Abstract
Introduction
The prognostic value of symptoms at disease presentation of advanced gastro-oesophageal cancer is unknown. Thus, the aim of this study was to characterise these symptoms and correlate them with the outcome, so new prognostic markers can be defined.
Methods
We analysed clinical data including symptoms, therapies and survival of patients with stage IV gastro-oesophageal cancer treated between 2002 and 2018 at the Vienna General Hospital, Austria. Initial symptoms as well as stenosis in endoscopy and HER2 positivity were evaluated in a cross-validation model to ascertain the impact of each variable on patient survival.
Results
In total, 258 patients were evaluated. Five factors (stenosis in endoscopy, weight loss, HER2 positivity, dyspepsia, ulcer or active bleeding) have proven to be statistically relevant prognostic factors and were given a count of +1 and −1, if applicable. The resulting score ranges between −3 and +2. The survival probability for 180 days with a score of −3/–2, −1, 0, +1 and +2 is 90%, 80%, 73%, 72% and 42%, whereas for 2 years, it is 30%, 30%, 8%, 7% and 3%, respectively. The median overall survival of a score of −3/–2, −1, 0, +1 and +2 was 579 (95% CI 274 to not measurable), 481 (95% CI 358 to 637), 297 (95% CI 240 to 346), 284 (95% CI 205 to 371), 146 (95% CI 120 to 229) days, respectively.
Conclusion
The data from this retrospective study indicate that the Viennese risk prediction score for Advanced Gastroesophageal carcinoma based on Alarm Symptoms score provides independent prognostic information that may support clinical decision making at diagnosis of advanced gastro-oesophageal cancer. Our findings should be evaluated in prospective studies.
Keywords: gastric, esophagus, gastroesophageal, score, prognosis
Key questions.
What is already known about this subject?
The prognostic value of symptoms at disease presentation of advanced gastro-oesophageal cancer is unknown. Thus, the aim of this study was to characterise these symptoms and correlate them with the outcome, so new prognostic markers can be defined.
What does this study add?
Five factors (stenosis in endoscopy, weight loss, HER2 positivity, dyspepsia, ulcer or active bleeding) have proven to be statistically relevant prognostic factors, and a prognostic score for the overall survival of patients with metastatic gastro-oesophageal cancer was developed in a cross-validation model.
How might this impact on clinical practice?
The data from this retrospective study indicate that the VAGAS score provides independent prognostic information that may support clinical decision making at diagnosis of advanced gastro-oesophageal cancer.
Introduction
Cancer of the upper gastrointestinal (GI) tract is a frequent disease and major contributor to global disease burden.1 Although it encompasses three entities (gastric cancer, esophageal cancer and gastro-oesophageal junction cancer) as well as two different histologies (adenocarcinoma and squamous cell carcinoma), the initial symptoms as well as the survival probabilities are similar, independent of tumour localisation and type. Gastro-oesophageal cancer is usually asymptomatic in early stages, and symptoms such as weight loss, dysphagia, dyspepsia, vomiting, early satiety and/or iron deficiency anaemia develop mostly in advanced tumour stages.2 Dysphagia, weight loss and age >55 years were found to be significant positive predictive factors for cancer compared with non-malignant diseases of the upper GI tract.3 Especially dysphagia and weight loss are known to be associated with higher stages of gastric cancer.4
These symptoms, also known as alarm symptoms, when identified, usually indicate that the cancer is already inoperable.5 In consequence, most patients in the western world are diagnosed very late during the course of the disease, at a locally advanced (stage III) or metastatic stage (stage IV). Even though the survival showed a steady increase during the past decades independently of the tumour stage, the prognosis remains poor especially in more advanced stages.6 7
Only few prognostic factors for gastric cancer are surmised to associate with a longer overall survival (OS).
Prognostic tools for the outcome of patients with cancer are often not feasible in patients with upper GI cancer, since their performance status is usually good despite their advanced tumour stages. Specific prognostic tools for the outcome of patients with advanced gastro-oesophageal cancer are therefore needed. Since the symptoms can reduce the treatment options as well as the survival (ie, weight loss leads to a reduced general condition and thereby to a reduced survival), we surmised that the initial symptoms of the patient diagnosed with advanced upper GI cancer could be used as a prognostic marker. The aim of this retrospective study was to define new symptom-based prognostic markers for the outcome of patients with gastro-oesophageal cancer and develop a prognostic score for the outcome.
Methods
Patient collection
Patients with histologically proven advanced or metastatic (stage IV) gastro-oesophageal cancer treated between March 2002 and June 2018 were identified from the patient database of the General Hospital Vienna, Austria. Clinical information including patient demographics, therapy regimens, adverse events, tumour marker profiles, symptoms and survival outcome was obtained.
All patients underwent tumour staging prior to therapy according to the local hospital standard, including history taking, physical examination, routine hematologic tests, upper GI endoscopy with histological biopsy and CT of the chest and abdomen.
Patients were treated according to the individual decision of an interdisciplinary tumour board, which ensured the best possible treatment according to the respective standard of knowledge at the time of diagnosis. As all treatments were in a palliative setting due to the advanced tumour stage, the prolongation of OS and the reduction of symptoms were the main goals.
The treatment included systemic (immuno)chemotherapy and/or palliative gastrectomy and/or radiation therapy of the primary tumour, lymph nodes or metastatic sites. Chemotherapy in almost all patients was fluoropyrimidine and platine based; some patients received chemotherapy with additional anticancer drugs. HER2 status was evaluated in all patients potentially eligible for trastuzumab treatment at the Medical University of Vienna after November 2009. Carcinomas with either immunohistochemical intensity score 3+ or 2+ with additional positive fluorescence in situ hybridisation were classified as HER2 positive and consequently eligible for anti-HER2 treatment. Patients with neoadjuvant treatment of the same tumour in an initially curative setting were excluded from the study.
