ABSTRACT
Aim
Post‐hoc analysis of the efficacy and safety of ertugliflozin in East/Southeast (E/SE) Asian patients with type 2 diabetes mellitus (T2DM).
Materials and Methods
Efficacy evaluations used data from randomized, double‐blind, phase 3 studies: a pool of two 26‐week placebo‐controlled studies and one 52‐week active‐comparator (glimepiride) study. Least squares mean change from baseline was calculated for HbA1c, fasting plasma glucose (FPG), body weight (BW) and systolic blood pressure (SBP). Safety evaluation included overall and prespecified adverse events based on pooled data (broad pool) from seven phase 3 studies (including studies in the efficacy analysis).
Results
Among 161 E/SE Asian patients in the placebo pool (ertugliflozin, n = 106), ertugliflozin reduced HbA1c, FPG, BW and SBP from baseline at week 26. The placebo‐adjusted changes from baseline for ertugliflozin 5 and 15 mg were: HbA1c, −0.9% and −1.0%; BW, −2.1 and −1.9 kg; and SBP, –3.3 and −3.5 mmHg, respectively. Among 174 E/SE Asian patients in the active‐comparator study (ertugliflozin, n = 118), HbA1c changes from baseline at week 52 were −0.6%, −0.6% and −0.7% for ertugliflozin 5 mg, 15 mg and glimepiride, respectively. Ertugliflozin 5 and 15 mg reduced BW from baseline by −4.3 and −4.1 kg, respectively, and SBP by −7.4 and −9.3 mmHg, respectively, compared with glimepiride. Safety findings were generally consistent with overall ertugliflozin safety data published to date.
Conclusions
Treatment with ertugliflozin was associated with reductions in HbA1c, FPG, BW and SBP, and was generally well tolerated in E/SE Asian patients with T2DM. https://ClinicalTrials.gov identifier: NCT01986855, NCT01999218, NCT01958671, NCT02099110, NCT02036515, NCT02033889, NCT02226003.
Keywords: sodium‐glucose cotransporter 2 inhibitor, type 2 diabetes mellitus
1. INTRODUCTION
The worldwide prevalence of diabetes in adults (aged 20–79 years) is expected to increase from 8.8% (~ 425 million people) in 2015 to an estimated 9.9% (~ 629 million people) by 2045.1 In Asian populations, including East Asia, the rate of diabetes has increased significantly over the past decade2; this may be related to increasing urbanization, a decrease in physical activity and a rise in obesity.2, 3, 4
East Asian patients with type 2 diabetes mellitus (T2DM) generally have a lower body mass index (BMI) compared with patients from other regions.4, 5, 6 Genetic factors vary between populations; there are significant differences between East Asian and European populations in the frequency of risk alleles associated with the development of T2DM.4 Environmental and lifestyle risk factors also have an impact on the development and management of T2DM. For example, white rice is an important part of the daily diet in East Asians and its consumption is associated with the risk of T2DM.6 As such, it is important to undertake an assessment of the efficacy and safety of antihyperglycaemic therapy in East Asian patients with T2DM.
Ertugliflozin, a selective sodium‐glucose cotransporter 2 (SGLT2) inhibitor,7, 8 has been evaluated for the treatment of adults with T2DM in the phase 3 VERTIS (eValuation of ERTugliflozin effIcacy and Safety) clinical trial programme.9, 10, 11, 12, 13, 14, 15 The results led to the approval of ertugliflozin as an adjunct to diet and exercise to improve glycaemic control in adults with T2DM, including in Hong Kong, Korea, Taiwan and several other East Asian countries.
This post‐hoc analysis of the phase 3 VERTIS programme included data from multinational studies that enrolled patients from East Asia and other regions. The safety and efficacy of ertugliflozin 5 mg and 15 mg were evaluated in East and Southeast (E/SE) Asian patients with T2DM. Non‐E/SE Asian patients were also analyzed for completeness.
2. MATERIALS AND METHODS
2.1. Study design: data sources
Three phase 3 VERTIS studies were included in the efficacy assessments (Figure 1). Two similarly designed placebo‐controlled 26‐week studies, VERTIS MET14 (ertugliflozin add‐on to metformin) and VERTIS SITA29 (ertugliflozin add‐on to metformin and sitagliptin), were pooled as the “placebo pool”. Data from VERTIS SU,11 a 52‐week active‐comparator study that compared ertugliflozin with glimepiride as add‐on to metformin, were analysed separately. Two other studies, VERTIS FACTORIAL13 and VERTIS RENAL,10 also enrolled patients from E/SE Asian countries, but they were not included in the efficacy analysis because of the relatively small numbers of E/SE Asian patients (<20 per treatment arm) and because the study designs were not appropriate for pooling data for analysis of ertugliflozin efficacy (ertugliflozin was co‐administered with sitagliptin in the VERTIS FACTORIAL study and VERTIS RENAL enrolled patients with chronic kidney disease). However, the safety data from these two studies were included in the safety analysis to provide a robust population appropriate for safety assessment.
Figure 1.
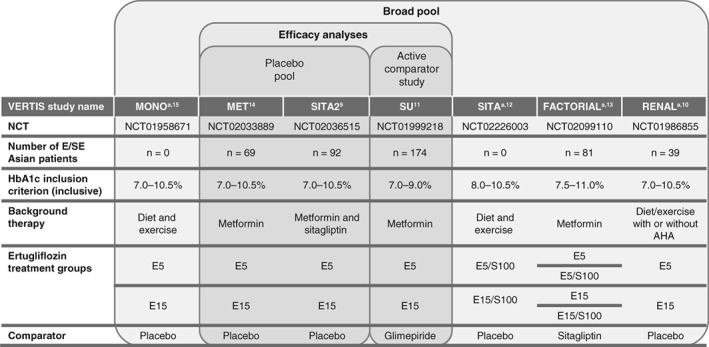
Phase 3 VERTIS studies included in the East and Southeast (E/SE) Asia post‐hoc analysis.9, 10, 11, 12, 13, 14, 15 AHA, antihyperglycaemic agent; E5, ertugliflozin 5 mg; E15, ertugliflozin 15 mg; NCT, http://clinicaltrials.gov identifier; S100, sitagliptin 100 mg.
