Abstract
Some therapies for diabetes increase the risk of hypoglycaemia, in particular all insulins and insulin secretagogues, including the glinides and sulfonylureas. Hypoglycaemia remains a major limiting factor to successful glycaemic management, despite the availability of prevention options such as insulin analogues, continuous glucose monitoring, insulin pumps, and dogs that have been trained to detect hypoglycaemia. Non‐severe (self‐treated) and severe (requiring assistance for recovery) hypoglycaemia rates are higher in people with type 1 diabetes, but those with insulin‐treated type 2 diabetes are also at risk. Education and regular review are essential between people with diabetes and their caregivers and healthcare professionals about symptoms, prevention and treatment. Awareness of the potential dangers of hypoglycaemia is fundamental to the optimal management of diabetes. When therapy is intensified to achieve glycaemic targets, it is important that people at risk of severe hypoglycaemia, and particularly their caregivers, have ready access to effective treatment for hypoglycaemia emergencies. The current and potential formulations of glucagon available for treatment of severe hypoglycaemia are reviewed.
Keywords: glucagon, severe hypoglycaemia, type 1 diabetes, type 2 diabetes
1. INTRODUCTION
Chronic hyperglycaemia associated with diabetes can result in long‐term vascular and tissue dysfunction and damage, and organ failure. Good glycaemic control is therefore a major aim in the treatment of diabetes. Therapeutic options that are currently available for diabetes include different types and formulations of insulin, glucagon‐like peptide‐1 receptor agonists, glinides, sulfonylureas, dipeptidyl peptidase‐4 inhibitors and thiazolidinediones. Some of these drug classes, particularly insulin and the insulin secretagogues, increase the risk of hypoglycaemia.
Hypoglycaemia associated with insulin therapy is much feared by people with diabetes, and also by their relatives, and is often a major barrier to maintaining good glycaemic control.1 People often do not discuss their fear of hypoglycaemia with their healthcare providers (HCPs), and it may be associated with behaviours like defensive snacking, decreased physical activity, reduced insulin dosage and suboptimal management of blood glucose.2, 3, 4 As a result, HCPs increase doses of oral drugs or insulin, or prescribe additional medications to improve glycaemic control. This may provoke further hypoglycaemia, causing people to become even more fearful about hypoglycaemia risks. New treatments for severe hypoglycaemia, which may help to alleviate ongoing concerns and fear of hypoglycaemia, have recently been approved or are in development and are included in this review.
1.1. Epidemiology of Hypoglycaemia
Hypoglycaemia is potentially one of the most serious acute adverse effects of therapies for diabetes. Clinical and functional criteria define different degrees of hypoglycaemia severity: non‐severe and severe.5 Non‐severe hypoglycaemia events usually generate autonomic and/or neuroglycopenic symptoms, which enable the individual to identify the onset, and to treat the falling blood glucose without requiring assistance. Severe hypoglycaemia is an event associated with impaired cognitive and/or physical functioning, and the progressive neuroglycopenia interferes with the ability to self‐treat so the assistance of another person is necessary for recovery.6
Reviews comparing randomized controlled trials (RCTs) and observational studies in real‐world settings in adults with diabetes who received insulin treatment have indicated that RCTs underestimate the incidence and prevalence of hypoglycaemia that are observed in real‐world settings.7, 8 Incidence rates of severe hypoglycaemia in people with type 1 diabetes (T1D) ranged from 70 to 159 events/100 person‐years.7 For people with type 2 diabetes (T2D), the frequency of hypoglycaemia varied depending on the type of insulin regimen employed. Incidence rates ranged from 0 to 12, 0 to 20, and 0 to 20 per 100 person‐years in people on a basal‐oral regimen, a basal‐bolus regimen, and a premix regimen, respectively.7 However, hypoglycaemia incidence rates in T2D, regardless of insulin regimen, were generally about one third of those observed in T1D.7
1.2. Severe hypoglycaemia in T1D
In unselected cohorts that included people with T1D who were at high risk of hypoglycaemia, the incidence of severe hypoglycaemia was much higher than that observed previously in the Diabetes Control and Complications Trial (DCCT),1 a widely cited prospective study, in which the participants were selected on the basis of having a low risk of severe hypoglycaemia. Compared with the DCCT, in which the intensively treated group experienced 0.62 events/person/year, in unselected cohorts the incidence rate ranged from 0.98 to 3.2 events/person/year.1 The incidence of severe hypoglycaemia also rose with increasing duration of T1D.1
1.3. Severe hypoglycaemia in T2D
In a global meta‐analysis, pooled incidence data from 19 observational studies with 76 254 people with T2D reported an incidence rate of severe hypoglycaemia of 80 (95% CI: 0, 215) events/100‐person years.8 When stratified by treatment type, pooled mean incidence rates of 105 (0, 369) and 1 (0, 55) event(s)/100‐person years were estimated for people on insulin and sulfonylureas, respectively. The frequencies of severe and non‐severe hypoglycaemia increased the longer patients with T2D were treated with multiple daily injections of insulin.9
2. SEVERE HYPOGLYCAEMIA
2.1. Pathophysiology
In β‐cell failure, as occurs in T1D and in advanced T2D, normal physiology is disrupted, whereby a decrease in plasma glucose does not suppress β‐cell insulin secretion, and the signal to increase pancreatic α‐cell glucagon secretion during hypoglycaemia is absent.10, 11 Additionally, the sympathoadrenal response is reduced with diminished release of catecholamines, which would normally be secreted to raise blood glucose and thus compensate for a deficiency of glucagon; this results in compromised glucose counter‐regulation and is also associated with impairment of hypoglycaemia awareness.10, 11 Although excess insulin per se is sufficient to cause hypoglycaemia, the influence of both relative or absolute therapeutic hyperinsulinaemia, and compromised physiological and behavioural defences against falling plasma glucose concentrations, ultimately contribute to iatrogenic hypoglycaemia.12
Hypoglycaemia can induce a myriad of pathophysiological adverse effects including oxidative stress,13 result in life‐threatening events such as cardiac arrhythmias,14 contribute to sudden cardiovascular death,14 and cause ischaemic cerebral damage.15, 16 These responses may potentially exert adverse effects via various mechanisms whereby acute and chronic hypoglycaemia events could increase cardiovascular risk.17 Evidence suggests an association between severe hypoglycaemia and mortality among patients with diabetes.18, 19 In the Action in Diabetes and Vascular Disease (ADVANCE) trial, death from cardiovascular and non‐cardiovascular causes was associated with severe hypoglycaemia.19 That trial included 11 140 patients with T2D, and 19.5% of those who reported severe hypoglycaemia died versus 9.0% of those who did not.19 Of those who died, 49% died from cardiovascular causes in those who reported severe hypoglycaemia compared with 53% in those who reported no hypoglycaemia.19 Possible reasons for the impact of hypoglycaemia upon cardiovascular death were assumed to be sympathoadrenal activation, increased thrombogenesis or inflammation.19 Additionally, while 16.8% and 11.5% of patients who had reported severe hypoglycaemia experienced major subsequent macro‐ and microvascular events, respectively, only 10.2% and 10.1% of patients who had no reported severe hypoglycaemia experienced events.19 However, the authors cautioned that hypoglycaemia should not be causally linked to the outcomes reported in the ADVANCE trial, rather that hypoglycaemia might instead indicate a vulnerability to adverse events.19 Similar results were reported in the Action to Control Cardiovascular Risk in Diabetes trial, whereby a history of severe hypoglycaemia was associated with higher mortality, and the authors cautioned that hypoglycaemia might be a risk factor for cardiovascular disease or an indicator of patient vulnerability.18
2.2. Risk factors
Impaired awareness of hypoglycaemia is one of the major risk factors for severe hypoglycaemia; awareness and the physiological responses to hypoglycaemia are diminished, increasing the risk of further hypoglycaemia events.11, 20 Additional hypoglycaemia risk factors can be categorized as clinical (eg, increased insulin sensitivity), medication‐related (eg, insulin therapy) and lifestyle factors (eg, delayed or missed meals) (Table 1).
