Summary
Awake tracheal intubation has a high success rate and a favourable safety profile but is underused in cases of anticipated difficult airway management. These guidelines are a comprehensive document to support decision making, preparation and practical performance of awake tracheal intubation. We performed a systematic review of the literature seeking all of the available evidence for each element of awake tracheal intubation in order to make recommendations. In the absence of high‐quality evidence, expert consensus and a Delphi study were used to formulate recommendations. We highlight key areas of awake tracheal intubation in which specific recommendations were made, which included: indications; procedural setup; checklists; oxygenation; airway topicalisation; sedation; verification of tracheal tube position; complications; management of unsuccessful awake tracheal intubation; post‐tracheal intubation management; consent; and training. We recognise that there are a range of techniques and regimens that may be effective and one such example technique is included. Breaking down the key practical elements of awake tracheal intubation into sedation, topicalisation, oxygenation and performance might help practitioners to plan, perform and address complications. These guidelines aim to support clinical practice and help lower the threshold for performing awake tracheal intubation when indicated.
Keywords: airway management, bronchoscopy, laryngoscopy, tracheal intubation, training, videolaryngoscopy
Recommendations
Awake tracheal intubation must be considered in the presence of predictors of difficult airway management.
A cognitive aid such as a checklist is recommended before and during performance of awake tracheal intubation.
Supplemental oxygen should always be administered during awake tracheal intubation.
Effective topicalisation must be established and tested. The maximum dose of lidocaine should not exceed 9 mg.kg−1 lean body weight.
Cautious use of minimal sedation can be beneficial. This should ideally be administered by an independent practitioner. Sedation should not be used as a substitute for inadequate airway topicalisation.
The number of attempts should be limited to three, with one further attempt by a more experienced operator (3 + 1).
Anaesthesia should only be induced after a two‐point check (visual confirmation and capnography) has confirmed correct tracheal tube position.
All departments should support anaesthetists to attain competency and maintain skills in awake tracheal intubation.
Why were these guidelines developed?
Awake tracheal intubation (ATI) has a high success rate and a low‐risk profile and has been cited as the gold standard in airway management for a predicted difficult airway. However, ATI is reported to be used in as few as 0.2% of all tracheal intubations in the UK 1. There are barriers preventing broad uptake and use of awake techniques for securing the airway. We aimed to produce generalisable guidelines to improve patient safety by making ATI more accessible to all clinicians, trainers and institutions. Rather than inform expert practice, these guidelines aim to support the use of ATI by more clinicians, with a particular focus on those that do not regularly perform ATI. There remains heterogeneity in clinical practice, underscoring the need for a more consistent approach using the available evidence, which these guidelines aim to deliver.
What other guidelines exist?
Although there are many guidelines on unanticipated difficult airway management 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, there are few that specifically focus on the anticipated difficult airway. The ASA, Canadian Airway Focus Group, French Society for Anesthesia and Intensive Care and German Society for Anesthesiology and Intensive Care describe clinical decision making in the anticipated difficult airway 6, 7, 10, 12.
How do these guidelines differ from existing guidelines?
At the time of writing, there were no nationally or internationally agreed guidelines on the practical performance of ATI.
Disclaimer
These guidelines are not intended to represent a minimum standard of practice, nor are they to be regarded as a substitute for good clinical judgement. They present key principles and suggested strategies for preparation, performance, consent and training to inform clinical practice. This document is intended to guide appropriately trained operators.
Introduction
A strategy for difficult airway management is necessary when facemask ventilation, supraglottic airway device (SAD) placement or ventilation, tracheal intubation or insertion of a front‐of‐neck airway (FONA) is predicted to be challenging. The incidence of difficult facemask ventilation is 0.66–2.5% 14, 15, 16, 17, difficult SAD placement or ventilation 0.5–4.7% 18, 19, 20, 21, 22, difficult tracheal intubation 1.9–10% 14, 16, 23, 24, 25 and combined difficulty in both facemask and tracheal intubation 0.3–0.4% 16. As a rescue technique after failed tracheal intubation, one study reported that SADs have a success rate as low as 65% in difficult airway management 26. The reported incidence of requirement for emergency FONA and death due to airway management are 0.002–0.07% (1:50,000–1:1400) 1, 27, 28 and 0.0006–0.04% (1:180,000–1:2800), respectively 1, 28. The risk and severity of adverse outcomes during difficult airway management is highlighted by the plethora of guidelines and cognitive aids for airway rescue 29.
Awake tracheal intubation involves placing a tracheal tube in an awake, spontaneously‐breathing patient, most commonly with flexible bronchoscopy (ATI:FB) or videolaryngoscopy (ATI:VL, Table 1). This allows the airway to be secured before induction of general anaesthesia, avoiding the potential risks and consequences of difficult airway management in an anaesthetised patient 30.
Table 1.
A summary of terms used in these guidelines
| Term | Definition |
|---|---|
| ATI | Awake tracheal intubation |
| ATI:FB | Awake tracheal intubation using flexible bronchoscopy |
| ATI:VL | Awake tracheal intubation using videolaryngoscopy |
| FONA | Front‐of‐neck airway |
| sTOP | Sedation, topicalisation, oxygenation, performance |
| Minimal sedation | Drug‐induced state during which the patient responds normally to verbal commands, while the airway, spontaneous ventilation and cardiovascular function are unaffected |
| Airway topicalisation | Topical application of local anaesthetic to the airway |
| Performance | The practical conduct of awake tracheal intubation |
| Two‐point check |
1. Visualisation of the tracheal lumen with ATI:FB or tracheal tube through the cords with ATI:VL to confirm tracheal placement 2. Capnography to exclude oesophageal intubation |
| Unsuccessful attempt | Unplanned removal of flexible bronchoscope, videolaryngoscope or tracheal tube from the airway |
| Unsuccessful ATI | Successful tracheal intubation not achieved after 3 + 1 attemptsa |
Three attempts by the primary operator and a fourth attempt by a more experienced operator.
Awake tracheal intubation has a favourable safety profile because both spontaneous ventilation and intrinsic airway tone are maintained until the trachea is intubated 31, 32, 33, 34, 35. Awake tracheal intubation can be unsuccessful in 1–2% of cases, but this rarely leads to airway rescue strategies or death 33, 34, 35. These guidelines aim to increase the use of ATI by providing clear guidance for clinicians to support decision making, preparation and performance of ATI in the setting of a predicted difficult airway.
Methods
The development of these guidelines followed the appraisal of guidelines for research and evaluation (AGREE) reporting checklist 36. To ensure these guidelines are supported by best evidence, a systematic review adhering to the preferred reporting items for systematic reviews and meta‐analyses (PRISMA) recommendations 37 was performed. We sought published data of relevance to ATI, including decision making, technical performance, complications, training and non‐technical aspects. Details of the search, screening and study selection are shown in the supplementary material (Supporting Information, Appendix S1).
Data from included studies were synthesised and consensus from all 10 members of the guideline group was sought to formulate guideline recommendations using a three‐round Delphi method 38, 39. The first round entailed an initially proposed longlist of recommendations, which were each reviewed and rated for content and clarity. The recommendations in which six or more members of the guideline group approved were shortlisted. A second round of rating was then undertaken, in which the highest rated recommendations were selected. Finally, a third round involving recommendation‐ratification in round‐table discussions was undertaken. These recommendations were based on a number of factors, including: volume and consistency of supportive evidence; applicability and generalisability of the evidence to current practice; and clinical and practical implications of recommendations.
We determined the level of evidence and graded the strength of subsequent recommendations using a modified version of the system developed by the Centre for Evidence‐based Medicine (Oxford, UK) (Table 2) 40. Each recommendation was graded A to D according to the strength of the available evidence 41.
Table 2.
Grading of recommendations based on the level of evidence available
| Grade | Level of evidence available |
|---|---|
| A |
|
| B |
|
| C |
|
| D |
|
RCT, randomised controlled trial.
Over 3 years the guideline group met 21 times in person and 14 times remotely in order to develop, draft and finalise these guidelines. Draft versions were presented at the 2017 and 2018 Difficult Airway Society (DAS) annual scientific meetings. We sent an electronic survey to DAS members (n = 2150) to capture their opinions, preferences and clinical experiences in ATI, of whom 632 (29%) responded. This survey highlighted the need for guidelines for ATI and the role of a standardised technique for training and clinical practice. We also performed a survey of 43 international experts, seeking details on commonly used strategies for oxygenation, topicalisation, sedation and performance of ATI. Patient and public involvement was also used to explore the views and experiences of patients who had undergone ATI. This was achieved by conducting a fully anonymised multicentre structured survey of 100 patients, where we explored the self‐reported experiences of the overall conduct of ATI. We consulted an anaesthetic assistant during the preparation of these guidelines and invited a senior anaesthetic nurse and two consultant head and neck surgeons to comment on the final draft. A draft manuscript of these guidelines was then sent to 13 international experts with clinical or academic experience related to ATI to gather specific comments and feedback on recommendations and to assess applicability and feasibility. The guideline group considered the responses from expert reviewers to inform the final recommendations. The final draft of the guideline was then submitted to DAS executive committee for ratification.
Indications
Prediction of difficult airway management is unreliable 14, 23, 42, but there are common features that have been identified in patients requiring ATI. These include, but are not limited to: patients with head and neck pathology (including malignancy, previous surgery or radiotherapy); reduced mouth opening; limited neck extension; obstructive sleep apnoea; morbid obesity; and progressive airway compromise 32, 33, 35, 43, 44. There is limited evidence for any individual, validated, predictive assessment tool developed specifically for ATI. Airway assessment including history, examination and appropriate investigations, is indicated for all patients 1, 2, 7, 45 (Grade D). Awake tracheal intubation must be considered in the presence of predictors of difficult airway management (Grade D). In an elective setting the patient should be appropriately fasted (Grade D). In the non‐fasted patient, the potential for regurgitation or aspiration of gastric contents still exists even with ATI. There are few relative contra‐indications to ATI (e.g. local anaesthetic allergy, airway bleeding, unco‐operative patients) but the only absolute contra‐indication is patient refusal.
Procedural setup
Awake tracheal intubation can be associated with the greatest operator‐related physical, mental and psychological stress of all elective airway management interventions 46. These stressors may be associated with suboptimal performance 47, 48, increasing the risk of complications including failure. Teamwork, good communication and appropriate preparation may mitigate these challenges 48, 49, 50 and the importance of well‐trained, competent assistants should not be underestimated. Safety should not be compromised by time pressures presented by other staff members; therefore planning and communication with anaesthetic assistants, operating theatre nursing staff, surgeons and skilled anaesthetic colleagues is essential (Grade D).
Consideration and planning of the appropriate location is essential. Awake tracheal intubation should ideally be performed in the operating theatre environment (Grade D). This setting has ready access to skilled assistance, drugs, equipment and space. For high‐risk patients, including those with significant airway obstruction, hypoxia, respiratory failure, challenging or failed ATI, the operating theatre may have advantages over an anaesthetic room 1, 51, such as greater space and immediate surgical assistance. When ATI is performed outside of the theatre environment (e.g. in the critical care unit or the emergency department), the same standards of care should apply (Grade D) 52.
Monitoring patients’ physiological parameters during anaesthetic care mitigates risks and may alert operators to impending complications 53, 54, 55, 56, 57. Frequently occurring avoidable complications in ATI that may be detected by monitoring are airway obstruction and hypoventilation secondary to over‐sedation 33, 34, 35, 58. Disturbances to cardiac rhythm and blood pressure following administration of pharmacological agents for topicalisation and sedation are possible 35, 59, 60, 61. In accordance with Association of Anaesthetists’ guidelines for patients receiving sedation 62, it is recommended that ECG, non‐invasive blood pressure, pulse oximetry and continuous end‐tidal carbon dioxide monitoring are used throughout the process of ATI (Grade C). It is acknowledged that end‐tidal carbon dioxide monitoring during ATI may be challenging in current practice.
