Abstract
Problem
For women of reproductive age, achieving a successful pregnancy requires both the normal functioning of reproductive endocrine and the health of the reproductive tract environment. We aimed to study how these fertility factors, such as female age, baseline sexual hormone levels, tubal patency, and vaginal pH, affect the composition of vaginal microbiome.
Method of study
The 16S rRNA sequencing was carried on vaginal microbiome samples from 85 women of reproductive age without vaginal infections or reproductive endocrine diseases. The detailed correlations between fertility factors and vaginal microbiome were quantified by Spearman's rank tests. A linear discriminant analysis was carried out to explore the effects of fertility factors on the relative abundances of vaginal bacterial species.
Results
The vaginal pH, levels of basal E2, LH, and FSH all had significant effects on the distribution of vaginal microbiome. The relative abundances of vaginal bacterial species, including Escherichia coli, Streptococcus agalactiae, and Prevotella intermedia, were significantly different due to the host's state of reproductive endocrine and tubal patency. It was worth noting that women with tubal obstruction, or prolonged menstrual cycle, or antral follicle count >15, or vaginal pH > 4.5 all had a higher abundance of Escherichia coli in vagina.
Conclusion
The fertility factors associated with the reproductive endocrine and the genital tract environment affected vaginal microbiome in women of reproductive age. The species Escherichia coli, Streptococcus agalactiae, Prevotella intermedia, etc could be used as biomarkers to reflect the pathological state of reproductive endocrine and genital tract.
Keywords: fertility, microbiota, vagina
The fertility factors associated with the reproductive endocrine and the genital tract environment affected vaginal microbiome in women of reproductive age, including the bacterial species Escherichia coli, Streptococcus agalactiae, and Prevotella intermedia, et al.

1. INTRODUCTION
For women of reproductive age, achieving a successful pregnancy requires both the normal functioning of reproductive endocrine and the health of the reproductive tract environment. Reproductive endocrine plays an essential role in promoting gonadal development, maintaining fertility, and ensuring reproductive health. Meanwhile, a healthy reproductive tract environment also helps support successful embryo implantation and maintains pregnancy.
As the age in women grows, the levels of sex hormones change and the vaginal microbiome is in constant flux.1 During a woman's reproductive years, the fluctuating levels of hormones that regulate the menstrual cycle are an important influence on the vaginal microbiome,2 especially the estradiol (E2) and progesterone (P).3, 4 The levels of sex hormones would affect the components of the female genital tract defensive barriers, including the mucous viscosity, epithelial barrier thickness, immune cell frequency, and resident vaginal microbes.3, 5
Previous studies have explored the physiological interaction between sex hormones and vaginal flora. Estrogen promotes hyperplasia and thickening of vaginal epithelia and the increase in glycogen.6 As the dominant bacteria in the vagina, Lactobacillus can change the glycogen into lactic acid, maintain the acidic environment of the vagina, inhibit the growth of other pathogens,7, 8 and strengthen the immune system.9, 10, 11 When a woman enters menopause, the level of estrogen will significantly reduce, while the level of follicle‐stimulating hormone (FSH) increases, and the vaginal pH environment changes from acidic to weakly acidic, which leads to the colonization of a large number of mixed bacteria.12, 13, 14 Meanwhile, the estradiol‐based hormone replacement therapy can maintain Lactobacillus dominance in post‐menopausal women, supporting a link between estradiol and lactobacilli.12, 15
The levels of sex hormones in the reproductive endocrine system are closely related to female fertility. Clinically, the levels of FSH, luteinizing hormone (LH), prolactin (PRL), testosterone (T), and antral follicle count (AFC) in the early follicular phase are used to assess the female fertility, as well as the female age, anti‐Müllerian hormone (AMH), and fallopian tube patency. However, there have been only a few studies about the correlation between these fertility factors and the vaginal microbiome.
Therefore, in this study, we examined the vaginal microbiome of women of reproductive age and tried to study how these fertility factors associated with the reproductive endocrine and reproductive tract environment affect the composition of the vaginal microbiome.
2. MATERIALS AND METHODS
2.1. Ethics statement
This study was approved by the Ethical Committee of Ren Ji Hospital, Shanghai Jiao Tong University School of Medicine (Approval No. 2016012904). All participants have provided written informed consent prior to their inclusion in the study.
2.2. Sample collection and clinical measurements
From December 2015 to April 2016, we recruited 85 women aged 24 to 43 years from the Center for Reproductive Medicine, Renji Hospital, Shanghai Jiao Tong University School of Medicine.
Among the 85 subjects recruited, 40 subjects were infertile due to the tubal obstruction. The other 45 subjects had normal fallopian tubes, who sought for the assisted reproductive technology support only due to the male sperm quality problems. Patients with reproductive endocrine diseases or had hormone replacement therapy in the past 3 months were excluded from this study.
Patients with bacterial vaginosis (Amsel criteria16), candidal vaginitis, trichomoniasis, mycoplasmal, chlamydial, or gonococcal infection were excluded. The subjects were given leukorrheal routine examinations. The pus cells of all subjects were 0‐15/HP, and the vaginal cleanliness was grade I–II. The Candida albicans, Trichomonas vaginalis, Neisseria gonorrhoeae, Mycoplasma hominis, Ureaplasma urealyticum, Chlamydia trachomatis, and clue cells were all not detected. The test results of hydrogen peroxide, leukocyte esterase, and sialic acid glucoside enzyme were all negative. Women who had taken any antibiotic drugs in the past 3 months were also excluded.
All participants reported their conditions of menstruation and fallopian tube patency. We recorded their age, height, and weight. The vaginal pH values were tested from the posterior fornix during the menstrual interval. We detected serum AMH levels using an ultrasensitive enzyme‐linked immunosorbent assay (Kangrun Biotech, catalog number KR‐AMH‐001). Since the levels of basic sexual hormones were regarded as stable at the baseline during the early follicular phase of the menstrual cycle,17 they were measured on day 1‐5 of the menstrual cycle by electrochemiluminescence immunoassay (ECLIA) (Roche Cobas E601), including FSH, LH, E2, PRL, and T. In the meantime, the antral follicle count (AFC), which means the sum of all follicles measuring 2‐10 mm in mean diameter in both ovaries, was done by a transvaginal ultrasound. The levels of clinical factors were displayed in histograms by SPSS software (version 22, SPSS Inc).