Table 1.
Risk factors for hypoglycaemia
| Type | Risk factor |
|---|---|
| Clinical86, 87, 88 | Increased insulin sensitivity |
|
Long duration of diabetes (type 1 diabetes) Increasing duration of insulin therapy (type 2 diabetes) Extremes of age (very young and very old) |
|
| Renal or hepatic impairment | |
| Previous severe hypoglycaemia events | |
|
Impaired awareness of hypoglycaemia C‐peptide negativity |
|
| Medication‐related22, 86, 87, 89, 90 | Insulin therapy |
| Insulin secretagogues (sulfonylureas, glinides) | |
| Concomitant medication that aggravates hypoglycaemia or masks hypoglycaemic symptoms | |
| Lifestyle22, 87 |
Delayed or missed meal Errors in administration of medication |
| Alcohol consumption | |
| Exercise |
Some patient groups have a higher risk of hypoglycaemia. Very young and older people have an increased risk as they may have difficulties in recognizing the onset of hypoglycaemia, and/or asking for assistance.21 People with diabetes who do not understand therapeutic principles, or do not have adequate education about the risk of hypoglycaemia, also have an increased risk of hypoglycaemia.22
2.3. Ways to limit hypoglycaemia
Several prevention and treatment options have been evaluated to reduce the occurrence of hypoglycaemia. In general, while insulin analogues reduce the frequency of non‐severe hypoglycaemia, they have not had a significant impact on minimizing the risk of severe hypoglycaemia. Both flash glucose monitoring and continuous glucose monitoring (CGM) have been shown to reduce the frequency of severe hypoglycaemia in people treated with insulin.23, 24, 25, 26, 27 Real‐time CGM can reduce the frequency of severe hypoglycaemia in people with an impaired awareness of hypoglycaemia28 and in those with long‐standing T1D.29 The use of continuous subcutaneous insulin infusion with insulin pumps has shown that the frequency of hypoglycaemia can be reduced,30 and increasing sophistication with the addition of insulin suspension has further lowered the risk of severe hypoglycaemia.31, 32 Dogs that have been trained to identify the onset of hypoglycaemia have been shown to reliably detect diurnal and nocturnal hypoglycaemia episodes and improve the clinical and psychosocial outcomes in people with insulin‐treated diabetes.33 Lastly, various adjunctive therapeutic agents, including beta‐adrenoreceptor agonists,34 adenosine‐receptor antagonists (ie, theophylline),35 modafinil36 and caffeine,37 have been tried for the prevention and treatment of hypoglycaemia. Despite advancements in technology and therapeutics to reduce the occurrence of hypoglycaemia, severe hypoglycaemia remains a major concern of successful glycaemic management.
2.4. Tools to identify people with diabetes and at risk of hypoglycaemia
The risk factors discussed above can be used to identify people with T2D who are at greater risk of developing hypoglycaemia. A validated risk stratification tool based upon six inputs is available to identify those with T2D at risk of hypoglycaemia.38 The tool identifies those at low, intermediate and high risk of hypoglycaemia, and it has the potential to facilitate targeted intervention management to reduce hypoglycaemia and improve patient safety.38 The International Hypoglycaemia Study Group (IHSG) has developed a tool for patients and their caregivers,39 and also a tool for HCPs40 to identify those with diabetes at risk of severe hypoglycaemia. Both tools identify those at low, moderate and high risk, and also provide treatment recommendations and risk‐reduction strategies for each risk range.39, 40
2.5. Education on how to treat a severe hypoglycaemia event
Several treatment guidelines are available to educate people and their caregivers to prevent or minimize the risk of hypoglycaemia. A working group of the American Diabetes Association (ADA) and the Endocrine Society has recommended that people with diabetes and their caregivers receive formal training and education on recognition of hypoglycaemia symptoms and effective treatment of hypoglycaemia.41 Recommendations for HCPs include assessment of the risk of hypoglycaemia by clinicians and educators at every consultation with people who are being treated with insulin and insulin secretagogues, and have knowledge of the pharmacokinetics (PK) of medications.41 People who have developed an impaired awareness of hypoglycaemia require specific therapeutic strategies, including frequent blood glucose testing or the use of CGM, frequent snacks and meals and the possible use of continuous subcutaneous insulin infusion, all of which may be valuable prophylactic measures.42 Training strategies are also available for people with an impaired awareness of hypoglycaemia to identify subtle warning symptoms of hypoglycaemia.41, 43
Psychoeducational interventions, like Blood Glucose Awareness Training and HypoAware, are available to help people with diabetes to effectively recognize, treat and prevent hypoglycaemia.43, 44 It is possible that psychosocial interventions can significantly reduce severe hypoglycaemia events, which would have a beneficial effect on psychosocial outcomes and healthcare costs. Additionally, these interventions, along with adequate self‐monitoring of blood glucose, regular discussion of treatment regimens and reviews of dietary intake, may contribute to better glycaemic control and subsequent reduction in fear of hypoglycaemia.
3. CURRENT TREATMENTS FOR SEVERE HYPOGLYCAEMIA
In people with insulin‐treated diabetes, plasma insulin concentrations cannot be regulated because of passive absorption of the injected insulin; as glucose levels fall, plasma insulin concentrations do not decline.45 Additionally, as blood glucose falls, glucagon secretion does not increase.46 Glucagon raises blood glucose concentration in the fasting state by increasing the hepatic production of glucose, initially via glycogenolysis and subsequently via gluconeogenesis. In the fed state in a person without diabetes, secretion of glucagon from α‐cells is inhibited by insulin, which signals to the liver to stop producing endogenous glucose.47 In people with T1D or advanced T2D, hypoglycaemia arises from a combination of relative or absolute insulin excess and impaired physiological defences against a fall in plasma glucose.48
Treatment of hypoglycaemia depends upon the severity and duration of the hypoglycaemia event, and is represented by a spectrum of increasing therapeutic complexity.49 At one end of the spectrum is self‐administration of oral carbohydrate, and at the other extreme is the administration of intravenous dextrose or glucagon, often in a hospital setting (Table 2). Current ADA standards of care for glycaemic control provide guidance regarding the prevention and treatment of hypoglycaemia. The ADA has recommended that people at risk of hypoglycaemia should be asked by their HCP about the occurrence of symptomatic and asymptomatic hypoglycaemia at each consultation.6 Oral glucose (15–20 g) is the preferred treatment for the fully conscious individual with impending hypoglycaemia, measured as a blood glucose concentration of ≤3.9 mmol/L (70 mg/dL).6 The ADA recommends that HCPs advise people to treat hypoglycaemia with fast‐acting carbohydrates.6 Furthermore, the acute glycaemic response is better associated with the glucose content of food rather than the carbohydrate content; thus pure glucose is the preferred treatment but any carbohydrate that contains glucose can increase blood glucose.6 Treatment of hypoglycaemia by consuming 15–20 g of fast‐acting carbohydrates will usually raise blood glucose levels within 15 minutes.50, 51, 52, 53 Fifteen minutes after treatment, if self‐monitored blood glucose shows persisting hypoglycaemia, the treatment should be repeated until capillary blood glucose returns to normal, and should be followed by a meal or snack containing complex carbohydrate (slow‐acting) to prevent hypoglycaemia recurring.6 When an individual is unconscious or unable to swallow, glucagon should be administered.6
Table 2.
