Abstract
Background
Oesophageal cancer is the sixth most common cause of cancer‐related mortality in the world. Currently surgery is the recommended treatment modality when possible. However, it is unclear whether non‐surgical treatment options is equivalent to oesophagectomy in terms of survival.
Objectives
To assess the benefits and harms of non‐surgical treatment versus oesophagectomy for people with oesophageal cancer.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, Science Citation Index, ClinicalTrials.gov, and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) up to 4th March 2016. We also screened reference lists of included studies.
Selection criteria
Two review authors independently screened all titles and abstracts of articles obtained from the literature searches and selected references for further assessment. For these selected references, we based trial inclusion on assessment of the full‐text articles.
Data collection and analysis
Two review authors independently extracted study data. We calculated the risk ratio (RR) with 95% confidence interval (CI) for binary outcomes, the mean difference (MD) or the standardised mean difference (SMD) with 95% CI for continuous outcomes, and the hazard ratio (HR) for time‐to‐event outcomes. We performed meta‐analyses where it was meaningful.
Main results
Eight trials, which included 1132 participants in total, met the inclusion criteria of this Cochrane review. These trials were at high risk of bias trials. One trial (which included five participants) did not contribute any data to this Cochrane review, and we excluded 13 participants in the remaining trials after randomisation; this left a total of 1114 participants, 510 randomised to non‐surgical treatment and 604 to surgical treatment for analysis. The non‐surgical treatment was definitive chemoradiotherapy in five trials and definitive radiotherapy in three trials. All participants were suitable for major surgery. Most of the data were from trials that compared chemoradiotherapy with surgery. There was no difference in long‐term mortality between chemoradiotherapy and surgery (HR 0.88, 95% CI 0.76 to 1.03; 602 participants; four studies; low quality evidence). The long‐term mortality was higher in radiotherapy than surgery (HR 1.39, 95% CI 1.18 to 1.64; 512 participants; three studies; very low quality evidence). There was no difference in long‐term recurrence between non‐surgical treatment and surgery (HR 0.96, 95% CI 0.80 to 1.16; 349 participants; two studies; low quality evidence). The difference between non‐surgical and surgical treatments was imprecise for short‐term mortality (RR 0.39, 95% CI 0.11 to 1.35; 689 participants; five studies; very low quality evidence), the proportion of participants with serious adverse in three months (RR 0.61, 95% CI 0.25 to 1.47; 80 participants; one study; very low quality evidence), and proportion of people with local recurrence at maximal follow‐up (RR 0.89, 95% CI 0.70 to 1.12; 449 participants; three studies; very low quality evidence). The health‐related quality of life was higher in non‐surgical treatment between four weeks and three months after treatment (Spitzer Quality of Life Index; MD 0.93, 95% CI 0.24 to 1.62; 165 participants; one study; very low quality evidence). The difference between non‐surgical and surgical treatments was imprecise for medium‐term health‐related quality of life (three months to two years after treatment) (Spitzer Quality of Life Index; MD −0.95, 95% CI −2.10 to 0.20; 62 participants; one study; very low quality of evidence). The proportion of people with dysphagia at the last follow‐up visit prior to death was higher with definitive chemoradiotherapy compared to surgical treatment (RR 1.48, 95% CI 1.01 to 2.19; 139 participants; one study; very low quality evidence).
Authors' conclusions
Based on low quality evidence, chemoradiotherapy appears to be at least equivalent to surgery in terms of short‐term and long‐term survival in people with oesophageal cancer (squamous cell carcinoma type) who are fit for surgery and are responsive to induction chemoradiotherapy. However, there is uncertainty in the comparison of definitive chemoradiotherapy versus surgery for oesophageal cancer (adenocarcinoma type) and we cannot rule out significant benefits or harms of definitive chemoradiotherapy versus surgery. Based on very low quality evidence, the proportion of people with dysphagia at the last follow‐up visit prior to death was higher with definitive chemoradiotherapy compared to surgery. Based on very low quality evidence, radiotherapy results in less long‐term survival than surgery in people with oesophageal cancer who are fit for surgery. However, there is a risk of bias and random errors in these results, although the risk of bias in the studies included in this systematic review is likely to be lower than in non‐randomised studies.
Further trials at low risk of bias are necessary. Such trials need to compare endoscopic treatment with surgical treatment in early stage oesophageal cancer (carcinoma in situ and Stage Ia), and definitive chemoradiotherapy with surgical treatments in other stages of oesophageal cancer, and should measure and report patient‐oriented outcomes. Early identification of responders to chemoradiotherapy and better second‐line treatment for non‐responders will also increase the need and acceptability of trials that compare definitive chemoradiotherapy with surgery.
Plain language summary
Non‐surgical treatment versus surgical treatment for oesophageal (gullet or food‐pipe) cancer
Review question
Is non‐surgical treatment equivalent to surgical treatment for treatment of people with oesophageal cancer (cancer of the gullet or food pipe)?
Background
Oesophageal cancer is the sixth most frequent cause of cancer‐related death in the world and is becoming more common. Treatment and survival depends upon the extent of cancer. When the cancer is limited to the gullet and the person is fit to undergo major surgery, surgical removal of the oesophagus (oesophagectomy) is currently the recommended treatment. Additional chemotherapy (use of chemicals to selectively destroy cancer) and radiotherapy (use of X‐rays to destroy cancer) may be given in addition to surgery in some people with oesophageal cancer. However chemotherapy, radiotherapy, or a combination of the two (chemoradiotherapy) can be used alone without surgery but are currently only recommended in people who are unfit for major surgery because of their general condition. Chemoradiotherapy on its own may cause such side effects as severe kidney damage, infection, and vomiting, but is less invasive than oesophagectomy, and may result in a shorter hospital stay and reduced risk of death. Oesophagectomy may carry the significant potential side effects of surgical site infection, the narrowing and breakdown of tissue where the cut end of the oesophagus is joined to the bowel, pneumonia, and difficulty swallowing. The death rate may also be higher, particularly when performed in smaller centres. It is unclear whether non‐surgical treatment may be as effective as surgery in cure of cancer.
Study characteristics
Eight studies met the inclusion criteria of this Cochrane review, and seven studies provided information for the review. The non‐surgical treatment was chemoradiotherapy only in five studies and radiotherapy only in three studies. We included a total of 1114 participants undergoing non‐surgical treatment (510 participants) or surgical treatment (604 participants) in the various analysis in the seven studies that provided information. Methods similar to tossing a coin were used to decide whether a participant received non‐surgical treatment or surgical treatment and ensure that the participants in the two groups were similar. Most trials included people who were healthy in aspects other than the condition requiring surgery. The evidence is current up to 4th March 2016.
Key results
Most information was from trials that compared chemoradiotherapy with surgery. There was no difference in long‐term deaths between chemoradiotherapy and surgery in people with oesophageal cancer who are fit for surgery. More people died in radiotherapy than surgery in people with oesophageal cancer who are fit for surgery in the long‐term. There was no difference in long‐term cancer recurrence between non‐surgical treatment and surgery. The difference between non‐surgical and surgical treatments were imprecise for short‐term deaths, the percentage of participants with serious adverse in three months, and the percentage of participants who had recurrence of cancer in and around the food‐pipe. The health‐related quality of life (covering aspects such as activity, daily living, health, support of family and friends, and outlook) was higher in non‐surgical treatment between four weeks and three months after treatment, although it is unclear what this difference means to the patient. The difference between non‐surgical and surgical treatments were imprecise for medium‐term health‐related quality of life (three months to two years after treatment). Chemoradiotherapy only appears to be at least equivalent to surgery in terms of short‐term and long‐term survival in people with one type of oesophageal cancer called squamous cell cancer and who are fit for surgery. There is more uncertainty in the comparison of chemoradiotherapy only versus surgery for another type of oesophageal cancer called adenocarcinoma, and we cannot rule out significant benefits or harms of definitive chemoradiotherapy versus surgery in this type of oesophageal cancer. More people had difficulty in swallowing prior to their death after chemoradiotherapy treatment compared to surgical treatment.
Radiotherapy only results in less long‐term survival than surgery (about 40% increase risk of deaths). Further well‐designed studies that measure outcomes that are important for patients are necessary.
Quality of the evidence
The quality of evidence was low or very low because the included studies were small and had errors in study design. As a result, there is a lot of uncertainty regarding the results.
Summary of findings
Background
Description of the condition
See Appendix 1 for a glossary of terms we have used in the Background.
Oesophageal cancer is the sixth most common cause of cancer‐related mortality in the world with an incidence varying from an age‐standardised annual incidence rate of less than one per 100,000 population in parts of Western Africa (Nigeria, Guinea, and Guinea‐Bissau) to an age‐standardised annual incidence rate of 17 to 24 per 100,000 population in parts of Eastern Africa, such as Malawi and Kenya, and parts of Central Asia (Turkmenistan) and East Asia (Mongolia) (IARC 2014); and worldwide incidence is thought to be increasing (Pennathur 2013). In 2012, there were about 455,000 new people diagnosed with oesophageal cancer and 400,000 deaths due to oesophageal cancer globally (IARC 2014). Treatment depends on the cancer stage. The American Joint Committee on Cancer (AJCC) oesophageal cancer staging system is used for this purpose (AJCC Cancer Staging Manual 2010; Rice 2010). The AJCC staging system takes into account several factors: involvement of tissue layers by the tumour (T), involvement of nodes (N), presence of metastases (M) (TNM classification), grade of the tumour (G), and histological type (AJCC Cancer Staging Manual 2010; Rice 2010; Stahl 2013). Whilst there are two histological types, namely squamous cell carcinoma and adenocarcinoma, there is only a marginal difference in their management (Berry 2014). Presence of metastases, on the other hand, has a large impact on treatment. If present, the cancer is stage IV and only palliative treatment is possible (Stahl 2013). In the absence of metastases and when the person is fit for surgery, endoscopic treatment or surgical treatment is recommended for carcinoma in situ and stage Ia oesophageal cancer (Stahl 2013). For other stages without metastases, surgical treatment with or without perioperative chemoradiotherapy depending upon the tumour stage and resection status is recommended when the person is fit for surgery (Stahl 2013). Five‐year survival depends on the stage, and ranges from 70% in Stage Ia squamous cell carcinoma and 80% in Stage Ia adenocarcinoma to 15% in Stage IV squamous cell carcinoma or adenocarcinoma (AJCC Cancer Staging Manual 2010; Rice 2010).
Description of the intervention
Surgical intervention involves mobilisation of the upper and lower oesophageal tract, oesophagectomy, and reconstruction of the oesophageal tube using a section of colon or bowel. There are two main methods to achieve this: transthoracic oesophagectomy and transhiatal oesophagectomy. Transthoracic oesophagectomy involves an abdominal incision followed by a thoracic incision to complete the oesophagectomy. A third incision may be used in the neck (McKeown 1976). Transhiatal oesophagectomy only requires an abdominal and cervical incision (Orringer 1978; Yamamoto 2013). Minimally‐invasive alternatives are laparoscopic or thoracoscopic adaptations of open oesophagectomy (Cuschieri 1992). Abdominal and thoracic incisions can be replaced by a series of 5 mm to 10 mm portholes for laparoscopic or thoracoscopic instruments (Luketich 2000; Yamamoto 2013). Chemoradiotherapy in conjunction with surgical intervention has been established as an effective treatment option for people with oesophageal cancer (van Hagen 2012), and is recommended for all people with node‐positive adenocarcinoma or adenocarcinoma that extends beyond muscularis propria undergoing surgical resection; and for people with squamous cell carcinoma who have undergone incomplete surgical resection (Berry 2014; Stahl 2013). Definitive chemoradiotherapy (chemoradiotherapy as the sole treatment with curative intent) is currently only advocated in people with localised cancer of the oesophagus who are unfit for surgery (Stahl 2013). Definitive chemoradiotherapy normally involves a 5‐fluorouracil and cisplatin chemotherapy treatment alongside a radiation dose of 46 to 65 grays (Bedenne 2007; Chiu 2005; Stahl 2005). There is a second alternative to surgical treatment for early stage oesophageal cancer (carcinoma in situ and stage Ia oesophageal cancer): endoscopic mucosal resection involves removing the cancerous tissue from within the oesophagus using endoscopic access (Stahl 2013). Only the affected tissue is removed, avoiding complete resection of the oesophagus which requires an open surgical procedure. A variation of the endoscopic mucosal resection is the endoscopic submucosal dissection, which involves injecting saline and dissecting the submucosal connective tissue just beneath the lesion from the muscularis propria (Ishihara 2008). The complication rates are considered to be low with endoscopic submucosal dissection (Sun 2014).
How the intervention might work
Surgery, endoscopic mucosal resection, and endoscopic submucosal dissection work by removal of the cancer tissue. Definitive chemoradiotherapy destroys the cancer cells using radiation and substances which are toxic to cells.
Why it is important to do this review
Definitive chemoradiotherapy may be comparable to surgery in locally‐advanced, non‐metastatic oesophageal squamous cell carcinoma (Bedenne 2007; Chiu 2005; Stahl 2005). Whilst associated with some side effects (renal toxicity, infection, and vomiting), definitive chemoradiotherapy is less invasive than oesophagectomy. Whether surgery is actually superior to definitive chemoradiotherapy is unclear (Yamashita 2008). Oesophagectomy carries a significant morbidity risk (such as surgical site infections, pneumonia, anastomotic stenosis, anastomotic dehiscence, intra‐abdominal abscess, and dysphagia) and mortality risk, particularly in smaller centres (Bedenne 2007; Goh 2015; Migliore 2007). Definitive chemoradiation may reduce mortality and may result in a shorter hospital stay compared to surgery. The same may be true for endoscopic mucosal resection. Since it does not rely on complete removal of the oesophagus, it is much less invasive. Therefore, these less invasive procedures may be preferred by people with oesophageal cancer, their families and carers, and healthcare providers if these procedures are as effective as surgery in terms of long‐term survival. The most recent National Comprehensive Cancer Network guidelines on treatment of oesophageal cancer reflect this and therefore allow a spectrum of treatment options that involve surgery, surgery with chemoradiotherapy, chemoradiotherapy alone, and endoscopic mucosal resection (Ajani 2011). This Cochrane review aims to assess the comparative roles of surgical and non‐surgical management in people with different stages of oesophageal cancer in order to develop treatment pathways to streamline clinical decisions.
Objectives
To assess the benefits and harms of non‐surgical treatment versus oesophagectomy for people with oesophageal cancer. In particular we planned to investigate the effects by participant groups (such as cancer stage and cancer type) and by intervention types (such as definitive chemoradiotherapy, definitive radiotherapy, and endoscopic treatment).
