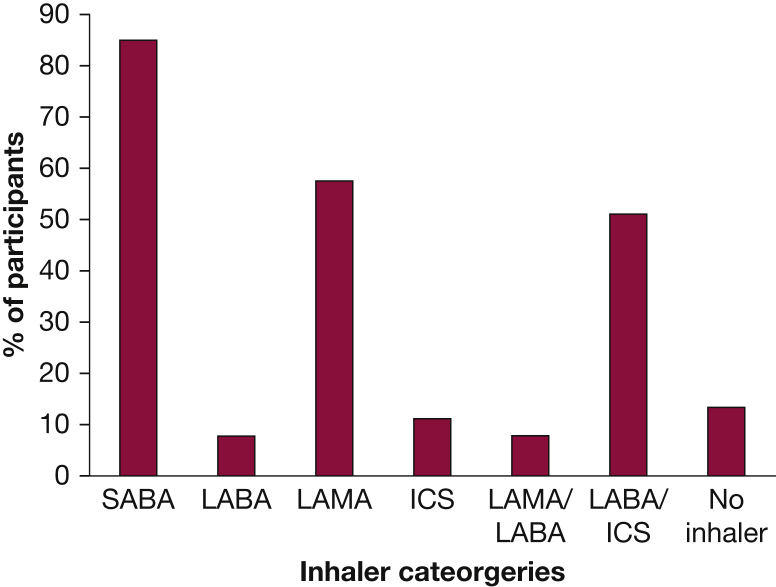
Most were prescribed an inhaler and were collecting prescriptions (defined as at least 50% pickup rate) from a pharmacy (n = 131; 81.4%). No inhalers were prescribed or collected for 21 participants (13.3%), and data were unavailable for 9 participants (5.5%). Of the participants with available data, 129 (84.9%), 88 (57.9%), and 78 (51.3%) collected prescriptions for short-acting beta 2 agonist, long-acting muscarinic antagonist, and an inhaled corticosteroid/long-acting beta 2 agonist combination, respectively (Fig 2). Three-quarters had attended a primary care practitioner for respiratory complaints within the preceding 2 years, with 18 (11%) requiring admission to hospital, staying for a mean of 11.5 days. Participants admitted to hospital were universally treated with bronchodilators, antibiotics, and steroids; three participants were offered noninvasive ventilation; two were treated in high-dependency areas; and none had level 3 care (invasive ventilation) (Table 2).
Figure 2.
Participants prescribed and picking up their inhalers (at least 50% of what was expected as recorded by the pharmacy team) as recorded on the primary care electronic prescribing system. Inhalers reviewed were SABA, LABA, LAMA, and ICS. ICS = inhaled corticosteroid; LABA = long-acting beta 2 agonist; LAMA = long-acting muscarinic antagonist; SABA = short-acting beta 2 agonist.
Table 2.
Health-Care Utilization From 2 Years Prior to Follow-Up Among Those Who Completed Follow-Up Questionnaires (N = 161)
| Variable | Value |
|---|---|
| Taking an inhaler regularly | |
| Yes | 131 (81.4) |
| No | 21 (13.0) |
| Not known | 9 (5.6) |
| Reported GP visits in last 2 y for respiratory conditions | |
| Yes | 121 (75.2) |
| No | 25 (15.5) |
| Not known | 15 (9.3) |
| No. of primary care visits (GP or nurse) | 8.6 ± 7.0 |
| Emergency hospital visits for respiratory conditions | |
| Yes | 17 (10.6) |
| No | 114 (70.8) |
| Not known | 30 (18.6) |
| Emergency hospital visits of those who did attend | 2.6 ± 1.9 |
| Admitted to hospital in last 2 y for respiratory conditions | |
| Yes | 17 (10.5) |
| No | 121 (74.7) |
| Not known | 24 (14.8) |
| Length of hospital stay, d | 11.5 ± 13.0 |
Values are presented as No. (%) or mean ± SD. Data were gathered from electronic medical records; participants not appearing on these systems are coded as not known, but might engage with extraregional, informal, or private health-care providers. GP = general practitioner.
