Abstract
Objective
In this study, we established a risk scoring system using easily obtained clinical characteristics at the time of initiating palliative chemotherapy to predict accurate overall survival of patients with advanced gastric cancer after first-line treatment with fluoropyrimidine–platinum combination chemotherapy.
Methods
A total of 1733 patients treated at the Samsung Medical Center, Korea were included in the study, and clinicopathological and laboratory data were retrospectively analysed. The dataset was split into a training set (n=1156, 67%) and a validation set (n=577, 33%). Top-ranked variables were identified using the random forest survival algorithm and integrated into a Cox regression model, thereby constructing the scoring system for predicting the overall survival of patients with advanced gastric cancer.
Results
The following five variables were finally included in the scoring system: serum neutrophil–lymphocyte ratio, alkaline phosphatase level, albumin level, performance status and histologic differentiation. The scoring system determined four distinct risk groups in the validation dataset with median overall survival of 17.1 months (95% CI=14.9 to 20.5 months), 12.9 months (95% CI=11.4 to 14.6 months), 8.1 months (95% CI=5.3 to 12.3 months) and 3.9 months (95% CI=1.5 to 8.2 months), respectively. The area under the curve to estimate the discrimination performance of the scoring system was 66.1 considering 1 year overall survival.
Conclusions
We developed a simple and clinically useful predictive scoring model in a homogeneous population with advanced gastric cancer treated with fluoropyrimidine-containing and platinum-containing chemotherapy. However, additional independent validation will be required before the scoring model can be used commonly.
Keywords: advanced gastric cancer, scoring model, prognostic factor
Key questions.
What is already known about this subject?
Several prognostic models based on clinicopathological factors have predicted survival in patients with advanced gastric cancer undergoing palliative chemotherapy.
What does this study add?
A homogeneous population by limiting first-line treatment to fluoropyrimidine and platinum combination regimen were analysed.
A clinically useful predictive scoring model in a total of 1733 patients with AGC (advanced gastric cancer) treated with palliative chemotherapy was developed.
The scoring system determined four distinct risk groups with a median overall survival of 17.1, 12.9, 8.1 and 3.9 months, respectively.
How might this impact on clinical practice?
This system could help to identify patients who could benefit from chemotherapy, provide prognostic information for patients and rapidly determine patients who should participate in later-line clinical trials.
Introduction
Gastric cancer is the fifth most common malignancy and the third most common cause of cancer-related deaths worldwide.1 Resection with curative intent is indicated for patients with early-stage gastric cancer. However, more than two-thirds of the patients with gastric cancer are diagnosed with unresectable or advanced-stage disease, for which systemic therapy is the only treatment option.2 Despite the recent improvement in therapeutic options,3 4 the clinical response of patients with advanced gastric cancer to systemic treatment is often poor.
Several biomarkers have been evaluated for predicting the response to targeted agents or checkpoint inhibitors, including human epidermal growth factor receptor 2 (HER2), programmed death-ligand-1 (PD-L1), Epstein-Barr virus and the microsatellite instability (MSI) status. However, only a small subset of patients is known to have these well-established biomarkers, and these biomarkers are usually used in second-line or subsequent-line treatment until today. Actually, current guidelines recommend a combination of cytotoxic chemotherapy for systemic first-line treatment of advanced gastric cancer and that targeted agents be added for tumours with HER2 overexpression.5 Currently, first-line chemotherapy regimens usually contain a combination of a fluoropyrimidine (eg, 5-fluorouracil and capecitabine) and a platinum agent as the backbone (eg, cisplatin and oxaliplatin).
The outcomes of advanced gastric cancer differ widely among patients treated with first-line chemotherapy. Accordingly, the estimation of the prognosis and approximate survival time of individual patients is crucial for patient stratification and for clinicians to make clinical decisions. Although current guidelines recommend the fluoropyrimidine plus platinum combination as the preferred regimen among various chemotherapeutic agents for the initial systemic treatment of advanced gastric cancer, other attenuated chemotherapeutic agents—such as fluoropyrimidines or taxanes alone—are usually administered to patients with various poor prognostic factors such as old age, poor performance status or underlying comorbidities in clinical practice. Therefore, most studies that reported prognostic models for advanced gastric cancer so far included a combination of chemotherapies other than preferred regimens as first-line treatment.