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs). We included studies reported as full‐text articles, those published as abstracts only and unpublished data, irrespective of the language in which they were published.
Types of participants
We included adults undergoing treatment for stages I to III oesophageal cancer (squamous cell carcinoma or adenocarcinoma) in the hospital setting (including palliative treatment centres such as hospices).
Types of interventions
We included trials that compared oesophagectomy (irrespective of whether it was performed by open method, laparoscopic method, or minimally invasive method and whether adjuvant chemotherapy, adjuvant radiotherapy, or adjuvant chemoradiotherapy was used) of any type with solely non‐surgical treatment for oesophageal cancer. Non‐surgical treatment included definitive chemoradiotherapy, endoscopic mucosal resection, and endoscopic submucosal dissection.
We aimed to perform the following comparisons and two meta‐analyses.
Definitive chemoradiotherapy, definitive chemotherapy, or definitive radiotherapy versus oesophagectomy.
Endoscopic treatment versus oesophagectomy.
However we only found trials that compared definitive chemoradiotherapy or definitive radiotherapy versus oesophagectomy. Therefore we only completed the first comparison.
Types of outcome measures
Primary outcomes
-
All‐cause mortality.
Short‐term mortality (in‐hospital mortality or mortality within three months).
Long‐term mortality.
-
Serious adverse events (within three months). We planned to accept the following definitions of serious adverse events.
ICH‐GCP International Conference on Harmonisation ‐ Good Clinical Practice guideline (ICH‐GCP 1996): serious adverse events defined as any untoward medical occurrence that results in death, is life‐threatening, requires inpatient hospitalisation or prolongation of existing hospitalisation, or results in persistent or significant disability/incapacity.
Other variations of ICH‐GCP classifications such as the U.S. Food and Drug Administration (FDA) classification (FDA 2006), or Medicines and Healthcare products Regulatory Agency (MHRA) classification (MHRA 2013).
-
Health‐related quality of life (using any validated scale).
Short‐term (four weeks to three months).
Medium‐term (more than three months to two years).
Long‐term (more than two years).
Secondary outcomes
-
Recurrence (local recurrence, surgical wound recurrence (also known as port site metastases in the endoscopic group) or distal metastases).
Short‐term recurrence (within six months).
Long‐term recurrence.
Adverse events (within three months). We accepted all adverse events reported by the study author(s) irrespective of the severity of the adverse event.
-
Measures of recovery.
Length of hospital stay (including the index admission for oesophagectomy (the hospital admission during which the oesophagectomy is performed) and any surgical complication‐related readmissions).
Time to return to normal activity (return to pretreatment mobility without any additional carer support; however defined by the trial authors).
Time to return to work (in those participants who were employed previously).
Dysphagia at maximal follow‐up (however defined by the trial authors).
We based the choice of the above clinical outcomes on the necessity to assess whether non‐surgical treatment is safe and effective in terms of short‐term results and long‐term cancer control.
Reporting of the outcomes listed here was not an inclusion criterion for this Cochrane review.
Search methods for identification of studies
Electronic searches
We conducted a literature search to identify all published and unpublished RCTs. The literature search identified potential studies in all languages. We translated the non‐English language papers and assessed them for potential inclusion in the review as necessary.
We searched the following electronic databases on 4th March 2016 for potential studies for inclusion.
The Cochrane Central Register of Controlled Trials (CENTRAL) (Issue 3, 2016) (Appendix 2).
MEDLINE (1966 to March 2016) (Appendix 3).
EMBASE (1988 to March 2016) (Appendix 4).
Science Citation Index (1982 to March 2016) (Appendix 5).
We conducted a search of ClinicalTrials.gov (Appendix 6) and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (Appendix 7) up to 4th March 2016.
Searching other resources
We checked reference lists of all included studies and review articles for additional references. We contacted authors of included trials and asked them to identify other published and unpublished studies.
We searched for errata or retractions from eligible trials on PubMed but did not find any.
Data collection and analysis
Selection of studies
Two review authors (LB and KG) independently screened for inclusion all the potential studies identified from the literature searches and coded them as either 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We retrieved the full‐text study reports and two review authors (LB and KG) independently screened the full‐text articles, identified studies for inclusion, and identified and recorded reasons for exclusion of ineligible studies. We resolved any disagreement through discussion. We identified and excluded duplicate references and collated multiple reports of the same study so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and a 'Characteristics of excluded studies' table.
Data extraction and management
We used a standardised data collection form for extraction of study characteristics and outcome data, which we had piloted on at least one study included in the review. Two review authors (LB and KG) extracted study characteristics from the included studies. We extracted the following study characteristics.
Methods: study design, total duration of study, number of study centres and location, study setting, withdrawals, and date of study.
Participants: number (N), mean age, age range, gender, tumour stage, tumour location, histological subtype, performance status, American Society of Anesthesiologists (ASA) status (ASA 2014), inclusion criteria, and exclusion criteria.
Interventions: intervention, comparison, and concomitant interventions.
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
Notes: funding for trial, and notable conflicts of interest of trial authors.
Two review authors (LB and KG) independently extracted outcome data from included studies. If the included studies reported outcomes multiple times for the same time frame (e.g. short‐term health‐related quality of life was reported at six weeks and three months), we chose the later time point (i.e. three months) for data extraction. For time‐to‐event outcomes, we extracted data to calculate the natural logarithm of the hazard ratio (HR) and its standard error (SE) using the methods suggested by Parmar 1998 if possible.
We included all randomised participants for medium‐ and long‐term outcomes (e.g. mortality or quality of life) and this was not conditional upon the short‐term outcomes (e.g. being alive at three months or having a low or high quality of life index at three months).
We have noted in the 'Characteristics of included studies' table if the included trials reported outcome data in an unusable way. We resolved disagreements by consensus. One review author (LB) copied the data from the data collection form into the Review Manager (RevMan) file (Review Manager 2014). We double‐checked that LB had entered the data correctly by comparing the study reports with how we presented the data in the systematic review.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each included study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion. We assessed the risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
We graded each potential source of bias as either 'high', 'low', or 'unclear' and provided a quote from the study report together with a justification for our judgement in the 'Risk of bias' tables. We summarised the 'Risk of bias' judgements across different studies for each listed domain. We acknowledge that blinding of participants and personnel is impossible but blinding of outcome assessors is possible. We considered blinding separately for different important outcomes where necessary (e.g. for unblinded outcome assessment, risk of bias for all‐cause mortality may be very different than for a patient‐reported quality of life scale since lack of blinding is unlikely to result in bias in all‐cause mortality while lack of blinding is likely to introduce a significant bias in quality of life). Where information on risk of bias relates to unpublished data or correspondence with a trial author, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took into account the risk of bias for the studies that contributed to that outcome.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol, Best 2015, and reported any deviations from it in the 'Differences between protocol and review' section.
Measures of treatment effect
We analysed dichotomous data (short‐term mortality, proportion of people with adverse and serious adverse events, short‐term recurrence) as risk ratios (RRs), and continuous data as mean differences (MDs) when all the trials reported the outcome or converted it to the same units (e.g. hospital stay), or standardised mean differences (SMDs) when trials used different scales to measure the outcome (e.g. quality of life). We have ensured that higher scores for continuous outcomes have the same meaning for the particular outcome, have explained the direction to the reader, and have reported where the directions were reversed if this was necessary. We calculated the rate ratio for outcomes such as adverse events and serious adverse events, where it was possible for the same person to develop more than one adverse event (or serious adverse event). If the trial authors calculated the rate ratio of adverse events (or serious adverse events) in the intervention versus control based on Poisson regression, we obtained the rate ratio by the Poisson regression method in preference to rate ratio calculated based on the number of adverse events (or serious adverse events) during a certain period. We calculated the HR for time‐to‐event outcomes such as long‐term mortality, long‐term recurrence, and time‐to‐first adverse event (or serious adverse event).
We undertook meta‐analyses only where meaningful i.e. if the treatments, participants, and the underlying clinical question were similar enough for pooling to make sense.
A common way that trial authors indicate they have skewed data is by reporting medians and interquartile ranges. When we encountered this we noted that the data were skewed and considered the implication of this. If the data were skewed, then we did not perform a meta‐analysis but performed a narrative summary instead.
Where a single trial reported multiple trial arms, we included only the relevant arms. If we had to enter two comparisons (e.g. laparoscopic oesophagectomy versus definitive chemoradiotherapy and open oesophagectomy versus definitive chemoradiotherapy) into the same meta‐analysis, we planned to half the control group to avoid double counting. The alternative way of including such trials with multiple arms is to pool the results of the oesophagectomy irrespective of the method and compare it with definitive chemoradiotherapy. We performed a sensitivity analysis to determine if the results of the two methods of dealing with multi‐arm trials led to different conclusions.
Unit of analysis issues
The unit of analysis was individual participants undergoing treatment for oesophageal cancer. We did not encounter any cluster‐RCTs for this comparison and therefore did not require any specific methodology for this trial type.
Dealing with missing data
We contacted investigators or study sponsors in order to verify key study characteristics and obtain missing numerical outcome data where possible (e.g. when a study is identified as abstract only). If we were unable to obtain the information from the trial authors or study sponsors, we imputed the mean from the median (i.e. consider median as the mean) and standard deviation (SD) from the SE, interquartile range, or P values according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) when the data did not appear to be skewed but we assessed the impact of including such studies as indicated in a sensitivity analysis. If we were unable to calculate the SD from SE, interquartile range, or P values, we imputed SD as the highest SD in the remaining trials included in the outcome, fully aware that this method of imputation decreases the weight of the studies in the meta‐analysis of MD and shifts the effect towards no effect for SMD.
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity among the trials in each analysis. If we identified substantial heterogeneity according to the Cochrane Handbook for Systematic Reviews of Interventions (greater than 50% to 60%), we explored it by prespecified subgroup analysis. We also assessed heterogeneity by evaluating whether there was good overlap of confidence intervals (CIs).
Assessment of reporting biases
We attempted to contact study authors by asking them to provide missing outcome data. Where this was not possible, and we thought the missing data may have introduced serious bias, we explored the impact of including such studies in the overall assessment of results by a sensitivity analysis. We also sought published protocols of trials to determine if outcomes mentioned in the protocol were not reported in order to determine selective outcome reporting bias. However, we were unable to locate the published protocol of any included trial.
If we were able to pool more than 10 trials, we planned to create and examine a funnel plot to explore possible publication biases. We planned to use Egger's test to determine the statistical significance of the reporting bias (Egger 1997). We considered a P value of less than 0.05 statistically significant reporting bias. However, we did not explore reporting biases since only eight trials met the inclusion criteria of this Cochrane review.
Data synthesis
We performed the analysis using RevMan (Review Manager 2014). We used the Mantel‐Haenszel method for dichotomous data, inverse variance method for continuous data, and generic inverse variance for count and time‐to‐event data. We used both a random‐effects model (DerSimonian 1986) and a fixed‐effect model (Demets 1987) for the analysis. In case of discrepancy between the two models, we reported both results; otherwise we only reported the results from the fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses.
Different cancer stages (stage I, stage II, stage III).
Different histological types (squamous cell carcinoma and adenocarcinoma).
Different locations (upper third, middle third, lower third).
Different non‐surgical treatments: endoscopic mucosal resection and endoscopic submucosal dissection.
Different anaesthetic risk patients (ASA I or II (a healthy participant or one with mild systemic disease) versus ASA III or more (a participant with severe systemic disease or worse) (ASA 2014).
We planned to use all the primary outcomes in the subgroup analysis.
We used the formal Chi² test for subgroup differences to test for subgroup interactions.
Sensitivity analysis
We planned to perform the following sensitivity analyses, which we defined a priori, to assess the robustness of our conclusions.
Exclusion of trials at unclear or high risk of bias (one of more of the risk of bias domains (other than blinding of surgeon) classified as unclear or high).
Exclusion of trials in which we imputed either mean or SD or both.
Exclusion of cluster RCTs in which the trial authors did not report the adjusted effect estimates.
Different methods of dealing with multi‐arm trials (please see the 'Measures of treatment effect' section).
'Summary of findings' table
We created a 'Summary of findings' table using all the outcomes. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence as it relates to the studies that contribute data to the meta‐analyses for the prespecified outcomes. We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and used GRADEpro Guidelines Development Tool (GDT) software (www.gradepro.org). We justified all decisions to downgrade or upgrade the quality of the evidence from included studies using footnotes, and we made comments to aid the reader's understanding of the review where necessary. We considered whether there was any additional outcome information that we could not incorporate into meta‐analyses, and planned to note this in the comments and state if it supported or contradicted the information from the meta‐analyses.
Reaching conclusions
We based our conclusions only on findings from the quantitative or narrative synthesis of included studies for this Cochrane review. We avoided making recommendations for practice and our implications for research will give the reader a clear sense of where the focus of any future research in the area should be and what the remaining uncertainties are.
Results
Description of studies
Results of the search
We identified 10,952 references through electronic searches of the Cochrane Central Register of Controled trials (CENTRAL), MEDLINE, EMBASE, Science Citation Index, ClinicalTrials.gov, and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP). After we removed duplicate references there were 7849 references. We excluded 7823 irrelevant references after we screened abstracts. We sought the full text of 26 references for further assessment. We did not identify any additional references to trials by searching the trial registry. We excluded six references because of the reasons mentioned in the 'Characteristics of excluded studies' table and 'Excluded studies' section. Eight trials (20 references) met the inclusion criteria (Badwe 1999; Bedenne 2007; Blazeby 2014; Carstens 2007; Chiu 2005; Fok 1994; Stahl 2005; Sun 2006). We have presented the study flow diagram in Figure 1.
1.
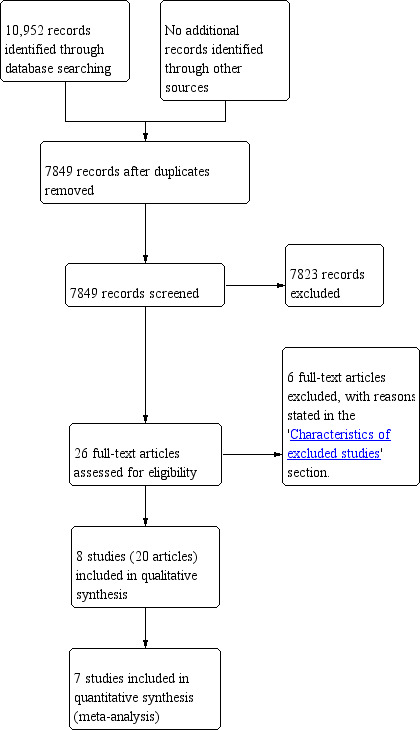
Study flow diagram.
