Abstract
Introduction
Ulcerative colitis (UC) is a chronic and inflammatory bowel disease. UC-associated colorectal cancer (UC-CRC) is one of the most severe complications of long-standing UC. In the present study, we explored the effects of miR-370-3p on UC-CRC in vivo and investigated its underlying mechanisms in vivo and in vitro.
Methods
Azoxymethane (AOM) and dextran sodium sulfate (DSS) were used to induce UC-CRC in C57BL/6 mice. AOM/DSS-induced mice were treated with 5×108 pfu miR-370-3p overexpressing-adenovirus via tail-vein injection every two weeks.
Results
We found that miR-370-3p significantly improved the body weights and survival rates and inhibited the tumorigenesis of UC-CRC in AOM/DSS mice. Mechanically, miR-370-3p inhibited AOM/DSS-induced inflammatory response by decreasing tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6) through targeting toll-like receptor 4 (TLR4), as demonstrated by down-regulation of TLR4, cyclooxygenase-2 (COX-2), prostaglandin E2 (PGE2), and phosphorylated epidermal growth factor receptor (pEGFR). miR-370-3p decreased the expression of tumor-associated proteins, including p53, β-catenin, and ki67 in AOM/DSS-treated mice. Additionally, miR-370-3p remarkably inhibited epithelial-mesenchymal transition (EMT) via increasing E-cadherin expression and reducing N-cadherin and Vimentin expression in vivo. Further studies showed that miR-370-3p repressed proliferation and EMT of colon cancer cells in vitro. Moreover, we proved that miR-370-3p decreased the expression of tumor-associated proteins and reversed EMT by regulating β-catenin in colon cancer cells.
Conclusion
Taken together, miR-370-3p alleviated UC-CRC by inhibiting the inflammatory response and EMT in mice, which suggested miR-370-3p as a novel potential target for UC-CRC therapy.
Keywords: ulcerative colitis-associated colorectal cancer, azoxymethane/dextran sodium sulfate, inflammatory response, epithelia-mesenchymal transition, carcinogenesis
Introduction
Ulcerative colitis (UC) is a disease of the colon that is characterized by chronic inflammation. The incidence of UC is increasing worldwide,1 however its etiology is still unclear. Generally, the clinical manifestations of UC are a complex course of continuous recurrence and remission, accompanied by pathological changes such as recurrent colonic mucosal ulcer and mucosal necrosis and regeneration. This process of repeated injury and repair increases the risk of developing colorectal cancer (CRC) in patients with UC.2 Epidemiological studies have shown that UC is one of the high-risk factors for CRC, which leads to a 10-fold increase in the risk of developing CRC.3,4 Despite modern screening procedures, only approximately half ulcerative colitis-related colorectal cancer (UC-CRC) patients are diagnosed at the advanced stage and have a poor prognosis. Due to this diagnostic dilemma, more effective diagnostic and therapeutic targets are needed for early clinical risk assessment and treatment.
MicroRNAs are a class of endogenous non-coding RNAs that are generally about 18–25 nt in length. They are capable of binding to the 3ʹ-untranslated region (UTR) of mRNAs to regulate the expression of downstream genes. miRNAs play crucial roles in cell proliferation, differentiation, and apoptosis. Aberrant expression of miRNAs is closely related to the progression of cancers,5 and miRNAs participate in the occurrence and development of tumors as tumor promoters or suppressors through directly regulating tumor suppressor genes or oncogenes.6 Moreover, miRNAs are important regulators of inflammatory signaling pathways and are involved in the development of a variety of human inflammatory diseases, including UC-CRC.7,8 miR-370-3p is located on human chromosome 14 and the DLK1-DIO3 imprinting genomic region of chromosome 12 of homologous mice. It is evolutionarily conserved and plays different regulatory roles in different tumors. For instance, miR-370-3p acted as a tumor inhibitor in ovarian cancer,9 cholangiocarcinoma,10 glioma,11 thyroid cancer12 and bladder cancer.13 On the contrary, some studies showed that up-regulated expression of miR-370-3p promoted the progression of prostate cancer,14 gastric cancer15 and Wilms tumor.16 It has been reported that miR-370-3p is significantly down-regulated in the biopsy tissues of colon mucosa of UC patients17 as well as in CRC tissues and cells.18 However, its biological roles and regulatory mechanisms in UC-CRC remain largely unknown.
Toll-like receptor 4 (TLR4) is an important receptor of endotoxin, which initiates inflammatory response. TLR4 is highly expressed in the intestinal mucosa of patients with inflammatory bowel disease19 and causes a continuously expanding inflammatory response. TLR4 signaling pathway plays a crucial role in the pathophysiological development of UC-CRC. A number of drugs that block or inhibit TLR4 signal have been studied and developed to treat inflammatory bowel disease,20,21 which provides new ideas for the treatment of UC-CRC.
Azoxymethane (AOM)/dextran sodium sulfate (DSS)-induced colitis-associated cancer animal models have been widely used to study UC-induced CRC in vivo. Our team for the first time illustrated the protection of miR-370-3p against UC-CRC in AOM/DSS model mice. We demonstrated that miR-370-3p inhibited inflammatory response and epithelial-mesenchymal transition (EMT) and thus alleviated mortality and tumorigenesis in mice with UC-CRC via targeting TLR4 and β-catenin. Our findings suggested that miR-370-3p might be a novel therapeutic target for UC-CRC treatment.
Materials and Methods
Mice and Experimental Models
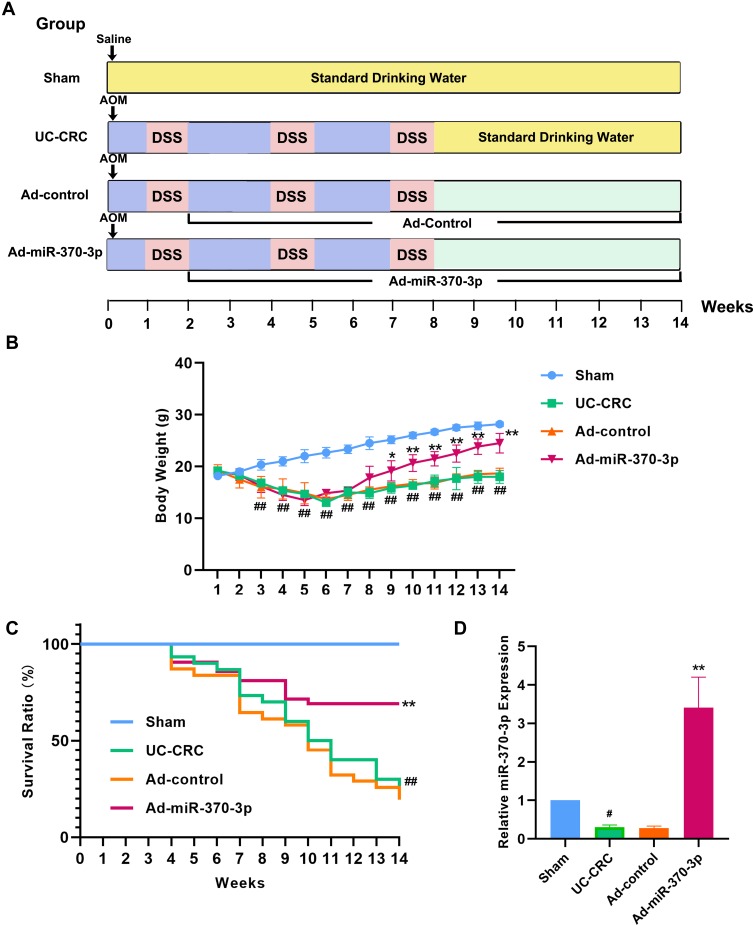
Male C57BL/6 mice (age 6–8 weeks, body weight 15–20 g) were purchased from Liaoning Changsheng biotechnology (Benxi, Liaoning, China). All animals were housed in a 25 ± 2 ° C environment with 12-h dark/light cycle and fed with standard diet and water. The mice were randomly divided into 4 groups (Sham group, UC-CRC group, adenovirus (Ad)-control group, and Ad-miR-370-3p group) after 1 week of adaptive feeding. The establishment of UC-CRC mouse model using AOM and DSS was shown in Figure 1A. They were intraperitoneally injected with 10 mg·kg−1 of AOM on the first day. After 1 week, mice were given 3% DSS (MPbio, California, USA) in drinking water for 1 week followed by standard drinking water for 2 weeks. This DSS cycle was repeated twice. After 2 weeks of AOM injection, mice in the Ad-control group or Ad-miR-370-3p group was injected with 5×108 pfu of control adenovirus (GenScript, Nanjing, China) or miR-370-3p overexpressing-adenovirus (GenScript, Nanjing, China) through the tail vein every two weeks for 12 weeks. Mice in the Sham group were injected with saline on the first day followed by feeding with standard drinking water throughout the process. After 14 weeks, the surviving mice were anesthetized and sacrificed, and the whole colon tissues were collected for subsequent experiments. This study was conformed to the Guide for the Care and Use of Laboratory Animals. All experimental procedures involving animals were approved by the Ethics Committee of Shengjing Hospital of China Medical University.
