Abstract
Large scale biopharmaceutical production of biologics relies on the overexpression of foreign proteins by cells cultivated in stirred tank bioreactors. It is well recognized and documented fact that protein overexpression may impact host cell metabolism and that factors associated with large scale culture, such as the hydrodynamic forces and inhomogeneities within the bioreactors, may promote cellular stress. The metabolic adaptations required to support the high‐level expression of recombinant proteins include increased energy production and improved secretory capacity, which, in turn, can lead to a rise of reactive oxygen species (ROS) generated through the respiration metabolism and the interaction with media components. Oxidative stress is defined as the imbalance between the production of free radicals and the antioxidant response within the cells. Accumulation of intracellular ROS can interfere with the cellular activities and exert cytotoxic effects via the alternation of cellular components. In this context, strategies aiming to alleviate oxidative stress generated during the culture have been developed to improve cell growth, productivity, and reduce product microheterogeneity. In this review, we present a summary of the different approaches used to decrease the oxidative stress in Chinese hamster ovary cells and highlight media development and cell engineering as the main pathways through which ROS levels may be kept under control.
Keywords: antioxidant, cell engineering, Chinese hamster ovary cells, CHO, oxidative stress
Reactive oxygen species are generated during recombinant protein production using CHO cells and can lead to oxidative stress. Consequently, cell growth, productivity and product quality can be negatively impacted. In order to alleviate oxidative stress, two main strategies can be used: media development and cell engineering. This review gives an overview of the different approaches available in literature.
1. INTRODUCTION
Over the last two decades, with 62 approved recombinant proteins between 2011 and 2016, protein‐based pharmaceuticals (biologics or biopharmaceuticals) strategy has proven to be a key player in the development of treatments for complex diseases (Lagassé et al., 2017). Mammalian cells are the preferred host to produce recombinant therapeutics, such as monoclonal antibodies. Their intracellular machinery is capable of producing posttranslationally modified proteins (mainly glycosylated forms) displaying similar modifications to those observed in human cells. Several mammalian cell lines have been used for production of recombinant proteins, including murine myeloma (NS0, Sp2/0) cells, baby hamster kidney (BHK21) cells, and human cell lines (HEK293 and HT‐1080), however, 70% of recombinant therapeutic proteins are produced in Chinese hamster ovary cells (CHO; Dumont, Euwart, Mei, Estes, & Kshirsagar, 2016; Kim, Kim, & Lee, 2012).
For safety and raw material control considerations, biopharmaceutical industries are now using serum‐free, chemically defined cell culture media. However, the impact of serum depletion with regard to growth performance and culture viability is considerable, and can, in severe cases, promote apoptosis (Yao & Asayama, 2017). Both the antioxidant properties of serum and the link between free radicals and cell death are well established, and it is therefore likely that oxidative stress resulting from the serum‐depletion is one of the root causes behind the decrease in cell viability (Halliwell, 2003; Lewinska, Wnuk, Slota, & Bartosz, 2007).
Oxidative stress is defined as the imbalance between the production of free radicals and the antioxidant response within the cells (Sies, Berndt, & Jones, 2017). Reactive oxygen species (ROS) are natural byproducts of aerobic metabolism, however, if their levels become too high, these compounds can impact cell health through their high reactivity towards biological components, including protein, lipids, RNA, and DNA (Halliwell, 2006). Protein is particularly vulnerable to oxidative modifications, notably by OH• which can react with all amino acid residues, or ROO• which shows higher affinity for sulfur side‐chain amino acids, such as l‐cysteine and l‐methionine, or aromatic amino acids such as l‐tryptophan and l‐tyrosine (Cai & Yan, 2013; Davies, 2016). When cells are not able to counterbalance these damages, apoptosis is activated to prevent necrosis. Although this phenomenon is a naturally‐occurring defense mechanism in organisms, it is not desirable in the context of bioprocesses. In addition to the negative impact on cell culture performance, the production of free radicals during the culture can also be detrimental through the recombinant protein microheterogeneity (He et al., 2018; Xu et al., 2014). Recombinant protein microheterogeneity corresponds to all the recombinant protein variants that can be generated during the production process, namely charge variants, size, variants bearing different N‐glycosylations, different levels of oxidation on specific amino acids such as tryptophan and methionine, and so forth. Overall, changes in proteins microheterogeneity may lead to changes in the coloration of the recombinant protein of interest. These variants can originate from different posttranslational modifications or nonenzymatic reactions (Beyer, Schuster, Jungbauer, & Lingg, 2018). Acidic variants of monoclonal antibody (mAb) have previously been shown to correlate with ROS levels, supporting the existence of a link between oxidative stress and recombinant protein microheterogeneity (Mallaney, Wang, & Sreedhara, 2014).
In the context of biopharmaceutical production, oxidative stress can be caused by a number of factors, including bioreactor oxygen inhomogeneity, rich cell culture media, high productivity, and waste accumulation. One important process parameter during bioproduction is oxygen level. Maintaining a constant and homogenous oxygen level in the bioreactor is challenging and can be influenced by many parameters, such as gas flow, stirring speed, gas transfer capacity of the fluid, and antifoam addition. In large bioreactors, local dO2 gradients can be formed (Xing, Kenty, Li, & Lee, 2009). This heterogeneity in oxygenation can result in localized hypoxic condition within the culture, and lead to an increase in the sensitivity of CHO cells to oxidative stress that is detrimental to cell growth, culture productivity and/or product quality (A. M. Lewis et al., 2016; Lin & Miller, 1992; Lin, Kimura, & Miller, 1993). On the other hand, hyperoxia can also be a problem in bioprocesses. Restelli et al. (2006) showed that an excessive dO2 concentration in the culture impacted the glycosylation profile of recombinant erythropoietin produced in CHO cells. The authors hypothesized that high dO2 concentrations promoted ROS production and ROS‐dependent protein alteration and that the energetic cost of detoxification of these toxic compounds could lead to a decrease in other critical metabolic processes.
Another potential source of oxidative stress is the elevated energetic demands required by the cell to produce recombinant proteins. The main source of ATP in aerobic organisms is oxidative phosphorylation (OXPHOS), which takes place in the mitochondria. Therefore an increase of metabolic fluxes towards OXPHOS, the TCA cycle, and related pathways is often observed in cells producing a recombinant protein (Templeton, Dean, Reddy, & Young, 2013). However, complex I and III activities within the electron transport chain are the main source of ROS within the cells (Turrens, 2003). An increase in the OXPHOS activity is, therefore, likely to generate ROS, among other pathway byproducts.
In addition to the increase of energy requirements, the combination of the normal production of host protein and recombinant protein production can lead to an accumulation of protein in the endoplasmic reticulum (ER; Mathias et al., 2018). ER is the central location for protein folding and the first step of the secretory pathway. Folding of proteins, in particular disulfide bond formation, requires an oxidative environment and, in certain cases, can induce ROS generation. For example, oxidation of ER oxidoreductin 1 (ERO1) is required to exchange its disulfide bond with its enzymatic partner, the protein disulfide isomerase (PDI). This exchange releases H2O2 as a by‐product in the ER (Tu & Weissman, 2004). In addition, when the ER is overloaded by unfolded proteins, a protection system called the unfolded protein response (UPR) is activated by a number of transcription factors and can also lead to apoptosis and ROS production (Hetz, 2012). Activation of UPR transcription factors, such as the proapoptotic transcription factor C/EBP‐homologous protein (CHOP) and the DNA damage‐inducible 34 protein (GADD34), coupled to altered calcium homeostasis is accompanied by an increase in ROS. Furthermore, CHOP‐dependent activation of GADD34 and ERO1α is reported to promote the accumulation of ROS in the ER due to an increase of oxidation events (Marciniak et al., 2004). Moreover, ROS activates inositol‐1,4,5 trisphosphate receptors (IP3Rs) which release calcium in the cytoplasm. This calcium signaling induces mitochondrial oxidative stress and further ROS production (Cao & Kaufman, 2014). Therefore, it is essential that the cells have efficient secretory machinery to avoid the accumulation of protein in ER and ultimately the overproduction of ROS. This relationship between accumulation of unfolded recombinant protein in the ER and oxidative stress has been evidenced in the biopharmaceutical production of blood coagulation factor VIII (Malhotra et al., 2008).
During the cultivation of mammalian cells, and especially in fed‐batch mode, a number of media components and cell metabolism byproducts are accumulating in the extracellular environment. Some cell culture media components such as vitamins, amino acids, glucose, and even antioxidant compounds such as polyphenolic compounds can generate ROS in the presence of oxygen (Halliwell, 2014; Kelts, Cali, Duellman, & Shultz, 2015; Schnellbaecher, Binder, Bellmaine, & Zimmer, 2019). Moreover, specific cell metabolism byproducts reported to be growth inhibitors can also lead to oxidative stress (Pereira, Kildegaard, & Andersen, 2018). For example, phenyl lactate, which was identified as a growth inhibitor in CHO cells, promotes lipid peroxidation in rat cortex cells tissue (C. G. Fernandes et al., 2010; Mulukutla, Kale, Kalomeris, Jacobs, & Hiller, 2017). Likewise, methylglyoxal, a byproduct of glucose and amino acid metabolism, has been shown to induce oxidative stress in rat cells (Fukunaga et al., 2005). In addition, this compound has also been shown to lead to increased levels of acidic species of mAbs produced in CHO cells (Chumsae et al., 2013).
Due to this large number of potential sources of oxidative stress during bioprocesses and given the deleterious impact of ROS on process performances, different strategies have been developed over the past few decades to counteract this issue. In particular, the approach consisting of altering cell culture media, mainly via supplementation of the medium with antioxidant molecules, is widely described in the literature. The decrease of oxidative stress in response to antioxidant supplementation, occurring through direct ROS scavenging or antioxidant cellular defense activation, has been shown to reduce cell death and the product microheterogeneity, and increase the productivity of the process. While cell culture media modification is the currently preferred option in the CHO cell scientific community, the emergence of CHO omics has also opened up new avenues to alleviate oxidative stress by cell engineering.
In this review, we first give an overview of the oxidative stress in mammalian cells by describing the potential sources of ROS during the cultivation as well as the cellular antioxidant defense. Thereafter, we provide a description of the two main strategies found in the literature to alleviate oxidative stress: supplementation of media formulations with antioxidant molecules, and cell line engineering.
2. OXIDATIVE STRESS IN MAMMALIAN CELLS
2.1. Sources of reactive species in mammalian cells
2.1.1. Intracellular generation of ROS
ROS refers to a group of molecules derived from oxygen, which, due to their oxygen content or the presence of unpaired electrons, display high reactivity towards a large array of biomolecules. Among ROS, the free radical forms, such as the superoxide anion radical, the hydrogen hydroxyl radical, or the peroxyl radical, are more reactive than the corresponding reduced forms, hydrogen peroxide or organic hydroperoxide. In addition to ROS, other radical species such as reactive nitrogen species (RNS), reactive sulfur species (RSS), reactive carbonyl species (RCS), and reactive selenium species can be generated (Sies et al., 2017). The production of ROS and RNS can be enzymatic or nonenzymatic (Dhawan, 2014). Moreover, in the context of bioprocesses, reactive species can be generated intracellularly due to the cell metabolism, or extracellularly in the cell culture media due to deterioration of media components.
The intracellular ROS mainly originate from the respiratory chain in the mitochondria (Turrens, Alexandre, & Lehninger, 1985). Superoxide anions produced in the mitochondrial matrix will be dismuted into H2O2 by the manganese‐dependent superoxide dismutase (MnSOD), while those generated in the intramembrane space are converted by the copper‐zinc superoxide dismutase (CuZnSOD; Fukai & Ushio‐Fukaimz, 2011; Turrens, 2003). Superoxide radicals can also form peroxynitrite after reaction with nitric oxide originating from arginine degradation by nitric oxide synthases in the mitochondria and in the peroxisomes (Ghafourifar & Cadenas, 2005; Schrader & Fahimi, 2006). In addition, the superoxide anion can react with H2O2 through the Haber‐Weiss reaction, to produce the hydroxyl radical OH• which is considered the most deleterious ROS (Nordberg & Arner, 2001). Indeed, due to its high reactivity, OH• nonpreferentially oxidizes amino acids, DNA, or lipids. The hydroxyl radical can also be generated in the presence of transition metal (Fe2+ or Cu2+) by the cleavage of H2O2 through the Fenton reaction. In contrast to the superoxide anion, H2O2 has less reactivity and can diffuse through membranes (D'Autreaux & Toledano, 2007). Due to its diffusion properties, H2O2 has an important role in signaling. For example, H2O2 modulates the activity of transcription factors such as activator protein‐1 (AP‐1) involved in cell proliferation, apoptosis, survival, and cell differentiation. It also regulates the activity of the sterol regulatory element‐binding protein 1 (SREBP1) involved in cholesterol, lipids, and fatty acids synthesis (Marinho, Real, Cyrne, Soares, & Antunes, 2014).