Included studies
The eight included trials compared definitive radiotherapy or definitive chemoradiotherapy with surgery (Badwe 1999; Bedenne 2007; Blazeby 2014; Carstens 2007; Chiu 2005; Fok 1994; Stahl 2005; Sun 2006). We did not identify any trials that compared endoscopic mucosal resection or endoscopic submucosal dissection with surgery. Seven trials were two armed randomised controlled trials (RCTs) (Badwe 1999; Bedenne 2007; Blazeby 2014; Carstens 2007; Chiu 2005; Stahl 2005; Sun 2006). The eighth trial was a four‐armed trial (Fok 1994). Three of the arms involved surgery (surgery alone, surgery with preoperative radiotherapy, and surgery with postoperative radiotherapy) and the fourth arm was non‐surgical (definitive radiotherapy). In total, the eight included trials randomised 1132 participants. One trial, which included five participants, did not contribute any data to this Cochrane review, because the trial authors did not report any of the outcomes included in this review (Blazeby 2014). Therefore, the seven trials that contributed data to this Cochrane review randomised 1127 participants, of which 13 participants were withdrawn postrandomisation, which left a total of 1114 participants for whom data were available. Of these 1114 participants, 510 were randomised to non‐surgical treatment and 604 to surgical treatment (Badwe 1999; Bedenne 2007; Carstens 2007; Chiu 2005; Fok 1994; Stahl 2005; Sun 2006).
Of the eight included trials, three used radiotherapy alone (Badwe 1999; Fok 1994; Sun 2006), five used chemoradiotherapy (Bedenne 2007; Blazeby 2014; Carstens 2007; Chiu 2005; Stahl 2005), and none used chemotherapy alone as the non‐surgical treatment. None of the trials compared endoscopic treatment with surgical treatment. Regarding the surgical arm, three trials included surgical treatment without any adjuvant therapy (Badwe 1999; Carstens 2007; Sun 2006), three trials used preoperative chemotherapy or chemoradiotherapy in addition to surgery (Bedenne 2007; Blazeby 2014; Stahl 2005), and one trial used postoperative chemoradiotherapy or radiotherapy (Chiu 2005). In one trial, surgical treatment consisted of three groups of which one group received preoperative radiotherapy, another received postoperative radiotherapy, and the last group received surgery alone (Fok 1994).
Three trials reported the cancer stage (Bedenne 2007; Blazeby 2014; Stahl 2005) and these all included T2‐4N0‐1M0 tumours (or a subset of this range). The remaining trials did not state the tumour stage of participants, but it is likely that these trials included only resectable cancers as surgical resection was one of the arms in the trials. Seven trials reported the histological classification of tumours, with five of these being squamous cell carcinoma only (Badwe 1999; Blazeby 2014; Chiu 2005; Fok 1994; Stahl 2005) and two either adenocarcinoma or squamous cell carcinoma (Bedenne 2007; Carstens 2007). The remaining trial, Sun 2006, did not report the histological cancer type. There was little concordance between studies in the terminology they used to describe the tumour location. We have stated the term the study authors used in the 'Characteristics of included studies' table. None of the studies specifically reported the American Society of Anesthesiologists (ASA) grade of participants, although it is likely that trials only included participants who were fit to undergo major surgery. In terms of the surgical treatment used, six trials used open oesophagectomy (Badwe 1999; Carstens 2007; Chiu 2005; Fok 1994; Stahl 2005; Sun 2006), and the remaining two trial did not report whether the surgery was performed by open method or laparoscopic method (Bedenne 2007; Blazeby 2014).
The follow‐up period in the trials were as follows.
Badwe 1999 had a maximum follow‐up of three years.
Bedenne 2007 had a median follow‐up of four years.
Blazeby 2014 did not state the follow‐up period.
Carstens 2007 did not specify the follow‐up period but reported four year follow‐up results.
Chiu 2005 had a median follow‐up 1.5 years.
Fok 1994 stated that long‐term follow‐up with partial data were available at 10 years, but did not specify details.
Stahl 2005 had a median follow‐up of six years.
Sun 2006 had a median follow‐up of 4.8 years.
Excluded studies
We excluded six full‐text articles (five studies) in total. We excluded two articles because they were not RCTs (Desai 1987; Ilson 2007). Two trials changed their protocol to a non‐randomised study following poor recruitment (Earlam 1991; Hainsworth 2007). We excluded one article because this trial is expected to completed in 2018 and the current report (a conference abstract) included details of the safety of surgery in participants randomised to surgical arm and the report included non‐randomised patients undergoing surgery as well (Nozaki 2014).
Risk of bias in included studies
All included trials were at high risk of bias, as we have shown in Figure 2 and Figure 3.
2.
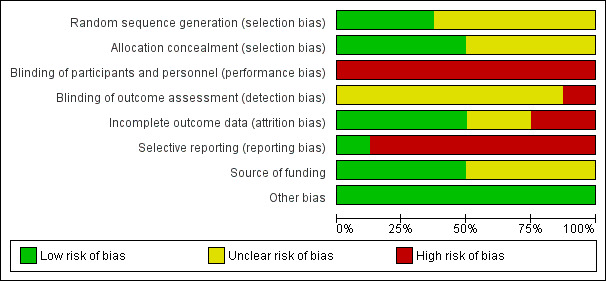
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.
3.
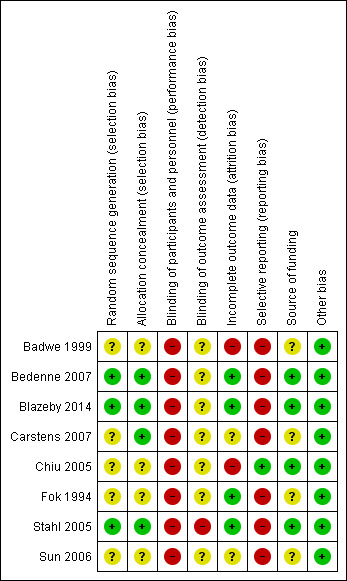
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
Allocation
Three trials were free from selection bias (Bedenne 2007; Blazeby 2014; Stahl 2005). These trials had low risk of bias regarding random sequence generation and allocation concealment. The remaining trials were at unclear risk of bias in at least one aspect of random sequence generation or allocation concealment.
Blinding
All eight trials were at high risk of performance bias because it is almost impossible to blind the participants and healthcare providers in a surgical versus non‐surgical trial. One trial reported that it was unblinded and therefore was at high risk of detection bias (Stahl 2005). The other seven trials did not address this aspect and we considered them to be at an unclear risk of detection bias.
Incomplete outcome data
We classified four trials as at low risk of attrition bias as they described no postrandomisation exclusions (Bedenne 2007; Blazeby 2014; Fok 1994; Stahl 2005). Two trials were at unclear risk of attrition bias as the reports do not clearly describe whether there were any postrandomisation exclusions (Carstens 2007; Sun 2006). Two trials were at high risk of attrition bias as they had postrandomisation exclusions, which was likely to affect the results (Badwe 1999; Chiu 2005).
Selective reporting
Protocols were not available for any of the included trials. Only one trial reported all important outcomes and we therefore considered it at low risk of reporting bias (Chiu 2005). The remaining seven trials were at high risk of reporting bias.
Other potential sources of bias
Four trials described an impartial source of funding, and were therefore at low risk of bias (Bedenne 2007; Blazeby 2014; Chiu 2005; Stahl 2005). The other four trials did not describe how they were funded and we therefore considered them at unclear risk of bias regarding the funding source (Badwe 1999; Carstens 2007; Fok 1994; Sun 2006). We considered all the included trials as free from any other sources of bias.
Effects of interventions
Summary of findings for the main comparison. Non‐surgical versus surgical treatment of oesophageal cancer (primary outcomes).
| Non‐surgical versus surgical treatment of oesophageal cancer | |||||
| Patient or population: people with oesophageal cancer Settings: secondary or tertiary care Intervention: non‐surgical treatment Comparison: surgical treatment | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Surgical treatment | Non‐surgical treatment | ||||
| Short‐term mortality All‐cause mortality either in‐hospital or within 3 months | 78 per 10001 | 30 per 1000 (9 to 105) | RR 0.39 (0.11 to 1.35) | 689 (5 studies) | ⊕⊝⊝⊝ very low2,3 |
| Long‐term mortality (binary outcome) All‐cause mortality for the duration of follow‐up | 691 per 10001 | 712 per 1000 (636 to 788) | RR 1.03 (0.92 to 1.14) | 511 (3 studies) | ⊕⊕⊝⊝ low2 |
| Long‐term mortality (time‐to‐event outcome): chemoradiotherapy versus surgery All‐cause mortality for the duration of follow‐up | 349 per 10001 | 314 per 1000 (278 to 357) | HR 0.88 (0.76 to 1.03) | 602 (4 studies) | ⊕⊕⊝⊝ low2 |
| Long‐term mortality (time‐to‐event outcome): radiotherapy versus surgery All‐cause mortality for the duration of follow‐up | 350 per 10001 | 451 per 1000 (398 to 507) | HR 1.39 (1.18 to 1.64) | 512 (3 studies) | ⊕⊝⊝⊝ very low2,3 |
|
Long‐term mortality (binary): definitive chemoradiotherapy versus surgery with neoadjuvant chemotherapy All‐cause mortality for the duration of follow‐up |
740 per 1000 | 769 per 1000 (688 to 858) | RR 1.04 (0.93 to 1.16) | 431 (2 studies) | ⊕⊝⊝⊝ low2 |
|
Long‐term mortality (time‐to‐event): definitive chemoradiotherapy versus surgery with neoadjuvant chemotherapy or chemoradiotherapy All‐cause mortality for the duration of follow‐up |
349 per 1000 | 346 per 1000 (284 to 418) | HR 0.99 (0.78 to 1.26) | 431 (2 studies) | ⊕⊝⊝⊝ very low2,4 |
| Proportion with a serious adverse event within 3 months Serious adverse event within 3 months as defined by ICH‐GCP International Conference on Harmonisation ‐ Good Clinical Practice guideline (ICH‐GCP 1996) or reasonable variations thereof | 273 per 10001 | 166 per 1000 (68 to 401) | RR 0.61 (0.25 to 1.47) | 80 (1 study) | ⊕⊝⊝⊝ very low2,4 |
| Short‐term health‐related quality of life Any validated scale | The mean short‐term health‐related quality of life in the control groups was 7.52 | The mean short‐term health‐related quality of life in the intervention groups was 0.93 higher (0.24 to 1.62 higher) | ‐ | 165 (1 study) | ⊕⊝⊝⊝ very low2,5 |
| Medium‐term health‐related quality of life Any validated scale | The mean medium‐term health‐related quality of life in the control groups was 8.76 | The mean medium‐term health‐related quality of life in the intervention groups was 0.95 lower (2.1 lower to 0.2 higher) | ‐ | 62 (1 study) | ⊕⊝⊝⊝ very low2,5 |
| *The basis for the assumed risk is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; HR: hazard ratio. | |||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | |||||
1The basis for control risk is the event rate across all studies (i.e. the sum of all events in the surgical group across all studies reporting the outcome divided by the sum of all people in the surgical group in the trials reporting the outcome) for all outcomes except long‐term mortality (time‐to‐event) where a control group risk of 0.35 was used (based on similar control group risks at 2 years in a number of trials included in this analysis) and long‐term recurrence (time‐to‐event) where a control group risk of 0.4 was used (based on similar control group risk at 2 year in a trial included for this analysis). 2Downgraded two levels due to significant bias within the trials. 3Downgraded two levels due to inconsistency in the results across the studies. 4Downgraded one level due to wide CIs (overlaps 1 and 0.75 or 1.25). 5Downgraded one level due to wide CIs (overlaps 0 and 0.25 and −0.25).
Summary of findings 2. Non‐surgical versus surgical treatment of oesophageal cancer (secondary outcomes).
| Non‐surgical versus surgical treatment of oesophageal cancer | ||||||
| Patient or population: people with oesophageal cancer Settings: secondary or tertiary care Intervention: non‐surgical treatment Comparison: surgical treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Surgical treatment | Non‐surgical treatment | |||||
| Long‐term recurrence (binary outcome) Local recurrence, surgical wound recurrence, or distal metastases | 526 per 10001 | 552 per 1000 (458 to 673) | RR 1.05 (0.87 to 1.28) | 339 (2 studies) | ⊕⊝⊝⊝ very low2,3 | ‐ |
| Long‐term recurrence (time‐to‐event outcome) Local recurrence, surgical wound recurrence, or distal metastases | 508 per 10001 | 494 per 1000 (433 to 561) | HR 0.96 (0.8 to 1.16) | 349 (2 studies) | ⊕⊕⊝⊝ low2 | ‐ |
| Local recurrence (binary) | 381 per 1000 | 339 per 1000 (267 to 427) | RR 0.89 (0.70 to 1.12) | 449 (3 studies) | ⊕⊝⊝⊝ very low2,3,4 | ‐ |
| Proportion with any adverse event within 3 months Any adverse event within 3 months of any severity | 386 per 10001 | 668 per 1000 (429 to 1000) | RR 1.73 (1.11 to 2.67) | 80 (1 study) | ⊕⊝⊝⊝ very low2,4 | ‐ |
| Length of hospital stay (days) Including the index admission for oesophagectomy (the hospital admission during which the oesophagectomy is performed) and any surgical complication‐related readmissions | See comment | See comment | Not estimable | 342 (2 studies) | ⊕⊝⊝⊝ very low2,5 | Significant heterogeneity present (I² statistic = 93%, P = 0.0001) making meta‐analysis inappropriate. The mean hospital stay was 16 days shorter (3 days shorter to 29 days shorter) in non‐surgical treatment than surgical treatment in 1 trial (Bedenne 2007) and 14 days longer (5 days longer to 23 days longer) in non‐surgical treatment than surgical treatment in another trial (Chiu 2005). |
| Dysphagia at maximal follow‐up | 367 per 1000 | 543 per 1000 (370 to 803) | RR 1.48 (1.01 to 2.19) | 139 (1 study) | ⊕⊝⊝⊝ very low2,4 | ‐ |
| *The basis for the assumed risk is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; HR: hazard ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1The basis for control risk is the event rate across all studies (i.e. the sum of all events in the surgical group across all studies reporting the outcome divided by the sum of all people in the surgical group in the trials reporting the outcome). 2Downgraded two levels due to significant bias within the trials. 3Downgraded one level due to inconsistency in the results across the studies. 4Downgraded one level due to wide CIs (overlaps 1 and 0.75 or 1.25). 5Downgraded one level due to wide CIs (overlaps 0 and 0.25 and −0.25).