Figure 1.
Effects of miR-370-3p on the body weights and survival rates of AOM/DSS-induced mice. (A) The schematic procedure of inducing UC-CRC mouse model by AOM/DSS. The details were described in “Materials and Methods”. (B) The body weights of mice in the Sham group, UC-CRC group, Ad-control group, and Ad-miR-370-3p group were measured once a week. (C) The survival of mice in each group was recorded weekly (thirty mice in each group). (D) The expression of miR-370-3p in the tumor colon tissues of mice from each group was measured by qRT-PCR. The comparison of body weights of mice among groups was performed by Repeated Measures ANOVA. The comparison among groups was performed by One‐way ANOVA. Data are presented as mean ± SD. #p<0.05 and ##p<0.01 compared with Sham group, *p<0.05 and **p<0.01compared with Ad-control group.
Quantitative Real-Time PCR (qRT-PCR)
Total RNAs were isolated by using RNAsimple Total RNA Kit (TIANGEN BIOTECH, Beijing, China). cDNAs were synthesized by M-MLV reverse transcriptase (TIANGEN BIOTECH, Beijing, China). The sequences f primers used in this experiment were shown in Table 1. Quantitative RT-PCR was performed using SYBR Green (Solarbio, Beijing, China) on ExicyclerTM 96 (BIONEER, Taejon, Korea). The data were analyzed using the 2−ΔΔCT method.
Table 1.
The Detail Sequences of Primers Used for qRT-PCR
| Gene Name | Sequence (5ʹ-3ʹ) |
|---|---|
| MUS TNF-α F | CAGGCGGTGCCTATGTCTCA |
| MUS TNF-α R | GCTCCTCCACTTGGTGGTTT |
| MUS IL-1β F | CTCAACTGTGAAATGCCACC |
| MUS IL-1β R | GAGTGATACTGCCTGCCTGA |
| MUS IL-6 F | ATGGCAATTCTGATTGTATG |
| MUS IL-6 R | GACTCTGGCTTTGTCTTTCT |
| MUS GAPDH F (internal control) | TGTTCCTACCCCCAATGTGTCCGTC |
| MUS GAPDH R (internal control) | CTGGTCCTCAGTGTAGCCCAAGATG |
| HOMO GAPDH F (internal control) | GACCTGACCTGCCGTCTAG |
| HOMO GAPDH R (internal control) | AGGAGTGGGTGTCGCTGT |
| mmu-miR-370-3p F | GCCTGCTGGGGTGGAACCTGGT |
| mmu-miR-370-3p R | GCAGGGTCCGAGGTATTC |
| U6 F (internal control) | CGCAAGGATGACACGCAAAT |
| U6 R (internal control) | GCAGGGTCCGAGGTATTC |
Abbreviation: F, forward; R, reverse.
Enzyme-Linked Immunosorbent Assays (ELISA)
The levels of TNF-α (tumor necrosis factor-α) and PGE2 (prostaglandin E2) were measured using Mouse TNF-α ELISA Kit (MULTI SCIENCES, Hangzhou, China) and Prostaglandin E2/PGE2 Competitive ELISA Kit (MULTI SCIENCES, Hangzhou, China), respectively, according to the protocols. In short, 100 μL of diluent standard substance or sample was added onto each well. Then 50 μL diluted antibodies were added and incubated for 2 h at room temperature. After washing, each well was added with 100 μL diluted horseradish peroxidase (HRP)-conjugated streptomycin and incubated for 45 min at room temperature. After repeated washing, 100 μL 3,3ʹ5,5ʹ-Tetramethylbenzidine (TMB) color liquid was added, and the mixture was incubated in the dark at room temperature for 20 min. Finally, 100 μL stop solution was used for stopping the reaction. The optical density (OD) values at the maximum absorption wavelength of 450 nm and the reference wavelength of 570 nm were measured by using a microplate reader (BIOTEK, ELX-800, Vermont, USA). The calibrated OD value was the measured value at 450 nm minus the measured value at 570 nm.
Immunohistochemistry (IHC) Staining
For immunohistochemical staining, paraffin-embedded slides with 5-μm-thick were deparaffinized in xylene (Aladdin, Shanghai, China) and hydrated in gradient concentration of alcohol. Antigen was retrieved by heating in the pre-boiling buffer for 10 min in a microwave. The sections were incubated with 3% H2O2 at room temperature for 15 min to eliminate the activity of endogenous peroxidase and blocked by goat serum (Solarbio, Beijing, China) at room temperature for 15 min. The specimens were subsequently incubated with primary antibodies at 4° C overnight: COX-2 (cyclooxygenase-2, 1:200, Abcam, Cambridge, UK), E-cadherin (1:200, CST, Boston, Massachusetts, USA), ki67 (1:200, Abcam, Cambridge, UK) and TLR4 (1:200, ABclonal, Wuhan, China). HRP-conjugated goat anti-rabbit IgG (1:500, Thermo Fisher, Waltham, Massachusetts, USA) was used as the secondary antibody. The sections were visualized by DAB (diaminobenzidine, Solarbio, Beijing, China) and counterstained with hematoxylin (Solarbio, Beijing, China). The results were observed under an optical microscope (OLYMPUS, DP73, Tokyo, Japan).
Hematoxylin-Eosin (HE) Staining
After deparaffinization and rehydration, the slides were stained with hematoxylin (Solarbio, Beijing, China) for 5 min and dipped in 1% acid alcohol (1% HCl in 70% alcohol) for 3 s. Then the slides were stained with eosin (Sangon Biotech, Shanghai, China) for 3 min, dehydrated with gradient alcohol, and vitrified in xylene. The mounted slides were then photographed using an optical microscope (OLYMPUS, DP73, Tokyo, Japan).
Cell Culture and Transient Transfection
HCT116 cell line, SW620 cell line, and HT29 cell line were purchased from Procell (Wuhan, China). HEK 293T cell line was purchased from Zhong Qiao Xin Zhou (Shanghai, China). HCT116 cells and HT29 cells were cultured in McCoy’s 5A medium (Procell, Wuhan, China) containing 10% fetal bovine serum (Hyclone, Logan, Utah, USA). SW620 cells were cultured in RPMI-1640 medium (Gibco, Grand Island, New York, USA) supplemented with 10% fetal bovine serum. HEK 293T cells were maintained in DMEM medium (Gibco, Grand Island, New York, USA) containing 10% fetal bovine serum. All cell lines were cultured in a humidified atmosphere at 37° C with 5% CO2.
NC agomir and miR-370-3p agomir were purchased from GenePharma (Shanghai, China). miR-370-3p inhibitor was purchased from JTS scientific (Wuhan, China). The sequences of β-catenin siRNA (JTS scientific, Wuhan, China) were as follows: forward, 5ʹ-GAUGGUGUCUGCUAUUGUATT-3ʹ, reverse 5ʹ-UACAAUAGCAGACACCAUCTT-3ʹ. The transfection was performed by using Lipofectamine 2000 (Invitrogen, Carlsbad, California, USA) according to the instruction.