Treatment of hypoglycaemia
| Duration of hypoglycaemia | Administrator | Treatment |
|---|---|---|
|
minutes |
Patient | Oral carbohydrate (>20 g) |
| hours | Caregiver |
|
| Primary healthcare setting |
|
|
| Hospital setting |
|
Oral carbohydrate (20–40 g) should be given when consciousness has been regained.49
Glucagon should be prescribed for all individuals at increased risk of level 2 hypoglycaemia, defined as blood glucose <3.0 mmol/L (54 mg/dL), so that it is available in emergency situations.6 In a study of people with insulin‐treated diabetes who were admitted to the emergency department with severe hypoglycaemia, less than 10% filled a glucagon prescription within 90 days of their emergency department visit.54 However, severe hypoglycaemia events may not be apparent to HCPs, because 33%–50% of people with diabetes do not discuss their most recent severe hypoglycaemia event with their HCP; additionally, more than 40% of people wait more than a month to discuss the severe hypoglycaemia event.55 Caregivers, school personnel or family members of these individuals should be informed as to where glucagon is stored and know when and how to administer it. Importantly, glucagon administration is not limited to HCPs.
In children and adolescents, severe hypoglycaemia is defined by the International Society for Paediatric and Adolescent Diabetes (ISPAD) as an event associated with severe cognitive impairment (including coma and convulsions) requiring external assistance from another person to actively administer carbohydrates, glucagon or other corrective actions.56 A subgroup of severe hypoglycaemia is severe hypoglycaemic coma, which is defined as a severe hypoglycaemia event resulting in coma or seizure requiring parenteral therapy. ISPAD guidelines recommend that glucagon should be readily accessible to all parents and caregivers, especially when there is a high risk of severe hypoglycaemia.56 Additionally, the guidelines note that education on how glucagon should be administered is essential.
Currently available treatments for severe hypoglycaemia, when a person is unable to safely swallow oral carbohydrates, are limited to intravenous dextrose, injectable glucagon emergency kits, and recently approved nasal glucagon (United States approval was granted in July 2019) and liquid glucagon rescue pen (FDA approval was granted in September 2019). Intravenous dextrose requires administration by HCPs or medically trained professionals within a hospital or emergency medical setting.57, 58 Different concentrations of dextrose are used in prehospital and hospital settings (ie, 5%–50%).57, 58 Stronger concentrations can cause rebound hyperglycaemia, and localized tissue damage if it leaks out of the vein59; thus, injectable glucagon and nasal glucagon are the only therapeutic options for caregivers to use outside of these settings. When delivered correctly, glucagon is efficacious as a rescue therapy for severe hypoglycaemia.60 The Glucagon Emergency Kit (Eli Lilly and Co, Indianapolis, IN)61 and the GlucaGen HypoKit (Novo Nordisk A/S, Bagsværd, Denmark)62 are the currently available glucagon kits administered by intramuscular or subcutaneous injection.48
Because aqueous glucagon is unstable it must be used immediately63; the currently available glucagon emergency kits contain powdered glucagon that must be reconstituted using a multiple‐step process before the drug can be administered parenterally.61, 62 During the stressful situation that surrounds the attempt to treat severe hypoglycaemia, caregivers may become overwhelmed and panic so that they do not know what to do, or they forget the necessary steps to be taken to prepare and administer glucagon by injection.64 Additionally, caregivers may be fearful or reluctant to administer glucagon injections with the concern that they may injure the person with hypoglycaemia.48 The glucagon dose requirement may vary based on factors like a person's body weight, which may place the added burden of dose calculation on those administering glucagon.61, 62 Overall, these glucagon kits require several steps61, 62 and are not user‐friendly65 (Figure 1), which can be perceived to be both an inconvenience and a serious risk to safety if the glucagon is not reconstituted correctly and the correct dose is not administered in an emergency. There continues to be an unmet need for an effective, safe and easily administered treatment for severe hypoglycaemia.
Figure 1.
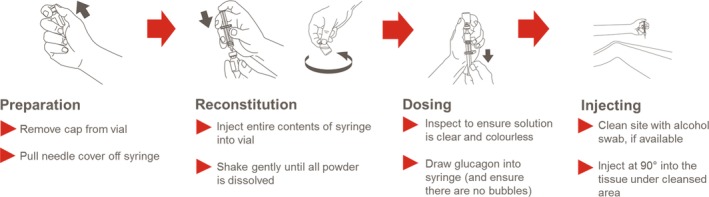
Injectable glucagon administration: steps required
4. STUDIES SUPPORTING NASAL GLUCAGON FOR TREATMENT OF SEVERE HYPOGLYCAEMIA
To date, nasal glucagon is the only newly approved glucagon treatment option since the launch of emergency glucagon kits. Nasal glucagon is a ready‐to‐use drug/device combination to treat severe hypoglycaemia in people with diabetes aged ≥4 years.66 A 3 mg dose of nasal glucagon dry powder, which does not require reconstitution, is administered by inserting the tip of the single‐use device into the patient's nostril and completely depressing the plunger until the line at the bottom of the plunger is no longer visible (Figure 2).66 Nasal glucagon is passively absorbed through the anterior nasal mucosa without the need for inhalation,67 making it suitable for a comatose person with profound neuroglycopenia. Key studies that were included in the development programme for nasal glucagon and the treatment of severe hypoglycaemia were a simulated usability study,68 adult and paediatric efficacy and safety studies,67, 69 adult and paediatric real‐world effectiveness studies,70, 71 and a study to evaluate the effect of nasal congestion on the PK/pharmacodynamics (PD) of nasal glucagon.72
Figure 2.
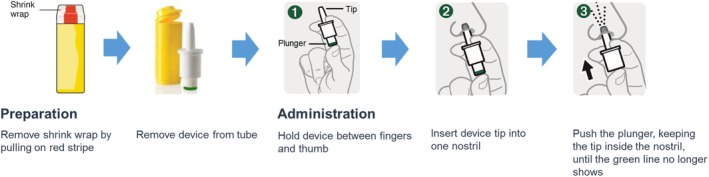
Nasal glucagon administration steps
4.1. Simulated usability studies
In a simulation study involving caregivers who were familiar with insulin‐treated diabetes and the treatment of hypoglycaemia, and untrained acquaintances (referred to as participants hereafter) with no previous experience, the use of nasal glucagon and a commercially available glucagon emergency kit, providing 1 mg glucagon by intramuscular injection, were compared for ease of use in treating simulated events of severe hypoglycaemia.68 Most participants (>90%) delivered full doses of nasal glucagon, whereas only 13% of the caregivers and none of the untrained participants delivered full doses of intramuscular glucagon, despite having access to a comprehensive description of the steps of administration for its use.68 The mean time to deliver nasal glucagon was <30 seconds compared with several minutes (full dose delivery, 1.89 minutes; partial dose delivery, 2.40 minutes) for the injectable form.68 It was particularly encouraging that the uninstructed participants were as successful as the instructed caregivers in administering nasal glucagon, showing the simplicity of this method and its ease of use.