The included trials reported the following outcomes: short‐term mortality, long‐term mortality, serious adverse events within three months, short‐term quality of life, medium‐term quality of life, long‐term recurrence, adverse events within three months, and length of hospital stay. None of the trials reported the remaining outcomes of interest in this Cochrane review, i.e. long‐term quality of life, recurrence within six months, time to return to normal activity, and time to return to work. We have summarised the results in 'Summary of findings' table 1 (Table 1) and 'Summary of findings' table 2 (Table 2).
Short‐term mortality
Five trials reported short‐term mortality, which is defined as mortality in hospital or within 30 days of treatment (Badwe 1999; Bedenne 2007; Carstens 2007; Chiu 2005; Stahl 2005). We pooled the trials and used a fixed‐effect model. There was a statistically significant lower proportion of participants who died within 30 days of treatment between the non‐surgical group (30 per 1000) compared to the surgical group (78 per 1000) (risk ratio (RR) 0.33, 95% confidence interval (CI) 0.16 to 0.69; 689 participants; five studies; I² statistic = 46%) . However we applied when a random‐effects model this became statistically non‐significant (RR 0.39, 95% CI 0.11 to 1.35; 689 participants; five studies; Analysis 1.1).
1.1. Analysis.
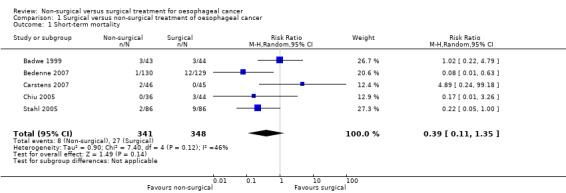
Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 1 Short‐term mortality.
Long‐term mortality
Three trials reported long‐term mortality, which included deaths at maximal follow‐up as binary outcomes (Bedenne 2007; Chiu 2005; Stahl 2005). We pooled the trials using a fixed‐effect model. There was no significant difference in the proportion of participants who died beyond three months after treatment between the non‐surgical group (712 per 1000) compared to the surgical group (691 per 1000) (RR 1.03, 95% CI 0.92 to 1.14; 511 participants; three studies; I² statistic = 0%; Analysis 1.2). There was no change to the conclusions when we used a random‐effects model.
1.2. Analysis.
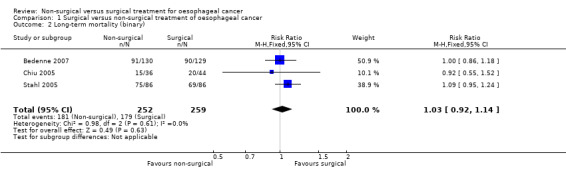
Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 2 Long‐term mortality (binary).
Seven trials reported long‐term mortality, which we could analyse as a time‐to‐event analysis (Badwe 1999; Bedenne 2007; Carstens 2007; Chiu 2005; Fok 1994; Stahl 2005; Sun 2006). We pooled the trials using a fixed‐effect model. There was no significant difference in long‐term mortality between the groups (hazard ratio (HR) 1.09, 95% CI 0.97 to 1.22; 1114 participants; seven studies; I² statistic = 79%; Analysis 1.3). There was no change to the conclusions when we used a random‐effects model.
1.3. Analysis.
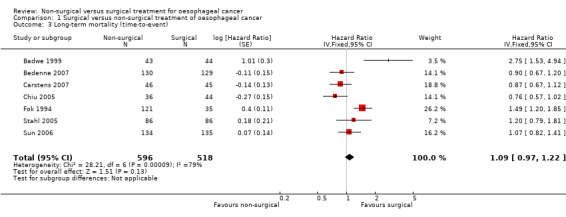
Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 3 Long‐term mortality (time‐to‐event).
Serious adverse events within three months
One trial reported serious adverse events within three months of treatment (Chiu 2005). There was no significant difference in the proportion of participants who suffered a serious adverse event within three months of treatment between the non‐surgical group (166 per 1000) compared to the surgical group (273 per 1000) (RR 0.61, 95% CI 0.25 to 1.47; 80 participants; one study; Analysis 1.4). Since only one trial reported this outcome, the issue of fixed‐effect model versus random‐effects model did not arise and studies of heterogeneity are irrelevant. None of the trials reported the number of serious adverse events within three months.
1.4. Analysis.
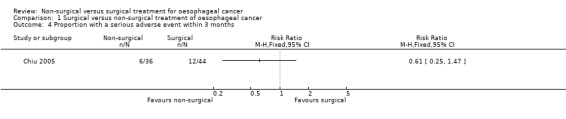
Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 4 Proportion with a serious adverse event within 3 months.
Short‐term health‐related quality of life
One trial reported short‐term health‐related quality of life, which is defined as any validated quality of life measurement (in this case Spitzer score) recorded four weeks to three months after treatment (Bedenne 2007). Lower scores indicate poorer quality of life (Spitzer 1981). There was a statistically significantly higher quality of life score in non‐surgical treatment between four weeks and three months after treatment (MD 0.93, 95% CI 0.24 to 1.62; 165 participants; one study; Analysis 1.5). Since only one trial reported this outcome, the issue of fixed‐effect model versus random‐effects model did not arise.
1.5. Analysis.
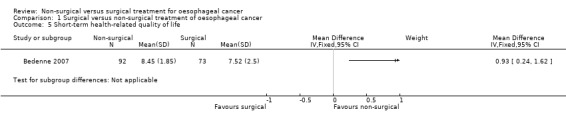
Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 5 Short‐term health‐related quality of life.
Medium‐term health‐related quality of life
One trial reported medium‐term health‐related quality of life, which is defined as any validated quality of life measurement (in this case Spitzer score) recorded three months to two years after treatment (Bedenne 2007). There was no significant difference in the quality of life score between three months and two years after treatment (MD −0.95, 95% CI −2.10 to 0.20; 62 participants; one study; Analysis 1.6). Since only one trial reported this outcome, the issue of fixed‐effect model versus random‐effects model did not arise.
1.6. Analysis.
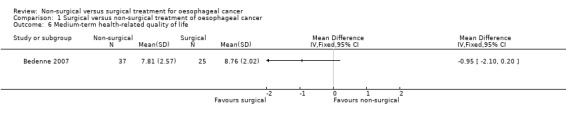
Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 6 Medium‐term health‐related quality of life.
Medium‐term health‐related quality of life
None of the trials reported this outcome.
Recurrence within six months
None of the trials reported this outcome.
Long‐term recurrence
Two trials reported recurrence as a binary outcome (Bedenne 2007; Chiu 2005). We pooled the trials using a fixed‐effect model. There was no significant difference in the proportion of participants who suffered recurrence more than six months after treatment between the non‐surgical group (552 per 1000) compared to the surgical group (526 per 1000) (RR 1.05, 95% CI 0.87 to 1.28; 339 participants; two studies; I² statistic = 0%; Analysis 1.7). There was no change to the conclusions when we used a random‐effects model.
1.7. Analysis.
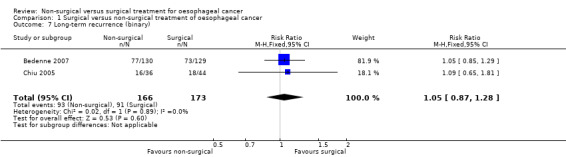
Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 7 Long‐term recurrence (binary).
Two trials reported long‐term recurrence as a time‐to‐event outcome (Chiu 2005; Sun 2006). We pooled the trials using a fixed‐effect model. There was no significant difference in the HR for recurrence after more than six months after treatment between the two groups (HR 0.96, 95% CI 0.80 to 1.16; 349 participants; two studies; I² statistic = 41%; Analysis 1.8). There was no change to the conclusions when we used a random‐effects model.
1.8. Analysis.
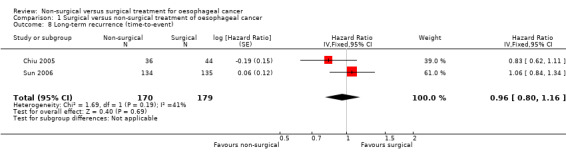
Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 8 Long‐term recurrence (time‐to‐event).
Three trials reported local recurrence as a binary outcome (Bedenne 2007; Chiu 2005; Fok 1994). There was no statistically significant difference in the local recurrence between the non‐surgical and surgical groups (RR 0.89, 95% CI 0.70 to 1.12; 449 participants; three studies; I² statistic = 90%; Analysis 1.9).
1.9. Analysis.
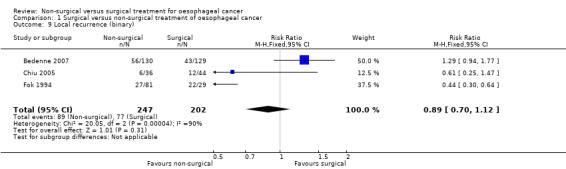
Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 9 Local recurrence (binary).
Adverse events within three months
One trial reported adverse events within three months of treatment (Chiu 2005). There were statistically significantly more adverse events within three months of treatment (RR 1.73, 95% CI 1.11 to 2.67; 80 participants; one study) in the non‐surgical group (668 per 1000) compared to the surgical group (386 per 1000) (Analysis 1.10). Since only one trial reported this outcome, the issue of fixed‐effect model versus random‐effects model did not arise and studies of heterogeneity are irrelevant. None of the included trials reported the number of adverse events within three months.
1.10. Analysis.
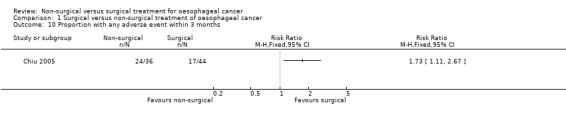
Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 10 Proportion with any adverse event within 3 months.
Length of hospital stay
Two trials reported length of hospital stay (Bedenne 2007; Chiu 2005). However these two trials showed completely opposite results and meta‐analysis had an I² statistic value of 93%. An I² statistic value of greater than 80% indicates significant underlying heterogeneity (Higgins 2011). The heterogeneity in the results between the trials was unexplained, although this could be because of the way the trials measured the length of hospital stay (please see the 'Discussion' section). Therefore meta‐analysis was invalid and we have only shown point estimates for individual studies (Analysis 1.11).
1.11. Analysis.
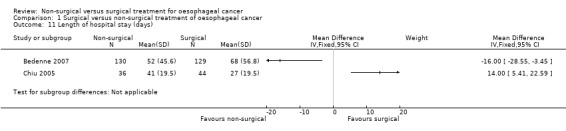
Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 11 Length of hospital stay (days).
Time to return to normal activity
None of the trials reported this outcome.
Time to return to work
None of the trials reported this outcome.
Dysphagia at maximal follow‐up
Only one trial reported dysphagia at maximal follow‐up (Bedenne 2007). People without dysphagia were asymptomatic or were able to eat solids with some dysphagia at the last clinic visit prior to death. The dysphagia at maximal follow‐up prior to death was statistically significantly higher in the non‐surgical group than surgical group (RR 1.48, 95% CI 1.01 to 2.19; 139 participants; one study; Analysis 1.12). Since only one trial reported this outcome, the issue of fixed‐effect model versus random‐effects model did not arise and studies of heterogeneity are irrelevant.
1.12. Analysis.
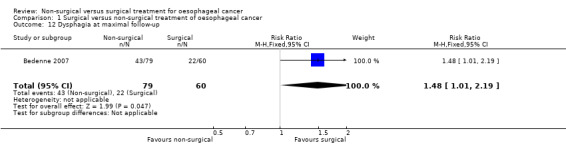
Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 12 Dysphagia at maximal follow‐up.
Subgroup analysis
We had planned to perform five subgroup analyses but only one was possible. The first planned subgroup analysis was not possible because only three trials reported cancer stage and they were all within a narrow range (T2‐4, N0‐1, M0). Analysis by histological type was not possible because five trials included squamous cell carcinoma only (Badwe 1999; Blazeby 2014; Chiu 2005; Fok 1994; Stahl 2005), two trials included either adenocarcinoma or squamous cell carcinoma (Bedenne 2007; Carstens 2007), and one trial did not report the histology (Sun 2006). The third analysis we had planned was by tumour location. However, only a few of the included trials reported this but did not report it in a standardised way, which made comparisons impossible.
Comparison of different types of non‐surgical treatment was the only subgroup analysis which was possible. The two different treatments were definitive chemoradiotherapy and definitive radiotherapy. The only outcome reported, which contained at least two trials for both definitive chemoradiotherapy and definitive radiotherapy, was long‐term mortality with time‐to‐event analysis (the presence of at least two trials allows meta‐analysis and assessment of heterogeneity within the subgroup to explore whether the heterogeneity in overall results could be explained because of the clinical differences). The results of the subgroup analysis show that there are statistically significant subgroup differences (test for subgroup differences: Chi² test = 16.15, df = 1 (P < 0.0001), I² statistic = 93.8%). There was a difference in both the magnitude and direction in the subgroups with definitive chemoradiotherapy having a HR of 0.88 (95% CI 0.76 to 1.03; 602 participants; four trials; Analysis 1.13) and definitive radiotherapy having a HR of 1.39 (95% CI 1.18 to 1.64; 512 participants; three trials; Analysis 1.13) compared to surgical treatment with respect to long‐term mortality. We performed a post‐hoc subgroup analysis in which we compared definitive chemoradiotherapy versus oesophagectomy with neoadjuvant chemotherapy or chemoradiotherapy. As shown in Analysis 1.14 and Analysis 1.15, there was no statistically significant difference in the long‐term survival between definitive chemoradiotherapy versus oesophagectomy with neoadjuvant chemotherapy or chemoradiotherapy whether analysed as binary outcome (RR 1.04, 95% CI 0.93 to 1.16; 431 participants; two trials; Analysis 1.14) or as time‐to‐event outcome (HR 0.99, 95% CI 0.78 to 1.26; 431 participants; two trials; Analysis 1.15).
1.13. Analysis.
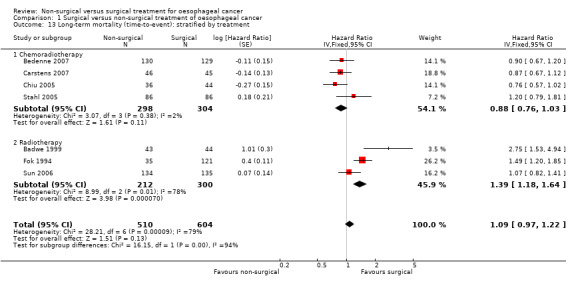
Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 13 Long‐term mortality (time‐to‐event): stratified by treatment.