Cell Counting Kit-8 (CCK-8) Assays
Before performing CCK-8 assay, cells were respectively cultured for 0 h, 24 h, 48 h, 72 h, and 96 h after transfection. After incubation with 10 μL CCK-8 (KeyGEN BioTECH, Nanjing, China) for 2 h at 37 ° C, the absorbance of cells in each well was measured by a microplate reader (BIOTEK, ELX-800, Vermont, USA). The OD value at a wavelength of 450 nm was used to calculate cell viability.
Luciferase Reporter Gene Assays
The putative binding sites of miR-370-3p in TLR4 and Ctnnb1 were predicted by Starbase. The wild type or mutant type of 3ʹ-UTR of TLR4 or Ctnnb1 was inserted into pmirGLO plasmid (Promega, Madison, Wisconsin, USA). Agomirs of miR-370-3p or negative control (NC) were transiently co-transfected with pmirGLO-wt-TLR4, pmirGLO-mut-TLR4, pmirGLO-wt-Ctnnb1 or pmirGLO-mut-Ctnnb1 into 293T cells. Cells were harvested after 48-h transfection for luciferase reporter assay.
Western Blot
The tissues and cells were lysed in RIPA lysis buffer (Solarbio, Beijing, China) with PMSF protease inhibitor (Solarbio, Beijing, China). After centrifuging at 10,000 g for 10 min at 4° C, the supernatant was collected as whole proteins. The concentrations of protein samples were determined by the BCA Protein Assay Kit (Solarbio, Beijing, China) according to the instruction.
For Western blot assay, 20 μg protein from each sample was separated by 8-12% sulfate-polyacrylamide gel and transferred onto PVDF (polyvinylidene difluoride, Millipore, Massachusetts, USA) membranes. After blocking with 5% skim milk (Sangon Biotech, Shanghai China) at room temperature for 1 h, the membranes were incubated with corresponding primary antibodies incuding TLR4 (1:1000, proteintech, Wuhan, China), COX-2 (1:1000, proteintech, Wuhan, China), pEGFR (phosphorylated epidermal growth factor receptor, 1:1000, Affinity, Changzhou, Jiangsu, China), β-catenin (1:5000, proteintech, Wuhan, China), p53 (1:3000, proteintech, Wuhan, China), ki67 (1:1000, Affinity, Changzhou, Jiangsu, China), E-cadherin (1:10,000, proteintech, Wuhan, China), N-cadherin (1:5000, proteintech, Wuhan, China), Vimentin (1:5000, proteintech, Wuhan, China) and GAPDH (1:10,000, proteintech, Wuhan, China) overnight at 4° C. Then the membranes were washed by TBST and incubated with appropriate secondary HRP-conjugated goat anti-rabbit or goat anti-mouse antibodies (1:3000, Solarbio, Beijing, China) for 1 h at 37 ° C. The proteins were visualized by using ECL Western Blotting Substrate (Solarbio, Beijing, China).
Statistics
Data were presented as mean ± standard deviation (SD) of at least three independent experiments. One-Way ANOVA or Two-Way ANOVA was used to analyze the data. The survival rate was evaluated by Kaplan-Meier survival analysis. All statistical analyses were performed by using GraphPad Prism 8.0, and p<0.05 was considered statistically significant.
Results
miR-370-3p Improves the Health and Survival of AOM/DSS-Induced Mice
As shown in Figure 1B, mice treated with AOM/DSS displayed a significant body weight loss compared with the mice in the sham group, while AOM/DSS-induced weight reduction was alleviated by injecting Ad-miR-370-3p. Additionally, miR-370-3p significantly improved the survival rate of AOM/DSS-treated mice according to the Kaplan-Meier survival curves (Figure 1C). The expression of miR-370-3p was declined in mice with UC-CRC and elevated following Ad-miR-370-3p treatment (Figure 1D). These results indicated that miR-370-3p improved the body weights and survival of UC-CRC model mice.
miR-370-3p Inhibits the Tumorigenesis in AOM/DSS-Induced Mice
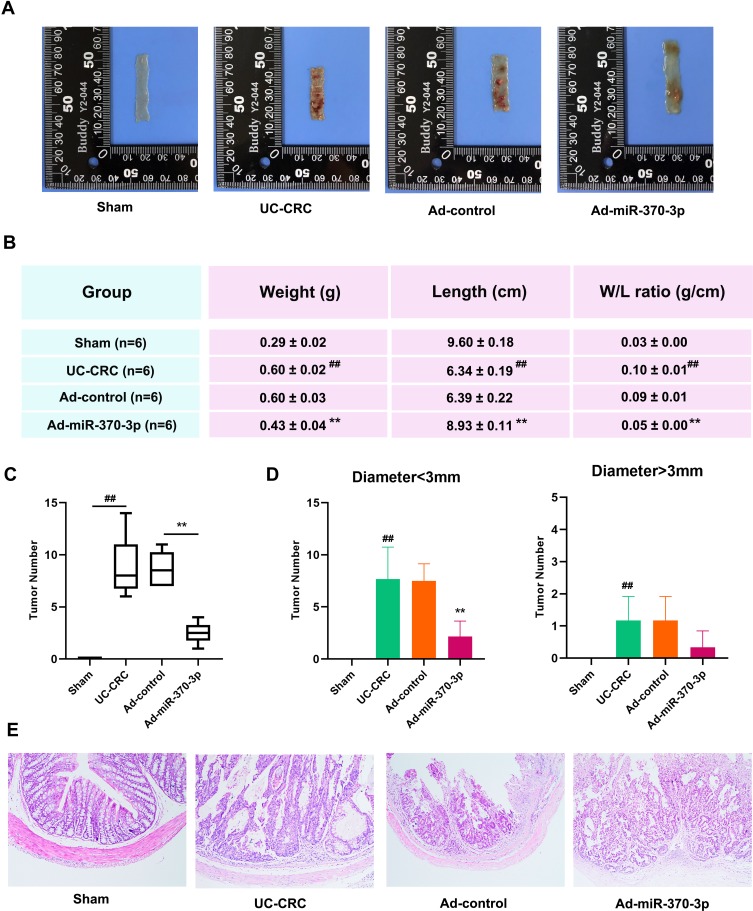
The representative image of mouse entire colon tissue in each group were shown in Figure 2A. AOM and DSS significantly increased the colon weight and reduced the colon length of mice, which was consistent with previous results.22 However, miR-370-3p remarkably reduced the weight/colon ratio (Figure 2B). Moreover, miR-370-3p significantly reduced the total number of tumors by more than 70% (Figure 2C), and caused a decrease in the number of both small tumors (diameter<3 mm) and big tumors (diameter>3 mm, Figure 2D). Pathological findings suggested that there were apparent crypt destruction and inflammatory cell infiltration in the colon tissues of AOM/DSS-treated mice, which were mitigated after Ad-miR-370-3p treatment (Figure 2E). These results suggested that miR-370-3p inhibited the tumorigenesis and relieved the clinical symptoms of UC-CRC in mice.
Figure 2.
