Abstract
Aims
To characterize efficacy of the Bacillus subtilis BSB3 (BSB3) strain in the prevention of excessive exercise‐induced side effects and in maintaining stability of the gut microbiota.
Methods and Results
Rats were pretreated by oral gavage with B. subtilis BSB3 (BSB3) or with phosphate‐buffered saline (PBS) twice a day for 2 days, and were either exposed forced treadmill running or remained sedentary. Histological analysis of intestine, immunofluorescence staining of tight junction (TJ) proteins, serum lipopolysaccharide and intestinal fatty acid‐binding protein assay, culture‐based analysis and pyrosequencing for the gut microbiota were performed for each rat. Forced running resulted in a substantial decrease in intestinal villi height and total mucosa thickness, the depletion of Paneth cells, an inhibition of TJ proteins expression. Short‐term treatment of rats with BSB3 before running prevented these adverse effects. Culture‐based analysis of the gut microbiota revealed significant elevation of pathogenic microorganisms only in treadmill‐exercised rats pretreated with PBS. High‐throughput 16S rRNA gene sequencing also revealed an increase in pathobionts in this group. Preventive treatment of animals with BSB3 resulted in predominance of beneficial bacteria.
Conclusions
BSB3 prevents excessive exercise‐associated complications by beneficial modulation of the gut microbiota.
Significance and Impact of the Study
Our study shows a new application of beneficial bacteria for prevention the adverse effects of excessive exercise.
Keywords: Bacillus subtilis, gut microbiota, I‐FABP, LPS, side effects of excessive exercise, tight junction proteins
Introduction
Physical activity induces distinct effects on health depending on intensity and duration. Regular moderate exercise reduces the incidence of inflammatory diseases, has protective effect against cancer, gastrointestinal disorders and obesity (Martin 2011; Kruk and Czerniak 2013; Allen et al. 2018). Physical activity is associated with a marked decrease in all‐cause mortality, cardiovascular mortality, cardiovascular disease (Kraus et al. 2019) and with a delay in onset of dementia in elderly persons (Larson et al. 2006). However, recent data suggest high level of physical activity results in increased risk of early mortality (Coenen et al. 2018). Excessive exercise has also been associated with a significant immunodepression, causing lower resistance to pathogens and an increase in the risk of infections and illnesses (Castell et al. 2019).
Intestinal complications are the most common among endurance athletes (Pires et al. 2017). To this end, 20–60% of athletes report various abdominal symptoms, such as bloating, nausea, vomiting, stomach pain, flatulence, diarrhoea, and constipation following a strenuous exercise bout (de Oliveira and Burini 2009; Pane et al. 2018). It has been suggested that endurance exercise reduces gastrointestinal blood flow, increases gut permeability and translocation of the gut microbiota and its metabolites into circulation (de Oliveira and Burini 2009). Recent scientific data indicate the significant role of the gut microbiota in host physiology, particularly in the maintenance of metabolism, immune homeostasis and mental health (Sommer and Backhed 2013; Naseribafrouei et al. 2014; Rieder et al. 2017; Cani 2018; Dicks et al. 2018). Stability of the gut microbiota is essential for the healthy status of the host, as disease conditions are accompanied with dysbiosis (Pham and Law 2014; Sommer et al. 2017). Significant changes in the gut microbiota with prevalence of conditionally pathogenic bacteria, similar to those induced by a high fat diet, have been observed after forced exercise (Kang et al. 2014; Allen et al. 2015). Balanced gut microbiota is responsible for normal gut morphology and function, whereas dysbiosis results in significant increase in gut permeability and inflammation (Dicks et al. 2018). Thus, maintaining the balanced gut microbiota can prevent various adverse effects associated with dysbiosis.
Probiotics have been suggested as a valuable approach for normalization of the gut microbiota with the intent of preventing and treating various pathological disorders (O'toole and Cooney 2008; Aureli et al. 2011). Special attention is paid for probiotic supplementation in sport medicine for prevention health issues, affecting sport performance, especially in endurance athletes. In clinical trials, some probiotics were effective in reducing incidence of upper respiratory symptoms and gastrointestinal problems in athletes, although other studies did not show efficacy (Leite et al. 2019). These clinical trials were focused on analysis of specific symptoms and immunological data, but no gut microbiota studies have been performed. Our previous results showed high efficacy of Bacillus subtilis BSB3 (BSB3) strain in prevention of heat stress‐related adverse events in rats (Moore et al. 2014; Sorokulova et al. 2016). However, it is currently unknown if BSB3 can affect biomarkers associated with gastrointestinal health following a rigorous bout of exercise. Therefore, the main aim of recent study was to characterize proficiency of this strain in mitigation side effects of excessive exercise and in maintaining stability of the gut microbiota in rats.
Materials and methods
Ethics statement
All animal procedures were approved by the Auburn University Institutional Animal Care and Use Committee (protocol number 2016‐2940 ‘Efficacy of a preexercise probiotic intervention on mitigating the adverse effects resulting from metabolic‐derived heat stress’, 09/08/2016). The study was performed in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
Animals
Adult male Sprague–Dawley rats (Harlan Laboratories, Indianapolis, IN) weighing 250–300 g were used in this study. Sprague–Dawley rats were used in our previous studies (Moore et al. 2014; Ducray et al. 2019) and in the studies of other authors (Lambert et al. 2002; Gong et al. 2012). Animals were housed two per cage under specific pathogen‐free conditions and were acclimatized for 2 days prior to experimentation at a temperature of 20 ± 1°C and standard lighting (12‐h day/12‐h night) with free access to water and standard food (2018 Teklad Global 18% Protein Rodent Diet; Harlan).
