Key Points
Question
Can a machine learning–derived predictive early warning system for pending intraoperative hypotension in combination with a hemodynamic diagnostic guidance and treatment protocol reduce the time-weighted average of hypotension during noncardiac surgery?
Findings
In this single-center preliminary randomized clinical trial that included 68 patients undergoing elective noncardiac surgery, the time-weighted average of hypotension for those randomized to the early warning system vs those receiving standard care was 0.10 mm Hg vs 0.44 mm Hg, a difference that was statistically significant.
Meaning
While the use of a machine learning–derived early warning system compared with standard care resulted in less intraoperative hypotension, further research with larger study populations in diverse settings is needed to understand the effect on patient outcomes and to fully assess safety and generalizability.
Abstract
Importance
Intraoperative hypotension is associated with increased morbidity and mortality. A machine learning–derived early warning system to predict hypotension shortly before it occurs has been developed and validated.
Objective
To test whether the clinical application of the early warning system in combination with a hemodynamic diagnostic guidance and treatment protocol reduces intraoperative hypotension.
Design, Setting, and Participants
Preliminary unblinded randomized clinical trial performed in a tertiary center in Amsterdam, the Netherlands, among adult patients scheduled for elective noncardiac surgery under general anesthesia and an indication for continuous invasive blood pressure monitoring, who were enrolled between May 2018 and March 2019. Hypotension was defined as a mean arterial pressure (MAP) below 65 mm Hg for at least 1 minute.
Interventions
Patients were randomly assigned to receive either the early warning system (n = 34) or standard care (n = 34), with a goal MAP of at least 65 mm Hg in both groups.
Main Outcomes and Measures
The primary outcome was time-weighted average of hypotension during surgery, with a unit of measure of millimeters of mercury. This was calculated as the depth of hypotension below a MAP of 65 mm Hg (in millimeters of mercury) × time spent below a MAP of 65 mm Hg (in minutes) divided by total duration of operation (in minutes).
Results
Among 68 randomized patients, 60 (88%) completed the trial (median age, 64 [interquartile range {IQR}, 57-70] years; 26 [43%] women). The median length of surgery was 256 minutes (IQR, 213-430 minutes). The median time-weighted average of hypotension was 0.10 mm Hg (IQR, 0.01-0.43 mm Hg) in the intervention group vs 0.44 mm Hg (IQR, 0.23-0.72 mm Hg) in the control group, for a median difference of 0.38 mm Hg (95% CI, 0.14-0.43 mm Hg; P = .001). The median time of hypotension per patient was 8.0 minutes (IQR, 1.33-26.00 minutes) in the intervention group vs 32.7 minutes (IQR, 11.5-59.7 minutes) in the control group, for a median difference of 16.7 minutes (95% CI, 7.7-31.0 minutes; P < .001). In the intervention group, 0 serious adverse events resulting in death occurred vs 2 (7%) in the control group.
Conclusions and Relevance
In this single-center preliminary study of patients undergoing elective noncardiac surgery, the use of a machine learning–derived early warning system compared with standard care resulted in less intraoperative hypotension. Further research with larger study populations in diverse settings is needed to understand the effect on additional patient outcomes and to fully assess safety and generalizability.
Trial Registration
ClinicalTrials.gov Identifier: NCT03376347
This unblinded randomized trial compares the effect of a machine learning–derived system that uses arterial waveform information to predict cardiovascular decompensation vs standard monitoring by anesthesiologists on the depth and duration of intraoperative hypotension in patients undergoing elective noncardiac surgery.
Introduction
An estimated 266 million operations were performed worldwide in 2015.1 One of the risks patients commonly face is intraoperative hypotension. A previous study involving 255 patients reported that 87% experienced 1 or more hypotensive episodes intraoperatively (with hypotension defined as a mean arterial pressure [MAP] <65 mm Hg).2 Reported causes are anesthetic drugs, existing comorbidities, and surgical manipulation.3,4
Clinical cohort studies have shown intraoperative hypotension in noncardiac surgery to be associated with postoperative complications such as renal insufficiency, myocardial injury, and increased mortality.5,6,7,8,9,10
Current management of intraoperative hypotensive episodes is predominantly reactive. Recently, Hatib et al11 developed an algorithm with the use of machine learning to predict hypotension minutes before blood pressure actually decreases, the Hypotension Prediction Index. This algorithm (hereafter referred to as the early warning system) was developed using the arterial waveform data of 1344 patients, and it has been internally and externally validated, showing a sensitivity of 88% and a specificity of 87%.2,11 This early warning system is fixed, meaning that it does not include dynamic learning changes evolving during patient care. Using an early warning system to predict hypotension does not necessarily lead to less hypotension. Associated factors to consider are feasibility of working with this tool and the possibility of performing a timely and correct intervention. Hemodynamic variables, in combination with a hemodynamic diagnostic guidance and treatment protocol, allow for determination of the underlying cause of the impending hypotension.
A preliminary single-center randomized clinical trial (RCT) was performed. It was hypothesized that use of the early warning system would reduce the amount of hypotension (MAP <65 mm Hg) as measured by time-weighted average during major noncardiac surgery.12
Methods
Participants
The Hypotension Prediction (HYPE) trial was a preliminary investigator-initiated single-center RCT. The study took place at the Amsterdam University Medical Centers, Location AMC, a tertiary academic center in the Netherlands. The study was approved by the institutional review board of the AMC (NL62115.018.17). Written informed consent was obtained from all patients the day prior to surgery by a designated researcher. The first participant was enrolled in May 2018, and the last follow-up was in March 2019. The trial protocol has been published previously13 and is available in Supplement 1.