Since Chau et al structured a prognostic index6 and validated the model prospectively in the population enrolled in the REAL-2 trial that evaluated the efficacy of capecitabine and oxaliplatin for untreated advanced esophagogastric cancer,7 a new prognostic index has been required to incorporate up-to-date laboratory and pathological test information in the era of trastuzumab. Accordingly, we aimed to construct and validate a risk scoring system based on easily obtained clinicopathological and laboratory parameters to predict the median overall survival and probability for 1 year survival in patients with advanced gastric cancer who initiated first-line chemotherapy with a combination of a fluoropyrimidine and a platinum agent according to the risk score.
Methods
Patients
Patients with advanced or metastatic gastric cancer who had received first-line chemotherapy containing fluoropyrimidine and platinum agents between January 2008 and October 2018 were identified from the patient database of the Samsung Medical Center, Seoul, South Korea (n=1733). Patients who received the following approved first-line regimens were eligible for inclusion: 5-fluorouracil plus leucovorin plus oxaliplatin; capecitabine or TS-1 plus cisplatin; and capecitabine plus oxaliplatin; as well as trastuzumab plus capecitabine plus cisplatin for HER2-positive gastric cancer. This study was approved by the Institutional Review Board of Samsung Medical Center (approval number: 2019-11-049) and the requirement for informed consent was waived.
Data extraction
Data was extracted from the Clinical Data Warehouse Darwin-C of Samsung Medical Center for this study. The following clinicopathological variables were extracted: age, sex, performance status, best overall response, HER2 positivity, histologic differentiation, previous gastrectomy history and the presence of peritoneal carcinomatosis. The following baseline laboratory results (ie, results obtained just before the administration of first-line chemotherapy) were extracted: white cell count; absolute neutrophil count (ANC); absolute lymphocyte count (ALC); neutrophil–lymphocyte ratio; platelet count; levels of haemoglobin, total protein, uric acid, alanine aminotransferase, blood urea nitrogen, creatinine, albumin, alkaline phosphatase (ALP) and total bilirubin; and the creatinine clearance rate (estimated from the Modification of Diet in Renal Disease equations). The chemotherapeutic regimen administered as first-line treatment was also identified. The whole data were split into two datasets by using the data-splitting method: two-thirds of the data were included in the training set and one-third in the validation set.
Data synthesis and statistical analysis
As most of the laboratory examination values were continuous variables, they needed to be converted into binary variables for easy analysis. The maximally selected rank statistics (maxstat) method was applied to determine the optimal cut-off values for the various laboratory results. The R package ‘maxstat’ repeatedly tests all possible cut-off points to identify the value with the maximum rank statistics, thereby providing the best separation into two groups in terms of contributing to the overall survival.
We selected the clinically relevant features by using a random survival forest algorithm to find predictive variables of overall survival after first-line chemotherapy. Variable importance (VIMP), which is a measure of how much worse the prediction would be if that variable was not available, was used to rank the predictors. A large VIMP value indicates that the variable has a significant predictive impact, and a VIMP value of 0 means that the variable contributes almost nothing to the predictive accuracy. The variable selection threshold in the VIMP method was set at 0.002 because any variable with a VIMP above 0.002 is unlikely to be noise.8 The most well-known predictive factor for overall survival, the best overall response, was introduced into the random survival forest model to verify the model’s performance. To reduce multicollinearity, among the factors with overlapping features (eg, neutrophil–lymphocyte ratio, ANC and ALC), only the variable with the highest VIMP value was included in the subsequent analysis.
Missing data were assumed to have occurred randomly, depending on the clinical parameters, and multiple imputations using chained equations were performed by using the ‘mice’ R package to generate 10 imputed datasets.9 After imputation, a multivariable Cox regression model was constructed using variables derived from the random forest survival algorithm.