Bacterial culture
Bacillus subtilis BSB3 was cultivated on plates with Difco sporulation medium (Difco Nutrient Broth; Becton, Dickinson and Company, Sparks, MD) at 37°C for 5 days. Bacteria were harvested by flooding the surface of the plates with sterile phosphate‐buffered saline (PBS) followed by scraping with a sterile cell spreader. The bacterial suspension was diluted in PBS to achieve 1 × 108 colony‐forming unit (CFU) per ml.
Antibodies
Primary rabbit polyclonal antibodies against zonula occludens (ZO‐1) (#61‐7300), occludin (#40‐4700), claudin (#37‐4900), Alexa Fluor 555 goat anti‐rabbit (#A32727) and Alexa Fluor 488 goat anti‐mouse (#A32723) secondary antibodies were acquired from ThermoFisher Scientific (Waltham, MA). Rabbit Anti‐Junctional Adhesion Molecule 1 (JAM‐A antibody, #ab125886) were from Abcam (Cambridge, MA).
Experimental design
Animals (n = 24) were treated twice a day with 6‐h dose intervals by oral gavage either with 1 ml of B. subtilis BSB3 suspension (n = 12) or 1 ml of PBS (n = 12) for 2 days. On day 3, rats in each group were further subdivided: BEx—pretreated with BSB3, undergoing forced running; BCont—pretreated with BSB3 remaining sedentary; PEx—pretreated with PBS, undergoing forced running; PCont—pretreated with PBS remaining sedentary. The forced running protocol was performed according to Przyborowski et al. (2017) with modifications. Briefly, animals started running on a treadmill (Exer‐3/6 Treadmill; Columbus Instruments, Columbus, OH) at 5 m min−1 followed by gradual increases in speed of 2 m min−1 until exhaustion. Rectal temperature was measured for each rat before and immediately after forced running using an electronic digital thermometer (Ducray et al. 2016). Four hours after the forced running experiments, rats were anaesthetized with isoflurane (2–4%) and killed by rapid decapitation. Trunk blood was collected from each rat into sterile apirogenic tubes to obtain serum. Sections of small intestine from each rat were taken for morphological analysis. Colon faecal matter was immediately placed in anaerobic broth for culture‐based microbiological analysis. For 16S rRNA sequencing of the gut microbiota faecal samples were placed into sterile tubes and stored at −80°C until the experiment.
Blood serum preparation
Blood collected in sterile tubes was allowed to clot for 30 min at room temperature. Tubes were centrifuged at 20°C, 7000 g for 10 min. Serum was collected and stored in 50 µl aliquots at −20°C until assay.
Histological analysis
Sample preparation
Small intestinal samples (0·5–2 cm in length) were completely immersed in fixative, Bouin's solution (Electron Microscopy Sciences, Hatfield, PA), immediately after harvesting. After 48 h of fixation at room temperature, the excess fixative was washed out in 70% ethanol (ETOH). Washed samples were placed into tissue embedding cassettes (VWR, Radnor, PA) and kept in 70% ETOH until processing in the Automated tissue processor (Tissue‐Tek VIP; Miles/Sakura, Torrance, CA). After processing, samples were embedded in paraffin blocks using embedding centre (Tissue‐Tek TEC; Sakura). Embedded tissues were sectioned at 6 mm using a microtome (Reichert‐Jung 2040 Autocut; Leica Biosystems Nussloch GmbH, Nussloch, Germany) and then mounted on slides until staining.
Sample staining
Histological sections were deparaffinized, dehydrated and stained with haematoxylin and eosin according to the standard protocol (Stevens 1990). After staining, sections were mounted using Eukitt Mounting Medium (Electron Microscopy Sciences, Hatfield, PA).
Measurements
Intestinal villi height and total mucosal thickness for each sample were measured as previously described (Ducray et al. 2016). Twenty measurements of each parameter in each sample were taken and expressed in micrometres. A total of eight sections were counted per rat for each rat in the treatment group. An average of these measurements was expressed as a mean villi height and mean total mucosal thickness for one treatment group.
Goblet cells count
Four sections of the small intestine from each rat were stained with Alcian Blue using a standard procedure, as previously described (Ducray et al. 2019). Enumeration of goblet cells was according the protocol proposed by Trevizan et al. (2016). Six images from each section were taken with a digital camera (Olympus BX50) coupled to an optical microscope with a 20x objective. The number of goblet cells present in a 0·96 mm2 in the mucosa of each animal were quantified using ImagePro 10 software (Media Cybernetics, Rockville, MD).
Paneth cells quantification
Paneth cells were visualized by phloxine‐tartrazine staining (Di Sabatino et al. 2008). Briefly, four sections of each rat were treated with Gill’s haematoxylin, phloxine B‐calcium carbonate, saturated solution of tartrazine. Quantification of Paneth cells was performed for each sample using a high‐resolution microscope system (Vainrub et al. 2006). Only crypts cut along the length of the crypt lumen were analysed.
Blood serum analysis
Lipopolysaccharide (LPS) serum concentration was analysed by the Pierce LAL Chromogenic Endotoxin Quantitation Kit (Thermo Scientific, Rockford, IL) using the limulus amebocyte lysate assay according to the manufacturer's recommendations. The sensitivity of the assay was 0·1 EU ml−1 (0·01 ng endotoxin per millilitres).
Concentration of intestinal fatty acid‐binding protein (I‐FABP) was determined by Rat I‐FABP/FABP2 ELISA Kit (LifeSpan BioSciences, Seattle, WA) with sensitivity <0·094 ng ml−1 according to the manufacturer's instruction.