2.3. Total bacterial genomic DNA extraction
For each participant, the vaginal microbiome sample was obtained by a sterile cotton swab from the posterior fornix of the vagina during the menstrual interval. The vaginal bacterial DNA was extracted by a PowerSoil‐htp 96‐well soil DNA isolation kit (Mo Bio Laboratories, catalog number 12888‐100). DNA quantification was carried out with a NanoDrop spectrophotometer (Nyxor Biotech).
2.4. PCR amplification and 16S rRNA gene sequencing
Amplification and sequencing were carried out as protocols described by Mao et al18 The V4 regions of bacterial 16S rRNA genes were amplified using the primers 515F (5′‐barcode‐GTGCCAGCMGCCGCGGTAA‐3′) and 806R (5′‐barcode‐GGACTACHVGGGTWTCTAAT‐3′). Amplicons were purified using a Qiagen QIAquick PCR purification kit (Qiagen) and quantified using the PicoGreen dsDNA reagent kit (Invitrogen Ltd.). The pooled product was paired‐end sequenced (2 × 250) on an Illumina MiSeq platform by the Department of NGS Sequencing, Tianyi Health Sciences Institute Co., Ltd.. Sequence data were archived in the NCBI Sequence Read Archive (SRA) under accession number SRP136384.
2.5. Sequence processing and data analysis
Raw Illumina FASTQ files were demultiplexed, quality filtered, and analyzed by Quantitative Insights Into Microbial Ecology (QIIME, version 1.8.0).19 The operational taxonomic units (OTUs) were clustered with a 97% similarity cutoff using UPARSE.20 Taxonomy was assigned to representative sequences using the Ribosomal Database Project (RDP) classifier in QIIME21 with a confidence value of 0.8 against the Silva bacterial 16S rRNA gene dataset (version 119) and Greengenes database for species‐level taxonomy information.
2.6. Gene function prediction
We used Canoco 5.0 software to perform redundancy analysis (RDA) at the genus level.22 For the multiple comparisons, the false discovery rate (FDR) adjusting method was employed to avoid type I errors. Provided by Benjamini and Hochberg, it advocated control of the expected proportion of falsely rejected hypotheses.23 The correlation coefficients between the relative abundance of vaginal bacteria and clinical factors were calculated by Spearman's rank tests. Linear discriminant analysis (LDA) effect size (LEfSe) method was used to discover the high‐dimensional biomarker and explain the genomic feature identification that characterizes the differences between various biological conditions.24
3. RESULTS
3.1. Vaginal microbial community composition of the women of reproductive age
The clinical results of fertility factors of 85 women of reproductive age were shown in frequency bar charts (Figure S1). 16S rRNA gene sequencing generated a total of 3 218 048 high‐quality reads, which were binned into 18 796 OTUs after sequence alignment with the Greengenes database at 3% dissimilarity. The detailed sequence data were archived in the NCBI Sequence Read Archive (SRA) under accession number SRP136384.
At the phylum level, Firmicutes were the principal strains in women of reproductive age, followed by Actinobacteria and Bacteroidetes (Figure 1A). At the genus level, the vaginal flora of women of reproductive age was dominated by Lactobacillus, followed by Gardnerella, Prevotella, Streptococcus, and Atopobium (Figure 1B). The heatmap showed the top 20 dominant genera in all samples (Figure 1C).
Figure 1.
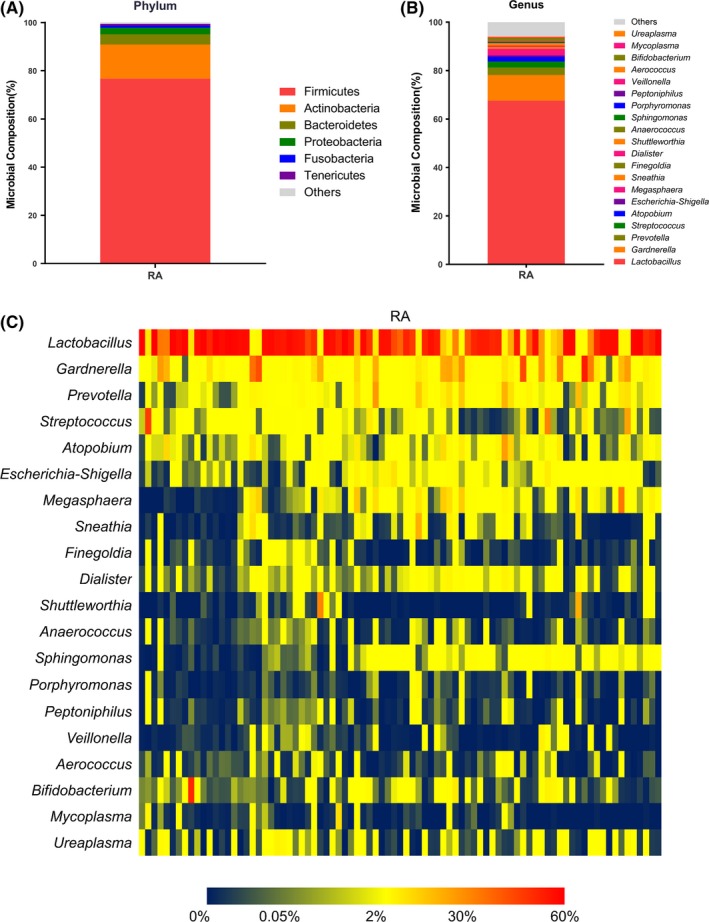
Results of 16S rRNA gene sequencing of the vaginal microbiome in women of reproductive age. The microbial composition of the vaginal microbiome at the phylum level (A) and the genus level (B). A heatmap was drawn from 20 dominant bacterial genera in all samples (C). RA, women of reproductive age
3.2. Analyses of correlations between the fertility factors and vaginal microbiome
We used the redundancy analysis (RDA) to visually examine the effect of fertility factors on the distribution of samples (Figure 2). The results showed that vaginal pH, levels of serum E2, LH, and FSH all had significant effects on the distribution of vaginal microbiota (P < .05). Among them, vaginal pH and the levels of serum E2 had the most significant effects (contribution of 29.5% and 21.7%, respectively).