The mean FEV1 was 2.05 ± 0.96 L at follow-up compared with 2.23 ± 0.97 L at baseline. Of the participants diagnosed with COPD/ACO at baseline and postbronchodilator spirometry at both time points, 94 (88.7%) had spirometry indicative of COPD at follow-up, with 38 (35.9%) having severe or very severe COPD (using GOLD guidelines) at follow-up compared with 26 (24.6%) at baseline. A further five participants (4.7%) had full reversibility (> 400 mL) and therefore were diagnosed with asthma, and seven (6.6%) had normal spirometry at follow-up (Table 3).
Table 3.
Diagnosis and Postbronchodilator Spirometry at Baseline and 2-Year Follow-Up of the 106 Participants Diagnosed With COPD or ACO at Baseline Who Completed Follow-Up
| Variable | Baseline | Follow-Up |
|---|---|---|
| FEV1, L | 2.23 ± 0.97 | 2.05 ± 0.95 |
| FEV1, % predicted | 69.1 ± 2.6 | 64.6 ± 2.7 |
| FVC, L | 4.07 ± 1.2 | 3.69 ± 1.1 |
| FVC, % predicted | 102.7 ± 23.7 | 95.5 ± 23.4 |
| FEV1/FVC ratio | 0.54 ± 0.13 | 0.53 ± 0.14 |
| Diagnosis (GOLD) | ||
| ACO | 4 (3.8) | … |
| Asthma | … | 5 (4.7) |
| Normal | … | 7 (6.6) |
| Severity (GOLD) | ||
| Mild | 37 (34.9) | 23 (21.7) |
| Moderate | 39 (36.8) | 33 (31.1) |
| Severe | 15 (14.2) | 24 (22.7) |
| Very severe | 11 (10.4) | 14 (13.2) |
Values are presented as No. (%) or mean ± SD. ACO = asthma-COPD overlap; GOLD = Global Initiative for Chronic Obstructive Lung Disease.
Participants reported a significant annualized increase in respiratory symptoms with the MRC and CAT scores increasing by a median of 0.48 (P < .001) and 1.60 (P < .001), respectively. They experienced a significant annualized decline in FEV1 and median oxygen saturation of 90 mL (P < .001) and 0.92% (P < .001), respectively (Table 4). Changes in smoking status and inhaler use were prehypothesized possible clinical factors that could influence FEV1 change. Since baseline, 49 participants (31.2%) reported a decrease in heroin smoking, and 73 (46.5%) reported unchanged usage (Fig 3). Change in drug use was not associated with change in FEV1. The final model showing change in drug and tobacco smoking status and inhaler use is presented in Table 5.
Table 4.
Annualized Change in Spirometry and Symptoms in the 106 Participants Diagnosed With COPD or ACO at Baseline Who Completed Follow-Up
| Variable | Baseline | Follow-Up | Change Per Year | Bootstrapping/95% CI | P Value |
|---|---|---|---|---|---|
| FEV1, L | 2.23 ± 97.12 | 2.05 ± 95.60 | −0.09 ± 0.19 | −0.05 to −0.13 | < .001 |
| MRC score | 3 (2-4) | 4 (3-5) | 0.46 (0.0 to 1.0) | 0.52 (0.36 to 0.67) | < .001 |
| CAT score | 25 (17-31) | 29 (23-33) | 1.60 (−0.48 to 4.32) | 0.46 (0.29 to 0.60) | < .001 |
| Spo2 (%) | 97 (96-98) | 95 (93-96) | −0.92 (−1.63 to 0.0) | 0.53 (0.38 to 0.66) | < .001 |
Values are mean ± SD, median (25th percentile-75th percentile), or as otherwise indicated. CAT = COPD Assessment Tool; MRC = Medical Research Council Dyspnea Scale; Spo2 = peripheral capillary oxygen saturation. See Table 3 legend for expansion of other abbreviation.
Figure 3.
Change in daily consumption of tobacco, heroin, and crack in 161 subjects over 2 y. If they have never smoked, their smoking status was recorded as stayed the same.
Table 5.
Linear Regression Model of Postbronchodilator FEV1 Change (n = 106)
| Variable | Coefficient (95% CI) for FEV1 Decrease (mL/y) | P Value (95% CI) |
|---|---|---|
| Change in reported heroin consumption | ||
| No change | Ref | |
| Increasea | 5.92 | 0.36 (−3.46 to 15.31) |
| Decreaseb | 5.35 | 0.21 (−6.31 to 17.03) |
| Change in reported crack consumption | ||
| No change | Ref | |
| Increasea | 0.18 | 0.96 (−9.00 to 7.68) |
| Decreaseb | 2.69 | 0.69 (−10.55 to 15.94) |
| Change in tobacco consumption | ||
| No change | Ref | |
| Increasea | 7.81 | 0.80 (−9.91 to 7.68) |
| Decreaseb | −1.11 | 0.34 (−8.51 to 24.14) |
| Change in inhaler use | ||
| No change | ||
| Increasea | −3.20 | 0.48 (−12.12 to 5.72) |
| Decreaseb | 1.79 | 0.79 (−11.61 to 15.20) |
Ref = reference.