Adult patients (≥18 years old) scheduled to undergo an elective noncardiac surgical procedure under general anesthesia with need for continuous invasive blood pressure monitoring per arterial line were included. A target MAP of at least 65 mm Hg during surgery was obligatory to ensure both study groups to be similar in this aspect. Patients for whom the attending anesthesiologists requested a target MAP higher or lower than 65 mm Hg were excluded prior to surgery. Patients undergoing emergency surgery were not eligible. Patients with cardiac failure, severe cardiac shunts, severe aortic stenosis, or severe cardiac arrhythmias were excluded in accordance with the summary of product characteristics of the early warning system. Patients with hypotension (MAP <65 mm Hg) before surgery and patients requiring dialysis were also excluded. Patients planned to undergo liver surgery or vascular surgery were excluded because of the use of vascular clamping. During the trial, anesthesiologists were not allowed to use perioperative goal-directed fluid therapy because the study hemodynamic diagnostic guidance and treatment protocol has blood pressure as a starting point, while a goal-directed fluid therapy protocol typically starts with flow evaluation as the main concept.
Randomization
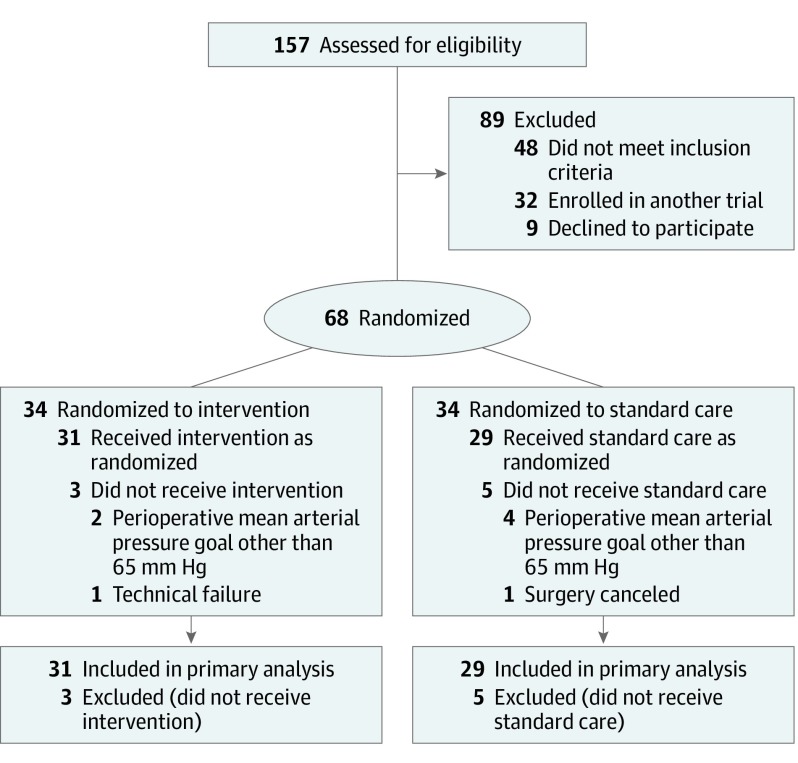
Patients were randomized to either the intraoperative early warning system (intervention group) or standard care (control group) (Figure 1). A computer-generated permutated block randomization (concealed and varying permutated block sizes of 4, 6, or 8 patients) was used with a 1:1 allocation ratio. The researcher performing statistical analyses was blinded to patient group allocation.
Figure 1. Participant Flow in the Hypotension Prediction (HYPE) Trial.
Intervention
In all study participants an arterial catheter was placed in the radial artery and connected to the Flotrac IQ sensor with the early warning system software (Edwards Lifesciences). The arterial pressure waveform was measured continuously with a sample frequency of 100 Hz. The HemoSphere monitor (Edwards Lifesciences) displayed hemodynamic parameters calculated from the waveform every 20 seconds. The value calculated by the early warning system was updated every 20 seconds as well.11
The Flotrac IQ pressure transducer was connected to the HemoSphere monitor (hereafter referred to as the study monitor), and the resulting electrical signal was transmitted to the Philips monitor (hereafter referred to as the standard monitor). The standard monitor displayed the MAP, systole, diastole, and pulse pressure variation per standard care in the study hospital. The quality of the arterial waveform signal was visually checked by the treating anesthesiologist for overdamping and underdamping after placement of the arterial line and during surgery.
Attending anesthesiologists and anesthesia nurses were informed about the study protocol and the use of the early warning system the day before surgery. Intraoperatively, an observer was present to record surgery- and anesthesia-related details.
Use of the study monitor was additional to standard care monitoring. The early warning system detects deteriorations in cardiovascular compensatory mechanisms that could lead to hypotension. The early warning system consists of 23 variables that are extracted from the arterial pressure waveform.11 When the value of the early warning system alarm (hereafter referred to as the alarm) exceeds 85, the likelihood of occurrence of a hypotensive event within the next 15 minutes is about 85%.2 The time to hypotension is not fixed; the progression into hypotension depends on the underlying physiological mechanisms causing the hypotension and on individual patient characteristics.11 The performance of the early warning system regarding prediction of hypotension was analyzed on patient data collected in the short observational study according to methods previously described by Hatib et al11 and presented in eFigure 1 in Supplement 2.