When asked which glucagon treatment they would prefer their friends or family members to use, 69% of people with diabetes preferred nasal glucagon to injectable glucagon, 19% preferred injectable glucagon over nasal glucagon, and 13% did not state a preference.68 Those who preferred nasal glucagon thought that the nasal glucagon device was easier to use, easier to teach, and easier to carry with no possibility of needle breakage or accidental needle stick injury.68 Those who preferred injectable glucagon stated that needles were better than nasal sprays, and that their families were already familiar with using needles.68 Eighty‐one per cent of caregivers preferred nasal glucagon to injectable glucagon, primarily because it was easier and less stressful to use, and less embarrassing compared with injectable glucagon.68 Injectable glucagon was preferred by two (13%) caregivers; one reported that injectable glucagon would be more effective than nasal glucagon, and another reported that although nasal glucagon was easier to administer, the syringe made it easier to see that the dose had been administered.68 Of note, neither of these caregivers who preferred injectable glucagon managed to administer a full dose of injectable glucagon during the simulation.68 One (6%) caregiver had no preference.68 For reasons such as ease of use of nasal glucagon and the fact that needles are intimidating, all uninstructed participants reported that they would recommend nasal glucagon, but not injectable glucagon.68 A similarly designed study evaluating user preference for nasal glucagon and injectable glucagon devices among trained and untrained participants reported similar results.73
4.2. Adult and paediatric efficacy and safety studies
In two randomized, crossover, multicentre, non‐inferiority studies in adults, the efficacy and safety of nasal glucagon (3 mg) was compared with that of glucagon emergency kit (1 mg intramuscular glucagon) in the treatment of insulin‐induced hypoglycaemia.69, 74 Treatment success was defined as an increase in plasma glucose to ≥3.9 mmol/L (70 mg/dL), or an increase of ≥1.1 mmol/L (20 mg/dL) from the glucose nadir within 30 minutes of receiving the study glucagon.69, 74 In both studies, nasal glucagon was shown to be non‐inferior to intramuscular glucagon for treatment of insulin‐induced hypoglycaemia in adults with T1D.69, 74 Reported mean times to treatment success were 16 minutes69 and 11.4 minutes74 for nasal glucagon and 13 minutes69 and 9.8 minutes74 for intramuscular glucagon. Commonly reported treatment‐emergent adverse events (≥10%) in those treated with nasal glucagon were nausea, vomiting and headache.69, 74 Upper respiratory tract irritation (eg, rhinorrhoea, nasal congestion and nasal discomfort events combined) was also frequently reported in the nasal glucagon‐treated patients.69, 74
In a paediatric study, the efficacy, safety and dose–response relationships of nasal glucagon were evaluated in children and adolescents aged 4 to <17 years with T1D.67 The study included three cohorts of children aged 4 to <8 (cohort 1), 8 to <12 (cohort 2) and 12 to <17 years (cohort 3).67 The evaluations in cohorts 1 and 2 were designed to compare the efficacy, safety and PK/PD of the 2 and 3 mg doses of nasal glucagon with one another, and with body weight‐based dose of intramuscular glucagon.67 Participants in cohort 3 were randomly assigned in a 1:1 ratio to receive either nasal glucagon 3 mg or glucagon emergency kit (1 mg intramuscular glucagon). Glucagon was given 5 minutes after the plasma blood glucose was <4.4 mmol/L (80 mg/dL).67 Treatment success was defined as an increase of ≥1.1 mmol/L (20 mg/dL) from the glucose nadir within 20 minutes of receiving the study glucagon.67 Treatment success was achieved with all 3 mg doses of nasal glucagon in those participants aged 4–16 years with T1D.67 The plasma glucose responses were similar for the 2 and 3 mg nasal glucagon doses and to that of intramuscular glucagon.67 Gastrointestinal adverse effects including nausea and vomiting were common with both intramuscular glucagon and nasal glucagon in those participants aged 4–16 years, which was consistent with the glucagon safety profile.67 Headache and nasal symptoms occurred more frequently with nasal glucagon across all age cohorts.67 These results support use of the 3 mg dose with no adjustment based on age or body weight. A single 3 mg intranasal dose appears to be appropriate for use across the entire age range from 4 to 16 years.
4.3. Adult and paediatric real‐world effectiveness studies
The effectiveness and ease of use of nasal glucagon (3 mg) in moderate or severe hypoglycaemia events were evaluated in two real‐world studies in adults, children and adolescents with T1D.70, 71 Nasal glucagon (3 mg) was effective in resolving 96% of hypoglycaemia events in adults with T1D within 30 minutes.71 All severe hypoglycaemia events (12 of 157 hypoglycaemia events) were resolved within 15 minutes.71 The time to nasal glucagon administration was <30 seconds for most (70%) hypoglycaemia events.71 The data from a caregiver‐reported questionnaire about each hypoglycaemia episode showed that the most common symptoms related to nasal glucagon administration were nasal irritation (59% of hypoglycaemia events) and headache (32% of hypoglycaemia events).71 Nausea and vomiting, well‐known adverse effects of glucagon, were reported in 13% and 7% of hypoglycaemia events, respectively.71 When surveyed, caregivers stated that they were satisfied with nasal glucagon use in 94% of hypoglycaemia events.71
Nasal glucagon (3 mg) was effective in resolving all moderate hypoglycaemia events in children and adolescents with T1D.70 The time to administer nasal glucagon was <30 seconds for most hypoglycaemia events.70 From the data obtained in the caregiver‐reported questionnaires about each hypoglycaemia episode, nasal discomfort was reported in 85% of hypoglycaemia events and headache in 54% of hypoglycaemia events.70 Nausea and vomiting were reported in 18% and 3% of hypoglycaemia events, respectively.70 Caregivers reported they were satisfied with nasal glucagon use in 93% of hypoglycaemia events.70
4.4. Effect of nasal congestion on nasal glucagon PK/PD
The effect of nasal congestion and decongestants on nasal glucagon have also been evaluated.72 PK and PD of nasal glucagon were not significantly affected by nasal congestion or nasal decongestants.72 Blood glucose increased within 5 minutes after nasal glucagon administration in all groups and peaked around 30–40 minutes.72 Incidence of adverse events was higher in participants with a common cold and was not affected by decongestant use.72
Nasal glucagon can be delivered by a caregiver of the person experiencing a severe hypoglycaemia event using a compact, portable, single‐use device with no reconstitution required. In addition, the data generated from the clinical development programme have shown a positive benefit/risk profile of nasal glucagon. An additional supportive study that evaluated user perceptions and preference for nasal glucagon and autoinjector devices suggests that people with diabetes prefer a device, like nasal glucagon, that is simple and ready to use.75 Nasal glucagon has the potential to substantially improve the treatment of severe hypoglycaemia, in which simplicity is important. Nasal glucagon might enable more caregivers to successfully administer rescue treatment for severe hypoglycaemia.