A positive change is an increase in use since baseline.
A negative change is a decrease in usage since baseline.
Discussion
In a population of heroin smokers, we found a high burden of lung disease. In the previously published baseline data, 50% of heroin users had COPD or ACO, with a mean MRC score of 3.1 and CAT score of 22.9.17 At follow-up, participants’ respiratory symptoms had worsened significantly from baseline, with annual increases in both CAT score (1.60) and MRC score (0.46), and mean oxygen saturation dropping from 97% to 95% from baseline to follow-up. We found that lung function measured by FEV1 declined by 90 mL annually, which was both statistically and clinically significant. The proportion of subjects classified as having severe or very severe disease increased from 25% to 36% over the 2-year follow-up period. Neither ongoing illicit drug use nor prescriptions of inhaled medication were associated with change in lung function.
The symptoms reported in this study are consistent with those of studies in this population, with increased dyspnea among heroin users being the common symptom.12,13,26 The decline in health status measured by a CAT score increase of 1.60 annually is greater than the 1 unit change seen in patients with stable COPD.27 The rate of decline in FEV1 is considerably higher than both the 30 mL/y age-related decline seen in nonsmokers and in people with tobacco-related COPD (which is reported at 35-79 mL/y, of which all but one paper reported an annual decline of ≤ 69 mL).26,28 To date, research on lung function in heroin smokers has focused on cross-sectional studies. The results from this longitudinal cohort study support and enhance previous cross-sectional studies that suggest heroin users are at a high risk of COPD and suggest that their decline is worse than that of tobacco smokers. Walker et al11 found heroin smokers developed early onset emphysema, with a mean age of diagnosis being 41 years, suggesting likely early progression of disease compared with nonheroin smokers. In Amsterdam, The Netherlands, Buster et al14 reported a difference in FEV1 from predicted values, finding that heroin smokers had an FEV1 of 260 mL less than predicted FEV1.
The rapid decline in FEV1 and the increase in respiratory symptoms in this population suggest heroin smoking is a driver of decline in lung function. Similarly, once established, this decline appears to continue even in people who stop smoking drugs.
Although COPD hospital admissions vary greatly across the United Kingdom, patients with COPD tend to have high health-care usage, particularly in areas of high deprivation.29,30 Previous research has also shown that heroin users with respiratory exacerbations are more likely to be readmitted with exacerbations than current/ex-tobacco smokers (OR, 1.00 vs 0.22/0.26, respectively).18 It is also clear that with the high levels of health-care access observed in this population, it is likely that ongoing trends toward inhaling heroin will further increase the use of, and burden on, the health system.4,6
The strengths of our study include that we followed-up the participants over a 18- to 24-month period in a community clinic setting. We have shown that it is feasible to engage this client group in both baseline and follow-up spirometry allowing for a diagnosis to be made. The lost to follow-up rate is a major limitation of this study, reducing the power of statistical analysis and making stratification of our results by age or GOLD stage unfeasible. Given a larger group, this information would potentially be helpful for targeting care, and is an area for future investigation. This population tends to smoke a mix of heroin, crack, and tobacco, establishing a causal relationship with therefore difficult. The participants in the study were generally from a poor socioeconomic background, and there is potential that their living condition environment could contribute to the rate of decline. Without significant heterogeneity of such potentially confounding factors, we have been unable to address this question further. There is also potential for selection bias, with people who regularly attend methadone clinics and have concerns about their respiratory system more likely to participate in the study.