aNot included in efficacy analysis as no patients (MONO and SITA) or <20 patients per treatment arm (FACTORIAL and RENAL) were enrolled from E/SE Asian countries
Safety analyses, except those related to documented and severe hypoglycaemia and estimated glomerular filtration rate (eGFR), were performed in the “broad pool”, which includes data from seven placebo‐controlled or active‐comparator studies: VERTIS MET, VERTIS SITA2, VERTIS MONO, VERTIS SU, VERTIS SITA, VERTIS FACTORIAL and VERTIS RENAL (http://clinicaltrials.gov identifiers NCT02033889, NCT02036515, NCT01958671, NCT01999218, NCT02226003, NCT02099110, and NCT01986855, respectively) (Figure 1). Data were collected for each patient from the time of randomization through each study's primary time point (week 26, or week 52 for VERTIS SU) and, for studies with a predefined extension period (all except VERTIS SITA), up to a prespecified data cut‐off (prior to completion of the extension period) to support regulatory submissions. The safety analysis for the broad pool represents mean durations of exposure to ertugliflozin 5 mg, ertugliflozin 15 mg and non‐ertugliflozin, of 356, 355 and 355 days, respectively. The incidences of documented and severe hypoglycaemia were assessed in the placebo pool only to avoid the confounding effects of the sulphonylurea glimepiride in the broad pool upon the analysis.
2.2. Patient population and treatments
The E/SE Asian population was defined as patients enrolled from Hong Kong, Korea, Malaysia, Philippines, Taiwan and Thailand. The placebo pool included 161 E/SE Asian patients and the active‐comparator study included 174 E/SE Asian patients. Data are also presented for the non‐E/SE Asian population for completeness. The non‐E/SE Asian population comprised patients enrolled from other countries. Details of inclusion criteria, exclusion criteria and the study design for the individual studies have been reported previously.9, 11, 14
Adult patients (aged ≥18 years) with T2DM according to American Diabetes Association criteria with baseline HbA1c levels of 7.0%–10.5% (range depending on the study) were randomized 1:1:1 to non‐ertugliflozin (placebo or glimepiride), ertugliflozin 5 mg or ertugliflozin 15 mg once daily in addition to existing metformin or metformin plus sitagliptin therapy (Figure 1).9, 11, 14 Background antihyperglycaemic agents other than protocol‐specified background therapy were stopped at the screening visit. Glycaemic rescue therapy was prescribed for patients who exceeded protocol‐specified glycaemic thresholds.9, 11, 14
All studies were conducted in accordance with the principles of Good Clinical Practice and were approved by the appropriate institutional review boards and regulatory agencies. Informed consent was obtained from all of the individuals in each study.
2.3. Efficacy endpoints and assessments
Efficacy endpoints were change from baseline to week 26 or week 52 in HbA1c, fasting plasma glucose (FPG), body weight and systolic blood pressure (SBP). The proportion of patients with HbA1c <7.0% at week 26 or week 52 was also evaluated. Body weight was measured in duplicate using a standardized digital scale. Sitting blood pressure was measured in triplicate with an automated oscillometric blood pressure measuring device.
2.4. Safety endpoints
Safety endpoints included incidences of adverse event (AE) summary measures (including incidence of AEs and serious AEs) and prespecified AEs of interest for SGLT2 inhibitors: genital mycotic infection (GMI) (by gender), urinary tract infection, volume depletion and documented and severe hypoglycaemia.
2.5. Statistical methods
The full analysis set (FAS) population consisted of all randomized patients who received at least one dose of study medication and had at least one measurement for the analysis endpoint (baseline or post‐randomization). Change from baseline in efficacy endpoints was analysed in the FAS population separately for each subgroup using a constrained longitudinal data analysis model to estimate least square (LS) mean changes.16 Analysis of the placebo pool used fixed effects for treatment, time, trial, baseline eGFR (continuous) and the interaction of time by treatment. Analysis of the active‐comparator study used fixed effects for treatment, time, prior antihyperglycaemic medication (monotherapy or dual therapy), baseline eGFR (continuous) and the interaction of time by treatment. Time was treated as a categorical variable. Efficacy results obtained after initiation of glycaemic rescue therapy were censored (referred to as excluding rescue approach).
Safety analyses were based on the all subjects as treated (ASaT) population, consisting of all randomized patients who received at least one dose of study medication. Safety data obtained after initiation of glycaemic rescue therapy were not censored (including rescue approach), except for hypoglycaemia data, which were assessed using the excluding rescue approach to avoid confounding the results by use of rescue medication.
Demographic and baseline disease characteristics were summarized descriptively in the ASaT population.
3. RESULTS
3.1. Patient disposition and baseline characteristics
The placebo pool comprised 161 E/SE Asian patients (placebo [n = 55], ertugliflozin 5 mg [n = 55] and ertugliflozin 15 mg [n = 51]) and 922 non‐E/SE Asian patients (placebo [n = 307], ertugliflozin 5 mg [n = 308] and ertugliflozin 15 mg [n = 307]). The active‐comparator study included 174 E/SE Asian patients (glimepiride [n = 56], ertugliflozin 5 mg [n = 54] and ertugliflozin 15 mg [n = 64]) and 1151 non‐E/SE Asian patients (glimepiride [n = 381], ertugliflozin 5 mg [n = 394] and ertugliflozin 15 mg [n = 376]). The broad pool included 4859 patients (455 E/SE Asian: non‐ertugliflozin [n = 135], ertugliflozin 5 mg [n = 155] and ertugliflozin 15 mg [n = 165]; and 4404 non‐E/SE Asian: non‐ertugliflozin [n = 1315], ertugliflozin 5 mg [n = 1561] and ertugliflozin 15 mg [n = 1528]).