Workspace ergonomics have an impact on performance and safety 51, 63, and should be considered before starting the procedure (Grade D; Fig. 1; Supporting Information, Appendix S2) 52. This includes optimising the position of patient, operator and assistants, as well as location of equipment and monitors, which should be in the direct line of sight of the operator. There is no consensus on the ideal operator or patient position 64, 65, 66, 67, but there are physiological and anatomical advantages to having patients sitting up 68, 69, 70.
Figure 1.
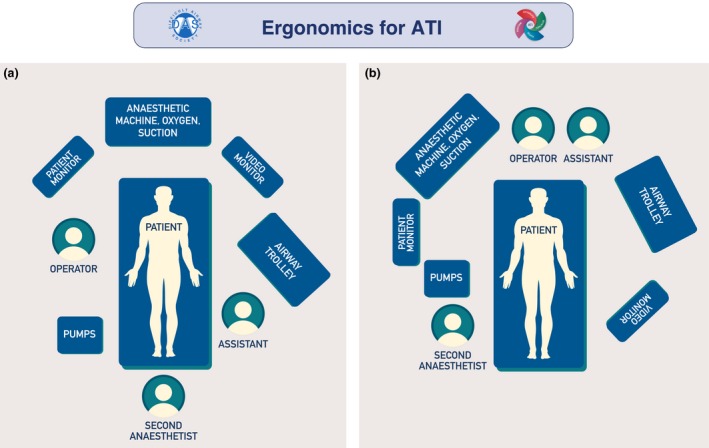
Examples of ergonomics for awake tracheal intubation (ATI). The primary operator should have a direct line of sight of the patient, video monitor and patient monitor, as well as immediate access to infusion pumps, anaesthetic machine, suction and oxygen delivery device. If a second anaesthetist is present, they should be positioned with a direct line of sight of the patient and have immediate access to infusion pumps, as well as be able to access all other equipment. The anaesthetic assistant's primary position should be with immediate access to the airway trolley, and in proximity to the operator. (a) Awake tracheal intubation performed with the operator positioned facing the patient who is in a sitting up position. (b) Awake tracheal intubation performed with the operator positioned behind the supine/semi‐recumbent patient. This figure forms part of the Difficult Airway Society guidelines for ATI in adults and should be used in conjunction with the text. ©Difficult Airway Society 2019.
Complications or unsuccessful ATI, although uncommon, should be prepared for 33, 34, 35, and immediate access to emergency drugs, staff and equipment is essential (Grade C). A plan for unsuccessful ATI, including possible postponement, FONA or high‐risk general anaesthesia, should be discussed explicitly and agreed on by all team members before beginning the procedure (Grade D).
It is important to select an appropriate route for tracheal intubation, visualisation device and tracheal tube. The route for tracheal intubation should take into account patient anatomy, surgical access and tracheal extubation plan (Grade D). For example, in patients with limited mouth opening, the nasal approach may be the only option, while in patients having nasal surgery, the oral approach may be the preferred route. There is no evidence or consensus among experts demonstrating superiority of one route if both are feasible 33, 34, 35, 64, 71.
Awake tracheal intubation using videolaryngoscopy has a comparable success rate and safety profile to ATI:FB (98.3% each) 31. Choosing between techniques is based on patient factors, operator skills and availability of equipment (Grade A). For example, in patients with limited mouth opening, a large tongue or fixed flexion deformity of the neck, ATI:FB may be more appropriate. Conversely, patients with airway bleeding may be more suitable for an ATI:VL technique. If the chosen ATI technique is unsuccessful, practitioners should consider using an alternative (e.g. ATI:FB if ATI:VL is unsuccessful or vice versa; Grade D). A combined approach to ATI using both VL and FB has been described 72, 73, 74 and could be considered in complex clinical scenarios (Grade D). In a well‐topicalised patient, insertion of an SAD as a conduit for ATI:FB has also been described 75, 76, and may provide the benefit of maintaining airway patency. Single‐use flexible bronchoscopes are associated with a similar safety profile to re‐usable ones 77. Operators should defer to local availability and personal experience in determining which flexible bronchoscope to use (Grade B). There is currently no evidence or consensus to support the safety or efficacy of any individual videolaryngoscope. For ATI:VL practitioners should use videolaryngoscopes with which they are most familiar (Grade B).
Careful selection of tracheal tube is integral to the success of any ATI technique. This should factor in size (internal and external diameter), shape, length, tip design and material. For ATI:FB, reinforced, Parker Flex‐Tip™ (Bridgewater, CN, USA) and intubating laryngeal mask airway tubes (LMA® Fastrach™ ETT, Teleflex, Beaconsfield, UK) have been shown to be superior to standard polyvinylchloride (PVC) tracheal tubes in terms of ease of tracheal intubation, railroading (advancing the tracheal tube over the flexible bronchoscope) and decreasing laryngeal impingement 78, 79, 80, 81, 82, 83, 84, 85, 86. Therefore, the use of a standard PVC tracheal tube is not recommended (Grade A). Using the smallest appropriate external diameter tracheal tube is advisable, as this may reduce the incidence of impingement 87 (Grade B). Positioning the bevel of the tracheal tube posteriorly is recommended 80, 82, 86 (Grade A). For ATI:VL, tracheal tube selection is similar to that in an asleep patient and is influenced by the VL selected.
Checklists
In the peri‐operative setting the use of cognitive aids, such as checklists, improves inter‐professional communication, teamwork and patient outcomes 88, 89, 90, 91. In anaesthetic practice, cognitive aids enhance performance in simulated emergency scenarios 92, 93, and their use been recommended in elective airway management 1. Given the potential benefits, we recommend a cognitive aid such as a checklist before and during performance of ATI (Grade D; Supporting Information, Appendix S2). The key components of ATI are sedation, topicalisation, oxygenation and performance (sTOP; Fig. 2). The ‘s’ is in lower case to emphasise the optional nature of sedation.
Figure 2.
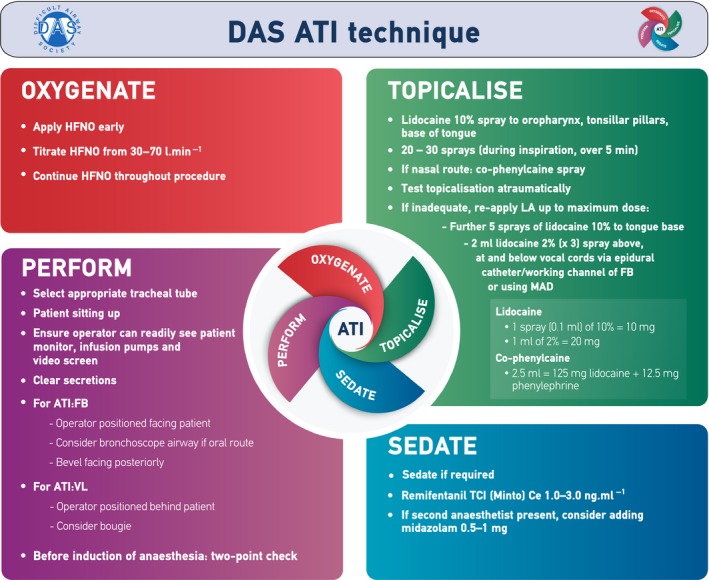
The Difficult Airway Society awake tracheal intubation (ATI) technique. This figure forms part of the Difficult Airway Society guidelines for ATI in adults and should be used in conjunction with the text. HFNO, high‐flow nasal oxygen; LA, local anaesthetic; FB, flexible bronchoscopy; MAD, mucosal atomising device; TCI, target‐controlled infusion; Ce, effect‐site concentration; VL, videolaryngoscopy. ©Difficult Airway Society 2019.
Oxygenation
The reported incidence of desaturation (SpO2 ≤ 90%) with low‐flow (< 30 l.min−1) oxygen techniques during ATI ranges between 12% and 16% 58, 94, 95. When warmed and humidified high‐flow nasal oxygen is used, the reported incidence of desaturation is 0–1.5% 33, 96; this was the most common oxygenation strategy used by experts responding to our survey. Although there are no randomised controlled trials comparing air vs. oxygen during ATI, data from bronchoscopy studies demonstrate that there is a significant difference in the incidence and severity of desaturation 97, 98. In patients receiving sedation in a variety of settings, administration of oxygen has been shown to reduce the incidence of desaturation when compared with air 97, 99, 100, 101, 102. United Kingdom, European and North American recommendations for sedation all suggest the use of supplemental oxygen 103, 104, 105. Whilst airway topicalisation alone may rarely be associated with desaturation and airway obstruction 59, 106, there is no significant difference in the incidence of desaturation between ATI:FB and ATI:VL techniques, and therefore the recommendations apply to both approaches 31. The administration of supplemental oxygen during ATI is recommended (Grade B). This should be started on patient arrival for the procedure and continued throughout (Grade D). If available, high‐flow nasal oxygen should be the technique of choice (Grade C).
Airway topicalisation
The success of ATI depends on effective topical application of local anaesthetic to the airway. Vasoconstriction of the nasal passage reduces the incidence of epistaxis 107, 108. The use of topical nasal vasoconstrictors before nasotracheal intubation is recommended (Grade A).
Lidocaine has theoretical safety benefits over other local anaesthetic agents due to a favourable cardiovascular and systemic toxicity risk profile 109; this is the most commonly used local anaesthetic agent for ATI. Following airway topicalisation, clinical evidence of toxicity or levels exceeding toxic plasma concentrations have been shown with lidocaine doses of 6.0–9.3 mg.kg−1 lean body weight 110, 111, 112, 113, 114. The dose of topical lidocaine should not exceed 9 mg.kg−1 lean body weight (Grade C) 115. The recommendation of 9 mg.kg−1 rather than 9.3 mg.kg−1 is a pragmatic decision to allow ease of calculation. Practitioners should recognise that this is not a target but a maximum dose, and in practice this is rarely required. The total dose of all local anaesthetics administered, regardless of route (e.g. regional anaesthesia or surgical infiltration), must also be considered (Grade D). Some studies have shown that lower concentrations of lidocaine are as effective as higher concentrations 112, 116, 117, 118, but higher concentrations may be associated with more rapid onset of airway anaesthesia. As with all local techniques, a high index of suspicion of the rare possibility of local anaesthetic toxicity with appropriate training, procedures and emergency drug provision (including lipid emulsion) should be in place 119, 120, 121, 122 (Grade D). The use of cocaine for topicalisation and vasoconstriction can be associated with toxic cardiovascular complications 123, 124, 125, 126, while its analgesic efficacy during nasotracheal tube insertion is no better than co‐phenylcaine (2.5 ml lidocaine 5%/phenylephrine 0.5%) 127. Cocaine in this setting is therefore not advised, and phenylephrine in combination with lidocaine is more appropriate (Grade A).
Depending on the delivery device used, there is variable local anaesthetic absorption 128 but this should not affect the maximal dose calculation. There is insufficient evidence to recommend any individual topicalisation technique (e.g. mucosal atomisation, spray‐as‐you‐go, transtracheal injection, nebulisation) 129. However, blocks of the glossopharyngeal and superior laryngeal nerves have been associated with higher plasma concentrations of local anaesthetic 130, local anaesthetic systemic toxicity 131 and lower patient comfort 132. Invasive techniques should therefore be reserved for those with expertise in their performance (Grade B). Nebulised lidocaine can be used but absorption is variable 133; consequently higher doses have been used to compensate for this 59. Regardless of technique used, the adequacy of topicalisation should be tested in an atraumatic manner before airway instrumentation 134 (Grade D), for example, with a soft suction catheter or Yankauer sucker.
The use of an antisialogogue is not mandatory in the performance of ATI and may be associated with undesirable clinical consequences (Grade D; Table 3) 135. There is limited evidence to support their use in ATI, but in anaesthetised patients the clarity of a visual field through a flexible bronchoscope may be improved 136. If used, intramuscular antisialogogues should be injected 40–60 min before performing ATI, for peak mucosal drying effect, but there are few data on the intravenous (i.v.) route in this setting.
Table 3.