Routine re-evaluation of the tumour status was performed at least every 3 months with CT or MRI. Evaluation of the response was done according to the current Response Evaluation Criteria in Solid Tumors (RECIST) criteria by experienced radiologists.8 9
Patients were followed up until death according to the hospital or public records or loss to follow-up.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1964 and later versions. Due to the retrospective design, no separate informed consent was necessary in the scope of this study.
Alarm symptoms and other parameters known at the time of initial diagnosis
We analysed the following parameters as alarm symptoms: dysphagia, dyspepsia, weight loss, stenosis and the localisation of the stenosis in the endoscopy, active bleeding and ulcers in the endoscopy and frailty. Dysphagia was classified as positive when the patient had moderate dysphagia (able to eat some solid foods) or severe dysphagia (able to swallow liquids only). Involuntary weight loss was classified as positive when it was documented in the patient’s history. The differentiation between different severities was done according to the amount of lost kilograms in percentage to the original weight (1%–10%: slight weight loss, 11%–20%: moderate weight loss, >21%: severe weight loss).
Frailty was assessed either by Eastern Cooperative Oncology Group (ECOG) performance status, which is routinely recorded in the patient’s history before starting chemotherapy or by some more detailed patient information recorded when first diagnosed (ie, too frail for chemotherapy, thus the patient received best supportive care). An ECOG performance status ≥2 as well as the documented word ‘frail’ was regarded as significant frailty in scope of this analysis.
We also analysed the treatment of dyspepsia, bleeding and dysphagia including proton-pump inhibitors, blood transfusions, stenting of the stenosis and surgery to treat the stenosis.
Furthermore, other parameters, which are already known at the time of diagnosis (age, grade, HER2 positivity, alcohol, nicotine, having a second cancer in patient history and positive family history) were included into the statistical analysis. We surmised that these initial parameters might have a prognostic impact as they are already known as prediction markers either in upper GI cancer or other cancer entities.10–16
These key, potential prognostic variables were selected because the data were nearly complete in our retrospective data set and could be widely representative, making the findings clinically applicable.
Statistical analysis
χ2 test was used for the analysis of the distribution of dichotomised variables. Metric values were compared between two groups with t-test, in case of thre or more groups with analysis of variance. Patients without an event (death) were censored at the date that they were last known to be alive.
OS was calculated from the date of the initial diagnosis to the death of the patient or the patient’s last follow-up date. Analyses of OS were done with Kaplan-Meier survival estimates with log-rank test and Cox regression.
Univariate Cox regression analysis was used to compare the survival times for age, gender, family history of cancer, family history of GI cancer, prior cancer, smoking, primary tumour site, histological carcinoma type, HER2, tumour grade, number and location of metastatic sites and the initial symptoms. Radiation therapy and chemotherapy were decided at baseline and therefore included in the Cox regression analyses as baseline variables, while gastrectomy was treated as time dependent variable with value 0 at baseline, switching to 1 on the day when gastrectomy was performed. Furthermore, the OS was also compared for the five defined alarm symptoms (dysphagia, dyspepsia, stenosis in endoscopy, weight loss, ulcers or active bleeding) using univariate Cox regression.
For the development of the prognostic score, a multivariable Cox regression model with stepwise model selection according to best Akaike Information Criterion (AIC) was used. Fivefold cross-validation was performed to get an impression of model overfit and the possible performance on independent data. Therefore, variable selection was conducted on each of the cross-validation sets separately, and Harrell‘s concordance statistic was used to evaluate goodness of fit of the resulting model on the trainings set and on the validation set. Selected variables, their parameter estimates and the two concordance statistics were presented for each of the five validation runs. The final model, containing all variables selected in at least 4 of the 5 validation runs, was then calculated on the full data set, still allowing for further model reduction with AIC. The resulting score was simplified for an easier use in daily practice, and the expected proportions of 6-month survivors, 1-year survivors and 2-year survivors were calculated for each possible score value. Kaplan-Meier curves for the resulting score groups are shown in a graph.
Two-tailed p values of ≤0.05 were considered to be statistically significant. All statistics were calculated using statistical package for the social sciences (SPSS) V.24.0 software or R 3.5.1
Results
Patient and tumour characteristics
Two hundred and fifty-eight patients with gastro-oesophageal cancer were included in further analysis. Online supplementary file 1 shows the demographics and the baseline characteristics.
esmoopen-2019-000623supp001.pdf (16.3KB, pdf)
Online supplementary file 2 shows the gastric cancer specific characteristics and treatment modalities. In 124 (48%) patients, the tumour was located in the stomach, in 70 (27%) patients at the gastro-oesophageal junction and in 64 (25%) patients in the oesophagus. There were 32 (12%) squamous cell carcinomas and 226 (88%) adenocarcinomas. Twenty-three patients (15% of 154 stage IV patients evaluated since November 2009) had HER2-positive tumours.