1.14. Analysis.
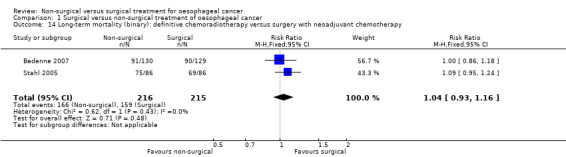
Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 14 Long‐term mortality (binary): definitive chemoradiotherapy versus surgery with neoadjuvant chemotherapy.
1.15. Analysis.
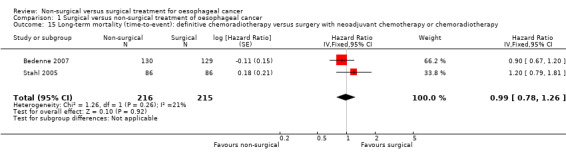
Comparison 1 Surgical versus non‐surgical treatment of oesophageal cancer, Outcome 15 Long‐term mortality (time‐to‐event): definitive chemoradiotherapy versus surgery with neoadjuvant chemotherapy or chemoradiotherapy.
Finally none of the included trials explicitly reported the ASA grade of included participants, which made subgroup analysis impossible.
Sensitivity analysis
We could not perform any of the planned sensitivity analyses. Regarding the first sensitivity analysis, all the trials were at unclear or high risk of bias and thus we could not perform the analysis. We could not perform the second sensitivity analysis since we did not impute the mean or SD for short‐term or medium‐term health‐related quality and imputed the mean or SD or both for all the trials included for the length of hospital stay. We did not perform the third sensitivity analysis since there were no cluster RCTs. Regarding the different ways of dealing with multi‐arm trials, one trial had four arms (three surgical treatments: surgery alone, surgery combined with preoperative radiotherapy, and surgery combined with postoperative radiotherapy; and definitive radiotherapy) (Fok 1994). Fok 1994 only reported the outcome of long‐term mortality, and presented the long‐term mortality for the three surgical groups together rather than for each subgroup individually. Thus, we could not perform this sensitivity analysis.
Discussion
Summary of main results
In this Cochrane review, we compared non‐surgical versus surgical treatment for people with non‐metastatic oesophageal cancer. The included trials compared the treatments of definitive chemoradiotherapy versus surgery and definitive radiotherapy versus surgery. There were no statistically significant differences in short‐term mortality, long‐term mortality, proportion of people with serious adverse events, medium‐term health‐related quality of life, proportion of people with recurrence after six months, long‐term recurrence (time‐to‐event), or proportion of people with local recurrence. However, the subgroup analysis of long‐term mortality showed that the results differed for chemoradiotherapy versus surgery and radiotherapy versus surgery. While radiotherapy treatment resulted in more long‐term mortality than surgery group (hazard ratio (HR) 1.39, 95% confidence interval (CI) 1.18 to 1.64), there was no statistically significant difference in long‐term mortality in the chemoradiotherapy group compared with the surgery group (HR 0.88, 95% CI 0.76 to 1.03). If anything, there was a trend that favoured chemoradiotherapy and there was good overlap of CIs in this subgroup (chemoradiotherapy versus surgery). We performed the post‐hoc subgroup analysis of definitive chemoradiotherapy versus neoadjuvant chemotherapy or chemoradiotherapy along with surgery since neoadjuvant chemotherapy or chemoradiotherapy along with surgery provides better survival than surgery alone (Sjoquist 2011) and the European Society for Medical Oncology (ESMO) guidelines recommend this treatment (Stahl 2013).
There was higher proportion of people with any adverse events in the non‐surgical treatment group than the surgical treatment group, while the short‐term health‐related quality of life was better in the non‐surgical treatment group than surgical treatment group. One might expect lower health‐related quality of life if there are more adverse events. However, we did not observe this. The conflicting results in this Cochrane review could be because of the way that trial authors reported the adverse events in the two groups or because of the inconsistent results in the studies that reported these outcomes. In either case, one cannot attach much clinical significance to the difference in the adverse events favouring surgical treatment since this was based on a single trial (Chiu 2005). Also there were no statistically significant differences between the groups for other outcomes such as mortality or recurrence. One cannot attach much clinical significance to the difference in the short‐term quality of life favouring non‐surgical treatment either since this was based on a single trial (Bedenne 2007) and it is unclear whether or not the difference was clinically significant. The length of hospital stay was lower in the non‐surgical treatment group than the surgical treatment group in one trial (Bedenne 2007), and higher in the non‐surgical treatment group than non‐surgical treatment group in another trial (Chiu 2005). The length of hospital stay was the mean hospital stay during the entire follow‐up period in Bedenne 2007, while this was the median hospital stay during the treatment in Chiu 2005. The inconsistent results in length of hospital stay between the studies are likely to be due to the different ways the two trials measured the length of hospital stay (Bedenne 2007; Chiu 2005).
Overall, chemoradiotherapy appears to be equivalent to surgery regarding long‐term survival (as the long‐term mortality of the non‐surgical treatment that was equivalent to surgery was chemoradiotherapy; radiotherapy resulted in higher long‐term mortality than non‐surgical treatment. In addition, most included trials, for the outcomes other than long‐term mortality, used chemoradiotherapy as the non‐surgical treatment). The major question is whether the lack of statistically significant difference in the outcomes between chemoradiotherapy and surgery was because of a lack of difference in the outcomes or the lack of evidence of difference. There was a trend that suggested that chemoradiotherapy resulted in less long‐term mortality but there was no statistical significance. We considered 25% relative change as clinically important since there was no evidence from literature regarding the clinically important difference in long‐term mortality. The CIs did not overlap a 25% relative increase in long‐term mortality (i.e. the confidence intervals did not overlap RR of 1.25) whether this was analysed as a binary outcome or a time‐to‐event outcome i.e. one can rule out a 25% relative increase in long‐term mortality with chemoradiotherapy based on the results reported in the study. The short‐term mortality was lower in the non‐surgical treatment group compared to surgical treatment group when we used the fixed‐effect model, but there was no statistically significant difference between the groups when we used the random‐effects model. Thus, there is nothing to suggest that the lack of statistical significance is because of lack of evidence of beneficial effect of surgery. The most likely interpretation of the data is that chemoradiotherapy is at least equivalent to surgery in terms of survival. In the absence of beneficial effect of surgery in other outcomes, there is no reason to prefer surgery over chemoradiotherapy based on the current data. Importantly, of the five trials that used chemoradiotherapy as the intervention arm (Bedenne 2007; Blazeby 2014; Carstens 2007; Chiu 2005; Stahl 2005), the surgical arm received adjuvant chemotherapy or chemoradiotherapy in three trials (Bedenne 2007; Blazeby 2014; Stahl 2005). Thus the control group is a contemporary control group. We performed another subgroup analysis to compare definitive chemoradiotherapy versus oesophagectomy with neoadjuvant therapy. There was no statistically significant difference in the long‐term mortality between the two groups irrespective of whether we analysed this as a binary outcome (RR 1.04, 95% CI 0.93 to 1.16) or as a time‐to‐event outcome (HR 0.99, 95% CI 0.78 to 1.26). When we analysed it as a binary outcome, the CIs did not overlap a 25% relative increase or decrease. When we analysed it as a time‐to‐event outcome, the CIs overlapped 25% relative increase. Overall, there does not appear to be any difference in the long‐term mortality between the two groups.
The proportion of people with dysphagia at the last follow‐up visit prior to death was higher with chemoradiotherapy than surgery in the only trial that reported dysphagia (Bedenne 2007). While dysphagia is an important patient‐oriented outcome and is likely to have a significant impact on the quality of people, we were unable to assess the impact of dysphagia on the quality of life since the trial (or any other trial) did not report long‐term quality of life. However, this must be confirmed in further trials before we can be definite that surgery is better than definitive chemoradiotherapy regarding dysphagia.
Overall completeness and applicability of evidence
This Cochrane review included participants either undergoing surgery of oesophageal cancer or non‐surgical treatment of different histological types and stages of oesophageal cancer. However, most participants had squamous cell carcinoma and thus, the results are applicable mainly to squamous cell carcinoma. Only two trials included adenocarcinoma (Bedenne 2007; Carstens 2007). In Bedenne 2007, only 10% of 259 participants had adenocarcinoma, while in Carstens 2007 50% of 91 participants had adenocarcinoma. The effect estimates observed in these trials did not differ from the other trials. So, there is no evidence to suggest that the effect estimates of definitive chemoradiotherapy versus surgery will be different for squamous cell carcinoma and adenocarcinoma. However, there is more uncertainty about equivalence of definitive chemoradiotherapy and surgery in terms of long‐term survival because of the low number of participants with adenocarcinoma. One of the trials that contributed significantly to the different meta‐analysis, only participants who responded to initial induction chemoradiotherapy (defined as least 30% decrease in tumour length following induction chemotherapy) were randomised to chemoradiotherapy and surgery. Those who did not respond were offered surgery. So, the findings of this review are applicable mainly to people who respond to chemoradiotherapy. However, failure of response to induction chemoradiotherapy does not prevent people with oesophageal cancer from having surgery; so, surgery can be offered to such people as definitive treatment.
Although the included trials did not report the American Society of Anesthesiologists (ASA) status, all participants must have been fit for major surgery if the randomisation procedures were performed adequately. Thus, the results of this review are applicable only to people with non‐metastatic oesophageal cancer (of different tumour histological types and stages) and are not applicable to people who are unsuitable for surgery either because of their anaesthetic risk or because of the location and extent of the cancer.
Quality of the evidence
All included trials were at high risk of bias. None of the included trials reported blinding of participants and personnel and it is unrealistic in trials that compare non‐surgical and surgical treatments. However, blinding of the outcome assessors can be performed and is necessary to decrease the detection bias. Only one trial, Stahl 2005, appeared to have blinded the outcome assessors. However, there is no evidence that lack of blinding will lead to bias in an outcome such as all‐cause mortality while it is likely to affect most of the other outcomes, including adverse events and quality of life. Every effort should be taken to ensure blinding of outcome assessors. All included trials except Chiu 2005 were at high risk of bias for selective reporting because they did not report treatment related complications, an important consideration when comparing treatment regimes. In addition two trials suffered high risk of attrition bias because of postrandomisation exclusions (Badwe 1999; Chiu 2005). The selection bias and attrition bias can be easily reduced by reporting the most important clinical outcomes in all randomised participants according to the group to which they were randomised (intention‐to‐treat analysis).
Another reason for low or very low quality of evidence was the small sample size for many outcomes. There was also inconsistency in the results for some outcomes. The heterogeneity in long‐term mortality for radiotherapy was only in the magnitude of effect and there was reasonable overlap of CIs for long‐term recurrence. However, the heterogeneity in the length of hospital stay was both in magnitude and in direction, and resulted in decreased confidence in the results of the length of hospital stay.
Potential biases in the review process
We followed the guidance of the Cochrane Handbook for Systematic Reviews of Intervention (Higgins 2011). There were no language, publication status, or sample size restrictions. Thus, we minimised the bias due to selection of trials. However, we used median values for the meta‐analyses when the mean value was unavailable. We also imputed the standard deviation from P values, according to the formulae stated in the Cochrane Handbook for Systematic Reviews of Intervention (Higgins 2011). This was only for the length of hospital stay which did not contribute to our conclusion because of the heterogeneity (and hence uncertainty) in the results. Thus, this did not impact on our conclusions. We were unable to assess the reporting bias because fewer than 10 trials met the inclusion criteria of this review. Since there was no restriction on the publication date, we included trials from the pre‐mandatory trial registration era. There is a possibility that some trials were not reported because of the direction of results. However, we have to be pragmatic and accept that it will be difficult to obtain useful data from these trials after such a long period of time. Therefore, we must base our conclusions on the trials that have been published or reported in conferences.
Agreements and disagreements with other studies or reviews
This is the first Cochrane review to assess non‐surgical versus surgical treatment for oesophageal cancer. We identified one previous systematic review on this topic (Pöttgen 2012). The authors of that systematic review concluded that surgery along with chemotherapy or chemoradiotherapy offers better results in terms of locoregional control than surgery alone or definitive chemoradiotherapy, and that definitive chemoradiotherapy is a reasonable choice especially in people with oesophageal squamous cell carcinoma and co‐morbidities. However, our conclusions are that chemoradiotherapy appears at least equivalent to surgery in terms of short‐term and medium‐term survival in people with oesophageal cancer (squamous type) who are fit for surgery. One possible reason for the difference in conclusions between the two systematic reviews is that Pöttgen 2012 did not perform a subgroup analysis of chemoradiotherapy versus surgery, which we had planned a priori in our review. However, it is unclear why the authors concluded that chemoradiotherapy is a reasonable choice in people with co‐morbidities, since the participants included in most included studies in Pöttgen 2012 were fit to undergo surgery.
Another question that has to be answered before arriving at any conclusions is whether the evidence from these trials is better than many observational studies that demonstrate that surgery (in combination with adjuvant therapy) offers the best outcome for most stages of operable oesophageal cancer. The major problem with such observational studies is the selection bias since the participants who receive chemoradiotherapy in such studies are unsuitable for surgery either in terms of their anaesthetic risk or in terms of the location or extent of cancer. This is because of the strong prejudice of surgeons in favouring surgery over other options. This prejudice was evident in a randomised controlled trial (RCT) where surgeons expressed preference to the surgery arm while recruiting participants in a RCT that compared definitive chemoradiotherapy with surgery (Blazeby 2014). This prejudice is also reflected in the current EMSO guidelines, which recommend definitive chemoradiotherapy only in those who are unfit for surgery (Stahl 2013). No statistical adjustment can account for the differences in the types of people who receive definitive chemoradiotherapy and surgery in such a prejudiced scenario because of the risk of residual confounding. The only study design that can overcome this prejudiced selection of participants for definitive chemoradiotherapy versus surgery is the RCT design. We have identified all RCTs on this topic. Despite the shortcomings in the studies included in this review, these studies constitute the best level of evidence that is currently available. Overall, the evidence from this systematic review is more trustworthy than observational studies and expert opinions.
Authors' conclusions
Implications for practice.