In addition to the mitochondria, ROS can also be produced in other locations within the cell. β‐Oxidation of fatty acids takes place in the mitochondria and in peroxisomes, which contain many types of oxidases such as acyl‐CoA oxidase and xanthine oxidase (Eaton, Bartlett, & Pourfarzam, 1996; Schrader & Fahimi, 2006). In consequence, ROS generation also occurs in peroxisomes as a product of these enzymatic reactions. Another location for ROS generation is the ER. The lumen of the ER is an oxidative environment that promotes the biochemical reactions required for protein folding (Tu & Weissman, 2004). Formation of disulfide bonds through the ERO1/PDI pathway, releases H2O2 in the ER lumen. Moreover, H2O2 is generated by NADPH oxidase 4 (NOX4) and by the microsomal monooxygenase system (MNO) in the ER membrane (Brandes, Weissmann, & Schröder, 2014; Zangar, Davydov, & Verma, 2004; Zeeshan, Lee, Kim, & Chae, 2016). ROS production in the ER can also activate the release of Ca2+ in the cytosol. In turn, this signaling cascade leads to a release of cytochrome c from the mitochondria and initiates apoptosis (Cao & Kaufman, 2014). This cascade of events shows the relationship between oxidative stress, ER stress, and apoptosis (Malhotra & Kaufman, 2007). Moreover, the recombinant protein produced can itself be a source of reactive species. A large protein with a large number of cysteine will have a higher propensity to accumulate in the ER due to a large number of disulfide bonds to be formed. The enzymes responsible for disulfide bond formation, ERO1, and PDI, will, therefore, generate more H2O2. This phenomenon was illustrated in yeast through the comparison of the expression of α amylase, a large protein with numerous cysteines but only four disulfide bonds, and a human insulin precursor, a small protein with three disulfide bonds (Tyo, Liu, Petranovic, & Nielsen, 2012). Because of the presence of many cysteines in the sequence of α amylase, the probability of incorrect disulfide bond formation is increased during protein folding. Along these lines, Tyo et al. (2012) demonstrated that more oxidative and osmotic stress transcription factors were activated upon α amylase expression than human insulin precursor expression. Incorrect disulfide bonds can be reduced by PDI thanks to its isomerase activity (Wilkinson & Gilbert, 2004). However, if the folding rate is too slow, misfolded proteins will accumulate in the ER leading to the unfolded protein response which, in turn, can promote oxidative stress (Malhotra & Kaufman, 2007).
2.1.2. Cell culture media‐derived ROS
In addition to intracellular sources, medium components can react with oxygen, light, and other components to generate ROS (Grzelak, Rychlik, & Bartosz, 2000). For example, riboflavin is light sensitive and can generate ROS by photooxidation. Grzelak, Rychlik, and Bartosz (2000) showed in their study that the riboflavin‐dependent ROS production is amplified in the presence of tryptophan, tyrosine, pyridoxine, and folic acid in medium. Folic acid is also light‐sensitive in presence of oxygen and can be degraded to 6‐formylpterin and pterin‐6‐carboxylic acid, which, in turn, generate ROS (Gazzali et al., 2016; Juzeniene, Grigalavicius, Ma, & Juraleviciute, 2016). Another vitamin, which is easily oxidized in cell culture media and generates H2O2, is ascorbic acid (Long & Halliwell, 2009). More generally, thiol compounds present un the media can also be a source of H2O2 following autoxidation reactions or interaction with other media components, such as metal ions (Grzelak et al., 2001; Hua Long & Halliwell, 2001). For example, reduced glutathione (GSH) can form complexes with copper in a reducing environment which leads to superoxide generation (Speisky et al., 2009). Glucose also reacts with oxygen and metal ions and produces ROS or reactive degradation products like methylglyoxal (Chumsae et al., 2013).
ROS and byproducts generated by media components oxidation can lead to degradation of the overexpressed protein and an increase in product microheterogeneity. For example, a recent study has demonstrated that ROS were generated in the medium at a high iron concentration (Xu et al., 2018). By lowering the iron concentration, they managed to decrease both free radicals generation and mAb microheterogeneity, especially tryptophan oxidation responsible for mAb coloration levels (Xu et al., 2014). However, as lowering iron led to a decrease of product titer, an adaptation of the process through cell line adaptation and further basal medium modifications were required to restore the original titer.
2.1.3. Antioxidant response in mammalian cells
To maintain reactive species at nondeleterious levels, mammalian cells have developed an array of antioxidant systems, including different detoxification enzymes and signaling pathways. One of the major players of this defense is GSH (Figure 1). GSH is a tripeptide (γ‐l‐glutamyl‐l‐cysteinyl glycine) which can be oxidized to form glutathione disulfide (GSSG). GSH acts as a ROS scavenger and as the substrate of detoxification enzymes such as glutathione peroxidases (GPXs) and glutathione‐S‐transferases (GSTs) (Espinosa‐Diez et al., 2015).
Figure 1.
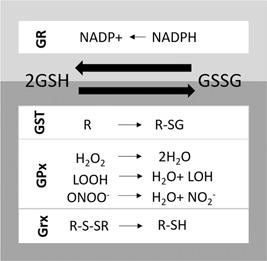
Major enzymatic reactions involving glutathione. Conjugation reactions with an electrophilic donor are catalyzed by glutathione‐S‐transferases (GST). Reduction of the substrate such as H2O2, lipid peroxide (LOOH), and peroxynitrite (ONOO−) are catalyzed by glutathione peroxidases (GPx). Disulfide bond reduction can be catalyzed by glutaredoxins (Grx). The reduction of glutathione disulfide (GSSG) to glutathione is catalyzed by the glutathione reductase (GR) and required NADPH
Eight GPXs have been identified so far in mammalian cells: GPX1–4 are selenoproteins, while GPX5–8 use cysteines to carry out their enzymatic activity (Brigelius‐Flohé & Maiorino, 2013). However, some of them, such as GPX5, are tissue‐specific and not present in CHO cells. These enzymes use GSH as a reducing substrate, but it has been shown that some of them can use other reducing substrates like thioredoxin or PDI. Their major substrate is H2O2, but they can also detoxify the peroxynitrite and oxidized chain of lipids (LOOH). Other enzymes such as catalase and peroxiredoxins also detoxify H2O2 (Nordberg & Arner, 2001).
Glutathione‐S‐transferases conjugate GSH with reactive electrophilic compounds to facilitate their removal from the cell through specific transporters (Salinas & Wong, 1999). GSSG is returned to its monomeric active form (GSH) through the activity of glutathione reductase and cofactor NADPH.
Two other proteins involved in antioxidant defense are glutaredoxins (Grx) and thioredoxins, which catalyze disulfide bond reductions. Thanks to this function, these proteins are capable of reversing posttranslational modifications caused by ROS such as sulfenylation, glutathionylation, and cysteinylation (Hanschmann, Godoy, Berndt, Hudemann, & Lillig, 2013). Thioredoxins also transfer electrons to peroxiredoxins (Prxs), which are H2O2 scavengers, and to methionine sulfoxide reductases which reverse methionine oxidation (Lu & Holmgren, 2014). Moreover, thioredoxins are involved in the modulation of some transcription factors activity such as nuclear factor‐κB (NF‐κB) and AP‐1 (Schenk, Klein, Erdbrugger, Droge, & Schulze‐Osthoff, 1994). Oxidized thioredoxins are reduced by thioredoxin reductases using NADPH as an electron donor while oxidized glutaredoxins are reduced by glutathione.
Another important aspect of cellular antioxidant response is the number of signaling pathways involved. In the presence of ROS, nuclear factor erythroid 2 (NRF2) dissociates from Kelch‐like ECH‐associated protein 1 (Keap 1). Free NRF2 will then activate the antioxidant response element (ARE) localized in genes coding for detoxifying enzymes such as glutathione‐S‐transferase and heme oxygenase‐1, which degrades free heme (Gong et al., 2001), but also enzymes involved in the biosynthesis of GSH such as the catalytic and regulatory subunits of the glutamate–cysteine ligase (GCL; Nguyen, Sherratt, & Pickett, 2003). Another example of a transcription factor involved in the antioxidant response is NF‐κB. The activation of this transcription factors has been shown to correlate with the expression of antioxidant enzymes, such as superoxide dismutases (MnSOD and ZnCuSOD), GST‐pi, and GPX‐1, in different tissues (Morgan & Liu, 2011). However, it has been reported that NF‐κB is also involved in the transcription of pro‐oxidant enzymes, such as xanthine oxidase/dehydrogenase and inducible nitric oxide synthase. The sterol regulatory element‐binding proteins 2 (SREBP‐2) involved in lipid homeostasis, also contributes to the antioxidant response through the regulation of paraoxonase‐2 expression in CHO cells (Gu, Lee, & Shen, 2014). This enzyme is a hydrolase with a wide range of substrates, that has been reported to exert antioxidant activity in several tissues, including brain, ovarian carcinoma, and intestinal epithelial cells (Devarajan et al., 2018; Giordano, Cole, Furlong, & Costa, 2011; Précourt et al., 2012).
In the context of biopharmaceutical production, several cellular adaptations to increased ROS generation and oxidative stress have been reported. Templeton et al. described a correlation between the production of mAb and increased fluxes into the TCA cycle during fed‐batch cell culture. Their metabolic flux analysis also showed that the ratio of NADPH/NADP+, which is high at the beginning of the process, decreased considerably during the late exponential and stationary phases (Templeton et al., 2013). Moreover, they observed the same effect on the intracellular GSH to GSSG ratio. As NADPH is required to recycle GSSG back to GSH, they suggested that the increased TCA cycle activity, which positively impacted mAb production, was a spontaneous cellular reaction to oxidative stress. Similarly, a rise in activity of the oxidative pentose phosphate pathway could also be a way for the cell to produce NADPH and strengthen its antioxidant defenses. The NADPH/NADP+ ratio is an indicator of the redox state of the cell and is often used as an oxidative stress marker (Blacker & Duchen, 2016). However, there are cellular pathways that do not involve glutathione regeneration and use NADPH as a cofactor, one example is lipid biosynthesis (C. A. Lewis et al., 2014). Although oxidative stress will tend to decrease the intracellular NADPH/NADP+ ratio, it can also be impacted by other metabolic adaptations.
3. MEDIUM DEVELOPMENT APPROACHES TO LIMIT OXIDATIVE STRESS
Despite the array of internal antioxidant defense mechanisms, cultivated cells may benefit from extracellular antioxidant activity to reduce production‐derived oxidative stress. Antioxidant components are extensively used in chemically defined serum‐free media to counterbalance the loss of serum and hydrolysate antioxidant properties (Saito et al., 2003). This section summarizes classes of compounds bearing antioxidant properties that were assessed for the culture of CHO cells (see Table 1).
Table 1.
