Abstract
A 64-year-old man presented to the emergency department with generalised fatigue and dizzy spells. His background history includes a previous admission with right leg deep vein thrombosis, provoked by pneumonia. Laboratory results showed evidence of hyponatremia and hyperkalaemia. A synacthen test was performed that indicated hypoadrenalism. CT of his abdomen revealed enlarged adrenal glands bilaterally. Adrenal antibodies and positron emission tomography (PET) scan were performed to assess the cause of enlarged adrenals. PET scan showed no evidence of increased uptake. Adrenal antibodies were found to be negative. Tuberculous (TB) adrenalitis was the principle differential diagnosis. TB QuantiFERON was strongly positive. Following 9 months of TB treatment, surveillance CT scan indicated a significant reduction in adrenal gland size. However, subsequent events culminated in a retrospective review of CT scans questioning the initial clinical diagnosis and suggesting that the observed adrenal gland enlargement was secondary to bilateral adrenal infarction and haemorrhage. Equally, the subsequently observed marked reduction in adrenal gland size was not secondary to an assumed response to TB therapy, but rather the sequela of infracted atrophied adrenal glands, as a manifestation of the underlying antiphospholipid syndrome (APS). The case highlights the importance of recognising adrenal insufficiency in patients with a history of APS. It also illustrates the role of multidisciplinary meetings in the management of such complex cases.
Keywords: adrenal disorders, tuberculosis, haematology (incl blood transfusion)
Background
Antiphospholipid syndrome (APS) is characterised by the presence of arterial or venous thrombosis. It is autoimmune in nature and manifests as a systemic disease. It can be primary in nature or in association with another autoimmune disease, usually systemic lupus erythematosus (SLE). Almost half of those presenting with APS have a primary disease, and around 35% show evidence of SLE.1 The diagnosis is made by the presence of antiphospholipid antibodies.2 These antibodies can be related to multiple thromboembolic events including the catastrophic variant of APS.3
In some rare instances, it may cause small vessel thrombosis hence leading to multiorgan failure. In this instance, it is referred to as catastrophic APS. It remains an extremely rare condition. A study concluded that of the 1000 patients with APS followed up over a 7-year period, it was found in only 0.8% of the cases.3
Adrenal vein haemorrhagic infarction, which is a sequel of adrenal vein thrombosis, ultimately leads to loss of adrenal function; it is one of the presentations of APS, especially with catastrophic type. In one analysis adrenal involvement was reported in only 13% of the reported cases of a catastrophic type of APS.4
Case presentation
A 64-year-old man presented to the emergency department with generalised fatigue, dizzy spells, nausea, vomiting and vague abdominal pain. He denied any preceding infections. He denied any history of weight loss or poor appetite.
His medical history included a previous admission with pneumonia complicated with right leg deep venous thrombosis. At that time a thrombophilia screen was performed and revealed positive anticardiolipin with serum level of >120 U/mL (0–12 U/mL) and anti-B-2 glycoprotein antibodies with serum level of 268 RU/mL (0–20 RU/mL). Hence, the patient was diagnosed with APS.
He had a family history of Reynaud’s phenomenon, affecting his biological daughter. He was a non-smoker and consumed up to 10 units of alcohol a week. From a functional status viewpoint, he was fully independent and worked as an administrator at a local university.
At the time of admission, he looked plethoric. His blood pressure was 90/45 mm Hg and a heart rate of 102 bpm. His cardiovascular examination was normal as was the respiratory examination. The abdominal exam was normal and there were no palpable masses. Lying and standing blood pressures were checked, no significant systolic drop in blood pressure was noted. The blood glucose level was 4.0 mmol/L (3.9–7.1 mmol/L).