Demographic and baseline characteristics in the placebo pool, active‐comparator study and broad pool were generally similar across treatment groups in both the E/SE Asian population (Table 1) and the non‐E/SE Asian population (Table S1). The E/SE Asian population had a lower mean body weight, BMI and FPG compared with the non‐E/SE Asian population.
Table 1.
Baseline demographics and treatment characteristics in the East and Southeast (E/SE) Asian population (all subjects as treated population)
| Placebo pool | Active comparator study | Broad pool | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Placebo (n = 55) | Ertugliflozin 5 mg (n = 55) | Ertugliflozin 15 mg (n = 51) | Glimepiride (n = 56) | Ertugliflozin 5 mg (n = 54) | Ertugliflozin 15 mg (n = 64) | Non‐ertugliflozin (n = 135) | Ertugliflozin 5 mg (n = 155) | Ertugliflozin 15 mg (n = 165) | |
| Male, n (%) | 30 (54.5) | 28 (50.9) | 28 (54.9) | 26 (46.4) | 22 (40.7) | 29 (45.3) | 69 (51.1) | 70 (45.2) | 75 (45.5) |
| Age (years) | 57.0 (9.9) | 57.6 (10.2) | 57.5 (9.3) | 56.9 (9.1) | 56.6 (9.5) | 53.6 (10.8) | 56.4 (9.9) | 56.1 (10.5) | 55.7 (10.0) |
| Duration of type 2 diabetes mellitus, years | 9.1 (5.1) | 9.3 (6.9) | 9.4 (4.8) | 7.6 (6.0) | 7.6 (6.1) | 6.3 (5.7) | 7.9 (5.4) | 8.0 (6.3) | 7.9 (5.9) |
| Body weight, kg | 69.8 (11.4) | 69.4 (15.3) | 71.3 (11.8) | 64.6 (13.2) | 69.3 (12.4) | 68.9 (12.4) | 67.5 (12.9) | 69.8 (15.6) | 68.9 (12.5) |
| BMI, kg/m2 | 26.3 (4.1) | 26.1 (4.4) | 26.7 (3.1) | 25.3 (3.4) | 26.9 (4.1) | 26.6 (3.8) | 26.1 (4.2) | 26.9 (4.7) | 26.8 (3.8) |
| HbA1c, % |
n = 54 8.2 (0.9) |
n = 55 8.1 (0.9) |
n = 50 8.0 (0.9) |
n = 56 7.8 (0.6) |
n = 54 7.7 (0.5) |
n = 64 7.9 (0.7) |
n = 133 8.1 (0.9) |
n = 152 8.1 (0.9) |
n = 163 8.2 (0.9) |
| FPG, mg/dL |
n = 54 155.6 (26.9) |
n = 55 158.7 (29.8) |
n = 50 158.6 (36.7) |
n = 56 145.1 (25.3) |
n = 54 147.1 (24.0) |
n = 64 155.8 (37.3) |
n = 134 153.7 (30.1) |
n = 155 153.9 (30.1) |
n = 163 157.1 (38.5) |
| SBP, mmHg |
n = 55 127.6 (14.4) |
n = 55 127.7 (11.4) |
n = 51 127.2 (12.8) |
n = 56 127.4 (12.3) |
n = 54 126.7 (12.6) |
n = 64 129.2 (11.1) |
n = 133 128.0 (13.1) |
n = 151 128.3 (12.2) |
n = 162 128.2 (11.8) |
| eGFR, mL/min/1.73 m2 |
n = 55 93.9 (18.0) |
n = 55 88.3 (18.0) |
n = 51 93.4 (21.4) |
n = 56 92.4 (17.4) |
n = 54 96.1 (24.0) |
n = 64 92.9 (17.6) |
n = 135 90.9 (19.4) |
n = 155 89.2 (24.3) |
n = 165 88.2 (22.6) |
| Enrolment region | |||||||||
| Asia | 55 (100) | 55 (100) | 51 (100) | 56 (100) | 54 (100) | 64 (100) | 135 (100) | 155 (100) | 165 (100) |
| Background AHA therapya, n (%) | |||||||||
| Currently on AHA therapy | 55 (100) | 55 (100) | 51 (100) | 56 (100) | 54 (100) | 63 (98.4)b | 134 (99.3) | 153 (98.7) | 162 (98.2) |
| Biguanides | 55 (100) | 55 (100) | 51 (100) | 56 (100) | 54 (100) | 64 (100) | 128 (94.8) | 139 (89.7) | 148 (89.7) |
| DPP4 inhibitors | 26 (47.3) | 22 (40.0) | 21 (41.2) | 0 (0) | 0 (0) | 2 (3.1) | 32 (23.7) | 35 (22.6) | 30 (18.2) |
| Sulphonylurea | 15 (27.3) | 19 (34.5) | 13 (25.5) | 6 (10.7) | 11 (20.4) | 7 (10.9) | 3 (2.2) | 11 (7.1) | 8 (4.8) |
| Other blood glucose‐lowering agents | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 4 (3.0) | 3 (1.9) | 7 (4.2) |
| Number of AHA agentsa, n (%) | |||||||||
| 1 | 14 (25.5) | 14 (25.5) | 17 (33.3) | 50 (89.3) | 43 (79.6) | 55 (85.9) | 102 (75.6) | 118 (76.1) | 131 (79.4) |
| 2 | 41 (74.5) | 41 (74.5) | 34 (66.7) | 6 (10.7) | 11 (20.4) | 9 (14.1) | 31 (23.0) | 35 (22.6) | 31 (18.8) |
| ≥3 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0.7) | 0 (0) | 0 (0) |
Abbreviations: AHA, antihyperglycaemic agent; BMI, body mass index; DPP4, dipeptidyl peptidase‐4; eGFR, estimated glomerular filtration rate;
FPG, fasting plasma glucose; SBP, systolic blood pressure.