Immunofluorescence staining of junctional proteins
Histological sections were deparaffinized in Hemo‐Di x 2 changes for 5 min, hydrated with 100, 95 and 70% ETOH and distilled water. Sections were blocked for 1 h in Odyssey blocking buffer (LiCor, Lincoln, CA) and incubated for 2 h at 4°C with primary antibodies against claudin, occludin, ZO‐1 and JAM‐A proteins. Sections were washed with PBS and incubated with secondary antibodies (1 : 100 dilution) for 1 h, then washed with PBS/0·01% Tween. Sections were mounted with VectaShield Mounting Media (Vector Laboratories, Burlingame, CA). Fluorescence images from each section were obtained with a digital camera (Olympus BX50) coupled to an optical microscope. The images were analysed with Image‐Pro10 (Media Cybernetics). Three fields per each of three sections from each rat were analysed.
Gut microbiota analysis
Culture‐based study
Faecal matter removed from the colon of each rat using sterile technique, was placed in sterile preweighted tubes with anaerobic broth, weighted and vortexed until homogenous. Serial tenfold dilutions from 10−1 to 10−7 were prepared and from the appropriate dilution, 0·1 ml aliquots were plated in four replicates on each of media: Anaerobic Basal Agar (Alfa Aesar, Ward Hill, MA) for total anaerobic bacteria; Brain Heart agar (Hardy Diagnostics, Santa Maria, CA) for total aerobic bacteria; Trypticase Soy Agar with Sheep Blood (Fisher Scientific, Hampton, NH) for haemolytic bacteria; Bifidobacterium Selective Agar (HiMedia Laboratories, Nashik, MH) for Bifibacterium sp.; Difco Lactobacilli MRS agar (Becton Dickinson) for Lactobacillus sp.; Brucella Agar w/Hemin and vitamin K1 (HiMedia Laboratories) for Bacteroides sp.: Reinforced Clostridial Medium (Hardy Diagnostics, Santa Maria, CA) for Clostridia sp.; Sabouraud Dextrose HiVeg Agar (HiMedia Laboratories) for yeasts. For isolation of anaerobic bacteria, plates were placed in an anaerobic chamber in a microaerophilic environment generated by a GasPak EZ Anaerobe Container System (Becton Dickinson and Co). All plates were incubated at 37°C and checked after 24 h for aerobic bacteria counting and after 48 h—for anaerobic bacteria. The number of CFU per gram of faecal matter was determined. Bacterial cultures and yeasts were identified by morphology of colonies, microscopical analysis of cells’ morphology, Gram staining, formation of spores, aerobic and anaerobic growth, as it was recommended elsewhere (Benno and Mitsuoka 1992; Sudo et al. 2004).
Pyrosequencing
Faecal samples were submitted to MR DNA (Shallowater, TX) for DNA isolation and sequencing. Analysis of the 16S rDNA was performed according to a previously published procedure (Dowd et al. 2008). Total genomic DNA was extracted from faecal samples using a QIAamp stool DNA (Qiagen, Germantown, MD) according the manufacture’s instruction. DNA samples were quantified using a Nanodrop spectrophotometer (Nyxor Biotech, Paris, France). The 16S rRNA gene V4 variable region PCR primers 515F GTGCCAGCMGCCGCGGTAA and 806R GGACTACHVGGGTWTCTAAT were utilized to evaluate the microbial ecology of each sample on the HiSeq 2500 with methods via the bTEFAP DNA analysis service. Each sample underwent a single‐step 30 cycle PCR using HotStarTaq Plus Master Mix Kit (Qiagen, Valencia, CA) under the following conditions: 94°C for 3 min, followed by 28 cycles of 94°C for 30 s; 53°C for 40 s and 72°C for 1 min; after which a final elongation step at 72°C for 5 min was performed. Following PCR, each stochastic replicate was combined and all amplicon products from different samples were mixed in equal concentrations and purified using Agencourt Ampure beads (Agencourt Bioscience Corporation, MA). Samples were sequenced utilizing the Illumina HiSeq chemistry following manufacturer’s protocols. The sequence data were processed using a proprietary analysis pipeline (MR DNA, Shallowater). Sequences smaller than 200 base pairs, contained ambiguous base calls, or contained homopolymer runs exceeding six base pairs were removed. Operational taxonomic units (OTU) were defined after removal of singleton sequences, clustering at 3% divergence (97% similarity). OTU were taxonomically classified using BLASTn against a curated National Center for Biotechnology Information database.
Statistics
All results were presented as a mean and standard deviation, unless otherwise indicated. Differences between groups were analysed by the one‐way anova, followed by the Fisher post hocs. Statistical analysis of sequence results was performed using a variety of computer packages including XLstat, NCSS 2007, ‘R’ and NCSS 2010. Significance reported for any analysis is defined as P < 0·05. Statistical calculations and graph plotting were carried out using Microcal Origin ver. 9.0 (Northampton, MA) and 2010 Microsoft Excel.
Results
Body temperature
Forced running resulted in significant elevation of rats’ body temperatures. Specifically, the mean body temperature was 36·7 ± 0·6°C prior to exercise and 39·3 ± 0·3°C immediately after exercise (P < 0·05). Body temperatures of the control (sedentary) rats were not altered (37·3 ± 0·4°C vs 37·6 ± 0·6°C).
Intestinal morphometry
Microscopic analysis of the small intestine revealed a significant decrease in villus height and total mucosa thickness in rats pretreated with PBS and exposed to forced running (PEx group) in comparison with rats from control PCont group (Fig. 1a,b). Villi height in animals from PEx and PCont groups were 435·57 ± 34·64 µm and 531·21 ± 26·11 µm (P < 0·05), and total mucosa thickness in these groups were 622·67 ± 15·27 µm and 715·83 ± 35·72 µm (P < 0·05) accordingly. Pretreatment of rats with BSB3 prior to forced running (BEx group) also resulted in significant reductions of the villi height in comparison with control groups (PCont and BCont), but values were significantly higher in comparison with animals from PEx group (531·21 ± 26·11 µm vs 435·57 ± 34·64 µm; P < 0·05). No significant reduction in the total mucosa thickness was observed in the BEx group in comparison with control groups.