Figure 2.
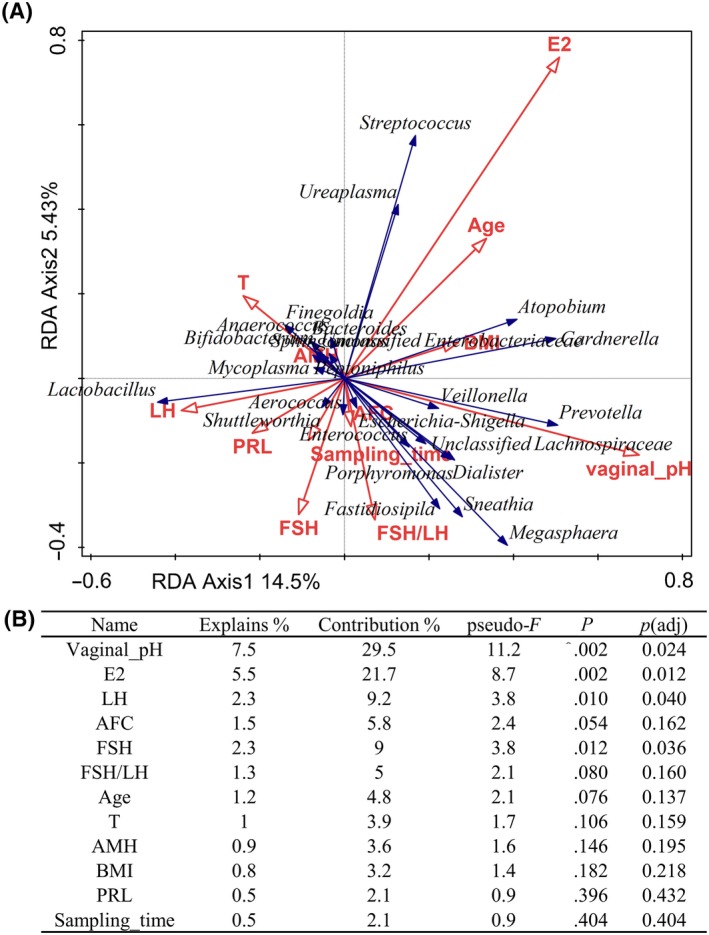
Redundancy analysis of the effects of fertility factors on the vaginal microbiome. A, A graphical result of redundancy analysis. Each point represented a single sample. The length of the arrow represented the correlation degree between the environmental factor and the sample distribution. An acute angle between the arrows indicated a positive correlation, while an obtuse angle indicated a negative correlation. B, The detailed contribution degree and the P‐value of each clinical factor. E2, estradiol. LH, luteinizing hormone. AFC, antral follicle count. FSH, follicle‐stimulating hormone. T, testosterone. AMH, anti‐Müllerian hormone. BMI, body mass index. PRL, prolactin
Next, we quantified the correlations between the relative abundance of vaginal flora and fertility factors by Spearman's rank tests. The results were presented in a heatmap (Figure 3). The vaginal bacteria were arranged according to the value of the correlation coefficient between the relative abundance of vaginal bacteria and the vaginal pH.
Figure 3.
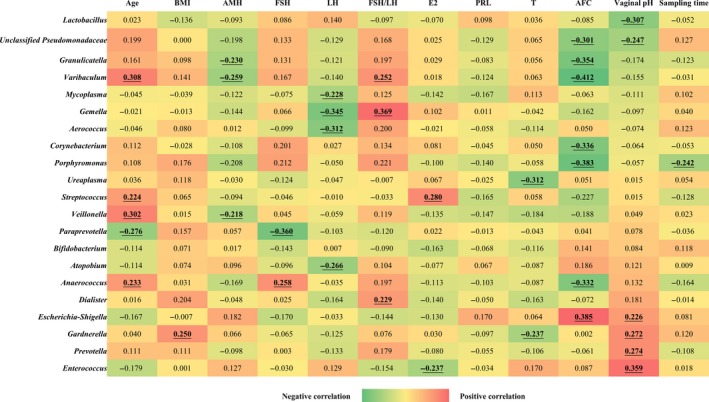
Spearman correlation analysis between the relative abundances of vaginal microbiome and clinical factors. The correlation coefficients were calculated between the relative abundances of vaginal bacterial genera and the clinical factors by Spearman's rank tests. The results with significant correlation (correlation coefficient > 0.2, P < .05) were presented in bold and underlined. Red indicated positive correlations while green indicated negative. BMI, body mass index. AMH, anti‐Müllerian hormone. FSH, follicle‐stimulating hormone. LH, luteinizing hormone. E2, estradiol. PRL, prolactin. T, testosterone. AFC, antral follicle count
As can be seen from the results, the relative abundance of Lactobacillus was negatively correlated with vaginal pH, suggesting that Lactobacillus was more likely to exist in a weakly acidic environment, which is a normal vaginal environment. Meanwhile, the relative abundances of Escherichia/Shigella, Gardnerella, Prevotella, and Enterococcus were positively correlated with vaginal pH. That means when the vaginal pH rises, the colonization of these bacteria would increase.
The relative abundance of Paraprevotella was negatively correlated with age and FSH. It was suggested that this bacterium was more commonly found in the vagina of women with normal ovarian function or younger. Meanwhile, the relative abundances of genera Varibaculum, Streptococcus, and Veillonella were positively related to age, indicating that the colonization of these bacteria in the vagina may increase with female age.