Antioxidant compounds used in CHO cells to reduce oxidative stress
| Component | Way of action described in literature | Effect observed | References |
|---|---|---|---|
| Thiol compounds | |||
| Glutathione |
ROS scavenger Substrate for ROS scanvenger enzymes Maintain high mitochondria potential |
Decrease cell death Increase titer |
Yun et al. (2001, 2003) |
|
N‐acetylcysteine and N‐acetylcysteine amine |
ROS scavenger Increase of intracellular glutathione pool |
Decrease cell death Increase titer Impact on sialylation |
Chang et al. (1999); Ercal, Treeratphan, Lutz, Hammond, and Matthews (1996); Issels, Nagele, Eckert, and Wllmanns (1988); Oh et al. (2005); Tanel and Averill‐Bates (2007); Wu et al. (2008) |
| S‐sulfocysteine |
Stabilized cysteine Up regulation of SODs transcription Increase of intracellular glutathione pool |
Decrease cell death Increase titer Decrease recombinant protein fragments level Decrease recombinant protein trisulfides level |
Hecklau et al. (2016); Seibel et al. (2017) |
| Thiazolidine |
Stabilization of the cell culture media Up regulation of SODs transcription Increase of intracellular glutathione pool |
Decrease cell death Increase titer |
Kuschelewski et al. (2017) |
| Taurine and it precursors |
Increase of intracellular glutathione pool Reduce lipid peroxidation Reduce catalase and erythrocyte G6PD activity Reduce mitochondrial superoxide generation |
Decrease cell death | Aruoma et al. (1988); Gurer et al. (2001); Jong, Azuma, and Schaffer (2012) |
| Lipoic acid |
ROS scavenger Iron and copper chelator |
Decrease cell death | Gurer et al. (1999); Maharjan et al. (2016) |
| α Ketoacids | |||
| Pyruvate | ROS scanvenger |
Decrease cell death Decrease recombinant protein trisulfides level |
Andrae et al. (1985); Kshirsagar, McElearney, Gilbert, Sinacore, and Ryll (2012); Long and Halliwell (2009) |
| α‐Ketoglutarate | ROS scanvenger Iron chelator | Decrease cell death | Andrae et al. (1985); Bayliak, Lylyk, Vytvytska, and Lushchak (2016) |
| Vitamins | |||
| Ascorbic acid and derivative | ROS scavenger |
Decrease cell death Increase titer |
Yun et al. (2001) |
| α‐Tocopherol | Lipid peroxyl radical scavenger | Decrease cell death | Chepda, Cadau, Chamson, Alexandre, and Frey (1999); Murati et al. (2017) |
| Trace elements | |||
| Selenium/selenocysteine |
Cofactor of antioxidant enzymes Regulate expression of glutathione peroxidase |
Decrease cell death | Aykin‐Burns an Ercal (2006); Gasser, Mulsant, and Gillois (1985); Hamilton and Ham (1977); Weiss and Sunde (1997) |
| Chelators | |||
| Defroxamine | Iron chelator | Decrease cell death | Yun et al. (2003) |
| Aurintricarboxylic acid | Iron chelator | Decrease cell death | Tabuchi, Sugiyama, Tanaka, and Tainaka (2010) |
| Polyamines | Iron chelator |
Decrease cell death Increase titer |
Gaboriau et al. (2004); Lovaas (1997) |
| Phenolic compounds | |||
| Butylated hydroxyanisole |
ROS scavenger Iron chelator |
Decrease cell death Increase titer |
Malhotra et al. (2008) |
| Baicalein | ROS scavenger | Increase titer | Ha et al. (2017); Hamada, Hiramatsu, Edamatsu, and Mori (1993) |
| Epicatechin gallate | ROS scavenger | Decrease recombinant protein charge variants level | Hossler et al. (2015) |
| Rutin | ROS scavenger | Decrease recombinant protein charge variants level | Hossler et al. (2015) |
Abbreviations: CHO, Chinese hamster ovary; ROS, reactive oxygen species; SOD, sodium oxide dismutase.
3.1. Thiol compounds
The majority of cellular antioxidant defense systems contain a thiol moiety. The mode of action of these compounds is broad: (a) They can act as a substrate for detoxifying enzymes (e.g., GSH), (b) they can directly scavenge ROS (e.g. N‐acetylcysteine [NAC]), or (c) they can be involved in metal chelation (e.g., lipoic acid; Biewenga, Haenen, & Bast, 1997; Deneke, 2000; Sun, 2010). In addition, they can participate in the thiol/disulfide intra or extracellular redox balance and/or in cell signaling (Deneke, 2000; Go & Jones, 2013). Studies carried out by Yun and colleagues indicate that the addition of GSH in serum‐free media reduces cell death and increases tissue plasminogen activator concentration (Yun, Takagi, & Yoshida, 2001). Besides GSH, a large number of thiol compounds with antioxidant properties can be supplemented to cell culture media to support cell defenses against oxidative stress. For instance, NAC is another a thiol antioxidant that is commonly used in CHO cell culture to prevent apoptosis induced by oxidative stress (Lord‐Fontaine & Averill, 1999; Wu et al., 2008; Xue et al., 2015). Some studies have demonstrated that NAC can improve the production of recombinant human interferon‐β‐1a and erythropoietin, especially in combination with a sodium butyrate treatment (Chang, Kim, & Kim, 1999; Oh et al., 2005). An alternative to NAC is N‐acetylcysteine amine, which has been shown to be less cytotoxic than NAC (Wu et al., 2008). Both compounds act as ROS scavengers and are precursors of cysteine and GSH that can contribute to increasing intracellular GSH content. Through direct interaction with thiol groups, NAC is capable of modulating the activity of signaling molecules, such as transcription factor NF‐κB and c‐Jun N‐terminal kinase (JNK). However, this activity has only be demonstrated in specific cell type and further investigation will be required to gain a better understanding of the range of activities and antioxidant potential of NAC (Zafarullah, Li, Sylvester, & Ahmad, 2003).
The use of S‐sulfocysteine as a replacement for cysteine in cell culture media has recently been shown to improve recombinant protein production by increasing the GSH pool in CHO cells (Hecklau et al., 2016). The use of S‐sulfocysteine in feed solutions was hypothesized to reduce the production of hydrogen sulfide by protecting thiol groups against oxidation. In an additional study, the same lab reported that this compound could also lower antibody low molecular weight species and trisulfide bond levels by decreasing free hydrogen sulfide in the medium (Seibel et al., 2017).
Similarly, supplementation of the culture with thiazolidine in feed solutions can help stabilize redox‐sensitive vitamins and amino acids, and decrease their ROS content in the feed. These molecules are obtained by condensation of cysteine with pyruvate or α ketoacids. In 1985, the antioxidant properties of pyruvate and α ketoacids, such as α ‐ketoglutarate, were highlighted in CHO cells exposed to H2O2 (Andrae, Singh, & Ziegler‐Skylakakis, 1985). Pyruvate has also been shown to scavenge H2O2 present in cell culture media, thereby ensuring its stability (Long & Halliwell, 2009; McCoy, Costa, & Morris, 2015). The addition of thiazolidine molecules during cell culture led to a decrease of cell death at the end of the production process and an increase of a recombinant mAb titer (Kuschelewski, Schnellbaecher, Pering, Wehsling, & Zimmer, 2017). Interestingly, the decrease of oxidative stress is observed upon thiazolidine supplementation appears to occur through an increase of intracellular GSH levels and expression of MnSOD and CuZnSOD.
Sulfur‐containing amino acid, taurine, has also been reported to reduce cell death by increasing the GSH content, reducing lipid peroxidation, and reducing catalase and the erythrocyte G6PD activity (Gurer, Ozgunes, Saygin, & Ercal, 2001). However, it has a low ROS scavenging activity compared with its precursors hypotaurine, s‐carboxymethylcysteine, cysteamine, and cysteine sulphinic acid (Aruoma, Halliwell, Hoey, & Butler, 1988).
Finally, lipoic acid, a fatty acid derivative with antioxidant properties, is another thiol compound tested in the context of CHO cell cultivation. It was used in an early chemically defined medium such as Ham's F12 developed for CHO cells (Hamilton & Ham, 1977). Lipoic acid is known to reduce lipid peroxidation, scavenge ROS, and chelate iron and copper, thereby contributing to a reduction of cell death (Gurer, Ozgunes, Oztezcan, & Ercal, 1999; Maharjan, Sakai, & Hoseki, 2016). However, due to its insolubility in aqueous solutions, other thiol‐containing molecules are generally preferred for medium supplementation.
3.2. Vitamins
Although the main function of vitamins is to act as enzyme cofactors, antioxidant properties have also been highlighted for some of these molecules. α‐Tocopherol (vitamin E), is well known for its ROS scavenging properties and its ability to counteract lipids peroxidation. However, despite some attempts, this compound is rarely included in cell culture media composition due to its poor solubility in water (Halliwell, 2014). Ascorbic acid (vitamin C), a cofactor of enzymes involved in acetyl CoA metabolism, displays high reactivity towards oxygen, which both confers it with antioxidant potential, but can also be detrimental to the culture if it is not stabilized by other molecules, such as magnesium, selenium, or GSH (Dolińska et al., 2012; Touitou, Alkabes, Memoli, & Alhaique, 1996). Vitamin C protects cells from lipid peroxidation, through the regeneration of oxidized vitamin E. However, vitamin C supplementation during the culture does fail to positively impact CHO cell growth as a result of its high instability in culture media (Kurano, Kurano, Leist, & Fiechter, 1990). The development of more stable derivatives of these molecules was considered to address these solubility and stability issues (Hata & Senoo, 1989). For example, the use of stabilized derivatives like l‐ascorbic 2‐phosphate can help to decrease cell death and improve recombinant protein titer (Yun et al., 2001). However, the use of vitamin derivatives comes with a risk of altered antioxidant potential that has to be carefully considered.
3.3. Trace elements and chelators
Trace elements are essential for cell function and survival, as they act as cofactors for key enzymes and play a major role in cell signaling and metabolism (Arigony et al., 2013; Hamilton & Ham, 1977). Metal ions may play a pro‐oxidant or antioxidant role depending on their concentration. For instance, iron and copper are key players in ROS generation as they are involved in Fenton and Fenton‐like reactions, respectively (Fenton, 1894; Pham, Xing, Miller, & Waite, 2013). Another element, selenium, can, at relatively low concentrations, activate cellular antioxidant defense as it is a cofactor of detoxifying enzymes (Brigelius‐Flohé & Maiorino, 2013; Fukai & Ushio‐Fukaimz, 2011). Selenium has been reported to activate antioxidant defense as it is a cofactor of some GSH peroxidases and thioredoxin reductase (Powis et al., 1997; Weiss & Sunde, 1997). In addition, it inhibits H2O2‐induced TRPM2 channels impacting the Ca2+ influx (Naziroglu et al., 2013). However, it has to be used carefully as, at high concentrations, selenium can display pro‐oxidant properties and generate ROS (Lee & Jeong, 2012). Concentrations of selenium below 1 µM have been shown to be safe for CHO‐K1 cells and are often included in cell culture medium (Zhang, Robinson, & Salmon, 2006; Zwolak, 2015). However, toxic concentrations of selenium have to be determined for each cell line. Moreover, the medium composition has to be considered when supplementing cultures with selenium as this compound can interact with other trace elements. Finally, selenocysteine supplementation has also been demonstrated to decrease cell death of CHO cells exposed to lead‐induced oxidative stress (Aykin‐Burns & Ercal, 2006).
To avoid the negative effect of trace element addition and facilitate their uptake by the cells, chelator molecules are usually used. Indeed, the chelation of reactive metal ions, like Fe2+ or Cu2+, can help decrease oxidative stress. Addition of components like transferrin, polyamines, and ferric citrate maintains iron in an inert state and promotes its transport into the cells, thereby improving cell viability and recombinant protein production (Bai et al., 2011; Gaboriau et al., 2004; Lovaas, 1997; Wang & Pantopoulos, 2011). Defroxamine and aurintricarboxylic acid in combination with GSH has also been shown to improve CHO cell viability during recombinant tissue plasminogen activator production (Yun, Takagi, & Yoshida, 2003).
3.4. (Poly)‐phenolic compounds
Phenolic compounds are well known for their antioxidant properties and their use in the treatment of diseases and aging has been the object of many studies (Mao, Gu, Chen, Yu, & He, 2017). The effects of (poly)‐phenol derivative addition in CHO cell cultures have been assessed in both academic and industrial research. Epigallocatechin gallate and rutin were used to decrease acidic variants of a mAb produced by CHO cells (Hossler et al., 2015). In addition, baicalein has been shown to decrease cell growth and increase recombinant mAb production (Ha, Hansen, Kol, Kildegaard, & Lee, 2017). Baicalein can decrease ROS levels and inhibit the activity of transcription factors involved in the ER stress response by interacting with immunoglobulin protein (BiP) and CHOP. Furthermore, the addition of butylated hydroxyanisole (BHA) has been reported to decrease apoptosis and reduce the accumulation of blood coagulation factor FVIII in the ER after the treatment of CHO cells with sodium butyrate (Malhotra et al., 2008).
