Abstract
Agricultural intensification is a leading threat to bird conservation. Highly diversified farming systems that integrate livestock and crop production might promote a diversity of habitats useful to native birds foraging across otherwise‐simplified landscapes. At the same time, these features might be attractive to nonnative birds linked to a broad range of disservices to both crop and livestock production. We evaluated the influence of crop–livestock integration on wild bird richness and density along a north‐south transect spanning the U.S. West Coast. We surveyed birds on 52 farms that grew primarily mixed vegetables and fruits alone or integrated livestock into production. Crop–livestock systems harbored higher native bird density and richness relative to crop‐only farms, a benefit more pronounced on farms embedded in nonnatural landscapes. Crop–livestock systems bolstered native insectivores linked to the suppression of agricultural pest insects but did not bolster native granivores that may be more likely to damage crops. Crop–livestock systems also significantly increased the density of nonnative birds, primarily European Starlings (Sturnus vulgaris) and House Sparrows (Passer domesticus) that may compete with native birds for resources. Models supported a small, positive correlation between nonnative density and overall native bird density as well as between nonnative density and native granivore density. Relative to crop‐only farms, on average, crop–livestock systems exhibited 1.5 times higher patch richness, 2.4 times higher density of farm structures, 7.3 times smaller field sizes, 2.4 times greater integration of woody crops, and 5.3 times greater integration of pasture/hay habitat on farm. Wild birds may have responded to this habitat diversity and/or associated food resources. Individual farm factors had significantly lower predictive power than farming system alone (change in C statistic information criterion (ΔCIC) = 80.2), suggesting crop–livestock systems may impact wild birds through a suite of factors that change with system conversion. Collectively, our findings suggest that farms that integrate livestock and crop production can attract robust native bird communities, especially within landscapes devoted to intensified food production. However, additional work is needed to demonstrate persistent farm bird communities through time, ecophysiological benefits to birds foraging on these farms, and net effects of both native and nonnative wild birds in agroecosystems.
Keywords: agricultural intensification, crop–livestock integration, European Starling, farming systems, House Sparrow, native birds, nonnative birds, organic farming
Introduction
Conversion of natural lands to agriculture represents a major threat to animal species worldwide, including to wild birds (Wilcove et al. 1998, Chamberlain et al. 2000, Geiger et al. 2010). Increasing losses of wild bird species are often seen as diverse, traditional farming systems are replaced by modern intensive agriculture centered on large monocultures of single crop species (Belfrage et al. 2005, Jones et al. 2005). Indeed, farming intensification has been associated with steep declines in bird species that were once relatively abundant on farmlands and thought to be tolerant of agriculture (Chamberlain et al. 2000, Stanton et al. 2018). At the same time, both a growing human population and increasing meat consumption lead to ever‐increasing pressure to convert wildlands to agriculture and to farm existing agricultural lands more intensively (Tilman et al. 2002, Kremen 2015). This suggests an inherent trade‐off between meeting human food needs and conserving bird populations.
This apparent conundrum has led to growing interest in farming practices that bring conservation benefits to birds and other wildlife (Bengtsson et al. 2005). That is, to look at land “sharing” as a complement to land “sparing” within regional wildlife‐conservation programs on arable lands (Kremen 2015). For example, agricultural practices that eschew or reduce the use of broad‐acting insecticides, such as some organic farming systems, can harbor relatively diverse and abundant insect prey for birds (Kennedy et al. 2013, Lichtenberg et al. 2017). Resulting enhanced wild bird diversity and abundance (Belfrage et al. 2005, Bengtsson et al. 2005) could in turn increase bird‐mediated suppression of pest insects (Philpott et al. 2009, Boesing et al. 2017). Wild birds often benefit from the addition of hedgerows or other seminatural habitats within farm fields, which provide roosting habitat, nesting sites, and refuge (Batáry et al. 2010, Heath et al. 2017, Wilson et al. 2017). Often these diversification practices are encouraged by government subsidies, in part because of their perceived benefits to wildlife (Dunn et al. 1993, Kleijn and Sutherland 2003). The ability of farm diversification practices to influence biodiversity, including birds, can be strongly mediated by the surrounding landscape, with the greatest benefits sometimes seen in more simplified landscapes (e.g., 1–20% non‐crop habitat) where the provisioning of local resources are not redundant with those already available in the surrounding landscape (Batáry et al. 2010, Tscharntke et al. 2012, Heath et al. 2017). In contrast, the most‐intensively farmed landscapes might lack source populations of birds to take advantage of enhanced resources that are provided on a particular farm (Geiger et al. 2010, Tscharntke et al. 2012).
One farm diversification scheme that could benefit wild birds is the integration of livestock with crop production (Šálek et al. 2017). Growers that adopt this crop–livestock farming system can replace chemical fertilizers purchased offsite with manure produced by resident livestock, while also generating both meat and produce sales to attract a wider customer base that stabilizes farm income (Herrero et al. 2010, Bell et al. 2014, Salton et al. 2014). Mixed livestock and crop production may benefit wild birds by diversifying habitat types on the farm (Benton et al. 2003), providing additional food resources through grain‐based livestock feed and insects associated with feces (Evans et al. 2006, Carlson et al. 2015, Hald et al. 2016), and by providing additional structures for nesting (Hiron et al. 2013, Šálek et al. 2017). However, livestock integration may also come with risks if it shifts wild bird communities towards species that damage crops (omnivores and granivores) or carry human enteric pathogens that might contaminate fresh produce (Hald et al. 2016, Dross et al. 2018). Further, species nonnative to North America and associated with livestock, such as European Starlings (Sturnus vulgaris) and House Sparrows (Passer domesticus), may dissuade native species through nest site competition or behavioral interference, damage crops, and disrupt pest control services (Weitzel 1988, Somers and Morris 2002, Peisley et al. 2015, Val et al. 2018).
Here, we test a suite of hypothesized relationships between land management (farming system and landscape context) and wild bird communities. We conducted a broad survey of wild bird communities on 52 diversified farms identified as organic (no synthetic pesticides, fertilizers, or herbicides) that grew either crops alone (primarily mixed vegetables, roots, fruits, and nuts; “crop systems”) or that grew these crops alongside livestock production (“crop–livestock systems”). Our study spanned the U.S. West Coast from northern Washington to southern California and included two Bird Conservation Regions (Fig. 1; Sauer et al. 2003). The study area is within the Fruitful Rim region where the majority of U.S. fruit and vegetable production is concentrated, in addition to 37% of U.S. dairy farming (Aguilar et al. 2015). We hypothesized that (1) crop–livestock systems would bolster native bird density, native bird richness, and nonnative bird density, with the greatest effects in more simplified landscapes, (2) crop–livestock systems would bolster native bird density across foraging guilds through grain‐based livestock feed and insects associated with feces, and (3) nonnative birds would reduce native bird density and richness (Appendix S1: Table S1).
Figure 1.
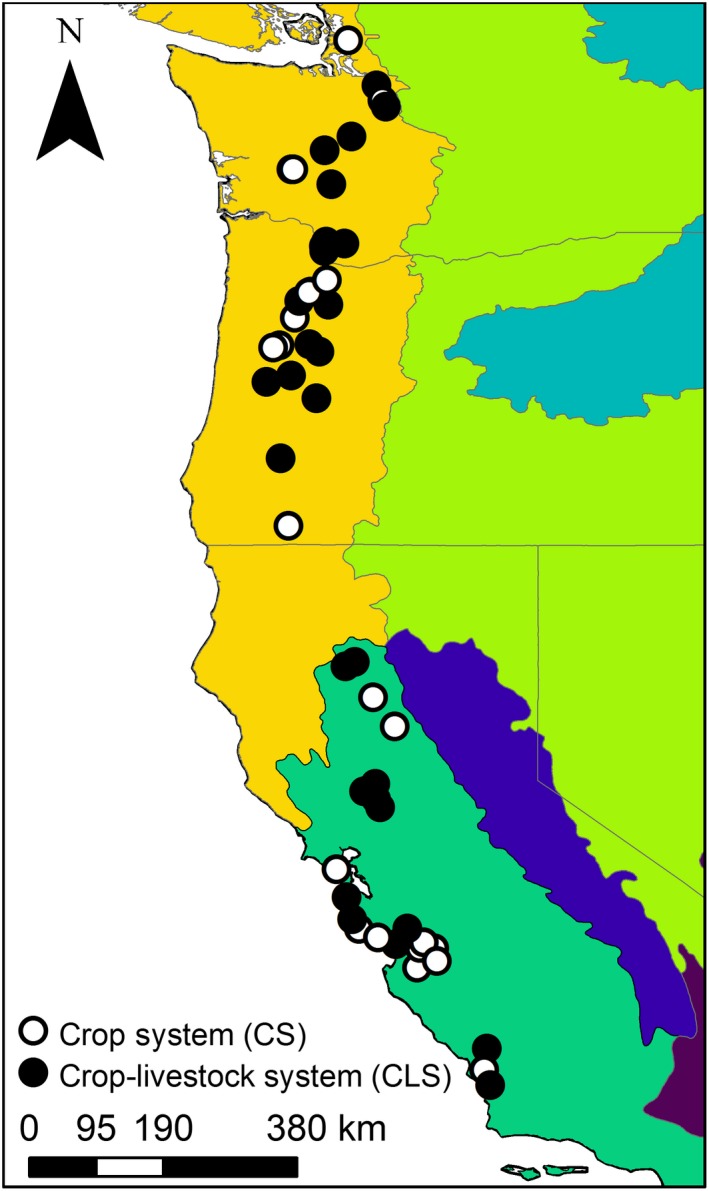
Map of 52 organic farms sampled across Washington, Oregon, and California, USA. White circles indicate crop systems and black circles indicate crop–livestock systems. Different colors denote different Bird Conservation Regions: yellow/orange, Northern Pacific Rainforest; lime green, Great Basin; teal, Northern Rockies; green, Coastal California; dark blue, Sierra Nevada; maroon, Sonoran and Mojave Desserts.