In addition, the relative abundances of genera Aerococcus and Atopobium were negatively correlated with LH. The relative abundance of Gemella was positively correlated with FSH/LH and that of Streptococcus was positively correlated with E2.
The vaginal genera negatively correlated with AFC included Varibaculum, Porphyromonas, Granulicatella, and Anaerococcus, while the relative abundance of Escherichia/Shigella was positively correlated with AFC.
3.3. Specific vaginal species in each subgroup of fertility factors
We then divided the women of reproductive age into multiple subgroups based on each fertility factor. It was found that there were significant differences in the abundances of some vaginal species between/among the subgroups of several fertility factors, including the age, E2, LH, T, fallopian tube patency, menstrual cycle length, AFC, and vaginal pH. The results of linear discriminant analysis (LDA) are shown in Figure 4 (the logarithmic LDA score ≥ 2.0, P < .05).
Figure 4.
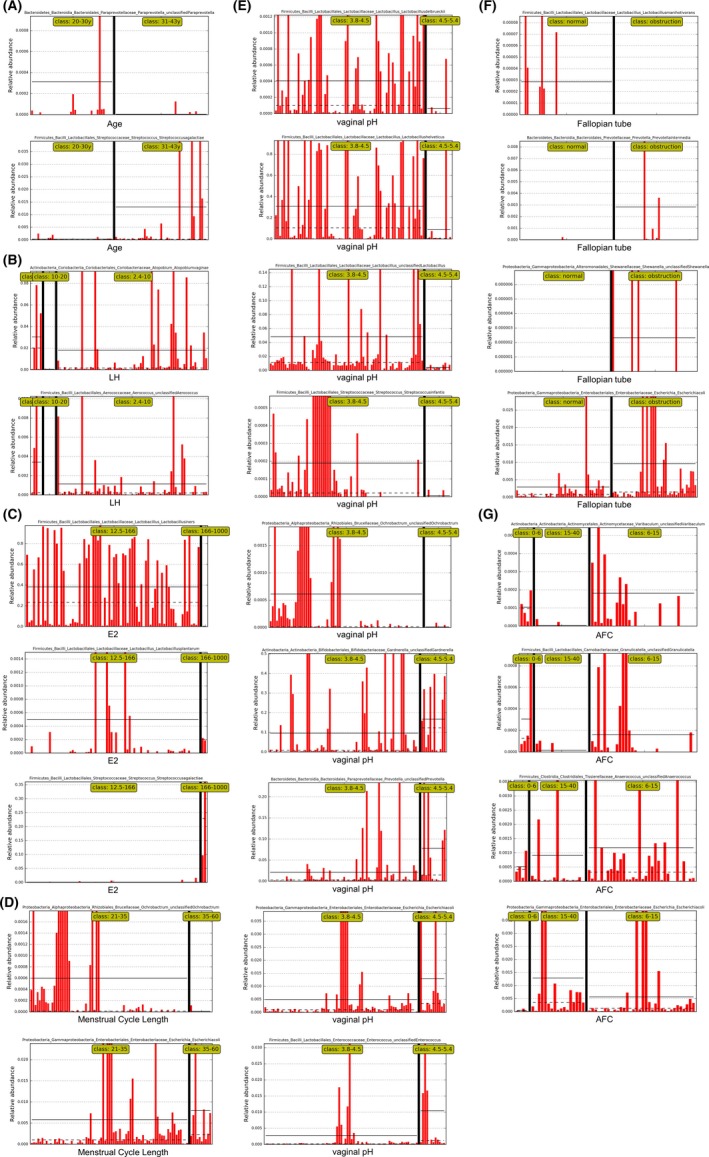
The relative abundances of specific vaginal species in each subgroup of fertility factors. The women of reproductive age were divided into multiple subgroups according to each fertility factor, including (A) age, (B) LH, (C) E2, (D) menstrual cycle length, (E) vaginal pH, (F) fallopian tube, and (G) AFC. The raw data of the features within each subgroup detected by LEfSe as biomarkers were plotted as abundance histograms (the logarithmic LDA score ≥ 2.0, P < .05). E2, estradiol. LH, luteinizing hormone. AFC, antral follicle count
We have concluded the results above in Figure 5, showing the specific vaginal species between/among subgroups of each fertility factor:
Figure 5.
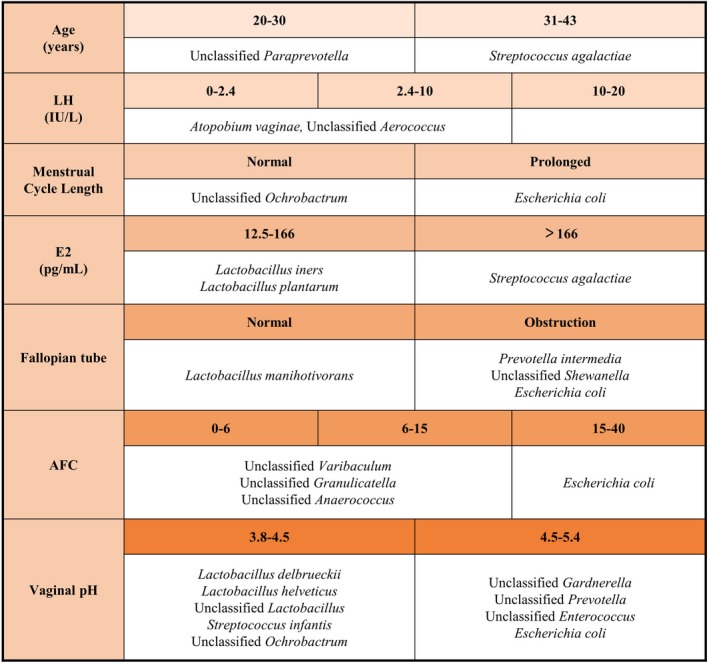
Summary table of specific vaginal species in each subgroup of fertility factors. A summary table based on the results above, listing the specific vaginal species between/among the different levels of the clinical indexes in women of reproductive age. E2, estradiol. LH, luteinizing hormone. AFC, antral follicle count
Compared with the younger women, the older women had a higher relative abundance of Streptococcus agalactiae in the vagina and a lower relative abundance of unclassified Paraprevotella. In women with higher levels of E2, there were fewer Lactobacillus iners and Lactobacillus plantarum in the vagina but more Streptococcus agalactiae. For women with the basal LH level < 10 IU/L, the abundances of Atopobium vaginae and unclassified Aerococcus in the vagina were significantly higher than that in women with basal LH level > 10 IU/L.