Investigations
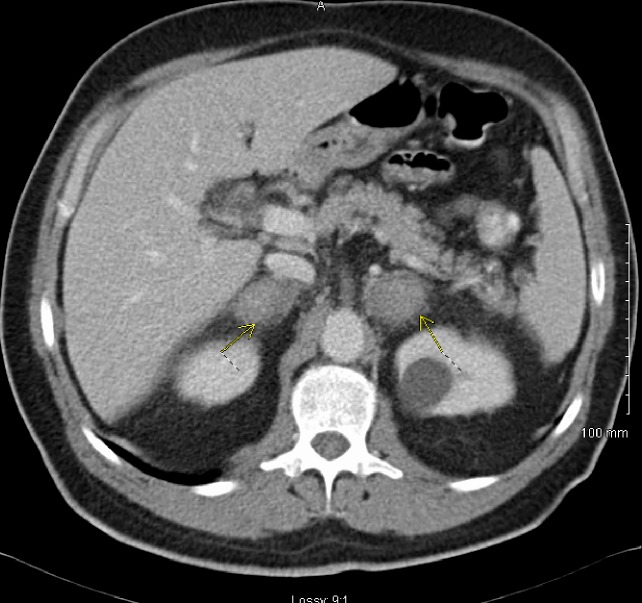
Initial investigations revealed a serum sodium level of 129 mmol/L (136–145 mmol/L) and potassium of 5.7 mmol/L (3.6–5.1). Activated partial thromboplastin time (APTT) of 72 s (28-40). Erythrocyte sedimentation rate of 73 mm/hour (1–14). Given the clinical presentation, the low sodium and the high potassium, a synacthen test was performed. Early morning (08:00) pre synacthen serum cortisol was 41 nmol/L (07:00–09:00 119–618 nmol/L) and post synacthen cortisol was 43 nmol/L (15:00–17:00 85–460 nmol/L), indicating no significant response to adrenocorticotropic hormone (ACTH). ACTH levels were recorded (08:00) to be 96 ng/L (<46 ng/L). Adrenal antibodies were negative. Abdominal ultrasound revealed a left kidney cyst measuring 3 cm in maximum diameter. This finding led to a CT scan of the abdomen that showed enlarged adrenal glands bilaterally (4.1 cm on the right and 3.9 cm on the left side) (figure 1). Urinary catecholamines and vanillylmandelic acid levels were requested; they were within the normal range.
Figure 1.
CT abdomen enlarged adrenal glands bilaterally measuring up to 4 cm.
A positron emission tomography scan was done to rule out a malignant cause of enlarged adrenal glands. It showed no fluorodeoxyglucose avid macroscopic disease.
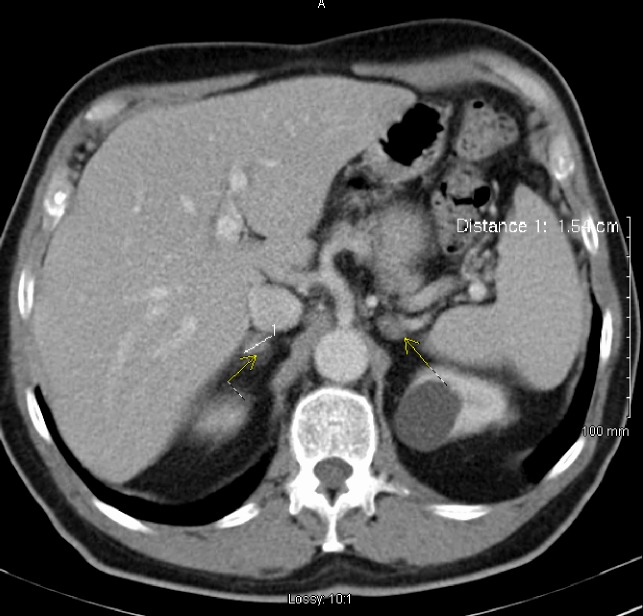
Serum QuantiFERON sample was requested as tuberculosis (TB) remained an important differential. It was strongly positive. Anti-TB treatment was started in view of evidence at hand. Regular follow-up plan was put in place. Five months after the initial scan, a repeat scan showed marked reduction in the size of adrenal glands from 4.1 cm on the right side to 1.3 cm and 3.9 cm to 1.1 cm on the left side (figure 2). After reviewing the serial scans at multidisciplinary team (MDT) discussion, the initial diagnosis was revised, and the MDT consensus led to a final diagnosis of haemorrhagic infarcts secondary to APS.
Figure 2.
CT abdomen dramatic reduction in size of adrenal glands bilaterally.