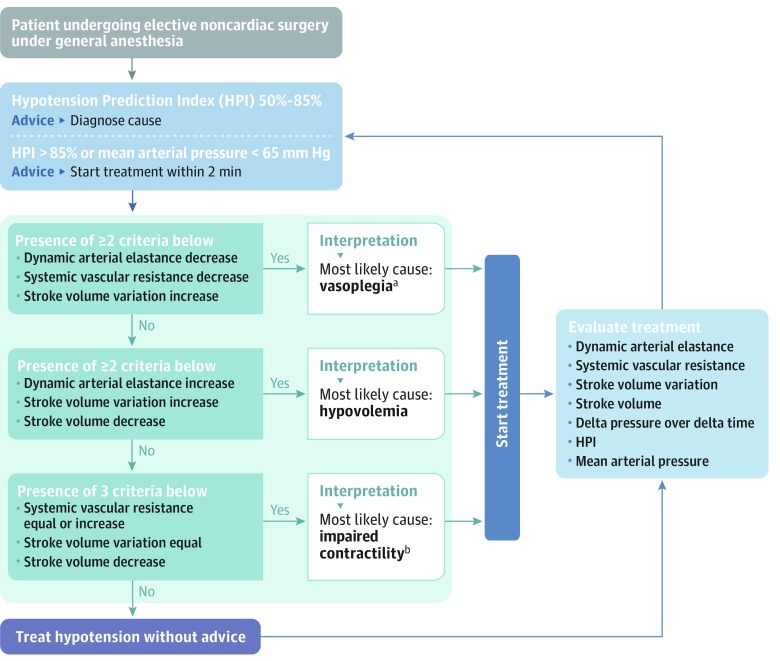
In this study, when the value of prediction of hypotension exceeded 85% (eFigure 2 in Supplement 2), which entailed both a sound and a flickering light, the anesthesiologist was reminded to act, preferably within 2 minutes. The study monitor with the early warning system software has a secondary screen (eFigure 2) with variables (heart rate, cardiac output, change in pressure over change in time, stroke volume, stroke volume variation, dynamic arterial elastance, and systemic vascular resistance) that provide information about the underlying cause of the predicted hypotension.14 The hemodynamic diagnostic guidance and treatment protocol (Figure 2 and eFigure 3 in Supplement 2) was designed by the authors to help treating anesthesiologists interpret the changes of the variables visible on the secondary screen.13 For example, the combination of an increase (arrow up) in stroke volume variation and a decrease (arrow down) in systemic vascular resistance results in the diagnosis of vasoplegia (Figure 2 and eFigure 3). The hemodynamic diagnostic guidance and treatment protocol was adapted from Pinsky and Payen.15
Figure 2. Hemodynamic Diagnostic Guidance and Treatment Protocol.
aVasoplegia indicates decreased systemic vascular resistance.
bImpaired left ventricular contractility.
Control
In the control group, the study monitor was connected, but the screen was fully covered and the alarms were silenced; anesthesiologists solely used the variables visible on the standard monitor to guide hemodynamic treatment.
Prior to launching the RCT, we conducted a short observational study to ensure that the care the control group received was representative of standard care in the study hospital (eTables 1 and 2 in Supplement 2). The only difference between the control group and the observational study group was that anesthesiologists were unaware of the aim of the study (to assess hypotension) in the observational study group.
All data were entered using an electronic clinical report form build in Castor EDC, a Good Clinical Practice–compliant data management system.16
Outcomes
The primary outcome measure was the time-weighted average of hypotension during surgery. The outcome assessor was blinded to participants’ group randomization. The time-weighted average combines the duration and severity (the minimal MAP reached) of hypotension, corrected for the total time of surgery.12 Hypotension was defined as a MAP less than 65 mm Hg for at least 1 minute. A hypotensive event ended when the value normalized (MAP ≥65 mm Hg) for at least 1 minute. The time-weighted average is measured by calculating the area under the threshold (AUT) divided by the total duration of surgery (eFigure 4 in Supplement 2)12,13: time-weighted average = (depth of hypotension in millimeters of mercury below a MAP of 65 mm Hg × time in minutes spent below a MAP of 65 mm Hg) ÷ total duration of operation in minutes. The units for AUT are millimeters of mercury × minutes and the units for time-weighted average are millimeters of mercury.13
As an example, a patient undergoes a surgery that lasts 180 minutes, in which they experience 10 episodes of hypotension, all lasting for 1 minute and all with a minimal MAP of 60 mm Hg. The AUT = 10 minutes × (65 − 60 = 5 mm Hg under the MAP threshold of 65 mm Hg) = 10 × 5 = 50 mm Hg per minute. The time-weighted average = 50 mm Hg per minute ÷ 180 minutes = 0.28 mm Hg.
The secondary outcome measures included incidence of hypotension (the number of hypotensive events per patient), total time with hypotension, and percentage of time spent with hypotension during surgery. To assess the risk of overtreatment, the above-mentioned variables were also assessed for hypertension (defined as a MAP >100 mm Hg for at least 1 minute). To be able to calculate the time-weighted average for hypertension, first the area above the curve needed to be calculated (eFigure 4 in Supplement 2). These time and incidence variables were also assessed post hoc for the alarms. An alarm was deemed present when the early warning system prediction value reached 85% or higher for at least 1 minute. An alarm ended when the value normalized (<85%) for at least 1 minute.
Clinicians’ behavior regarding treatment of alarms (intervention group) and hypotension (control group) was assessed. We noted (1) treatment choice (ie, vasopressor, fluids, inotropes, Trendelenburg position, and decrease in anesthetics); (2) cumulative dose; and (3) time from alarm to start of treatment in the intervention group and from onset of hypotension to start of treatment in the control group. All alarms or hypotensive events per patient were used for this analysis. If an alarm or hypotensive event had more than 1 treatment, the time to first treatment was used. In post hoc analyses, control group clinicians’ treatment behavior after silent alarms to which they were blinded was also assessed and compared with treatment behavior after alarms in the intervention group. We calculated (1) total number of silent alarms; (2) number of alarms per patient; (3) number of alarms that led to treatment; (4) number of treatments per alarm; (5) time from alarm to treatment in the intervention group compared with time from silent alarm to treatment in the control group (all alarms per patient were used for this analysis; if an alarm had more than 1 treatment, the time to first treatment was used); and (6) time from first alarm to first treatment in the intervention group compared with time from first silent alarm to first treatment in the control group. The last analysis was performed because all actions after the first alarm might be influenced by and correlated with the first alarm.
The feasibility of working with the hemodynamic diagnostic guidance and treatment protocol was analyzed based on the number of protocol violations.
Primary and secondary end points were analyzed for the intraoperative period only. Intraoperative and postoperative adverse events and serious adverse events were documented (for definitions, see eTable 3 in Supplement 2).