Figure 1.
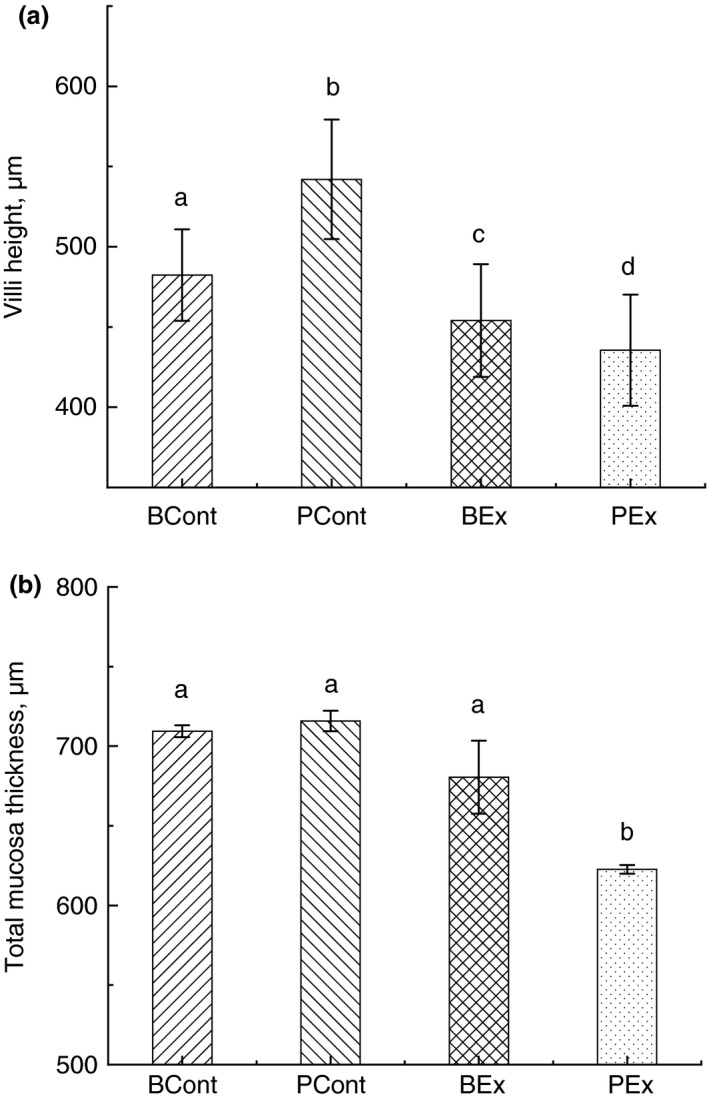
Intestinal villi height (a) and total mucosa thickness (b) in rats from different experimental groups. Rats were pretreated by oral gavage with Bacillus subtilis BSB3 (BSB3) or with PBS and were either exposed forced treadmill running or remained sedentary (n = 6 per condition). BEx, pretreated with BSB3, undergoing forced running; BCont, pre‐treated with BSB3 remaining sedentary; PEx, pretreated with PBS, undergoing forced running; PCont, pretreated with PBS remaining sedentary. Data with different superscript letters signify groups are significantly different (P < 0·05).
Paneth cells
Forced running resulted in significant reductions of Paneth cells number in rats pretreated with PBS (PEx group) in comparison with control PCont group (0·27 ± 0·03 and 0·67 ± 0·06 respectively; P < 0·05) (Fig. 2a). Rats administered BSB3 before forced running (BEx group) demonstrated significantly higher counts of Paneth cells in comparison with PEx group (0·93 ± 0·06 vs 0·27 ± 0·03, P < 0·05). Pretreatment of control rats with BSB3 (BCont) resulted in significantly higher numbers of Paneth cells when compared with rats from the PCont group (1·78 ± 0·07 and 0·67 ± 0·06 accordingly, P < 0·05).
Figure 2.