Characteristics of drugs used commonly during ATI
| Class | Drug | Onset | Duration of action | Terminal elimination half‐life | Dosing | Notes |
|---|---|---|---|---|---|---|
| Antisialogogue | Glycopyrronium bromide | 20 min (i.m.) | 30–60 min | 40–80 min | 0.2–0.4 mg | Administer 30–60 min pre‐procedure |
| 3–5 min (i.v.) | 30–60 min | 40–80 min | 0.1–0.2 mg | May produce significant tachycardia | ||
| Atropine | 20 min (i.m.) | 30–60 min | 2 h | 0.3–0.6 mg | Administer 30–60 min pre‐procedure – less commonly used than glycopyrronium bromide due to tachycardia | |
| 2–3 min (i.v.) | 30–60 min | 2 h | 0.2–0.3 mg | May produce significant tachycardia | ||
| Hyoscine hydrobromide |
30 min (i.m.) 5–10 min (i.v.) |
4 h | 5 h | 0.2–0.6 mg |
Administer 30–60 min pre‐procedure Longer lasting systemic effects than glycopyrronium bromide and atropine May produce tachycardia, dizziness and sedation |
|
| Topical anaesthesia | Co‐phenylcaine spray | 2–5 min | 30 min | 1.5–2 h |
Lidocaine 125 mg Phenylephrine 12.5 mg |
1 bottle = 2.5 ml of lidocaine 50 mg.ml−1 and phenylephrine 5 mg.ml−1 |
| Lidocaine 1–10% | 5 min | 30–60 min | 1.5–2 h | Total dose not > 9 mg.kg−1 LBW |
1 ml of 1% = 10 mg 1 spray of 10% = 10 mg |
|
| Cocaine 10% | 1–3 min | 30–60 min | 1 h | < 1.5 mg.kg−1 |
LD50 1.2 g, but significant toxic effects have been reported at doses as low as 20 mg in adults Particular care in older patients and/or those with cardiac disease |
|
| Sedatives | Propofol | 30 s | 5–10 min | 1.5–3 h | TCI (effect‐site) 0.5–1 μg.ml−1 |
Caution with doses in excess of 1.5 μg.ml−1: risk of over‐sedation and hypoventilation, particularly with concomitant opioid use Avoid bolus dosing |
| Midazolam | 3–5 min | 1–2 h | 1.5–3 h | Bolus 0.5–1 mg |
Titrate to effect Peak effect at 5–10 min so care with multiple doses |
|
| Dexmedetomidine | 1–2 min | 5–10 min | 2 h | Bolus 0.5–1 μg.kg−1 over 5 min followed by infusion (0.3–0.6 μg.kg−1.h−1) | Caution with bolus dosing as associated with hypertension and bradycardia | |
| Analgesia | Remifentanil | 1 min | 3–5 min | 1–20 min | TCI (effect‐site) 1–3 ng.ml−1 |
Caution with respiratory depression. Avoid bolus dosing. |
| Fentanyl | 2–5 min | 30–60 min | 6–10 min | Bolus 0.5–1 μg.kg−1, subsequent doses of 0.5 μg.kg−1 as required | ||
| Alfentanil | 2–3 min | 15 min | 90–120 min | Bolus 5 μg.kg−1, subsequent doses of 1–3 μg.kg−1 as required |
ATI, awake tracheal intubation; i.m., intramuscular; i.v., intravenous; TCI, target‐controlled infusion; LD50, median lethal dose; LBW, lean body weight.
Sedation
Awake tracheal intubation may be safely and effectively performed without sedation 33, 59, 60. However, its use during ATI can reduce patient anxiety and discomfort and increase procedural tolerance 137. Minimal sedation is defined as “a drug‐induced state during which the patient responds normally to verbal commands, whilst the airway, spontaneous ventilation and cardiovascular function are unaffected” 138. Sedative drugs can produce a number of effects which may be considered desirable (e.g. amnesia) or detrimental (e.g. over‐sedation). The risk of over‐sedation and its sequelae, including respiratory depression, airway loss, hypoxia, aspiration and cardiovascular instability, make the presence of an independent anaesthetist delivering, monitoring and titrating sedation desirable 1 (Grade D). In certain patient populations, the risk of over‐sedation is particularly hazardous, thus an independent practitioner delivering sedation is strongly recommended (Grade D; Table 4). If required, we recommend the cautious use of minimal sedation (Grade D).
Table 4.
Special circumstances that may affect standard performance of ATI with suggested management options
| Special circumstance | Considerations | Modification | Potential management options |
|---|---|---|---|
| Critically ill | Limited physiological reserve and greater adverse consequences associated with sedation | Sedation | Avoid or minimise sedation |
|
Higher risk of local anaesthetic systemic toxicity Increased secretions |
Topicalisation |
Cautious use of local anaesthetic Suction airway before instrumentation |
|
| Increased oxygen demand and reduced oxygen reserves | Oxygenation | Supplemental oxygen essential | |
| Unstable for transfer to operating theatre | Performance |
Do not transfer patient out of critical care settings Maintain same standards of equipment and monitoring Time‐critical performance of ATI Early consideration for high‐risk general anaesthesia |
|
| Obstetrics | Fetal sedation with benzodiazepines, long‐acting opioids or propofol | Sedation |
Sedation with dexmedetomidine or remifentanil Warn neonatologists |
| Higher risk of local anaesthetic systemic toxicity; concomitant use of local anaesthetics via epidural analgesia | Topicalisation | Cautious dosing of local anaesthetic; consider using pre‐pregnancy body weight for dosing | |
| Increased oxygen demand and reduced oxygen reserves | Oxygenation | Supplemental oxygen essential | |
|
Increased upper airway oedema and perfusion thus increasing risk of nasal haemorrhage FONA more difficult |
Performance |
Oral approach to ATI Identify and mark cricothyroid membrane early Airway ultrasound to identify cricothyroid membrane |
|
| Obesity | Critical adverse consequences of over‐sedation | Sedation | Avoid or minimise sedation |
| Risk of local anaesthetic overdose | Topicalisation | Local anaesthetic dosing on lean body weight | |
| Increased oxygen demand and reduced oxygen reserves | Oxygenation | Supplemental oxygen essential | |
| Diaphragmatic splinting and reduced functional residual capacity | Performance |
Sitting position or reverse Trendelenburg Operator facing patient |
|
| FONA more difficult |
Identify and mark cricothyroid membrane early Airway ultrasound to identify cricothyroid membrane |
||
| Trauma | Critical adverse consequences of over‐sedation | Sedation | Avoid or minimise sedation |
| Difficult administration due to airway soiling | Topicalisation | Clear soiled airway before topicalisation | |
| Increased oxygen demand and reduced oxygen reserves | Oxygenation | Supplemental oxygen essential | |
| Unstable for transfer to operating theatre | Performance |
Do not transfer patient out of critical care settings Maintain same standards of equipment and monitoring |
|
| Airway soiling from haemorrhage, secretions, vomitus and tissue oedema |
ATI: VL Tracheal intubation via SAD |
||
| Suspected base of skull or facial fracture |
Avoid HFNO Oral approach to ATI |
||
| Trismus | Critical adverse consequences of over‐sedation | Sedation | Avoid or minimise sedation |
| Limited pharyngeal access | Topicalisation |
Nebulised lidocaine Spray‐as‐you‐go Transtracheal lidocaine injection Insertion of mucosal atomiser and patient gargling |
|
| Potentially increased oxygen demand | Oxygenation | Supplemental oxygen essential | |
| Limited mouth opening | Performance | Nasal approach to ATI: FB | |
| Stridor | Critical adverse consequences of over‐sedation | Sedation | Avoid or minimise sedation |
| Risk of laryngospasm | Topicalisation | Consider nebulised and/or lower concentrations of lidocaine | |
| Airway obstruction | Oxygenation | HFNO highly recommended | |
| Narrowed airway | Performance |
Recognise that airway narrowing may preclude oral or nasal tracheal intubation Prime for emergency FONA Use smaller tracheal tube Most experienced practitioner to perform May require combined technique |
ATI, awake tracheal intubation; VL, videolaryngoscopy; SAD, supraglottic airway device; HFNO, high‐flow nasal oxygen; FB, flexible bronchoscopy; FONA, front‐of‐neck airway.
Remifentanil and dexmedetomidine are associated with high levels of patient satisfaction and low risk of over‐sedation and airway obstruction when used for ATI 137. A single‐agent strategy is safest for the non‐expert, and if used, remifentanil or dexmedetomidine are appropriate (Grade A). As a sole sedative agent, propofol is associated with a greater risk of over‐sedation, coughing and airway obstruction than remifentanil 139, 140, 141 and is therefore not advisable in this setting (Grade A) 137. If co‐administration of sedative agents is to be performed, remifentanil and midazolam are both reversible and therefore appropriate, recognising the increased risk of over‐sedation (Grade D). Sedation should not be used as a substitute for inadequate airway topicalisation (Grade D) 129. A suggested sedation regimen is presented in Fig. 2.
Two‐point check of tracheal tube placement
Awake tracheal intubation can result in incorrect tracheal tube placement, including pharyngeal, oesophageal or bronchial intubation. Oesophageal intubation occurs in 2.3% of procedures with ATI:FB and 4.9% with ATI:VL 58. Capnography has 100% sensitivity and specificity in identifying correct tracheal tube positioning in patients who lungs are ventilated 142, 143. However, in a patient who is spontaneously breathing, a capnographic trace may also be seen with supraglottic or bronchial placement of the tracheal tube. A two‐point check is therefore required to confirm the position of the tracheal tube:
visualisation of the tracheal lumen with ATI:FB or the tracheal tube through the vocal cords with ATI:VL to confirm tracheal placement; and
capnography to exclude oesophageal intubation (Grade C)
Anaesthesia should be induced only when the two‐point check has confirmed correct tracheal tube placement (Grade D). Once the flexible bronchoscope is in the trachea, the carina should be identified before advancing the tracheal tube to minimise the risk of misplacement (Grade D). The distance from the tracheal tube tip to the carina should be confirmed as appropriate before removing the bronchoscope (Grade D). On removal of the flexible bronchoscope or videolaryngoscope, care must be taken to maintain the correct position of the tracheal tube. The tip of the bronchoscope should be in the neutral position and the tracheal tube held firmly in position (Grade D). The tracheal tube cuff can be gently inflated before, during or after induction of anaesthesia. The decision around timing of cuff inflation should be guided by the relative risks of aspiration, patient movement, coughing and tracheal tube displacement (Grade D). If there is suspicion of a cuff tear, gentle inflation of the cuff to check integrity before induction of anaesthesia is recommended (Grade D).
Difficult Airway Society ATI technique
An example of a practical approach to the sTOP ATI technique is shown in Fig. 2. This technique has been specifically considered for simplicity and generalisability. We recognise that there are a range of different techniques and regimens which also address the key sTOP components that will be equally effective.
Special circumstances
Specific patient pathophysiology may dictate modifications to the performance of ATI that must be considered and planned for. As with all other aspects of ATI, these modifications can be categorised based on sTOP. Examples of suggested changes to technique are presented in Table 4.
Managing complications
The reported overall complication rate in patients undergoing ATI either flexible bronchoscopic or videolaryngoscopic, is up to 18% 33, 34, 35, 58, 144, 145, 146. Complications during ATI occur due to inadequate sTOP. In the event of a complication, its aetiology should be determined and appropriately managed (Grade D; Fig. 3).
Figure 3.
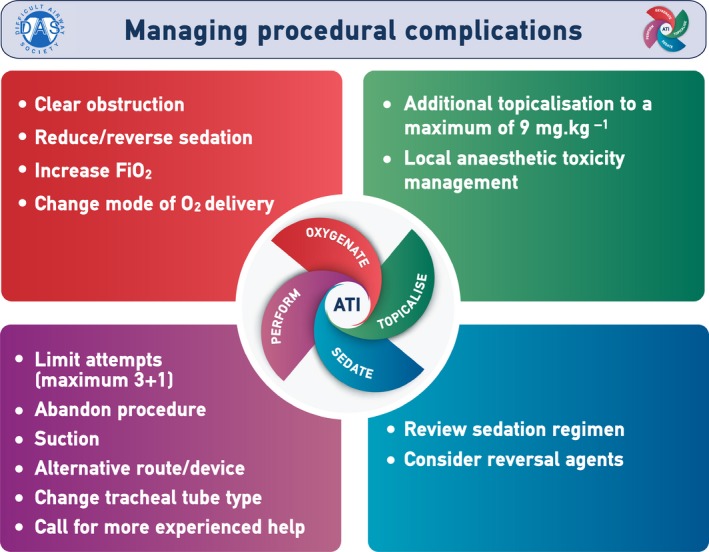
Managing procedural complications during awake tracheal intubation (ATI). This provides a framework for managing complications, but is not meant to be a comprehensive guide. This figure forms part of the Difficult Airway Society guidelines for ATI in adults and should be used in conjunction with the text. FIO2, fractional inspired concentration of oxygen; O2, oxygen ©Difficult Airway Society 2019.