Moreover, women with tubal obstruction had a higher relative abundance of Escherichia coli, Prevotella intermedia, and unclassified Shewanella in the vagina compared with the women with the normal fallopian tubes. Meanwhile, there were more Lactobacillus manihotivorans in the latter group compared to the previous group. It was worth noting that women with prolonged menstrual cycle also had more Escherichia coli and less unclassified Ochrobactrum compared to women with normal menstrual cycles.
We also found out that when the number of AFC was at different levels, the abundances of vaginal bacterial species in women of reproductive age also had specific differences. For women whose AFC was between 0‐6 and 6‐15, the relative abundances of unclassified Varibaculum, unclassified Granulicatella, and unclassified Anaerococcus in the vagina were higher than that in AFC > 15 group. Meanwhile, the relative abundance of Escherichia coli in the posterior group was higher than in the former two groups. Women with higher vaginal pH had less Lactobacillus delbrueckii, Lactobacillus helveticus, unclassified Lactobacillus, Streptococcus infantis, and unclassified Ochrobactrum in the vagina, while the colonization of unclassified Prevotella, unclassified Enterococcus, unclassified Gardnerella, and Escherichia coli increased compared to the women with normal vaginal pH.
4. DISCUSSION
The results above suggested that the fertility factors associated with reproductive endocrine and the reproductive tract environment could affect the vaginal microbiome in women of reproductive age. The relative abundances of several vaginal bacterial species were significantly different due to the host's age, levels of basic sexual hormones, antral follicle count, fallopian tube patency, menstrual cycle length, and vaginal pH.
We found that in older women, or women with higher levels of basal E2, the abundance of Streptococcus agalactiae in the vagina was higher than the normal group. Streptococcus agalactiae, also known as Group B Streptococcus (GBS), is an important pathogen in aerobic vaginitis. It rarely causes infections in healthy adults; however, occasionally it may cause morbidity in older women, pregnant women, or the patients with underlying predisposing conditions.25
Elderly adults account for >40% of persons with invasive GBS disease in the United States, colonized at vaginal and rectal sites. It causes skin and soft‐tissue infections, and bacteremia with no identified focus.26, 27 In pregnant and postpartum women, GBS is a frequent cause of asymptomatic bacteriuria, urinary tract infection, and upper genital tract infection et al.28, 29 It is noted that invasive maternal infection with GBS is associated with pregnancy loss and preterm delivery.30, 31
In the early follicular phase of women of reproductive age, high levels of basal E2 (>60‐80 pg/mL) with normal FSH levels may indicate a poor ovarian response. Early follicular E2 levels are better used with basal FSH to assess ovarian reserve.32 However, the relationship between GBS and the ovarian response has not been studied, and our study was the first to find that GBS infection might be associated with the decline of ovarian function.
In women with higher levels of basal E2 (>166 pg/mL), there were fewer Lactobacillus iners and Lactobacillus plantarum in the vagina than women with normal levels of basal E2 (12.5‐166 pg/mL). Lactobacillus iners is the most prevalent lactobacilli in most women.33 However, L iners lacks the gene coding for D‐lactic acid dehydrogenase,34 only producing the L‐lactic acid isomer.35 Lactobacillus iners dominated vaginal community type seems to be less stable or more in transition than the other community types and more associated with vaginal dysbiosis.36 Our results suggested that high level of basal E2 may have an impact on the colonization of Liners.
Lactobacillus plantarum strains produce anti‐infective agents, including hydrogen peroxide, and are able to co‐aggregate efficiently with vaginal pathogens.8 It helps treat vulvovaginal candidiasis and bacterial vaginosis, and restore a normal vaginal microbiota.37, 38
Moreover, it was first found that the abundances of Atopobium vaginae and unclassified Aerococcus in the vagina were correlated with the basal LH level. LH activity had previously been deemed potentially detrimental to reproductive function.39 However, Sun et al40 found that a high basal LH level did not have any adverse effects on oocyte quality and embryo quality and did not lead to poor outcomes of IVF/ICSI treatment. Moreover, more embryos and more top‐quality embryos developed in the group with LH > 10 mIU/mL than in the other groups.
The genera Prevotella, Atopobium, and Gardnerella were associated with bacterial vaginosis (BV), which may be linked to an increased risk of preterm labor.11 Abnormal vaginal microbiota, such as a high abundance of Gardnerella vaginalis or Atopobium vaginae, may negatively affect the clinical pregnancy rate in IVF patients.41
Based on our results, it was also first discovered that the number of antral follicles was also associated with the colonization of genera Granulicatella, Anaerococcus, and Varibaculum in the vagina. Previous researches have shown that the relative abundances of genera Granulicatella and Anaerococcus were increased in bacterial vaginosis patients.42 Varibaculum cambriense has been isolated from intrauterine devices and human vagina and abscess specimens.43
It was interesting to notice that women with AFC > 15 or prolonged menstrual cycle all had a higher abundance of Escherichia coli in the vagina. When the number of antral follicles is too high, it is often suggested that the subject has high levels of serum androgen, resulting in hyperandrogenic anovulation.44 Prolonged menstrual cycle, increased number of antral follicles, and the elevated serum androgen all suggest the occurrence of polycystic ovary syndrome (PCOS). Studies have found that the abundance of Escherichia/Shigella was higher in the intestinal flora of PCOS patients compared with the control group, and it was positively correlated with the levels of serum testosterone of the subjects,45 suggesting the pathological relationship between Escherichia/Shigella and PCOS. A study investigated the inflammatory associated microbial dysbiosis that occurred in a primate model of PCOS. They found that both diet and androgen treatment result in alterations in the genital tract microbiome, such as Sneathia and Mobiluncus, suggesting that inflammatory associated cervicovaginal microbiome dysbiosis plays a role in adverse fertility and pregnancy outcomes seen with PCOS.46
Moreover, we also found that women with tubal obstruction had a higher abundance of Escherichia coli, Prevotella intermedia, and unclassified Shewanella in the vagina than women with normal fallopian tubes. A previous study showed that the women in the stage 3/4 endometriosis group had more Escherichia/Shigella in the cervical and stool microbiome,47 which suggested that Escherichia coli may cause inflammatory reactions both in the reproductive tract and intestine. Virulence factor analysis showed that vaginal Escherichia coli was a reservoir along the “fecal‐vaginal‐urinary/neonatal” course of transmission in the extraintestinal E coli infections.48 Moreover, the vaginal colonization by E coli was regarded as a risk factor for very low birth weight delivery and other perinatal complications.49 E coli was also present in the vagina of subjects with elevated vaginal pH. The results above suggested that E coli might have a negative impact on female reproductive tract infection, tubal obstruction, and fertility, which deserves serious attention.