esmoopen-2019-000623supp002.pdf (28.6KB, pdf)
Regarding metastasis, 150 patients (58%) had only one site of metastasis and 85 patients (33%) had two sites, and there were also patients with three (20 (8%)), four (2 (1%)) and five (1%) sites of metastasis when they were first diagnosed. Around 10% of the patients had a second cancer either before or at the same time of the diagnosis of gastro-oesophageal cancer. Concerning treatment options, 228 (88%) patients received palliative chemotherapy to reduce the tumour load and help with the symptoms, and only 30 (12%) patients received best supportive care without any chemotherapy. Chemotherapy usually was fluropyrimidine and platine based; sometimes triple chemotherapy was administered. Regimens used in our cohort of patients with gastro-oesophageal cancer are docetaxel/cisplatin/5-FU, epirubicin/oxaliplatin/capecitabine, cisplatin/5-FU, leucovorin/5-FU/oxaliplatin, capecitabine/oxaliplatin, etoposide/leucovorin/5-FU, irinotecan/mitomycin, cisplatin/docetaxel, docetaxel mono, 5-FU/leucovorin/epirubicin/cisplatin, 5-FU/leucovorin/oxaliplatin/docetaxel, oxaliplatin/docetaxel and capecitabine. Trastuzumab-containing regimens were also administered; the most widely used combination includes cisplatin/capecitabine/trastuzumab according to the Trastuzumab for Gastric Cancer (ToGA) protocol.17 Radiation therapy was used in 48 patients (19%), and 39 patients (15%) received a palliative gastrectomy.
Frequency of symptoms and the treatment
One hundred and twelve (43%) patients experienced dysphagia, and 120 (47%) patients experienced dyspepsia as a first symptom. Frailty was a first symptom in 54 (21%) patients and bleeding in 47 (18%, 33 patients with active bleeding, 14 patients with ulcers). Weight loss was a first symptom in 155 (60.1%) patients, 61 (39.4%) of them suffering from slight, 71 (45.8%) from moderate and 23 (14.8%) from severe weight loss. Eighty patients had an initial stenosis in the GI tract (proximal oesophagus: 5 patients (6.3%), middle oesophagus: 3 patients (3.8%), distal oesophagus 41 patients (51.2%), Gastroesophageal Junction Tumor (GEJ): 12 patients (15%), pylorus: 19 patients (23.8%)).
There were 32 stents implanted due to stenosis (5 in the stomach, 10 in the GEJ and 17 in the oesophagus), and there occurred several complications (11 dislocations, 11 residual stenoses and 5 other complications). Other treatments for dysphagia included two gastroenteromies, three gastrectomies, two percutaneous endoscopic gastrostomies, one nasogastric feeding tube and one local radiation therapy. Only 29 patients with dysphagia experienced an improvement of the symptoms after the intervention.
In our cohort, 39 palliative gastrectomies were performed, 11 due to active bleeding, 2 due to dysphagia. All other gastrectomies were performed in clinically fit patients either with clinically good response to several cycles of chemotherapy or regarding the patient’s wish to have the primary tumour removed.
Correlation of symptoms with other parameters
These results of symptoms depending on gender, tumour location, histology and sites of metastasis are shown in online supplementary file 3. The difference between histology concerning dysphagia (p<0.001), dyspepsia (p=0.023), weight loss (p=0.049) and stenosis (p=0.014) is statistically significant, whereas the difference concerning bleeding (p=0.501) and frailty (p=0.821) are not. Dysphagia occurred only in 21 patients with gastric cancer (17%) but in 43 patients with GEJ cancer (61%) and in 48 patients with oesophageal cancer (75%, p<0.001). Stenosis was most common in patients with oesophageal cancer (p<0.001). In contrast, bleeding was more common in patients with gastric cancer, although the difference is not statistically significant (23 patients (19%) to 4 patients (6%) and 6 patients (9%), respectively; p=0.103).
esmoopen-2019-000623supp003.pdf (21.8KB, pdf)
Correlation between symptoms and survival
The median OS of the study population was 286 days (95% CI 238 to 334).
Median OS of patients who received systemic therapy, irrespective of other additional treatment modalities, was 332 days (95% CI 289 to 375) in comparison with a median OS of 80 days (95% CI 55 to 105) of patients without systemic therapy (p<0.001).
The survival analysis of the time-dependent variable gastrectomy showed a slightly risk lowering influence but it was not significant (β=−0.307, p=0.117). In the univariate model, HER2 positivity was not associated with a longer OS (p=0.257 in all 258 patients, p=0.277 in 154 patients who were routinely evaluated for HER2 positivity since November 2009).
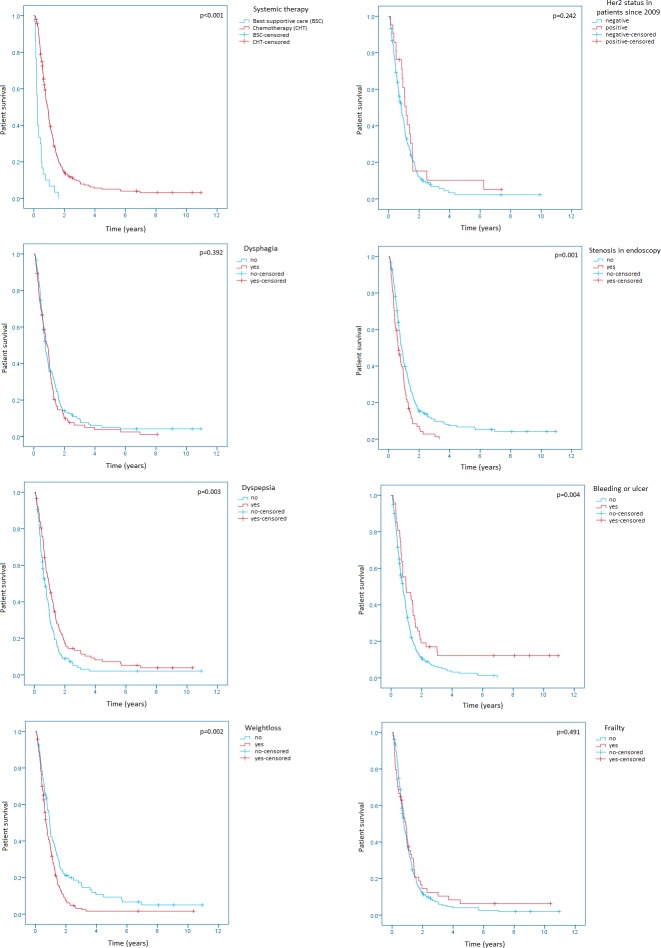
Patients without an initial stenosis in the upper GI tract had a longer OS (313 days; 95% CI 264 to 362) than patients with a stenosis (229 days; 95% CI 160 to 298; p=0.001). There was also a benefit in the OS for patients with dyspepsia as an initial symptom (p=0.003; median OS without dyspepsia 247 days; 95% CI 198 to 296; median OS with dyspepsia 351 days; 95% CI 266 to 436) and a benefit for patients without initial weight loss (p=0.002, median OS without weight loss 348 days; 95% CI 308 to 388; median OS with weight loss 252 days; 95% CI 212 to 292). Active bleeding or an ulcer in the initial endoscopy was also correlated with a longer OS (patients with bleeding/ulcers 358 days; 95% CI 100 to 616; patients without bleeding/ulcers 284 days; 95% CI 237 to 331; p=0.004). The Kaplan-Meier curves of the univariate models are shown in figure 1.