Sample Size Calculation
Time-weighted average is a relatively novel end point such that only an estimation could be made of what difference would be clinically relevant. Prior to the study, an expert panel familiar with the potential effect of intraoperative hypotension was consulted, and it was decided that a 75% reduction of hypotension in terms of combined depth and duration (time-weighted average) was considered to be clinically relevant. Prior to this study, the mean time-weighted average of hypotension in the study clinic was estimated to be 0.5 mm Hg. Thus, the estimated mean difference between groups for the calculation of the sample size was considered to be 0.38 mm Hg. Based on preliminary results from a trial,12 the standard deviation of time-weighted average of hypotension was estimated to be 0.51 mm Hg. Dividing the mean difference by the standard deviation resulted in an effect size of 0.74. It was calculated that a sample size of 60 patients, 30 in each group, would have 80% power to detect this effect using a 2-group t test with an α = .05 2-sided significance level. R version 3.3.3 (R Foundation) was used to perform these calculations.17
Patients who were randomized but for whom no study measurements were performed were excluded (Figure 1).
Statistical Analysis
All patients who met the inclusion criteria at the end of the study period were analyzed according to their randomization group. If data were missing, the amount of missing data and the reasons were assessed.
Continuous data are presented as medians with interquartile ranges (IQRs) or as means with standard deviations when normally distributed. The confidence intervals for the median differences were calculated with the Hodges-Lehmann method. Normality of distribution was assessed visually with histograms and Q-Q plots. Categorical data are presented as frequencies with percentages. Differences between categorical data were analyzed using the χ2 test.
For each of the analyses, a 2-sided probability value of P < .05 was considered to be statistically significant. An exploratory regression analysis was performed to assess possible effects of confounders on the primary end point (eFigure 5 in Supplement 2). Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary end points and post hoc end points should be interpreted as exploratory.
All analyses were performed using Matlab version R2018b (MathWorks Inc) and SPSS version 25 (IBM Corp).
Results
Study Population
For the preliminary RCT, 157 patients were assessed for eligibility. A total of 68 patients were enrolled, of whom 34 were randomly assigned to the intervention group and 34 to the control group (Figure 1). Of these 68 patients, 60 (88%) completed the trial. The median age was 64 (interquartile range, 57-70) years); 26 patients (43%) were women. The majority of patients completing the trial (n = 54 [90%]) underwent oncologic gastrointestinal surgery. Table 1 shows the baseline characteristics of the intervention group and the control group. The short observational study conducted prior to launching the RCT consisted of 40 patients (eTables 1 and 2 in Supplement 2). None of the analyzed determinants had any missing values.
Table 1. Baseline Characteristics.
| Characteristics | Intervention (n = 31) | Control (n = 29) |
|---|---|---|
| Age, median (IQR), y | 68 (61-73) | 62 (56-67) |
| Sex, No. (%) | ||
| Men | 21 (68) | 13 (45) |
| Women | 10 (32) | 16 (55) |
| Body mass index, median (IQR)a | 24.2 (21-26) | 24.7 (22-27) |
| ASA classification, No. (%)b | ||
| 1 | 1 (3) | 3 (10) |
| 2 | 24 (77) | 24 (83) |
| 3 | 6 (19) | 2 (7) |
| 4 | 0 | 0 |
| WHO classification, No. (%)c | ||
| 0 | 20 (65) | 17 (59) |
| 1 | 6 (19) | 6 (21) |
| 2 | 4 (13) | 5 (17) |
| 3 | 1 (3) | 1 (3) |
| 4 | 0 | 0 |
| MAP, median (IQR), mm Hg | ||
| Outpatient clinic | 100 (93-106) | 92 (81-98) |
| Day before | 98 (88-105) | 92 (82-102) |
| Before induction | 104 (95-112) | 93 (85-104) |
| Type of surgery, No. (%) | ||
| Gynecological | 1 (3) | 3 (10) |
| Gastrointestinal | 30 (97) | 24 (83) |
| Pancreas | 19 (63) | 9 (38) |
| Esophagus | 9 (30) | 8 (33) |
| Otherd | 0 | 2 (7) |
| Surgical approach, No. (%) | ||
| Laparotomy | 19 (61) | 13 (45) |
| Laparoscopic | 2 (7) | 5 (17) |
| Conversion | 1 (3) | 3 (10) |
| Combined | 9 (29) | 8 (28) |
| Duration, median (IQR), min | ||
| Surgerye | 256 (194-425) | 259 (223-442) |
| Anesthesia administrationf | 302 (230-475) | 300 (259-487) |
Abbreviations: ASA, American Society of Anesthesiologists; IQR, interquartile range; MAP, mean arterial pressure; WHO, World Health Organization.
Calculated as weight in kilograms divided by height in meters squared.
The ASA classifications were as follows: 1: a healthy person; 2: a patient with mild systemic disease; 3: a patient with severe systemic disease; and 4: a patient with severe systemic disease that is a constant threat to life.18,19
The WHO classifications were as follows: 0: fully active, able to carry on all predisease performance without restriction; 1: restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature, eg, light housework, office work; 2: ambulatory and capable of all self-care but unable to carry out any work activities, mobile for more than 50% of waking hours; 3: capable of only limited self-care, confined to bed or chair more than 50% of waking hours; 4: completely disabled, unable to carry out any self-care, totally confined to bed or chair.20,21
Including excision of a recurrent abdominal wall carcinoma and a deep inferior epigastric perforator breast reconstruction.
Duration of surgery was calculated in minutes from the time of incision until closure of the surgical wound.
Calculated in minutes from the time of first anesthetic drug administration (sufentanil, lidocaine, or propofol) until extubation. If extubation was not in the operating room but in the intensive care unit or postanesthesia care unit, the time of leaving the operating room was noted as the end of anesthesia administration.
Primary End Point
The median time-weighted average of hypotension was 0.10 mm Hg (IQR, 0.01-0.43 mm Hg) in the intervention group vs 0.44 mm Hg (IQR, 0.23-0.72 mm Hg) in the control group, for a median difference of 0.38 mm Hg (95% CI, 0.14-0.43 mm Hg; P = .001) (Table 2).