Autoantibody and vasculitis screen was requested to look for underlying associated autoimmune conditions. Antinuclear antibody was positive (titre 400). Cardiolipin IgG was positive with a serum level of >120 U/mL (0–12 U/mL) and anti-B2GP-1 IgG was also positive with a level of 268 RU/mL (0–20 RU/mL). Although anti-Sjogren syndrome-related antigen A (anti-SSa/RO) antibodies were weakly positive, there were no clinical signs to suggest SLE. The remaining autoantibodies screen including anti-double stranded DNA (anti-dsDNA), anti-Sjogren syndrome-related antigen B (anti-SSB/LA), anti-centromere protein B, anti-smith/ribonucleoprotein antibodies (anti-sm/RNP), anti-ribosomal-P-protein, anti-histones and anti-topoisomerase antibodies (anti-Scl70) were all negative.
Differential diagnosis
Initial symptoms of abdominal pain, dizziness and low blood pressure suggested underlying sepsis, likely biliary. However, CT of the abdomen did not show any gallbladder stone or inflammation, but it did reveal bilateral adrenal gland enlargement. The presence of hyponatremia and hyperkalaemia along with hypotension raised suspicion of hypoadrenalism which was confirmed with a failed synacthen test. Differential diagnosis at that point included Addison’s disease and TB adrenalitis.
Addison disease was ruled out as adrenal antibodies were not detected. TB adrenalitis, however, was considered given the strongly positive QuantiFERON. Although adrenal gland biopsy would have been more diagnostic, it was excluded due to the high risk of haemorrhage. As treatment started, subsequent scans showed a significant reduction in the size of adrenal glands bilaterally in a relatively short period.
The CT scans were then revisited. MDT concluded initial findings were representative of haemorrhagic infarcts within the adrenal glands. The greatly reduced size on subsequent scans were a sequela of thrombosis secondary to APS which was the most likely cause of adrenal insufficiency. Possible precipitating factors included infections and medications.
Treatment
Immediately following the confirmation of adrenal insufficiency diagnosis, the patient was started on high-dose intravenous hydrocortisone 200 mg four times a day which was switched after 48 hours to oral hydrocortisone and he remained on lifelong steroids thereafter. He was also educated about sick day rules and advised to wear a medical alert bracelet for future emergency situations.
Once the diagnosis of TB adrenalitis was made, he was started on anti-TB therapy.
He was followed up in the outpatients with regular surveillance scans. Subsequent scans showed a significant reduction in adrenal glands size. It was considered to be a result of successful anti-TB treatment. He continued on anti-TB therapy for 9 months. However, unfortunately, during this period, he developed peripheral neuropathy, which was attributed to the anti-TB treatment.
Two years later, he was diagnosed with a new right-sided femoral venous thrombosis. He was started on lifelong warfarin. Given the recurrent thrombotic events all previous scans were revisited in an MDT meeting. The consensus confirmed that initially enlarged adrenal glands were secondary to haemorrhagic infarcts within the glands.
Treatment also included hydroxychloroquine for the underlying APS, as recommended by the rheumatology team. He also received four cycles of rituximab along with intravenous immunoglobulins at a dose of 0.4 g/kg when he presented a few years later with multiorgan failure secondary to catastrophic APS with multiple shooting thrombi.
Outcome and follow-up
Our patient was placed on lifelong warfarin following recurrent thrombosis events. However, he presented 2 years later with a third event of unprovoked deep vein thrombosis (DVT) on warfarin. Doppler ultrasound revealed an extension of the previously documented left femoral vein thrombus. He was placed on low molecular weight heparin for 6 months and subsequently switched back to warfarin.
Four years later, he presented to the emergency department with cholelithiasis/choledocholithiasis, a diagnosis which was confirmed on ultrasound of his abdomen. Subsequently, he underwent endoscopic retrograde cholangiopancreatography (ERCP) for stone retrieval. However, that admission was complicated with multiorgan failure including acute kidney injury, severe liver injury, acute coronary syndrome and bilateral subsegmental pulmonary emboli. This presentation was all attributed at the time to a flare-up of underlying APS causing multiple shooting thrombi. This admission was also complicated further by a large retroperitoneal haemorrhage requiring 6 units of blood transfusion.
Three years later he presented again with dysarthria and left-sided motor deficit. CT brain showed subacute right thalamic and cerebral peduncle infarction. He was managed according to the national stroke management protocol.
He is currently on lifelong warfarin therapy with a target international normalised ratio 3.0–3.5. As warfarin remains the safest therapeutic option. To this date there are no randomised control trials available regarding the use of direct oral anticoagulant in the management of anti-phospholipid syndrome, and international guidelines continue to recommend warfarin in the majority of circumstances.
He is followed up in the anticoagulation clinic, endocrinology, rheumatology and cardiology clinics.