The top-performing predictors extracted from the VIMP method were integrated into a Cox regression model if the p values of the variables were <0.1. The HR estimates were pooled from 10 imputed derivation datasets by using Rubin's rules.10 The score for each variable was determined according to the HR: the score was 1 if the HR was 1–1.49; the score was 2 if the HR was 1.5–2.49; and the score was 3 if the HR was 2.5–3.49.11 To assess the quality of the constructed score, calibration plots were generated for the predicted survival probability versus the observed survival frequencies as indicated by 1 year survival rate using the ‘riskRegression’ R package. The probability for 1 year overall survival and median overall survival according to the risk score was estimated based on randomly selected imputed training and validation datasets by using the Kaplan-Meier method. Pairwise comparisons among the risk score groups were also conducted by using the multiple log-rank tests.
Results
Patient characteristics
The baseline characteristics of the 1733 included patients are listed in online supplementary table S1. The descriptive statistics were not significantly different between the training set (n=1156) and the validation set (n=577). The characteristics of the randomly selected imputed dataset and the determined cut-off values for the continuous variables are shown in online supplementary table S2. In the training set, the mean patient age was 57.3 years, and the proportion of HER2 positivity was 11.8%. Most patients (58.1%) were treated with capecitabine plus oxaliplatin as first-line chemotherapy, and 7.1% of patients received chemotherapy plus trastuzumab, a HER2-targeting agent. The overall response rate, that is, the proportion of patients who achieved a complete or partial response as their best overall response, was 32.3%.
esmoopen-2020-000670supp001.pdf (551.3KB, pdf)
Variable identification
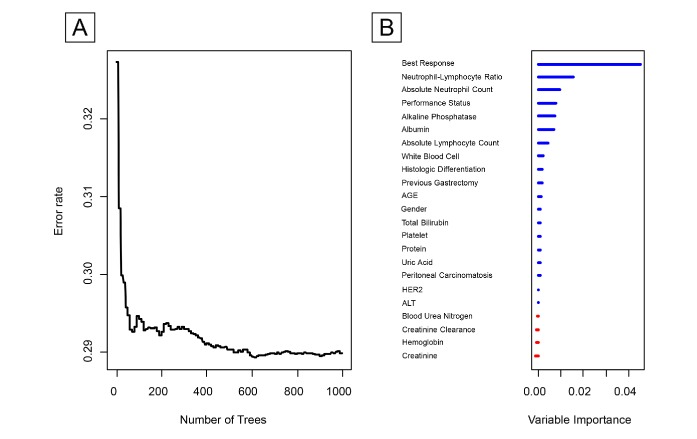
The variable selection process using the random survival forest is shown in figure 1. Plot A revealed that the error rate started to stabilise at approximately 600 generated trees. Plot B ranked the VIMP values of the covariates from high to low. The strongest predictor was the best overall response, as expected, which was included in the model to check the performance of the algorithm. The predictors with VIMP values above the threshold (>0.002) were the neutrophil–lymphocyte ratio, ANC, performance status, ALP level, albumin level, ALC, white cell count, histologic differentiation and previous gastrectomy, in that order.
Figure 1.
Random survival forests and the corresponding VIMP values for variables used for predicting overall survival of patients with advanced gastric cancer. (A) Error rates according to the number of trees generated in the simplified random forest survival algorithm. (B) VIMP value of the predictors ALT, alanine aminotransferase; HER2, human epidermal growth factor receptor 2; VIMP, variable importance.
Risk score construction
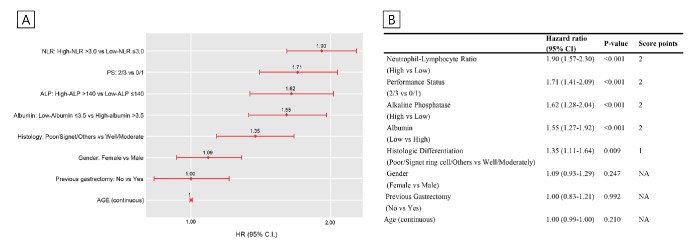
As the neutrophil–lymphocyte ratio, ANC, ALC and white cell count are related and can be derived from each other, only the top-ranked variable, that is, the neutrophil–lymphocyte ratio, was introduced in the Cox model. The next ranked covariates, that is, performance status, ALP level, albumin level, histologic differentiation and previous gastrectomy, were also included in the model. Moreover, age and sex were also integrated into the Cox model as clinically meaningful and adjusting covariates. The risk score was obtained from pooled estimates of the Cox regression models for multiple imputed datasets (figure 2). Among the included variables, the neutrophil–lymphocyte ratio (2 points), performance status (2 points), ALP level (2 points), albumin level (2 points) and histologic differentiation (1 point) were finally selected for the risk score construction, as they showed significant associations with overall survival.