Effect of miR-370-3p on tumorigenesis in AOM/DSS-induced mice. (A) The entire colon tissues were removed from mice in the Sham group, UC-CRC group, Ad-control group, and Ad-miR-370-3p group at the end of the experiment. Representative photos showed the colons that were longitudinally cut. (B) The weight and length of the entire colon tissues in mice from each group were measured. (C) Effect of miR-370-3p on the total number of tumors in the entire colon tissues of AOM/DSS-treated mice. (D) Effect of miR-370-3p on the tumor numbers of different sizes (diameter<3 mm and diameter>3 mm) in the entire colon tissues in AOM/DSS-treated mice. (E) The colonic sections of the tumor colon tissues in mice from each group were stained with HE. Scale bar=200 μm. The comparison among groups was performed by One way ANOVA. Data are presented as mean ± SD. ##p<0.01 compared with the Sham group, **p<0.01 compared with the Ad-control group.
miR-370-3p Inhibits the Inflammatory Response and TLR4 Signaling Pathway in AOM/DSS-Induced Mice
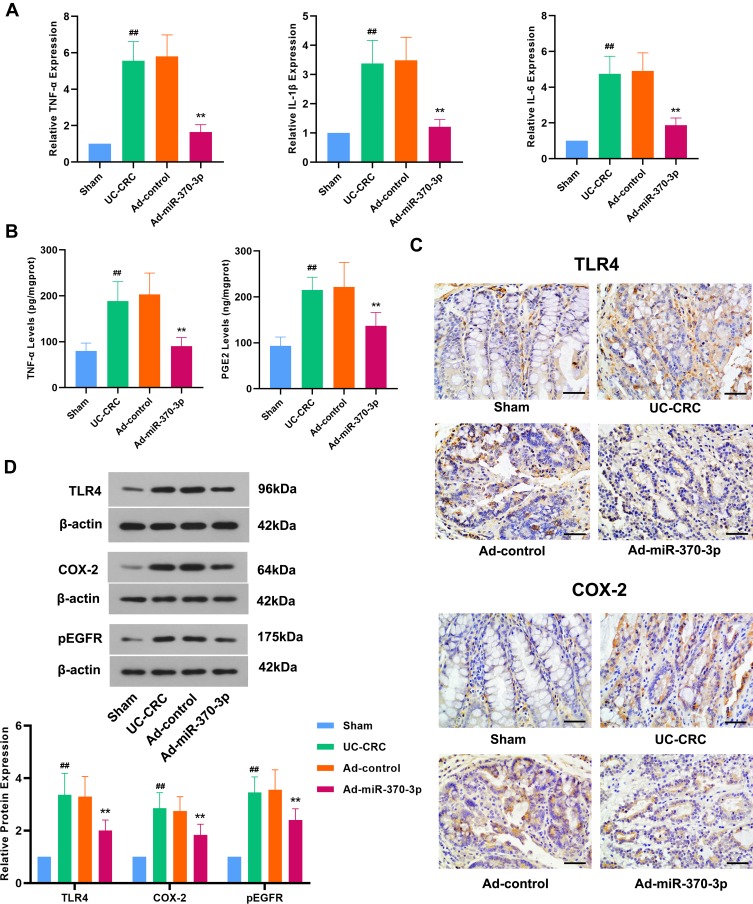
Next, we investigated the effect of miR-370-3p on the inflammation in UC-CRC model mice. The overproduction of some inflammatory cytokines such as TNF-α, IL-1β, and IL-6 contributes to the pathogenesis of UC.23 As shown in Figure 3A, the expression of TNF-α, IL-1β, and IL-6 was significantly elevated in the colon tissues of mice with UC-CRC. As indicated by ELISA, the production of TNF-α in UC-CRC colon tissues was notably increased (Figure 3B). However, miR-370-3p remarkably reversed AOM/DSS-induced elevations in these inflammatory cytokines (Figure 3A and B). In recent years, scholars found that the TLR4-COX-2-PGE2 axis plays a vital role in the development of tumors.45 In this regard, we found that AOM/DSS dramatically increased the level of PGE2 in the colon tissues of mice with UC-CRC; nevertheless, it was significantly attenuated by miR-370-3p (Figure 3B). The results of Western blot and IHC showed that miR-370-3p attenuated AOM/DSS-induced increased TLR4 and COX-2 expression (Figure 3C and D). Additionally, the expression of COX-2 and PGE2 induced by TLR4 is associated with enhanced EGFR signaling. We found that the exposure of mice to AOM/DSS caused a significant increase in the expression of pEGFR, which was markedly reduced by miR-370-3p (Figure 3D). Taken together, miR-370-3p inhibited the inflammatory response and TLR4 signaling pathway in UC-CRC model mice.
Figure 3.
Effect of miR-370-3p on inflammatory response and TLR4 signaling pathway in AOM/DSS-induced mice. (A) The expression of TNF-α, IL-1β, and IL-6 in colonic tissues of mice from the Sham group, UC-CRC group, Ad-control group, and Ad-miR-370-3p group was detected by qRT-PCR. (B) The production of TNF-α and PGE2 in the lysate of the tumor colon tissues were detected by ELISA. (C) The expression of TLR4 and COX-2 in the tumor colon tissues from each group was evaluated by using IHC staining. Scale bar=50 μm. (D) The expression levels of TLR4, COX-2, and pEGFR in the tumor colon tissues from each group were determined by Western blot, and the relative band intensity was analyzed.The comparison among groups was performed by One‐way ANOVA. Data are presented as mean ± SD. ##p<0.01 compared with the Sham group, **p<0.01 compared with the Ad-control group.
miR-370-3p Inhibits the Expression of Tumor-Associated Proteins and EMT Induced by AOM/DSS in Mice
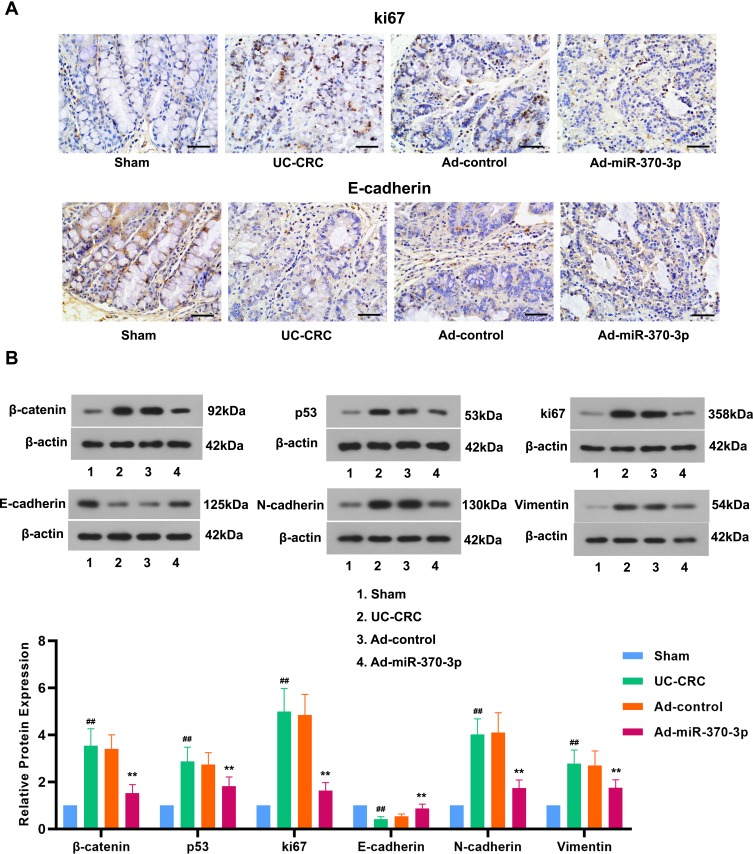
To investigate the underlying mechanism of tumor suppression of miR-370-3p in UC-CRC, we determined the expression of several oncogenic proteins. The results showed that the expression of oncogenic proteins, β-catenin and ki67, was dramatically up-regulated in the UC-CRC group compared with that in the Sham group (Figure 4A and B). A variety of evidence indicated that p53 expression was closely related to UC-CRC development.24 In our work, the expression of p53 protein was significantly increased by AOM/DSS in colonic tissues of mice (Figure 4B). However, miR-370-3p notably inhibited the elevations in these tumor markers in AOM/DSS-induced mice (Figure 4A and B). It was well known that the combination of β-catenin and E-cadherin was closely related to tumor invasion. The results showed that exposure of mice to AOM/DSS decreased the expression of E-cadherin, the epithelial marker, and increased the expression of N-cadherin and Vimentin, the mesenchymal markers, suggesting the occurrence of EMT. Nevertheless, miR-370-3p treatment counteracted AOM/DSS induced EMT in mice (Figure 4A and B). These results illustrated that miR-370-3p inhibited the expression of tumor-associated proteins and EMT in UC-CRC model mice.