The addition of antioxidant molecules to cell culture media with the aim of reducing oxidative stress is an easily implemented and relatively successful approach used in the industrial sector. However, such supplementations are to be investigated on a case‐by‐case basis, considering the different modes of action of the potential candidates, the chemistry of culture media, and the selected cell clones or cell lines used in the culture. In particular, potential interactions between the antioxidant supplements and components of the medium is a critical aspect of the optimization of the supplementation process. As they can both positively or negatively impact the culture depending on cases, it is important for such interactions them to be fully characterized, a fact that is often complicated by the nondisclosure of media formulations used in commercial processes. Due to the variability factors associated with supplementation, the use of empirical statistical analysis and high throughput assays is recommended. In bioproduction, it is generally assumed that the cell lines have different historical backgrounds (origin, clone, selection procedure) and, as a direct consequence, display differences in metabolism and sensitivity to oxidative stress (Reinhart et al., 2018). A better understanding of these differences can be obtained through the use of omics techniques and can help experimenters highlight reactions leading to oxidative stress and adapt antioxidant supplementation to specific cell lines.
4. USE OF CELL ENGINEERING TO REDUCE OXIDATIVE STRESS
Thanks to recent technical advances in genetics, cell engineering can be used to upregulate or downregulate pathways of interest. Considerable effort has been put into developing strategies to reduce apoptosis, with several efforts resulting in increased viability and, indirectly, improved process productivity (Meents, Enenkel, Eppenberger, Werner, & Fussenegger, 2002). Notably, the overexpression of bcl‐2 or bcl‐xL has been successfully used to activate antiapoptotic pathways in CHO cells (Tey, Singh, Piredda, Piacentini, & Al‐Rubeai, 2000; Zustiak, Jose, Xie, Zhu, & Betenbaugh, 2014). A similar approach can be considered to relieve oxidative stress by increasing cellular defense or decreasing ROS‐generating cellular activities. As redox reactions are the basis of cellular energy production and protein folding processes, any attempts to reduce these fundamental reactions may negatively impact the production process. For this reason, the strategy aiming to increase the antioxidant defenses is generally seen as preferable for the purpose of recombinant protein production.
The interest in genetic manipulation of the GSH biosynthesis pathway is not new. Tamura, McMicken, Smith, and Hansen (1996) overexpressed human glutathione reductase in CHO cells and were able to increase their resistance to oxidative stress. Likewise, in 2002, the overexpression of the GCL catalytic subunit was demonstrated to increase the resistance to lead‐induced oxidative stress in CHO cells (C. J. Fernandes et al., 2002). More recently, in the bioprocess field, a high intracellular concentration of GSH in high producers was observed in a metabolome comparison of CHO cells (Chong et al., 2012). These results were then confirmed by a proteome comparison of high and low producer cultivated in bioreactors using a batch process. This study highlighted an upregulation of genes related to the GSH pathway (Orellana et al., 2015). Similarly, GSH‐related amino acid transporters have been reported to have higher expression during stationary phase, when the specific productivity is higher (Kyriakopoulos, Polizzi, & Kontoravdi, 2013). Moreover, it has been shown that GSH plays a role in the maintenance of the redox status within CHO cell ER by preventing the formation of nonnative disulfide bonds (Chakravarthi & Bulleid, 2004). In this context, several studies have examined the biosynthesis and turnover of GSH in CHO cell factories. First, the overexpression of the regulatory subunit of GCL in CHO‐K1 cells was observed to promote clone productivity. Interestingly, this study also showed that the overexpression of the GCL catalytic subunit does not impact recombinant protein production; suggesting that the intracellular GSH content is not the direct cause of higher productivity (Orellana, Marcellin, Gray, & Nielsen, 2017). In another study, it was demonstrated that partial inhibition of GCL by methionine sulfoximine (MSX) or buthionine sulfoximine (BSO) in GS–CHOK1SV cells leads to an increase in productivity (Feary, Racher, Young, & Smales, 2017). The authors hypothesized that partial inhibition of GSH synthesis helps to oxidize the ER environment. This modulation of the redox status of the ER increases the oxidized form of Ero1, thereby promoting activation of PDI enzymes and improving protein folding. Moreover, they suggest that the partial inhibition of GSH synthesis can be a selection method for high producer clones. Although there are still many unknowns concerning the involvement of the GSH synthesis pathway in recombinant protein production in CHO cells, and a certain degree of contradictory results, the aforementioned studies illustrate this pathway's potential.
The expression of other antioxidant enzymes or transcription factors involved in oxidative stress has also been a target of cell engineering approaches. Overexpression of human peroxiredoxin 5 and human MnSOD in CHO cells was reported to lead to a decrease in cell death caused by oxidative stress (Banmeyer et al., 2004; Warner, Papes, Heile, Spitz, & Wispe, 1993). However, it is worth noting that these studies were performed in CHO cells with the aim of mimicking cancer cell metabolism and growth, not in the context of heterologous protein expression. With regard to transcription factors, ATF4, which is activated during the UPR response and in oxidative stress conditions, was shown to protect fibroblasts against oxidative stress (Harding et al., 2003). Moreover, overexpression of ATF4 or GADD34, an activator of ATF4, were both reported to lead to an increase in titer in several CHO cell lines (Haredy et al., 2013; Ohya et al., 2008; Omasa et al., 2008).
More recently, miRNA has been used to specifically target oxidative stress in CHO cells. In other cell lines, several miRNAs, including miRNA‐145, miRNA‐451 in erythroid cells and miR‐1 and miR‐133 in rat cells, have been shown to modulate oxidative (Jadhav et al., 2013). Although the use of miRNA in CHO cells has mostly been focused on the modulation of ER stress or, more generally, apoptosis, the potential of this approach for preventing oxidative stress is garnering more interest. Following depletion of miR‐23 using a microRNA sponge, Kelly et al. (2015) observed an increase in production of the recombinant protein. In parallel, mitochondrial activity was boosted and the production of antioxidative proteins Thioredoxin 1 and peroxiredoxin 6 was increased.
So far, due to the long lead times and heavy workloads associated with cell engineering, a limited number of studies have been published on improving the CHO cells chassis by genetic engineering to alleviate oxidative stress. However, the engineering of cell line has unquestionably proven its value as supported by the studies reporting its potential to reduced apoptosis and ER stress (Borth, Mattanovich, Kunert, & Katinger, 2005; Mohan & Lee, 2009; Pieper, Strotbek, Wenger, Olayioye, & Hausser, 2017; Prashad & Mehra, 2015). Oxidative stress cell engineering is complex due to the number of pathways involved and their interconnections of these pathways with other cellular functions. Despite this complexity and the long process needed to generate an appropriate clone, the work published so far is promising with regard to the use of cell engineering to alleviate oxidative stress in bioprocesses.
5. DISCUSSION
With the development of high biomass and high productivity CHO bioprocesses, scientists faced a new hurdle in the increase of cellular stresses resulting from boosted metabolism, higher resource demands (e.g., for dissolved oxygen), and higher waste accumulation. In this context, oxidative stress, which occurs when there is an imbalance between oxidant molecules accumulation and antioxidant response, can become an issue due to its detrimental impact on cell viability, productivity, and the integrity of the recombinant protein being produced. Dissolved oxygen, can be controlled to limit oxidative stress. However, maintaining dissolved oxygen homogeneity in the bioreactor is complicated to study and requires particular attention during scale up as large scale bioreactors often present different geometries to those used during process development. High dO2 usually has to be maintained in the bioreactor to avoid an oxygen dead zone even if simulation tools are now available to optimize process parameters depending on bioreactor size and dimensions (Dhanasekharan, Sanyal, Jain, & Haidari, 2005; Koynov, Tryggvason, & Khinast, 2007).
In light of the challenges encountered in trying to control all aspects of large scale bioreactor processes, the approaches consisting in making the cells and the culture process more resistant to potential sources of oxidative stress have to be considered. Although, increased energy metabolism and a consequent activity of the folding/secretion machinery have been observed or modeled in high producer cell lines, it remains possible to limit ROS byproducts of these cellular processes (Borth et al., 2005; Ghorbaniaghdam, Chen, Henry, & Jolicoeur, 2014; Mathias et al., 2018; Prashad & Mehra, 2015; Templeton et al., 2013). Two of the main strategies considered to date consist in (a) the supplementation of the cell culture media with antioxidant molecules, and (b) the engineering of metabolic pathways associated with oxidative stress. Supplementation of cell culture media with antioxidant compounds appears to be the easiest and fastest solution from an industrial point‐of‐view. It requires a few changes to the process and can be implemented at later stages of process development. However, due to the possible interactions of antioxidant molecules with other cell culture media components and variability between CHO cell lines, considerable time and effort have to be put into component screening to identify the best match for the combination of the cell line, media, and process. For this reason, cell engineering appears as an interesting alternative to supplementation. However, this approach requires early implementation and evaluation during process development, and a change in the cell line will be more complicated to put in place once the first clinical phases have been carried out for a given recombinant protein production process. This strategy remains to be extensively studied in CHO cells as cell engineering research has thus far mainly focused on the modulation of secretory pathways, apoptosis, and the unfolded protein response pathways. However, compelling evidence from microbial strains, such as Escherichia coli, Saccharomyces cerevisiae, and Yarrowia lipolytica, highlight the potential of this approach for controlling oxidative stress and promoting cell growth in cultures (Basak & Jiang, 2012; Davy, Kildegaard, & Andersen, 2017; Ukibe, Hashida, Yoshida, & Takagi, 2009; Xu, Qiao, & Stephanopoulos, 2017).
Most of the studies cited in this review were performed on fed‐batch processes where the accumulation of waste and media components may be additional sources of oxidative stress. As described above, the supplementation of antioxidants can be used to scavenge ROS produced. However, removal or reduction of unstable components from the cell culture medium, such as ascorbic acid, is an alternative way to reduce ROS (Halliwell, 2014). The use of cell engineering to reduce byproduct generation is another option. For example, it has recently been shown that phenyl lactate production could be reduced by cell engineering of the phenylalanine‐tyrosine catabolic pathway (Mulukutla et al., 2019). Finally, continuous processes are another potential solution to issues arising from the accumulation of waste in the medium. This type of cultivation had gained in popularity in the industry as an efficient way both increase process yields and better control product quality, however, the ability of this approach to reduce oxidative stress remains to be investigated (Kunert & Reinhart, 2016).
To date, both supplementation and cell engineering strategies have focused on decreasing cell death and increasing productivity, with little consideration going towards product quality. Recently, researchers have started to explore the impact of heightened antioxidant activity on product microheterogeneity. However, the possibility of reducing product microheterogeneity upon oxidative stress engineering in CHO cells remains undocumented.
While media development is likely to remain the dominant strategy for the time being, oxidative stress engineering has shown promising results and offers a credible alternative to support recombinant protein production in CHO cells. Moreover, new tools, such as genome‐scale models, might provide insight into limitations and potential improvements to these strategies, and may open the door to the use of a combined approach, using both antioxidant supplementation and cell engineering, to control ROS production and oxidative stress, and simultaneously increase productivity and maintain product quality.
CONFLICT OF INTERESTS
V. Chevallier and L. Malphettes are employees of UCB Nordic A/S and UCB Pharma S.A., both of which carry out production activities in the area of interest. Mikael R Andersen does not have any conflict of interest.
ACKNOWLEDGMENTS
The authors thank Gregory Mathy for his valuable comments that helped to improve the manuscript. This work was supported by Innovationsfonden (5189‐00037B) and UCB Nordic A/S.