Data are mean (SD) unless otherwise stated.
At screening for the placebo pool and active comparator study or at randomization for the broad pool.
One patient was reported in the category of “not on an AHA” at screening; however, this patient was taking metformin at screening.
3.2. Glycaemic control
In the E/SE Asian population, ertugliflozin provided a greater reduction in HbA1c than placebo at week 26 with a placebo‐corrected LS mean reduction (95% confidence interval [CI]) of −0.9% (−1.2, −0.6) and −1.0% (−1.3, −0.7) for ertugliflozin 5 and 15 mg, respectively (Figure 2A). More patients who received ertugliflozin 5 mg (45.3%) and 15 mg (47.9%) compared with placebo (10.0%) had HbA1c <7.0% at week 26 (Figure 2B). In the active‐comparator study, a reduction in HbA1c was observed in the ertugliflozin and glimepiride treatment groups at week 52. The LS mean reduction in HbA1c was −0.6%, −0.6% and −0.7% for ertugliflozin 5 mg, 15 mg and glimepiride, respectively (Figure 2A). The proportion of patients who had HbA1c <7.0% at week 52 was 39.6%, 39.3% and 45.3% for ertugliflozin 5 mg, 15 mg and glimepiride, respectively (Figure 2B).
Figure 2.
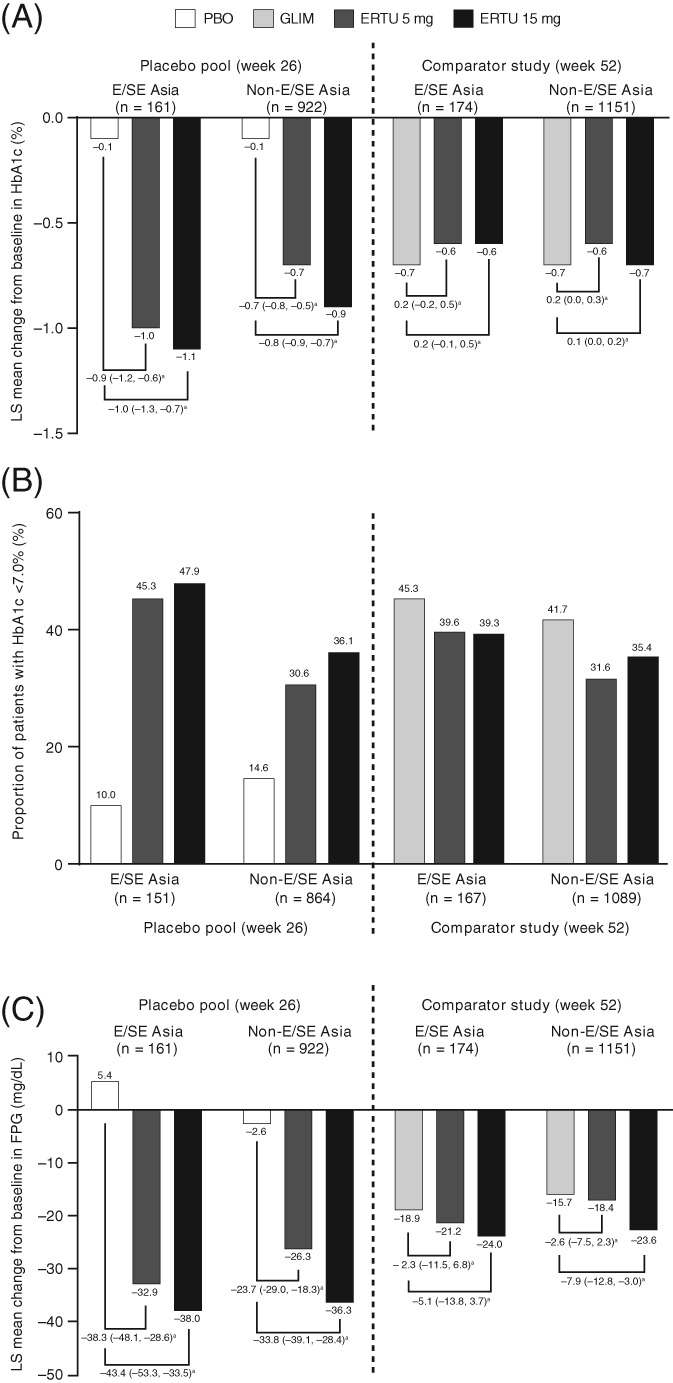
(A) Least squares (LS) mean change from baseline in HbA1c; (B) proportion of patients with HbA1c <7%; (C) LS mean change from baseline in fasting plasma glucose (FPG) at week 26 and week 52 for East and Southeast (E/SE) Asian and non‐E/SE Asian patients (full analysis set [FAS] population, excluding rescue approach). CI, confidence interval; ERTU, ertugliflozin; GLIM, glimepiride; PBO, placebo.
aLS mean (95% CI) difference vs. placebo or glimepiride
In the E/SE Asian population, ertugliflozin provided a reduction in FPG from baseline at week 26, whereas there was an increase from baseline with placebo. The placebo‐corrected LS mean reduction (95% CI) was −38.3 mg/dL (−48.1, −28.6) and −43.4 mg/dL (−53.3, −33.5) for ertugliflozin 5 and 15 mg, respectively. In the active‐comparator study, a reduction from baseline in FPG was observed in the ertugliflozin and glimepiride treatment groups at week 52. The LS mean reduction in FPG was −21.2 mg/dL, −24.0 mg/dL and −18.9 mg/dL for ertugliflozin 5 mg, 15 mg and glimepiride, respectively (Figure 2C).
Reductions from baseline in HbA1c and FPG were also observed in the ertugliflozin groups in the non‐E/SE Asian population (Figure 2).