Figure 4.
Effects of miR-370-3p in the expression of tumor-associated proteins and EMT in mice treated with AOM/DSS. (A) IHC assessed the expression levels of ki67 and E-cadherin in the tumor colon tissues from each group. Scale bar=50 μm. (B) The expression of β-catenin, p53, ki67, E-cadherin, N-cadherin, and Vimentin in the tumor colon tissues was detected by Western blot. The relative band intensity ratio was analyzed. The comparison among groups was performed by One‐way ANOVA. Data are presented as mean ± SD. ##p<0.01 compared with the Sham group, **p<0.01 compared with the Ad-control group.
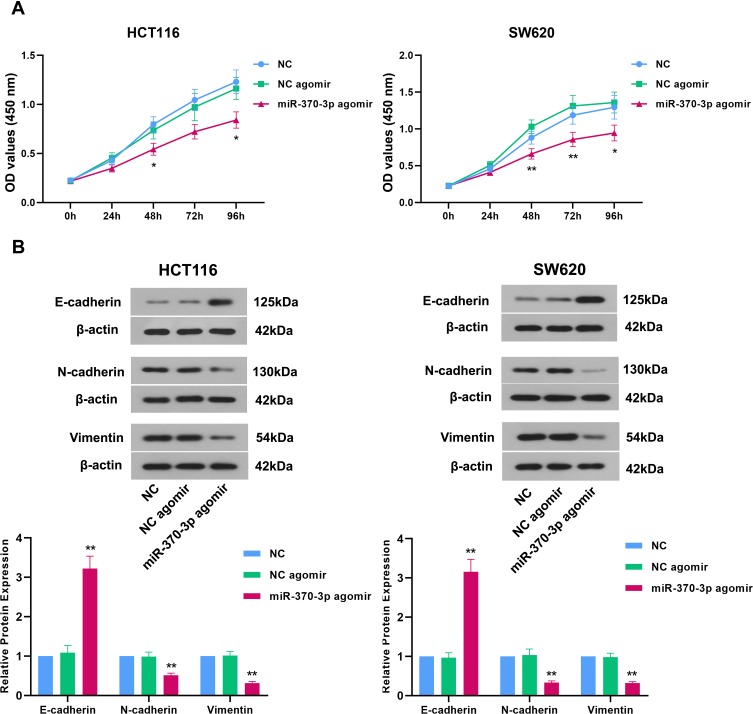
miR-370-3p Suppresses Proliferation and EMT of Colon Cancer Cells
The previous study has demonstrated the role of miR-370 in inhibiting proliferation and inducing apoptosis in CRC cells.25 Here, we focused on the effect of miR-370-3p on the proliferation and EMT of colon cancer cells. HCT116 and SW620 cells with the lowest expression of miR-37025 were selected for this study. The regulatory effect of miR-370-3p on the proliferation of HCT116 cells and SW620 cells was evaluated by using CCK-8 assay. As shown in Figure 5A, miR-370-3p significantly decreased the proliferation of these two cells compared with the corresponding controls, which was in line with the prior study.25 The expression changes in epithelial and mesenchymal markers were determined by Western blot. miR-370-3p markedly elevated E-cadherin expression and reduced N-cadherin and Vimentin expression in HCT116 cells and SW620 cells (Figure 5B), which indicated that miR-370-3p inhibited EMT of colon cancer cells.
Figure 5.
Effects of miR-370-3p on proliferation and EMT of colon cancer cells in vitro. (A) After transfection for 48 h, CCK-8 assay was used to detect the effect of miR-370-3p on the proliferation of HCT116 cells and SW620 cells. (B) The expression of E-cadherin, N-cadherin, and Vimentin in HCT116 cells and SW620 cells was detected by Western blot.The comparison among groups was performed by One‐way ANOVA. Data are presented as mean ± SD.*p<0.05 and **p<0.01 compared with NC agomir control.
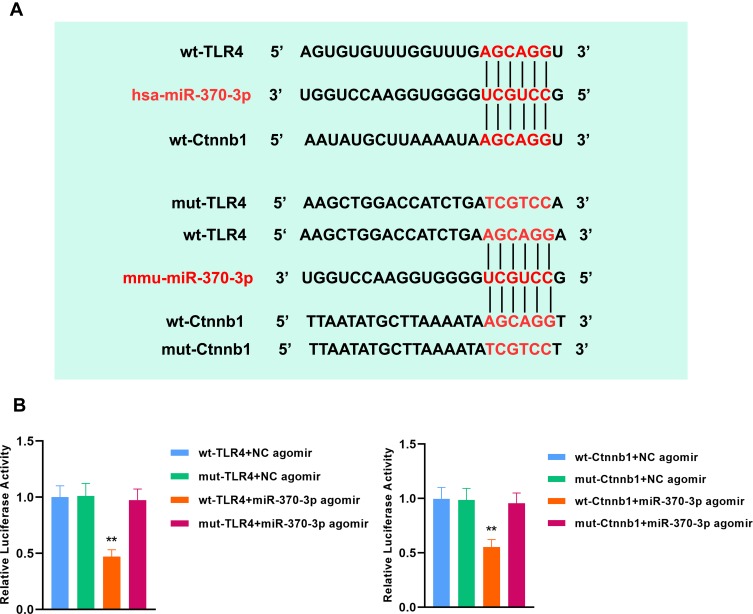
mmu-miR-370-3p Directly Targets TLR4 and β-Catenin in Mice
The regulatory effects of hsa-miR-370-3p on human TLR4 and β-catenin have been reported11,26 (Figure 6A). Here, we verified that mmu-miR-370-3p targeted TLR4 and β-catenin in mice. The predicted binding sites of mmu-miR-370-3p in the 3ʹUTR sequence of murine TLR4 and Ctnnb1 were shown in Figure 6A, respectively. The co-transfection of miR-370-3p agomir and the luciferase reporter plasmid containing wt-TLR4 or wt-Ctnnb1 led to a significant decrease in the relative luciferase activity in 293T cells compared with the corresponding negative control (Figure 6B). These findings implied that mmu-miR-370-3p directly targeted TLR4 and β-catenin in mice.
Figure 6.
TLR4 and β-catenin serve as direct targets of mmu-miR-370-3p in mice. (A) The binding sites of hsa-miR-370-3p in human wild type TLR4 (wt-TLR4) and wild type Ctnnb1 (wt-Ctnnb1) have been confirmed. The sequences of mmu-miR-370-3p binding sites in the 3ʹUTR of TLR4 miRNA or β-catenin miRNA in mice were shown. The luciferase reporter constructs contained the wild type (wt-TLR4, wt-Ctnnb1) or mutant type (mut-TLR4, mut-Ctnnb1) sequence. (B) Luciferase reporter gene assay showed the direct targeting of mmu-miR-370-3p to murine TLR4 and β-catenin. Firefly/Renilla was used to evaluate the relative luciferase activity.The comparison among groups was performed by One‐way ANOVA. Data are presented as mean ± SD. **p<0.01.