Chevallier V, Andersen MR, Malphettes L. Oxidative stress‐alleviating strategies to improve recombinant protein production in CHO cells. Biotechnology and Bioengineering. 2020;117:1172–1186. 10.1002/bit.27247
REFERENCES
- Andrae, U. , Singh, J. , & Ziegler‐Skylakakis, K. (1985). Pyruvate and related alpha‐ketoacids protect mammalian cells in culture against hydrogen peroxide‐induced cytotoxicity. Toxicology Letters, 28, 28–98. 10.1016/0378-4274(85)90015-3 [DOI] [PubMed] [Google Scholar]
- Arigony, A. L. V. , de Oliveira, I. M. , Machado, M. , Bordin, D. L. , Bergter, L. , Prá, D. , & Pêgas Henriques, J. A. (2013). The influence of micronutrients in cell culture: A reflection on viability and genomic stability. BioMed Research International, 2013, 597282–22. 10.1155/2013/597282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruoma, O. I. , Halliwell, B. , Hoey, B. M. , & Butler, J. (1988). The antioxidant action of taurine, hypotaurine and their metabolic precursors. Biochemical Journal, 256(1), 251–255. 10.1042/bj2560251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aykin‐Burns, N. , & Ercal, N. (2006). Effects of selenocystine on lead‐exposed Chinese hamster ovary (CHO) and PC‐12 cells. Toxicology and Applied Pharmacology, 214(2), 136–143. 10.1016/j.taap.2005.12.002 [DOI] [PubMed] [Google Scholar]
- Bai, Y. , Wu, C. , Zhao, J. , Liu, Y. H. , Ding, W. , & Ling, W. L. (2011). Role of iron and sodium citrate in animal protein‐free CHO cell culture medium on cell growth and monoclonal antibody production. Biotechnology Progress, 27(1), 209–219. 10.1002/btpr.513 [DOI] [PubMed] [Google Scholar]
- Banmeyer, I. , Marchand, C. , Verhaeghe, C. , Vucic, B. , Rees, J. F. , & Knoops, B. (2004). Overexpression of human peroxiredoxin 5 in subcellular compartments of Chinese hamster ovary cells: Effects on cytotoxicity and DNA damage caused by peroxides. Free Radical Biology and Medicine, 36(1), 65–77. 10.1016/j.freeradbiomed.2003.10.019 [DOI] [PubMed] [Google Scholar]
- Basak, S. , & Jiang, R. (2012). Enhancing E. coli tolerance towards oxidative stress via engineering its global regulator cAMP receptor protein (CRP). PLOS One, 7(12), e51179 10.1371/journal.pone.0051179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliak, M. M. , Lylyk, M. P. , Vytvytska, O. M. , & Lushchak, V. I. (2016). Assessment of antioxidant properties of alpha‐keto acids in vitro and in vivo. European Food Research and Technology, 242(2), 179–188. 10.1007/s00217-015-2529-4 [DOI] [Google Scholar]
- Beyer, B. , Schuster, M. , Jungbauer, A. , & Lingg, N. (2018). Microheterogeneity of recombinant antibodies: Analytics and functional impact. Biotechnology Journal, 13(1), 1700476 10.1002/biot.201700476 [DOI] [PubMed] [Google Scholar]
- Biewenga, G. P. , Haenen, G. R. , & Bast, A. (1997). The pharmacology of the antioxidant lipoic acid. General Pharmacology, 29(3), 315–331. 10.1016/S0306-3623(96)00474-0 [DOI] [PubMed] [Google Scholar]
- Blacker, T. S. , & Duchen, M. R. (2016). Investigating mitochondrial redox state using NADH and NADPH autofluorescence. Free Radical Biology and Medicine, 100, 53–65. 10.1016/j.freeradbiomed.2016.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borth, N. , Mattanovich, D. , Kunert, R. , & Katinger, H. (2005). Effect of increased expression of protein disulfide isomerase and heavy chain binding protein on antibody secretion in a recombinant CHO cell line. Biotechnology Progress, 21(1), 106–111. 10.1021/bp0498241 [DOI] [PubMed] [Google Scholar]
- Brandes, R. P. , Weissmann, N. , & Schröder, K. (2014). Nox family NADPH oxidases: Molecular mechanisms of activation. Free Radical Biology and Medicine, 76(Suppl C), 208–226. 10.1016/j.freeradbiomed.2014.07.046 [DOI] [PubMed] [Google Scholar]
- Brigelius‐Flohé, R. , & Maiorino, M. (2013). Glutathione peroxidases. Biochimica et Biophysica Acta (BBA) ‐ General Subjects, 1830(5), 3289–3303. 10.1016/j.bbagen.2012.11.020 [DOI] [PubMed] [Google Scholar]
- Cai, Z. , & Yan, L. J. (2013). Protein oxidative modifications: Beneficial roles in disease and health. J Biochem Pharmacol Res, 1(1), 15–26. [PMC free article] [PubMed] [Google Scholar]
- Cao, S. S. , & Kaufman, R. J. (2014). Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxidants & Redox Signaling, 21(3), 396–413. 10.1089/ars.2014.5851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarthi, S. , & Bulleid, N. J. (2004). Glutathione is required to regulate the formation of native disulfide bonds within proteins entering the secretory pathway. Journal of Biological Chemistry, 279(38), 39872–39879. 10.1074/jbc.M406912200 [DOI] [PubMed] [Google Scholar]
- Chang, K. H. , Kim, K. S. , & Kim, J. H. (1999). N‐acetylcysteine increases the biosynthesis of recombinant EPO in apoptotic Chinese hamster ovary cells. Free Radical Research, 30(2), 85–91. 10.1080/10715769900300091 [DOI] [PubMed] [Google Scholar]
- Chepda, T. , Cadau, M. , Chamson, A. , Alexandre, C. , & Frey, J. (1999). Alpha‐tocopherol as a protective agent in cell culture. In Vitro Cellular & Developmental Biology‐Animal, 35(9), 491–492. 10.1007/s11626-999-0058-9 [DOI] [PubMed] [Google Scholar]
- Chong, W. P. , Thng, S. H. , Hiu, A. P. , Lee, D. Y. , Chan, E. C. , & Ho, Y. S. (2012). LC‐MS‐based metabolic characterization of high monoclonal antibody‐producing Chinese hamster ovary cells. Biotechnology and Bioengineering, 109(12), 3103–3111. 10.1002/bit.24580 [DOI] [PubMed] [Google Scholar]
- Chumsae, C. , Gifford, K. , Lian, W. , Liu, H. , Radziejewski, C. H. , & Zhou, Z. S. (2013). Arginine modifications by methylglyoxal: Discovery in a recombinant monoclonal antibody and contribution to acidic species. Analytical Chemistry, 85(23), 11401–11409. 10.1021/ac402384y [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Autreaux, B. , & Toledano, M. B. (2007). ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nature Reviews Molecular Cell Biology, 8(10), 813–824. 10.1038/nrm2256 [DOI] [PubMed] [Google Scholar]
- Davies, Michael J. (2016). Protein oxidation and peroxidation. Biochemical Journal, 473(Pt 7), 805–825. 10.1042/BJ20151227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davy, A. M. , Kildegaard, H. F. , & Andersen, M. R. (2017). Cell factory engineering. Cell Systems, 4(3), 262–275. 10.1016/j.cels.2017.02.010 [DOI] [PubMed] [Google Scholar]
- Deneke, S. M. (2000). Thiol‐based antioxidants. Current Topics in Cellular Regulation, 36, 151–180. 10.1016/S0070-2137(01)80007-8 [DOI] [PubMed] [Google Scholar]
- Devarajan, A. , Su, F. , Grijalva, V. , Yalamanchi, M. , Yalamanchi, A. , Gao, F. , … Reddy, S. T. (2018). Paraoxonase 2 overexpression inhibits tumor development in a mouse model of ovarian cancer. Cell Death & Disease, 9(3), 392 10.1038/s41419-018-0395-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanasekharan, K. M. , Sanyal, J. , Jain, A. , & Haidari, A. (2005). A generalized approach to model oxygen transfer in bioreactors using population balances and computational fluid dynamics. Chemical Engineering Science, 60(1), 213–218. 10.1016/j.ces.2004.07.118 [DOI] [Google Scholar]
- Dhawan, V. (2014). Reactive oxygen and nitrogen species: General considerations In Ganguly N. K., Jindal S. K., Biswal S., Barnes P. J. & Pawankar R. (Eds.), Studies on respiratory disorders (pp. 27–47). New York, NY: Springer New York. [Google Scholar]
- Dolińska, B. , Ostróżka‐Cieślik, A. , Caban, A. , Rimantas, K. , Leszczyńska, L. , & Ryszka, F. (2012). Influence of trace elements on stabilization of aqueous solutions of ascorbic acid. Biological Trace Element Research, 150(1‐3), 509–512. 10.1007/s12011-012-9524-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont, J. , Euwart, D. , Mei, B. , Estes, S. , & Kshirsagar, R. (2016). Human cell lines for biopharmaceutical manufacturing: History, status, and future perspectives. Critical Reviews in Biotechnology, 36(6), 1110–1122. 10.3109/07388551.2015.1084266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton, S. , Bartlett, K. , & Pourfarzam, M. (1996). Mammalian mitochondrial beta‐oxidation. Biochemical Journal, 320(Pt 2), 345–357. 10.1042/bj3200345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercal, N. , Treeratphan, P. , Lutz, P. , Hammond, T. C. , & Matthews, R. H. (1996). N‐acetylcysteine protects Chinese hamster ovary (CHO) cells from lead‐induced oxidative stress. Toxicology, 108(1‐2), 57–64. 10.1016/S0300-483X(95)03273-I [DOI] [PubMed] [Google Scholar]
- Espinosa‐Diez, C. , Miguel, V. , Mennerich, D. , Kietzmann, T. , Sánchez‐Pérez, P. , Cadenas, S. , & Lamas, S. (2015). Antioxidant responses and cellular adjustments to oxidative stress. Redox Biology, 6, 183–197. 10.1016/j.redox.2015.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feary, M. , Racher, A. J. , Young, R. J. , & Smales, C. M. (2017). Methionine sulfoximine supplementation enhances productivity in GS‐CHOK1SV cell lines through glutathione biosynthesis. Biotechnology Progress, 33(1), 17–25. 10.1002/btpr.2372 [DOI] [PubMed] [Google Scholar]
- Fenton, H. J. H. (1894). Oxidation of tartaric acid in presence of iron. Journal of the Chemical Society, Transactions, 65(0), 899–910. 10.1039/CT8946500899 [DOI] [Google Scholar]
- Fernandes, C. G. , Leipnitz, G. , Seminotti, B. , Amaral, A. U. , Zanatta, A. , Vargas, C. R. , … Wajner, M. (2010). Experimental evidence that phenylalanine provokes oxidative stress in hippocampus and cerebral cortex of developing rats. Cellular and Molecular Neurobiology, 30(2), 317–326. 10.1007/s10571-009-9455-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes, C. J. , Rong, L. , Tamura, T. , Stewart, K. D. , Rogers, L. K. , McMicken, H. W. , … Smith, C. V. (2002). Stable transfection of Chinese hamster ovary cells with glutamate‐cysteine ligase catalytic subunit cDNA confers increased resistance to tert‐butyl hydroperoxide toxicity. Toxicology Letters, 136(2), 107–120. 10.1016/S0378-4274(02)00287-4 [DOI] [PubMed] [Google Scholar]
- Fukai, T. , & Ushio‐Fukai, M. (2011). Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxidants & redox signaling, 15(6), 1583–1606. 10.1089/ars.2011.3999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga, M. , Miyata, S. , Higo, S. , Hamada, Y. , Ueyama, S. , & Kasuga, M. (2005). Methylglyoxal induces apoptosis through oxidative stress‐mediated activation of p38 mitogen‐activated protein kinase in rat Schwann cells. Annals of the New York Academy of Sciences, 1043(1), 151–157. 10.1196/annals.1333.019 [DOI] [PubMed] [Google Scholar]
- Gaboriau, F. , Kreder, A. , Clavreul, N. , Moulinoux, J. P. , Delcros, J. G. , & Lescoat, G. (2004). Polyamine modulation of iron uptake in CHO cells. Biochemical Pharmacology, 67(9), 1629–1637. 10.1016/j.bcp.2003.12.033 [DOI] [PubMed] [Google Scholar]
- Gasser, F. , Mulsant, P. , & Gillois, M. (1985). Long‐term multiplication of the Chinese hamster ovary (CHO) cell line in a serum‐free medium. In Vitro Cellular amp; Developmental Biology, 21(10), 588–592. 10.1007/bf02620890 [DOI] [PubMed] [Google Scholar]