5. STUDIES SUPPORTING LIQUID GLUCAGON FOR TREATMENT OF SEVERE HYPOGLYCAEMIA
5.1. Liquid glucagon rescue pen
A novel, ready‐to‐use, body temperature stable, rescue pen containing liquid glucagon was recently approved for the treatment of severe hypoglycaemia in people with diabetes aged 2 years and older.76 The glucagon rescue pen is available in two premeasured doses: 0.5 mg for paediatric use and 1.0 mg for use in adolescents and adults.77, 78, 79 Administration of glucagon with the rescue pen is a two‐step process, with no need for reconstitution.77, 78, 79 The clinical development programme was conducted to evaluate efficacy and safety of the glucagon rescue pen, and to characterize the PK/PD. Key investigations included adult and paediatric efficacy and safety studies,77, 78, 79, 80 and a usability study.81
The time‐to‐treatment and usability of the glucagon rescue pen were evaluated and compared with currently available emergency glucagon kits in four separate studies: formative usability, summative human factors and two phase 3 studies. In the formative usability study, 87.5% of users successfully administered a complete glucagon injection using the glucagon rescue pen compared with only 31.3% of those using glucagon emergency kits.81 Most users (98.7%) were able to administer the rescue pen successfully in the summative human factors study.81 A significant improvement in preparation time with the glucagon rescue pen in comparison with the glucagon emergency kit users was also reported.81 In the formative usability study, the mean total administration time was 48 and 109 seconds with the glucagon rescue pen and the glucagon emergency kit, respectively.81 In the phase 3 study, drug preparation and administration time by trained HCPs was significantly shorter with the glucagon rescue pen (27 vs. 97 seconds).82
A phase 3, randomized, controlled, double‐blind, crossover clinical trial was conducted in adults with T1D to compare a subcutaneous 1 mg dose of glucagon administered by the glucagon rescue pen with glucagon given with a glucagon emergency kit to treat insulin‐induced hypoglycaemia.79 Efficacy was evaluated as either an increase in plasma glucose to ≥3.9 mmol/L (70 mg/dL) or an increase in plasma glucose of ≥1.1 mmol/L (20 mg/dL) from baseline glucose of <2.8 mmol/L (50 mg/dL) within 30 minutes of dosing.79 Efficacy was comparable between groups (glucagon rescue pen, 97.4%; glucagon emergency kit, 100%), and all participants were rescued from severe hypoglycaemia without requiring any additional measures.79 Glucose Cmax, Tmax and area under the curve (AUC, 0–90 minutes) were also comparable between groups.79 At each treatment visit, serial assessments of four autonomic and four neuroglycopenic symptoms and awareness of hypoglycaemia were also performed.78 The mean time to symptom relief and mean time to resolution of the general feeling of hypoglycaemia were comparable between the glucagon rescue pen and the glucagon emergency kit.78 The incidence of adverse events was low in both groups; and nausea (glucagon rescue kit, 20.5%; glucagon emergency kit, 12.7%) was the most commonly reported adverse event, followed by vomiting and headache.78
A second phase 3, randomized, controlled, single‐blind, crossover clinical trial was also conducted in adults with T1D to compare subcutaneous 1 mg doses of glucagon administered by the glucagon rescue pen with the glucagon emergency kit for the treatment of insulin‐induced severe hypoglycaemia. The study objective was to evaluate symptom relief during rescue treatment of severe hypoglycaemia. The mean time to symptom relief following glucagon administration was comparable between glucagon rescue pen and glucagon emergency kit for autonomic symptoms (9.9 ± 6.45 and 9.8 ± 6.86 minutes), neuroglycopenic symptoms (10.3 ± 8.92 and 9.9 ± 7.22 minutes), for average total symptom scores (13.0 ± 9.23 and 11.9 ± 7.57 minutes) and mean time to resolution of the general feeling of hypoglycaemia (11.6 ± 6.51 and 13.1 ± 7.93 minutes), respectively.80 Plasma glucose recovery was achieved for all participants with both treatments.80 The incidence of all adverse events was comparable among treatments; the most commonly reported event was mild to moderate nausea (glucagon rescue pen, 38.2%; glucagon emergency kit, 33.3%) followed by vomiting (glucagon rescue pen, 6.3%; glucagon emergency kit, 14.1%).80
Glucagon rescue pen has been evaluated for the treatment of hypoglycaemia in children with two age‐specific doses (2 to <6 years [0.5 mg dose], 6 to <12 years [0.5 mg dose] and 12 to <18 years [1.0 mg dose]).77 Plasma glucose <4.5 mmoL/L (80 mg/dL) was induced by intravenous infusion of insulin or by using an insulin pump. The primary efficacy endpoint was an increase in mean plasma glucose from baseline (the hypoglycaemic nadir) to 30 minutes after glucagon administration. Secondary endpoints were assessment of the time from dosing to achieve a 1.4 mmol/L (25 mg/dL) increase in plasma glucose and measurement of other PK/PD variables. Across the three age groups, statistically significant increments in mean plasma glucose from baseline to 30 minutes postglucagon administration were observed.77 All evaluable participants achieved the target glucose elevation of ≥1.4 mmol/L (25 mg/dL) from baseline.77 No pronounced differences across groups with respect to mean glucose AUC (0–90 minutes), Cmax and Tmax or median time to achieve the specified glucose increment were observed.77 Plasma glucagon AUC (0–240 minutes), Cmax and Tmax were similar across groups.77 The most commonly reported adverse events were nausea and vomiting.77
In summary, glucagon rescue pen is a ready‐to‐use, body temperature stable device with no reconstitution required, which can be used to treat a severe hypoglycaemia event quickly and effectively. The clinical development programme has generated data that are supportive of a positive benefit/risk profile for the glucagon rescue pen. The glucagon rescue pen has been shown to provide prompt relief of neuroglycopenic symptoms, which is critical in the treatment of severe hypoglycaemia.
6. EMERGING RESCUE THERAPIES FOR SEVERE HYPOGLYCAEMIA
Additional glucagon options are currently in development for the treatment of severe hypoglycaemia that offer unique formulation and administration compared with the currently approved glucagon kits. To date, data for some of these emerging therapies are not available as full peer‐reviewed reports, but only in limited form as abstracts and posters presented at scientific meetings. Thus detailed information regarding study design, patient characteristics and other relevant details is not available.
6.1. BioChaperone glucagon
A stable, ready‐to‐inject, aqueous formulation of human glucagon, BioChaperone glucagon (BCG), is currently being developed to treat severe hypoglycaemia. In a randomized, double‐blind, crossover study, the safety and efficacy of two BCG formulations (BCG1 and BCG2) were compared with GlucaGen in people with T1D.83 Participants received single subcutaneous doses of 1 mg of BCG1, BCG2 or GlucaGen during insulin‐induced hypoglycaemia (plasma glucose <3.3 mmol/L; <60 mg/dL).83 Both BCGs quickly restored plasma glucose to ≥3.9 mmol/L (70 mg/dL) within 30 minutes of the hypoglycaemic nadir in all but one individual.83 Similar results were also observed with GlucaGen. Both BCG formulations were safe and well tolerated; nausea was the most frequent adverse event reported with both BCGs and GlucaGen.83
6.2. Dasiglucagon
Dasiglucagon is a novel stable peptide analogue of human glucagon in an aqueous solution at neutral pH, with improved physical and chemical stability compared with currently available glucagon formulations.84 While dosing and administration of dasiglucagon are currently in development, completed studies have reported on single subcutaneous administration of dasiglucagon at doses ranging from 0.03 to 1.0 mg.84 The clinical development programme has included safety and tolerability evaluation, and characterization of PK/PD.
In a single‐centre, randomized, double‐blind study, the PK/PD properties of 0.1, 0.3, 0.6 and 1.0 mg dasiglucagon, as well as its safety and tolerability, were compared with those of 0.5 and 1.0 mg GlucaGen in the treatment of insulin‐induced hypoglycaemia in patients with T1D.84 Dasiglucagon produced a dose‐dependent and rapid increase in plasma glucose concentrations. Similar to GlucaGen, dasiglucagon rapidly increased plasma glucose by ≥1.1 mmol/L (20 mg/dL) to plasma glucose ≥3.9 mmol/L (70 mg/dL), but had a greater and more sustained effect on raising plasma glucose. Median tmax values were numerically higher with dasiglucagon compared with GlucaGen, but no significant differences were observed in the median time to reach plasma glucose ≥3.9 mmol/L (70 mg/dL) (dasiglucagon dose ≥0.3 mg, 6 minutes; 0.1 mg, 10 minutes; GlucaGen [both doses], 6–7 minutes).84 All participants in both groups reached these endpoints within 30 minutes (predefined success criteria). Similar median times to achieve a plasma glucose increment of 1.1 mmol/L (20 mg/dL) were observed with dasiglucagon in doses of ≥0.3 mg (9–10 minutes) and with both doses of GlucaGen (10 minutes); the median time for dasiglucagon 0.1 mg was 14 minutes.84 Both treatments were well tolerated, and adverse events were mild to moderate in severity and did not appear to be dose‐related.84 Gastrointestinal adverse events occurred with similar frequency following dasiglucagon and GlucaGen administration. Nausea was the most common adverse event followed by headache in those treated with dasiglucagon.84 Vomiting was also common and generally occurred 2–3 hours following dasiglucagon administration.84, 85
In a phase 3, randomized, double‐blind study, the efficacy and safety of dasiglucagon 0.6 mg, GlucaGen 1.0 mg and placebo were compared in the treatment of insulin‐induced hypoglycaemia in adults with T1D.85 When comparing the median time to blood glucose recovery, dasiglucagon 0.6 mg (10 minutes) was superior to placebo (40 minutes) (P < 0.001) but not to GlucaGen 1.0 mg (12 minutes).85 Following dasiglucagon administration, blood glucose recovery occurred within 15 minutes in 99% of participants, compared with 2% with placebo and 95% with GlucaGen.85 No new safety concerns for dasiglucagon emerged in this study; nausea and vomiting were common and occurred with similar frequencies with dasiglucagon and GlucaGen (nausea: 55% and 53%, vomiting: 23% and 19%, respectively).85
7. CONCLUSIONS
Hypoglycaemia is a major limiting factor in the glycaemic management of T1D and T2D. Risk factors for severe hypoglycaemia are well known and risk assessment tools are available for use in clinical practice. However, severe hypoglycaemia remains a substantial clinical problem. It is important that those who are at risk of severe hypoglycaemia have access to rescue treatments for emergency use.