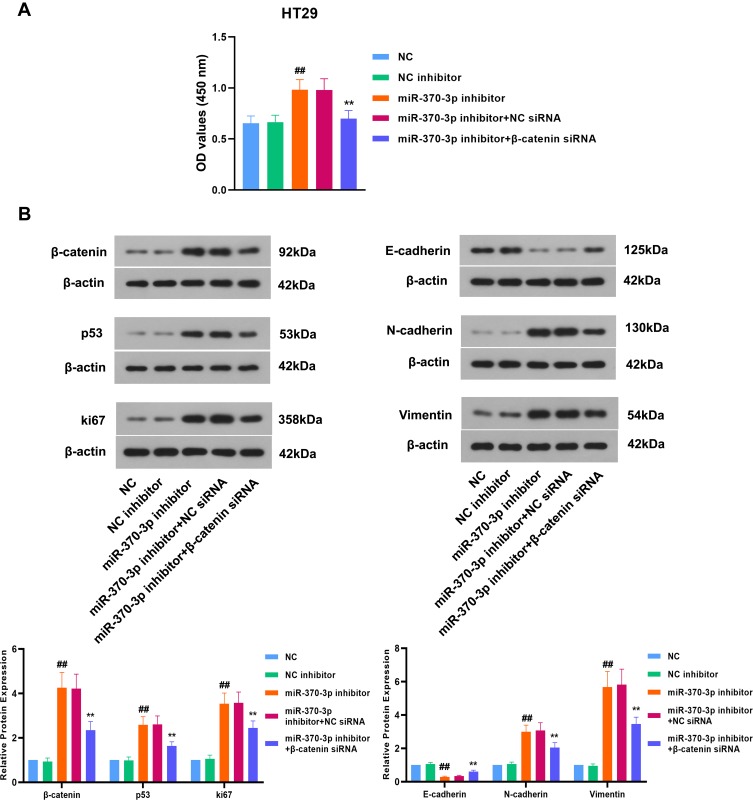
miR-370-3p Suppresses the Proliferation, the Expression of Tumor-Associated Proteins and EMT of Colon Cancer Cells by Regulating β-Catenin
Based on the interaction of miR-370-3p with β-catenin, we further explored whether miR-370-3p affected the progression of colon cancer through regulating β-catenin. According to the previous study,25 HT29 cells with the highest expression of miR-370 were used for further experiments. The results of CCK-8 assay showed that inhibiting miR-370-3p led to a significant increase in the proliferation of colon cancer cells, while knockdown of β-catenin notably suppressed this effect (Figure 7A). As shown in Figure 7B, the expression of β-catenin, p53, and ki67 was up-regulated in HT29 cells transfected with miR-370-3p inhibitor, and downregulated by β-catenin siRNA. E-cadherin expression was obviously down-regulated, and N-cadherin and Vimentin expression was markedly increased by silencing miR-370-3p in HT29 cells. However, inhibition of β-catenin was shown to inhibit EMT in colon cancer cells (Figure 7B). Collectively, miR-370-3p suppressed the proliferation, the expression of tumor-associated proteins, and EMT of colon cancer cells by regulating β-catenin.
Figure 7.
miR-370-3p inhibits the progression of colon cancer cells by regulating β-catenin. (A) After transfection for 48 h, the proliferation of HT29 cells was assessed by CCK-8 assay. (B) After transfection for 48 h, Western blot was used to detect the expression of β-catenin, p53, ki67, E-cadherin, N-cadherin, and Vimentin in HT29 cells. The relative band intensity ratio was analyzed.The comparison among groups was performed by One‐way ANOVA. Data are presented as mean ± SD. ##p<0.01 compared with NC inhibitor control, **p<0.01 compared with miR-370-3p inhibitor + NC siRNA control.
Discussion
UC-CRC is a malignant colonic disease with high mortality. Accumulating studies suggested that the maintenance of treatment for chronic UC might be an important strategy to reduce the risk of developing CRC in UC patients. At present, an increasing number of people are committed to exploring the potential pathogenesis mechanism of UC-CRC and developing novel therapeutic agents. The transition from UC to UC-CRC is a complex process, which remains elusive. AOM/DSS-induced colitis-associated cancer mouse are widely used animal models to study UC-CRC. In our work, miR-370-3p led to a significant increase in the survival ratio of AOM/DSS-treated mice. UC-CRC-induced a decrease in body weight gain and colon length, and an increase in colon weight, which were considered to be caused by apparent mucosal thickening,22 were markedly alleviated by the treatment of miR-370-3p. The previous study showed that miR-370 reduced CRC tumor growth in vivo.27 Here, we demonstrated that miR-370-3p remarkably inhibited the tumorigenesis and mitigated the pathological changes in mice with UC-CRC. These findings indicated that miR-370-3p could be considered as a new potential therapeutic target to alleviate UC-CRC.
The inflammatory environment is believed to play a crucial role in the pathogenesis of UC-CRC.28 Chronic inflammation can lead to repeated stimulation of epithelial cells and infiltration of immune cells and soluble mediators, which provides an ideal microenvironment for tumor development.29 The anti–inflammatory effect of miR-370-3p in vitro and in vivo has been widely reported,10,30,31 which was in line with our findings. TNF-α signaling promotes the expression of downstream inflammatory mediators associated with abnormal cell proliferation and differentiation.32 An elevated TNF-α level increased the risk of CRC,33 and a decreased IL-1β level reduced the risk of advanced CRC.34 IL-6, an important downstream inflammatory mediator of TNF-α signaling pathway, is associated with tumor occurrence, tumor size, advanced tumor stage and the survival of CRC patients.35 In our work, miR-370-3p could remarkably reduce the expression of these pro-inflammatory cytokines in AOM/DSS-treated mice. The incidence of colon cancer is enhanced with the increase of TLR4 expression in UC-CRC animal models induced by AOM/DSS, suggesting that TLR4 is closely related to the occurrence of UC-CRC.36 TLR4 known as a colonic tumor promoter increased the mucosal expression of TNF-α, epithelial expression of COX-2 protein, and mucosal production of PGE2.36 In our study, miR-370-3p via directly targeting TLR4 suppressed the expression of TLR4, COX-2, and PGE2, which suggested the inhibition of TLR4-COX-2-PEG2 axis in AOM/DSS model mice. TLR4 was also responsible for the activation of EGFR signaling in chronic colitis. Several studies demonstrated that PGE2, as a downstream gene of TLR4, promoted colorectal carcinogenesis at least in part by activating EGFR signaling.37,38 PGE2 could mediate EGFR phosphorylation through inducing intracellular mediators or EGFR ligands. EGFR phosphorylation results in various biological events, such as dysregulation of cell proliferation and apoptosis.39,40 Besides, studies showed that EGFR signaling increased the expression of COX-2 and the secretion of PGE2, resulting in a positive feedback loop41 in colon carcinogenesis. Here, miR-370-3p targets TLR4 to decrease pEGFR expression in UC-CRC model mice, which was consistent with the previous conclusion. In a word, miR-370-3p can inhibit the inflammatory response induced by UC-CRC through targeting TLR4 in mice.
As a tumor suppressor, p53 plays a crucial role in the cell cycle, apoptosis and senescence, and is highly expressed in patients with UC-CRC.42 In our study, the expression of p53 was significantly enhanced by AOM/DSS in mice. p53 is normally low-expressed in cells and rapidly degraded. The accumulation of p53 in UC-CRC animal models represents the early loss of p53 function.43 Nevertheless, the treatment of miR-370-3p notably down-regulated the expression of p53 induced by AOM/DSS. In addition, the increases in the expression of β-catenin and ki67 were markedly suppressed by miR-370-3p. These data suggested that miR-370-3p could prevent the carcinogenesis of UC-CRC.
EMT is a critical mechanism of regulating tumor invasion, migration, and metastasis and involves in the occurrence and development of various malignancies, including CRC.44 EMT endows cells with migratory and invasive capacity, prevents apoptosis and senescence, and contributes to immunosuppression.45 It also disrupts cell-cell adherence and triggers matrix remodeling to promote pathogenesis and metastasis of CRC.46 However, the effect of miR-370-3p on EMT of UC-CRC is still unclear. Studies showed that miR-370 repressed cell adhesion in colon adenocarcinoma cells,47 and its enhanced expression inhibited CRC migration and invasion in vitro.27 However, its effect on EMT of UC-CRC has not been described yet. In this work, we found a dramatic increase in the expression of E-cadherin and a significant decrease in the expression of N-cadherin and Vimentin in AOM/DSS-induced mice, which were reversed by miR-370-3p in vivo. Besides, we identified that miR-370-3p suppressed the proliferation and EMT of colon cancer cells in vitro, which might be a key link in the transformation from UC to CRC. The β-catenin signaling pathway plays a vital role in the process of EMT.48 β-catenin binds to E-cadherin on cell membranes to participate in the intercellular adhesion. Upon downregulation of β-catenin, the intercellular adhesion was disrupted leading to the invasion and metastasis of tumor cells. Here, we confirmed that mmu-miR-370-3p targets β-catenin and negatively regulated its expression in mice. Moreover, miR-370-3p inhibited the proliferation, EMT, and the expression of tumor-associated proteins through regulating β-catenin. Additionally, TLR4 exerts a certain role in the EMT of tumor cells. It was found that TLR4 signaling could promote EMT induced by lipopolysaccharide in human hepatocellular carcinoma,49 and TLR4 knockdown attenuated lipopolysaccharide-induced EMT in intrahepatic biliary epithelial cells.50 Studies also showed that the increase of pro-inflammatory cytokines could trigger the activation of EMT.51 Therefore, we hypothesized that miR-370-3p might inhibit EMT of UC-CRC in part by regulating TLR4 and its downstream factors.