Figure 2.
Clinical scoring system according to the HR using a multivariable COX regression model. (A) Association between variables extracted from RSF (random survival forest) and overall survival within 10 imputed derivation datasets. (B) The risk score construction according to HRs of the variables. ALP, alkaline phosphatase; NLR, neutrophil–lymphocyte ratio; PS, performance status.
Simplified risk score and validation
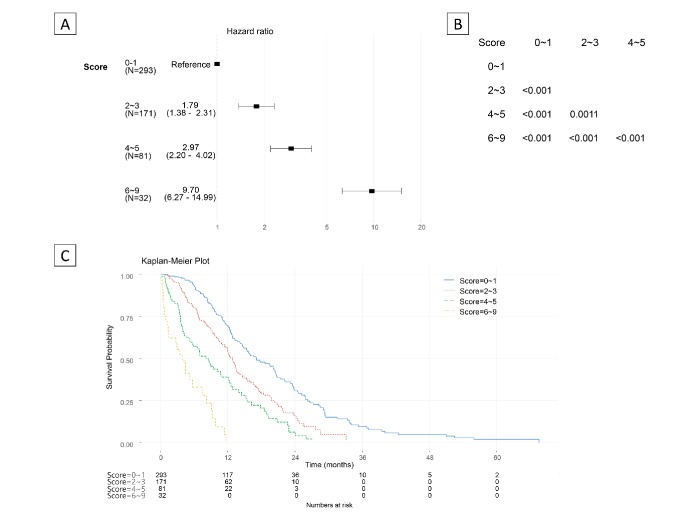
In order to simplify the scoring system, thereby making it clinically convenient to use, the score points with a range of 0– 9 were categorised into four groups, score 0–1, 2–3, 4–5 and 6–9, which maximised the log-rank statistics of pairwise comparison (figure 3A, B). Cox regression analysis using the risk score group as a covariate revealed that an increasing score was associated with an increasing HR in both the training and the validation datasets (Figure 3A, online supplementary figure S1) : score 2–3 (HR=1.79; 95% CI=1.38 to 2.31), score 4–5 (HR=2.97; 95% CI=2.20 to 4.02) and score 6–9 (HR=9.70; 95% CI=6.27 to 14.99), compared with score 0–1 as the reference group in the validation set. The Kaplan-Meier survival curve demonstrated clear separation among the four risk groups (Figure 3C, online supplementary figure S2). An increasing group score also corresponded with a decreasing duration of median survival and decreasing probability of 1 year overall survival (table 1). For a randomly selected training and validation dataset, the median overall survival ranged from 14.83 to 3.27 months and 17.13 to 3.87 months, respectively, according to the risk score group. The 1 year survival rate ranged from 62.8% to 12.9% in the training dataset and from 68.79% to not applicable in the validation dataset. The risk score group-based prediction of 1 year overall survival appeared to be well calibrated (online supplementary figure S3), and the area under the curve of the time-dependent receiver operating characteristic curve was 0.661 for the 1 year overall survival (online supplementary figure S4).
Figure 3.
HR by the risk score and pairwise comparisons using the log-rank test (validation set). (A) HR by the risk score group using the score 0–1 as the reference group. (B) P values for pairwise comparisons with the log-rank test. (C) Kaplan-Meier survival curve by the risk score of the validation dataset.
Table 1.