- Gazzali, A. M. , Lobry, M. , Colombeau, L. , Acherar, S. , Azaïs, H. , Mordon, S. , … Frochot, C. (2016). Stability of folic acid under several parameters. European Journal of Pharmaceutical Sciences, 93(Suppl C), 419–430. 10.1016/j.ejps.2016.08.045 [DOI] [PubMed] [Google Scholar]
- Ghafourifar, P. , & Cadenas, E. (2005). Mitochondrial nitric oxide synthase. Trends In Pharmacological Sciences, 26(4), 190–195. 10.1016/j.tips.2005.02.005 [DOI] [PubMed] [Google Scholar]
- Ghorbaniaghdam, A. , Chen, J. , Henry, O. , & Jolicoeur, M. (2014). Analyzing clonal variation of monoclonal antibody‐producing CHO cell lines using an in silico metabolomic platform. PLoS One, 9(3), e90832 10.1371/journal.pone.0090832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano, G. , Cole, T. B. , Furlong, C. E. , & Costa, L. G. (2011). Paraoxonase 2 (PON2) in the mouse central nervous system: A neuroprotective role? Toxicology and Applied Pharmacology, 256(3), 369–378. 10.1016/j.taap.2011.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go, Y. ‐M. , & Jones, D. P. (2013). Thiol/disulfide redox states in signaling and sensing. Critical Reviews in Biochemistry and Molecular Biology, 48(2), 173–181. 10.3109/10409238.2013.764840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong, P. , Hu, B. , Stewart, D. , Ellerbe, M. , Figueroa, Y. G. , Blank, V. , … Alam, J. (2001). Cobalt induces heme oxygenase‐1 expression by a hypoxia‐inducible factor‐independent mechanism in Chinese hamster ovary cells: Regulation by Nrf2 and MafG transcription factors. Journal of Biological Chemistry, 276(29), 27018–27025. 10.1074/jbc.M103658200 [DOI] [PubMed] [Google Scholar]
- Grzelak, A. , Rychlik, B. , & Bartosz, G. (2000). Reactive oxygen species are formed in cell culture media. Acta Biochimica Polonica, 47(4), 1197–1198. [PubMed] [Google Scholar]
- Grzelak, A. , Rychlik, B. , & Bartosz, G. (2001). Light‐dependent generation of reactive oxygen species in cell culture media. Free Radical Biology and Medicine, 30(12), 1418–1425. 10.1016/S0891-5849(01)00545-7 [DOI] [PubMed] [Google Scholar]
- Gu, Y. , Lee, W. , & Shen, J. (2014). Site‐2 protease responds to oxidative stress and regulates oxidative injury in mammalian cells. Scientific Reports, 4, 6268 10.1038/srep06268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurer, H. , Ozgunes, H. , Oztezcan, S. , & Ercal, N. (1999). Antioxidant role of α‐lipoic acid in lead toxicity. Free Radical Biology and Medicine, 27(1), 75–81. 10.1016/S0891-5849(99)00036-2 [DOI] [PubMed] [Google Scholar]
- Gurer, H. , Ozgunes, H. , Saygin, E. , & Ercal, N. (2001). Antioxidant effect of taurine against lead‐induced oxidative stress. Archives of Environmental Contamination and Toxicology, 41(4), 397–402. 10.1007/s002440010265 [DOI] [PubMed] [Google Scholar]
- Ha, T. K. , Hansen, A. H. , Kol, S. , Kildegaard, H. F. , & Lee, G. M. (2017). Baicalein reduces oxidative stress in CHO cell cultures and improves recombinant antibody productivity. Biotechnology Journal, 13, 1700425 10.1002/biot.201700425 [DOI] [PubMed] [Google Scholar]
- Halliwell, B. (2003). Oxidative stress in cell culture: An under‐appreciated problem? FEBS Letters, 540(1), 3–6. 10.1016/S0014-5793(03)00235-7 [DOI] [PubMed] [Google Scholar]
- Halliwell, B. (2006). Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiology, 141(2), 312–322. 10.1104/pp.106.077073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell, B. (2014). Cell culture, oxidative stress, and antioxidants: Avoiding pitfalls. Biomed Journals, 37(3), 99–105. 10.4103/2319-4170.128725 [DOI] [PubMed] [Google Scholar]
- Hamada, H. , Hiramatsu, M. , Edamatsu, R. , & Mori, A. (1993). Free radical scavenging action of baicalein. Archives of Biochemistry and Biophysics, 306(1), 261–266. 10.1006/abbi.1993.1509 [DOI] [PubMed] [Google Scholar]
- Hamilton, W. G. , & Ham, R. G. (1977). Clonal growth of Chinese hamster cell lines in protein‐free media. In Vitro, 13(9), 537–547. 10.1007/BF02627849 [DOI] [PubMed] [Google Scholar]
- Hanschmann, E. ‐M. , Godoy, J. R. , Berndt, C. , Hudemann, C. , & Lillig, C. H. (2013). Thioredoxins, glutaredoxins, and peroxiredoxins—molecular mechanisms and health significance: From cofactors to antioxidants to redox signaling. Antioxidants & Redox Signaling, 19(13), 1539–1605. 10.1089/ars.2012.4599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, H. P. , Zhang, Y. , Zeng, H. , Novoa, I. , Lu, P. D. , Calfon, M. , … Ron, D. (2003). An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Molecular Cell, 11(3), 619–633. [DOI] [PubMed] [Google Scholar]
- Haredy, A. M. , Nishizawa, A. , Honda, K. , Ohya, T. , Ohtake, H. , & Omasa, T. (2013). Improved antibody production in Chinese hamster ovary cells by ATF4 overexpression. Cytotechnology, 65(6), 993–1002. 10.1007/s10616-013-9631-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata, R. , & Senoo, H. (1989). l‐Ascorbic acid 2‐phosphate stimulates collagen accumulation, cell proliferation, and formation of a three‐dimensional tissuelike substance by skin fibroblasts. Journal of Cellular Physiology, 138(1), 8–16. 10.1002/jcp.1041380103 [DOI] [PubMed] [Google Scholar]
- He, L. , Desai, J. X. , Gao, J. , Hazeltine, L. B. , Lian, Z. , Calley, J. N. , & Frye, C. C. (2018). Elucidating the impact of CHO cell culture media on tryptophan oxidation of a monoclonal antibody through gene expression analyses. Biotechnology Journal, 13):e1700254 10.1002/biot.201700254 [DOI] [PubMed] [Google Scholar]
- Hecklau, C. , Pering, S. , Seibel, R. , Schnellbaecher, A. , Wehsling, M. , Eichhorn, T. , … Zimmer, A. (2016). S‐sulfocysteine simplifies fed‐batch processes and increases the CHO specific productivity via anti‐oxidant activity. Journal of Biotechnology, 218, 53–63. 10.1016/j.jbiotec.2015.11.022 [DOI] [PubMed] [Google Scholar]
- Hetz, C. (2012). The unfolded protein response: Controlling cell fate decisions under ER stress and beyond. Nature Reviews Molecular Cell Biology, 13, 89–102. 10.1038/nrm3270 [DOI] [PubMed] [Google Scholar]
- Hossler, P. , Wang, M. , McDermott, S. , Racicot, C. , Chemfe, K. , Zhang, Y. , … Manuilov, A. (2015). Cell culture media supplementation of bioflavonoids for the targeted reduction of acidic species charge variants on recombinant therapeutic proteins. Biotechnology Progress, 31(4), 1039–1052. 10.1002/btpr.2095 [DOI] [PubMed] [Google Scholar]
- Hua Long, L. , & Halliwell, B. (2001). Oxidation and generation of hydrogen peroxide by thiol compounds in commonly used cell culture media. Biochemical and Biophysical Research Communications, 286(5), 991–994. 10.1006/bbrc.2001.5514 [DOI] [PubMed] [Google Scholar]
- Issels, R. D. , Nagele, A. , Eckert, K. ‐G. , & Wllmanns, W. (1988). Promotion of cystine uptake and its utilization for glutathione biosynthesis induced by cysteamine and N‐acetylcysteine. Biochemical Pharmacology, 37(5), 881–888. 10.1016/0006-2952(88)90176-1 [DOI] [PubMed] [Google Scholar]
- Jadhav, V. , Hackl, M. , Druz, A. , Shridhar, S. , Chung, C. ‐Y. , Heffner, K. M. , … Borth, N. (2013). CHO microRNA engineering is growing up: Recent successes and future challenges. Biotechnology Advances, 31(8), 1501–1513. 10.1016/j.biotechadv.2013.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong, C. J. , Azuma, J. , & Schaffer, S. (2012). Mechanism underlying the antioxidant activity of taurine: Prevention of mitochondrial oxidant production. Amino Acids, 42(6), 2223–2232. 10.1007/s00726-011-0962-7 [DOI] [PubMed] [Google Scholar]
- Juzeniene, A. , Grigalavicius, M. , Ma, L. W. , & Juraleviciute, M. (2016). Folic acid and its photoproducts, 6‐formylpterin and pterin‐6‐carboxylic acid, as generators of reactive oxygen species in skin cells during UVA exposure. Journal of Photochemistry and Photobiology. B, Biology, 155, 116–121. 10.1016/j.jphotobiol.2016.01.001 [DOI] [PubMed] [Google Scholar]
- Kelly, P. S. , Breen, L. , Gallagher, C. , Kelly, S. , Henry, M. , Lao, N. T. , … Barron, N. (2015). Re‐programming CHO cell metabolism using miR‐23 tips the balance towards a highly productive phenotype. Biotechnology Journal, 10(7), 1029–1040. 10.1002/biot.201500101 [DOI] [PubMed] [Google Scholar]
- Kelts, J. L. , Cali, J. J. , Duellman, S. J. , & Shultz, J. (2015). Altered cytotoxicity of ROS‐inducing compounds by sodium pyruvate in cell culture medium depends on the location of ROS generation. SpringerPlus, 4, 269–269. 10.1186/s40064-015-1063-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J. Y. , Kim, Y. G. , & Lee, G. M. (2012). CHO cells in biotechnology for production of recombinant proteins: Current state and further potential. Applied Microbiology and Biotechnology, 93(3), 917–930. 10.1007/s00253-011-3758-5 [DOI] [PubMed] [Google Scholar]
- Koynov, A. , Tryggvason, G. , & Khinast, J. G. (2007). Characterization of the localized hydrodynamic shear forces and dissolved oxygen distribution in sparged bioreactors. Biotechnology and Bioengineering, 97(2), 317–331. 10.1002/bit.21281 [DOI] [PubMed] [Google Scholar]
- Kshirsagar, R. , McElearney, K. , Gilbert, A. , Sinacore, M. , & Ryll, T. (2012). Controlling trisulfide modification in recombinant monoclonal antibody produced in fed‐batch cell culture. Biotechnology and Bioengineering, 109(10), 2523–2532. 10.1002/bit.24511 [DOI] [PubMed] [Google Scholar]
- Kunert, R. , & Reinhart, D. (2016). Advances in recombinant antibody manufacturing. Applied Microbiology and Biotechnology, 100, 3451–3461. 10.1007/s00253-016-7388-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurano, S. , Kurano, N. , Leist, C. , & Fiechter, A. (1990). Utilization and stability of vitamins in serum‐containing and serum‐free media in CHO cell culture. Cytotechnology, 4(3), 243–250. 10.1007/BF00563784 [DOI] [PubMed] [Google Scholar]
- Kuschelewski, J. , Schnellbaecher, A. , Pering, S. , Wehsling, M. , & Zimmer, A. (2017). Antioxidant effect of thiazolidine molecules in cell culture media improves stability and performance. Biotechnology Progress, 33(3), 759–770. 10.1002/btpr.2458 [DOI] [PubMed] [Google Scholar]
- Kyriakopoulos, S. , Polizzi, K. M. , & Kontoravdi, C. (2013). Comparative analysis of amino acid metabolism and transport in CHO variants with different levels of productivity. Journal of Biotechnology, 168(4), 543–551. 10.1016/j.jbiotec.2013.09.007 [DOI] [PubMed] [Google Scholar]
- Lagassé, H. A. D. , Alexaki, A. , Simhadri, V. L. , Katagiri, N. H. , Jankowski, W. , Sauna, Z. E. , & Kimchi‐Sarfaty, C. (2017). Recent advances in (therapeutic protein) drug development. F1000Research, 6, 113–113. 10.12688/f1000research.9970.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, K. H. , & Jeong, D. (2012). Bimodal actions of selenium essential for antioxidant and toxic pro‐oxidant activities: The selenium paradox (Review). Molecular Medicine Reports, 5(2), 299–304. 10.3892/mmr.2011.651 [DOI] [PubMed] [Google Scholar]