Prevotella intermedia was isolated from endometrial cultures of women with acute salpingitis.50 Since the pelvic inflammatory disease is caused by microorganisms that ascend from the vagina and endocervix to the endometrium and fallopian tubes, P intermedia may cause inflammation of the endometrium and fallopian tubes in succession, affecting female reproductive health.
Studies also have found that Shewanella colonized in the female reproductive tract in women of reproductive age, including the peritoneal fluid from the pouch of Douglas (5.6%), the endometrium (3.38%), and the fallopian tubes (1.81%).51 There are several reports describing the soft‐tissue and wound infection caused by Shewanella spp.52 However, the effects of Shewanella on the female reproductive tract have not been thoroughly studied.
The results above suggest that vaginal bacteria may affect the patency of fallopian tubes by causing the female genital infection.
In addition, we found that women with normal menstrual cycles have more unclassified Ochrobactrum in the vagina than women with the prolonged menstrual cycle, which is an emerging opportunistic pathogen in immunocompromised patients.53 It should be noted that although subjects with mycoplasma or chlamydia infection were excluded according to the clinical laboratory tests, some subjects were found to had Mycoplasma or Ureaplasma colonized in the vagina with a relative abundance of <5%. This may be due to the higher sensitivity and precision of 16S rRNA sequencing.
The vaginal microbiome played a central role in protecting and influencing the health of the vaginal environment. It was clinically meaningful to study how the fertility factors affect the vaginal microbiome in women of reproductive age. These results were progressive and mutually confirmed, proving that the pathological state of reproductive endocrine and genital tract could affect the composition of the vaginal microbiome. It also suggested that we can simultaneously detect the vaginal microbiome as an auxiliary assessment when testing the fertility‐related clinical indexes for the evaluation of female fertility. However, the gonadal axis involves a complex interaction between the hypothalamus, pituitary gland, and the gonads. These glands often act in concert to regulate development, reproduction, aging, and many other body processes. In the analysis of clinical trials, it was difficult to completely control the multiple variables and only study the effect of one single variable.
It should be noted that the levels of basic sexual hormones were measured during the early follicular phase (day 1‐5), not fixed on the 3rd of the menstrual cycle. It may have slight fluctuations during this period, which may affect the accuracy of correlation analysis between the reproductive endocrine and vaginal microbiome. Furthermore, the samples of vaginal microbiome were obtained during the menstrual interval. Though the previous study showed that the vaginal bacterial communities seemed resilient or almost invariant during the menstrual cycle,54 the sex hormone levels on the day of sampling may have an effect on the vaginal microbiome. Moreover, with the small sample size, cautions must be applied, and the actual relevance and clinical significance remain to be verified through a series of clinical control studies and physiological experiments.
5. CONCLUSIONS
The fertility factors associated with reproductive endocrine and the genital tract environment had effects on the vaginal microbiome in women of reproductive age. The bacterial species Escherichia coli, Lactobacillus iners, Streptococcus agalactiae, Prevotella intermedia, etc could be used as biomarkers to reflect the pathological state of reproductive endocrine and genital tract.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
HUMAN SUBJECTS APPROVAL
This study was approved by the Ethical Committee of Ren Ji Hospital, Shanghai Jiao Tong University School of Medicine (Approval No. 2016012904).
PUBLIC SHARING OF DATA
Sequence data were archived in the NCBI Sequence Read Archive (SRA) under accession number SRP136384.
Supporting information
ACKNOWLEDGMENTS
This study was supported by the National Key Research and Development Program of China (2017YFC1001002 and 2018YFC1003202), by the National Natural Science Foundation of China (NSFC)(81671414)(81490743), by the Chinese University of Hong Kong‐Shanghai Jiao Tong University Joint Research Collaboration Fund (4750357), and by the Shanghai Commission of Science and Technology (17DZ2271100).
Xu J, Bian G, Zheng M, et al. Fertility factors affect the vaginal microbiome in women of reproductive age. Am J Reprod Immunol. 2020;83:e13220 10.1111/aji.13220
Jieying Xu, Gaorui Bian and Min Zheng should be considered joint first authors.
Yanzhi Du, Kaiping Yang and Zi‐Jiang Chen should be considered joint senior authors.
Funding information
Dr Chen has support from the National Natural Science Foundation of China (NSFC) (81490743) and Shanghai Commission of Science and Technology (17DZ2271100). Dr Li has support from the National Key Research and Development Program of China (2017YFC1001002) and the Chinese University of Hong Kong‐Shanghai Jiao Tong University Joint Research Collaboration Fund (4750357). Dr Du has support from the National Natural Science Foundation of China (NSFC) (81671414) and the National Key Research and Development Program of China (2018YFC1003202). The above funding sources had no role in the study design, collection/analysis/interpretation of data, or manuscript preparation.