Median OS and probability for 1 year OS according to the risk score groups
| Training set | ||
| Median OS, month (95% CI) | 1 Year OS, % (95% CI) | |
| Score 0–1 | 14.83 (14.1 to 16.10) | 62.8 (58.4 to 67.6) |
| Score 2–3 | 11.93 (10.6 to 13.40) | 48.7 (42.6 to 55.6) |
| Score 4–5 | 7.9 (6.6 to 9.23) | 26.5 (19.1 to 36.7) |
| Score 6–9 | 3.27 (2.5 to 4.2) | 12.9 (6.9 to 23.9) |
| Validation set | ||
| Median OS, month (95% CI) | 1 Year OS, % (95% CI) | |
| Score 0–1 | 17.13 (14.93–20.53) | 68.79 (62.5 to 75.5) |
| Score 2–3 | 12.87 (11.40–14.63) | 53.0 (45.0 to 62.5) |
| Score 4–5 | 8.13 (5.27–12.30) | 37 (27.3 to 50.4) |
| Score 6–9 | 3.87 (1.53–8.23) | NA (NA-NA) |
OS, overall survival
Discussion
In the current study, we developed and validated a risk scoring system using the data of 1778 patients who received first-line systemic chemotherapy with a combination of a fluoropyrimidine and a platinum agent. We discovered the top-ranked predictors by using the variable selection method with the random survival forest algorithm and introduced these variables into the Cox regression model to construct the risk scoring system. The median overall survival and 1 year survival rate were clearly distinguished by the four risk score groups: 0–1, 2–3, 4–5 and 6–9, with significant survival differences.
In the current study, the Cox proportional hazard model and random survival forest algorithm were used in order to develop the risk score grouping. As recent studies on prediction modelling in oncology field mainly used high-throughput genomic data, the use of a random survival forest analysis for selecting features has started to attract attention. The random forest survival algorithm has outstanding performance among survival prediction models with high-dimensional variables by reducing the dimensionality of datasets, and it can manage complex interaction structures consisting of highly correlated variables.12 In addition, feature selection performance of the algorithm is a crucial advantage when the input dataset of the prediction model consists of complex or high-throughput data.13 We used this machine-learning technique for selecting the features and incorporated the selected top-ranked variables into the Cox regression model, which is frequently used in the clinical field and produces results that are easy to interpret.
To date, the HER2 status is the only validated predictive biomarker for molecular-targeted treatment for advanced gastric cancer. Several studies have evaluated the association between HER2 overexpression and prognosis of patients with advanced gastric cancer.14–16 Inconsistent findings of the prognostic effect of HER2 positivity on gastric cancer have been reported to date, unlike that observed for breast cancer. In the current study, HER2 positivity was not included in the construction process of the risk scoring system, as it was not a top-ranked predictor considering the VIMP results, which was attributed to the fact that 82 (60.2%) of 136 patients with HER2-positive disease received the trastuzumab-containing regimen as first-line treatment in the training dataset. Thus, it is considered that HER2 positivity is not a significant prognostic factor when patients with HER2-positive tumours receive chemotherapy containing HER2-targeting agent.
The following five significant poor prognostic factors were identified for predicting overall survival: high neutrophil–lymphocyte ratio (>3.0), poor performance status (2 or 3), high ALP level (>140 IU), low albumin level (≤3.5 g/dL) and poor/signet ring cell/others histology. The inflammatory response to tumours is a prognostic indicator in various cancer types including advanced gastric cancer.17 In the variable selection process used in the current study, other inflammatory markers including ANC, ALC and total white cell count were also ranked high, but the neutrophil–lymphocyte ratio was the highest performing variable in the process, consistent with previous findings. However, the result regarding the significance of the neutrophil–lymphocyte ratio should be interpreted cautiously in patients with concurrent infection and in those receiving corticosteroid treatment. In addition, the performance status is a well-known important factor for predicting response and survival in most cancer types, with the pooled analysis from three randomised controlled trials showing this result in patients with metastatic gastric cancer.6 Finally, the ALP level, albumin level, and histologic differentiation were other factors identified even in previous studies.18–20
In the study by Chau et al, the model divided prognostic group into good, moderate and poor risk groups and the median survival times were 12.7, 8.6 and 4.3 months, respectively.7 In another study by Custodio et al, median overall survival was 5.7, 9.4, 14 months for high-risk, intermediate-risk and low-risk groups, respectively.19 The scoring system in our study generated four risk groups and median overall survival of 17.1, 12.9, 8.1, 3.9 months, respectively, which was comparable with above studies considering that supportive care as well as chemotherapy have evolved over time.