Table 2. Primary and Secondary End Points.
| Median (Interquartile Range)a | Median Difference (95% CI)b | P Valuec | ||
|---|---|---|---|---|
| Intervention (n = 31) | Control (n = 29) | |||
| Primary End Point | ||||
| Time-weighted average of hypotension, mm Hg | 0.10 (0.01-0.43) | 0.44 (0.23-0.72) | 0.38 (0.14 to 0.43) | .001 |
| Secondary End Points | ||||
| Hypotension | ||||
| Area under the threshold, mm Hg/mind | 20.0 (2.2-148.3) | 142.2 (64.67-258.92) | 74.0 (33.0 to 137.7) | .002 |
| Incidence | 3.0 (1.0-8.0) | 8.0 (3.5-12.0) | 4.0 (1.0 to 7.0) | .004 |
| Total time, min | 8.0 (1.3-26.0) | 32.7 (11.5-59.7) | 16.7 (7.7 to 31.0) | .001 |
| Surgery time, % | 2.8 (0.8-6.6) | 10.3 (4.6-15.6) | 5.6 (3.0 to 9.4) | <.001 |
| Hypertension | ||||
| Time-weighted average, mm Hg | 0.09 (0.00-0.21) | 0.05 (0.00-0.13) | 0.00 (−0.85 to 0.17) | .47 |
| Area above the threshold, mm Hg/mind | 33.3 (0.0-88.0) | 13.3 (0.0-44.3) | −3.5 (−29.0 to 5.5) | .40 |
| Incidence | 2.0 (0.0-3.0) | 1.0 (0.0-2.0) | 0.0 (−1.0 to 0.0) | .23 |
| Total time, min | 4.0 (0.0-10.7) | 3.0 (0.0-6.8) | −0.7 (−4.3 to 0.7) | .40 |
| Surgery time, % | 1.5 (0.0-3.3) | 0.9 (0.0-1.9) | −0.2 (−1.4 to 0.3) | .40 |
| Treatment behavior | ||||
| Reaction time, se | 53.0 (24.0-99.0) | 87.3 (53.0-172.5) | 34.3 (22.8 to 47.3) | <.001 |
| Post Hoc End Points | ||||
| Treatment behavior | ||||
| No. of treatments per patientf | 15.0 (5.0-29.0) | 9.0 (3.5-13.0) | −6.0 (−13.0 to −1.0) | .02 |
| Early warning system alarms | ||||
| Time-weighted average, Hypotension Prediction Indexg | 1.99 (1.12-3.17) | 4.31 (2.50-5.79) | 1.79 (0.74 to 2.95) | .001 |
| Area above the threshold, Hypotension Prediction Index/mind | 529.7 (196.3-1315.0) | 1231.0 (701.5-1966.3) | 629.3 (229.3 to 1012.3) | .002 |
| Incidence | 11.0 (7.0-16.0) | 11.0 (8.0-14.5) | 0.0 (−4.0 to 3.0) | .84 |
| Total time, min | 56.7 (21.7-122.7) | 116.3 (68.3-170.3) | 51.7 (20.7 to 91.0) | .002 |
| Time, % | 20.9 (14.5-35.6) | 41.1 (23.9-56.4) | 15.8 (5.8 to 25.9) | .002 |
All end points are medians per patient. Incidence rates of hypotension, hypertension, and early warning system alarms are median number of events per patient.
Median differences and their 95% confidence intervals were calculated with the Hodges-Lehmann method.
P values were measured with the Mann-Whitney U test.
See Supplement 7 for details on calculation of area under the threshold and area above the threshold.
In the intervention group, reaction time was measured as the time (in seconds) from the onset of the early warning system alarm to treatment. In the control group, reaction time was defined as the time from start of hypotension to treatment.
Treatments per patient were calculated as median number of treatments related to hypotension or early warning system alarm per patient.
The Hypotension Prediction Index (referred to in this article as the early warning system) is an algorithm developed with the use of machine learning to predict hypotension.11
Secondary End Points
Hypotension
In the intervention group, 26 patients (84%) experienced 1 or more hypotensive episode during surgery compared with 28 (97%) in the control group, for a difference of 13% (95% CI, −2% to 28%; P = .09). The median incidence of hypotension was 3.00 (IQR, 1.00-8.00) hypotensive episodes per patient in the intervention group vs 8.00 (IQR, 3.50-12.00) in the control group, for a median difference of 4.00 (95% CI, 1.00-7.00) episodes per patient (P = .004). The median incidence of hypotension was calculated including patients who had 0 hypotensive episodes. The median total time of having hypotension per patient was 8.00 (IQR, 1.33-26.00) minutes in the intervention group vs 32.67 (IQR, 11.50-59.67) minutes in the control group, for a median difference of 16.67 (95% CI, 7.67-31.00) minutes (P < .001).
There were no significant differences for hypotension end points between the control group and the observational study group (eTable 2 in Supplement 2).
Treatment Behavior
Comparing treatment choice, ephedrine was chosen 38 of 596 times (6%) in the intervention group vs 37 of 258 times (14%) in the control group, for a difference of 8% (95% CI, 6%-14%; P < .001). Phenylephedrine was chosen 110 of 596 times (19%) in the intervention group compared with 61 of 258 times (24%) in the control group, for a difference of 5% (95% CI, 1% to 11%; P = .04). Fluid boluses were chosen 96 of 596 times (16%) as treatment of choice in the intervention group compared with 16 of 258 times (6%) in the control group, for a difference of 10% (95% CI, 6%-14%; P < .001) (eTable 4 in Supplement 2).
There was no significant difference in the cumulative dose of vasopressors or fluids given during surgery (Table 3). The median cumulative dose of noradrenaline was 1034 μg (IQR, 770-1720 μg) in the intervention group compared with 925 μg (IQR, 428-2131 μg) in the control group (median difference, 118 μg; 95% CI, −418 to 534 μg; P = .67). The median dose of fluids was 1800 mL (IQR, 1500-2700 mL) in the intervention group vs 1800 mL (IQR, 1450-2650 mL) in the control group (median difference, 150 mL; 95% CI, −470 to 600 mL; P = .58).