Methods
Our study had three components aimed at testing the hypothesized relationships between real‐world farm management, landscape context, and wild bird communities. First, we surveyed bird communities on commercial farms that produced crops only or that integrated livestock with these crops. Second, we mapped farm features using aerial imagery and quantified the landscape context using the National Landcover Database (Homer et al. 2012). Third, we used structural equation modeling (SEM; Shipley 2009, Lefcheck 2016) to examine the linkages among farming practices, surrounding landscape, Bird Conservation Region, and wild bird communities. We separated wild bird communities into native bird (birds found in North America pre‐European colonization) density and richness and nonnative bird (birds introduced to North America post‐European colonization) density. We only considered nonnative bird density because of the low nonnative species richness in our study region (four possible species). We further characterized native species as granivores, insectivores, omnivores, cavity nesters, and species of concern for additional sets of analyses.
Study area and design
Across two years (2016 and 2017), we surveyed bird communities on a total of 52 diversified organic farms in California (2016, n = 12; 2017, n = 23), Oregon (2016, n = 14; 2017, n = 15), and Washington (2016, n = 14; 2017, n = 13; Fig. 1; Data S1 from Smith et al. 2019). These farms were located between 35°18′ to 48°27′ N and 123°27′ to 120°40′ W, spanned two Bird Conservation Regions (Coastal California and Northern Pacific Rainforest), and ranged in size from 0.38 to 272 ha (2016, 29.95 ± 8.55 ha [mean ± SE]; 2017, 24.6 ± 6.41 ha). Farms only grew crops (crop system [CS]; 2016, n = 21; 2017, n = 23) or integrated livestock into farming operations (crop–livestock system [CLS]; 2016, n = 19; 2017, n = 29). We surveyed all farms from 2016 again in 2017, except for one that went out of business. We added 13 new farms in California in 2017 that were not surveyed in 2016, with farms selected to better balance landscape context by farming system and region (Appendix S1: Fig. S1).
Farms were highly diversified and grew a range of crops including cereals (e.g., corn, wheat, barley), vegetables and melons (e.g., brassicas, leafy vegetables), fruits and nuts (e.g., citrus fruits, grapes, berries, walnuts), oilseed crops (e.g., olives, sunflower), roots (e.g., potatoes), spice crops (e.g., chilies, peppers, fennel), beverage crops (e.g., tea), medicinal crops, commercial flowers, and grasses and fodder crops, among others. Livestock were integrated into farming operations in a variety of forms including full crop–livestock field rotations, use of horses to plow and fertilize fields, and permanent housing outside of crop fields, among other management strategies. Chickens were the most frequently integrated livestock (n = 25 farms, mean = 73 chickens/farm with chickens), followed by horses (n = 9 farms, mean = 2 horses/farm with horses), sheep (n = 8 farms, mean = 25 sheep/farm with sheep), pigs (n = 7 farms, mean = 6 pigs/farm with pigs), goats (n = 6 farms, mean = 4 goats/farm with goats), cattle (n = 6 farms, mean = 22 cows/farm with cattle), other types of fowl (peafowl, turkeys, ducks, geese, Guinea fowl; n = 7 farms, mean = 14 fowl/farm with other fowl), and other types of ungulates (alpacas, donkeys; n = 2 farms, mean = 3.5 ungulates/farm with other ungulates; see Data S1 from Smith et al. 2019 for full data).
Bird survey design and guild classification
Bird surveys were conducted twice per farm each year between 27 April and 8 August 2016–2017 to coincide with produce harvest in both climatically distinct regions (see Data S1 from Smith et al. 2019 for survey dates). For each survey, we moved in a south‐north transect, which we repeated immediately upon conclusion of the first survey. Thus, survey 1 each year roughly corresponded with the nesting season along the south‐north transect, while survey 2 roughly corresponded with the fledging and flocking periods for gregarious species. The broad geographic range of our study limited our data collection to only two surveys per year. Our surveys were conducted outside of peak migration periods for each region. One point with 100 m radius was surveyed for every 4 ha of farmed land and stratified to capture the range of land usages present on farms (Smith et al. 2010). We used the point count as the replicate for our analyses with farm included as a random effect. Points were at least 200 m apart to avoid double counting. Points were surveyed in a different order each visit to reduce detection bias due to time‐of‐day effects. If structures interfered with visual detectability of birds, the observer moved within survey points to see around structures if necessary (Šálek et al. 2017). In 2016, we conducted point counts at 179 locations on 41 farms (range 1–15 points per farm, mean 4.5 points per farm, proportional to farm area). In 2017, we conducted point counts at 210 locations on 51 farms. In total, we surveyed 125 points across 23 crop‐only farms (5.4 points/farm) and 107 points across 29 crop–livestock farms (3.9 points/farm). While surveying each point, we recorded if livestock were physically present within the point at the time of the survey (“livestock at point”).
Surveys were conducted between sunrise and 10:45 only in the absence of heavy rain, and all were conducted by the same skilled observer (O. Smith). At each point, we recorded the number of unique individuals per species seen or heard during a 10‐min period. Individuals flying over sites were excluded from analysis. Aerial foragers (swallows, swifts) were included with a note “aerial foraging” and included in analyses (Šálek et al. 2017). We used the number of individuals per survey point for species density estimates and the number of species per survey point for species richness estimates in our structural equation models, with farm included as a random effect. This allowed us to examine per unit area changes in wild bird communities, the most relevant metric to farmers, and account for species‐area relationships across variably sized farms. Each survey was included as a repeated measures replicate. We only considered nonnative bird density because of the low nonnative bird richness in our study region (four possible species). All bird species detected were assigned to a diet guild following the Birds of North America Online database (Rodewald 2015), Wilman et al. (2014), and De Graaf et al. (1985). Nonnative birds observed were European Starling, House Sparrow, Rock Pigeon (Columba livia), and Eurasian Collared‐Dove (Streptopelia decaocto). The full list of bird species included in the analyses, their diet guilds, nesting guilds, and conservation statuses are given in Data S2 in Smith et al. (2019).
Farm and landscape characteristics
To characterize local farm attributes, we recorded habitat types on aerial images during farm visits and manually digitized land use on each farm (Appendix S1: Fig. S2) in ArcGIS (version 10.2; ESRI, Redlands, California, USA) using high spatial resolution ortho‐photographs (1 m; National Agriculture Imagery Program, courtesy of the U.S. Geological Survey, Smith 2017). We classified cover types by combining the National Land Cover Database codes, habitat mapping codes in the Cornell Lab of Ornithology's HabitatNetwork, and farm bird habitat classification in Smith (2017; Data S3 from Smith et al. 2019). We calculated total farm size, average crop field size, patch richness (number of unique habitat types), structure density (number of structures/ha), and percentage of total farm area in row crops (e.g., brassicas, squash, leafy greens, cereals), woody crops (e.g., citrus trees, pome and stone fruits, nut trees, shrub fruit, vineyards), pasture/hay (e.g., pasture for livestock or grass and fodder crops), and natural/seminatural habitat (e.g., hedgerows, wetlands, etc.) using the PatchGrid FRAGSTATS interface in ArcGIS (Smith 2017). We used Mann‐Whitney U tests to test for differences in total farm size, crop field mean patch size, patch richness, structure density, and percentage of total farm area in row crops, woody crops, pasture/hay, and natural/seminatural habitat between farms with only crops and those that integrated livestock into operations (Data S1 from Smith et al. 2019).