We define an unsuccessful attempt at ATI as the unplanned removal of flexible bronchoscope, videolaryngoscope or tracheal tube from the airway. Patients in whom ATI is indicated are at greater risk of the adverse consequences of multiple attempts, such as airway trauma, airway obstruction, bleeding and unsuccessful ATI 1. It is therefore advisable to minimise the number of attempts at ATI (Grade D). Operators should consider if they require more experienced support before commencing ATI (Grade D). Operators should ensure sTOP is optimised before the first attempt (Grade D). If unsuccessful with the first attempt, operators should re‐assess, correct any inadequate sTOP components, and call for help before proceeding with a second attempt (Grade D). If unsuccessful with the second attempt, a third may be considered only if conditions can be further optimised (Grade D). A fourth and final attempt (3 + 1) should only be undertaken by a more experienced operator, which may include a surgeon (Grade D). Each attempt subsequent to the first should involve a change in the elements of performance to improve the likelihood of success (Grade D). The use of an alternative device (e.g. FB to VL or vice versa) should be counted in the total number of attempts. Each failed attempt may adversely affect patient and operator confidence. Seeking expert help at the earliest opportunity is recommended (Grade D). If unsuccessful after 3 + 1 attempts, the unsuccessful ATI algorithm should be followed (Grade D; Fig. 4).
Figure 4.
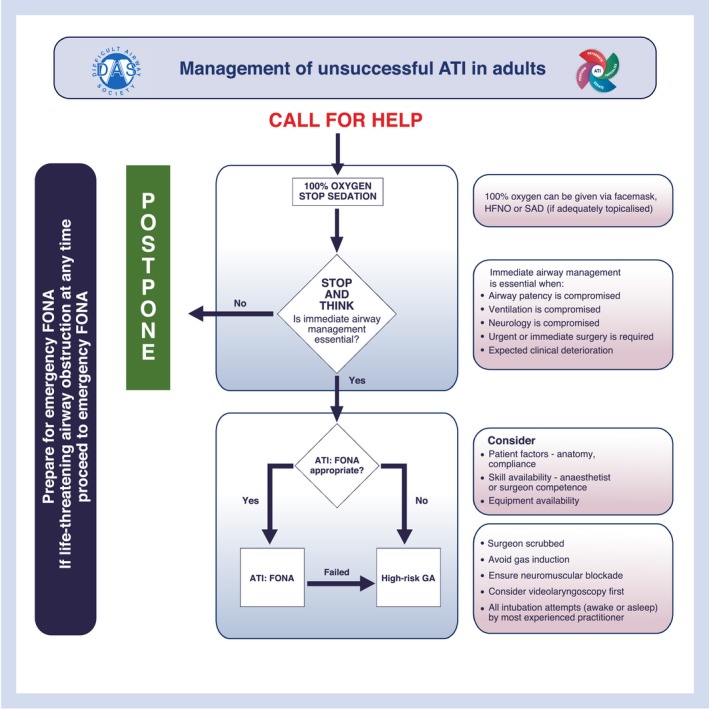
The Difficult Airway Society management of unsuccessful awake tracheal intubation (ATI) in adults. This algorithm forms part of the Difficult Airway Society guidelines for ATI in adults and should be used in conjunction with the text. HFNO, high‐flow nasal oxygen; SAD, supraglottic airway device; FONA, front‐of‐neck airway; GA, general anaesthesia. ©Difficult Airway Society 2019.
Management of unsuccessful ATI
The unsuccessful ATI algorithm is a guide for the rare occasions where successful tracheal intubation has not been achieved in 3 + 1 attempts. Immediate actions should include a call for help, ensuring 100% oxygen is applied and stopping (if necessary, reversing) any sedative drugs (Grade D). Operators should ‘stop and think’ to determine subsequent airway management, while also ‘priming’ for emergency FONA 2, 52 (Grade D). The default action in the event of unsuccessful ATI should be to postpone the procedure (Grade D). Operators should only proceed with immediate airway management if essential (e.g. if airway patency, ventilation or neurology is compromised; urgent or immediate surgery is required; or clinical deterioration is expected) (Grade D).
If airway management is deemed essential, the preferred option for securing the airway after unsuccessful ATI:VL or ATI:FB should be ATI using FONA (ATI:FONA), which includes cricothyroidotomy or tracheostomy (Grade D). The most appropriately skilled clinician available should perform this (Grade C). The considerations for the appropriateness of ATI:FONA include: patient factors; skill; and equipment availability. If inappropriate or unsuccessful, a high‐risk general anaesthetic is the only remaining option. In this scenario, the operator should formulate an achievable A to D airway management strategy informed by the unsuccessful attempts at ATI and based on the 2015 DAS guidelines 2, recognising that they are primarily for the unanticipated difficult tracheal intubation (Grade D). This strategy should include an i.v. induction of anaesthesia with full neuromuscular blockade 2, 52 (Grade D). Videolaryngoscopes may improve tracheal intubation success rates in cases of difficult tracheal intubation 147; therefore, the first attempt at tracheal intubation in this scenario should be with a videolaryngoscope (Grade A). All attempts with any device should be performed by the most appropriately skilled clinician present (Grade C).
Post‐tracheal intubation management
Patients who have had ATI due to predicted difficult airway management are at high risk of complications at tracheal extubation 1, 148, and require an appropriate tracheal extubation strategy. Planning, preparation, performing and post‐tracheal extubation care should follow DAS guidelines 148 (Grade D).
Before tracheal extubation, laryngoscopy, either with a direct laryngoscope or videolaryngoscope, may provide useful information for risk stratification of tracheal extubation and any subsequent airway management. The view at laryngoscopy may be altered by the presence of a tracheal tube 148. Therefore, verification of laryngoscopy grade may rule out, but not rule in, easy subsequent asleep tracheal intubation.
Topical lidocaine has a dose‐dependent duration of analgesic action of up to 40 min although this may vary with concentration and method of administration 149, 150. However, the time to return of laryngeal reflexes can be longer 151. Given that the terminal elimination half‐life of lidocaine is up to 2 h, patients should remain nil by mouth for at least 2 h following airway topicalisation for ATI (Grade D).
Documentation of ATI in clinical records is necessary to inform and guide future patient management 1, 152. This should include: documentation of oxygenation; topicalisation; sedation strategy; device and tracheal tube used; approach (e.g. right nasal, left nasal, oral); number of attempts; and any complications or notes (Grade D; Supporting Information, Appendix S3).
Consent
Clinicians should adhere to the Association of Anaesthetists’ guidelines on consent for anaesthesia 153 (Grade D). Informed consent must be taken, with patients being given information (ideally including a patient information leaflet 154) in a timely manner (Grade D). The risks of ATI and its alternative (induction of general anaesthesia before securing the airway) should be discussed (Table 5) (Grade D). Appropriate explanation is vital and a good rapport can increase the confidence and co‐operation of the patient in the procedure and is strongly encouraged (Grade D). The consent process should be documented (Grade D).
Table 5.
Incidence of complications when asleep or awake tracheal intubation is performed. The rates reported for asleep tracheal intubation include data for all patients, and patients who are predicted to have difficult airway management. The rates reported for awake tracheal intubation are only for patients who are predicted to be at risk of difficult airway management
| Asleep tracheal intubation | Awake tracheal intubation | ||
|---|---|---|---|
| All patients | Predicted difficult tracheal intubation | ||
| Difficult facemask ventilation | 2.2–2.5% | 18.6–22% | Not applicable |
| Impossible facemask ventilation | 0.15% | Not currently available | Not applicable |
| Difficult tracheal intubation | 1.9–10% | 25% | Not applicable |
| Failed tracheal intubation | 0.15% | 0.36%a | 1–2% |
| CICO | 0.04% | 0.75%a | 0–0.06% |
| Front‐of‐neck airway | 0.002–0.07% | 0.12%a | 0–0.38% |
| Death | 0.0006–0.04% | Not currently available | Not currently available |
CICO, cannot intubate, cannot oxygenate.
Unpublished data from the Danish Anaesthesia Database.
Training
Successful performance of ATI has been shown to be independent of seniority, but related to experience 33. There are many strategies used for training in the technical aspects of ATI, including the use of manikins, simulators, cadavers and patients 59, 155, 156, 157, 158, 159, 160, 161. All anaesthetists should seek every opportunity to attain and maintain skills in ATI and all departments should support this 1 (Grade C). Awake tracheal intubation is a skill in the compulsory higher training curriculum of the Royal College of Anaesthetists 162, but opportunities for training are known to be limited 163, 164, 165, 166, 167. These guidelines provide a common stem for sedation, topicalisation, oxygenation and performance to encourage training in ATI. Experience using a range of tools should be sought, complementing active clinical practice to develop non‐technical aspects of ATI (Grade B). We recognise this may be difficult to achieve 163, 164, 165, 166, 167, but local hospital airway leads are ideally placed to facilitate training and provision of ATI skills and equipment. Team training in ATI should include anaesthetic assistants, operating department practitioners and theatre staff. Awake tracheal intubation may be performed solely for the purposes of training provided appropriate consent is taken (Grade D).
Future directions
These guidelines highlight the paucity of high‐quality evidence in ATI, as demonstrated by the need for expert opinion for the majority of recommendations. This presents an opportunity for further research to be undertaken to improve both clinical and patient‐centred outcomes 168. In particular, the ideal topicalisation and sedation strategies are yet to be elucidated, with a limited evidence base for individual drugs, administration methods (e.g. infusion vs. bolus, combinations of sedatives, mucosal atomiser vs. nebulisation) and their related outcomes 129. There remains uncertainty regarding many aspects of procedural performance such as ideal patient and operator positioning, the role of checklists and cognitive aids and immediate management of complications. Moreover, training in ATI has thus far focussed on technical aspects, primarily with FB, but training with alternative devices and non‐technical skills have had little attention in the published literature and warrant further investigation. Novel technology for ATI must also be developed, such as improved capnography and monitoring, safer sedation delivery devices and better image visualisation and guidance technology. Finally, the impact these guidelines have on clinical practice should be examined to allow further iterations to be improved upon. This will require updates of these guidelines using similar methodology when a more robust evidence base becomes available.
Discussion
The primary aims of these guidelines are to provide practitioners with a comprehensive document on ATI. These guidelines should support clinical practice and lower the performance threshold thereby increasing the use of ATI when indicated. The quality of evidence supporting many recommendations is limited, with interventions and outcomes being highly heterogeneous. This is likely influenced by the fact that ATI can be successfully performed in a wide range of settings and patients with varying techniques 129. For example, the use of SADs as a conduit to ATI or optical stylets in awake patients have not been well‐described but warrant future investigation as their role becomes more defined. Similarly, the lack of previously published specific guidelines means that research in ATI is disparate and inconsistent 169. However, we have sought and appraised the available evidence in ATI, and in its absence we have incorporated the practical and theoretical experience of international experts. We have involved patients, DAS members and international experts in order to further understand current practice and the need for these guidelines. Formal resource implication analysis has not been conducted; however, the tools to practically perform ATI are available widely, and thus we expect the resource impact to be modest.
These guidelines prioritise patient safety and provide recommendations for best clinical practice. It is hoped that they will lead to a paradigm shift in clinical practice and improve the care of patients with predicted difficult airway management in the UK and beyond.
Supporting information
Appendix S1. Summary of the systematic review methodology.