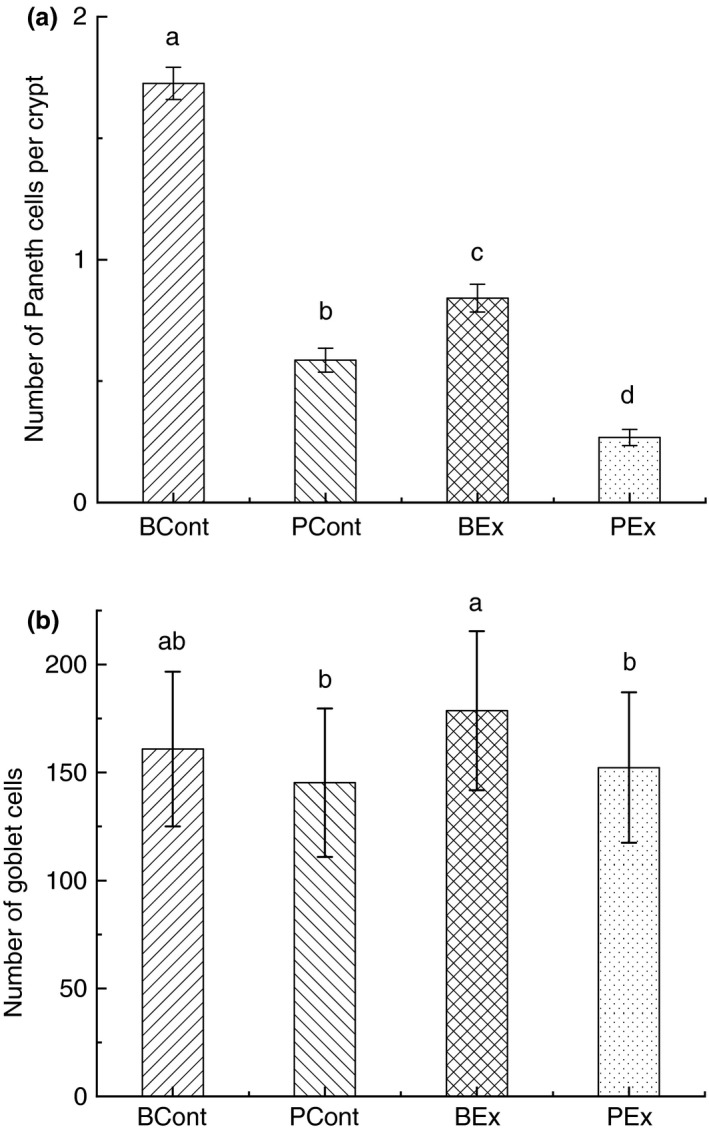
Paneth (a) and goblet (b) cells in intestine of rats from different experimental groups. Rats were pretreated by oral gavage with Bacillus subtilis BSB3 (BSB3) or with PBS and were either exposed forced running or remained sedentary (n = 6 rats per condition). BEx, pretreated with BSB3, undergoing forced running; BCont, pretreated with BSB3 remaining sedentary; PEx, pretreated with PBS, undergoing forced running; PCont, pretreated with PBS remaining sedentary. Data with different superscript letters signify groups are significantly different (P < 0·05).
Goblet cells
Pretreatment of rats with BSB3 prior to forced running (BEx) resulted in significant increase in goblet cells in comparison with rats pretreated with PBS (PEx and PCont groups). Specifically, the number of goblet cells in BEx, PEx and PCont animals was 178·63 ± 36·82, 152·29 ± 34·79 and 145·29 ± 34·43 respectively (Fig. 2b).
Expression of TJ proteins
Levels of all assayed tight junction (TJ) proteins were significantly lower in PEx rats in comparison with all other groups (Fig. 3). Pretreatment with BSB3 before forced running resulted in a considerable increase in the expression of TJ proteins compared to the PEx group, though these values were lower than control groups.
Figure 3.
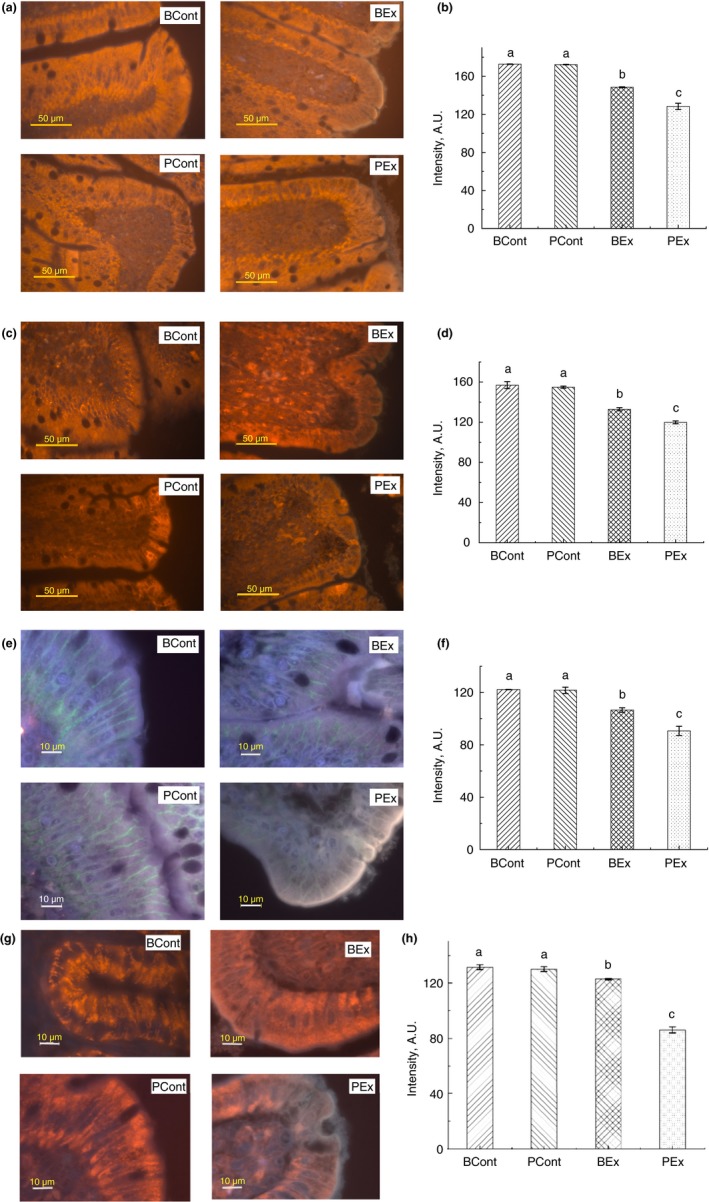
Immunofluorescence staining of TJ proteins in the intestine of rats from different experimental groups. (a) Images of occludin immunostaining, Bar 50 µm; (b) Analysis of occludin expression with immunostaining; (c) Images of ZO‐1 immunostaining, bar 50 µm; (d) Analysis of ZO‐1 expression with immunostaining; (e) Images of claudin immunostaining, bar 10 µm; (f) Analysis of claudin expression with immunostaining; (g) Images of JAM‐A immunostaining, bar 10 µm; (h) Analysis of JAM‐A expression with immunostaining. A.U., arbitrary units. Rats were pretreated by oral gavage with B. subtilis BSB3 (BSB3) or with PBS and were either exposed forced running or remained sedentary (n = 6 rats per condition). BEx, pretreated with BSB3, undergoing forced running; BCont, pretreated with BSB3 remaining sedentary; PEx, pretreated with PBS, undergoing forced running; PCont, pretreated with PBS remaining sedentary. Data with different superscript letters signify groups are significantly different (P < 0·05). [Colour figure can be viewed at https://www.wileyonlinelibrary.com]
Serum LPS concentration
Significant elevation of serum LPS level was observed only in treadmill‐exercised rats pretreated with PBS (PEx group). Treatment with BSB3 prevented rise of LPS in BEx animals. Specifically, serum LPS concentrations in PEx and BEx rats were 0·85 ± 0·14 and 0·55 ± 0·12 EU ml−1 accordingly (P < 0·05). (Fig. 4a).