We used the average territory size of birds observed during our surveys (1.5 km) as our landscape scale metric to delineate a biologically relevant landscape scale (Seavy et al. 2009, Jackson and Fahrig 2015). We used the National Land Cover Database buffered to 1.5 km to calculate percent natural/seminatural (hereafter “natural,” which included forest, scrubland, herbaceous, and wetland categories) habitat using Program R and FRAGSTATS 4.1 (McGarigal and Marks 1994). We buffered points at the center of the farm as well as at each point, which were highly correlated (R 2 = 0.99; see Appendix S1: Figs. S3–S5 for pairwise correlations between variables examined). Percent natural habitat in the landscape was highly correlated from 100 to 2,500 m, suggesting our results are robust to the choice in landscape scale (Appendix S1: Fig. S6). Percent natural habitat in the landscape was not correlated with landscape field size (Appendix S1: Fig. S7; Pearson's R 2 = −0.074, P = 0.60; Spearman's R 2 = −0.094, P = 0.51), landscape field size was not correlated with the size of the farm surveyed (Pearson's R 2 = −0.21, P = 0.14; Spearman's R 2 = −0.08, P = 0.57), and landscape field size was not correlated with size of the farm surveyed (Pearson's R 2 = −0.19, P = 0.19; Spearman's R 2 = −0.16, P = 0.25). Thus, examining field size in the landscape was beyond the scope of our study, and we included both farm size and percent natural habitat in the landscape in our models.
Data analysis
We used nonmetric multidimensional scaling (NMDS) to describe the variation in the composition of farm bird communities between farming systems within each Bird Conservation Region (Kennedy et al. 2010). The NMDS was performed in the vegan package of program R version 3.4.3 (R Core Team 2017, Oksanen et al. 2019) using a Bray‐Curtis dissimilarity matrix derived from average species density per survey point across the 2 yr at the farm level. We used the maximum density for each survey point for each species out of the four visits to each farm and averaged the values across survey points within a farm as the density metric in the NMDS (Batáry et al. 2010). Statistical significance between the four groups (farming system within region) was determined using analysis of similarities (ANOSIM).
We generated a series of hypotheses using the existing literature to construct structural equation models (SEMs) to test relationships between farm management, landscape context, native birds, and nonnative birds (see Appendix S1: Table S1 for our variables and predictions; see Appendix S1: Table S2 for our full model list), which could interact through various direct and indirect pathways. SEM offers a flexible way to account for Poisson error distributions for count data, log link functions for nonlinear relationships, and interactions specific to different variables within the overall structure of the path model (Shipley 2009, Lefcheck 2016). We therefore built a series of generalized multilevel path models based on linear mixed‐effects models (Appendix S1: Table S2). The models generally differed in the description of how the effects of livestock influence bird communities (overall native communities, nonnative birds, diet guilds, cavity nesters, and species of concern) via (1) the direct effects of the farming system, (2) the direct effects of the physical presence of livestock, and (3) the indirect effects of the farming system driven by changes in particular farm characteristics (increased farm structures, more pasture/hay, etc.).
We used point location nested within farm as a random effect to account for spatial dependence and used an observation level random effect to account for overdispersion in the data (Warton and Hui 2011). We tested for overdispersion by examining the ratio of deviance to degrees of freedom. We examined spatial residuals to check for spatial autocorrelation (Appendix S1: Fig. S8). Continuous fixed effects were standardized prior to fitting the model using a generic scale function that calculated the mean and standard deviation of the entire vector for each fixed effect, subtracted each element of the fixed effect by the mean, and then divided by the standard deviation. To calculate the percentage of variation explained by each mixed‐effect model, we calculated both the marginal pseudo R 2 (based solely on fixed effects) and conditional pseudo R 2 (incorporating the random effects) (Nakagawa and Schielzeth 2013, Lefcheck 2016). D‐separation tests of independence claims allowed us to assess overall model fit (Shipley 2009). These analyses were performed using the lme4 and piecewiseSEM packages in program R (R Core Team 2017, Bates et al. 2015, Lefcheck 2016). Although field data are made available, geographic location is masked to protect grower privacy (Data S4 from Smith et al. 2019).
Results
We detected 11,597 individual birds from 134 species (Data S2 from Smith et al. 2019). The most abundant were European Starling, Brewer's Blackbird (Euphagus cyanocephalus), and American Robin (Turdus migratorius). Species with the highest occurrence were American Robin, European Starling, and House Finch (Haemorhous mexicanus). Only 20% of the species made up 81% of all observations. We detected one species that is red‐listed, the Olive‐sided Flycatcher (Contopus cooperi), and 31 that are listed as sensitive, threatened, or endangered by the U.S. federal government or by state agencies in at least one of the states in the study region (Data S2 from Smith et al. 2019). We observed one or more individuals classified as sensitive, threatened, or endangered on all farms on at least one survey occasion.
Community composition
The NMDS ordination resulted in a two‐axis solution with a final stress of 0.24. The two axes represented 95.0% of the variation in bird communities using a nonmetric fit R 2 and 73.6% using a linear fit R 2. Analyses of community similarity confirmed that Bird Conservation Regions and farming systems (four group comparison; CS in Coastal California, CS in the Pacific Northwest Rainforest, CLS in Coastal California, and CLS in the Pacific Northwest Rainforest) had unique communities (Fig. 2; R = 0.37, P = 0.001). Farms within the same Bird Conservation Region had strong groupings in ordination space. Systems within regions overlapped but tended to exhibit unique communities. Species at the center were common across the range such as European Starling and Red‐winged Blackbird (Agelaius phoeniceus). Species falling out of convex hulls tended to be uncommon species associated with uncommon landscape features such as marshes (e.g., Yellow‐headed Blackbird [Xanthocephalus xanthocephalus]) or whose range limits fell at the edge of the study area (e.g., California Thrasher [Toxostoma redivivum]).
Figure 2.
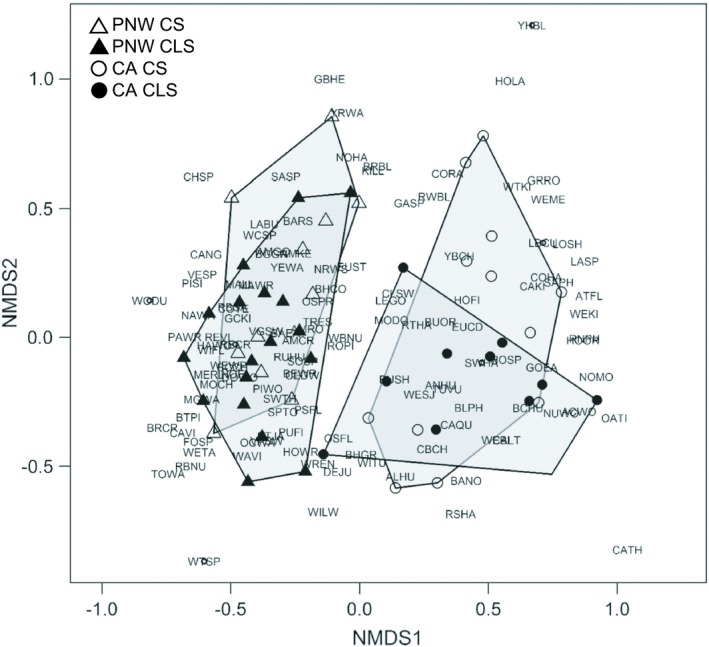
Nonmetric multidimensional scaling (NMDS) of farm bird community abundances in Pacific Northwest crop systems (PNW CS; n = 11), Pacific Northwest crop–livestock systems (PNW CLS; n = 18), California crop systems (CA CS; n = 12), and California crop–livestock systems (CA CLS; n = 11) showing differences in community composition between groups. See Data S2 from Smith et al. (2019) for species codes.
Relationship between region, landscape context, livestock integration, farm size, native bird density, native bird richness, and nonnative bird density
The first model set revealed strong direct effects and interactions between Bird Conservation Region, landscape, and local drivers on wild bird density and richness (Fig. 3a; Appendix S1: Table S3). Though community composition differed by Bird Conservation Region (Fig. 2) and native bird richness was higher in Coastal California (β = −0.15 ± 0.06 [mean ± SE], P = 0.015), it was a poor predictor of native (β = 0.043 ± 0.10, P = 0.67) and nonnative bird density (link not supported; Test of directed separation β = −0.21 ± 0.35, P = 0.55). Amount of natural habitat in the landscape had a strong direct, negative impact on nonnative bird density (β = −0.86 ± 0.16, P < 0.0001). Native bird richness (β = −0.13 ± 0.053, P = 0.011) and density (β = −0.22 ± 0.091, P = 0.016) were higher on diversified crop–livestock farms but only in simplified landscapes (Appendix S1: Fig. S10). Livestock integration had a strong, direct positive influence on nonnative bird density (β = 1.29 ± 0.31, P < 0.0001). Farm size had a negative influence on native bird richness (β = −0.29 ± 0.035, P < 0.0001), density (β = −0.27 ± 0.050, P < 0.0001), and nonnative (β = −1.12 ± 0.18, P < 0.0001) density. Nonnative birds had a positive link with native density (β = 0.010 ± 0.004, P = 0.011) but no significant link with native richness (β = −0.0005 ± 0.003, P = 0.84). The global model provided a good fit to the data (Fisher's C = 1.21, P = 0.55). The second model set substituted farm‐level livestock integration for presence of livestock within a bird survey point (Fig. 3b; Appendix S1: Table S4). Models supported the same trends, and the global model provided a good fit to the data (Fisher's C = 0.60, P = 0.74).