Table 3. Cumulative Doses of Medications Given During Surgery.
| Medications | Median (Interquartile Range) | Median Difference (95% CI) | P Value | |
|---|---|---|---|---|
| Intervention (n = 31) | Control (n = 29) | |||
| Noradrenaline, μg | 1034.0 (770.7-1720.0) | 925.7 (428.7-2131.0) | −118.0 (−534.3 to 418.0) | .67 |
| Noradrenaline, μg/kg/mina | 0.057 (0.033-0.071) | 0.040 (0.021-0.066) | −0.010 (−0.025 to 0.005) | .18 |
| Ephedrine, mg | 10.0 (0.0-22.5) | 10.0 (10.0-16.3) | 0.0 (−5.0 to 5.0) | .52 |
| Phenylephrine, μg | 200.0 (100.0-600.0) | 300.0 (100.0-500.0) | 0.0 (−200.0 to 100.0) | .84 |
| Fluids, mL | 2100.0 (1750.0-3000.0) | 1800.0 (1550.0-2950.0) | −150 (−600.0 to 470.0) | .58 |
| Colloids, mL | 250.0 (0.0-500.0) | 0.0 (0.0-500.0) | 0.0 (−250.0 to 0.0) | .09 |
| Crystalloids, mL | 1800.0 (1500.0-2700.0) | 1800.0 (1450.0-2650.0) | 100.0 (−550.0 to 300.0) | .72 |
| Fluid balance, mLb | 1180.0 (680.0-1650.0) | 1150.0 (582.5-1552.5) | −80.00 (−480.00 to 300.00) | .62 |
| Propofol, mgc | 100 (60-1647) | 125 (50-2568) | 20 (−1430 to 1467) | .90 |
| Sevoflurane, % volume | 1.67 (1.48-1.73) | 1.57 (1.42-1.75) | −0.02 (−0.80 to 0.17) | .66 |
| Sufentanil (intravenous), μg | 80.0 (50.0-85.0) | 70.0 (53.8-85.0) | −5.0 (−25.0 to 15.0) | .51 |
| Morphine, mgd | 10.00 (8.50-11.25) | 10.00 (8.75-15.00) | 0.00 (−2.50 to 6.00) | .83 |
| Epidural analgesia, No. (%) of patients | 21 (68) | 13 (45) | .07 | |
Noradrenaline dose corrected for patient body weight.
Fluid balance at the end of surgery.
Calculated for patients who received propofol (n = 7 in the intervention group and n = 12 in the control group).
Calculated for patients who received morphine (n = 6 in the intervention group and n = 13 in the control group).
The median time from alarm (intervention group) or hypotension (control group) to first treatment was 53 seconds (IQR, 24-99 seconds) in the intervention group vs 87 seconds (IQR, 53-173 seconds) in the control group (median difference, 34 seconds; 95% CI, 23-47 seconds; P < .001).
In post hoc analyses, the median time from alarm to treatment (intervention group) or the median time from silent alarm (blinded for clinicians) to treatment (control group) was 53 seconds (IQR, 24-99 seconds) in the intervention group vs 161 seconds (IQR, 73-391 seconds) in the control group (median difference, 48 seconds; 95% CI, 13-97 seconds; P < .001). The median time from solely the first alarm in all patients (silent alarm in the control group) to first treatment was 57 seconds (IQR, 22-81 seconds) in the intervention group vs 108 (IQR, 44-204 seconds) in the control group (median difference, 91 seconds; 95% CI, 70-117 seconds; P = .01) (eTable 5 in Supplement 2).
Hemodynamic Diagnostic Guidance and Treatment Protocol Violations
In total, 377 predictive alarms with a duration of more than 1 minute were present in the intervention group. Among the 377 alarms, 81% (304 alarms) led to treatment within 2 minutes. In 5% (20 alarms), treatment was not according to study treatment protocol, and 14% of alarms (53 alarms) were ignored by anesthesiologists.
There were several reasons for ignoring alarms (protocol violations). In 36% (19 alarms), the current treatment modality was exhausted or treatment was provided just before the alarm (indicating a high [>85%] chance of hypotension occurring) was triggered. In 36% (19 alarms), the anesthesiologist did not want to act on the alarm because of alarm fatigue, ie, the anesthesiologist refused to treat because of the frequency of the alarms. Alarm fatigue is a phenomenon described in more detail in the literature.22 In 26% (14 alarms), there was a temporary reason for the (predicted) hypotension, such as lung recruitment or brief surgical obstruction of the vena cava. In the remaining 2% (1 alarm), the anesthesiologist had a different priority, namely an airway problem.
Hypertension
The median time-weighted average of hypertension was 0.09 mm Hg (IQR, 0.00-0.21 mm Hg) in the intervention group vs 0.05 mm Hg (IQR, 0.00-0.13 mm Hg) in the control group (median difference, 0.00 mm Hg; 95% CI, −0.85 to 0.17 mm Hg; P = .47).
Adverse Events
In the intervention group, 0 serious adverse events resulting in death occurred vs 2 (7%) in the control group. In total, 33 adverse events occurred in the intervention group vs 30 in the control group (eTable 3 in Supplement 2)
Discussion
This preliminary study demonstrated that application of a machine learning–derived early warning system for pending intraoperative hypotension in combination with a hemodynamic diagnostic guidance and treatment protocol significantly reduced the time-weighted average of hypotension during surgery. Hypotension was prevented without increasing the number of hypertensive events. Neither the cumulative dose of vasoactive medication given nor the fluid balance was significantly higher in the intervention group. Among all alarms, 81% were treated according to protocol. In post hoc analyses, the time from alarm to treatment was significantly lower in the intervention group.
This study extends the work by Hatib et al11 and Davies et al,2 who showed that the early warning system was able to predict hypotension with good sensitivity and specificity. This study adds the translation from prediction to actual prevention of hypotension.