3.3. Body weight and SBP
In the placebo pool, ertugliflozin 5 and 15 mg provided a greater reduction from baseline in body weight and SBP compared with placebo at week 26 in the E/SE Asian population. For ertugliflozin 5 and 15 mg, the placebo‐corrected LS mean reduction (95% CI) in body weight was −2.1 kg (−2.9, −1.3) and −1.9 kg (−2.8, −1.1), respectively, and in SBP it was −3.3 mmHg (−7.7, 1.1) and −3.4 mmHg (−7.9, 1.0), respectively (Figure 3).
Figure 3.
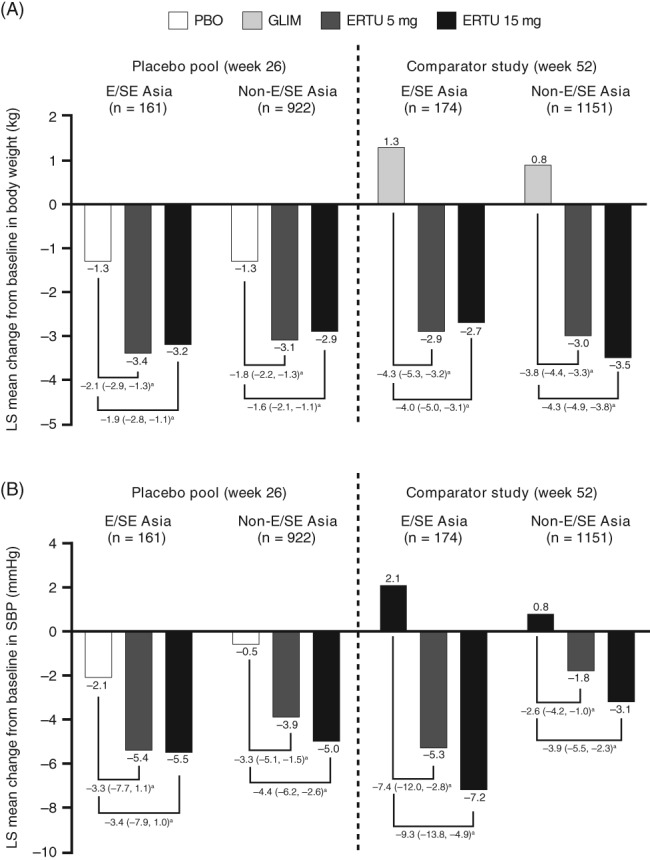
Least squares (LS) mean change from baseline in (A) body weight and (B) systolic blood pressure (SBP) at week 26 and week 52 for East and Southeast (E/SE) Asian and non‐E/SE Asian patients (full analysis set [FAS] population, excluding rescue approach). CI, confidence interval; ERTU, ertugliflozin; GLIM, glimepiride; PBO, placebo.
aLS mean (95% CI) difference vs. placebo or glimepiride
In the E/SE Asian population of the active‐comparator study, a reduction from baseline to week 52 in body weight and SBP was observed with ertugliflozin 5 and 15 mg, whereas an increase from baseline in body weight and SBP was observed in the glimepiride treatment group. The difference in LS mean reduction (95% CI) in body weight between ertugliflozin and glimepiride was −4.3 kg (−5.3, −3.2) and −4.0 kg (−5.0, −3.1) for ertugliflozin 5 and 15 mg, respectively, and in SBP it was −7.4 mmHg (−12.0, −2.8) and −9.3 mmHg (−13.8, −4.9) for ertugliflozin 5 and 15 mg, respectively (Figure 3).
Reductions from baseline in body weight and SBP were also observed in the ertugliflozin groups in the non‐E/SE Asian population (Figure 3).
3.4. Safety
In the ertugliflozin groups, there were no notable differences in the incidences of AEs, serious AEs or AEs leading to discontinuation of study medication compared with the non‐ertugliflozin group in both populations (Table 2). There was one death (acute myocardial infarction) in the E/SE Asian population in the ertugliflozin 5 mg group, and a low incidence of death across groups in the non‐E/SE Asian population.
Table 2.
Summary of overall safety and prespecified adverse events (AEs) in the East and Southeast (E/SE) Asian and non‐E/SE Asian populations: broad pool (all subjects as treated population, including rescue approacha)
| E/SE Asian | Non‐E/SE Asian | |||||
|---|---|---|---|---|---|---|
| Patients, n (%) | Non‐ertugliflozin (n = 135) | Ertugliflozin 5 mg (n = 155) | Ertugliflozin 15 mg (n = 165) | Non‐ertugliflozin (n = 1315) | Ertugliflozin 5 mg (n = 1561) | Ertugliflozin 15 mg (n = 1528) |
| ≥1 AEs | 96 (71.1) | 97 (62.6) | 103 (62.4) | 844 (64.2) | 977 (62.6) | 946 (61.9) |
| Serious AEs | 11 (8.1) | 15 (9.7) | 12 (7.3) | 69 (5.2) | 95 (6.1) | 86 (5.6) |
| Died | 0 (0) | 1 (0.6) | 0 (0) | 3 (0.2) | 9 (0.6) | 8 (0.5) |
| Discontinued treatment due to an AE | 4 (3.0) | 4 (2.6) | 5 (3.0) | 56 (4.3) | 66 (4.2) | 69 (4.5) |
| Discontinued due to a serious AE | 2 (1.5) | 1 (0.6) | 0 (0) | 8 (0.6) | 16 (1.0) | 15 (1.0) |
| Prespecified AEs of interest | ||||||
| UTI | 9 (6.7) | 7 (4.5) | 7 (4.2) | 106 (8.1) | 111 (7.1) | 112 (7.3) |
| Volume depletion | 1 (0.7) | 2 (1.3) | 2 (1.2) | 16 (1.2) | 30 (1.9) | 21 (1.4) |
| GMI (female)b | 0/66 (0) | 3/85 (3.5) | 3/90 (3.3) | 20/597 (3.4) | 68/746 (9.1) | 85/759 (11.2) |
| GMI (male)b | 0/69 (0) | 1/70 (1.4) | 1/75 (1.3) | 2/718 (0.3) | 40/815 (4.9) | 32/769 (4.2) |
| Documented hypoglycaemia (placebo pool)a , b | 0/55 (0) | 4/55 (7.3) | 2/51 (3.9) | 14/307 (4.6) | 18/308 (5.8) | 17/307 (5.5) |
| Severe hypoglycaemia (placebo pool)a , b | 0/55 (0) | 0/55 (0) | 0/51 (0) | 2/307 (0.7) | 2/308 (0.6) | 0/307 (0) |
Abbreviations: GMI, genital mycotic infection; UTI, urinary tract infection.