Figure 3.
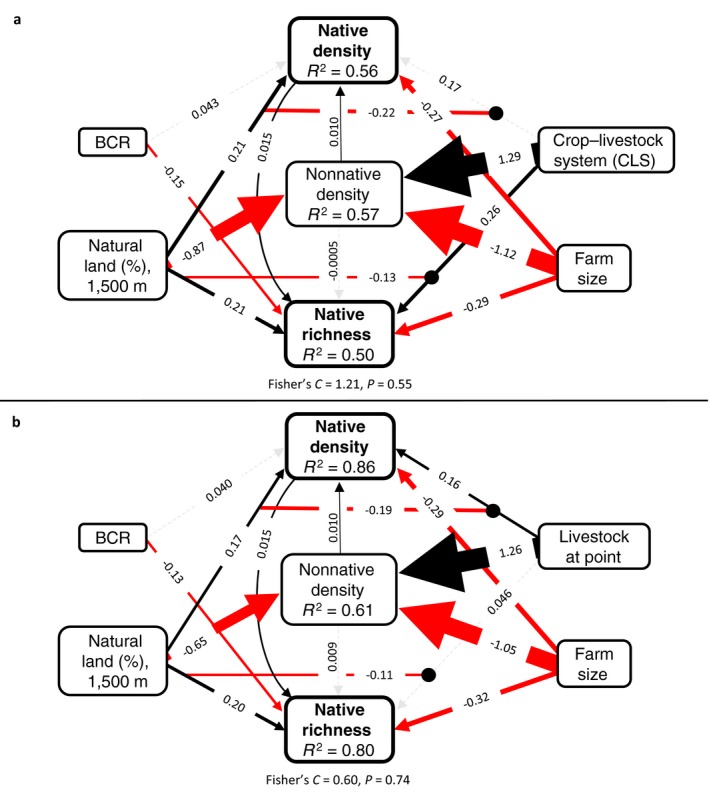
Structural equation model showing links between (a) Bird Conservation Region (BCR), percent natural land cover (1,500 m radius), crop–livestock system (CLS), farm size, nonnative bird density, native bird density, and native bird richness and (b) percent natural land cover buffered around a point (1,500 m radius), livestock presence at point, farm size, nonnative bird density, native bird density, and native bird richness. Black solid lines indicate positive relationships, red solid arrows indicate negative relationships, solid circles indicate interactions, and dashed gray lines indicate nonsignificant relationships. Lines are scaled to coefficients.
Mediators of the relationships between farm system, native bird density, native bird richness, and nonnative bird density
To better understand specific farm attributes that may be impacting wild birds, we conducted analyses examining differences in farm management between crop systems and crop–livestock systems (Fig. 4; Appendix S1: Figs. S2, S4). Crop–livestock farms tended to be smaller than crop‐only farms, but the trend was not significant (Fig. 4; Mann‐Whitney U Test, W = 376; P = 0.44). Crop–livestock farms had smaller on‐farm crop field sizes (Mann‐Whitney U Test, W = 462.5, P = 0.018), higher on‐farm patch richness (Mann‐Whitney U test, W = 97.5, P < 0.001), and greater structure density on‐farm than crop‐only farms (Mann‐Whitney U test, W = 139, P < 0.001). Crop–livestock farms also had a smaller percentage of farm area in row crops (Mann‐Whitney U test, W = 529, P < 0.001), more farm area in woody crops (Mann‐Whitney U test, W = 187, P = 0.007), and more farm area in pasture/hay (Mann‐Whitney U test, W = 69, P < 0.001). Both systems had similar amounts of natural/seminatural habitat on‐farm (Mann‐Whitney U test, W = 246, P = 0.11).
Figure 4.
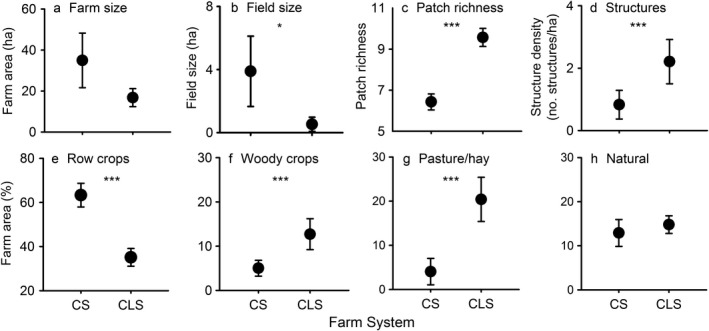
On‐farm habitat attributes (mean ± SE) of 52 crop systems (CS) and crop–livestock systems (CLS) showing (a) farm size (ha), (b) average farm crop field size, (c) patch richness (number of unique patch types), (d) structure density (no. structures/ha), (e) percentage of farm area in row crops, (f) percentage of farm area in woody crops, (g) percentage of farm area in pasture/hay, and (h) percentage of farm area natural/seminatural habitat. Asterisks indicate significant differences determined through a Mann‐Whitney U test: *P < 0.05; ***P < 0.001.
We next attempted to isolate how bird communities responded to individual farm characteristics that differed between crop–livestock vs. crop‐only systems. To do this we built a series of SEMs (Appendix S1: Fig. S11; Appendix S1: Tables S2, S5) with an indirect link between livestock integration and native bird richness, native bird density, and nonnative bird density. The best‐supported model using the change in C statistic information criterion (ΔCIC) included only a direct link between crop–livestock system and bird communities. The second‐best‐supported model included the direct effect of structure density on wild bird communities with a direct link between structure density and farming system and thus an indirect link between farming system and bird communities (ΔCIC = 80.2; Appendix S1: Table S5; Appendix S1: Fig. S11). All global models including indirect effects of livestock systems were poor fits to the data (D‐sep P < 0.0001 for all), with suggested direct links missing between farming system and nonnative density/native richness. We added missing direct links into the second‐best model (structure density), but the more complex model was still not well supported (ΔCIC = 14.4). We, therefore, did not explore further ways to improve indirect effects models.
Relationship between region, landscape context, livestock integration, farm size, native diet guilds, and nonnative bird density
Next, we explored the relative influence of region, landscape context, farm size, and farming system on density of native birds from the three most abundant diet guilds (insectivores, omnivores, and granivores [Appendix S1: Table S2]; other diet guilds did not have sufficient density to converge; see Data S4 from Smith et al. 2019 for densities) and nonnative bird density (Fig. 5a; Appendix S1: Table S6). Region influenced granivore density (higher in Coastal California; β = −0.81 ± 0.20, P < 0.0001) but was a poor predictor of insectivore density (link not supported; Test of directed separation β = 0.14 ± 0.13, P = 0.27), omnivore density (link not supported; test of directed separation β = −0.10 ± 0.15, P = 0.48), and nonnative bird density (link not supported; test of directed separation β = −0.21 ± 0.35, P = 0.55). Amount of natural habitat in the landscape had a direct positive effect on omnivore density (β = 0.28 ± 0.066, P < 0.0001) and a direct negative effect on nonnative bird density (β = −0.86 ± 0.16, P < 0.0001) but no effect on insectivore density (β = 0.085 ± 0.057, P = 0.13) nor granivore density (β = −0.11 ± 0.096, P = 0.27). Livestock integration had a direct positive influence on insectivore density (β = 0.26 ± 0.11, P = 0.023) and nonnative bird density (β = 1.29 ± 0.31, P < 0.0001) but no impact on granivore density (β = 0.066 ± 0.18, P = 0.72) nor omnivore density (β = −0.046 ± 0.13, P = 0.73). Farm size had a negative influence on insectivore (β = −0.26 ± 0.058, P < 0.0001), granivore (β = −0.79 ± 0.11, P < 0.0001), omnivore (β = −0.28 ± 0.070, P < 0.0001), and nonnative bird (β = −1.12 ± 0.18, P < 0.0001) density. Nonnative bird density had a positive correlation with granivore density (β = 0.027 ± 0.007, P = 0.0003) but no significant correlation with insectivore (β = 0.003 ± 0.005, P = 0.56) nor omnivore density (β = 0.009 ± 0.006, P = 0.18). The global model provided a good fit to the data (Fisher's C = 15.2, P = 0.23). The second model set substituted farm livestock integration for the presence of livestock within a bird survey point (Fig. 5b; Appendix S1: Table S7). Models indicated the same trends, except that the link between natural habitat in the landscape and insectivore density became significant (β = 0.14 ± 0.053, P = 0.009), and the link between insectivore density and livestock presence at a point became nonsignificant (β = 0.055 ± 0.10, P = 0.59). The global model provided a good fit to the data (Fisher's C = 14.6, P = 0.26).