Based on low to very low quality evidence, chemoradiotherapy appears to be at least equivalent to surgery in terms of short‐term and long‐term survival in people with oesophageal cancer (squamous cell carcinoma type) who are fit for surgery and are responsive to induction chemoradiotherapy. However, there is uncertainty in the comparison of definitive chemoradiotherapy versus surgery for oesophageal cancer (adenocarcinoma type) and we cannot rule out significant benefits or harms of definitive chemoradiotherapy versus surgery. Based on very low quality evidence, the proportion of people with dysphagia at the last follow‐up visit prior to death was higher with definitive chemoradiotherapy compared to surgery. Based on very low quality evidence, radiotherapy results in less long‐term survival than surgery in people with oesophageal cancer who are fit for surgery. Notably, there is a risk of bias and random errors in these results, although the risk of bias in the included studies is likely to be less than in non‐randomised studies.
Implications for research.
Further trials at low risk of bias are necessary. Such trials need to compare endoscopic treatment with surgical treatment in early stage oesophageal cancer (carcinoma in situ and Stage Ia) and definitive chemoradiotherapy with surgical treatments in other stages of non‐metastatic oesophageal cancer. It may be inappropriate to compare definitive radiotherapy alone with surgical treatment given evidence of benefit of surgery over definitive radiotherapy. Early identification of responders to chemoradiotherapy and better second‐line treatment for non‐responders will also increase the need and acceptability of trials comparing definitive chemoradiotherapy with surgery. Trials of non‐surgical treatment versus surgical treatment should measure and report the mortality (with a follow‐up period of at least two to three years), health‐related quality of life using validated quality of life measures, treatment‐related adverse events including the severity, length of hospital stay during the entire follow‐up period, return to normal activity, and return to work. Trials need to be designed according to the SPIRIT statement (SPIRIT 2013), and need to be conducted and reported according to the CONSORT statement (CONSORT 2010).
Acknowledgements
We thank Karin Dearness, Managing Editor of the Cochrane Upper Gastrointestinal and Pancreatic Diseases Group (UGPD), for providing administrative and logistical support for this Cochrane review.
We thank the peer reviewers, copy editors, and the Cochrane Editorial Unit for their comments.
We thank the study authors who provided further information.
Appendices
Appendix 1. Glossary of terms
Adenocarcinoma: cancer arising from glandular cells. Anastomosis: joining of cut end of the bowel after removing part of oesophagus (food pipe) (in this context). Anastomotic dehiscence: breakdown of the anastomosis. Anastomotic stenosis: narrowing of the anastomosis. Cervical: of the 'neck' (in this context). Dissection: separation of different tissues during surgery. Dysphagia: difficulty in swallowing. Endoscopic: the insertion of a tube with a camera and light through the mouth to allow visual examination and perform procedures in the oesophagus (in this context). Endoscopic mucosal resection: removal of cancer using endoscope. Grade: in this context, indicates how aggressive the cancer cells appear under the microscope. Histological: examination under microscope. Incision: surgical cut. Induction chemoradiotherapy: starting dose of chemoradiotherapy. Laparoscopic: key hole surgery in the tummy. Metastases: cancer spread from site of origin to other parts of the body. Mobilisation: separating the oesophagus from the surrounding structures so that it can be removed (in this context). Morbidity: complications. Mortality: death. Mucosa: lining of the inner wall of the gut. Muscularis propria: muscle layer of the gut wall. Nodes: lymph glands. Oesophageal: of the 'food pipe'. Oesophagectomy: removal of oesophagus. Palliative: treatment that reduces the symptoms of a disease without curing it. Port‐site metastases: metastasis at the site of the surgical incisions (ports or holes) through which instruments are introduced into the body during laparoscopic surgery (key hole surgery). Renal: of the 'kidney'. Resection: surgical removal. Squamous cell carcinoma: cancer arising from squamous cells (a type of cell which appears flat under the microscope). Submucosa: layer underneath the mucosa. Thoracoscopic: key hole surgery in the chest. Transhiatal: through an opening in the diaphragm, the structure that separates the organs in the abdomen from the chest. Transthoracic: through the chest.
Appendix 2. CENTRAL search strategy
#1 MeSH descriptor: [Esophagectomy] explode all trees
#2 (esophagectomy or esophagectomies or oesophagectomy or oesophagectomies or ((esophag* or oesophag*) and (resection or resections or removal or operation or operations)))
#3 #1 or #2
#4 MeSH descriptor: [Esophageal Neoplasms] explode all trees
#5 (esophag* near/5 neoplas*)
#6 (oesophag* near/5 neoplas*)
#7 (esophag* near/5 cancer*)
#8 (oesophag* near/5 cancer*)
#9 (esophag* near/5 carcin*)
#10 (oesophag* near/5 carcin*)
#11 (esophag* near/5 tumo*)
#12 (oesophag* near/5 tumo*)
#13 (esophag* near/5 malig*)
#14 (oesophag* near/5 malig*)
#15 MeSH descriptor: [Esophagogastric Junction] explode all trees
#16 #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15
#17 #3 and #16
Appendix 3. MEDLINE search strategy
1. exp Esophagectomy/
2. (esophagectomy or esophagectomies or oesophagectomy or oesophagectomies or ((esophag* or oesophag*) and (resection or resections or removal or operation or operations))).mp.
3. 1 or 2
4. exp esophageal neoplasms/
5. (esophag* adj5 neoplas*).tw.
6. (oesophag* adj5 neoplas*).tw.
7. (esophag* adj5 cancer*).tw.
8. (oesophag* adj5 cancer*).tw.
9. (esophag* adj5 carcin*).tw.
10. (oesophag* adj5 carcin*).tw.
11. (esophag* adj5 tumo*).tw.
12. (oesophag* adj5 tumo*).tw.
13. (esophag* adj5 malig*).tw.
14. (oesophag* adj5 malig*).tw.
15. exp esophagogastric junction/
16. 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15
17. 3 and 16
18. randomized controlled trial.pt.
19. controlled clinical trial.pt.
20. randomized.ab.
21. placebo.ab.
22. drug therapy.fs.
23. randomly.ab.
24. trial.ab.
25. groups.ab.
26. 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25
27. exp animals/ not humans.sh.
28. 26 not 27
29. 17 and 28
Appendix 4. EMBASE search strategy
1. exp esophagus resection/
2. (esophagectomy or esophagectomies or oesophagectomy or oesophagectomies or ((esophag* or oesophag*) and (resection or resections or removal or operation or operations))).mp.
3. 1 or 2
4. exp esophagus tumor/
5. (esophag* adj5 neoplas*).tw.
6. (oesophag* adj5 neoplas*).tw.
7. (esophag* adj5 cancer*).tw.
8. (oesophag* adj5 cancer*).tw.
9. (esophag* adj5 carcin*).tw.
10. (oesophag* adj5 carcin*).tw.
11. (esophag* adj5 tumo*).tw.
12. (oesophag* adj5 tumo*).tw.
13. (esophag* adj5 malig*).tw.
14. (oesophag* adj5 malig*).tw.
15. exp lower esophagus sphincter/
16. 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15
17. 3 and 16
18. Clinical trial/
19. Randomized controlled trial/
20. Randomization/
21. Single‐Blind Method/
22. Double‐Blind Method/
23. Cross‐Over Studies/
24. Random Allocation/
25. Placebo/
26. Randomi?ed controlled trial$.tw.
27. Rct.tw.
28. Random allocation.tw.
29. Randomly allocated.tw.
30. Allocated randomly.tw.
31. (allocated adj2 random).tw.
32. Single blind$.tw.
33. Double blind$.tw.
34. ((treble or triple) adj blind$).tw.
35. Placebo$.tw.
36. Prospective study/
37. or/18‐36
38. Case study/
39. Case report.tw.
40. Abstract report/ or letter/
41. or/38‐40
42. 37 not 41
43. 17 and 42
Appendix 5. Science Citation Index search strategy
# 1 TS=(esophagectomy or esophagectomies or oesophagectomy or oesophagectomies or ((esophag* or oesophag*) and (resection or resections or removal or operation or operations)))
# 2 TS=(esophag* near/5 neoplas*)
# 3 TS=(oesophag* near/5 neoplas*)
# 4 TS=(esophag* near/5 cancer*)
# 5 TS=(oesophag* near/5 cancer*)
# 6 TS=(esophag* near/5 carcin*)
# 7 TS=(oesophag* near/5 carcin*)
# 8 TS=(esophag* near/5 tumo*)
# 9 TS=(oesophag* near/5 tumo*)
# 10 TS=(esophag* near/5 malig*)
# 11 TS=(oesophag* near/5 malig*)
# 12 #11 OR #10 OR #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2
# 13 #12 AND #1
# 14 TS=(random* OR rct* OR crossover OR masked OR blind* OR placebo* OR meta‐analysis OR systematic review* OR meta‐analys*)
# 15 #16 AND #15
Appendix 6. ClinicalTrials.gov search strategy
Interventional Studies | esophageal cancer | esophagectomy or resection | Phase 2, 3, 4
Appendix 7. World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) search strategy
oesphagectomy or esophagectomy or oesophageal resection or esophageal resection
Data and analyses
Comparison 1. Surgical versus non‐surgical treatment of oesophageal cancer.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term mortality | 5 | 689 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.11, 1.35] |
| 2 Long‐term mortality (binary) | 3 | 511 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.92, 1.14] |
| 3 Long‐term mortality (time‐to‐event) | 7 | 1114 | Hazard Ratio (Fixed, 95% CI) | 1.09 [0.97, 1.22] |
| 4 Proportion with a serious adverse event within 3 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Short‐term health‐related quality of life | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6 Medium‐term health‐related quality of life | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Long‐term recurrence (binary) | 2 | 339 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.87, 1.28] |
| 8 Long‐term recurrence (time‐to‐event) | 2 | 349 | Hazard Ratio (Fixed, 95% CI) | 0.96 [0.80, 1.16] |
| 9 Local recurrence (binary) | 3 | 449 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.70, 1.12] |
| 10 Proportion with any adverse event within 3 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11 Length of hospital stay (days) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12 Dysphagia at maximal follow‐up | 1 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [1.01, 2.19] |
| 13 Long‐term mortality (time‐to‐event): stratified by treatment | 7 | 1114 | Hazard Ratio (Fixed, 95% CI) | 1.09 [0.97, 1.22] |
| 13.1 Chemoradiotherapy | 4 | 602 | Hazard Ratio (Fixed, 95% CI) | 0.88 [0.76, 1.03] |
| 13.2 Radiotherapy | 3 | 512 | Hazard Ratio (Fixed, 95% CI) | 1.39 [1.18, 1.64] |
| 14 Long‐term mortality (binary): definitive chemoradiotherapy versus surgery with neoadjuvant chemotherapy | 2 | 431 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.93, 1.16] |
| 15 Long‐term mortality (time‐to‐event): definitive chemoradiotherapy versus surgery with neoadjuvant chemotherapy or chemoradiotherapy | 2 | 431 | Hazard Ratio (Fixed, 95% CI) | 0.99 [0.78, 1.26] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Badwe 1999.
| Methods | Randomised controlled trial (RCT) | |
| Participants | Country: India Number randomised: 99 Postrandomisation exclusions: 12 (12%) Number analysed: 87 Average age: 52 years Females: 26 (26%) Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 2 groups
Group 1: radiotherapy (N = 43) Further details: 50 gray of external radiation in 28 fractions followed by an external boost of 15 gray in 8 fractions or intraluminal radiotherapy of 15 gray with a 200 centigray/hour dose rate at 1 cm off axis Group 2: oesophagectomy (N = 44) Further details: standard Ivor‐Lewis procedure |
|
| Outcomes | Outcomes reported were short‐term mortality and long‐term mortality | |
| Cancer stage/histology | Not reported/squamous cell carcinoma | |
| Tumour location | Infra‐aortic | |
| American Society of Anaesthesiologists (ASA) physical status score | Not reported | |
| Notes | We attempted to contact the study authors in February 2015 Reasons for postrandomisation exclusions: 2 participants from the surgery arm due to direct spread to the bronchus, 10 participants from the radiotherapy arm as 7 received radiotherapy at other treatment centres and 3 did not take any treatment Participants were followed‐up for a maximum of 3 years |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised by closed envelope method". Comment: further details were unavailable. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "randomised by closed envelope method". Comment: further details were unavailable. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: it was impossible to blind the participants and healthcare providers. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was unavailable. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there was an imbalance in postrandomisation exclusions. |
| Selective reporting (reporting bias) | High risk | Comment: treatment‐related complications were not reported. |
| Source of funding | Unclear risk | Comment: this information was unavailable. |
| Other bias | Low risk | Comment: there was no other source of bias. |
Bedenne 2007.
| Methods | RCT | |
| Participants | Country: France Number randomised: 259 Postrandomisation exclusions: 0 (0%) Number analysed: 259 Average age: 58 years Females: 17 (6.7%) Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 2 groups Group 1: chemoradiotherapy (N = 130) Further details: external radiotherapy of 15 gray over 4 days and fluorouracil (FU) 800 mg/m² daily and cisplatin 15mg/m² daily for 5 days (x 3 cycles with a 2 week interval between cycles) Group 2: oesophagectomy (N = 129) Further details: different methods of oesophagectomy as required. Preoperative chemoradiotherapy was administered |
|
| Outcomes | Outcomes reported were short‐term mortality, long‐term mortality, recurrence, length of hospital stay, short‐term health‐related quality of life, and medium‐term health‐related quality of life. | |
| Cancer stage/histology | T3N0‐1M0/squamous cell carcinoma or adenocarcinoma | |
| Tumour location | Thoracic oesophagus | |
| American Society of Anaesthesiologists (ASA) physical status score | Not reported | |
| Notes | We attempted to contact the study authors in February 2015 Both groups received external radiotherapy (30 gray over 10 days with a 2 week interval between day 5 and day 6) and fluorouracil (FU) 800 mg/m² daily and cisplatin 15 mg/m² daily for 5 days (x 2 cycles with a 2 week interval between cycles) prior to randomisation Participants were followed up starting at 4 months after starting the treatment (in the surgical arm, 2 months after resection, in the non‐surgical arm, 3 weeks after the end of chemotherapy). Follow‐up was carried out every 3 months for 2 years and then every 6 months thereafter Median follow‐up: 4 years |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomly assigned by telephone at the FFCD Data Center through a minimization program". |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomly assigned by telephone at the FFCD Data Center through a minimization program". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: it was impossible to blind the participants and healthcare providers. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was unavailable. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no postrandomisation exclusions. |
| Selective reporting (reporting bias) | High risk | Comment: treatment‐related complications were not reported. |
| Source of funding | Low risk | Quote: "Supported by grants from the Ligue Nationale Contre le Cancer, the Fonds de Recherche de la Société Nationale Francaise de Gastroentérologie, the Programme Hospitalier pour la Recher‐ che Clinique, and the Association pour la Recherche Contre le Cancer". |
| Other bias | Low risk | Comment: there was no other source of bias. |
Blazeby 2014.