In summary, our findings show the significant respiratory impairment with which heroin smoking is implicated, and a concerning accelerated rate of decline over time. Future studies with larger cohorts, possibly in the context of a targeted public health intervention, are needed to understand if specific subgroups are especially vulnerable, and how the personal and health-care costs associated with chronic respiratory illness could be best averted. The study methodology is in support of it being feasible to colocate respiratory and drug services to one community location. Future studies may benefit from a parallel group of heroin users without spirometric abnormalities at baseline to determine their rate decline compared with patients with COPD. These results combined with previous studies support the call for enhanced screening for inhaled drug users.19 A pilot followed by clinical trial would be needed to assess if screening and treatment services would be clinically and cost-effective in this population.
Acknowledgments
Author contributions: All authors wrote the manuscript, approved the version to be published, and agreed to be accountable for all aspects of the work. R. N., K. M., P. P. W., T. B., S. M.-G., S. R., J. R., and H. B. contributed to study design. R. N., K. M., M. S., T. B., S. M.-G., and H. B. contributed to acquisition of data. R. N., E. G., J. R., and H. B. contributed to analysis of data. R. N., K. M., P. P. W., J. R., and H. B. contributed to interpretation of data.
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Additional information: The e-Table can be found in the Supplemental Materials section of the online article.
Footnotes
Drs Rylance and Burhan contributed equally to this manuscript.
FUNDING/SUPPORT: This project was funding by the Medical Research Council Doctoral Training Programme (MRC DTP). The Liverpool Clinical Commissioning Group funded the baseline data collection and a Health Foundation charity grant assisted with administrative support for repeat spirometry.
Supplementary Data
References
- 1.NHS. Statistics on Drug Misuse: England, 2018 [PAS]. https://digital.nhs.uk/data-and-information/publications/statistical/statistics-on-drug-misuse/2018. Accessed December 3, 2019.
- 2.Hickman M., Higgins V., Hope V. Injecting drug use in Brighton, Liverpool, and London: best estimates of prevalence and coverage of public health indicators. J Epidemiol Community Health. 2004;58(9):766–771. doi: 10.1136/jech.2003.015164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strang J., Griffiths P., Powis B., Gossop M. First use of heroin: changes in route of administration over time. BMJ. 1992;304(6836):1222. doi: 10.1136/bmj.304.6836.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hartgers C., Van Den Hoek A., Krijnen P., Van Brussel G.H., Coutinho R.A. Changes over time in heroin and cocaine use among injecting drug users in Amsterdam, The Netherlands, 1985–1989. Br J Addict. 1991;86(9):1091–1097. doi: 10.1111/j.1360-0443.1991.tb01876.x. [DOI] [PubMed] [Google Scholar]
- 5.Crawford C., Gohel R., Heneghan M., Thompson F., Wright C. United Kingdom Focal Point at Public Health England; London, UK: 2016. United Kingdom Drug Situation: Focal Point Annual Report 2016. [Google Scholar]
- 6.Swift W., Maher L., Sunjic S. Transitions between routes of heroin administration: a study of Caucasian and Indochinese heroin users in south-western Sydney, Australia. Addiction. 1999;94(1):71–82. doi: 10.1046/j.1360-0443.1999.941714.x. [DOI] [PubMed] [Google Scholar]
- 7.Pizzey R., Hunt N. Distributing foil from needle and syringe programmes (NSPs) to promote transitions from heroin injecting to chasing: an evaluation. Harm Reduct J. 2008;5(1):24. doi: 10.1186/1477-7517-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stöver H.J., Schäffer D. SMOKE IT! Promoting a change of opiate consumption pattern-from injecting to inhaling. Harm Reduct J. 2014;11(1):18. doi: 10.1186/1477-7517-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi H., Krantz A., Smith J., Trick W. Medical diagnoses associated with substance dependence among inpatients at a large urban hospital. PLoS One. 2015;10(6) doi: 10.1371/journal.pone.0131324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cygan J., Trunsky M., Corbridge T. Inhaled heroin-induced status asthmaticus: five cases and a review of the literature. Chest. 2000;117(1):272–275. doi: 10.1378/chest.117.1.272. [DOI] [PubMed] [Google Scholar]