Figure 1.
Kaplan-Meier survival analyses regarding chemotherapy, HER2 positivity, dysphagia, dyspepsia, stenosis in endoscopy, active bleeding or ulcers, weight loss and frailty.
There was no significant difference in the OS regarding age (p=0.096), gender (p=0.795), family history (p=0.526), family history of the upper GI tract (p=0.806), second oncologies (p=0.832), smoking (p=0.561), alcohol (p=0.268), body mass index (p=0.330), the number of metastatic sites (p=0.658), the palliative treatment with radiotherapy (p=0.738), dysphagia as an initial symptom (p=0.392) or frailty (p=0.491) (see online supplementary files 1 and 2).
Development of the VAGAS score
Thirteen predefined variables (dysphagia, dyspepsia, stenosis in endoscopy, weight loss, frailty, GI bleeding, age, grade, HER2 positivity, alcohol, nicotine, having a second cancer in patient history and positive family history), which were recorded at the time of the diagnosis, were evaluated in a cross-validation model to ascertain the impact of each variable on patient survival. In the final prognostic regression model, five factors (stenosis in endoscopy, weight loss, HER2 positivity, dyspepsia and ulcer or active bleeding) have proven to be statistically relevant prognostic factors in patients with stage IV cancer of the upper GI tract with the following parameter estimates: (stenosis in endoscopy: b1=0.286; weight loss: b2=0.442; HER2 positivity: b3=-384; dyspepsia: b4=−0.443; ulcer or active bleeding: b5=−0.465). Cross-validation showed stable results with similar Harrell’s concordance statistics in the validation sets as in the training sets. The cross-validation and prognostic regression model can be seen in online supplementary files 4 and 5.
esmoopen-2019-000623supp005.pdf (18.7KB, pdf)
esmoopen-2019-000623supp004.pdf (18.5KB, pdf)
To increase feasibility of the score in daily routine, the estimates were reduced to their presence by giving the prognostic parameters a count of +1 and −1, if the factor is not applicable the count is 0. The score is shown table 1.
Table 1.
Viennese risk prediction score for advanced Gastroesophageal carcinoma based on Alarm Symptoms (VAGAS score)
| Prognostic factor | Yes | No |
| Stenosis in endoscopy | +1 | 0 |
| Weight loss | +1 | 0 |
| Her2 positivity | -1 | 0 |
| Dyspepsia | -1 | 0 |
| Ulcer or active bleeding | -1 | 0 |
| Total count |
The OS based on the VAGAS score in out cohort is shown in figure 2. The resulting score ranges between −3 and +2. Thirty-three patients had a prognostic score of +2, 71 a score of +1, 93 a score of 0, 51 a score of −1, 9 a score of −2, respectively. There was only one patient in the data set with score of −3, therefore he was included in the −2 group.
Figure 2.
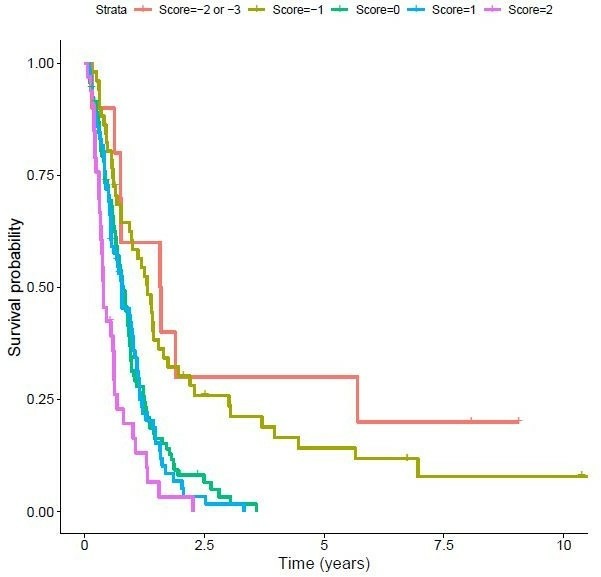
Overall survival based on the VAGAS score in our cohort of 258 patients with stage IV upper gastrointestinal cancer. VAGAS, Viennese risk prediction score for advanced Gastroesophageal carcinoma based on Alarm Symptoms.