| Methods | RCT | |
| Participants | Country: UK Sample size: 5 Postrandomisation exclusions: 0 (0%) Number analysed: 5 Average age: not reported Females: not reported Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 2 groups Group 1: chemoradiotherapy (N = 2) Further details: chemotherapy was given for a total of 84 days (including induction therapy) of 21 days cycles of either cisplatin 80 mg/m² by intravenous infusion on days 1 and 5 followed by fluorouracil 1 g/m² per day intravenous infusion for 4 days or cisplatin 80 mg/m² by intravenous infusion on day 1 and capecitabine 625 mg/m² orally twice daily continuously and radiotherapy total 50 gray in 25 fractions Group 2: oesophagectomy (N = 3) Further details: different methods of oesophagectomy as required |
|
| Outcomes | The trial did not report any of the outcomes of interest | |
| Cancer stage/histology | T2‐4N0‐1M0/Squamous cell carcinoma | |
| Tumour location | Any part of the oesophagus up to 2 cm away from the cricopharyngeus | |
| American Society of Anaesthesiologists (ASA) physical status score | Not reported | |
| Notes | We attempted to contact the study authors in February 2015 Both groups received induction chemotherapy given as 21 days of either cisplatin 80 mg/m² by intravenous infusion on days 1 and 5 followed by fluorouracil 1 g/m² per day intravenous infusion for 4 days or cisplatin 80 mg/m² by intravenous infusion on day 1 and capecitabine 625 mg/m² orally twice daily continuously |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "When eligible patients consented to randomisation, treatment allocation was determined by an automated randomisation web‐based system". |
| Allocation concealment (selection bias) | Low risk | Quote: "When eligible patients consented to randomisation, treatment allocation was determined by an automated randomisation web‐based system". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: it was impossible to blind the participants and healthcare providers. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was unavailable. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no postrandomisation exclusions. |
| Selective reporting (reporting bias) | High risk | Comment: mortality and complications were not reported. |
| Source of funding | Low risk | Quote: "This article summarises independent research funded by the National Institute for Health Research (NIHR) under its Research for Patient Benefit (RfPB) Program (Grant reference PB‐PG‐0807– 14131)". |
| Other bias | Low risk | Comment: there was no other source of bias. |
Carstens 2007.
| Methods | RCT | |
| Participants | Country: Norway and Sweden Number randomised: 91 Postrandomisation exclusions: 0 (0%) Number analysed: 91 Average age: not reported Females: not reported Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 2 groups Group 1: chemoradiotherapy (N = 46) Further details: external radiotherapy total of 64 gray given in 32 fractions over 9 weeks and three 3‐weekly cycles of cisplatin 100 mg/m² on day 1 and 5‐fluorouracil chemotherapy 750 mg/m²/day from day 1 to 5 Group 2: oesophagectomy (N = 45) Further details: Ivor‐Lewis procedure |
|
| Outcomes | Outcomes reported were: short‐term mortality and long‐term mortality | |
| Cancer stage/histology | Not reported/squamous cell carcinoma or adenocarcinoma | |
| Tumour location | Not specified | |
| American Society of Anaesthesiologists (ASA) physical status score | Not reported | |
| Notes | We attempted to contact the study authors in February 2015, and the authors replied in March 2015 The trial authors did not specify the follow‐up period but reported 4 year follow‐up results. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was unavailable. |
| Allocation concealment (selection bias) | Low risk | Comment: participants were centrally allocated (information retrieved directly from the trial author). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: it was impossible to blind the participants and healthcare providers. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was unavailable. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was unavailable. |
| Selective reporting (reporting bias) | High risk | Comment: treatment‐related complications were not reported. |
| Source of funding | Unclear risk | Comment: this information was unavailable. |
| Other bias | Low risk | Comment: there was no other source of bias. |
Chiu 2005.
| Methods | RCT | |
| Participants | Country: China Number randomised: 81 Postrandomisation exclusions: 1 (1.2%) Number analysed: 80 Average age: 62 years Females: 14 (17.3%) Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 2 groups Group 1: chemoradiotherapy (N = 36) Further details: external radiotherapy total of 50 to 60 gray given in 25 to 30 fractions over 5 to 6 weeks and two 3‐weekly cycles of cisplatin 60 mg/m² on day 1 and day 22 and 5‐fluorouracil chemotherapy 200 mg/m²/day from day 1 to day 42 Group 2: oesophagectomy (N = 44) Further details: 2‐ or 3‐stage oesophagectomy |
|
| Outcomes | Outcomes reported were short‐term mortality, long‐term mortality, short‐term adverse events, short‐term serious adverse events, long‐term recurrence, and length of hospital stay | |
| Cancer stage/histology | Not reported (must be deemed resectable and have no metastases)/squamous cell carcinoma | |
| Tumour location | Mid‐ or lower‐thorax | |
| American Society of Anaesthesiologists (ASA) physical status score | Not reported | |
| Notes | We attempted to contact the study authors in February 2015 Reason for postrandomisation drop‐out: initially deemed resectable but later considered unresectable Patients were followed up at 6 to 8 week intervals in the 1st year and at 3‐month intervals thereafter Median follow‐up: 1.5 years |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was unavailable. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was unavailable. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: it was impossible to blind the participants and healthcare providers. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was unavailable. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there was an imbalance in postrandomisation exclusions. |
| Selective reporting (reporting bias) | Low risk | Comment: the trial reported all important outcomes. |
| Source of funding | Low risk | Quote: "This study was supported by the Research Grant Council (RGC) of Hong Kong Special Administrative Region, China". |
| Other bias | Low risk | Comment: there was no other source of bias. |
Fok 1994.
| Methods | RCT | |
| Participants | Country: China Number randomised: 156 Postrandomisation exclusions: 0 (0%) Number analysed: 156 Average age: 55 years Females: not reported Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 4 groups Group 1: radiotherapy (N = 35): Further details: 43 to 53 gray over 4 to 5 weeks Group 2: oesophagectomy with neoadjuvant radiotherapy (N = 40) Group 3: oesophagectomy with adjuvant radiotherapy (N = 42) Group 4: oesophagectomy without radiotherapy (N = 39) |
|
| Outcomes | Outcomes reported were long‐term mortality only | |
| Cancer stage/histology | Not reported ('potentially curable')/squamous cell carcinoma | |
| Tumour location | Middle third of the oesophagus | |
| American Society of Anaesthesiologists (ASA) physical status score | Not reported | |
| Notes | We attempted to contact the study authors in February 2015 Long‐term follow‐up with partial data were available at 10 years, however details were not specified |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was unavailable. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was unavailable. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: it was impossible to blind the participants and healthcare providers. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was unavailable. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no postrandomisation exclusions. |
| Selective reporting (reporting bias) | High risk | Comment: treatment‐related complications were not reported. |
| Source of funding | Unclear risk | Comment: this information was unavailable. |
| Other bias | Low risk | Comment: there was no other source of bias. |
Stahl 2005.
| Methods | RCT | |
| Participants | Country: Germany Number randomised: 172 Postrandomisation exclusions: 0 (0%) Number analysed: 172 Average age: 57 years Females: 34 (19.8%) Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 2 groups Group 1: chemoradiotherapy (N = 86) Further details: additional external radiotherapy with 2 x 1.5 gray/day for a total dose of at least 20 gray Group 2: oesophagectomy (N = 86) |
|
| Outcomes | Outcomes reported were short‐term mortality and long‐term mortality | |
| Cancer stage/histology | T3‐4 N0‐1 M0/squamous cell carcinoma | |
| Tumour location | Upper and mid third of the oesophagus | |
| American Society of Anaesthesiologists (ASA) physical status score | Not reported (WHO performance status grade of 0 to 1) | |
| Notes | We attempted to contact the study authors in February 2015 Both groups received external radiotherapy with 2 x 1.5 gray/day for a total dose of 40 gray and chemotherapy (bolus fluorouracil 500 mg/m², leucovorin 300 mg/m², etoposide 100 mg/m², and cisplatin 30 mg/m² on days 1 to 3 every 3 weeks) and cisplatin 50 mg/m² on days 2 to 8 and etoposide 80 mg/m² on days 3 to 5 concomitant with radiotherapy Participants were seen for the first follow‐up 8 to 12 weeks after the end of treatment and, thereafter, every 3 months up to 2 years. Afterwards, follow‐up was planned every 6 months up to 5 years Median follow‐up: 6 years |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Allocation to treatment groups was performed...using a computerized randomization program". |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation to treatment groups was performed...using a computerized randomization program". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "This was an unblinded, prospectively randomized phase III trial". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "This was an unblinded, prospectively randomized phase III trial". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no postrandomisation exclusions. |
| Selective reporting (reporting bias) | High risk | Comment: treatment related complications were not reported. |
| Source of funding | Low risk | Quote: "Supported by the Stiftung Deutsche Krebshilfe (German Cancer Aid)". |
| Other bias | Low risk | Comment: there was no other source of bias. |
Sun 2006.
| Methods | RCT | |
| Participants | Country: China Number randomised: 269 Postrandomisation exclusions: 0 (0%) Number analysed: 269 Average age: 56 years Females: 67 (24.9%) Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 2 groups Group 1: radiotherapy (N = 134) Further details: external radiotherapy conventionally fractionated at 1.8 to 2.0 Gray/day for the 1st two thirds of treatment course to a dose of about 50 to 50.4 gray followed by late course accelerated hyperfractionated radiotherapy, twice daily at 1.5 gray per fraction (with a minimal interval of 6 hours between fractions) to a dose of 18 to 21 gray. The total dose whole radiotherapy was 68.4 to 71.0 gray Group 2: oesophagectomy (N = 135) Further details: abdominothoracic approach |
|
| Outcomes | Outcomes reported were long‐term mortality and long‐term recurrence | |
| Cancer stage/histology | Not reported | |
| Tumour location | Upper, middle, and lower oesophagus | |
| American Society of Anaesthesiologists (ASA) physical status score | Not reported | |
| Notes | We attempted to contact the study authors in February 2015 Median follow‐up: 4.8 years |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was unavailable. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was unavailable. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: it was impossible to blind the participants and healthcare providers. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was unavailable. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was unavailable. |
| Selective reporting (reporting bias) | High risk | Comment: treatment‐related complications were not reported. |
| Source of funding | Unclear risk | Comment: this information was unavailable. |
| Other bias | Low risk | Comment: there was no other source of bias. |
Abbreviations: RCT: randomised controlled trial; TNM = tumour stage, nodal stage, and metastasis; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Desai 1987 | Participants were not randomised. |
| Earlam 1991 | The trial was stopped due to poor recruitment in May 1988 16 months after the start because only 31 participants had been entered. No randomised data was generated. |
| Hainsworth 2007 | The protocol for this trial was changed so allocation was not randomised. |
| Ilson 2007 | This was not a randomised controlled trial. However, we retrieved and screened the full‐text article as this was unclear based on the title alone. |
| Nozaki 2014 | This trial is expected to be completed in 2018 and the current report (a conference abstract) includes details of the safety of surgery in participants randomised to the surgical arm. The report included non‐randomised participants undergoing surgery as well. |
Differences between protocol and review
We reversed the intervention and control since surgical treatment is currently considered the standard treatment for oesophagectomy.
We revised the search strategy since the original search did not identify some trials.
We included dysphagia at maximal follow‐up as one of the secondary outcomes as this is an important patient symptom.
We performed a further subgroup analysis in which we compared definitive chemoradiotherapy versus oesophagectomy with neoadjuvant chemotherapy or chemoradiotherapy. This is because neoadjuvant chemotherapy or chemoradiotherapy along with surgery provides better survival than surgery alone (Sjoquist 2011) and is the treatment recommended by the European Society for Medical Oncology (ESMO) guidelines (Stahl 2013).
Contributions of authors
KG conceived the protocol and review. LB and KG designed the protocol and review. LB and KG co‐ordinated the protocol and review. KG designed the search strategies. LB wrote the protocol and review. KG and MM provided critical comments on the design and content of the review. KG secured funding for the protocol. Performed previous work that was the foundation of the current study: not applicable.
Sources of support
Internal sources
University College London, UK.
External sources
-
National Institute for Health Research, UK.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure, Cochrane Programme Grant or Cochrane Incentive funding to the Upper Gastro‐intestinal and Pancreatic Diseases group (UGPD) and Cochrane‐Hepato Biliary Group (CHBG). The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Declarations of interest
This report is independent research and is funded by the National Institute for Health Research (NIHR Cochrane Programme Grants, 13/89/03 ‐ Evidence‐based diagnosis and management of upper digestive, hepato‐biliary, and pancreatic disorders). The views expressed in this publication are those of the review authors and not necessarily those of the NHS, the National Institute for Health Research, or the Department of Health.