- Lewinska, A. , Wnuk, M. , Slota, E. , & Bartosz, G. (2007). Total anti‐oxidant capacity of cell culture media. Clinical and Experimental Pharmacology and Physiology, 34(8), 781–786. 10.1111/j.1440-1681.2007.04637.x [DOI] [PubMed] [Google Scholar]
- Lewis, A. M. , Croughan, W. D. , Aranibar, N. , Lee, A. G. , Warrack, B. , Abu‐Absi, N. R. , … Li, Z. J. (2016). Understanding and controlling sialylation in a CHO Fc‐fusion process. PLOS One, 11(6), e0157111 10.1371/journal.pone.0157111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, C. A. , Parker, S. J. , Fiske, B. P. , McCloskey, D. , Gui, D. Y. , Green, C. R. , … Metallo, C. M. (2014). Tracing compartmentalized NADPH metabolism in the cytosol and mitochondria of mammalian cells. Molecular Cell, 55(2), 253–263. 10.1016/j.molcel.2014.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, A. A. , & Miller, W. M. (1992). CHO cell responses to low oxygen: Regulation of oxygen consumption and sensitization to oxidative stress. Biotechnology and Bioengineering, 40(4), 505–516. 10.1002/bit.260400409 [DOI] [PubMed] [Google Scholar]
- Lin, A. A. , Kimura, R. , & Miller, W. M. (1993). Production of tPA in recombinant CHO cells under oxygen‐limited conditions. Biotechnology and Bioengineering, 42(3), 339–350. 10.1002/bit.260420311 [DOI] [PubMed] [Google Scholar]
- Long, L. H. , & Halliwell, B. (2009). Artefacts in cell culture: Pyruvate as a scavenger of hydrogen peroxide generated by ascorbate or epigallocatechin gallate in cell culture media. Biochemical and Biophysical Research Communications, 388(4), 700–704. 10.1016/j.bbrc.2009.08.069 [DOI] [PubMed] [Google Scholar]
- Lord‐Fontaine, S. , & Averill, D. A. (1999). Enhancement of cytotoxicity of hydrogen peroxide by hyperthermia in chinese hamster ovary cells: Role of antioxidant defenses. Archives of Biochemistry and Biophysics, 363(2), 283–295. 10.1006/abbi.1998.1087 [DOI] [PubMed] [Google Scholar]
- Lovaas, E. (1997). Antioxidative and metal‐chelating effects of polyamines. Advances in Pharmacology, 38, 119–149. 10.1016/S1054-3589(08)60982-5 [DOI] [PubMed] [Google Scholar]
- Lu, J. , & Holmgren, A. (2014). The thioredoxin antioxidant system. Free Radical Biology and Medicine, 66, 75–87. 10.1016/j.freeradbiomed.2013.07.036 [DOI] [PubMed] [Google Scholar]
- Maharjan, S. , Sakai, Y. , & Hoseki, J. (2016). Screening of dietary antioxidants against mitochondria‐mediated oxidative stress by visualization of intracellular redox state. Bioscience, Biotechnology, and Biochemistry, 80(4), 726–734. 10.1080/09168451.2015.1123607 [DOI] [PubMed] [Google Scholar]
- Malhotra, J. D. , & Kaufman, R. J. (2007). Endoplasmic reticulum stress and oxidative stress: A vicious cycle or a double‐edged sword? Antioxidants & Redox Signaling, 9(12), 2277–2293. 10.1089/ars.2007.1782 [DOI] [PubMed] [Google Scholar]
- Malhotra, J. D. , Miao, H. , Zhang, K. , Wolfson, A. , Pennathur, S. , Pipe, S. W. , & Kaufman, R. J. (2008). Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proceedings of the National Academy of Sciences, 105(47), 18525–18530. 10.1073/pnas.0809677105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallaney, M. , Wang, S. H. , & Sreedhara, A. (2014). Effect of ambient light on monoclonal antibody product quality during small‐scale mammalian cell culture process in clear glass bioreactors. Biotechnology Progress, 30(3), 562–570. 10.1002/btpr.1920 [DOI] [PubMed] [Google Scholar]
- Mao, X. , Gu, C. , Chen, D. , Yu, B. , & He, J. (2017). Oxidative stress‐induced diseases and tea polyphenols. Oncotarget, 8(46), 81649–81661. 10.18632/oncotarget.20887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciniak, S. J. , Yun, C. Y. , Oyadomari, S. , Novoa, I. , Zhang, Y. , Jungreis, R. , … Ron, D. (2004). CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes & Development, 18(24), 3066–3077. 10.1101/gad.1250704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinho, H. S. , Real, C. , Cyrne, L. , Soares, H. , & Antunes, F. (2014). Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biology, 2, 535–562. 10.1016/j.redox.2014.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias, S. , Fischer, S. , Handrick, R. , Fieder, J. , Schulz, P. , Bradl, H. , … Otte, K. (2018). Visualisation of intracellular production bottlenecks in suspension‐adapted CHO cells producing complex biopharmaceuticals using fluorescence microscopy. Journal of Biotechnology, 271, 47–55. 10.1016/j.jbiotec.2018.02.009 [DOI] [PubMed] [Google Scholar]
- McCoy, R. E. , Costa, N. A. , & Morris, A. E. (2015). Factors that determine stability of highly concentrated chemically defined production media. Biotechnology Progress, 31(2), 493–502. 10.1002/btpr.2047 [DOI] [PubMed] [Google Scholar]
- Meents, H. , Enenkel, B. , Eppenberger, H. M. , Werner, R. G. , & Fussenegger, M. (2002). Impact of coexpression and coamplification of sICAM and antiapoptosis determinants bcl‐2/bcl‐x(L) on productivity, cell survival, and mitochondria number in CHO‐DG44 grown in suspension and serum‐free media. Biotechnology and Bioengineering, 80(6), 706–716. 10.1002/bit.10449 [DOI] [PubMed] [Google Scholar]
- Mohan, C. , & Lee, G. M. (2009). Calnexin overexpression sensitizes recombinant CHO cells to apoptosis induced by sodium butyrate treatment. Cell Stress & Chaperones, 14(1), 49–60. 10.1007/s12192-008-0054-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan, M. J. , & Liu, Z. ‐g (2011). Crosstalk of reactive oxygen species and NF‐κB signaling. Cell Research, 21(1), 103–115. 10.1038/cr.2010.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulukutla, B. C. , Kale, J. , Kalomeris, T. , Jacobs, M. , & Hiller, G. W. (2017). Identification and control of novel growth inhibitors in fed‐batch cultures of Chinese hamster ovary cells. Biotechnology and Bioengineering, 114(8), 1779–1790. 10.1002/bit.26313 [DOI] [PubMed] [Google Scholar]
- Mulukutla, B. C. , Mitchell, J. , Geoffroy, P. , Harrington, C. , Krishnan, M. , Kalomeris, T. , … Hiller, G. W. (2019). Metabolic engineering of Chinese hamster ovary cells towards reduced biosynthesis and accumulation of novel growth inhibitors in fed‐batch cultures. Metabolic Engineering, 54, 54–68. 10.1016/j.ymben.2019.03.001 [DOI] [PubMed] [Google Scholar]
- Murati, T. , Šimić, B. , Pleadin, J. , Vukmirović, M. , Miletić, M. , Durgo, K. , … Kmetič, I. (2017). Reduced cytotoxicity in PCB‐exposed Chinese hamster ovary (CHO) cells pretreated with vitamin E. Food and Chemical Toxicology, 99, 17–23. 10.1016/j.fct.2016.11.014 [DOI] [PubMed] [Google Scholar]
- Naziroglu, M. , Ozgul, C. , Kucukayaz, M. , Cig, B. , Hebeisen, S. , & Bal, R. (2013). Selenium modulates oxidative stress‐induced TRPM2 cation channel currents in transfected Chinese hamster ovary cells. Basic & Clinical Pharmacology & Toxicology, 112(2), 96–102. 10.1111/j.1742-7843.2012.00934.x [DOI] [PubMed] [Google Scholar]
- Nguyen, T. , Sherratt, P. J. , & Pickett, C. B. (2003). Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annual Review of Pharmacology and Toxicology, 43, 233–260. 10.1146/annurev.pharmtox.43.100901.140229 [DOI] [PubMed] [Google Scholar]
- Nordberg, J. , & Arner, E. S. (2001). Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radical Biology and Medicine, 31(11), 1287–1312. 10.1016/S0891-5849(01)00724-9 [DOI] [PubMed] [Google Scholar]
- Oh, H. K. , So, M. K. , Yang, J. , Yoon, H. C. , Ahn, J. S. , Lee, J. M. , … Byun, T. H. (2005). Effect of N‐acetylcystein on butyrate‐treated Chinese hamster ovary cells to improve the production of recombinant human interferon‐β‐1a. Biotechnology Progress, 21(4), 1154–1164. 10.1021/bp050057v [DOI] [PubMed] [Google Scholar]
- Ohya, T. , Hayashi, T. , Kiyama, E. , Nishii, H. , Miki, H. , Kobayashi, K. , … Ohtake, H. (2008). Improved production of recombinant human antithrombin III in Chinese hamster ovary cells by ATF4 overexpression. Biotechnology and Bioengineering, 100(2), 317–324. 10.1002/bit.21758 [DOI] [PubMed] [Google Scholar]
- Omasa, T. , Takami, T. , Ohya, T. , Kiyama, E. , Hayashi, T. , Nishii, H. , … Ohtake, H. (2008). Overexpression of GADD34 enhances production of recombinant human antithrombin III in Chinese hamster ovary cells. Journal of Bioscience and Bioengineering, 106(6), 568–573. 10.1263/jbb.106.568 [DOI] [PubMed] [Google Scholar]
- Orellana, C. A. , Marcellin, E. , Gray, P. P. , & Nielsen, L. K. (2017). Overexpression of the regulatory subunit of glutamate‐cysteine ligase enhances monoclonal antibody production in CHO cells. Biotechnology and Bioengineering, 114(8), 1825–1836. 10.1002/bit.26316 [DOI] [PubMed] [Google Scholar]
- Orellana, C. A. , Marcellin, E. , Schulz, B. L. , Nouwens, A. S. , Gray, P. P. , & Nielsen, L. K. (2015). High‐antibody‐producing Chinese hamster ovary cells up‐regulate intracellular protein transport and glutathione synthesis. Journal of Proteome Research, 14(2), 609–618. 10.1021/pr501027c [DOI] [PubMed] [Google Scholar]
- Pereira, S. , Kildegaard, H. F. , & Andersen, M. R. (2018). Impact of CHO metabolism on cell growth and protein production: An overview of toxic and inhibiting metabolites and nutrients. Biotechnology Journal, 13(3), e1700499 10.1002/biot.201700499 [DOI] [PubMed] [Google Scholar]
- Pham, A. N. , Xing, G. , Miller, C. J. , & Waite, T. D. (2013). Fenton‐like copper redox chemistry revisited: Hydrogen peroxide and superoxide mediation of copper‐catalyzed oxidant production. Journal of Catalysis, 301, 54–64. 10.1016/j.jcat.2013.01.025 [DOI] [Google Scholar]
- Pieper, L. A. , Strotbek, M. , Wenger, T. , Olayioye, M. A. , & Hausser, A. (2017). ATF6beta‐based fine‐tuning of the unfolded protein response enhances therapeutic antibody productivity of Chinese hamster ovary cells. Biotechnology and Bioengineering, 114(6), 1310–1318. 10.1002/bit.26263 [DOI] [PubMed] [Google Scholar]
- Powis, G. , Gasdaska, J. R. , Gasdaska, P. Y. , Berggren, M. , Kirkpatrick, D. L. , Engman, L. , … Baker, A. (1997). Selenium and the thioredoxin redox system: Effects on cell growth and death. Oncology Research, 9(6‐7), 303–312. [PubMed] [Google Scholar]
- Prashad, K. , & Mehra, S. (2015). Dynamics of unfolded protein response in recombinant CHO cells. Cytotechnology, 67(2), 237–254. 10.1007/s10616-013-9678-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Précourt, L. ‐P. , Marcil, V. , Ntimbane, T. , Taha, R. , Lavoie, J. ‐C. , Delvin, E. , … Levy, E. (2012). Antioxidative properties of paraoxonase 2 in intestinal epithelial cells. American Journal of Physiology Gastrointestinal and Liver Physiology, 303(5), G623–G634. 10.1152/ajpgi.00039.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart, D. , Damjanovic, L. , Kaisermayer, C. , Sommeregger, W. , Gili, A. , Gasselhuber, B. , … Kunert, R. (2018). Bioprocessing of recombinant CHO‐K1, CHO‐DG44, and CHO‐S: CHO Expression hosts favor either mAb production or biomass synthesis. Biotechnology Journal, 14, e1700686 10.1002/biot.201700686 [DOI] [PubMed] [Google Scholar]