The VAGAS score can predict the survival probability using these five prognostic factors. The survival probabilities for 2 years, 1 year and 180 days are shown in figure 3 and table 2.
Figure 3.
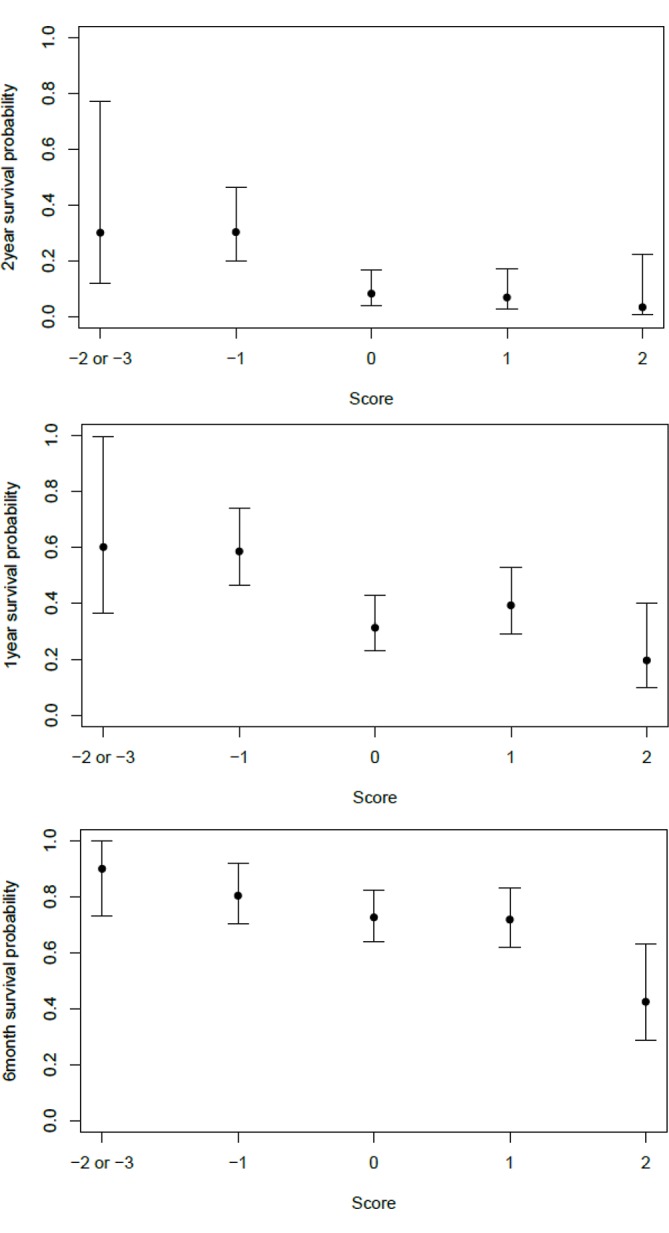
Survival probabilities in regard of the VAGAS score for 2 years, 1 year and 180 days. VAGAS, Viennese risk prediction score for advanced Gastroesophageal carcinoma based on Alarm Symptoms.
Table 2.
Estimated survival probability for 180 days, 1 year and 2 years
| Score | N | 180 days | 1 year | 2 years | |||
| Survival in % | 95% CI | Survival in % | 95% CI | Survival in % | 95% CI | ||
| −3/−2 | 10 | 90 | 73 to 100 | 60 | 36 to 100 | 30 | 12 to 77 |
| −1 | 51 | 80 | 70 to 92 | 58 | 46 to 74 | 30 | 20 to 46 |
| 0 | 93 | 73 | 64 to 82 | 31 | 23 to 43 | 8 | 4 to 17 |
| +1 | 71 | 72 | 62 to 83 | 39 | 29 to 53 | 7 | 3 to 17 |
| +2 | 33 | 42 | 29 to 63 | 20 | 9 to 40 | 3 | 0 to 22 |
The survival probability for 180 days with a score of −3/–2, −1, 0, +1 and +2 is 90%, 80%, 73%, 72% and 42%, respectively. Whereas the survival probability for 1 year with a score of −3/–2, −1, 0, +1 and +2 is 60%, 58%, 31%, 39% and 20%, respectively. The survival probability for 2 years with a score of −3/–2, −1, 0, +1 and +2 is 30%, 30%, 8%, 7% and 3%, respectively. The median OS of patients with a score of −3/–2, −1, 0, +1 and +2 was 579 (95% CI 274 to not measurable), 481 (95% CI 358 to 637), 297 (95% CI 240 to 346), 284 (95% CI 205 to 371) and 146 (95% CI 120 to 229) days, respectively.
Discussion
There are several known alarm symptoms and signs for gastro-oesophageal cancer. When identified, they usually indicate that the cancer is already advanced and therefore inoperable.5 18 For the multidisciplinary treatment of these patients, it is essential to have clinically feasible prognostic tools to help with the decision of treatment options and supportive treatment arrangements.19–21 Commonly used performance scores in patients with cancer, such as the ECOG and the Karnofsky score, are used in everyday life because of their feasibility and because they are essential tools in clinical trials.22–24 Although frequently used, both scores seem to fail to provide a useful prognostic score for patients with gastro-oesophageal cancer and show a considerable variability depending on the physician who evaluates the score.25–27 Since the usual alarm symptoms such as dysphagia or dyspepsia comprise a huge burden for everyday life but do not influence the overall performance of the patient right away, they do not have any effect on the results of the ECOG or Karnofsky score. That is why we surmised that these symptoms and signs may have great prognostic value for the performance and OS of patients with metastatic gastro-oesophageal cancer.