Hypoglycaemia was assessed in the placebo pool only, excluding rescue approach.
Data are shown as n/N (%).
GMI AEs were reported in males and females with ertugliflozin but not with non‐ertugliflozin in the E/SE Asian population. In the non‐E/SE Asian population, the incidence of GMI in males and females was higher with ertugliflozin compared with non‐ertugliflozin (Table 2).
In the placebo pool, few patients in the E/SE Asian population had documented hypoglycaemia and the incidence with ertugliflozin in the E/SE Asian population was not notably different from the non‐E/SE Asian population. Of the six patients in the E/SE Asian population with documented hypoglycaemia (four in the ertugliflozin 5 mg group and two in the ertugliflozin 15 mg group), precipitating factors were identified in five of the patients, including skipped meals or increases in physical activity. There were no episodes of severe hypoglycaemia in the E/SE Asian population. The incidence of documented and severe hypoglycaemia was low across treatment groups in the non‐E/SE Asian population (Table 2).
In both the placebo pool and the active‐controlled study, a small early transient decrease in eGFR was observed with ertugliflozin (week 6), which returned to baseline by the end of the studies. In the placebo pool, the mean (standard deviation [SD]) change from baseline in eGFR with ertugliflozin 5 mg, 15 mg and placebo at week 6 was −2.0 (9.2), −1.6 (10.9) and −2.1 (8.8) mL/min/1.73 m2, respectively, and at week 26 it was 3.3 (11.1), 3.1 (11.2) and 0.7 (9.5) mL/min/1.73m2, respectively. In the active‐controlled study, the mean (SD) change in eGFR with ertugliflozin 5 mg, 15 mg and glimepiride at week 6 was −1.5 (10.5), −0.7 (10.8) and −2.2 (11.1) mL/min/1.73 m2, respectively, and at week 52 it was 2.5 (18.0), 2.3 (12.5) and −0.5 (17.7) mL/min/1.73 m2, respectively.
4. DISCUSSION
This post‐hoc analysis of multinational randomized phase 3 VERTIS studies that enrolled East Asian patients evaluated the efficacy and safety of ertugliflozin in patients with T2DM from E/SE Asian and non‐E/SE Asian countries.
The results reported here show that ertugliflozin 5 and 15 mg provide robust glycaemic efficacy in E/SE Asian patients with T2DM. A greater reduction in HbA1c and FPG was observed with ertugliflozin compared with placebo at week 26 and a greater proportion of patients had HbA1c <7% relative to placebo at week 26. Although this post‐hoc analysis was not designed to compare treatment groups, the reduction from baseline in HbA1c and FPG was similar to that with ertugliflozin and glimepiride. An incremental lowering of HbA1c and FPG in the placebo pool and an incremental lowering of FPG in the glimepiride‐controlled study was observed with ertugliflozin 15 mg compared with the 5 mg dose. The HbA1c reduction in the glimepiride‐controlled study was similar for both ertugliflozin doses. These results are generally consistent with the findings from the total cohort in the VERTIS phase 3 clinical trial programme, where ertugliflozin 15 mg had an incremental glycaemic effect relative to ertugliflozin 5 mg across studies. In a pooled analysis of three placebo‐controlled studies, the difference in HbA1c reduction between the two doses was ~ 0.15%.17 Although the difference in glycaemic efficacy between doses is small, ertugliflozin 15 mg can provide additional glycaemic efficacy for patients if needed. On the basis of the results from the phase 3 programme, the approved ertugliflozin labelling in the United States and European Union recommends a starting dose of 5 mg, increasing to 15 mg if therapy is well tolerated and if additional glycaemic control is needed.