Figure 5.
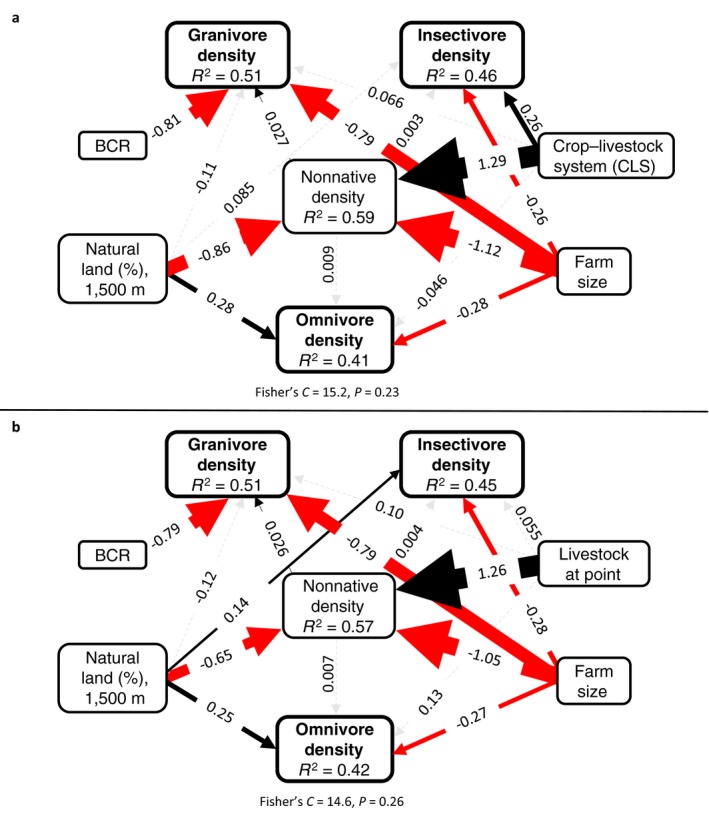
Structural equation model showing links between (a) Bird Conservation Region (BCR), percent natural land cover (1,500 m radius), crop–livestock system, farm size, nonnative bird density, native granivore density, native insectivore density, and native omnivore density and (b) percent natural land cover buffered around a point (1,500 m radius), livestock presence at point, farm size, nonnative bird density, native granivore density, native insectivore density, and native omnivore density. Black solid lines indicate positive relationships, red solid arrows indicate negative relationships, solid circles indicate interactions, and dashed gray lines indicate nonsignificant relationships. Lines are scaled to coefficients.
Relationship between region, landscape context, livestock integration, farm size, native cavity nester density, and nonnative cavity nester density
Next, we explored the relative influence of Bird Conservation Region, landscape context, farm size, and farming system on density of native cavity nesters and density of nonnative cavity nesters (Appendix S1: Fig. S12a; Appendix S1: Table S2 and S8). Bird Conservation Region was a poor predictor of native cavity nester density (β = −0.24 ± 0.21, P = 0.26). Amount of natural habitat in the landscape had a direct positive effect on native cavity nester density (β = 0.38 ± 0.098, P = 0.0001) and a direct negative effect on nonnative density (β = −0.75 ± 0.16, P < 0.0001). Livestock integration had a direct positive influence on native cavity nester density (β = 1.08 ± 0.19, P < 0.0001) and nonnative cavity nester density (β = −0.75 ± 0.16, P < 0.0001). Farm size had a negative influence on native cavity nester density (β = −0.32 ± 0.12, P = 0.008) and nonnative cavity nester density (β = −1.03 ± 0.19, P < 0.0001). Nonnative cavity nester density was not significantly correlated with native cavity nester density (β = 0.013 ± 0.008, P = 0.099). The global model provided a good fit to the data (Fisher's C = 0.56, P = 0.76). The second model set substituted farm livestock integration for the presence of livestock within a survey point (Appendix S1: Fig. S12b; Appendix S1: Table S9). Models indicated the same trends, except that the link between livestock in the point and native cavity nester density was not significant (β = −0.003 ± 0.16, P = 0.99), and the link between native and nonnative cavity nester density were positively correlated (β = 0.018 ± 0.008, P = 0.027). The global model provided a good fit to the data (Fisher's C = 0.004, P = 0.99).
Relationship between region, landscape context, livestock integration, farm size, species of concern density, and nonnative density
Our final model sets explored the relative influence of Bird Conservation Region, landscape context, farm size, and farming system on density of species of concern and density of nonnative birds (Appendix S1: Fig. S13a; Appendix S1: Table S2 and S10). Bird Conservation Region was a poor predictor of species of concern density (β = −0.15 ± 0.18, P = 0.39). Amount of natural habitat in the landscape had a positive effect on species of concern density (β = 0.41 ± 0.13, P = 0.002) and a direct negative effect on nonnative density (β = −0.86 ± 0.16, P < 0.0001). Livestock integration had a positive influence on species of concern density (β = 0.48 ± 0.16, P = 0.002) and nonnative density (β = 1.29 ± 0.31, P < 0.0001). However, livestock integration had the highest impact on species of concern density compared to crop‐only farms when there was less natural habitat in the landscape (β = −0.37 ± 0.16, P = 0.023). Farm size did not impact species of concern density (β = −0.042 ± 0.090, P = 0.64) but did influence nonnative density (β = −1.12 ± 0.18, P < 0.0001). Nonnative density was not significantly correlated with species of concern density (β = −0.005 ± 0.009, P = 0.59). The global model provided a good fit to the data (Fisher's C = 1.21, P = 0.55). The second model set substituted farm livestock integration for the presence of livestock within a survey point (Appendix S1: Fig. S13b; Appendix S1: Table S11). Models indicated the same trends, and the global model provided a good fit to the data (Fisher's C = 0.44, P = 0.80).
Discussion
We found potential for crop–livestock systems to support more native birds than crop‐only systems across two Bird Conservation Regions (Fig. 3, 5). Integrating livestock yielded the greatest increase in bird density and richness in landscapes with the least natural habitat (Fig. 3; Appendix S1: Fig. S10). Further, crop–livestock systems bolstered numbers of native insectivorous birds, which could provide valuable pest control services to growers (Karp et al. 2013, Boesing et al. 2017) but did not increase the density of granivorous or omnivorous native species that may impose greater damage to crops than insectivores (Fig. 5; Gebhardt et al. 2011).
The effect of farm size and farming system
With the exception of species of concern, smaller farms supported higher bird density and richness for all bird guilds in all models and was the only factor that consistently benefited wild birds (Figs. 3, 5; Appendix S1: Figs. S12–13). Smaller farms may benefit birds through smaller field sizes, smaller amounts of land in row crops that experience greater disturbance than woody crops, lower mechanization, fewer inputs, and greater crop diversification per area (Tscharntke et al. 2012, Frei et al. 2018b). We predicted that integrated crop–livestock production would have greater benefits to wild birds than crop‐only systems by diversifying habitat types on the farm (Benton et al. 2003), which may provide additional food resources (Evans et al. 2006, Carlson et al. 2015, Hald et al. 2016) and offer additional structures for nesting (Hiron et al. 2013, Šálek et al. 2017). Indeed, we found that crop–livestock systems promoted higher density and richness of wild birds (Fig. 3). Both farm livestock integration and physical livestock presence similarly impacted native and nonnative birds with two exceptions: native insectivore and cavity nester density were higher on crop–livestock farms but were not impacted by the presence of livestock within a survey point (Fig. 5a vs. b and Appendix S1: Fig. S12a vs. b). Contrary to our expectations, this suggests insectivores and cavity nesters may respond more to whole‐farm changes rather than to resources physically proximate to livestock (e.g., flies on feces; Evans et al. 2006).