- Restelli, V. , Wang, M. D. , Huzel, N. , Ethier, M. , Perreault, H. , & Butler, M. (2006). The effect of dissolved oxygen on the production and the glycosylation profile of recombinant human erythropoietin produced from CHO cells. Biotechnology and Bioengineering, 94(3), 481–494. 10.1002/bit.20875 [DOI] [PubMed] [Google Scholar]
- Saito, K. , Jin, D. H. , Ogawa, T. , Muramoto, K. , Hatakeyama, E. , Yasuhara, T. , & Nokihara, K. (2003). Antioxidative properties of tripeptide libraries prepared by the combinatorial chemistry. Journal of Agricultural and Food Chemistry, 51(12), 3668–3674. 10.1021/jf021191n [DOI] [PubMed] [Google Scholar]
- Salinas, A. E. , & Wong, M. G. (1999). Glutathione S‐transferases‐‐a review. Current Medicinal Chemistry, 6(4), 279–309. [PubMed] [Google Scholar]
- Schenk, H. , Klein, M. , Erdbrugger, W. , Droge, W. , & Schulze‐Osthoff, K. (1994). Distinct effects of thioredoxin and antioxidants on the activation of transcription factors NF‐kappa B and AP‐1. Proceedings of the National Academy of Sciences of the United States of America, 91(5), 1672–1676. 10.1073/pnas.91.5.1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnellbaecher, A. , Binder, D. , Bellmaine, S. , & Zimmer, A. (2019). Vitamins in cell culture media: Stability and stabilization strategies. Biotechnology and Bioengineering, 0(0), 1537–1555. 10.1002/bit.26942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader, M. , & Fahimi, H. D. (2006). Peroxisomes and oxidative stress. Biochimica et Biophysica Acta (BBA) ‐ Molecular Cell Research, 1763(12), 1755–1766. 10.1016/j.bbamcr.2006.09.006 [DOI] [PubMed] [Google Scholar]
- Seibel, R. , Maier, S. , Schnellbaecher, A. , Bohl, S. , Wehsling, M. , Zeck, A. , & Zimmer, A. (2017). Impact of S‐sulfocysteine on fragments and trisulfide bond linkages in monoclonal antibodies. mAbs, 9(6), 889–897. 10.1080/19420862.2017.1333212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sies, H. , Berndt, C. , & Jones, D. P. (2017). Oxidative stress. Annual Review of Biochemistry, 86, 715–748. 10.1146/annurev-biochem-061516-045037 [DOI] [PubMed] [Google Scholar]
- Speisky, H. , Gómez, M. , Burgos‐Bravo, F. , López‐Alarcón, C. , Jullian, C. , Olea‐Azar, C. , & Aliaga, M. E. (2009). Generation of superoxide radicals by copper–glutathione complexes: Redox‐consequences associated with their interaction with reduced glutathione. Bioorganic & Medicinal Chemistry, 17(5), 1803–1810. 10.1016/j.bmc.2009.01.069 [DOI] [PubMed] [Google Scholar]
- Sun, S. ‐Y. (2010). N‐acetylcysteine, reactive oxygen species and beyond. Cancer Biology & Therapy, 9(2), 109–110. 10.4161/cbt.9.2.10583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuchi, H. , Sugiyama, T. , Tanaka, S. , & Tainaka, S. (2010). Overexpression of taurine transporter in Chinese hamster ovary cells can enhance cell viability and product yield, while promoting glutamine consumption. Biotechnology and Bioengineering, 107(6), 998–1003. 10.1002/bit.22880 [DOI] [PubMed] [Google Scholar]
- Tamura, T. , McMicken, H. W. , Smith, C. V. , & Hansen, T. N. (1996). Overexpression of human glutathione reductase in Chinese hamster ovary cells protects cells from oxidant injury. Pediatric Research, 39, 248 10.1203/00006450-199604001-01497 8825795 [DOI] [Google Scholar]
- Tanel, A. , & Averill‐Bates, D. A. (2007). Inhibition of acrolein‐induced apoptosis by the antioxidant N‐acetylcysteine. Journal of Pharmacology and Experimental Therapeutics, 321(1), 73–83. 10.1124/jpet.106.114678 [DOI] [PubMed] [Google Scholar]
- Templeton, N. , Dean, J. , Reddy, P. , & Young, J. D. (2013). Peak antibody production is associated with increased oxidative metabolism in an industrially relevant fed‐batch CHO cell culture. Biotechnology and Bioengineering, 110(7), 2013–2024. 10.1002/bit.24858 [DOI] [PubMed] [Google Scholar]
- Tey, B. T. , Singh, R. P. , Piredda, L. , Piacentini, M. , & Al‐Rubeai, M. (2000). Influence of bcl‐2 on cell death during the cultivation of a Chinese hamster ovary cell line expressing a chimeric antibody. Biotechnology and Bioengineering, 68(1), 31–43. [DOI] [PubMed] [Google Scholar]
- Touitou, E. , Alkabes, M. , Memoli, A. , & Alhaique, F. (1996). Glutathione stabilizes ascorbic acid in aqueous solution. International Journal of Pharmaceutics, 133(1‐2), 85–88. 10.1016/0378-5173(95)04419-1 [DOI] [Google Scholar]
- Tu, B. P. , & Weissman, J. S. (2004). Oxidative protein folding in eukaryotes: Mechanisms and consequences. Journal of Cell Biology, 164(3), 341–346. 10.1083/jcb.200311055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrens, J. F. (2003). Mitochondrial formation of reactive oxygen species. Journal of Physiology, 552(Pt 2), 335–344. 10.1113/jphysiol.2003.049478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrens, J. F. , Alexandre, A. , & Lehninger, A. L. (1985). Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Archives of Biochemistry and Biophysics, 237(2), 408–414. 10.1016/0003-9861(85)90293-0 [DOI] [PubMed] [Google Scholar]
- Tyo, K. E. , Liu, Z. , Petranovic, D. , & Nielsen, J. (2012). Imbalance of heterologous protein folding and disulfide bond formation rates yields runaway oxidative stress. BMC Biology, 10(1), 16 10.1186/1741-7007-10-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukibe, K. , Hashida, K. , Yoshida, N. , & Takagi, H. (2009). Metabolic engineering of Saccharomyces cerevisiae for astaxanthin production and oxidative stress tolerance. Applied and Environmental Microbiology, 75(22), 7205–7211. 10.1128/AEM.01249-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. , & Pantopoulos, K. (2011). Regulation of cellular iron metabolism. Biochemical Journal, 434(Pt 3), 365–381. 10.1042/BJ20101825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner, B. , Papes, R. , Heile, M. , Spitz, D. , & Wispe, J. (1993). Expression of human Mn SOD in Chinese hamster ovary cells confers protection from oxidant injury. American Journal of Physiology, 264(6 Pt 1), L598–L605. 10.1152/ajplung.1993.264.6.L598 [DOI] [PubMed] [Google Scholar]
- Weiss, S. L. , & Sunde, R. A. (1997). Selenium regulation of classical glutathione peroxidase expression requires the 3′ untranslated region in Chinese hamster ovary cells. Journal of Nutrition, 127(7), 1304–1310. 10.1093/jn/127.7.1304 [DOI] [PubMed] [Google Scholar]
- Wilkinson, B. , & Gilbert, H. F. (2004). Protein disulfide isomerase. Biochimica et Biophysica Acta/General Subjects, 1699(1‐2), 35–44. 10.1016/j.bbapap.2004.02.017 [DOI] [PubMed] [Google Scholar]
- Wu, W. , Abraham, L. , Ogony, J. , Matthews, R. , Goldstein, G. , & Ercal, N. (2008). Effects of N‐acetylcysteine amide (NACA), a thiol antioxidant on radiation‐induced cytotoxicity in Chinese hamster ovary cells. Life Sciences, 82(21‐22), 1122–1130. 10.1016/j.lfs.2008.03.016 [DOI] [PubMed] [Google Scholar]
- Xing, Z. , Kenty, B. M. , Li, Z. J. , & Lee, S. S. (2009). Scale‐up analysis for a CHO cell culture process in large‐scale bioreactors. Biotechnology and Bioengineering, 103(4), 733–746. 10.1002/bit.22287 [DOI] [PubMed] [Google Scholar]
- Xu, J. , Jin, M. , Song, H. , Huang, C. , Xu, X. , Tian, J. , … Li, Z. J. (2014). Brown drug substance color investigation in cell culture manufacturing using chemically defined media: A case study. Process Biochemistry, 49(1), 130–139. 10.1016/j.procbio.2013.10.015 [DOI] [Google Scholar]
- Xu, J. , Rehmann, M. S. , Xu, X. , Huang, C. , Tian, J. , Qian, N. X. , & Li, Z. J. (2018). Improving titer while maintaining quality of final formulated drug substance via optimization of CHO cell culture conditions in low‐iron chemically defined media. mAbs, 10(3), 488–499. 10.1080/19420862.2018.1433978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, P. , Qiao, K. , & Stephanopoulos, G. (2017). Engineering oxidative stress defense pathways to build a robust lipid production platform in Yarrowia lipolytica. Biotechnology and Bioengineering, 114(7), 1521–1530. 10.1002/bit.26285 [DOI] [PubMed] [Google Scholar]
- Xue, L. , Li, J. , Li, Y. , Chu, C. , Xie, G. , Qin, J. , … Fu, X. (2015). N‐acetylcysteine protects Chinese hamster ovary cells from oxidative injury and apoptosis induced by microcystin‐LR. International Journal of Clinical and Experimental Medicine, 8(4), 4911–4921. [PMC free article] [PubMed] [Google Scholar]
- Yao, T. , & Asayama, Y. (2017). Animal‐cell culture media: History, characteristics, and current issues. Reproductive Medicine and Biology, 16(2), 99–117. 10.1002/rmb2.12024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun, Z. , Takagi, M. , & Yoshida, T. (2001). Effect of antioxidants on the apoptosis of CHO cells and production of tissue plasminogen activator in suspension culture. Journal of Bioscience and Bioengineering, 91(6), 581–585. 10.1016/S1389-1723(01)80177-8 [DOI] [PubMed] [Google Scholar]
- Yun, Z. , Takagi, M. , & Yoshida, T. (2003). Combined addition of glutathione and iron chelators for decrease of intracellular level of reactive oxygen species and death of Chinese hamster ovary cells. Journal of Bioscience and Bioengineering, 95(2), 124–127. 10.1016/S1389-1723(03)80116-0 [DOI] [PubMed] [Google Scholar]
- Zafarullah, M. , Li, W. Q. , Sylvester, J. , & Ahmad, M. (2003). Molecular mechanisms of N‐acetylcysteine actions. Cellular and Molecular Life Science, 60(1), 6–20. 10.1007/s000180300001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangar, R. C. , Davydov, D. R. , & Verma, S. (2004). Mechanisms that regulate production of reactive oxygen species by cytochrome P450. Toxicology and Applied Pharmacology, 199(3), 316–331. 10.1016/j.taap.2004.01.018 [DOI] [PubMed] [Google Scholar]
- Zeeshan, H. M. A. , Lee, G. H. , Kim, H. ‐R. , & Chae, H. ‐J. (2016). Endoplasmic reticulum stress and associated ROS. International Journal of Molecular Sciences, 17(3), 327 10.3390/ijms17030327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. , Robinson, D. , & Salmon, P. (2006). A novel function for selenium in biological system: Selenite as a highly effective iron carrier for Chinese hamster ovary cell growth and monoclonal antibody production. Biotechnology and Bioengineering, 95(6), 1188–1197. 10.1002/bit.21081 [DOI] [PubMed] [Google Scholar]
- Zustiak, M. P. , Jose, L. , Xie, Y. , Zhu, J. , & Betenbaugh, M. J. (2014). Enhanced transient recombinant protein production in CHO cells through the co‐transfection of the product gene with Bcl‐x(L). Biotechnology Journal, 9(9), 1164–1174. 10.1002/biot.201300468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwolak, I. (2015). Increased cytotoxicity of vanadium to CHO‐K1 cells in the presence of inorganic selenium. Bulletin of Environmental Contamination and Toxicology, 95(5), 593–598. 10.1007/s00128-015-1615-4 [DOI] [PMC free article] [PubMed] [Google Scholar]