In the E/SE Asian population, greater reductions in body weight were observed with ertugliflozin 5 and 15 mg compared with placebo or glimepiride. Although mean baseline body weight was over 10 kg lower in the E/SE Asian population compared with the overall population,11, 18 the absolute magnitude of weight loss with ertugliflozin treatment was similar. Previous reports have noted that Asian populations have a lower BMI compared with Western populations, but the increased risk of T2DM starts at a lower BMI in Asian compared with Western patients.5, 6 The baseline BMI in the E/SE Asian population in this current analysis is similar to studies of other SGLT2 inhibitors in Asian and E/SE Asian populations (25.5 to 26.0 kg/m2).19, 20
A greater reduction from baseline in SBP was observed with ertugliflozin treatment compared with placebo and glimepiride in the E/SE Asian population. The SBP lowering may be of particular relevance in East Asia, given the high burden of stroke in this region.21, 22 The ongoing VERTIS CV trial, which enrolled 522 (6%) patients from Asia, will evaluate the impact of ertugliflozin on cardiovascular outcomes in patients with T2DM.23
Overall, the efficacy of ertugliflozin in the E/SE Asian and non‐E/SE Asian populations was generally consistent with results for the total cohorts for the placebo pool18 and the active‐comparator study,11 as well as with the overall population of patients with T2DM in the phase 3 VERTIS programme9, 11, 12, 13, 14, 15 (excluding the VERTIS RENAL study, which assessed patients with T2DM and stage 3 chronic kidney disease). These findings are in line with pharmacokinetic analyses of ertugliflozin, which found that there was no meaningful difference in ertugliflozin exposure between races (data not shown). The efficacy findings are also consistent with those reported in a meta‐analysis of 33 randomized controlled studies that have assessed SGLT2 inhibitors (dapagliflozin, canagliflozin, empagliflozin, ipragliflozin, tofogliflozin and luseogliflozin) in E/SE Asian patients with T2DM.24
This analysis focused on E/SE Asian patients as defined in the methodology. Treatment with ertugliflozin was previously studied in Asian patients with T2DM in a dedicated placebo‐controlled, randomized, double‐blind, phase 3 study that enrolled 506 Asian patients with T2DM inadequately controlled with metformin (VERTIS Asia).25 All the patients in that study were from East Asia, with ~ 80% from mainland China. Consistent with the results reported here, VERTIS Asia reported clinically meaningful reductions in HbA1c, FPG, body weight and SBP with ertugliflozin 5 and 15 mg.25
Both doses of ertugliflozin were generally well tolerated among E/SE Asian and non‐E/SE Asian patients with T2DM. In the non‐E/SE Asian population, there was a higher incidence of male and female GMI with ertugliflozin compared with non‐ertugliflozin; however, a lower incidence was observed across all treatment groups in the E/SE Asian population and data were insufficient to draw conclusions. It is not anticipated that there would be a difference in the risk of GMIs in E/SE Asian patients receiving SGLT2 inhibitors relative to other ethnic populations. A possible explanation for the lower incidence in E/SE Asians compared with non‐E/SE Asians may be that it is in part a result of cultural reporting bias, which was proposed as a possible explanation for similar findings in a study with canagliflozin in Asian patients with T2DM.20 A recent meta‐analysis of East Asian patients also reported a higher incidence of genital tract infections in those receiving SGLT2 inhibitors compared with the control treatment arms (placebo or non‐SGLT2 inhibitor antihyperglycaemic drugs).24 Few patients in the E/SE Asian population had documented hypoglycaemia. In the E/SE Asian population, the incidence of hypoglycaemia in the ertugliflozin groups was similar to that observed in the non‐E/SE Asian population, and the lower incidence observed in the placebo group for the E/SE Asian population compared with the non‐E/SE Asian population should be interpreted with caution given the small numbers of patients. There were no reports of severe hypoglycaemia in the E/SE Asian population. The observed safety profile is consistent with the overall safety data that have been published for ertugliflozin to date.9, 11, 12, 13, 14, 15, 25
East/SE Asian patients included in the current analysis of ertugliflozin efficacy had normal baseline renal function with a baseline eGFR similar to studies of other SGLT2 inhibitors in Asian and E/SE Asian populations (85.9 to 95.3 mL/min/m2).19, 20 A small initial decrease from baseline in eGFR was observed with ertugliflozin in the E/SE Asian population in the placebo pool and active‐comparator study that returned to baseline over time. These results are consistent with findings from other studies in the VERTIS phase 3 programme and this pattern of eGFR change from baseline is common among SGLT2 inhibitors.
The safety and tolerability of another SGLT2 inhibitor, empagliflozin, in East Asian patients with T2DM are reported in a pooled analysis based on >2100 patient‐years’ exposure.19 Empagliflozin was generally well tolerated in this population with a similar safety profile in terms of general AEs and prespecifed AEs to those described here with ertugliflozin. Incidences of hypoglycaemia differed according to baseline antihyperglycaemic medication, with a higher risk in patients on background sulphonylurea therapy as expected because of the associated stimulation of insulin secretion.26 Similar to the observations in our analysis, the overall rate of genital infections was lower in East Asian trial participants compared with the overall analysis population, irrespective of gender.
In conclusion, treatment with ertugliflozin 5 mg and 15 mg was associated with clinically meaningful reductions in HbA1c, FPG, body weight and SBP, and was generally well tolerated in patients with T2DM from E/SE Asian countries.
CONFLICT OF INTEREST
J. L., S. P., L. W., S. H., A. P. and I. G. are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and own stock in Merck & Co., Inc., Kenilworth, NJ, USA. N. B. C., S. G. T., A. H. and A. D. are employees of Pfizer Inc. and own stock in Pfizer Inc, New York, NY, USA.
AUTHOR CONTRIBUTIONS
All of the authors critically reviewed the draft manuscript and approved the final version of the manuscript for publication.
DATA ACCESSIBILITY
Upon request, and subject to certain criteria, conditions and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de‐identified participant data from Pfizer‐sponsored global interventional clinical studies conducted for medicines, vaccines and medical devices (1) for indications that have been approved in the United States and/or the European Union or (2) in programmes that have been terminated (ie, development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de‐identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
Supporting information
Supplementary Table S1 Baseline demographics and treatment characteristics in the non‐E/SE Asian population (ASaT population)
ACKNOWLEDGMENTS
This study was sponsored by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA in collaboration with Pfizer Inc., New York, NY, USA. Editorial support was provided by Marion James, PhD, of Engage Scientific Solutions (Horsham, UK) and was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, in collaboration with Pfizer Inc., New York, NY, USA.