Appendix S2. The Difficult Airway Society checklist for awake tracheal intubation (ATI) in adults. SAD, supraglottic airway device.
Appendix S3. The Difficult Airway Society documentation sticker for awake tracheal intubation (ATI) in adults. FB, flexible bronchoscopy; VL, videolaryngoscopy; L, left; R, right.
Acknowledgements
The systematic review was registered at PROSPERO (registration ID CRD42017072707). We thank Ms M. Hillier (librarian, UK) for her assistance with the literature search. We thank Mr A. Diver (anaesthetic assistant, UK), for his contribution to the development of the recommendations. We thank Mr A. Fry (consultant surgeon, UK), Mr R. Oakley (consultant surgeon, UK), Mrs I. Anastasescu (anaesthetic nurse, UK) and Mrs E. Jacovou (anaesthetic nurse, UK) for their independent review of the manuscript. For their contribution to the expert surveys and/or manuscript review, we thank Prof. M. Aziz (USA), Dr P. Baker (New Zealand), Dr E. Burdett (UK), Dr S. Charters (UK), Dr N. Chrimes (Australia), Dr S. Clarke (UK), Professor T. Cook (UK), Professor R. Cooper (Canada), Professor P. Diemunsch (France), Dr J. Doyle (UAE), Professor C. Frerk (UK), Professor K. Greenland (Australia), Professor R. Greif (Switzerland), Dr P. Groom (UK), Professor T. Heidegger (Austria), Dr A. Higgs (UK), Dr E. Hodgson (South Africa), Dr R. Hoffmeyer (South Africa), Dr J. Huitink (The Netherlands), Dr F. Kelly (UK), Dr M. Kristensen (Denmark), Professor J. A. Law (Canada), Dr B. McGuire (UK), Professor C. Mendonca (UK), Professor M. Mushambi (UK), Professor S. Myatra (India), Dr R. Coloma Navarro (Chile), Professor V. Nekhendzy (USA), Dr H. Osses (Chile), Professor J. Pandit (UK), Dr B. Patel (UK), Professor W. Rosenblatt (USA), Dr N. Shallik (Qatar), Professor A. Smith (UK), Dr M. Sorbello (Italy) and Dr N. Woodall (UK). We thank Drs A. Nørskov, C. Rosenstock and L. Lundstrøm for data provided from the Danish Anaesthesia Database in Table 5. Costs related to Guideline Group meetings and graphic design were met by DAS. IA has previously received honoraria for consulting for Ambu, honoraria and funding for travel and accommodation from Fisher & Paykel Healthcare, Ambu and Verathon Medical to give lectures at international meetings. KE is an Editor for Anaesthesia and this manuscript underwent external review. KE has previously received honoraria for consulting for Ambu. RB has received products for departmental and workshop use by Ambu, Armstrong, Cook Medical, Fisher & Paykel Healthcare, Karl Storz, PROACT and Teleflex. AM has received equipment for evaluation and teaching (including the running of workshops) from Ambu, Cook Medical, Fisher & Paykel Healthcare, Medtronic, Karl Storz, Teleflex, VBM/Freelance. AM has acted as an advisor to the Medicines and Healthcare products Regulatory Agency (MHRA) and participated on an advisory board and speaker panel for Medtronic. He has received travel expenses for teaching sessions from Fisher & Paykel Healthcare. IH has been given trial products for clinical use and evaluation from Ambu, Cook Medical, Storz, Verathon, Venner Medical and Fannin. IH has also received funding for travel and accommodation to give lectures from Covidien and has received equipment to conduct airway workshops from Storz, Ambu, Verathon and Fannin. AP has helped to develop a videolaryngoscope. He has received travel, consulting and research support from Fisher & Paykel Healthcare. FM has received funding for travel and accommodation from Fisher & Paykel Healthcare. There has been no involvement of any industry in any aspect of this project. No external funding or competing interest declared.
‘[Correction added on 20 February 2020, after first online publication: In the Acknowledgements section, Mr A. Diver's name was previously wrong and has been corrected in this version.]
This article is accompanied by an editorial by Aziz and Kristensen, Anaesthesia, 2020; 75:https://doi.org/10.1111/anae.14947.
Contributor Information
I. Ahmad, https://twitter.com/dr_imranahmad.
K. El‐Boghdadly, Email: elboghdadly@gmail.com, https://twitter.com/elboghdadly.
R. Bhagrath, https://twitter.com/ravibhagrath.
A. F. McNarry, https://twitter.com/altgm.
E. P. O'Sullivan, https://twitter.com/ProfEllenO.
D. Vaughan, https://twitter.com/oldandbaffled.
References
- 1. Cook TM, Woodall NM, Frerk CM. 4th National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. British Journal of Anaesthesia 2011; 106: 617–31. [DOI] [PubMed] [Google Scholar]
- 2. Frerk C, Mitchell VSS, McNarry AFF, et al. Difficult Airway Society 2015 guidelines for management of unanticipated difficult intubation in adults. British Journal of Anaesthesia 2015; 115: 827–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mushambi MC, Kinsella SM, Popat M, et al. Obstetric Anaesthetists’ Association and Difficult Airway Society guidelines for the management of difficult and failed tracheal intubation in obstetrics. Anaesthesia 2015; 70: 1286–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Petrini F, Accorsi A, Adrario E, et al. Recommendations for airway control and difficult airway management. Minerva Anestesiologica 2005; 71: 617–57. [PubMed] [Google Scholar]
- 5. Australian and New Zealand College of Anaesthetists . Guidelines for the management of evolving airway obstruction: transition to the can't intubate can't oxygenate airway emergency. Australian and New Zealand College of Anaesthetists (ANZCA) 2016.; 2016.
- 6. Law JA, Broemling N, Cooper RM, et al. The difficult airway with recommendations for management – Part 1 – Intubation encountered in an unconscious/induced patient. Canadian Journal of Anesthesia 2013; 60: 1089–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Apfelbaum JL, Hagberg CA, Caplan RA, et al. Practice guidelines for management of the difficult airway: an updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology 2013; 118: 251–70. [DOI] [PubMed] [Google Scholar]
- 8. Myatra SN, Shah A, Kundra P, et al. All India Difficult Airway Association 2016 guidelines for the management of unanticipated difficult tracheal intubation in adults. Indian Journal of Anaesthesia 2016; 60: 885–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ramkumar V, Dinesh E, Shetty SR, et al. All India difficult airway association 2016 guidelines for the management of unanticipated difficult tracheal intubation in obstetrics. Indian Journal of Anaesthesia 2016; 60: 899–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Langeron O, Bourgain J‐LL, Francon D, et al. Difficult intubation and extubation in adult anaesthesia. Anaesthesia Critical Care and Pain Medicine 2018; 37: 639–51. [DOI] [PubMed] [Google Scholar]
- 11. Rehn M, Hyldmo PK, Magnusson V, et al. Scandinavian SSAI clinical practice guideline on pre‐hospital airway management. Acta Anaesthesiologica Scandinavica 2016; 60: 852–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Piepho T, Cavus E, Noppens R, et al. S1‐Leitlinie Atemwegsmanagement: Leitlinie der Deutschen Gesellschaft für Anästhesiologie und Intensivmedizin (DGAI). Anaesthesist 2015; 64: 27–40.26727936 [Google Scholar]
- 13. Anon JSA. airway management guideline 2014: to improve the safety of induction of anesthesia. Journal of Anaesthesia 2014; 28: 482–93. [DOI] [PubMed] [Google Scholar]
- 14. Nørskov AK, Rosenstock CV, Wetterslev J, Astrup G, Afshari A, Lundstrøm LH. Diagnostic accuracy of anaesthesiologists’ prediction of difficult airway management in daily clinical practice: a cohort study of 188 064 patients registered in the Danish Anaesthesia Database. Anaesthesia 2015; 70: 272–81. [DOI] [PubMed] [Google Scholar]
- 15. Nørskov AK, Wetterslev J, Rosenstock CV, et al. Prediction of difficult mask ventilation using a systematic assessment of risk factors vs. existing practice – a cluster randomised clinical trial in 94,006 patients. Anaesthesia 2017; 72: 296–308. [DOI] [PubMed] [Google Scholar]
- 16. Kheterpal S, Healy D, Aziz MF, et al. Incidence, predictors, and outcome of difficult mask ventilation combined with difficult laryngoscopy: a report from the multicenter perioperative outcomes group. Anesthesiology 2013; 119: 1360–9. [DOI] [PubMed] [Google Scholar]
- 17. Kheterpal S, Martin L, Shanks AM, Tremper KK. Prediction and outcomes of impossible mask ventilation: a review of 50,000 anesthetics. Anesthesiology 2009; 110: 891–7. [DOI] [PubMed] [Google Scholar]
- 18. Verghese C, Brimacombe JR. Survey of laryngeal mask airway usage in 11,910 patients: safety and efficacy for conventional and non‐conventional usage. Anesthesia and Analgesia 1996; 82: 129–33. [DOI] [PubMed] [Google Scholar]
- 19. Saito T, Liu W, Chew STH, Ti LK. Incidence of and risk factors for difficult ventilation via a supraglottic airway device in a population of 14,480 patients from South‐East Asia. Anaesthesia 2015; 70: 1079–83. [DOI] [PubMed] [Google Scholar]
- 20. Francksen H, Renner J, Hanss R, Scholz J, Doerges V, Bein B. A comparison of the i‐gel with the LMA‐Unique in non‐paralysed anaesthetised adult patients. Anaesthesia 2009; 64: 1118–24. [DOI] [PubMed] [Google Scholar]
- 21. Rose DK, Cohen MM. The airway: problems and predictions in 18,500 patients. Canadian Journal of Anesthesia 1994; 41: 372–83. [DOI] [PubMed] [Google Scholar]
- 22. Cook TM, Trümpelmann P, Beringer R, Stedeford J. A randomised comparison of the Portex SoftsealTM laryngeal mask airway with the LMA‐UniqueTM during anaesthesia. Anaesthesia 2005; 60: 1218–25. [DOI] [PubMed] [Google Scholar]
- 23. Shiga T, Wajima Z, Inoue T, Sakamoto A. Predicting difficult intubation in apparently normal patients: a meta‐analysis of bedside screening test performance. Anesthesiology 2005; 103: 429–37. [DOI] [PubMed] [Google Scholar]
- 24. Lundstrøm LH, Møller AM, Rosenstock C, Astrup G, Gätke MR, Wetterslev J. A documented previous difficult tracheal intubation as a prognostic test for a subsequent difficult tracheal intubation in adults. Anaesthesia 2009; 64: 1081–8. [DOI] [PubMed] [Google Scholar]
- 25. Detsky ME, Jivraj N, Adhikari NK, et al. Will this patient be difficult to intubate? the rational clinical examination systematic review. Journal of the American Medical Association 2019; 321: 493–503. [DOI] [PubMed] [Google Scholar]
- 26. Thomsen JLD, Nørskov AK, Rosenstock CV. Supraglottic airway devices in difficult airway management: a retrospective cohort study of 658,104 general anaesthetics registered in the Danish Anaesthesia Database. Anaesthesia 2019; 74: 151–7. [DOI] [PubMed] [Google Scholar]
- 27. Rosenstock CV, Nørskov AK, Wetterslev J, Lundstrøm LH. Danish Anaesthesia Database. Emergency surgical airway management in Denmark: a cohort study of 452,461 patients registered in the Danish Anaesthesia Database. British Journal of Anaesthesia 2016; 117(Suppl): i75–82. [DOI] [PubMed] [Google Scholar]