- 11.Walker P.P., Thwaite E., Amin S., Curtis J.M., Calverley P.M. The association between heroin inhalation and early onset emphysema. Chest. 2015;148(5):1156–1163. doi: 10.1378/chest.15-0236. [DOI] [PubMed] [Google Scholar]
- 12.Pierce M., Bird S.M., Hickman M., Millar T. National record linkage study of mortality for a large cohort of opioid users ascertained by drug treatment or criminal justice sources in England, 2005–2009. Drug Alcohol Depend. 2015;146:17–23. doi: 10.1016/j.drugalcdep.2014.09.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winklhofer S., Surer E., Ampanozi G. Post-mortem whole body computed tomography of opioid (heroin and methadone) fatalities: frequent findings and comparison to autopsy. Eur Radiol. 2014;24(6):1276–1282. doi: 10.1007/s00330-014-3128-7. [DOI] [PubMed] [Google Scholar]
- 14.Buster M.C., Rook L., van Brussel G.H., van Ree J., van den Brink W. Chasing the dragon, related to the impaired lung function among heroin users. Drug Alcohol Depend. 2002;68(2):221–228. doi: 10.1016/s0376-8716(02)00193-x. [DOI] [PubMed] [Google Scholar]
- 15.Lewis-Burke N., Vlies B., Wooding O., Davies L., Walker P.P. A screening study to determine the prevalence of airway disease in heroin smokers. COPD. 2016;13(3):333–338. doi: 10.3109/15412555.2015.1082999. [DOI] [PubMed] [Google Scholar]
- 16.Nelson P., Bartu A., de Klerk N., Aborgye-Sarfo P., Musk B. High prevalence of smoking and respiratory symptoms of patients on opioid substitution treatment. Intern Med J. 2013;43(3):19. [Google Scholar]
- 17.Burhan H., Young R., Byrne T. Screening heroin smokers attending community drug services for COPD. Chest. 2019;155(2):279–287. doi: 10.1016/j.chest.2018.08.1049. [DOI] [PubMed] [Google Scholar]
- 18.Yadavilli R., Collins A., Ding W.Y., Garner N., Williams J., Burhan H. Hospital readmissions with exacerbation of obstructive pulmonary disease in illicit drug smokers. Lung. 2014;192(5):669–673. doi: 10.1007/s00408-014-9632-3. [DOI] [PubMed] [Google Scholar]
- 19.Tashkin D.P. Heroin smoking and COPD: a case for targeted screening spirometry. Chest. 2019;155(2):247–248. doi: 10.1016/j.chest.2018.08.1039. [DOI] [PubMed] [Google Scholar]
- 20.UK Data Service. https://ukdataservice.ac.uk/. Accessed December 3, 2019.
- 21.Jones P., Harding G., Berry P., Wiklund I., Chen W., Leidy N.K. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648–654. doi: 10.1183/09031936.00102509. [DOI] [PubMed] [Google Scholar]
- 22.Bestall J., Paul E., Garrod R., Garnham R., Jones P., Wedzicha J. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581–586. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller M.R., Crapo R., Hankinson J. General considerations for lung function testing. Eur Respir J. 2005;26(1):153–161. doi: 10.1183/09031936.05.00034505. [DOI] [PubMed] [Google Scholar]
- 24.Quajer P., Tammeling G., Cotes J., Pedersen O., Perlin R., Yernault J. Lung volumes and forced ventilatory flows. Report working party. Standardization of lung function test European Community for steel and Coal official statement of the European Respiratory Society. Eur Respir J. 1993;6(5):40. [PubMed] [Google Scholar]
- 25.Global Initiative for Chronic Obstructive Lung Disease. http://goldcopd.org/. Accessed December 3, 2019.
- 26.Lange P., Celli B., Agustí A. Lung-function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med. 2015;373(2):111–122. doi: 10.1056/NEJMoa1411532. [DOI] [PubMed] [Google Scholar]
- 27.CAT Governance Board. The COPD Assessment Test (CAT) Healthcare Professional User Guide, Issue 4. 2018. https://www.catestonline.org/hcp-homepage/clinical-practice.html. Accessed December 3, 2019.
- 28.Tantucci C., Modina D. Lung function decline in COPD. Int J Chron Obstruct Pulmon Dis. 2012;7:95. doi: 10.2147/COPD.S27480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Britton M. The burden of COPD in the UK: results from the confronting COPD survey. Respir Med. 2003;97(suppl C):S71–S79. doi: 10.1016/s0954-6111(03)80027-6. [DOI] [PubMed] [Google Scholar]
- 30.Calderón-Larrañaga A., Carney L., Soljak M. Association of population and primary healthcare factors with hospital admission rates for chronic obstructive pulmonary disease in England: national cross-sectional study. Thorax. 2011;66(3):191–196. doi: 10.1136/thx.2010.147058. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.