Several studies have demonstrated intraoperative hypotension to be associated with myocardial injury, acute kidney injury, and mortality.5,6,7,8,9,10,23 Based on these studies, the r2019 Perioperative Quality Initiative consensus statement concluded with the notion that anesthesiologists should maintain a MAP threshold of greater than 60 to 70 mm Hg during surgery.24 Furthermore, it states that that postoperative injury is a function of both time spent having hypotension and depth of hypotension, making the time-weighted average of hypotension an end point of particular interest.24 Futier et al25 demonstrated in an RCT that maintaining a higher MAP during abdominal surgery reduced the risk of postoperative organ dysfunction. In all cases, a vasopressor (norepinephrine) was used to maintain the higher MAP. In the current study, hypotension prevention was taken a step further by predicting hypotension and preventing it through diagnosing and treating the specific cause of the impeding hypotension (preload, afterload, or contractility).
Limitations
This study has several limitations. First, the definition of hypotension (MAP <65 mm Hg) was similar for all patients. Maintaining this threshold is current best practice.23,24 However, every patient may have a personal minimal MAP to be maintained during surgery.26,27 In the future, the optimal hypotension threshold per patient might be determined using a machine learning tool, further personalizing intraoperative hemodynamic treatment.
Second, in the trial, the depth of anesthesia was not measured. In a recent RCT, a significant reduction in norepinephrine dose was observed by using electroencephalographic monitoring in patients under anesthesia.17 However, because patients were randomized, the anesthesia depth was expected to be similar between the groups. Indeed, the cumulative dose of propofol and sevoflurane did not statistically differ between the groups.
Third, because the early warning system is validated only for invasive continuous blood pressure monitoring, patients in this study were more severely ill and had a higher risk of hypotension than in a more general population. The study population mainly entailed oncologic gastrointestinal patients. In addition, the time-weighted average of 0.44 mm Hg in the control group is quite high compared with a US study reporting a time-weighted average of hypotension of 0.30 mm Hg in noncardiac surgery.23 The selection of patients in this trial, and possibly a lack of awareness of the importance of intraoperative hypotension in the study hospital, might explain this difference. Accordingly, a different study population might not—or not to this extent—benefit from the use of the early warning system.
Fourth, an observer being present in the operating room may have influenced protocol adherence. In future trials, a more pragmatic approach without an observer present in the operating room should be used.
Fifth, this was a preliminary study, a single-center RCT with a small sample size. In this trial, a physiological rather than clinical outcome was assessed. Future trials should be powered on clinical and economic outcomes such as disability-free survival (World Health Association Disability Assessment Schedule 2.0), organ injury, mortality, and costs.20
Conclusions
In this single-center preliminary study of patients undergoing elective noncardiac surgery, the use of a machine learning–derived early warning system for pending hypotension compared with standard care resulted in less intraoperative hypotension. However, further research with larger study populations in diverse settings is needed to understand the effect on additional patient outcomes and to fully assess safety and generalizability.
Trial Protocol
eFigure 1. Performance of the Early Warning System in the Observational Study Group (ROC Analysis)
eFigure 2. HemoSphere Monitor and Secondary Screen
eFigure 3. Hemodynamic Diagnostic Guidance and Treatment Protocol Explanation
eTable 1. Observational Study Baseline Characteristcs
eTable 2. Observational Study vs Control Group RCT
eFigure 4. AAT and AUT to Calculate TWA
eTable 3. Adverse and Serious Adverse Events
eFigure 5. Post Hoc Regression Analysis
eTable 4. Treatment Choice RCT
eTable 5. Treatment Behavior Silent Alarms
Data Sharing Statement
Section Editor: Derek C. Angus, MD, MPH, Associate Editor, JAMA (angusdc@upmc.edu).
References
- 1.Holmer H, Bekele A, Hagander L, et al. . Evaluating the collection, comparability and findings of six global surgery indicators. Br J Surg. 2019;106(2):e138-e150. doi: 10.1002/bjs.11061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies SJ, Vistisen ST, Jian Z, Hatib F, Scheeren TWL. Ability of an arterial waveform analysis-derived hypotension prediction index to predict future hypotensive events in surgical patients [published online ahead of print March 20, 2019]. Anesth Analg. 2020;130(2):352-359. doi: 10.1213/ANE.0000000000004121 [DOI] [PubMed] [Google Scholar]
- 3.Tritapepe L. Hypotension during surgery for high risk patients: cause or consequence of pathology? Minerva Anestesiol. 2013;79(9):978-990. [PubMed] [Google Scholar]
- 4.Cheung CC, Martyn A, Campbell N, et al. . Predictors of intraoperative hypotension and bradycardia. Am J Med. 2015;128(5):532-538. doi: 10.1016/j.amjmed.2014.11.030 [DOI] [PubMed] [Google Scholar]
- 5.van Waes JA, van Klei WA, Wijeysundera DN, van Wolfswinkel L, Lindsay TF, Beattie WS. Association between intraoperative hypotension and myocardial injury after vascular surgery. Anesthesiology. 2016;124(1):35-44. doi: 10.1097/ALN.0000000000000922 [DOI] [PubMed] [Google Scholar]
- 6.Xu L, Yu C, Jiang J, et al. . Major adverse cardiac events in elderly patients with coronary artery disease undergoing noncardiac surgery: a multicenter prospective study in China. Arch Gerontol Geriatr. 2015;61(3):503-509. doi: 10.1016/j.archger.2015.07.006 [DOI] [PubMed] [Google Scholar]