Prognostic factors
We evaluated known alarm symptoms as well as other parameters that are available at first diagnosis in our large European retrospective cohort to get a clinically feasible prognostic score. The alarm symptoms, which were analysed in this study, encompassed subjective experiences such as dysphagia and dyspepsia, as well as objective evidences of a disease, also called signs, such as stenosis and ulcers in the endoscopy, and parameters that can be experienced by the patient and observed by the physician at the same time such as weight loss, active bleeding and frailty.
Interestingly initial dysphagia turned out to have no correlation with the OS, but stenosis in the endoscopy does. This may be due to the fact that an irritation of the oesophagus or stomach without stenosis may lead to some discomfort while swallowing but does not cause any obstruction in the endoscopy, thereby causing the feeling of dysphagia without disruption of the digestion. This feeling of dysphagia without stenosis was already described more than 30 years ago.28 Thus, stenosis in endoscopy might be a more accurate prognostic factor, and it is also an objective parameter, which is not biased by the history taking. Furthermore, the severity of dysphagia was not assessed in this retrospective analysis. Since this factor might also influence the OS, a more thoroughly assessment of this symptom should be performed in further prospective studies.
Weight loss is also known to have prognostic value on the OS of patients with GI cancer,29 yet until now failed to be used as a prognostic tool. Furthermore, nutritional therapy, which, as it is an outpatient treatment, is not documented regularly in the hospital records of the Medical University of Vienna and therefore not included in this analysis, might also influence the outcome and OS. Thus, the longitudinal effects of initial weight loss should be evaluated in further prospective studies.29
Interestingly, dyspepsia as an initial symptom was associated with longer OS. This may be due to the fact that dyspepsia is a commonly known medical problem, and we surmise that patients often seek the help of a medical professional soon after it occurs. This finding is in accordance with the finding of Maconi et al,30 who reported that the risk of death is nearly threefold in patients with at least one alarm symptom compared with that in patients with uncomplicated dyspepsia.
There are several studies that evaluated the correlation of gastric ulcers and bleeding with the outcome of patients with gastro-oesophageal cancer yet have failed to establish this symptom as a prognostic factor.18 31 In our cohort, active bleeding as well as visible ulcers at the time of cancer diagnosis was associated with a longer OS. This might be due to the effect that these symptoms cause anaemia and fatigue as well as pain, thereby urging patients to seek help from medical professionals and specialists.
HER2 positivity was not statistically associated with a longer OS but still is an important prognostic factor in the scoring system. HER2, which initially was associated with a worse prognosis, nowadays is associated with a favourable outcome due to targeted therapy, and patients are screened routinely for positivity since 2009.32–37 The cross-validation model then assessed it to be an important prognostic factor, which is consistent with known literature.
VAGAS prognostic score
Throughout the last decades, several retrospective studies evaluated alarm symptoms as prognostic factors18 31 38; however, to our best knowledge, we are the first to establish a prognostic score that can be used at the time of the initial diagnosis in patients with metastatic gastro-oesophageal cancer.
The feasibility of the VAGAS score with only five factors, which are already known at the time of the initial diagnosis, is essential for clinical use. The results of the prognostic score can easily be interpreted, and the score can be divided into three risk groups. A total count of +2 is associated with high risk patients, a total count of +1 or 0 is associated with medium risk patients and a count of −1, −2 or −3 is associated with low risk patients. This prognostic tool might be an important assistance for deciding the further antitumor treatment such as supportive measurements such as psycho-oncological support and palliative care facilities.
Since this prognostic score was established in a cohort of patients with metastatic upper GI cancer, it might be more accurate than general performance scores like the ECOG or Karnofsky score. This may be an important asset for clinical patient care and to improve the consistency of the conduct of clinical trials. The VAGAS score will be evaluated and compared with commonly used performance scores in further prospective analyses.
Online supplementary file 6 gives an example how the VAGAS score might be implemented in everyday clinical routine.
esmoopen-2019-000623supp006.pdf (51.8KB, pdf)
Strengths and limitations of the study
The patient population was homogenous: all patients had metastatic gastro-oesophageal cancer at presentation and were therefore in a palliative treatment setting. All patients were treated according to the individual decision of an interdisciplinary tumour board, which ensured the best possible treatment according to the respective standard of knowledge at the time of diagnosis. Due to this individual decision, no comparison between used regimen is feasible in scope of this analysis.
One important limitation of this study, as it is a retrospective analysis, is the accuracy and reliability of the data collection. Since detailed documentation of the patient history including the evaluation of alarm symptoms is obligatory and standardised at the General Hospital Vienna, the results were retrievable from the medical records. If an alarm symptom was not mentioned specifically in the medical records, it was classified as negative in this retrospective analysis. Although the under-reporting of symptoms is a known problem in retrospectively collected data, alarm symptoms are severe impairments of everyday life and thereby are less likely to be under-reported than other symptoms, which do not interfere with everyday routine. Nevertheless, the results of this retrospective analysis still have to be verified in a prospective cohort to optimise the data collection.
Since our analyses were performed at the time of cancer diagnosis, the investigation of the longitudinal effects of these alarm symptoms, for example, the effect of nutritional therapy on weight loss and its correlation with the OS, and the VAGAS score on cancer progression is an important objective in future studies.