Liu J, Patel S, Cater NB, et al. Efficacy and safety of ertugliflozin in East/Southeast Asian patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2020;22:574–582. 10.1111/dom.13931
Funding information Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA; Pfizer Inc., New York, NY, USA; Merck
REFERENCES
- 1. International Diabetes Federation . IDF Diabetes Atlas. 8th ed., Brussels, Belgium: International Diabetes Federation; 2017. [Google Scholar]
- 2. Noh J. The diabetes epidemic in Korea. Endocrinol Metab. 2016;31:349‐353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li H, Oldenburg B, Chamberlain C, et al. Diabetes prevalence and determinants in adults in China mainland from 2000 to 2010: a systematic review. Diabetes Res Clin Pract. 2012;98:226‐235. [DOI] [PubMed] [Google Scholar]
- 4. Cho YS, Lee JY, Park KS, Nho CW. Genetics of type 2 diabetes in East Asian populations. Curr Diab Rep. 2012;12:686‐696. [DOI] [PubMed] [Google Scholar]
- 5. Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors and pathophysiology. JAMA. 2009;301:2129‐2140. [DOI] [PubMed] [Google Scholar]
- 6. Ma RC, Chan JC. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci. 2013;1281:64‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kalgutkar AS, Tugnait M, Zhu T, et al. Preclinical species and human disposition of PF‐04971729, a selective inhibitor of the sodium‐dependent glucose cotransporter 2 and clinical candidate for the treatment of type 2 diabetes mellitus. Drug Metab Dispos. 2011;39:1609‐1619. [DOI] [PubMed] [Google Scholar]
- 8. Miao Z, Nucci G, Amin N, et al. Pharmacokinetics, metabolism, and excretion of the antidiabetic agent ertugliflozin (PF‐04971729) in healthy male subjects. Drug Metab Dispos. 2013;41:445‐456. [DOI] [PubMed] [Google Scholar]
- 9. Dagogo‐Jack S, Liu J, Eldor R, et al. Efficacy and safety of the addition of ertugliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sitagliptin: the VERTIS SITA2 placebo‐controlled randomized study. Diabetes Obes Metab. 2018;20:530‐540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grunberger G, Camp S, Johnson J, et al. Ertugliflozin in patients with stage 3 chronic kidney disease and type 2 diabetes mellitus: the VERTIS RENAL randomized study. Diabetes Ther. 2018;9:49‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hollander P, Liu J, Hill J, et al. Ertugliflozin compared with glimepiride in patients with type 2 diabetes mellitus inadequately controlled on metformin: the VERTIS SU randomized study. Diabetes Ther. 2018;9:193‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Miller S, Krumins T, Zhou H, et al. Ertugliflozin and sitagliptin co‐initiation in patients with type 2 diabetes: the VERTIS SITA randomized study. Diabetes Ther. 2018;9:253‐268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pratley RE, Eldor R, Raji A, et al. Ertugliflozin plus sitagliptin versus either individual agent over 52 weeks in patients with type 2 diabetes mellitus inadequately controlled with metformin: the VERTIS FACTORIAL randomized trial. Diabetes Obes Metab. 2018;20:1111‐1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rosenstock J, Frias J, Páll D, et al. Effect of ertugliflozin on glucose control, body weight, blood pressure and bone density in type 2 diabetes mellitus inadequately controlled on metformin monotherapy (VERTIS MET). Diabetes Obes Metab. 2018;20:520‐529. [DOI] [PubMed] [Google Scholar]
- 15. Terra SG, Focht K, Davies M, et al. Phase III, efficacy and safety study of ertugliflozin monotherapy in people with type 2 diabetes mellitus inadequately controlled with diet and exercise alone. Diabetes Obes Metab. 2017;19:721‐728. [DOI] [PubMed] [Google Scholar]
- 16. Liang K‐Y, Zeger SL. Longitudinal data analysis of continuous and discrete responses for pre‐post designs. Sankhyā Ser B. 2000;62:134‐148. [Google Scholar]
- 17. Liu J, Tarasenko L, Terra SG, et al. Efficacy of ertugliflozin in monotherapy or combination therapy in patients with type 2 diabetes: a pooled analysis of placebo‐controlled studies. Diab Vasc Dis Res. 2019;16:415‐423. [DOI] [PubMed] [Google Scholar]
- 18. Calle RA, Liu J, Huyck S, et al. A pooled analysis of the efficacy and safety of ertugliflozin as add‐on therapy to metformin. 78th Scientific Sessions of the American Diabetes Association; June 22–26, 2018, Orlando, FL. Poster 1140P. [Google Scholar]
- 19. Yabe D, Yasui A, Ji L, et al. Safety and tolerability of empagliflozin in East Asian patients with type 2 diabetes: pooled analysis of phase I‐III clinical trials. J Diabetes Investig. 2019;10:418‐428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ji L, Han P, Liu Y, et al. Canagliflozin in Asian patients with type 2 diabetes on metformin alone or metformin in combination with sulphonylurea. Diabetes Obes Metab. 2015;17:23‐31. [DOI] [PubMed] [Google Scholar]
- 21. Kim YD, Jung YH, Saposnik G. Traditional risk factors for stroke in East Asia. J Stroke. 2016;18:273‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mehndiratta MM, Khan M, Mehndiratta P, Wasay M. Stroke in Asia: geographical variations and temporal trends. J Neurol Neurosurg Psychiatry. 2014;85:1308‐1312. [DOI] [PubMed] [Google Scholar]
- 23. Cannon CP, McGuire DK, Pratley R, et al. Design and baseline characteristics of the eValuation of ERTugliflozin effIcacy and Safety CardioVascular outcomes trial (VERTIS‐CV). Am Heart J. 2018;206:11‐23. [DOI] [PubMed] [Google Scholar]
- 24. Yang L, Zhang L, He H, Zhang M, An Z. Efficacy and safety of sodium‐glucose cotransporter 2 inhibitors in East Asians with type 2 diabetes: a systematic review and meta‐analysis. Diabetes Ther. 2019;10:1921‐1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ji L, Liu Y, Miao H, et al. Safety and efficacy of ertugliflozin in Asian patients with type 2 diabetes mellitus inadequately controlled with metformin monotherapy: VERTIS Asia. Diabetes Obes Metab. 2019;21:1474‐1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient‐centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35:1364‐1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1 Baseline demographics and treatment characteristics in the non‐E/SE Asian population (ASaT population)
Data Availability Statement
Upon request, and subject to certain criteria, conditions and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de‐identified participant data from Pfizer‐sponsored global interventional clinical studies conducted for medicines, vaccines and medical devices (1) for indications that have been approved in the United States and/or the European Union or (2) in programmes that have been terminated (ie, development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de‐identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.