Figure 4.
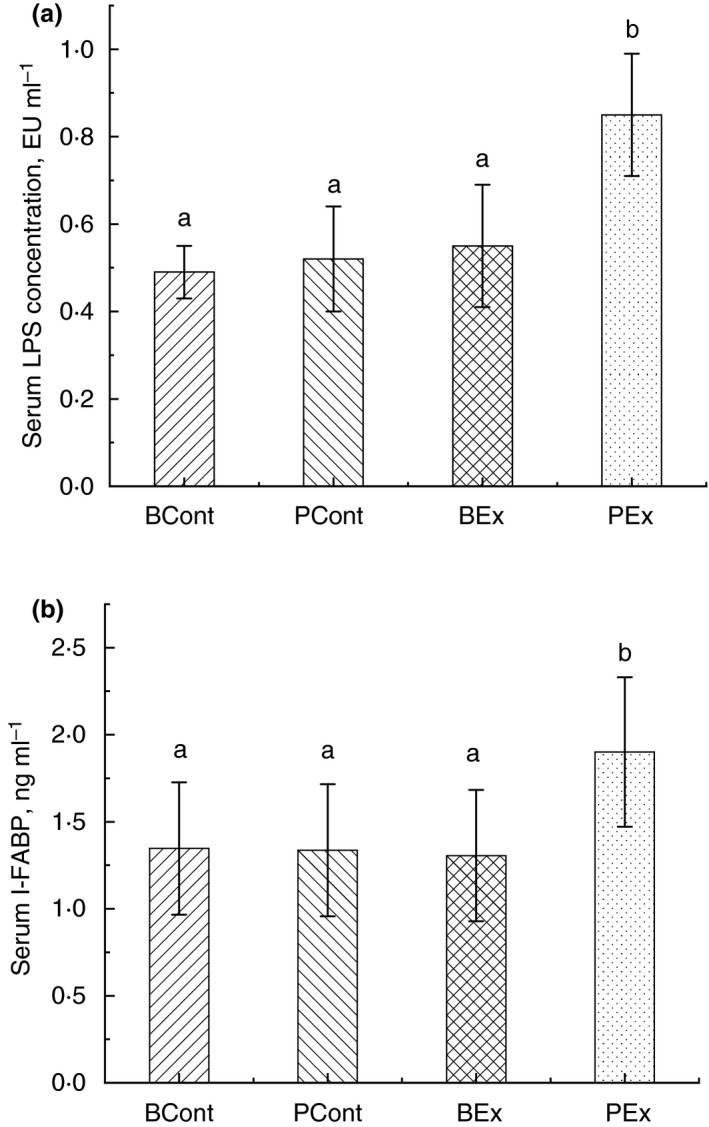
Serum LPS (a) and I‐FABP (b) concentration in rats from different experimental groups. Rats were pretreated by oral gavage with Bacillus subtilis BSB3 (BSB3) or with PBS and were either exposed forced running or remained sedentary (n = 6 rats per condition). BEx, pretreated with BSB3, undergoing forced running; BCont, pretreated with BSB3 remaining sedentary; PEx, pretreated with PBS, undergoing forced running; PCont, pretreated with PBS remaining sedentary. Data with different superscript letters signify groups are significantly different (P < 0·05).
I‐FABP
Considerably elevated level of serum I‐FABP (1·9 ± 0·4 ng ml−1) detected only in rats, received PBS before forced running. Concentration of I‐FABP in animals from BEx group was the same as in control rats (1·3 ± 0·3 ng ml−1) (Fig. 4b).
Gut microbiota analysis
Culture‐based analysis
Culture‐based analysis of the gut microbiota showed a significant elevation of haemolytic bacteria, yeasts and Bacteroides sp. in rats from the PEx group compared with other groups (Fig. 5a). Yeasts and Bacteroides sp. counts was higher in control rats, pretreated with PBS (PCont group) compared to rats that obtained BSB3 (BCont and BEx groups) (Fig. 5b,c). Considerably higher numbers of Lactobacillus sp. and Bifidobacterium sp. were revealed in rats from the PEx group (Fig. 5d,e). Treatment with BSB3 did not affect these bacteria counts in comparison with control PCont rats.
Figure 5.