There are considerable evidences that described the complicated relationship between inflammation and EMT. EMT can stimulate cancer cells to produce pro-inflammatory factors. In contrast, inflammation is a powerful inducer of EMT in tumors.52 Our findings suggest that miR-370-3p can attenuate the development of UC-CRC in mice through regulating multiple mechanisms, including inhibiting inflammation response, suppressing carcinogenesis, and repressing EMT, which reveals the potential application of miR-370-3p in the treatment for UC-CRC.
Conclusion
In a word, our work demonstrated that miR-370-3p could inhibit the inflammatory response and EMT to alleviate UC-CRC via regulating TLR4 and β-catenin respectively in mice. Based on the data presented in this study, we demonstrates that miR-370-3p might become a worthy therapeutic target for UC-CRC in the future.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142(1):46–54.e42. doi: 10.1053/j.gastro.2011.10.001 [DOI] [PubMed] [Google Scholar]
- 2.Okayasu I, Ohkusa T, Kajiura K, Kanno J, Sakamoto S. Promotion of colorectal neoplasia in experimental murine ulcerative colitis. Gut. 1996;39(1):87–92. doi: 10.1136/gut.39.1.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48(4):526–535. doi: 10.1136/gut.48.4.526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernstein CN, Blanchard JF, Kliewer E, Wajda A. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer. 2001;91(4):854–862. doi: [DOI] [PubMed] [Google Scholar]
- 5.Lu J, Getz G, Miska EA, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435(7043):834–838. doi: 10.1038/nature03702 [DOI] [PubMed] [Google Scholar]
- 6.Iorio MV, Croce CM. MicroRNAs in cancer: small molecules with a huge impact. J Clin Oncol. 2009;27(34):5848–5856. doi: 10.1200/JCO.2009.24.0317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Viennois E, Zhao Y, Han MK, et al. Serum miRNA signature diagnoses and discriminates murine colitis subtypes and predicts ulcerative colitis in humans. Sci Rep. 2017;7(1):1–16. doi: 10.1038/s41598-017-02782-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woalder A. Panel of methylated microRNA biomarkers for identifying high-risk patients with ulcerative colitis-associated colorectal cancer. Physiol Behav. 2017;153(6):1634–1646.e8. doi:doi: 10.1053/j.gastro.2017.08.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen XP, Chen YG, Lan JY, Shen ZJ. MicroRNA-370 suppresses proliferation and promotes endometrioid ovarian cancer chemosensitivity to cDDP by negatively regulating ENG. Cancer Lett. 2014;353(2):201–210. doi: 10.1016/j.canlet.2014.07.026 [DOI] [PubMed] [Google Scholar]
- 10.Meng F, Wehbe-janek H, Henson R, Smith H, Patel T. Epigenetic regulation of microRNA-370 by interleukin-6 in malignant human cholangiocytes. Oncogene. 2008;27(3):378–386. doi: 10.1038/sj.onc.1210648 [DOI] [PubMed] [Google Scholar]
- 11.Peng Z, Wu T, Li Y, et al. MicroRNA-370-3p inhibits human glioma cell proliferation and induces cell cycle arrest by directly targeting β-catenin. Brain Res. 2016;1644:53–61. doi: 10.1016/j.brainres.2016.04.066 [DOI] [PubMed] [Google Scholar]
- 12.Chen F, Feng Z, Zhu J, et al. Emerging roles of circRNA_NEK6 targeting miR-370-3p in the proliferation and invasion of thyroid cancer via Wnt signaling pathway. Cancer Biol Ther. 2018;19(12):1139–1152. doi: 10.1080/15384047.2018.1480888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang X, Zhu H, Gao Z, et al. Wnt7a activates canonical Wnt signaling, promotes bladder cancer cell invasion, and is suppressed by MIR-370-3p. J Biol Chem. 2018;293(18):6693–6706. doi: 10.1074/jbc.RA118.001689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Z, Sun H, Zeng W, He J, Mao X. Upregulation of mircoRNA-370 induces proliferation in human prostate cancer cells by downregulating the transcription factor FOXO1. PLoS One. 2012;7(9). doi: 10.1371/journal.pone.0045825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lo SS, Hung PS, Chen JH, et al. Overexpression of miR-370 and downregulation of its novel target TGFΒ-RII contribute to the progression of gastric carcinoma. Oncogene. 2012;31(2):226–237. doi: 10.1038/onc.2011.226 [DOI] [PubMed] [Google Scholar]
- 16.Cao X, Liu D, Yan X, et al. Stat3 inhibits WTX expression through up-regulation of microRNA-370 in Wilms tumor. FEBS Lett. 2013;587(6):639–644. doi: 10.1016/j.febslet.2013.01.012 [DOI] [PubMed] [Google Scholar]
- 17.Min M, Peng L, Yang Y, Guo M, Wang W, Sun G. MicroRNA-155 is involved in the pathogenesis of ulcerative colitis by targeting FOXO3a. Inflamm Bowel Dis. 2014;20(4):652–659. doi: 10.1097/MIB.0000000000000009 [DOI] [PubMed] [Google Scholar]
- 18.Bandrés E, Cubedo E, Agirre X, et al. Identification by real-time PCR of 13 mature microRNAs differentially expressed in colorectal cancer and non-tumoral tissues. Mol Cancer. 2006;5(1):1–10. doi: 10.1186/1476-4598-5-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rakoff-nahoum S, Paglino J, Eslami-varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118(2):229–241. doi: 10.1016/j.cell.2004.07.002 [DOI] [PubMed] [Google Scholar]
- 20.Kesharwani SS, Rajput M, Tummala H. Developing polymer-drug complex based toll-like receptor (TLR-2/TLR-4) antagonist for modulating gut innate immune system to prevent/treat IBD and colon cancer. J Immunol. 2017;198(1):81-20. [Google Scholar]
- 21.Kesharwani SS, Ahmad R, Bakkari MA, et al. Site-directed non-covalent polymer-drug complexes for inflammatory bowel disease (IBD): formulation development, characterization and pharmacological evaluation. J Control Release. 2018;290:165–179. doi: 10.1016/j.jconrel.2018.08.004 [DOI] [PubMed] [Google Scholar]
- 22.Li H, Wu WKK, Li ZJ, et al. 2,3′,4,4′,5′-Pentamethoxy-trans-stilbene, a resveratrol derivative, inhibits colitis-associated colorectal carcinogenesis in mice. Br J Pharmacol. 2010;160(6):1352–1361. doi: 10.1111/j.1476-5381.2010.00785.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murano M, Maemura K, Hirata I, et al. Therapeutic effect of intracolonically administered nuclear factor κB (p65) antisense oligonucleotide on mouse dextran sulphate sodium (DSS)- induced colitis. Clin Exp Immunol. 2000;120(1):51–58. doi: 10.1046/j.1365-2249.2000.01183.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lashner BA, Bauer WM, Rybicki LA, Goldblum JR. Abnormal p53 immunohistochemistry is associated with an increased colorectal cancer-related mortality in patients with ulcerative colitis. Am J Gastroenterol. 2003;98(6):1423–1427. doi: 10.1016/S0002-9270(03)00422-2 [DOI] [PubMed] [Google Scholar]
- 25.Shen X, Zuo X, Zhang W, Bai Y, Qin X, Hou N. MiR‑370 promotes apoptosis in colon cancer by directly targeting MDM4. Oncol Lett. 2018;15(2):1673–1679. doi: 10.3892/ol.2017.7524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tian D, Sha Y, Lu JM, Du XJ. MiR-370 inhibits vascular inflammation and oxidative stress triggered by oxidized low-density lipoprotein through targeting TLR4. J Cell Biochem. 2018;119(7):6231–6237. doi: 10.1002/jcb.26851 [DOI] [PubMed] [Google Scholar]