- 28. Huitink JM, Lie PP, Heideman I, et al. A prospective, cohort evaluation of major and minor airway management complications during routine anaesthetic care at an academic medical centre. Anaesthesia 2017; 72: 42–8. [DOI] [PubMed] [Google Scholar]
- 29. Edelman DA, Perkins EJ, Brewster DJ. Difficult airway management algorithms: a directed review. Anaesthesia 2019; 74: 1175–85. [DOI] [PubMed] [Google Scholar]
- 30. Cook TM. Strategies for the prevention of airway complications – a narrative review. Anaesthesia 2018; 73: 93–111. [DOI] [PubMed] [Google Scholar]
- 31. Alhomary M, Ramadan E, Curran E, Walsh SR. Videolaryngoscopy vs. fibreoptic bronchoscopy for awake tracheal intubation: a systematic review and meta‐analysis. Anaesthesia 2018; 73: 1151–61. [DOI] [PubMed] [Google Scholar]
- 32. Ajay S, Singhania A, Akkara AG, Shah A, Adalja M. A study of flexible fiberoptic bronchoscopy aided tracheal intubation for patients undergoing elective surgery under general anesthesia. Indian Journal of Otolaryngology and Head and Neck Surgery 2013; 65: 116–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. El‐Boghdadly K, Onwochei DN, Cuddihy J, et al. A prospective cohort study of awake fibreoptic intubation practice at a tertiary centre. Anaesthesia 2017; 72: 694–703. [DOI] [PubMed] [Google Scholar]
- 34. Law JA, Morris IR, Brousseau PA, de la Ronde S, Milne AD. The incidence, success rate, and complications of awake tracheal intubation in 1,554 patients over 12 years: an historical cohort study. Canadian Journal of Anesthesia 2015; 62: 736–44. [DOI] [PubMed] [Google Scholar]
- 35. Joseph TT, Gal JS, DeMaria SJ, Lin H‐M, Levine AI, Hyman JB. A retrospective study of success, failure, and time needed to perform awake intubation. Anesthesiology 2016; 125: 105–14. [DOI] [PubMed] [Google Scholar]
- 36. Brouwers MC, Kerkvliet K, Spithoff K, Consortium ANS. The AGREE Reporting Checklist: a tool to improve reporting of clinical practice guidelines. British Medical Journal 2016; 352: i1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, John PA. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. British Medical Journal 2009; 339: b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Boulkedid R, Abdoul H, Loustau M, Sibony O, Alberti C. Using and reporting the Delphi method for selecting healthcare quality indicators: a systematic review. Wright JM, ed. PLoS ONE 2011; 6: e20476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Diamond IR, Grant RC, Feldman BM, et al. Defining consensus: a systematic review recommends methodologic criteria for reporting of Delphi studies. Journal of Clinical Epidemiology 2014; 67: 401–9. [DOI] [PubMed] [Google Scholar]
- 40. Pandit JJ, Popat MT, Cook TM, et al. The Difficult Airway Society “ADEPT” Guidance on selecting airway devices: the basis of a strategy for equipment evaluation. Anaesthesia 2011; 66: 726–37. [DOI] [PubMed] [Google Scholar]
- 41. Du Rand IA, Blaikley J, Booton R, et al. Summary of the British Thoracic Society guideline for diagnostic flexible bronchoscopy in adults. Thorax 2013; 68: 786–7. [DOI] [PubMed] [Google Scholar]
- 42. Roth D, Pace NL, Lee A, et al. Airway physical examination tests for detection of difficult airway management in apparently normal adult patients. Cochrane Database of Systematic Reviews 2018; 2018: CD008874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Benumof JL. Obstructive sleep apnea in the adult obese patient: implications for airway management. Anesthesiology Clinics of North America 2002; 20: 789–811. [DOI] [PubMed] [Google Scholar]
- 44. Petrini F, Di Giacinto I, Cataldo R, et al. Perioperative and periprocedural airway management and respiratory safety for the obese patient: 2016 SIAARTI Consensus. Minerva Anestesiologica 2016; 82: 1314–35. [PubMed] [Google Scholar]
- 45. Law JA, Broemling N, Cooper RM, et al. The difficult airway with recommendations for management – Part 2 – Difficult airway. Canadian Journal of Anesthesia 2013; 60: 1119–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Weinger MB, Vredenburgh AG, Schumann CM, et al. Quantitative description of the workload associated with airway management procedures. Journal of Clinical Anaesthesia 2000; 12: 273–82. [DOI] [PubMed] [Google Scholar]
- 47. Reason J. Human error: models and management. British Medical Journal 2000; 320: 768–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rall M, Dieckmann P. Safety culture and crisis resource management in airway management: general principles to enhance patient safety in critical airway situations. Best Practice and Research: Clinical Anesthesiology 2005; 19: 539–57. [DOI] [PubMed] [Google Scholar]
- 49. Smith AF, Mishra K. Interaction between anaesthetists, their patients, and the anaesthesia team. British Journal of Anaesthesia 2010; 105: 60–8. [DOI] [PubMed] [Google Scholar]
- 50. Flin R, Fioratou E, Frerk C, Trotter C, Cook TM. Human factors in the development of complications of airway management: preliminary evaluation of an interview tool. Anaesthesia 2013; 68: 817–25. [DOI] [PubMed] [Google Scholar]
- 51. Davis M, Hignett S, Hillier S, Hames N, Hodder S. Safer anaesthetic rooms: human factors/ergonomics analysis of work practices. Journal of Perioperative Practice 2016; 26: 274–80. [DOI] [PubMed] [Google Scholar]
- 52. Higgs A, McGrath BA, Goddard C, et al. Guidelines for the management of tracheal intubation in critically ill adults. British Journal of Anaesthesia 2018; 120: 323–52. [DOI] [PubMed] [Google Scholar]
- 53. Eichhorn JH, Cooper JB, Cullen DJ, Maier WR, Philip JH, Seeman RG. Standards for patient monitoring during anesthesia at Harvard Medical School. Journal of American Medical Association 1986; 256: 1017–20. [PubMed] [Google Scholar]
- 54. Webb RK, Currie M, Morgan CA, et al. The Australian incident monitoring study: an analysis of 2000 incident reports. Anaesthesia and Intensive Care 1993; 21: 520–8. [DOI] [PubMed] [Google Scholar]
- 55. Williamson JA, Webb RK, Sellen A, Runciman WB, Van der Walt JH. The Australian incident monitoring study. Human failure: an analysis of 2000 incident reports. Anaesthesia and Intensive Care 1993; 21: 678–83. [DOI] [PubMed] [Google Scholar]
- 56. Keenan RL, Boyan CP. Decreasing frequency of anesthetic cardiac arrests. Journal of Clinical Anaesthesia 1991; 3: 354–7. [DOI] [PubMed] [Google Scholar]
- 57. McKay WPS, Noble WH. Critical incidents detected by pulse oximetry during anaesthesia. Canadian Journal of Anesthesia 1988; 35: 265–9. [DOI] [PubMed] [Google Scholar]
- 58. Rosenstock CV, Thogersen B, Afshari A, Christensen AL, Eriksen C, Gatke MR. Awake fiberoptic or awake video laryngoscopic tracheal intubation in patients with anticipated difficult airway management: a randomized clinical trial. Anesthesiology 2012; 116: 1210–16. [DOI] [PubMed] [Google Scholar]
- 59. Woodall NM, Harwood RJ, Barker GL, et al. Complications of awake fibreoptic intubation without sedation in 200 healthy anaesthetists attending a training course. British Journal of Anaesthesia 2008; 100: 850–5. [DOI] [PubMed] [Google Scholar]
- 60. Patil V, Barker GL, Harwood RJ, Woodall NM. Training course in local anaesthesia of the airway and fibreoptic intubation using course delegates as subjects. British Journal of Anaesthesia 2002; 89: 586–93. [DOI] [PubMed] [Google Scholar]
- 61. Sun Y, Liu J‐X, Jiang H, Zhu Y‐S, Xu H, Huang Y. Cardiovascular responses and airway complications following awake nasal intubation with blind intubation device and fibreoptic bronchoscope: a randomized controlled study. European Journal of Anesthesiology 2010; 27: 461–7. [DOI] [PubMed] [Google Scholar]
- 62. Checketts MR, Alladi R, Ferguson K, et al. Recommendations for standards of monitoring during anaesthesia and recovery 2015: association of Anaesthetists. Anaesthesia 2016; 71: 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Carayon P, Xie A, Kianfar S. Human factors and ergonomics as a patient safety practice. British Medical Journal Quality and Safety 2014; 23: 196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ovassapian A, Tuncbilek M, Weitzel EK, Joshi CW. Airway management in adult patients with deep neck infections: a case series and review of the literature. Anesthesia and Analgesia 2005; 100: 585–9. [DOI] [PubMed] [Google Scholar]
- 65. Meghjee SPL, Marshall M, Redfern EJ, McGivern DV. Influence of patient posture on oxygen saturation during fibre‐optic bronchoscopy. Respiratory Medicine 2001; 95: 5–8. [DOI] [PubMed] [Google Scholar]
- 66. van Zwam JP, Kapteijns EFG, Lahey S, Smit HJM. Flexible bronchoscopy in supine or sitting position. Journal of Bronchology and Interventional Pulmonology 2010; 17: 29–32. [DOI] [PubMed] [Google Scholar]
- 67. Ling IT, Piccolo F, Mulrennan SA, Phillips MJ. Posture influences patient cough rate, sedative requirement and comfort during bronchoscopy: an observational cohort study. Cough 2011; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Altermatt FR, Muñoz HR, Delfino AE, Cortínez LI. Pre‐oxygenation in the obese patient: effects of position on tolerance to apnoea. British Journal of Anaesthesia 2005; 95: 706–9. [DOI] [PubMed] [Google Scholar]
- 69. Isono S, Ishikawa T, Nishino T, Tanaka A, Tagaito Y. Sitting posture decreases collapsibility of the passive pharynx in anesthetized paralyzed patients with obstructive sleep apnea. Anesthesiology 2010; 113: 812–18. [DOI] [PubMed] [Google Scholar]
- 70. Ikeda H, Ayuse T, Oi K. The effects of head and body positioning on upper airway collapsibility in normal subjects who received midazolam sedation. Journal of Clinical Anesthesia 2006; 18: 185–93. [DOI] [PubMed] [Google Scholar]
- 71. Heidegger T, Gerig HJ, Ulrich B, Schnider TW. Structure and process quality illustrated by fibreoptic intubation: analysis of 1612 cases. Anaesthesia 2003; 58: 734–9. [DOI] [PubMed] [Google Scholar]
- 72. Gómez‐Ríos MÁ, Nieto SL. Combined use of an Airtraq® optical laryngoscope, Airtraq video camera, Airtraq wireless monitor, and a fibreoptic bronchoscope after failed tracheal intubation. Canadian Journal of Anesthesia 2011; 58: 411–12. [DOI] [PubMed] [Google Scholar]
- 73. Chung MY, Park B, Seo J, Kim CJ. Successful airway management with combined use of McGrath MAC video laryngoscope and fiberoptic bronchoscope in a severe obese patient with huge goiter ‐a case report. Korean Journal of Anesthesiology 2018; 71: 232–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Liew GHC, Wong TGL, Lu A, Kothandan H. Combined use of the glidescope and flexible fibrescope as a rescue technique in a difficult airway. Proceedings of Singapore Healthcare 2015; 24: 117–20. [Google Scholar]
- 75. Shiraishi T. Awake insertion of the air‐QTM intubating laryngeal airway device that facilitates safer tracheal intubation in morbidly obese patients. British Journal of Anaesthesia 2013; 111: 1024–5. [DOI] [PubMed] [Google Scholar]