We evaluated if variables covarying with livestock integration (Fig. 4) were drivers of observed trends. Interestingly, we found that models with indirect links between farming system and bird communities had poor predictive power relative to a model with a direct link with farming system (Appendix S1: Table S5). We hypothesize that this is because crop–livestock systems bring together a diversity of farm attributes, subsets of which benefit different species. Therefore, the farming system leads to cumulative benefits for bird communities that are greater than any single farm attribute. For example, farms with livestock have greater density of structures, which can promote nesting by Barn Swallows (Hirundo rustica), Cliff Swallows (Petrochelidon pyrrhonata), and Black Phoebes (Saynoris nigricans), among other species (Šálek et al. 2017), but these structures would not impact species such as Cassin's Vireo (Vireo cassinii) that build open cup nests in trees. Addition of pasture habitat could benefit grassland‐nesting birds such as Ring‐necked Pheasants (Phasianus colchicus) and Savannah Sparrows (Passerculus sandwichensis) but would likely not benefit species that nest in forest habitat. Addition of grain resources could attract large numbers of European Starlings, House Sparrows, or native blackbirds that utilize this food source (Carlson et al. 2015) but would likely not benefit obligate insectivores, such as Barn Swallows. Indeed, it appeared that the impacts of each individual component that covaried with livestock integration were small, but when combined, the effects can promote diverse and abundant wild bird populations.
The effects of landscape context
Tscharntke et al. (2005) proposed that on‐farm diversity provides the greatest benefit to biodiversity when the surrounding landscape is simple (e.g., 1–20% non‐crop habitat) rather than complex because intensively farmed landscapes lack source populations to benefit from resources provided on a single farm, while farms surrounded by natural habitat may provide redundant resources. The model is often supported for beneficial insects and birds (Smith et al. 2010, Winqvist et al. 2011, Tuck et al. 2014), although numerous exceptions exist (Kennedy et al. 2013, Lichtenberg et al. 2017). We found native bird density and richness were higher in crop–livestock systems in the most simplified landscapes in accordance with the predictions of Tscharntke et al. (2005). In contrast, native density and richness were higher on crop‐only farms in landscapes with high amounts of natural habitat (Fig. 3; Appendix S1: Fig. S10). Density of nonnative birds, on the other hand, increased with livestock integration and lower amounts of natural habitat in the landscape, but there was no interaction between the two scales. Therefore, livestock integration may be most effective at bolstering native birds in the least natural landscapes, but it may come with the cost of simultaneously attracting nonnative birds that may be undesirable (Weitzel 1988, Somers and Morris 2002). More broadly, the differing responses of native vs. nonnative birds may provide another reason why relationships between landscape structure and the benefits of on‐farm habitats to species conservation vary from one study to another (as reviewed by Tscharntke et al. 2016). That these differing effects on native vs. nonnative birds were seen across two distinct Bird Conservation Regions suggests possible generality of these patterns for bird species.
Ecosystem service and conservation implications
Understanding net effects of birds in agroecosystems is crucial for developing and promoting management practices to create win‐win scenarios for biodiversity and farmers (Pejchar et al. 2018). One approach is to understand how functional groups, such as diet guilds, respond to land management (Otieno et al. 2011) and how these shifts in diet guilds or individual species impact ecosystem service provisioning, but there are limitations to broad classification approaches. For example, insectivorous birds can increase crop yields through consumption of pest insects (Karp et al. 2013, Garfinkel and Johnson 2015, Kross et al. 2016) but are intraguild predators that have also been found to negatively impact crop yields through consumption of arthropod natural enemies (Martin et al. 2013). Omnivorous or granivorous guilds represent other contrasting effects. Birds in these guilds could include crop pests (Gebhardt et al. 2011); however, granivorous or omnivorous species could also benefit growers if they consume weed seeds and help control weed populations, which are a significant barrier to crop production for organic farmers (Bond and Grundy 2001). Therefore, some caution is warranted before concluding that more robust populations of specific bird guilds or species would necessarily lead to more effective biological control of pests or increase damage to crops.
We predicted nonnative birds would reduce native bird populations through competition for limiting nest cavities, behavioral interference, or numerical dominance (Appendix S1: Table S1; Weitzel 1988, Crozier et al. 2006, Val et al. 2018). Surprisingly, however, we found a positive relationship between density of nonnative birds and total density of native birds, native granivores, and native cavity nesters (Figs. 3, 5; Appendix S1: Fig. S12). We hypothesize the correlation is due to similar traits between nonnative birds and native granivores: three of the four nonnative bird species in our system are classified as granivores, while the fourth (European Starling) is omnivorous and a common visitor to cattle troughs, where both native and nonnative birds can reach high foraging density (De Graaf et al. 1985, Wilman et al. 2014, Carlson et al. 2015).
Cavity exclusion is the primary mechanism by which nonnative birds are thought to be detrimental to native birds (Weitzel 1988); however, our study and Koenig (2003) both failed to find significant negative impacts of nonnative birds on native birds at the community or population levels. Surprisingly, a small positive link was supported between native and nonnative cavity nesters. It is possible this is due to nonnatives utilizing cavities in livestock barns and sheds rather than nest boxes or natural cavities that may be more suitable for most cavity‐nesting natives. Nonnative birds were also most common on farms in the least natural landscapes, while native cavity nesters had higher density on farms in natural landscapes, suggesting the nonnative birds may simply utilize habitat of lower quality than native birds. Thus, we suggest crop–livestock integration could benefit native bird populations in landscapes with otherwise little habitat without great risk of interference from nonnatives.
Though most of the species we observed were common, we detected one species that is red‐listed, the Olive‐sided Flycatcher (Contopus cooperi), and 31 that are listed as endangered, threatened, or as a species of concern by the U.S. federal government or by state agencies in at least one of the states in the study region. This suggests that diversified organic agriculture could provide a refuge for threatened or endangered wildlife and supports a “land‐sharing” approach that promotes conservation (Kremen 2015). Crop–livestock integration may be a good way to support grassland birds, which is the fastest declining habitat guild in North America (Stanton et al. 2018), through the increased amount of on‐farm pasture/hay habitat. Farmer willingness to uptake crop–livestock integration may be higher than other common grassland bird management approaches such as the Conservation Reserve Program because of the direct profitability benefits to growers and ability to keep land in production (Herrero et al. 2010, Bell et al. 2014, Salton et al. 2014).
Study limitations
In our study, we strived for broad geographic coverage, which we acknowledge introduced certain limitations. First, we conducted only two point count surveys per farm per year: once during peak breeding and then again during fledgling and flocking periods. This per‐farm sampling intensity restricted our ability to robustly model species detection probabilities (Mackenzie and Royle 2005). However, we suspect that detection probabilities were lower in crop–livestock farms because they tended to occur in more natural landscapes (Appendix S1: Fig. S1) and had more woody crops and seminatural habitat (Fig. 4). Thus, accounting for detection probability would have likely strengthened effect sizes. Second, our study spanned several ecosystem types, a wide variety of land‐use contexts, and cropping systems. We depended upon the willingness of growers to participate in this study, which did not allow for recruiting large numbers of growers across wide regions with little variation between farming practices. However, we accounted for most of this variation within our modeling framework or examined in post‐hoc correlations. Regardless, we acknowledge that variation due to crop types grown or other management factors may have influenced bird activity and confounded our results.
Conclusions
The apparent attractiveness of integrated crop–livestock farming to birds that we report here may reflect this farming system's integration of greater food diversity alongside greater structural diversity. In support of this interpretation, Hiron et al. (2013) found that farmsteads with farm buildings housing livestock nearby crop fields attracted more diverse and abundant bird communities than similar sites where farming had ceased. Further, Šálek et al. (2017) found that the benefits of active farmsteads to birds persisted through the winter and extended to species of conservation concern. The integration of livestock production alongside diversified organic crop production may provide additional benefits to birds beyond those provided by organic cropping alone.
Wild bird populations have continued to decline in agricultural landscapes due to intensified production (Geiger et al. 2010, Jeliazkov et al. 2016, Frei et al. 2018a), so identifying in what systems and landscapes various agricultural diversification strategies are most effective for which species is crucial to inform agricultural policy aimed at conservation. Across two broad regions and unique biomes, we found crop–livestock systems promoted a greater richness and density of native bird species compared to crop‐only systems and found no evidence for negative impacts of nonnative birds on native birds. Our results also suggest that converting to crop–livestock systems may have greater impact than integration of single diversification practices. Overall, our study suggests small‐scale, crop–livestock systems may be an effective strategy to promote wild bird conservation and bolster ecosystem services provided to growers with minimal negative impacts.