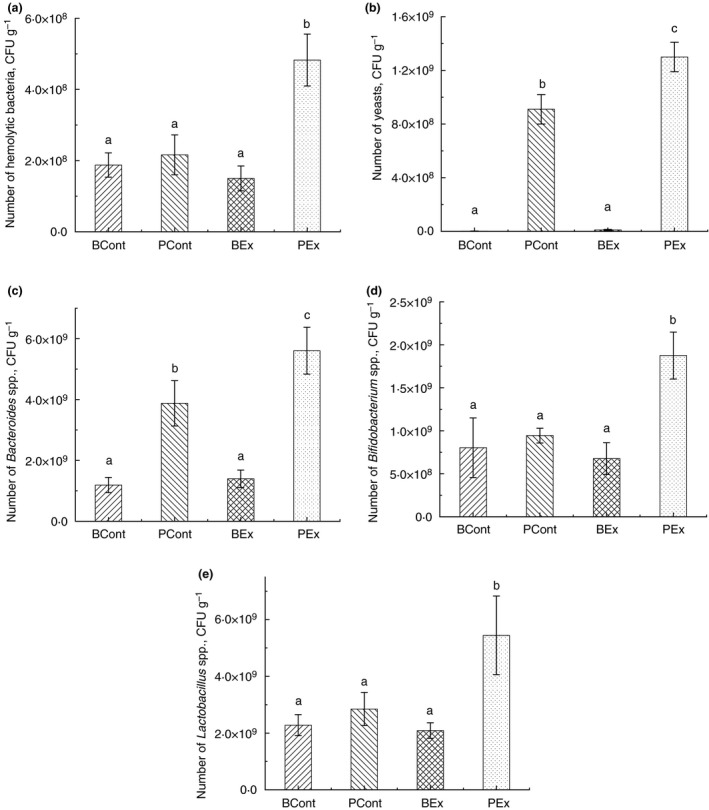
Results of the gut microbiota analysis by a culture‐based method: (a) Number of hemolytic bacteria; (b) Number of yeasts; (c) Number of Bacteroides spp.; (d) Number of Bifidobacterium spp.; (e) Number of Lactobacillus spp. Rats were pretreated by oral gavage with Bacillus subtilis BSB3 (BSB3) or with PBS and were either exposed forced running or remained sedentary (n = 6 rats per condition). BEx, pretreated with BSB3, undergoing forced running; BCont, pretreated with BSB3 remaining sedentary; PEx, pretreated with PBS, undergoing forced running; PCont, pretreated with PBS remaining sedentary. Data with different superscript letters signify groups are significantly different (P < 0·05).
Sequencing
After stringent quality sequence curation, a total of 2 251 607 sequences were parsed and 2 074 232 were then clustered. 2 073 720 sequences identified within the Bacteria domain were utilized for final microbiota analyses. The average reads per sample was 129 607.
Ten bacterial phyla were detected in rats from all groups (Fig. 6a) with three dominant phyla: Firmicutes, Bacteroidetes and Actinobacteria. No significant difference between phyla was found, but the Firmicutes to Bacteroidetes ratio in the BEx group showed marked distinction from all other groups (Fig. 6b). More notable changes were observed at the genus level (Table 1). Comparison of the gut microbiota in PEx rats with the PCont group revealed significant depletion of Candidatus arthromitus, elevation of Veillonella and appearance of Facklamia. Pretreatment of rats with BSB3 before forced running (BEx group) resulted in considerable increase in Allobaculum, Faecalibacterium, Olsenella and decrease in C. arthromitus and Flavonifractor in comparison with PCont rats. Substantial elevation of Olsenella was found also in control rats, pretreated with BSB3 (BCont group). Microbial composition of the gut microbiota in this group was different from the PCont group by significant decrease in Parasporobacterium.
Figure 6.
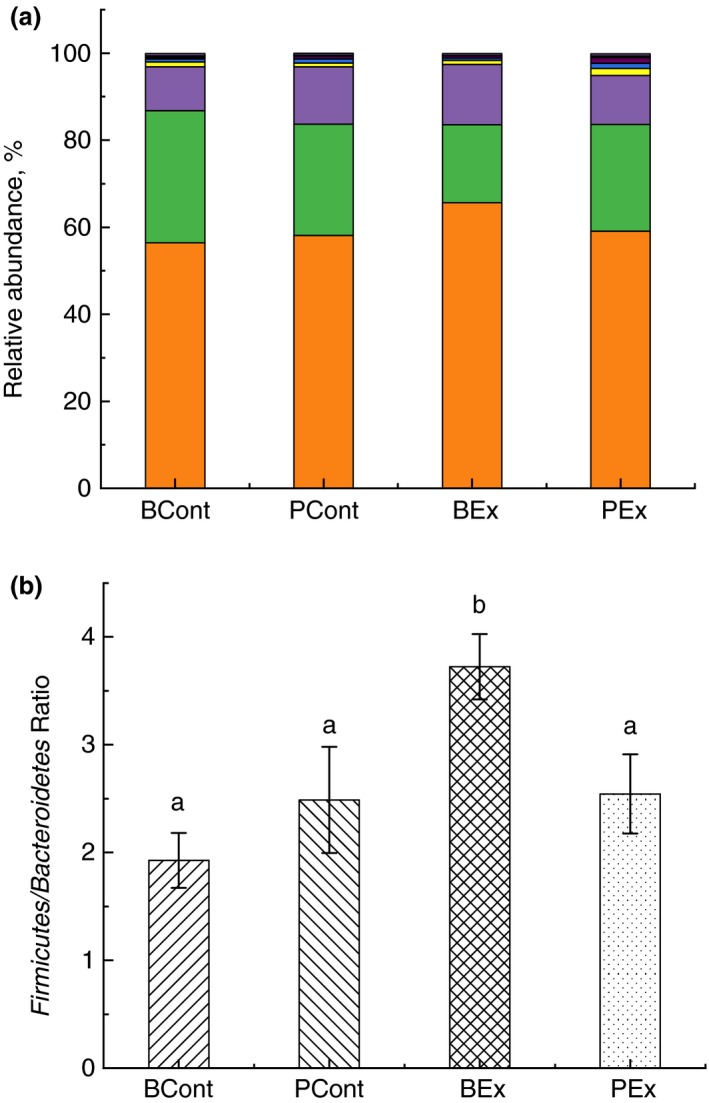
Analysis of the gut microbiota composition of rats at the phylum level. (a) Relative abundance of phyla. All phyla present in abundance of <0·1% are presented as other ( Others;
Others;  Cyanobacteria;
Cyanobacteria;  TM7;
TM7;  Elusimicrobio;
Elusimicrobio;  Deferribacteres;
Deferribacteres;  Verrucomicrobia;
Verrucomicrobia;  Proteobacteria;
Proteobacteria;  Tenericutes;
Tenericutes;  Actinobacteria;
Actinobacteria;  Bacteroidetes;
Bacteroidetes;  Firmicutes); (b) Firmicutes to Bacteroidetes ratio. Rats were pretreated by oral gavage with Bacillus subtilis BSB3 (BSB3) or with PBS and were either exposed forced running or remained sedentary (n = 6 rats per condition). BEx, pretreated with BSB3, undergoing forced running; BCont, pretreated with BSB3 remaining sedentary; PEx, pretreated with PBS, undergoing forced running; PCont, pretreated with PBS remaining sedentary. Data with different superscript letters signify groups are significantly different (P < 0·05). [Colour figure can be viewed at https://www.wileyonlinelibrary.com]
Firmicutes); (b) Firmicutes to Bacteroidetes ratio. Rats were pretreated by oral gavage with Bacillus subtilis BSB3 (BSB3) or with PBS and were either exposed forced running or remained sedentary (n = 6 rats per condition). BEx, pretreated with BSB3, undergoing forced running; BCont, pretreated with BSB3 remaining sedentary; PEx, pretreated with PBS, undergoing forced running; PCont, pretreated with PBS remaining sedentary. Data with different superscript letters signify groups are significantly different (P < 0·05). [Colour figure can be viewed at https://www.wileyonlinelibrary.com]