- 7.Sun LY, Wijeysundera DN, Tait GA, Beattie WS. Association of intraoperative hypotension with acute kidney injury after elective noncardiac surgery. Anesthesiology. 2015;123(3):515-523. doi: 10.1097/ALN.0000000000000765 [DOI] [PubMed] [Google Scholar]
- 8.Hallqvist L, Granath F, Huldt E, Bell M. Intraoperative hypotension is associated with acute kidney injury in noncardiac surgery: an observational study. Eur J Anaesthesiol. 2018;35(4):273-279. doi: 10.1097/EJA.0000000000000735 [DOI] [PubMed] [Google Scholar]
- 9.Monk TG, Bronsert MR, Henderson WG, et al. . Association between intraoperative hypotension and hypertension and 30-day postoperative mortality in noncardiac surgery. Anesthesiology. 2015;123(2):307-319. doi: 10.1097/ALN.0000000000000756 [DOI] [PubMed] [Google Scholar]
- 10.Bijker JB, van Klei WA, Vergouwe Y, et al. . Intraoperative hypotension and 1-year mortality after noncardiac surgery. Anesthesiology. 2009;111(6):1217-1226. doi: 10.1097/ALN.0b013e3181c14930 [DOI] [PubMed] [Google Scholar]
- 11.Hatib F, Jian Z, Buddi S, et al. . Machine-learning algorithm to predict hypotension based on high-fidelity arterial pressure waveform analysis. Anesthesiology. 2018;129(4):663-674. doi: 10.1097/ALN.0000000000002300 [DOI] [PubMed] [Google Scholar]
- 12.Maheshwari K, Khanna S, Bajracharya GR, et al. . A randomized trial of continuous noninvasive blood pressure monitoring during noncardiac surgery. Anesth Analg. 2018;127(2):424-431. doi: 10.1213/ANE.0000000000003482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wijnberge M, Schenk J, Terwindt LE, et al. . The use of a machine-learning algorithm that predicts hypotension during surgery in combination with personalized treatment guidance: study protocol for a randomized clinical trial. Trials. 2019;20(1):582. doi: 10.1186/s13063-019-3637-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205-213. doi: 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Protocolized cardiovascular management based on ventricular-arterial coupling In: Pinsky MR, Payen D, eds. Functional Hemodynamic Monitoring: Update in Intensive Care and Emergency Medicine. Springer-Verlag; 2004:381-395. [Google Scholar]
- 16.Castor Electronic Data Capture. https://castoredc.com.
- 17.Sponholz C, Schuwirth C, Koenig L, et al. . Intraoperative reduction of vasopressors using processed electroencephalographic monitoring in patients undergoing elective cardiac surgery: a randomized clinical trial [published online ahead of print February 19, 2019]. J Clin Monit Comput. 2020;34(1):71-80. doi: 10.1007/s10877-019-00284-1 [DOI] [PubMed] [Google Scholar]
- 18.Doyle DJ, Garmon EH. American Society of Anesthesiologists Classification (ASA Class). StatPearls Publishing LLC; 2019. [PubMed] [Google Scholar]
- 19.Knuf KM, Maani CV, Cummings AK. Clinical agreement in the American Society of Anesthesiologists physical status classification. Perioper Med (Lond). 2018;7:14. doi: 10.1186/s13741-018-0094-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Federici S, Bracalenti M, Meloni F, Luciano JV. World Health Organization Disability Assessment Schedule 2.0: an international systematic review. Disabil Rehabil. 2017;39(23):2347-2380. doi: 10.1080/09638288.2016.1223177 [DOI] [PubMed] [Google Scholar]
- 21.Oken MM, Creech RH, Tormey DC, et al. . Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649-655. doi: 10.1097/00000421-198212000-00014 [DOI] [PubMed] [Google Scholar]
- 22.Wilken M, Hüske-Kraus D, Klausen A, Koch C, Schlauch W, Röhrig R. Alarm fatigue: causes and effects. Stud Health Technol Inform. 2017;243:107-111. [PubMed] [Google Scholar]
- 23.Salmasi V, Maheshwari K, Yang D, et al. . Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology. 2017;126(1):47-65. doi: 10.1097/ALN.0000000000001432 [DOI] [PubMed] [Google Scholar]
- 24.Sessler DI, Bloomstone JA, Aronson S, et al. ; Perioperative Quality Initiative-3 Workgroup; POQI Chairs; Physiology Group; Preoperative Blood Pressure Group; Intraoperative Blood Pressure Group; Postoperative Blood Pressure Group . Perioperative Quality Initiative consensus statement on intraoperative blood pressure, risk and outcomes for elective surgery. Br J Anaesth. 2019;122(5):563-574. doi: 10.1016/j.bja.2019.01.013 [DOI] [PubMed] [Google Scholar]
- 25.Futier E, Lefrant JY, Guinot PG, et al. ; INPRESS Study Group . Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA. 2017;318(14):1346-1357. doi: 10.1001/jama.2017.14172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wesselink EM, Kappen TH, Torn HM, Slooter AJC, van Klei WA. Intraoperative hypotension and the risk of postoperative adverse outcomes: a systematic review. Br J Anaesth. 2018;121(4):706-721. doi: 10.1016/j.bja.2018.04.036 [DOI] [PubMed] [Google Scholar]
- 27.Ackland GL, Brudney CS, Cecconi M, et al. ; Perioperative Quality Initiative-3 Workgroup; POQI Chairs; Physiology Group; Preoperative Blood Pressure Group; Intraoperative Blood Pressure Group; Postoperative Blood Pressure Group . Perioperative Quality Initiative consensus statement on the physiology of arterial blood pressure control in perioperative medicine. Br J Anaesth. 2019;122(5):542-551. doi: 10.1016/j.bja.2019.01.011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eFigure 1. Performance of the Early Warning System in the Observational Study Group (ROC Analysis)
eFigure 2. HemoSphere Monitor and Secondary Screen
eFigure 3. Hemodynamic Diagnostic Guidance and Treatment Protocol Explanation
eTable 1. Observational Study Baseline Characteristcs
eTable 2. Observational Study vs Control Group RCT
eFigure 4. AAT and AUT to Calculate TWA
eTable 3. Adverse and Serious Adverse Events
eFigure 5. Post Hoc Regression Analysis
eTable 4. Treatment Choice RCT
eTable 5. Treatment Behavior Silent Alarms
Data Sharing Statement