- 27.El-daly SM, Abba ML, Patil N, Allgayer H. MiRs-134 and-370 function as tumor suppressors in colorectal cancer by independently suppressing EGFR and PI3K signalling. Sci Rep. 2016;6(November2015):1–11. doi: 10.1038/srep24720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yashiro M. Ulcerative colitis-associated colorectal cancer. World J Gastroenterol. 2014;20(44):16389–16397. doi: 10.3748/wjg.v20.i44.16389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yao D, Dong M, Dai C, Wu S. Inflammation and inflammatory cytokine contribute to the initiation and development of ulcerative colitis and its associated cancer. Inflamm Bowel Dis. 2019;25(10):1595–1602. doi: 10.1093/ibd/izz149 [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Zhu Y, Gao G, Zhou Z. Knockdown XIST alleviates LPS-induced WI-38 cell apoptosis and inflammation injury via targeting miR-370-3p/TLR4 in acute pneumonia. Cell Biochem Funct. 2019;37(5):348–358. doi: 10.1002/cbf.3392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J, Mao F, Zhao G, Wang H, Yan X, Zhang Q. Long non-coding RNA SNHG16 promotes lipopolysaccharides-induced acute pneumonia in A549 cells via targeting miR-370-3p/IGF2 axis. Int Immunopharmacol. 2020;78(1):106065. doi: 10.1016/j.intimp.2019.106065 [DOI] [PubMed] [Google Scholar]
- 32.Long TM, Raufman JP. The diagnostic and prognostic role of cytokines in colon cancer. Gastrointest Cancer Targets Ther. 2011;27. doi: 10.2147/gictt.s18423 [DOI] [Google Scholar]
- 33.Chan AT, Ogino S, Giovannucci EL, Fuchs CS. Inflammatory markers are associated with risk of colorectal cancer and chemopreventive response to anti-inflammatory drugs. Gastroenterology. 2011;140(3):799–808.e2. doi: 10.1053/j.gastro.2010.11.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bobe G, Murphy G, Albert PS, et al. Serum cytokine concentrations, flavonol intake and colorectal adenoma recurrence in the polyp prevention trial. Br J Cancer. 2010;103(9):1453–1461. doi: 10.1038/sj.bjc.6605915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knüpfer H, Preiss R. Serum interleukin-6 levels in colorectal cancer patients-a summary of published results. Int J Colorectal Dis. 2010;25(2):135–140. doi: 10.1007/s00384-009-0818-8 [DOI] [PubMed] [Google Scholar]
- 36.Fukata M, Shang L, Santaolalla R, et al. Constitutive activation of epithelial TLR4 augments inflammatory responses to mucosal injury and drives colitis-associated tumorigenesis. Inflamm Bowel Dis. 2011;17(7):1464–1473. doi: 10.1002/ibd.21527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pai R, Soreghan B, Szabo IL, Pavelka M, Baatar D, Tarnawski AS. Prostaglandin E2, transactivates EGF receptor: a novel mechanism for promoting colon cancer growth and gastrointestinal hypertrophy. Nat Med. 2002;8(3):289–293. doi: 10.1038/nm0302-289 [DOI] [PubMed] [Google Scholar]
- 38.Buchanan FG, Wang D, Bargiacchi F, DuBois RN. Prostaglandin E2 regulates cell migration via the intracellular activation of the epidermal growth factor receptor. J Biol Chem. 2003;278(37):35451–35457. doi: 10.1074/jbc.M302474200 [DOI] [PubMed] [Google Scholar]
- 39.Stern LE, Erwin CR, O’brien DP, Huang F, Warner BW. Epidermal growth factor is critical for intestinal adaptation following small bowel resection. Microsc Res Tech. 2000;51(2):138–148. doi: [DOI] [PubMed] [Google Scholar]
- 40.Dannenberg AJ, Lippman SM, Mann JR, Subbaramaiah K, DuBois RN. Cyclooxygenase-2 and epidermal growth factor receptor: pharmacologic targets for chemoprevention. J Clin Oncol. 2005;23(2):254–266. doi: 10.1200/JCO.2005.09.112 [DOI] [PubMed] [Google Scholar]
- 41.Coffey RJ, Hawkey CJ, Damstrup L, et al. Epidermal growth factor receptor activation induces nuclear targeting of cyclooxygenase-2, basolateral release of prostaglandins, and mitogenesis in polarizing colon cancer cells. Proc Natl Acad Sci U S A. 1997;94(2):657–662. doi: 10.1073/pnas.94.2.657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu X, Yu Y, Tan S. p53 expression in patients with ulcerative colitis - associated with dysplasia and carcinoma: a systematic meta-analysis. BMC Gastroenterol. 2017;17(1):1–8. doi: 10.1186/s12876-017-0665-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yazlovitskaya EM, DeHaan RD, Persons DL. Prolonged wild-type p53 protein accumulation and cisplatin resistance. Biochem Biophys Res Commun. 2001;283(4):732–737. doi: 10.1006/bbrc.2001.4849 [DOI] [PubMed] [Google Scholar]
- 44.Spaderna S, Schmalhofer O, Hlubek F, et al. A transient, EMT-linked loss of basement membranes indicates metastasis and poor survival in colorectal cancer. Gastroenterology. 2006;131(3):830–840. doi: 10.1053/j.gastro.2006.06.016 [DOI] [PubMed] [Google Scholar]
- 45.Thiery JP, Acloque H, Huang RYJ, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890. doi: 10.1016/j.cell.2009.11.007 [DOI] [PubMed] [Google Scholar]
- 46.Wang H, Wang HS, Zhou BH, et al. Epithelial-Mesenchymal Transition (EMT) induced by TNF-α requires AKT/GSK-3β-mediated stabilization of snail in colorectal cancer. PLoS One. 2013;8(2). doi: 10.1371/journal.pone.0056664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wei Y, Shao J, Wang Y, et al. Hsa-miR-370 inhibited P-selectin-induced cell adhesion in human colon adenocarcinoma cells. Mol Cell Biochem. 2019;450(1–2):159–166. doi: 10.1007/s11010-018-3382-0 [DOI] [PubMed] [Google Scholar]
- 48.Stanczak A, Stec R, Bodnar L, et al. Prognostic significance of Wnt-1, β-catenin and E-cadherin expression in advanced colorectal carcinoma. Pathol Oncol Res. 2011;17(4):955–963. doi: 10.1007/s12253-011-9409-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jing YY, Han ZP, Sun K, et al. Toll-like receptor 4 signaling promotes epithelial-mesenchymal transition in human hepatocellular carcinoma induced by lipopolysaccharide. BMC Med. 2012;10(1):98. doi: 10.1186/1741-7015-10-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang S, Jiang X, Wu L, et al. Toll-like receptor 4 shRNA attenuates lipopolysaccharide-induced epithelial-mesenchymal transition of intrahepatic biliary epithelial cells in rats. Biomed Pharmacother. 2018;107(June):1210–1217. doi: 10.1016/j.biopha.2018.08.071 [DOI] [PubMed] [Google Scholar]
- 51.Landskron G, De La Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res. 2014;2014:1–19. doi: 10.1155/2014/149185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suarez-carmona M, Lesage J, Cataldo D, Gilles C. EMT and inflammation: inseparable actors of cancer progression. Mol Oncol. 2017;11(7):805–823. doi: 10.1002/1878-0261.12095 [DOI] [PMC free article] [PubMed] [Google Scholar]