Currently available treatments for severe hypoglycaemia are limited to intravenous dextrose, injectable glucagon, nasal glucagon and liquid glucagon. Intravenous dextrose requires administration by HCPs or medically trained professionals, so injectable glucagon, nasal glucagon and liquid glucagon are the only treatment options for caregivers outside hospital or emergency medical settings. However, injectable glucagon is currently not available in a ready‐to‐use formulation and can be cumbersome for caregivers to administer in emergency situations. Nasal glucagon is a ready‐to‐use drug/device combination that is simple and easy for the caregivers of people with diabetes to use. Other treatments currently in development, such as dasiglucagon, may also potentially provide a portable, easy‐to‐use drug/device requiring no reconstitution, which caregivers can administer during emergency severe hypoglycaemia events. Simplifying the treatment of severe hypoglycaemia will greatly improve the emergency management of this potentially life‐threatening adverse effect of insulin therapy.
CONFLICT OF INTEREST
B.M.F. has served on advisory boards for Lilly, Novo Nordisk, Locemia Solutions and Zucara, and participated as a speaker at meetings for Lilly, Novo Nordisk, MSD, Abbott, Roche and Boehringer Ingelheim. V.T.T., B.D.M. and O.J.V. are employees of Eli Lilly and own stock in the company. All of the named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
AUTHOR CONTRIBUTIONS
All named authors take responsibility for the integrity and accuracy of the work as a whole. All authors participated in critical drafting of the manuscript. All authors had full access to all of the data in the manuscript and had final responsibility for the decision to submit for publication.
Supporting information
Supplementary Table 1: Overview of Glucagon Published Reports
ACKNOWLEDGMENTS
This work was funded by Eli Lilly and Company.
Thieu VT, Mitchell BD, Varnado OJ, Frier BM. Treatment and prevention of severe hypoglycaemia in people with diabetes: Current and new formulations of glucagon. Diabetes Obes Metab. 2020;22:469–479. 10.1111/dom.13941
Peer Review The peer review history for this article is available at https://publons.com/publon/10.1111/dom.13941.
Funding information This work was funded by Eli Lilly and Company
REFERENCES
- 1. Frier BM. Hypoglycaemia in diabetes mellitus: epidemiology and clinical implications. Nat Rev Endocrinol. 2014;10:711‐722. [DOI] [PubMed] [Google Scholar]
- 2. Ahola AJ, Saraheimo M, Freese R, et al. Fear of hypoglycaemia and self‐management in type 1 diabetes. J Clin Transl Endocrinol. 2016;4:13‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Martyn‐Nemeth P, Quinn L, Penckofer S, Park C, Hofer V, Burke L. Fear of hypoglycemia: Influence on glycemic variability and self‐management behavior in young adults with type 1 diabetes. J Diabetes Complications. 2017;31:735‐741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pinsker JE, Kraus A, Gianferante D, et al. Techniques for exercise preparation and management in adults with type 1 diabetes. Can J Diabetes. 2016;40:503‐508. [DOI] [PubMed] [Google Scholar]
- 5. Canadian Diabetes Association Clinical Practice Guidelines Expert Committee , Clayton D, Woo V, Yale JF. Hypoglycemia. Can J Diabetes. 2013;37:S69‐S71. [DOI] [PubMed] [Google Scholar]
- 6. American Diabetes Association . 6. Glycemic targets: Standards of Medical Care in Diabetes ‐ 2019. Diabetes Care. 2019;42:S61‐S70. [DOI] [PubMed] [Google Scholar]
- 7. Elliott L, Fidler C, Ditchfield A, Stissing T. Hypoglycemia event rates: a comparison between real‐world data and randomized controlled trial populations in insulin‐treated diabetes. Diabetes Ther. 2016;7:45‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Edridge CL, Dunkley AJ, Bodicoat DH, et al. Prevalence and incidence of hypoglycaemia in 532 542 people with type 2 diabetes on oral therapies and insulin: a systematic review and meta‐analysis of population based studies. PLoS One. 2015;10:e0126427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. United Kingdom Hypoglycaemia Study Group . Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia. 2007;50:1140‐1147. [DOI] [PubMed] [Google Scholar]
- 10. Cryer PE. Minireview: Glucagon in the pathogenesis of hypoglycemia and hyperglycemia in diabetes. Endocrinology. 2012;153:1039‐1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cryer PE. Mechanisms of hypoglycemia‐associated autonomic failure in diabetes. N Engl J Med. 2013;369:362‐372. [DOI] [PubMed] [Google Scholar]
- 12. Cryer PE. The barrier of hypoglycemia in diabetes. Diabetes. 2008;57:3169‐3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Monnier L, Colette C, Owens DR. Integrating glycaemic variability in the glycaemic disorders of type 2 diabetes: a move towards a unified glucose tetrad concept. Diabetes Metab Res Rev. 2009;25:393‐402. [DOI] [PubMed] [Google Scholar]
- 14. Frier BM, Schernthaner G, Heller SR. Hypoglycemia and cardiovascular risks. Diabetes Care. 2011;34(Suppl 2):S132‐S137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dave KR, Tamariz J, Desai KM, et al. Recurrent hypoglycemia exacerbates cerebral ischemic damage in streptozotocin‐induced diabetic rats. Stroke. 2011;42:1404‐1411. [DOI] [PubMed] [Google Scholar]
- 16. Wright RJ, Frier BM. Vascular disease and diabetes: is hypoglycaemia an aggravating factor? Diabetes Metab Res Rev. 2008;24:353‐363. [DOI] [PubMed] [Google Scholar]
- 17. Snell‐Bergeon JK, Wadwa RP. Hypoglycemia, diabetes, and cardiovascular disease. Diabetes Technol Ther. 2012;14:S51‐S58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ. 2010;340:b4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zoungas S, Patel A, Chalmers J, et al. Severe hypoglycemia and risks of vascular events and death. N Engl J Med. 2010;363:1410‐1418. [DOI] [PubMed] [Google Scholar]
- 20. Barendse S, Singh H, Frier BM, Speight J. The impact of hypoglycaemia on quality of life and related patient‐reported outcomes in Type 2 diabetes: a narrative review. Diabet Med. 2012;29:293‐302. [DOI] [PubMed] [Google Scholar]
- 21. American Diabetes Association . 5. Glycemic targets. Diabetes Care. 2016;39:S39‐S46. [DOI] [PubMed] [Google Scholar]
- 22. Briscoe VJ, Davis SN. Hypoglycemia in type 1 and type 2 diabetes: physiology, pathophysiology, and management. Clin Diabetes. 2006;24:115‐121. [Google Scholar]
- 23. Bolinder J, Antuna R, Geelhoed‐Duijvestijn P, Kroger J, Weitgasser R. Novel glucose‐sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non‐masked, randomised controlled trial. Lancet. 2016;388:2254‐2263. [DOI] [PubMed] [Google Scholar]