Biomarker-driven systemic treatment, which targets a driver mutation of tumours, has been visualised in various types of cancer including gastric cancer. In a recent large-scale umbrella trial, researchers classified patients with advanced gastric cancer on the basis of the clinical sequencing results, assigned patients to each biomarker group and compared the outcomes between the biomarker group and the conventional chemotherapy group.21 Patients who received biomarker-driven treatment for the specific genetic change in their tumour showed significantly longer survival than those who received conventional chemotherapy. This result was encouraging because biomarker-driven treatment has reached palliative second-line treatment for advanced gastric cancer. Therefore, it is increasingly important to screen patients who are expected to be unresponsive to first-line chemotherapy and to actively enrol those patients in biomarker-driven trials, and our study results could be helpful for clinical decision-making in this context.
The current study has some inherent limitations. First, despite the large number of patients analysed, the study was limited in the scalability of the result interpretation owing to the single-centre, retrospective design, and single ethnicity of the study population. In order to achieve more generality of the model, additional independent validation from larger advanced gastric cancer cohorts should be required. Second, although the survival gain after second-line treatment was proven in patients with advanced gastric cancer,4 22 our model did not consider the effect and type of second-line or subsequent-line chemotherapy. However, as the factors associated with survival at the start of first-line chemotherapy were the major features of the study, second-line or subsequent-line treatment was an undefined factor and was not included in the model construction. Third, we did not include other crucial factors that could affect the prognosis, such as genetic mutational profiles, although the purpose of this study was to construct a prognostic model using clinically easy-to-use variables.
In conclusion, we developed and validated a newly constructed risk scoring system to predict the overall survival of patients with advanced gastric cancer undergoing first-line fluoropyrimidine and platinum-based combination chemotherapy. This system could help with clinical decision-making, such as in identifying patients who could benefit from chemotherapy, providing prognostic information for patients and rapidly determining patients who should participate in later-line clinical trials. Moreover, we plan to use the scoring system in our electronic medical record system to validate whether the score can predict survival accurately in a prospective cumulative registry, which could result in the use of this scoring classification as a computerised clinical decision support system in clinical settings. The MSI status, tumour mutational burden, and PD-L1 expression will also be integrated into the scoring system in future to construct a more robust prediction system.
Footnotes
Contributors: All authors had full access to all of the study data and take responsibility for the integrity of the data and accuracy of the data analysis. JK and JL helped in the study concept and design. JK, JYJ, STK and SHP performed the drafting of the manuscript. All authors helped in the acquisition, analysis or interpretation of data and critical revision of the manuscript for importantintellectual content. Statistical analysis was performed by JK, SYJ, JSC, DKC and SWS. Study supervision was done by WKK, SWS and JL.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request. The data that support the findings of this study are available on request from the corresponding author, JL.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. . Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Wöhrer SS, Raderer M, Hejna M. Palliative chemotherapy for advanced gastric cancer. Ann Oncol 2004;15:1585–95. 10.1093/annonc/mdh422 [DOI] [PubMed] [Google Scholar]
- 3.Bang Y-J, Van Cutsem E, Feyereislova A, et al. . Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687–97. 10.1016/S0140-6736(10)61121-X [DOI] [PubMed] [Google Scholar]
- 4.Wilke H, Muro K, Van Cutsem E, et al. . Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (rainbow): a double-blind, randomised phase 3 trial. Lancet Oncol 2014;15:1224–35. 10.1016/S1470-2045(14)70420-6 [DOI] [PubMed] [Google Scholar]