Conclusion
In conclusion, a prognostic score for the survival of patients with stage IV gastro-oesophageal cancer, which is mainly based on the alarm symptoms of patients and was established by using prognostic factors that are already known at the time of first diagnosis, could be established in this retrospective analysis. Since prognostic scores for the outcome of patients with gastro-oesophageal cancer are scarce, the VAGAS score is a clinically feasible tool to estimate the survival probability and could easily be established in all hospitals that treat patients with metastatic gastro-oesophageal cancer. To confirm these results and validate the score, a prognostic study implementing the score in a clinical setting is needed and will be an objective for further studies.
Acknowledgments
The authors gratefully acknowledge Medical University of Vienna’s core funding to the Department of Internal Medicine I and the Center for Medical Statistics.
Footnotes
Contributors: The manuscript has been read and approved by all authors. All authors contributed to the preparation of the manuscript significantly and are in agreement with the content of the manuscript. All authors were involved in creating the study design and concept.
Funding: This study was funded by Medical University of Vienna.
Competing interests: HCP has received travel support from Eli Lilly, MSD, Novartis, Pfizer and Roche. ASB has research support from Daiichi Sankyo and Roche, honoraria for lectures, consultation or advisory board participation from Roche Bristol-Meyers Squibb, Merck, Daiichi Sankyo as well as travel support from Roche, Amgen, Daiichi Sankyo and AbbVie. MP has received honoraria for lectures, consultation or advisory board participation from the following for-profit companies: Bayer, Bristol-Myers Squibb, Novartis, Gerson Lehrman Group (GLG), CMC Contrast, GlaxoSmithKline, Mundipharma, Roche, MedMedia, Astra Zeneca, AbbVie, Lilly, Medahead, Daiichi Sankyo and Merck Sharp & Dome. AI-M participated in advisory boards from MSD and Servier, received lecture honoraria from Eli Lilly and Servier and is the local principal investigator for clinical trials sponsored by BMS and Astellas.
Patient consent for publication: Not required.
Ethics approval: All procedures were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1964 and later versions. Due to the retrospective design, no separate informed consent was necessary in the scope of this study. The study was approved by the ethics committee of the Medical University of Vienna (signee Jürgen Zezula, reference number: 2267/2016).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available on reasonable request. The data that support the findings of this study are available from the corresponding author, AIM, on reasonable request.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. . Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86. 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 2.Smyth EC, Verheij M, Allum W, et al. . Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v38–49. 10.1093/annonc/mdw350 [DOI] [PubMed] [Google Scholar]
- 3.Kapoor N, Bassi A, Sturgess R, et al. . Predictive value of alarm features in a rapid access upper gastrointestinal cancer service. Gut 2005;54:40–5. 10.1136/gut.2004.039438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee S-W, Chang C-S, Yeh H-J, et al. . The diagnostic value of alarm features for identifying types and stages of upper gastrointestinal malignancies. Gastroenterology Res 2017;10:120–5. 10.14740/gr826w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Axon A. Symptoms and diagnosis of gastric cancer at early curable stage. Best Pract Res Clin Gastroenterol 2006;20:697–708. 10.1016/j.bpg.2006.03.015 [DOI] [PubMed] [Google Scholar]
- 6.National Cancer Institute SEER Cancer Stat Facts: Esophageal Cancer, 2019. Available: https://seer.cancer.gov/statfacts/html/esoph.html
- 7.National Cancer Institute Seer cancer STAT facts: stomach cancer, 2019. Available: https://seer.cancer.gov/statfacts/html/stomach.html
- 8.Eisenhauer EA, Therasse P, Bogaerts J, et al. . New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 9.Therasse P, Arbuck SG, Eisenhauer EA, et al. . New guidelines to evaluate the response to treatment in solid tumors. European organization for research and treatment of cancer, National cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205–16. 10.1093/jnci/92.3.205 [DOI] [PubMed] [Google Scholar]
- 10.Liedtke C, Rody A, Gluz O, et al. . The prognostic impact of age in different molecular subtypes of breast cancer. Breast Cancer Res Treat 2015;152:667–73. 10.1007/s10549-015-3491-3 [DOI] [PubMed] [Google Scholar]
- 11.Jørgensen JT, Hersom M. HER2 as a Prognostic Marker in Gastric Cancer - A Systematic Analysis of Data from the Literature. J Cancer 2012;3:137–44. 10.7150/jca.4090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu X, Cai H, Yu L, et al. . Prognostic significance of cancer family history for patients with gastric cancer: a single center experience from China. Oncotarget 2016;7:37305–18. 10.18632/oncotarget.9032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rochefort MM, Ankeny JS, Kadera BE, et al. . Impact of tumor grade on pancreatic cancer prognosis: validation of a novel TNMG staging system. Ann Surg Oncol 2013;20:4322–9. 10.1245/s10434-013-3159-3 [DOI] [PubMed] [Google Scholar]