Contributor Information
Kaiping Yang, Email: kyang@uwo.ca.
Zi‐Jiang Chen, Email: Chenzijiang@hotmail.com.
Yanzhi Du, Email: duyz@sjtu.edu.cn.
REFERENCES
- 1. Zhou X, Bent SJ, Schneider MG, et al. Characterization of vaginal microbial communities in adult healthy women using cultivation‐independent methods. Microbiology. 2004;150(Pt 8):2565‐2573. [DOI] [PubMed] [Google Scholar]
- 2. Farage MA, Miller KW, Sobel JD. Dynamics of the vaginal ecosystem‐hormonal influences. Infect Dis Res Treat. 2010;3:IDRT.S3903. [Google Scholar]
- 3. Wira CR, Rodriguez‐Garcia M, Patel MV. The role of sex hormones in immune protection of the female reproductive tract. Nat Rev Immunol. 2015;15(4):217‐230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wessels JM, Felker AM, Dupont HA, Kaushic C. The relationship between sex hormones, the vaginal microbiome and immunity in HIV‐1 susceptibility in women. Dis Model Mech. 2018;11(9):dmm035147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vitali D, Wessels JM, Kaushic C. Role of sex hormones and the vaginal microbiome in susceptibility and mucosal immunity to HIV‐1 in the female genital tract. Aids Res Ther. 2017;14(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Silva C, Rey R, Elena NM. Effects of estrogen administration on the colonization capability of lactobacilli and Escherichia coli in the urinary tracts of mice. Methods Mol Biol. 2004;268:387‐399. [DOI] [PubMed] [Google Scholar]
- 7. Osset J, Bartolome RM, Garcia E, Andreu A. Assessment of the capacity of Lactobacillus to inhibit the growth of uropathogens and block their adhesion to vaginal epithelial cells. J Infect Dis. 2001;183(3):485‐491. [DOI] [PubMed] [Google Scholar]
- 8. Mastromarino P, Brigidi P, Macchia S, et al. Characterization and selection of vaginal Lactobacillus strains for the preparation of vaginal tablets. J Appl Microbiol. 2002;93(5):884‐893. [DOI] [PubMed] [Google Scholar]
- 9. Reid G, Younes JA, Van der Mei HC, et al. Microbiota restoration: natural and supplemented recovery of human microbial communities. Nat Rev Microbiol. 2011;9(1):27‐38. [DOI] [PubMed] [Google Scholar]
- 10. Reid G, Dols J, Miller W. Targeting the vaginal microbiota with probiotics as a means to counteract infections. Curr Opin Clin Nutr Metab Care. 2009;12(6):583‐587. [DOI] [PubMed] [Google Scholar]
- 11. Li J, McCormick J, Bocking A, Reid G. Importance of vaginal microbes in reproductive health. Reprod Sci. 2012;19(3):235‐242. [DOI] [PubMed] [Google Scholar]
- 12. Brotman RM, Shardell MD, Gajer P, et al. Association between the vaginal microbiota, menopause status, and signs of vulvovaginal atrophy. Menopause. 2014;21(5):450‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mirmonsef P, Modur S, Burgad D, et al. Exploratory comparison of vaginal glycogen and Lactobacillus levels in premenopausal and postmenopausal women. Menopause. 2015;22(7):702‐709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Farage MA, Miller KW, Song Y, Sobel J. The Vaginal Microbiota in Menopause. 2017;1417‐1431.
- 15. Shen J, Song N, Williams CJ, et al. Effects of low dose estrogen therapy on the vaginal microbiomes of women with atrophic vaginitis. Sci Rep. 2016;6:24380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Amsel R, Totten PA, Spiegel CA, et al. Nonspecific vaginitis: Diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983;74(1):14‐22. [DOI] [PubMed] [Google Scholar]
- 17. Zhao Y, Kolp L, Yates M, Zacur H. Clinical evaluation of female factor infertility. 2010;133‐146.
- 18. Mao S, Zhang M, Liu J, Zhu W. Characterising the bacterial microbiota across the gastrointestinal tracts of dairy cattle: membership and potential function. Sci Rep. 2015;5:16116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high‐throughput community sequencing data. Nat Methods. 2010;7(5):335‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods. 2013;10(10):996‐998. [DOI] [PubMed] [Google Scholar]
- 21. Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73(16):5261‐5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Šmilauer P,Lepš J. Multivariate Analysis of Ecological Data using CANOCO 5. Cambridge, England: Cambridge University Press; 2014. [Google Scholar]
- 23. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc: Ser B. 1995;57(1):289‐300. [Google Scholar]
- 24. Segata N, Izard J, Waldron L, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12(6):R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kaambo E, Africa C, Chambuso R, Passmore JS. Vaginal microbiomes associated with aerobic vaginitis and bacterial vaginosis. Front Public Health. 2018;6:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. High KP, Edwards MS, Baker CJ. Group B streptococcal infections in elderly adults. Clin Infect Dis. 2005;41(6):839‐847. [DOI] [PubMed] [Google Scholar]
- 27. Edwards MS, Rench MA, Palazzi DL, Baker CJ. Group B streptococcal colonization and serotype‐specific immunity in healthy elderly persons. Clin Infect Dis. 2005;40(3):352‐357. [DOI] [PubMed] [Google Scholar]
- 28. Regan JA, Klebanoff MA, Nugent RP, et al. Colonization with group B streptococci in pregnancy and adverse outcome. VIP Study Group. Am J Obstet Gynecol. 1996;174(4):1354‐1360. [DOI] [PubMed] [Google Scholar]
- 29. Krohn MA, Hillier SL, Baker CJ. Maternal peripartum complications associated with vaginal group B streptococci colonization. J Infect Dis. 1999;179(6):1410‐1415. [DOI] [PubMed] [Google Scholar]