- 76. Lim WY, Wong P. Awake supraglottic airway guided flexible bronchoscopic intubation in patients with anticipated difficult airways: a case series and narrative review. Korean Journal of Anesthesiology 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kristensen MS, Fredensborg BB. The disposable Ambu aScope vs. a conventional flexible videoscope for awake intubation – a randomised study. Acta Anaesthesiologica Scandinavica 2013; 57: 888–95. [DOI] [PubMed] [Google Scholar]
- 78. Brull SJ, Wiklund R, Ferris C, Connelly NR, Ehrenwerth J, Silverman DG. Facilitation of fiberoptic orotracheal intubation with a flexible tracheal tube. Anesthesia and Analgesia 1994; 78: 746–8. [DOI] [PubMed] [Google Scholar]
- 79. Hakala P, Randell T, Valli H. Comparison between tracheal tubes for orotracheal fibreoptic intubation. British Journal of Anaesthesia 1999; 82: 135–6. [DOI] [PubMed] [Google Scholar]
- 80. Jafari A, Gharaei B, Reza Kamranmanesh M, et al. Wire reinforced endotracheal tube compared with Parker Flex‐Tip tube for oral fiberoptic intubation: a randomized clinical trial. Minerva Anestesiologica 2014; 80: 324–9. [PubMed] [Google Scholar]
- 81. Lucas DN, Yentis SM. A comparison of the intubating laryngeal mask tracheal tube with a standard tracheal tube for fibreoptic intubation. Anaesthesia 2000; 55: 358–61. [DOI] [PubMed] [Google Scholar]
- 82. Sharma D, Bithal PK, Rath GP, Pandia MP. Effect of orientation of a standard polyvinyl chloride tracheal tube on success rates during awake flexible fibreoptic intubation. Anaesthesia 2006; 61: 845–8. [DOI] [PubMed] [Google Scholar]
- 83. Kristensen MS. The Parker Flex‐Tip tube versus a standard tube for fiberoptic orotracheal intubation: a randomized double‐blind study. Anesthesiology 2003; 98: 354–8. [DOI] [PubMed] [Google Scholar]
- 84. Rai MR, Scott SH, Marfin AG, Popat MT, Pandit JJ. A comparison of a flexometallic tracheal tube with the intubating laryngeal mask tracheal tube for nasotracheal fibreoptic intubation using the two‐scope technique. Anaesthesia 2009; 64: 1303–6. [DOI] [PubMed] [Google Scholar]
- 85. Barker KF, Bolton P, Cole S, Coe PA. Ease of laryngeal passage during fibreoptic intubation: a comparison of three endotracheal tubes. Acta Anaesthesiologica Scandinavica 2001; 45: 624–6. [DOI] [PubMed] [Google Scholar]
- 86. Lomax SL, Johnston KD, Marfin AG, Yentis SM, Kathawaroo S, Popat MT. Nasotracheal fibreoptic intubation: a randomised controlled trial comparing the GlideRite® (Parker‐Flex® Tip) nasal tracheal tube with a standard pre‐rotated nasal RAETM tracheal tube. Anaesthesia 2011; 66: 180–4. [DOI] [PubMed] [Google Scholar]
- 87. Koga K, Asai T, Latto IP, Vaughan RS. Effect of the size of a tracheal tube and the efficacy of the use of the laryngeal mask for fibrescope‐aided tracheal intubation. Anaesthesia 1997; 52: 512. [DOI] [PubMed] [Google Scholar]
- 88. Arriaga AF, Bader AM, Wong JM, et al. Simulation‐based trial of surgical‐crisis checklists. New England Journal of Medicine 2013; 368: 246–53. [DOI] [PubMed] [Google Scholar]
- 89. Ziewacz JE, Arriaga AF, Bader AM, et al. Crisis checklists for the operating room: development and pilot testing. Journal of the American College of Surgeons 2011; 213: 212.e10–217.e10. [DOI] [PubMed] [Google Scholar]
- 90. Russ S, Rout S, Sevdalis N, Moorthy K, Darzi A, Vincent C. Do safety checklists improve teamwork and communication in the operating room? A systematic review Annals of Surgery 2013; 258: 856–71. [DOI] [PubMed] [Google Scholar]
- 91. Haynes AB, Weiser TG, Berry WR, et al. A surgical safety checklist to reduce morbidity and mortality in a global population. New England Journal of Medicine 2009; 360: 491–9. [DOI] [PubMed] [Google Scholar]
- 92. Harvey R, Foulds L, Housden T, et al. The impact of didactic read‐aloud action cards on the performance of cannula cricothyroidotomy in a simulated ‘can't intubate can't oxygenate’ scenario. Anaesthesia 2017; 72: 343–9. [DOI] [PubMed] [Google Scholar]
- 93. Marshall SD, Mehra R. The effects of a displayed cognitive aid on non‐technical skills in a simulated “can't intubate, can't oxygenate” crisis. Anaesthesia 2014; 69: 669–77. [DOI] [PubMed] [Google Scholar]
- 94. Sidhu VS, Whitehead EM, Ainsworth QP, Smith M, Calder I. A technique of awake fibreoptic intubation. Experience in patients with cervical spine disease. Anaesthesia 1993; 48: 910–13. [DOI] [PubMed] [Google Scholar]
- 95. Fuchs G, Schwarz G, Baumgartner A, Kaltenböck F, Voit‐Augustin H, Planinz W. Fiberoptic intubation in 327 neurosurgical patients with lesions of the cervical spine. Journal of Neurosurgical Anesthesiology 1999; 11: 11–16. [DOI] [PubMed] [Google Scholar]
- 96. Badiger S, John M, Fearnley RA, Ahmad I, Asai T. Optimizing oxygenation and intubation conditions during awake fibre‐optic intubation using a high‐flow nasal oxygen‐delivery system. British Journal of Anaesthesia 2015; 115: 629–32. [DOI] [PubMed] [Google Scholar]
- 97. Rozario L, Sloper D, Sheridan MJ. Supplemental oxygen during moderate sedation and the occurrence of clinically significant desaturation during endoscopic procedures. Gastroenterology Nursing 2008; 31: 281–5. [DOI] [PubMed] [Google Scholar]
- 98. Milman N, Faurschou P, Grode G, Jørgensen A. Pulse oximetry during fibreoptic bronchoscopy in local anaesthesia: frequency of hypoxaemia and effect of oxygen supplementation. Respiration 1994; 61: 342–7. [DOI] [PubMed] [Google Scholar]
- 99. Reed MWR, O'leary DP, Duncan JL, Majeed AW, Wright B, Reilly CS. Effects of sedation and supplemental oxygen during upper alimentary tract endoscopy. Scandinavian Journal of Gastroenterology 1993; 28: 319–22. [DOI] [PubMed] [Google Scholar]
- 100. Reshef R, Shiller M, Kinberg R, et al. A prospective study evaluating the usefulness of continuous supplemental oxygen in various endoscopic procedures. Israel Journal of Medical Sciences 1996; 32: 736–40. [PubMed] [Google Scholar]
- 101. Deitch K, Chudnofsky CR, Dominici P, Latta D, Salamanca Y. The utility of high‐flow oxygen during emergency department procedural sedation and analgesia with propofol: a randomized, controlled trial. Annals of Emergency Medicine 2011; 58: 360–4. [DOI] [PubMed] [Google Scholar]
- 102. Deitch K, Chudnofsky CR, Dominici P. The utility of supplemental oxygen during emergency department procedural sedation and analgesia with midazolam and fentanyl: a randomized. Controlled Trial. Annals of Emergency Medicine 2007; 49: 1–8. [DOI] [PubMed] [Google Scholar]
- 103. Hinkelbein J, Lamperti M, Akeson J, et al. European Society of Anaesthesiology and European Board of Anaesthesiology guidelines for procedural sedation and analgesia in adults. European Journal of Anesthesiology 2018; 35: 6–24. [DOI] [PubMed] [Google Scholar]
- 104. Surgeons M. Practice guidelines for moderate procedural sedation and analgesia 2018: a Report by the American Society of Anesthesiologists Task Force on Moderate Procedural Sedation and Analgesia, the American Association of Oral and Maxillofacial Surgeons, American. Anesthesiology 2018; 128: 437–79. [DOI] [PubMed] [Google Scholar]
- 105. Newton M, Blightman K. Guidance on the provision of sedation services 2016 In: Royal College of Anaesthetists. Guidelines for the Provision of Anaesthesia Services. London: Royal College of Anaesthetists, 2016: Chapter 19. [Google Scholar]
- 106. Ho AMH, Chung DC, To EWH, Karmakar MK. Total airway obstruction during local anesthesia in a non‐sedated patient with a compromised airway. Canadian Journal of Anesthesia 2004; 51: 838–41. [DOI] [PubMed] [Google Scholar]
- 107. Song J. A comparison of the effects of epinephrine and xylometazoline in decreasing nasal bleeding during nasotracheal intubation. Journal of Dental Anesthesia and Pain Medicine 2017; 17: 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. O'Hanlon J, Harper KW. Epistaxis and nasotracheal intubation–prevention with vasoconstrictor spray. Irish Journal of Medical Science 1994; 163: 58–60. [DOI] [PubMed] [Google Scholar]
- 109. El‐Boghdadly K, Pawa A, Chin KJ. Local anesthetic systemic toxicity: current perspectives. Local and Regional Anesthesia 2018; 11: 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Milman N, Laub M, Munch EP, Angelo HR. Serum concentrations of lignocaine and its metabolite monoethylglycinexylidide during fibre‐optic bronchoscopy in local anaesthesia. Respiratory Medicine 1998; 92: 40–3. [DOI] [PubMed] [Google Scholar]
- 111. Hasmoni MH, Rani MFA, Harun R, Manap RA, Tajudin NAA, Anshar FM. Randomized‐controlled trial to study the equivalence of 1% versus 2% lignocaine in cough suppression and satisfaction during bronchoscopy. Journal of Bronchology 2008; 15: 78–82. [Google Scholar]
- 112. Mainland PA, Kong AS, Chung DC, Chan CH, Lai CK. Absorption of lidocaine during aspiration anesthesia of the airway. Journal of Clinical Anaesthesia 2001; 13: 440–6. [DOI] [PubMed] [Google Scholar]
- 113. Efthimiou J, Higenbottam T, Holt D, Cochrane GM. Plasma concentrations of lignocaine during fibreoptic bronchoscopy. Thorax 1982; 37: 68–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Williams KA, Barker GL, Harwood RJ, Woodall NM. Combined nebulization and spray‐as‐you‐go topical local anaesthesia of the airway. British Journal of Anaesthesia 2005; 95: 549–53. [DOI] [PubMed] [Google Scholar]
- 115. Ingrande J, Lemmens HJM. Dose adjustment of anaesthetics in the morbidly obese. British Journal of Anaesthesia 2010; 105: i16–23. [DOI] [PubMed] [Google Scholar]
- 116. Woodruff C, Wieczorek PM, Schricker T, Vinet B, Backman SB. Atomised lidocaine for airway topical anaesthesia in the morbidly obese: 1% compared with 2%. Anaesthesia 2010; 65: 12–17. [DOI] [PubMed] [Google Scholar]
- 117. Xue FS, Liu HP, He N, et al. Spray‐as‐you‐go airway topical anesthesia in patients with a difficult airway: a randomized, double‐blind comparison of 2% and 4% lidocaine. Anesthesia and Analgesia 2009; 108: 536–43. [DOI] [PubMed] [Google Scholar]
- 118. Wieczorek PM, Schricker T, Vinet B, et al. Airway topicalisation in morbidly obese patients using atomised lidocaine: 2% compared with 4%. Anaesthesia 2007; 62: 984–8. [DOI] [PubMed] [Google Scholar]
- 119. Haldar R, Gyanesh P, Samanta S. Lignocaine toxicity from topical anaesthesia of airway during awake fibreoptic intubation. Journal of Neuro Anesthesiology and Critical Care 2014; 1: 146–7. [Google Scholar]
- 120. Giordano D, Panini A, Pernice C, Raso MG, Barbieri V. Neurologic toxicity of lidocaine during awake intubation in a patient with tongue base abscess. Case report. American Journal of Otolaryngology – Head and Neck Medicine and Surgery 2014; 35: 62–5. [DOI] [PubMed] [Google Scholar]
- 121. El‐Boghdadly K, Chin KJ. Local anesthetic systemic toxicity: continuing professional development. Canadian Journal of Anesthesia 2016; 63: 330–49. [DOI] [PubMed] [Google Scholar]
- 122. Association of Anaesthetists . Safety Guideline: management of severe local anaesthetic toxicity, 2010. https://anaesthetists.org/Home/Resources-publications/Guidelines/Management-of-severe-local-anaesthetic-toxicity (accessed 17/10/2019).