- 14.Zheng Y, Cao X, Wen J, et al. . Smoking affects treatment outcome in patients with resected esophageal squamous cell carcinoma who received chemotherapy. PLoS One 2015;10:e0123246 10.1371/journal.pone.0123246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phipps AI, Shi Q, Limburg PJ, et al. . Alcohol consumption and prognosis in patients with stage III colon cancer: a correlative analysis of phase III trial NCCTG N0147 (Alliance). J Clin Oncol 2015;33:1508 10.1200/jco.2015.33.15_suppl.1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanba K, Chinen Y, Uchiyama H, et al. . Prognostic impact of a past or synchronous second cancer in diffuse large B cell lymphoma. Blood Cancer J 2018;8:1 10.1038/s41408-017-0043-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bang Y-J, Van Cutsem E, Feyereislova A, et al. . Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687–97. 10.1016/S0140-6736(10)61121-X [DOI] [PubMed] [Google Scholar]
- 18.Stephens MR, Lewis WG, White S, et al. . Prognostic significance of alarm symptoms in patients with gastric cancer. Br J Surg 2005;92:840–6. 10.1002/bjs.4984 [DOI] [PubMed] [Google Scholar]
- 19.Chau I, Fuchs CS, Ohtsu A, et al. . Association of quality of life with disease characteristics and treatment outcomes in patients with advanced gastric cancer: exploratory analysis of rainbow and regard phase III trials. Eur J Cancer 2019;107:115–23. 10.1016/j.ejca.2018.11.013 [DOI] [PubMed] [Google Scholar]
- 20.Diamantis G, Scarpa M, Bocus P, et al. . Quality of life in patients with esophageal stenting for the palliation of malignant dysphagia. World J Gastroenterol 2011;17:144–50. 10.3748/wjg.v17.i2.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim JW, Kim JG, Kang BW, et al. . Treatment patterns and changes in quality of life during first-line palliative chemotherapy in Korean patients with advanced gastric cancer. Cancer Res Treat 2019;51:223-239 10.4143/crt.2018.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ECOG-ACRIN ECOG performance status, 2019. Available: https://ecog-acrin.org/resources/ecog-performance-status
- 23.Kelly CM, Shahrokni A. Moving beyond Karnofsky and ECOG performance status assessments with new technologies. J Oncol 2016;2016:1–13. 10.1155/2016/6186543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Péus D, Newcomb N, Hofer S. Appraisal of the Karnofsky performance status and proposal of a simple algorithmic system for its evaluation. BMC Med Inform Decis Mak 2013;13:72 10.1186/1472-6947-13-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan S-Q, Nie R-C, Chen Y-M, et al. . Glasgow prognostic score is superior to ECOG PS as a prognostic factor in patients with gastric cancer with peritoneal seeding. Oncol Lett 2018;15:4193–200. 10.3892/ol.2018.7826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Datta SS, Ghosal N, Daruvala R, et al. . How do clinicians rate patient's performance status using the ECOG performance scale? A mixed-methods exploration of variability in decision-making in oncology. Ecancermedicalscience 2019;13:913 10.3332/ecancer.2019.913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sørensen JB, Klee M, Palshof T, et al. . Performance status assessment in cancer patients. An inter-observer variability study. Br J Cancer 1993;67:773–5. 10.1038/bjc.1993.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halpert RD, Spickler E, Feczko PJ. Dysphagia in patients with gastric cancer and a normal esophagram. Radiology 1985;154:589–91. 10.1148/radiology.154.3.3969457 [DOI] [PubMed] [Google Scholar]
- 29.Andreyev HJ, Norman AR, Oates J, et al. . Why do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies? Eur J Cancer 1998;34:503–9. 10.1016/S0959-8049(97)10090-9 [DOI] [PubMed] [Google Scholar]
- 30.Maconi G, Kurihara H, Panizzo V, et al. . Gastric cancer in young patients with no alarm symptoms: focus on delay in diagnosis, stage of neoplasm and survival. Scand J Gastroenterol 2003;38:1249–55. 10.1080/00365520310006360 [DOI] [PubMed] [Google Scholar]
- 31.Maconi G, Manes G, Porro G-B. Role of symptoms in diagnosis and outcome of gastric cancer. World J Gastroenterol 2008;14:1149–55. 10.3748/wjg.14.1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shitara K, Yatabe Y, Matsuo K, et al. . Prognosis of patients with advanced gastric cancer by HER2 status and trastuzumab treatment. Gastric Cancer 2013;16:261–7. 10.1007/s10120-012-0179-9 [DOI] [PubMed] [Google Scholar]
- 33.Ilhan-Mutlu A, Taghizadeh H, Beer A, et al. . Correlation of trastuzumab-based treatment with clinical characteristics and prognosis in HER2-positive gastric and gastroesophageal junction cancer: a retrospective single center analysis. Cancer Biol Ther 2018;19:169–74. 10.1080/15384047.2017.1414759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boku N. HER2-positive gastric cancer. Gastric Cancer 2014;17:1–12. 10.1007/s10120-013-0252-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol 2008;19:1523–9. 10.1093/annonc/mdn169 [DOI] [PubMed] [Google Scholar]
- 36.Maresch J, Schoppmann SF, Thallinger CMR, et al. . Her-2/neu gene amplification and over-expression in stomach and esophageal adenocarcinoma: from pathology to treatment. Crit Rev Oncol Hematol 2012;82:310–22. 10.1016/j.critrevonc.2011.06.003 [DOI] [PubMed] [Google Scholar]
- 37.Begnami MD, Fukuda E, Fregnani JHTG, et al. . Prognostic implications of altered human epidermal growth factor receptors (HERS) in gastric carcinomas: HER2 and HER3 are predictors of poor outcome. J Clin Oncol 2011;29:3030–6. 10.1200/JCO.2010.33.6313 [DOI] [PubMed] [Google Scholar]
- 38.Bowrey DJ, Griffin SM, Wayman J, et al. . Use of alarm symptoms to select dyspeptics for endoscopy causes patients with curable esophagogastric cancer to be overlooked. Surg Endosc 2006;20:1725–8. 10.1007/s00464-005-0679-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2019-000623supp001.pdf (16.3KB, pdf)
esmoopen-2019-000623supp002.pdf (28.6KB, pdf)
esmoopen-2019-000623supp003.pdf (21.8KB, pdf)
esmoopen-2019-000623supp005.pdf (18.7KB, pdf)
esmoopen-2019-000623supp004.pdf (18.5KB, pdf)
esmoopen-2019-000623supp006.pdf (51.8KB, pdf)