Discussion
In this report, we present a complicated case of primary adrenal insufficiency caused by bilateral adrenal glands thrombosis secondary to a catastrophic anti-phospholipid syndrome.
Primary adrenal insufficiency was described in early 1855 by Thomas Addison as destruction or dysfunction of the entire adrenal cortex which can affect the adrenal cortex synthetic function.5
TB was the most common cause, however, currently, TB accounts for only 7%–20%,6 of cases, while autoimmune diseases account for almost 70% of cases.7 Other causes include infections, malignancies and adrenal haemorrhage or infarction, which can be a complication of an underlying anti-phospholipids syndrome. However, this remains rare. In a retrospective study of primary adrenal insufficiency in children, adrenal haemorrhage contributed to only 3% of cases.8
This low prevalence, along with the misleading, false positive QuantiFERON led in our case to inappropriate initial diagnosis and treatment.
QuantiFERON test is useful screening test for TB. However a manufacturing error in the testing apparatus has been identified as a cause of false positive results in the USA in the last decade.9
Another case report highlighted cross reactivity with environmental bacteria mycobacterium gordonae.10
American centre for disease control in their fact sheet has stated that there are many limitations in the use of QuantiFERON test. These include improper handling or storage of samples leading to inaccurate results. It also states that the results cannot be accurate in the setting of immune compromised patients.
Anti-phospholipid syndrome is an autoimmune disorder characterised by circulating antiphospholipid antibodies, which can alter the coagulation pathway causing thrombosis by vascular inflammation. It tends to cause venous thrombosis more than arterial, especially veins of the calf, but the venous system in other organs can also be affected such as cerebral sinuses, hepatic and axillary; however, the adrenal vein and artery are rarely affected.11
Catastrophic APS is a small subset of APS characterised by a widespread thrombotic manifestation associated with multiorgan failure typically involving small blood vessels in multiple organs.12
Although adrenal insufficiency is a well-known rare manifestation of catastrophic anti-phospholipids, in our case, the APS preceded other events of the syndrome by several years. There is limited evidence in the literature about the prevalence of adrenal insufficiency presenting as an initial presentation of catastrophic APS. Espinosa and his colleagues reported in a literature review that in only 36% of cases, adrenal insufficiency was the initial presentation of anti-phospholipid syndrome.13
Abdominal pain was the main presentation in these patients 55%, followed by hypotension 54%, fever and vomiting (40%, 31%).14 Radiological finding in primary adrenal insufficiency caused by APS usually illustrates adrenal gland enlargement secondary to haemorrhage and histopathology may confirm the finding of haemorrhagic infarction and thrombosis in most of these patients.14
A computer-assisted literature search carried by presotto et al identified 20 patients presenting with primary adrenal failure as the first presentation of APS. Seventy-five per cent of those patients were man and the mean age was 41.6±20.5 and only one patient had adrenal failure as a component of catastrophic APS.15
The destruction of the adrenal cortex in APS is likely to be related to thrombosis of adrenal veins which subsequently leads to haemorrhagic infarction. The limited venous drainage of the adrenal gland drained by a single vein along with its complex vasculature makes it more susceptible to haemorrhagic damage with progressive increase in arterial blood pressure during vein thrombosis.16 Other possible mechanisms include autoimmune adrenal failure.
Our case highlights the importance of multidisciplinary teamwork in diagnosing such conditions. Our patient initially received anti-TB treatment for a provisional diagnosis of TB adrenalitis; however, multidisciplinary input by colleagues from radiology, haematology, rheumatology and endocrinology helped to achieve the appropriate diagnosis and management.
Moreover, early clinical recognition and having a high index of clinical suspicion is of significant importance in cases with an atypical history of progressive adrenal failure and abdominal pain or in patients with adrenal insufficiency and prolongation of the APTT on routine blood tests.
Learning points.
Physicians should have a high index of suspicion regarding the rare causes of hypoadrenalism as the correct diagnosis is essential.
Antiphospholipid syndrome should be considered as a possible cause of hypoadrenalism even though it is a very rare cause.
Haemorrhagic infarction should be considered as part of the differential of bilaterally enlarged adrenal glands
Tuberculous adrenalitis in immunocompetent patients is rare, and false positive QuantiFERON tests are not uncommon.
The need for a multidisciplinary approach is essential as highlighted in this case.