- 123. Osula S, Stockton P, Abdelaziz MM, Walshaw MJ. Intratracheal cocaine induced myocardial infarction: an unusual complication of fibreoptic bronchoscopy. Thorax 2003; 58: 733–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Chiu YC, Brecht K, Dasgupta DS, Mhoon E. Myocardial infarction with topical cocaine anesthesia for nasal surgery. archives of otolaryngology‐head and neck. Surgery 1986. [DOI] [PubMed] [Google Scholar]
- 125. Higgins TS, Hwang PH, Kingdom TT, Orlandi RR, Stammberger H, Han JK. Systematic review of topical vasoconstrictors in endoscopic sinus surgery. Laryngoscope 2011; 121: 422–32. [DOI] [PubMed] [Google Scholar]
- 126. Lenders GD, Jorens PG, De Meyer T, Vandendriessche T, Verbrugghe W, Vrints CJ. Coronary spasm after the topical use of cocaine in nasal surgery. American Journal of Case Reports 2013; 14: 76–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Cara DM, Norris AM, Neale LJ. Pain during awake nasal intubation after topical cocaine or phenylephrine/lidocaine spray. Anaesthesia 2003; 58: 777–80. [DOI] [PubMed] [Google Scholar]
- 128. Takaenoki Y, Masui K, Oda Y, Kazama T. The pharmacokinetics of atomized lidocaine administered via the trachea: a randomized trial. Anesthesia and Analgesia 2016; 123: 74–81. [DOI] [PubMed] [Google Scholar]
- 129. Cabrini L, Baiardo Redaelli M, Ball L, et al. Awake fiberoptic intubation protocols in the operating room for anticipated difficult airway: a systematic review and meta‐analysis of randomized controlled trials. Anesthesia and Analgesia 2019; 128: 971–80. [DOI] [PubMed] [Google Scholar]
- 130. Reasoner DK, Warner DS, Todd MM, Hunt SW, Kirchner J. A comparison of anesthetic techniques for awake intubation in neurosurgical patients. Journal of Neurosurgical Anesthesiology 1995; 7: 94–9. [DOI] [PubMed] [Google Scholar]
- 131. Wiles JR, Kelly J, Mostafa SM. Hypotension and bradycardia following superior laryngeal nerve block. British Journal of Anaesthesia 1989; 63: 125–7. [DOI] [PubMed] [Google Scholar]
- 132. Sitzman BT, Rich GF, Rockwell JJ, Leisure GS, Durieux ME, DiFazio CA. Local anesthetic administration for awake direct laryngoscopy. Are glossopharyngeal nerve blocks superior? Anesthesiology 1997; 86: 34–40. [DOI] [PubMed] [Google Scholar]
- 133. O'Callaghan C, Barry PW. The science of nebulised drug delivery. Thorax 1997; 52: S31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Kundra P, Kutralam S, Ravishankar M, Scand AA, Care C, Education PM. Local anaesthesia for awake fibreoptic nasotracheal intubation. Acta Anaesthesiologica Scandinavica 2000; 44: 511–16. [DOI] [PubMed] [Google Scholar]
- 135. Ali‐Melkkila T, Kaila T, Kanto J. Glycopyrrolate; pharmacokinetics and some pharmacodynamics findings. Acta Anaesthesiologica Scandinavica 1989; 33: 513–17. [DOI] [PubMed] [Google Scholar]
- 136. Brookman CA, Teh HP, Morrison LM. Anticholinergics improve fibreoptic intubating conditions during general anaesthesia. Canadian Journal of Anaesthesia 1997; 44: 165–7. [DOI] [PubMed] [Google Scholar]
- 137. Johnston KD, Rai MR. Conscious sedation for awake fibreoptic intubation: a review of the literature. Canadian Journal of Anesthesia 2013; 60: 584–99. [DOI] [PubMed] [Google Scholar]
- 138. Academy of Medical Royal Colleges . Safe sedation practice for healthcare procedures, 2013. https://www.rcoa.ac.uk/system/files/PUB-SafeSedPrac2013.pdf (accessed 17/10/2019).
- 139. Rai MR, Parry TM, Dombrovskis A, Warner OJ. Remifentanil target‐controlled infusion vs propofol target‐controlled infusion for conscious sedation for awake fibreoptic intubation: a double‐blinded randomized controlled trial. British Journal of Anaesthesia 2008; 100: 125–30. [DOI] [PubMed] [Google Scholar]
- 140. Lallo A, Billard V, Bourgain JL. A comparison of propofol and remifentanil target‐controlled infusions to facilitate fiberoptic nasotracheal intubation. Anesthesia and Analgesia 2009; 108: 852–7. [DOI] [PubMed] [Google Scholar]
- 141. Zhang X, He W, Wu X, Zhou X, Huang W, Feng X. TCI remifentanil vs. TCI propofol for awake fiber‐optic intubation with limited topical anesthesia. International Journal of Clinical Pharmacology and Therapeutics 2012; 50: 10–16. [DOI] [PubMed] [Google Scholar]
- 142. Silvestri S, Ladde JG, Brown JF, et al. Endotracheal tube placement confirmation: 100% sensitivity and specificity with sustained four‐phase capnographic waveforms in a cadaveric experimental model. Resuscitation 2017; 115: 192–8. [DOI] [PubMed] [Google Scholar]
- 143. Grmec Š. Comparison of three different methods to confirm tracheal tube placement in emergency intubation. Intensive Care Medicine 2002; 28: 701–4. [DOI] [PubMed] [Google Scholar]
- 144. Merry AF, Mitchell SJ. Complications of anaesthesia. Anaesthesia 2018; 73: 7–11. [DOI] [PubMed] [Google Scholar]
- 145. Wahba SS, Tammam TF, Saeed AM. Comparative study of awake endotracheal intubation with Glidescope video laryngoscope versus flexible fiber optic bronchoscope in patients with traumatic cervical spine injury. Egyptian Journal of Anaesthesia 2012; 28: 257–60. [Google Scholar]
- 146. Kramer A, Müller D, Pförtner R, Mohr C, Groeben H. Fibreoptic vs. videolaryngoscopic (C‐MAC® D‐BLADE) nasal awake intubation under local anaesthesia. Anaesthesia 2015; 70: 400–6. [DOI] [PubMed] [Google Scholar]
- 147. Lewis SR, Butler AR, Parker J, Cook TM, Smith AF. Videolaryngoscopy versus direct laryngoscopy for adult patients requiring tracheal intubation. Cochrane Database of Systematic Reviews 2016; 2016: CD011136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Popat M, Mitchell V, Dravid R, Patel A, Swampillai C, Higgs A. Difficult Airway Society Guidelines for the management of tracheal extubation. Anaesthesia 2012; 67: 318–40. [DOI] [PubMed] [Google Scholar]
- 149. Kirkpatrick MB, Sanders RV, Bass JB. Physiologic effects and serum lidocaine concentrations after inhalation of lidocaine from a compressed gas‐powered jet nebulizer. American Review of Respiratory Disease 1987; 136: 447–9. [DOI] [PubMed] [Google Scholar]
- 150. Schønemann NK, van der Burght M, Arendt‐Nielsen L, Bjerring P. Onset and duration of hypoalgesia of lidocaine spray applied to oral mucosa – a dose response study. Acta Anaesthesiologica Scandinavica 1992; 36: 733–5. [DOI] [PubMed] [Google Scholar]
- 151. Roberts MH, Gildersleve CD. Lignocaine topicalization of the pediatric airway. Pediatric Anesthesia 2016; 26: 337–44. [DOI] [PubMed] [Google Scholar]
- 152. General Medical Council . Good medical practice Domain 1: Knowledge skills and performance Manchester, UK. 2019.
- 153. Yentis SM, Hartle AJ, Barker IR, et al. Association of Anaesthetists: consent for anaesthesia 2017. Anaesthesia 2017; 72: 93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Difficult Airway Society . DAS ATI Patient Information Leaflet. https://das.uk.com/files/awake_leaflet.pdf (accessed 21/02/2019).
- 155. Martin KM, Larsen PD, Segal R, Marsland CP. Effective nonanatomical endoscopy training produces clinical airway endoscopy proficiency. Anesthesia and Analgesia 2004; 99: 938–44. [DOI] [PubMed] [Google Scholar]
- 156. Baker PA, Weller JM, Baker MJ, et al. Evaluating the ORSIM ® simulator for assessment of anaesthetists’ skills in flexible bronchoscopy: aspects of validity and reliability. British Journal of Anaesthesia 2016; 117: i87–91. [DOI] [PubMed] [Google Scholar]
- 157. Casso G, Schoettker P, Savoldelli GL, Azzola A, Cassina T. Development and initial evaluation of a novel, ultraportable, virtual reality bronchoscopy simulator. Anesthesia and Analgesia 2018; 1. [DOI] [PubMed] [Google Scholar]
- 158. Jiang B, Ju H, Zhao Y, Yao L, Feng Y. Comparison of the efficacy and efficiency of the use of virtual reality simulation with high‐fidelity mannequins for simulation‐based training of fiberoptic bronchoscope manipulation. Simulation in Healthcare: Journal of the Society for Simulation in Healthcare 2018; 13: 83–7. [DOI] [PubMed] [Google Scholar]
- 159. Marsland C, Larsen P, Segal R, et al. Proficient manipulation of fibreoptic bronchoscope to carina by novices on first clinical attempt after specialized bench practice. British Journal of Anaesthesia 2010; 104: 375–81. [DOI] [PubMed] [Google Scholar]
- 160. Cole AFD, Mallon JS, Rolbin SH, Ananthanarayan C. Fiberoptic intubation using anesthetized, paralyzed, apneic patients results of a resident training program. Anesthesiology 1996; 84: 1101–6. [DOI] [PubMed] [Google Scholar]
- 161. De Oliveira GS, Glassenberg R, Chang R, Fitzgerald P, McCarthy RJ. Virtual airway simulation to improve dexterity among novices performing fibreoptic intubation. Anaesthesia 2013; 68: 1053–8. [DOI] [PubMed] [Google Scholar]
- 162. The Royal College of Anaesthetists . CCT in Anaesthetics – Advanced Level Training (Annex E) Edition 2, 2017. https://www.rcoa.ac.uk/system/files/TRG-CCT-ANNEXE.pdf (accessed 29/03/2019).
- 163. McNarry AF, Dovell T, Dancey FML, et al. Perception of training needs and opportunities in advanced airway skills: a survey of British and Irish trainees. European Journal of Anesthesiology 2007; 24: 498–504. [DOI] [PubMed] [Google Scholar]
- 164. Ezri T, Szmuk P, Warters RD, Katz J, Hagberg CA. Difficult airway management practice patterns among anesthesiologists practicing in the United States: have we made any progress? Journal of Clinical Anaesthesia 2003; 15: 418–22. [DOI] [PubMed] [Google Scholar]
- 165. Wiles MD, McCahon RA, Armstrong JAM. An audit of fibreoptic intubation training opportunities in a UK teaching hospital. Journal of Anesthesiology 2014; 2014: 1–4. [Google Scholar]
- 166. Dawson AJ, Marsland C, Baker P, Anderson BJ. Fibreoptic intubation skills among anaesthetists in New Zealand. Anaesthesia and Intensive Care 2005; 33: 777–83. [DOI] [PubMed] [Google Scholar]
- 167. Goldmann K, Braun U. Airway management practices at German university and university‐affiliated teaching hospitals – Equipment, techniques and training: results of a nationwide survey. Acta Anaesthesiologica Scandinavica 2006; 50: 298–305. [DOI] [PubMed] [Google Scholar]
- 168. Ahmad I, Onwochei DN, Muldoon S, Keane O, El‐Boghdadly K. Airway management research: a systematic review. Anaesthesia 2019; 74: 225–36. [DOI] [PubMed] [Google Scholar]
- 169. Ahmad I, El‐Boghdadly K. From evidence based on practice to evidence‐based practice: time for a difficult airway management research strategy. Anaesthesia 2019; 74: 135–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Summary of the systematic review methodology.
Appendix S2. The Difficult Airway Society checklist for awake tracheal intubation (ATI) in adults. SAD, supraglottic airway device.
Appendix S3. The Difficult Airway Society documentation sticker for awake tracheal intubation (ATI) in adults. FB, flexible bronchoscopy; VL, videolaryngoscopy; L, left; R, right.