- 5.Smyth EC, Verheij M, Allum W, et al. . Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v38–49. 10.1093/annonc/mdw350 [DOI] [PubMed] [Google Scholar]
- 6.Chau I, Norman AR, Cunningham D, et al. . Multivariate prognostic factor analysis in locally advanced and metastatic esophago-gastric cancer--pooled analysis from three multicenter, randomized, controlled trials using individual patient data. J Clin Oncol 2004;22:2395–403. 10.1200/JCO.2004.08.154 [DOI] [PubMed] [Google Scholar]
- 7.Chau I, Ashley S, Cunningham D. Validation of the Royal Marsden hospital prognostic index in advanced esophagogastric cancer using individual patient data from the REAL 2 study. J Clin Oncol 2009;27:e3–4. 10.1200/JCO.2009.22.0863 [DOI] [PubMed] [Google Scholar]
- 8.Ishwaran H, Kogalur UB, Blackstone EH, et al. . Random survival forests. Ann Appl Stat 2008;2:841–60. 10.1214/08-AOAS169 [DOI] [Google Scholar]
- 9.Gelman A, Hill J. Multiple imputation with diagnostics (MI) in R: opening windows into the black box. J Stat Softw 2011;45. [Google Scholar]
- 10.Marshall A, Altman DG, Holder RL, et al. . Combining estimates of interest in prognostic modelling studies after multiple imputation: current practice and guidelines. BMC Med Res Methodol 2009;9:57 10.1186/1471-2288-9-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta HB, Mehta V, Girman CJ, et al. . Regression coefficient-based scoring system should be used to assign weights to the risk index. J Clin Epidemiol 2016;79:22–8. 10.1016/j.jclinepi.2016.03.031 [DOI] [PubMed] [Google Scholar]
- 12.Boulesteix A-L, Janitza S, Kruppa J, et al. . Overview of random forest methodology and practical guidance with emphasis on computational biology and bioinformatics. WIREs Data Mining Knowl Discov 2012;2:493–507. 10.1002/widm.1072 [DOI] [Google Scholar]
- 13.Wang W, Liu W. Integration of gene interaction information into a reweighted random survival forest approach for accurate survival prediction and survival biomarker discovery. Sci Rep 2018;8:13202 10.1038/s41598-018-31497-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park DI, Yun JW, Park JH, et al. . HER-2/neu amplification is an independent prognostic factor in gastric cancer. Dig Dis Sci 2006;51:1371–9. 10.1007/s10620-005-9057-1 [DOI] [PubMed] [Google Scholar]
- 15.Janjigian YY, Werner D, Pauligk C, et al. . Prognosis of metastatic gastric and gastroesophageal junction cancer by HER2 status: a European and USA international collaborative analysis. Ann Oncol 2012;23:2656–62. 10.1093/annonc/mds104 [DOI] [PubMed] [Google Scholar]
- 16.Shitara K, Yatabe Y, Matsuo K, et al. . Prognosis of patients with advanced gastric cancer by HER2 status and trastuzumab treatment. Gastric Cancer 2013;16:261–7. 10.1007/s10120-012-0179-9 [DOI] [PubMed] [Google Scholar]
- 17.Cho IR, Park JC, Park CH, et al. . Pre-treatment neutrophil to lymphocyte ratio as a prognostic marker to predict chemotherapeutic response and survival outcomes in metastatic advanced gastric cancer. Gastric Cancer 2014;17:703–10. 10.1007/s10120-013-0330-2 [DOI] [PubMed] [Google Scholar]
- 18.Lee J, Lim T, Uhm JE, et al. . Prognostic model to predict survival following first-line chemotherapy in patients with metastatic gastric adenocarcinoma. Ann Oncol 2007;18:886–91. 10.1093/annonc/mdl501 [DOI] [PubMed] [Google Scholar]
- 19.Custodio A, Carmona-Bayonas A, Jiménez-Fonseca P, et al. . Nomogram-based prediction of survival in patients with advanced oesophagogastric adenocarcinoma receiving first-line chemotherapy: a multicenter prospective study in the era of trastuzumab. Br J Cancer 2017;116:1526–35. 10.1038/bjc.2017.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ter Veer E, van Kleef JJ, Schokker S, et al. . Prognostic and predictive factors for overall survival in metastatic oesophagogastric cancer: a systematic review and meta-analysis. Eur J Cancer 2018;103:214–26. 10.1016/j.ejca.2018.07.132 [DOI] [PubMed] [Google Scholar]
- 21.Lee J, Kim ST, Kim K, et al. . Tumor genomic profiling guides patients with metastatic gastric cancer to targeted treatment: the VIKTORY umbrella trial. Cancer Discov 2019;9:CD-19-0442–1405. 10.1158/2159-8290.CD-19-0442 [DOI] [PubMed] [Google Scholar]
- 22.Kim HS, Kim HJ, Kim SY, et al. . Second-Line chemotherapy versus supportive cancer treatment in advanced gastric cancer: a meta-analysis. Ann Oncol 2013;24:2850–4. 10.1093/annonc/mdt351 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2020-000670supp001.pdf (551.3KB, pdf)