New
References
References to studies included in this review
Badwe 1999 {published data only}
- Badwe RA, Sharma V, Bhansali MS, Dinshaw KA, Patil PK, Dalvi N, et al. The quality of swallowing for patients with operable esophageal carcinoma: a randomized trial comparing surgery with radiotherapy. Cancer 1999;85(4):763‐8. [PubMed] [Google Scholar]
Bedenne 2007 {published data only}
- Bedenne L, Michel P, Bouché O, Milan C, Mariette C, Conroy T, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. Journal of Clinical Oncology 2007;25(10):1160‐8. [DOI] [PubMed] [Google Scholar]
- Bedenne L, Michel P, Bouché O, Triboulet JP, Conroy T, Pezet D, et al. Randomized phase III trial in locally advanced esophageal cancer: radiochemotherapy followed by surgery versus radiochemotherapy alone (FFCD 9102). Proceedings of the American Society of Clinical Oncology 2002;21(1):130. [Google Scholar]
- Bonnetain F, Bedenne L, Michel P, Bouche O, Triboulet JP, Conroy T, et al. Definitive results of a comparative longitudinal quality of life study using the Spitzer index in the randomized multicentric phase III trial FFCD 9102 (surgery vs radiochemotherapy in patients with locally advanced esophageal cancer). Proceedings of the American Society of Clinical Oncology 2003;39th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL:250. [Google Scholar]
- Bonnetain F, Bouché O, Michel P, Mariette C, Conroy T, Pezet D, et al. A comparative longitudinal quality of life study using the Spitzer quality of life index in a randomized multicenter phase III trial (FFCD 9102): chemoradiation followed by surgery compared with chemoradiation alone in locally advanced squamous resectable thoracic esophageal cancer. Annals of Oncology 2006;17(5):827‐34. [DOI] [PubMed] [Google Scholar]
Blazeby 2014 {published data only}
- Blazeby JM, Strong S, Donovan JL, Wilson C, Hollingworth W, Crosby T, et al. Feasibility RCT of definitive chemoradiotherapy or chemotherapy and surgery for oesophageal squamous cell cancer. British Journal of Cancer 2014;111(2):234‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carstens 2007 {published and unpublished data}
- Carstens H, Albertsson M, Friesland S, Adell G, Frykholm G, Wagenius G, et al. A randomized trial of chemoradiotherapy versus surgery alone in patients with resectable esophageal cancer. Journal of Clinical Oncology 2007;25(18 Suppl 1):4530. [Google Scholar]
Chiu 2005 {published data only}
- Chan AC, Lee DW, Leung SF, Griffith J, Siu WT, Li MK, et al. A multi‐center randomized controlled trial comparing standard esophagectomy versus primary chemo‐irradiation as the treatment for squamous esophageal cancer ‐ early results. Gastroenterology 2003;124(4):A298‐9. [Google Scholar]
- Chiu PW, Cheung FK, Mui WL, Yung MY, Lam CC, Chan AC, et al. The effect of definitive chemoradiotherapy (ChemoRT) against esophagectomy on pulmonary function in patients with squamous esophageal cancer ‐ results from a prospective randomized trial by Chinese University research group for esophageal cancer (CURE). Gastroenterology 2006;130(4):A454‐5. [Google Scholar]
- Chiu PWY, Chan ACW, Leung SF, Leong HT, Kwong KH, Li MKW, et al. Multicenter prospective randomized trial comparing standard esophagectomy with chemoradiotherapy for treatment of squamous esophageal cancer: early results from the Chinese University Research Group for Esophageal Cancer (CURE). Journal of Gastrointestinal Surgery 2005;9(6):794‐802. [DOI] [PubMed] [Google Scholar]
- Teoh AYB, Chiu PWY, Yeung WK, Liu SYW, Wong SKH, Ng EKW. Long‐term survival outcomes after definitive chemoradiation versus surgery in patients with resectable squamous carcinoma of the esophagus: results from a randomized controlled trial. Annals of Oncology 2013;24(1):165‐71. [DOI] [PubMed] [Google Scholar]
Fok 1994 {published data only}
- Fok M, Sham JST, Choy D, Cheng SWK, Wong J. Postoperative radiotherapy for carcinoma of the esophagus: a prospective, randomized controlled study. Surgery 1994;113(2):138‐47. [PubMed] [Google Scholar]
Stahl 2005 {published data only}
- Stahl M, Stuschke M, Lehmann N, Meyer HJ, Walz MK, Seeber S, et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. Journal of Clinical Oncology 2005;23(10):2310‐7. [DOI] [PubMed] [Google Scholar]
- Stahl M, Wilke H, Lehmann N, Stuschke M, German Oesophageal Cancer Study Group. Long‐term results of a phase III study investigating chemoradiation with and without surgery in locally advanced squamous cell carcinoma (LA‐SCC) of the esophagus. Journal of Clinical Oncology 2008;26(15 Suppl 1):4530. [Google Scholar]
- Stahl M, Wilke H, Preusser P, Stuschke M, Walz MK, Betzler M, et al. Preoperative chemoradiation followed by surgery versus definitive chemoradiation without surgery in the treatment of patients with locally advanced squamous cell carcinoma (LA‐SCC) of the esophagus: first report of a German multicenter study. Proceedings of the American Society of Clinical Oncology 2001;20:163. [Google Scholar]
- Stahl M, Wilke H, Walz MK, Seeber S, Klump B, Budach W, et al. Randomized phase III trial in locally advanced squamous cell carcinoma (SCC) of the esophagus: chemoradiation with and without surgery [abstract]. Proceedings of the American Society of Clinical Oncology 2003;39th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 31‐June 3, 2003:Abstract No. 1001. [Google Scholar]
- Stuschke M, Stahl M, Wilke H, Walz M, Seeber S, Klump B, et al. Randomized phase III trial in locally advanced squamous cell carcinoma of the esophagus: neoadjuvant radiochemotherapy followed by surgery vs. definitive radiochemotherapy. Radiotherapy and Oncology 2004;73 Suppl 1:148. [Google Scholar]
- Stuschke M, Stahl M, Wilke H, Walz MK, Seeber S, Klump B, et al. Randomized phase III trial in locally advanced squamous cell carcinoma of the esophagus: neoadjuvant radiochemotherapy followed by surgery vs. definitive radiochemotherapy. International Journal of Radiation Oncology, Biology, Physics 2004;60(1 Suppl 1):139. [Google Scholar]
Sun 2006 {published data only}
- Sun XD, Yu JM, Fan XL, Ren RM, Li MH, Zhang GL. Randomized clinical study of surgery versus radiotherapy alone in the treatment of resectable esophageal cancer in the chest. Zhonghua Zhong Liu Za Zhi [Chinese Journal of Oncology] 2006;28(10):784‐7. [PubMed] [Google Scholar]
- Yu J, Ren R, Sun X, Yin Y, Fu Z. A randomized clinical study of surgery versus radiotherapy in the treatment of resectable esophageal cancer. Journal of Clinical Oncology 2006;24(18 Suppl 1):4013. [PubMed] [Google Scholar]
References to studies excluded from this review
Desai 1987 {published data only}
- Desai PB, Advani SH, Sharma S, Saikia T, Gopal R. A comparison of surgery, radiotherapy and combination chemotherapy (followed by radiotherapy or surgery) in the treatment of locally advanced esophageal carcinoma. Proceedings of the American Society of Clinical Oncology. 1987:Abstract 289.
Earlam 1991 {published data only}
- Earlam R. An MRC prospective randomised trial of radiotherapy versus surgery for operable squamous cell carcinoma of the oesophagus. Annals of the Royal College of Surgeons of England 1991;73(1):8‐12. [PMC free article] [PubMed] [Google Scholar]
Hainsworth 2007 {published data only}
- Gray JR, Hainsworth JD, Meluch AA, Spigel DR, Rosenoff SH, Bradof JE, et al. Concurrent paclitaxel/carboplatin/infusional 5‐FU/radiation therapy (RT) with or without subsequent esophageal resection in patients with localized esophageal cancer: A Minnie Pearl Cancer Research Network trial. Journal of Clinical Oncology 2005;23(16 Suppl 1):4018. [Google Scholar]
- Hainsworth JD, Meluch AA, Gray JR, Spigel DR, Meng C, Bearden JD, et al. Concurrent chemoradiation followed by esophageal resection vs chemoradiation alone for localized esophageal cancer. Community Oncology 2007;4(7):431‐9. [Google Scholar]
Ilson 2007 {published data only}
- Ilson DH. Surgery after primary chemoradiotherapy in squamous cancer of the esophagus: is the photon mightier than the sword?. Journal of Clinical Oncology 2007;25(10):1155‐6. [DOI] [PubMed] [Google Scholar]
Nozaki 2014 {published data only}
- Nozaki I, Kato K, Igaki H, Ito Y, Daiko H, Yano M, et al. Safety profile of thoracoscopic esophagectomy for esophageal cancer compared with traditional thoracotomy from the results of JCOG0502: a randomized trial of esophagectomy versus chemoradiotherapy. Journal of Clinical Oncology 2014;32(1 Suppl 3):Abstract 82. [Google Scholar]
Additional references
Ajani 2011
- Ajani J, Barthel JS, Bentrem DJ, D'Amico T, Das P, Denlinger CS, et al. Esophageal and esophagogastric junction cancers. Journal of the National Comprehensive Cancer Network 2011;9(8):830‐87. [DOI] [PubMed] [Google Scholar]
AJCC Cancer Staging Manual 2010
- Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. 7th Edition. New York: Springer, 2010. [Google Scholar]
ASA 2014
- American Society of Anaesthesiologists. ASA physical status classification system. https://www.asahq.org/clinical/physicalstatus.htm (accessed 14 September 2014).
Berry 2014
- Berry M. Esophageal cancer: staging system and guidelines for staging and treatment. Journal of Thoracic Disease 2014;6 Suppl 3:289‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
CONSORT 2010
- Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Annals of Internal Medicine 2010;152(11):726‐32. [DOI] [PubMed] [Google Scholar]
Cuschieri 1992
- Cuschieri A, Shimi S, Banting S. Endoscopic oesophagectomy through a right thoracoscopic approach. Journal of the Royal College of Surgeons of Edinburgh 1992;37(1):7‐11. [PubMed] [Google Scholar]
Demets 1987
- Demets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
FDA 2006
- Center for Biologics Evaluation and Research, U.S. Food, Drug Administration. Guidance for industry. Adverse reactions section of labeling for human prescription drug and biological products — content and format. January 2006. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm075057.pdf (accessed on 4 July 2014).
Goh 2015
- Goh SL, Silva RP, Dhital K, Gett RM. Is low serum albumin associated with postoperative complications in patients undergoing oesophagectomy for oesophageal malignancies?. Interactive Cardiovascular and Thoracic Surgery 2015;20(1):107‐13. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. Chichester: John Wiley & Sons.
IARC 2014
- International Agency for Research on Cancer. GLOBOCAN 2012. http://globocan.iarc.fr/Default.aspx 2014 (accessed 5 September 2014).
ICH‐GCP 1996
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Code of Federal Regulation & ICH Guidelines. Media: Parexel Barnett, 1996. [Google Scholar]
Ishihara 2008
- Ishihara R, Iishi H, Uedo N, Takeuchi Y, Yamamoto S, Yamada T, et al. Comparison of EMR and endoscopic submucosal dissection for en bloc resection of early esophageal cancers in Japan. Gastrointestinal Endoscopy 2008;68(6):1066‐72. [DOI] [PubMed] [Google Scholar]
Luketich 2000
- Luketich JD, Schauer PR, Christie NA, Weigel TL, Raja S, Fernando HC, et al. Minimally invasive esophagectomy. Annals of Thoracic Surgery 2000;70(3):906‐11; discussion 911‐2. [DOI] [PubMed] [Google Scholar]
McKeown 1976
- McKeown KC. Total three‐stage oesophagectomy for cancer of the oesophagus. The British Journal of Surgery 1976;63(4):259‐62. [DOI] [PubMed] [Google Scholar]
MHRA 2013
- Medicines and Healthcare products Regulatory Agency (MHRA). Clinical trials for medicines: Safety reporting ‐ SUSARs and DSURs. 2013. http://www.mhra.gov.uk/Howweregulate/Medicines/Licensingofmedicines/Clinicaltrials/Safetyreporting‐SUSARsandASRs/ (accessed on 4 July 2014).
Migliore 2007
- Migliore M, Choong C, Lim E, Goldsmith K, Ritchie A, Wells F. A surgeon's case volume of oesophagectomy for cancer strongly influences the operative mortality rate. European Journal of Cardio‐Thoracic Surgery 2007;32(2):375‐80. [DOI] [PubMed] [Google Scholar]
Orringer 1978
- Orringer MB, Sloan H. Esophagectomy without thoracotomy. Journal of Thoracic and Cardiovascular Surgery 1978;76(5):643‐54. [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
Pennathur 2013
- Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. The Lancet 2013;381(9864):400‐12. [DOI] [PubMed] [Google Scholar]
Pöttgen 2012
- Pöttgen C, Stuschke M. Radiotherapy versus surgery within multimodality protocols for esophageal cancer‐‐a meta‐analysis of the randomized trials. Cancer Treatment Reviews 2012;38(6):599‐604. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (Revman). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rice 2010
- Rice TW, Blackstone EG, Rusch VW. 7th edition of the AJCC cancer staging manual: esophagus and esophagogastric junction. Annals of Surgical Oncology 2010;17(7):1721‐4. [DOI] [PubMed] [Google Scholar]
Sjoquist 2011
- Sjoquist KM, Burmeister BH, Smithers BM, Zalcberg JR, Simes RJ, Barbour A, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta‐analysis. The Lancet Oncology 2011;12(7):681‐92. [DOI] [PubMed] [Google Scholar]
SPIRIT 2013
- Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža‐Jerić K, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Annals of Internal Medicine 2013;158(3):200‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spitzer 1981
- Spitzer WO, Dobson AJ, Hall J, Chesterman E, Levi J, Shepherd R, et al. Measuring the quality of life of cancer patients: a concise QL‐index for use by physicians. Journal of Chronic Diseases 1981;34(12):585‐97. [DOI] [PubMed] [Google Scholar]
Stahl 2013
- Stahl M, Mariette C, Haustermans K, Cervantes A, Arnold D, EMSO Guidelines Working Group. Oesophageal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Annals of Oncology 2013;24(Suppl 6):vi51‐6. [DOI] [PubMed] [Google Scholar]
Sun 2014
- Sun F, Yuan P, Chen T, Hu J. Efficacy and complication of endoscopic submucosal dissection for superficial esophageal carcinoma: a systematic review and meta‐analysis. Journal of Cardiothoracic Surgery 2014;9:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Hagen 2012
- Hagen P, Hulshof MCMM, Lanschot JJB, Steyerberg EW, Berge Henegouwen MI, Wijnhoven BPL, et al. the CROSS Group. Preoperative chemoradiotherapy for esophageal or junctional cancer. The New England Journal of Medicine 2012;366(22):2074‐84. [DOI] [PubMed] [Google Scholar]
Yamamoto 2013
- Yamamoto M, Weber JM, Karl RC, Meredith KL. Minimally invasive surgery for esophageal cancer: review of the literature and institutional experience. Cancer Control 2013;20(2):130‐7. [DOI] [PubMed] [Google Scholar]
Yamashita 2008
- Yamashita H, Nakagawa K, Yamada K, Kaminishi M, Mafune K, Ohtomo K. A single institutional non‐randomized retrospective comparison between definitive chemoradiotherapy and radical surgery in 82 Japanese patients with resectable esophageal squamous cell carcinoma. Diseases of the Esophagus 2008;21(5):430‐6. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Best 2015
- Best LMJ, Gurusamy KS. Surgical versus non‐surgical treatment for oesophageal cancer. Cochrane Database of Systematic Reviews 2015, Issue 1. [DOI: 10.1002/14651858.CD011498] [DOI] [PMC free article] [PubMed] [Google Scholar]