Supporting information
Acknowledgments
Funding for this research was provided by USDA‐NIFA‐OREI grant 2015‐51300‐24155 and the Carl H. Elling Endowment in the Washington State University School of Biological Sciences. We are grateful to the many growers who allowed us access to their farms and took time to facilitate this research, especially V. Alexander. We thank J. Brunner, J. Piovia‐Scott, and H. Watts for helpful comments on the project. A. Tormanen and J. Taylor facilitated field research. M. Jones, J. Taylor, and C. Blubaugh assisted in analyses. N. A. F. Smith and D. F. Sacks provided logistical support. O. Smith, C. Kennedy, J. Owen, and W. Snyder conceived the ideas and designed methodology; O. Smith collected the data; O. Smith and T. Northfield analyzed the data; O. Smith and C. Latimer wrote code; O. Smith and W. Snyder led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Smith O. M., Kennedy C. M., Owen J. P., Northfield T. D., Latimer C. E., and Snyder W. E.. 2020. Highly diversified crop–livestock farming systems reshape wild bird communities. Ecological Applications 30(2):e02031 10.1002/eap.2031
Corresponding Editor: Dianne Brunton.
Data Availability
Data on farm and landscape information, species traits, and data used in SEMs are deposited in the Dryad Digital Repository: https://doi.org/10.5061/dryad.rjdfn2z62
Literature Cited
- Aguilar, J. , Gramig G. G., Hendrickson J. R., Archer D. W., Forcella F., and Liebig M. A.. 2015. Crop species diversity changes in the United States: 1978–2012. PLoS ONE 10:e0136580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batáry, P. , Matthiesen T., and Tscharntke T.. 2010. Landscape‐moderated importance of hedges in conserving farmland bird diversity of organic vs. conventional croplands and grasslands. Biological Conservation 143:2020–2027. [Google Scholar]
- Bates, D. , Maechler M., Bolker B., and Walker S.. 2015. Fitting linear mixed‐effects models using lme4. Journal of Statistical Software 67:1–48. [Google Scholar]
- Belfrage, K. , Björklund J., and Salomonsson L.. 2005. The effects of farm size and organic farming on diversity of birds, pollinators, and plants in a Swedish landscape. AMBIO: A Journal of the Human Environment 34:582–588. [PubMed] [Google Scholar]
- Bell, L. W. , Moore A. D., and Kirkegaard J. A.. 2014. Evolution in crop – livestock integration systems that improve farm productivity and environmental performance in Australia. European Journal of Agronomy 57:10–20. [Google Scholar]
- Bengtsson, J. , Ahnström J., and Weibull A. C.. 2005. The effects of organic agriculture on biodiversity and abundance: a meta‐analysis. Journal of Applied Ecology 42:261–269. [Google Scholar]
- Benton, T. G. , Vickery J. A., and Wilson J. D.. 2003. Farmland biodiversity: is habitat heterogeneity the key? Trends in Ecology and Evolution 18:182–188. [Google Scholar]
- Boesing, A. L. , Nichols E., and Metzger J. P.. 2017. Effects of landscape structure on avian‐mediated insect pest control services: a review. Landscape Ecology 32:931–944. [Google Scholar]
- Bond, W. , and Grundy A. C.. 2001. Non‐chemical weed management in organic farming systems. Weed Research 41:383–405. [Google Scholar]
- Carlson, J. C. , Hyatt D. R., Bentler K., Mangan A. M., Russell M., Piaggio A. J., and Linz G. M.. 2015. Molecular characterization of Salmonella enterica isolates associated with starling–livestock interactions. Veterinary Microbiology 179:109–118. [DOI] [PubMed] [Google Scholar]
- Chamberlain, D. E. , Fuller R. J., Bunce R. G. H., Duckworth J. C., and Shrubb M.. 2000. Changes in the abundance of farmland birds in relation to the timing of agricultural intensification in England and Wales. Journal of Applied Ecology 37:771–788. [Google Scholar]
- Crozier, A. M. L. , Seamans M. E., Gutiérrez R. J., Peter J., and Horn R. B.. 2006. Does the presence of Barred Owls suppress the calling behavior of Spotted Owls? Condor 108:760–769. [Google Scholar]
- De Graaf, R. M. , Tilghman N. G., and Anderson S. H.. 1985. Foraging guilds of North American birds. Environmental Management 9:493–536. [Google Scholar]
- Dross, C. , Princé K., Jiguet F., and Tichit M.. 2018. Contrasting bird communities along production gradients of crops and livestock in French farmlands. Agriculture, Ecosystems and Environment 253:55–61. [Google Scholar]
- Dunn, C. P. , Stearns F., Guntenspergen G. R., and Sharpe D. M.. 1993. Ecological benefits of the Conservation Reserve Program. Conservation Biology 7:132–139. [Google Scholar]
- Evans, D. M. , Redpath S. M., Evans S. A., Elston D. A., Gardner C. J., Dennis P., and Pakeman R. J.. 2006. Low intensity, mixed livestock grazing improves the breeding abundance of a common insectivorous passerine. Biology Letters 2:636–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei, B. , Bennett E. M., and Kerr J. T.. 2018a. Cropland patchiness strongest agricultural predictor of bird diversity for multiple guilds in landscapes of Ontario, Canada. Regional Environmental Change 18:2105–2115. [Google Scholar]
- Frei, B. , Renard D., Mitchell M. G. E., Seufert V., Jeanine R. C., and Elena M. R.. 2018b. Bright spots in agricultural landscapes: identifying areas exceeding expectations for multifunctionality and biodiversity. Journal of Applied Ecology 55:2731–2743. [Google Scholar]
- Garfinkel, M. , and Johnson M.. 2015. Pest‐removal services provided by birds on small organic farms in northern California. Agriculture, Ecosystems and Environment 211:24–31. [Google Scholar]
- Gebhardt, K. , Anderson A. M., Kirkpatrick K. N., and Shwiff S. A.. 2011. A review and synthesis of bird and rodent damage estimates to select California crops. Crop Protection 30:1109–1116. [Google Scholar]
- Geiger, F. , et al. 2010. Landscape composition influences farm management effects on farmland birds in winter: a pan‐European approach. Agriculture, Ecosystems and Environment 139:571–577. [Google Scholar]
- Hald, B. , Skov M. N., Nielsen E. M., Rahbek C., Madsen J. J., Wainø M., Chriél M., Nordentoft S., Baggesen D. L., and Madsen M.. 2016. Campylobacter jejuni and Campylobacter coli in wild birds on Danish livestock farms. Acta Veterinaria Scandinavica 58:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath, S. K. , Soykan C. U., Velas K. L., Kelsey R., and Kross S. M.. 2017. A bustle in the hedgerow: woody field margins boost on farm avian diversity and abundance in an intensive agricultural landscape. Biological Conservation 212:153–161. [Google Scholar]
- Herrero, M. , et al. 2010. Smart investments in sustainable food production: revisiting mixed crop‐livestock systems. Science 327:822–825. [DOI] [PubMed] [Google Scholar]
- Hiron, M. , Berg Å., Eggers S., Josefsson J., and Pärt T.. 2013. Bird diversity relates to agri‐environment schemes at local and landscape level in intensive farmland. Agriculture, Ecosystems and Environment 176:9–16. [Google Scholar]
- Homer, C. , Fry J., and Barnes C.. 2012. The National Land Cover Database. U.S. Geological Survey Fact Sheet 2012‐3020:4. https://pubs.usgs.gov/fs/2012/3020
- Jackson, H. B. , and Fahrig L.. 2015. Are ecologists conducting research at the optimal scale? Global Ecology and Biogeography 24:52–63. [Google Scholar]
- Jeliazkov, A. , Mimet A., Charge R., Jiguet F., Devictor V., and Chiron F.. 2016. Impacts of agricultural intensification on bird communities: new insights from a multi‐level and multi‐facet approach of biodiversity. Agriculture, Ecosystems and Environment 216:9–22. [Google Scholar]
- Jones, G. A. , Sieving K. E., and Jacobson S. K.. 2005. Avian diversity and functional insectivory on North‐Central Florida farmlands. Conservation Biology 19:1234–1245. [Google Scholar]
- Karp, D. S. , Mendenhall C. D., Sandí R. F., Chaumont N., Ehrlich P. R., Hadly E. A., and Daily G. C.. 2013. Forest bolsters bird abundance, pest control and coffee yield. Ecology Letters 16:1339–1347. [DOI] [PubMed] [Google Scholar]
- Kennedy, C. M. , Marra P. P., Fagan W. F., and Neel M. C.. 2010. Landscape matrix and species traits mediate responses of Neotropical resident birds to forest fragmentation in Jamaica. Ecological Monographs 80:651–669. [Google Scholar]
- Kennedy, C. M. , et al. 2013. A global quantitative synthesis of local and landscape effects on wild bee pollinators in agroecosystems. Ecology Letters 16:584–599. [DOI] [PubMed] [Google Scholar]