- 24. Choudhary P, Ramasamy S, Green L, et al. Real‐time continuous glucose monitoring significantly reduces severe hypoglycemia in hypoglycemia‐unaware patients with type 1 diabetes. Diabetes Care. 2013;36:4160‐4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline JP, Rayman G. Flash glucose‐sensing technology as a replacement for blood glucose monitoring for the management of insulin‐treated type 2 diabetes: a multicenter, open‐label randomized controlled trial. Diabetes Ther. 2017;8:55‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liebl A, Davidson J, Mersebach H, Dykiel P, Tack CJ, Heise T. A novel insulin combination of insulin degludec and insulin aspart achieves a more stable overnight glucose profile than insulin glargine: results from continuous glucose monitoring in a proof‐of‐concept trial. J Diabetes Sci Technol. 2013;7:1328‐13 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Beers CA, DeVries JH, Kleijer SJ, et al. Continuous glucose monitoring for patients with type 1 diabetes and impaired awareness of hypoglycaemia (IN CONTROL): a randomised, open‐label, crossover trial. Lancet Diabetes Endocrinol. 2016;4:893‐902. [DOI] [PubMed] [Google Scholar]
- 28. Heinemann L, Freckmann G, Ehrmann D, et al. Real‐time continuous glucose monitoring in adults with type 1 diabetes and impaired hypoglycaemia awareness or severe hypoglycaemia treated with multiple daily insulin injections (HypoDE): a multicentre, randomised controlled trial. Lancet. 2018;391:1367‐1377. [DOI] [PubMed] [Google Scholar]
- 29. Rickels MR, Peleckis AJ, Dalton‐Bakes C, et al. Continuous glucose monitoring for hypoglycemia avoidance and glucose counterregulation in long‐standing type 1 diabetes. J Clin Endocrinol Metab. 2018;103:105‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pickup JC, Sutton AJ. Severe hypoglycaemia and glycaemic control in Type 1 diabetes: meta‐analysis of multiple daily insulin injections compared with continuous subcutaneous insulin infusion. Diabet Med. 2008;25:765‐774. [DOI] [PubMed] [Google Scholar]
- 31. Bergenstal RM, Klonoff DC, Garg SK, et al. Threshold‐based insulin‐pump interruption for reduction of hypoglycemia. N Engl J Med. 2013;369:224‐232. [DOI] [PubMed] [Google Scholar]
- 32. Choudhary P, Shin J, Wang Y, et al. Insulin pump therapy with automated insulin suspension in response to hypoglycemia: reduction in nocturnal hypoglycemia in those at greatest risk. Diabetes Care. 2011;34:2023‐2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Weber KS, Roden M, Mussig K. Do dogs sense hypoglycaemia? Diabet Med. 2016;33:934‐938. [DOI] [PubMed] [Google Scholar]
- 34. Raju B, Arbelaez AM, Breckenridge SM, Cryer PE. Nocturnal hypoglycemia in type 1 diabetes: an assessment of preventive bedtime treatments. J Clin Endocrinol Metab. 2006;91:2087‐2092. [DOI] [PubMed] [Google Scholar]
- 35. de Galan BE, Tack CJ, Lenders JW, et al. Theophylline improves hypoglycemia unawareness in type 1 diabetes. Diabetes. 2002;51:790‐796. [DOI] [PubMed] [Google Scholar]
- 36. Smith D, Pernet A, Rosenthal JM, et al. The effect of modafinil on counter‐regulatory and cognitive responses to hypoglycaemia. Diabetologia. 2004;47:1704‐1711. [DOI] [PubMed] [Google Scholar]
- 37. Debrah K, Sherwin RS, Murphy J, Kerr D. Effect of caffeine on recognition of and physiological responses to hypoglycaemia in insulin‐dependent diabetes. Lancet. 1996;347:19‐24. [DOI] [PubMed] [Google Scholar]
- 38. Karter AJ, Warton EM, Lipska KJ, et al. Development and validation of a tool to identify patients with type 2 diabetes at high risk of hypoglycemia‐related emergency department or hospital use. JAMA Intern Med. 2017;177:1461‐1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. International Hypoglycaemia Study Group . IHSG Patient Hypoglycaemia Risk Tool. 2018. https://ihsgonline.com/wp-content/uploads/2018/05/180525_IHSG_Patient_Hypoglycaemia_Risk_Tool_tabloid_2018.pdf.
- 40. International Hypoglycaemia Study Group . IHSG Physician Hypoglycaemia Risk Tool. 2018. https://ihsgonline.com/wp-content/uploads/2018/05/180525_IHSG_Physician_Hypoglycaemia_Risk_Tool_tabloid_2018.pdf.
- 41. Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36:1384‐1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Graveling AJ, Frier BM. Impaired awareness of hypoglycaemia: a review. Diabetes Metab. 2010;36:S64‐S74. [DOI] [PubMed] [Google Scholar]
- 43. Cox D, Ritterband L, Magee J, Clarke W, Gonder‐Frederick L. Blood glucose awareness training delivered over the internet. Diabetes Care. 2008;31:1527‐1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rondags SM, de Wit M, Snoek FJ. HypoAware: development and pilot study of a brief and partly web‐based psychoeducational group intervention for adults with Type 1 and insulin‐treated Type 2 diabetes and problematic hypoglycaemia. Diabet Med. 2016;33:184‐191. [DOI] [PubMed] [Google Scholar]
- 45. Cryer PE, Davis SN, Shamoon H. Hypoglycemia in diabetes. Diabetes Care. 2003;26:1902‐1912. [DOI] [PubMed] [Google Scholar]
- 46. Gerich JE, Langlois M, Noacco C, Karam JH, Forsham PH. Lack of glucagon response to hypoglycemia in diabetes: evidence for an intrinsic pancreatic alpha cell defect. Science. 1973;182:171‐173. [DOI] [PubMed] [Google Scholar]
- 47. Triplitt CL. Examining the mechanisms of glucose regulation. Am J Manag Care. 2012;18:S4‐S10. [PubMed] [Google Scholar]
- 48. Kedia N. Treatment of severe diabetic hypoglycemia with glucagon: an underutilized therapeutic approach. Diabetes Metab Syndr Obes. 2011;4:337‐346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. MacCuish AC. Treatment of Hypoglycaemia In: Frier BM, Fisher BM, eds. Hypoglycaemia and Diabetes: Clinical and Physiological Aspects. London, UK: Edward Arnold; 1993. [Google Scholar]
- 50. Canadian Diabetes Association . The role of dietary sugars in diabetes mellitus. Beta Release. 1991;15:117‐123. [Google Scholar]
- 51. Brodows RG, Williams C, Amatruda JM. Treatment of insulin reactions in diabetics. JAMA. 1984;252:3378‐3381. [PubMed] [Google Scholar]
- 52. Slama G, Traynard PY, Desplanque N, et al. The search for an optimized treatment of hypoglycemia. Carbohydrates in tablets, solution, or gel for the correction of insulin reactions. Arch Intern Med. 1990;150:589‐593. [PubMed] [Google Scholar]
- 53. Wiethop BV, Cryer PE. Alanine and terbutaline in treatment of hypoglycemia in IDDM. Diabetes Care. 1993;16:1131‐1136. [DOI] [PubMed] [Google Scholar]
- 54. Fendrick AM, He X, Liu D, Buxbaum JD, Mitchell BD. Glucagon prescriptions for diabetes patients after emergency department visits for hypoglycemia. Endocr Pract. 2018;24:861‐866. [DOI] [PubMed] [Google Scholar]
- 55. Snoek FJ, Jiletcovici A, Bushnell DMI, et al. Conversations and reactions around severe hypoglycemia (CRASH): U.S. results from a global survey of people with T1DM or insulin‐treated T2DM and caregivers. Diabetes. 2019;68(Suppl 1):285‐OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Abraham MB, Jones TW, Naranjo D, et al. ISPAD Clinical Practice Consensus Guidelines 2018: Assessment and management of hypoglycemia in children and adolescents with diabetes. Pediatr Diabetes. 2018;19(Suppl 27):178‐192. [DOI] [PubMed] [Google Scholar]
- 57. Mahadevan SM, Garmel GM. An Introduction to Clinical Emergency Medicine . 2nd ed. New York, NY: Cambridge University Press; 2012. [Google Scholar]
- 58. Diabetes Canada Clinical Practice Guidelines Expert Committee , Yale JF, Paty B, Senior PA. Hypoglycemia. Can J Diabetes. 2018;42:S104‐S108. [DOI] [PubMed] [Google Scholar]
- 59. Hospira I. 50% Dextrose Injection package insert. 2018. http://labeling.pfizer.com/ShowLabeling.aspx?id=4422. Accessed July 18. 2019.