Acknowledgments
I acknowledge the participation of the patient and his family and other healthcare providers who have been involved in the care over the years.
Footnotes
Contributors: MW headed up the case report. DO helped manage the patient. MW and DO monitored patient follow up. SAW and MM summarised the patients note and wrote the manuscript. MW and DO analysed and revised the paper.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Cervera R, Serrano R, Pons-Estel GJ, et al. Morbidity and mortality in the antiphospholipid syndrome during a 10-year period: a multicentre prospective study of 1000 patients. Ann Rheum Dis 2015;74:1011–8. 10.1136/annrheumdis-2013-204838 [DOI] [PubMed] [Google Scholar]
- 2.Negrini S, Pappalardo F, Murdaca G, et al. The antiphospholipid syndrome: from pathophysiology to treatment. Clin Exp Med 2017;17:257–67. 10.1007/s10238-016-0430-5 [DOI] [PubMed] [Google Scholar]
- 3.Mendoza-Pinto C, García-Carrasco M, Cervera R. Role of infectious diseases in the antiphospholipid syndrome (including its catastrophic variant). Curr Rheumatol Rep 2018;20:62 10.1007/s11926-018-0773-x [DOI] [PubMed] [Google Scholar]
- 4.Cervera R, Piette J-C, Font J, et al. Antiphospholipid syndrome: clinical and immunologic manifestations and patterns of disease expression in a cohort of 1,000 patients. Arthritis Rheum 2002;46:1019–27. 10.1002/art.10187 [DOI] [PubMed] [Google Scholar]
- 5.Smans LCCJ, Zelissen PMJ. [Thomas Addison and the adrenal gland]. Ned Tijdschr Geneeskd 2012;156:A4788. [PubMed] [Google Scholar]
- 6.Soedarso MA, Nugroho KH, Meira Dewi KA. A case report: Addison disease caused by adrenal tuberculosis. Urol Case Rep 2018;20:12–14. 10.1016/j.eucr.2018.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elshimy G, Alghoula F, Jeong JM. Adrenal crisis. Treasure Island (FL): StatPearls, 2020. [Google Scholar]
- 8.Hsieh S, White PC. Presentation of primary adrenal insufficiency in childhood. J Clin Endocrinol Metab 2011;96:E925–8. 10.1210/jc.2011-0015 [DOI] [PubMed] [Google Scholar]
- 9.Slater M, Parsonnet J, Banaei N. Investigation of false-positive results given by the QuantiFERON-TB gold in-tube assay. J Clin Microbiol 2012;50:3105–7. 10.1128/JCM.00730-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gajurel K, Subramanian AK. False-Positive quantiferon TB-Gold test due to Mycobacterium gordonae. Diagn Microbiol Infect Dis 2016;84:315–7. 10.1016/j.diagmicrobio.2015.10.020 [DOI] [PubMed] [Google Scholar]
- 11.Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006;4:295–306. 10.1111/j.1538-7836.2006.01753.x [DOI] [PubMed] [Google Scholar]
- 12.Erkan D, Espinosa G, Cervera R. Catastrophic antiphospholipid syndrome: updated diagnostic algorithms. Autoimmun Rev 2010;10:74–9. 10.1016/j.autrev.2010.08.005 [DOI] [PubMed] [Google Scholar]
- 13.Espinosa G, Santos E, Cervera R, et al. Adrenal involvement in the antiphospholipid syndrome: clinical and immunologic characteristics of 86 patients. Medicine 2003;82:106–18. 10.1097/00005792-200303000-00005 [DOI] [PubMed] [Google Scholar]
- 14.Bicer B, Sisman P, Cander S, et al. Acute adrenal insufficiency due to adrenal hemorrhage complicating colorectal surgery: report of two cases and correlation with the antiphospholipid antibody syndrome. Endocrine Abstracts 2017;49:EP131 10.1530/endoabs.49.EP131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Presotto F, Fornasini F, Betterle C, et al. Acute adrenal failure as the heralding symptom of primary antiphospholipid syndrome: report of a case and review of the literature. Eur J Endocrinol 2005;153:507–14. 10.1530/eje.1.02002 [DOI] [PubMed] [Google Scholar]
- 16.Fox B. Venous infarction of the adrenal glands. J Pathol 1976;119:65–89. 10.1002/path.1711190202 [DOI] [PubMed] [Google Scholar]