- Kleijn, D. , and Sutherland W. J.. 2003. How effective are European agri‐environment schemes in conserving and promoting biodiversity? Journal of Applied Ecology 40:947–969. [Google Scholar]
- Koenig, W. D. 2003. European Starlings and their effect on native cavity‐nesting birds. Conservation Biology 17:1134–1140. [Google Scholar]
- Kremen, C. 2015. Reframing the land‐sparing/land‐sharing debate for biodiversity conservation. Annals of the New York Academy of Sciences 1355:52–76. [DOI] [PubMed] [Google Scholar]
- Kross, S. M. , Kelsey T. R., McColl C. J., and Townsend J. M.. 2016. Field‐scale habitat complexity enhances avian conservation and avian‐mediated pest‐control services in an intensive agricultural crop. Agriculture, Ecosystems and Environment 225:140–149. [Google Scholar]
- Lefcheck, J. S. 2016. piecewiseSEM: Piecewise structural equation modelling in R for ecology, evolution, and systematics. Methods in Ecology and Evolution 7:573–579. [Google Scholar]
- Lichtenberg, E. M. , et al. 2017. A global synthesis of the effects of diversified farming systems on arthropod diversity within fields and across agricultural landscapes. Global Change Biology 23:4946–4957. [DOI] [PubMed] [Google Scholar]
- Mackenzie, D. I. , and Royle J. A.. 2005. Designing occupancy studies: general advice and allocating survey effort. Journal of Applied Ecology 42:1105–1114. [Google Scholar]
- Martin, E. A. , Reineking B., Seo B., and Steffan‐Dewenter I.. 2013. Natural enemy interactions constrain pest control in complex agricultural landscapes. Proceedings of the National Academy of Sciences USA 110:5534–5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarigal, K. , and Marks B.. 1994. FRAGSTATS: spatial pattern analysis program for quanityfing landscape structure. U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station, Corvallis, Oregon, USA. [Google Scholar]
- Nakagawa, S. , and Schielzeth H.. 2013. A general and simple method for obtaining R2 from generalized linear mixed‐effects models. Methods in Ecology and Evolution 4:133–142. [Google Scholar]
- Oksanen, J. , Blanchet F. G., Kindt R., Legendre P., Minchin P. R., Hara R. B. O., Simpson G. L., Solymos P., Stevens M. H. H., and Wagner H.. 2019. vegan: Community Ecology Package. R package version 2.5‐4. https://CRAN.R-project.org/package=vegan
- Otieno, N. E. , Gichuki N., Farwig N., and Kiboi S.. 2011. The role of farm structure on bird assemblages around a Kenyan tropical rainforest. African Journal of Ecology 49:410–417. [Google Scholar]
- Peisley, R. K. , Saunders M. E., and Luck G. W.. 2015. A systematic review of the benefits and costs of bird and insect activity in agroecosystems. Springer Science Reviews 3:113–125. [Google Scholar]
- Pejchar, L. , Clough Y., Ekroos J., Nicholas K. A., Olsson O. L. A., Ram D., Tschumi M., and Smith H. G.. 2018. Net effects of birds in agroecosystems. BioScience 68:896–904. [Google Scholar]
- Philpott, S. M. , Soong O., Lowenstein J. H., Pulido A. L., Lopez T., Flynn D. F. B., Declerck F., and Lopez D. T.. 2009. Functional richness and ecosystem services: bird predation in tropical on arthropods agroecosystems. Ecological Applications 19:1858–1867. [DOI] [PubMed] [Google Scholar]
- R Core Team . 2017. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: http://www.R-project.org [Google Scholar]
- Rodewald, P. 2015. The birds of North America. Cornell Laboratory of Ornithology, Ithaca, New York, USA. [Google Scholar]
- Šálek, M. , Bažant M., and Zmihorski M.. 2017. Active farmsteads are year‐round strongholds for farmland birds. Journal of Applied Ecology 55:1908–1918. [Google Scholar]
- Salton, J. C. , Mercante F. M., Tomazi M., Zanatta J. A., Concenço G., Silva W. M., and Retore M.. 2014. Integrated crop‐livestock system in tropical Brazil: toward a sustainable production system. Agriculture, Ecosystems and Environment 190:70–79. [Google Scholar]
- Sauer, J. , Fallon J., and Johnson R.. 2003. Use of North American Breeding Bird Survey data to estimate population change for bird conservation regions. Journal of Wildlife Management 67:372–389. [Google Scholar]
- Seavy, N. E. , Viers J. H., and Wood J. K.. 2009. Riparian bird response to vegetation structure: a multiscale analysis using LiDAR measurements of canopy height. Ecological Applications 19:1848–1857. [DOI] [PubMed] [Google Scholar]
- Shipley, B. 2009. Confirmatory path analysis in a generalized multilevel context. Ecology 90:363–368. [DOI] [PubMed] [Google Scholar]
- Smith, O. M. 2017. Population responses of the Northern Bobwhite (Colinus virginianus) to land use changes in the agricultural landscapes of Ohio, USA. Population Ecology 59:363–370. [Google Scholar]
- Smith, H. , Dänhardt J., Lindström Å., and Rundlöf M.. 2010. Consequences of organic farming and landscape heterogeneity for species richness and abundance of farmland birds. Oecologia 162:1071–1079. [DOI] [PubMed] [Google Scholar]
- Smith, O. , Kennedy C., Owen J., Northfield T., Latimer C., and Snyder W.. 2019. Data from: Highly diversified crop‐livestock farming systems reshape wild bird communities. Dryad. 10.5061/dryad.rjdfn2z62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers, C. M. , and Morris R. D.. 2002. Birds and wine grapes: foraging activity causes small‐scale damage patterns in single vineyards. Journal of Applied Ecology 39:511–523. [Google Scholar]
- Stanton, R. L. , Morrissey C. A., and Clark R. G.. 2018. Analysis of trends and agricultural drivers of farmland bird declines in North America: a review. Agriculture, Ecosystems and Environment 254:244–254. [Google Scholar]
- Tilman, D. , Cassman K. G., Matson P. A., Naylor R., and Polasky S.. 2002. Agricultural sustainability and intensive production practices. Nature 418:671. [DOI] [PubMed] [Google Scholar]
- Tscharntke, T. , Klein A., Kruess A., Steffan‐Dewenter I., and Thies C.. 2005. Landscape perspectives on agricultural intensification and biodiversity–ecosystem service management. Ecology Letters 8:857–874. [Google Scholar]
- Tscharntke, T. , et al. 2012. Landscape moderation of biodiversity patterns and processes—eight hypotheses. Biological Reviews 87:661–685. [DOI] [PubMed] [Google Scholar]
- Tscharntke, T. , et al. 2016. When natural habitat fails to enhance biological pest control—five hypotheses. Biological Conservation 204:449–458. [Google Scholar]
- Tuck, S. L. , Winqvist C., Mota F., Ahnström J., Turnbull L. A., and Bengtsson J.. 2014. Land‐use intensity and the effects of organic farming on biodiversity: a hierarchical meta‐analysis. Journal of Applied Ecology 51:746–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Val, J. , Eldridge D. J., Travers S. K., and Oliver I.. 2018. Livestock grazing reinforces the competitive exclusion of small‐bodied birds by large aggressive birds. Journal of Applied Ecology 55:1919–1929. [Google Scholar]
- Warton, D. I. , and Hui F. K. C.. 2011. The arcsine is asinine: the analysis of proportions in ecology. Ecology 92:3–10. [DOI] [PubMed] [Google Scholar]
- Weitzel, N. H. 1988. Nest‐site competition between the European Starling and native breeding birds in Northwestern Nevada. Condor 90:515–517. [Google Scholar]
- Wilcove, D. S. , Rothstein D., Dubow J., Phillips A., and Losos E.. 1998. Quantifying threats to imperiled species in the United States. BioScience 48:607–615. [Google Scholar]
- Wilman, H. , Belmaker J., Jennifer S., de la Rosa C., Rivadeneira M. M., and Jetz W.. 2014. EltonTraits 1.0: species‐level foraging attributes of the world's birds and mammals. Ecology 95:2027. [Google Scholar]
- Wilson, S. , Mitchell G. W., Pasher J., McGovern M., Hudson M. A. R., and Fahrig L.. 2017. Influence of crop type, heterogeneity and woody structure on avian biodiversity in agricultural landscapes. Ecological Indicators 83:218–226. [Google Scholar]
- Winqvist, C. , et al. 2011. Mixed effects of organic farming and landscape complexity on farmland biodiversity and biological control potential across Europe. Journal of Applied Ecology 48:570–579. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data on farm and landscape information, species traits, and data used in SEMs are deposited in the Dryad Digital Repository: https://doi.org/10.5061/dryad.rjdfn2z62