- 60. Pearson T. Glucagon as a treatment of severe hypoglycemia: safe and efficacious but underutilized. Diabetes Educ. 2008;34:128‐134. [DOI] [PubMed] [Google Scholar]
- 61. Eli Lilly and Company . Glucagon Emergency Kit. http://www.lillyglucagon.com. Accessed October 29, 2018.
- 62. Nordisk Novo. GlucaGen® Hypokit®. http://www.glucagenhypokit.com.
- 63. Pontiroli AE. Intranasal glucagon: a promising approach for treatment of severe hypoglycemia. J Diabetes Sci Technol. 2015;9:38‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Harris G, Diment A, Sulway M, Wilkinson M. Glucagon administration – underevaluated and undertaught. Pract Diabetes Int. 2001;18:22‐25. [Google Scholar]
- 65. Pontiroli AE, Tagliabue E. Therapeutic use of intranasal glucagon: resolution of hypoglycemia. Int J Mol Sci. 2019;20:3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Eli Lilly and Company . Baqsimi US Prescribing Information. http://pi.lilly.com/us/baqsimi-uspi.pdf. Accessed July 30, 2019.
- 67. Sherr JL, Ruedy KJ, Foster NC, et al. Glucagon nasal powder: a promising alternative to intramuscular glucagon in youth with type 1 diabetes. Diabetes Care. 2016;39:555‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yale JF, Dulude H, Egeth M, et al. Faster use and fewer failures with needle‐free nasal glucagon versus injectable glucagon in severe hypoglycemia rescue: a simulation study. Diabetes Technol Ther. 2017;19:423‐432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rickels MR, Ruedy KJ, Foster NC, et al. Intranasal glucagon for treatment of insulin‐induced hypoglycemia in adults with type 1 diabetes: a randomized crossover noninferiority study. Diabetes Care. 2016;39:264‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Deeb LC, Dulude H, Guzman CB, et al. A phase 3 multicenter, open‐label, prospective study designed to evaluate the effectiveness and ease of use of nasal glucagon in the treatment of moderate and severe hypoglycemia in children and adolescents with type 1 diabetes in the home or school setting. Pediatr Diabetes. 2018;19:1007‐1013. [DOI] [PubMed] [Google Scholar]
- 71. Seaquist ER, Dulude H, Zhang XM, et al. Prospective study evaluating the use of nasal glucagon for the treatment of moderate to severe hypoglycaemia in adults with type 1 diabetes in a real‐world setting. Diabetes Obes Metab. 2018;20:1316‐1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Guzman CB, Dulude H, Piche C, et al. Effects of common cold and concomitant administration of nasal decongestant on the pharmacokinetics and pharmacodynamics of nasal glucagon in otherwise healthy participants: A randomized clinical trial. Diabetes Obes Metab. 2018;20:646‐653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Settles J, Child CJ, Bajpai SK, Spaepen E, Suico JG. Nasal vs. injected glucagon: user experience results of a simulated severe hypoglycemia study. Diabetes. 2019;68(Suppl 1):13‐LB. [Google Scholar]
- 74. Suico J, Hövelmann U, Zhang S, et al. Nasal glucagon: a viable alternative to treat insulin‐induced hypoglycaemia in adults with type 1 diabetes. Diabetologia. 2018;61(Suppl 1):77‐78. [Google Scholar]
- 75. Bajpai S, Cambron‐Mellott MJ, Peck E, et al. Factors influencing preferences for glucagon delivery devices among patients with diabetes, caregivers, and acquaintances. Value Health. 2019;22(Suppl 2):S156. [Google Scholar]
- 76. Xeris Pharmaceuticals . GVOKE US Prescribing Information. 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212097s000lbl.pdf.
- 77. Buckingham B, Cummins MJ, Prestrelski SJ, Strange P. Liquid room temperature stable glucagon—glucose response in pediatric type 1 diabetes patients. Diabetes. 2018;67(Suppl 1):1241. [Google Scholar]
- 78. Christiansen MP, Cummins MJ, Prestrelski SJ, Strange P. A phase 3 comparison of a novel liquid glucagon autoinjector to glucagon emergency kit for the symptomatic relief of severe hypoglycemia. Diabetes. 2018;67(Suppl 1):304‐OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Christiansen MP, Cummins MJ, Prestrelski SJ, Strange P. A phase 3 comparison of a novel liquid glucagon autoinjector to glucagon emergency kit for the treatment of severe hypoglycemia. Diabetes. 2018;67(Suppl 1):1239. [Google Scholar]
- 80. Christiansen MP, Cummins M, Prestrelski S, Junaidi MK. A phase 3 comparison of a ready‐to‐use liquid glucagon rescue pen to glucagon emergency kit for the symptomatic relief of severe hypoglycemia. J Diabetes Sci Technol. 2019;13:293‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Valentine V, Newswanger B, Prestrelski S, Andre AD, Garibaldi M. Human factors usability and validation studies of a glucagon autoinjector in a simulated severe hypoglycemia rescue situation. Diabetes Technol Ther 2019;21(9):522‐530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Cummins M, Andre A, Christiansen MP, Newswanger B, Prestrelski S, Junaidi MK. An assessment of usability and drug preparation time for a ready‐to‐use liquid glucagon pen. J Diabetes Sci Technol. 2019;13:293‐409. [Google Scholar]
- 83. Glezer S, Hovelmann U, Teng S, et al. BioChaperone glucagon (BCG), a stable ready‐to‐use liquid glucagon formulation, is well tolerated and quickly restores euglycemia after insulin‐induced hypoglycemia. Diabetes. 2018;67(Suppl 1):305‐OR. [Google Scholar]
- 84. Hovelmann U, Bysted BV, Mouritzen U, et al. Pharmacokinetic and pharmacodynamic characteristics of dasiglucagon, a novel soluble and stable glucagon analog. Diabetes Care. 2018;41:531‐537. [DOI] [PubMed] [Google Scholar]
- 85. Pieber TR, Hovelmann U, Willard J, et al. 286‐OR: Phase 3 results for dasiglucagon as a fast and effective treatment for severe hypoglycemia. Diabetes. 2019;68(Suppl. 1). [Google Scholar]
- 86. Dunning T, Sinclair A, Colagiuri S. New IDF Guideline for managing type 2 diabetes in older people. Diabetes Res Clin Pract. 2014;103:538‐540. [DOI] [PubMed] [Google Scholar]
- 87. Oyer DS. The science of hypoglycemia in patients with diabetes. Curr Diabetes Rev. 2013;9:195‐208. [DOI] [PubMed] [Google Scholar]
- 88. Wohland T, Holstein JD, Patzer OM, et al. New risk and protective factors for severe hypoglycaemia in people with type 1 diabetes. Nutr Metab Cardiovasc Dis. 2017;27:407‐414. [DOI] [PubMed] [Google Scholar]
- 89. Lipska KJ, Yao X, Herrin J, et al. Trends in drug utilization, glycemic control, and rates of severe hypoglycemia, 2006–2013. Diabetes Care. 2017;40:468‐475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Geller AI, Shehab N, Lovegrove MC, et al. National estimates of insulin‐related hypoglycemia and errors leading to emergency department visits and hospitalizations. JAMA Intern Med. 2014;174:678‐686. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: Overview of Glucagon Published Reports