- 30. Phares CR, Lynfield R, Farley MM, et al. Epidemiology of invasive group B streptococcal disease in the United States, 1999–2005. JAMA. 2008;299(17):2056‐2065. [DOI] [PubMed] [Google Scholar]
- 31. Zaleznik DF, Rench MA, Hillier S, et al. Invasive disease due to group B Streptococcus in pregnant women and neonates from diverse population groups. Clin Infect Dis. 2000;30(2):276‐281. [DOI] [PubMed] [Google Scholar]
- 32. Smotrich DB, Widra EA, Gindoff PR, et al. Prognostic value of day 3 estradiol on in vitro fertilization outcome. Fertil Steril. 1995;64(6):1136‐1140. [PubMed] [Google Scholar]
- 33. Ravel J, Gajer P, Abdo Z, et al. Vaginal microbiome of reproductive‐age women. Proc Natl Acad Sci. 2011;108(Supplement_1):4680‐4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mendes‐Soares H, Suzuki H, Hickey RJ, Forney LJ. Comparative functional genomics of Lactobacillus spp. reveals possible mechanisms for specialization of vaginal lactobacilli to their. Environment. J Bacteriol. 2014;196(7):1458‐1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Witkin SS, Mendes‐Soares H, Linhares IM, et al. Influence of vaginal bacteria and D‐and L‐lactic acid isomers on vaginal extracellular matrix metalloproteinase inducer: implications for protection against upper genital tract infections. MBio. 2013;4(4):e00460‐e513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Petrova MI, Reid G, Vaneechoutte M, Lebeer S. Lactobacillus iners: Friend or Foe? Trends Microbiol. 2017;25(3):182‐191. [DOI] [PubMed] [Google Scholar]
- 37. Mastromarino P, Macchia S, Meggiorini L, et al. Effectiveness of Lactobacillus‐containing vaginal tablets in the treatment of symptomatic bacterial vaginosis. Clin Microbiol Infec. 2009;15(1):67‐74. [DOI] [PubMed] [Google Scholar]
- 38. De Seta F, Parazzini F, De Leo R, et al. Lactobacillus plantarum P17630 for preventing Candida vaginitis recurrence: a retrospective comparative study. Eur J Obstet Gynecol Reprod Biol. 2014;182:136‐139. [DOI] [PubMed] [Google Scholar]
- 39. Dumitrescu R, Mehedintu C, Briceag I, Purcarea VL, Hudita D. The polycystic ovary syndrome: an update on metabolic and hormonal mechanisms. J Med Life. 2015;8(2):142‐145. [PMC free article] [PubMed] [Google Scholar]
- 40. Sun L, Ye J, Wang Y, et al. Elevated basal luteinizing hormone does not impair the outcome of human menopausal gonadotropin and medroxyprogesterone acetate treatment cycles. Sci Rep. 2018;8(1):13835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Haahr T, Jensen JS, Thomsen L, et al. Abnormal vaginal microbiota may be associated with poor reproductive outcomes: a prospective study in IVF patients. Hum Reprod. 2016;31(4):795‐803. [DOI] [PubMed] [Google Scholar]
- 42. Xu S, Zong L, Liu M, et al. Illumina sequencing 16S rRNA tagging reveals diverse vaginal microbiomes associated with bacterial vaginosis. Nan Fang Yi Ke Da Xue Xue Bao. 2013;33(5):672‐677. [PubMed] [Google Scholar]
- 43. Verhelst R. Characterization of the vaginal microflora. 2006.
- 44. Martins WP, Kollmann M, Raine‐Fenning N. Counting ovarian follicles: updated threshold for diagnosis of hyperandrogenic anovulation. Ultrasound Obstet Gynecol. 2014;44(2):131‐134. [DOI] [PubMed] [Google Scholar]
- 45. Liu R, Zhang C, Shi Y, et al. Dysbiosis of Gut Microbiota Associated with Clinical Parameters in Polycystic Ovary Syndrome. Front Microbiol. 2017;8:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Megli C, Prince A, Slayden O, et al. A distinct cervicovaginal microbiome dysbiosis precedes adverse reproductive outcomes in a primate model of PCOS. Am J Obstet Gynecol. 2019;220(1):S643. [Google Scholar]
- 47. Ata B, Yildiz S, Turkgeldi E, et al. The Endobiota Study: Comparison of Vaginal, Cervical and Gut Microbiota Between Women with Stage 3/4 Endometriosis and Healthy Controls. Sci Rep. 2019;9(1):2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Obata‐Yasuoka M, Ba‐Thein W, Tsukamoto T, Yoshikawa H, Hayashi H. Vaginal Escherichia coli share common virulence factor profiles, serotypes and phylogeny with other extraintestinal E. coli. Microbiology+. 2002;148(9):2745‐2752. [DOI] [PubMed] [Google Scholar]
- 49. Krohn MA, Thwin SS, Rabe LK, Brown Z, Hillier SL. Vaginal colonization by escherichia coli as a risk factor for very low birth weight delivery and other perinatal complications. J Infect Dis. 1997;175(3):606‐610. [DOI] [PubMed] [Google Scholar]
- 50. Soper DE, Brockwell NJ, Dalton HP, Johnson D. Observations concerning the microbial etiology of acute salpingitis. Am J Obstet Gynecol. 1994;170(4):1008‐1017. [DOI] [PubMed] [Google Scholar]
- 51. Chen C, Song X, Wei W, et al. The microbiota continuum along the female reproductive tract and its relation to uterine‐related diseases. Nat Commun. 2017;8(1):875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sharma KK, Kalawat U. Emerging infections: shewanella ‐ a series of five cases. J Lab Physicians. 2010;2(2):61‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mudshingkar SS, Choure AC, Palewar MS, Dohe VB, Kagal AS. Ochrobactrum anthropi: an unusual pathogen: are we missing them? Indian J Med Microbiol. 2013;31(3):306‐308. [DOI] [PubMed] [Google Scholar]
- 54. Gajer P, Brotman RM, Bai G, et al. Temporal dynamics of the human vaginal microbiota. Sci Transl Med. 2012;4(132):132ra52‐132ra52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