Table 1.
Changes of the genera in the gut microbiota of rats from different experimental groups
| Genus | PCont | PEx | PEx vs PCont changes, % | P value | ||
|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | |||
| Candidatus arthromitus | 0·0575 | 0·0119 | 0·0061 | 0·0038 | −89·4505 | 0·0147 |
| Enterorhabdus | 0·0083 | 0·0005 | 0·0164 | 0·0020 | 97·2746 | 0·0176 |
| Facklamia | 0·0016 | 0·0008 | PEx* | |||
| Veillonella | 0·0022 | 0·0015 | PEx* | |||
| Genus | PCont | BEx | BEx vs PCont changes, % | P value | ||
|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | |||
| Allobaculum | 0·0289 | 0·0092 | 0·4302 | 0·0326 | 1390·7571 | 0·0002 |
| Candidatus arthromitus | 0·0575 | 0·0119 | 0·0181 | 0·0075 | −68·5834 | 0·0487 |
| Faecalibacterium | 0·0143 | 0·0056 | 0·0331 | 0·0038 | 131·3225 | 0·0411 |
| Flavonifractor | 0·0469 | 0·0051 | 0·0224 | 0·0072 | −52·3085 | 0·0499 |
| Olsenella | 0·0104 | 0·0024 | 0·1998 | 0·0501 | 1830·1272 | 0·0241 |
| Genus | PCont | BCont | BCont vs PCont changes, % | P value | ||
|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | |||
| Olsenella | 0·0104 | 0·0024 | 0·1729 | 0·0281 | 1569·6716 | 0·0045 |
| Parasporobacterium | 0·4358 | 0·0763 | 0·1613 | 0·0326 | −62·9872 | 0·0297 |
Rats were pretreated by oral gavage with B. subtilis BSB3 (BSB3) or with PBS and were either exposed forced running or remained sedentary (n = 6 rats per condition).
BEx, pretreated with BSB3, undergoing forced running; BCont, pretreated with BSB3 remaining sedentary; PEx, pretreated with PBS, undergoing forced running; PCont, pretreated with PBS remaining sedentary.
Genus was found only in this group.
Discussion
The results of this study demonstrated that pretreatment of rats with B. subtilis BSB3 bacteria prevented adverse effects, associated with an exhaustive treadmill exercise bout through beneficial modulation of the gut microbiota.
Our data showed that core body temperature in rats, exposed to exhaustive treadmill exercise exceeded 39°C, which was significantly higher than in sedentary controls. These data indicate that heat stress occurred during exercise without an elevation in environmental temperatures, and these elevations were similar to values obtained in our previous study, which involved increasing core body temperatures via environmental heat stress without exercise (Moore et al. 2014).
We found the changes in gut morphology in rats, pretreated with PBS before forced running (PEx group). Thus, villi height and total mucosa thickness in this group significantly decreased in comparison with control sedentary animals. BSB3 treatment of rats before exhaustive treadmill exercise (BEx group) prevented substantial gut injury: mucosa thickness did not change compared to control BCont and PCont groups; villi height significantly increased in comparison with PEx group. These results agree with our previous findings, in that elevated core body temperature is accompanied by considerable decrease in villi height and BSB3 administration can prevent this damage to the gut of rats (Moore et al. 2014).
Further analysis revealed reduction in Paneth cells in PEx rats, but BSB3 administration prevented the exercise‐induced depletion of these cells. Paneth cells are specialized secretory cells which produce antimicrobial peptides and other compounds, that contribute to intestinal homeostasis and rejuvenation of the intestinal epithelium (Bevins and Salzman 2011; Clevers and Bevins 2013). A reduction in Paneth cells may result in development of epithelial barrier defects, multiorgan dysfunction and systemic inflammation (Estienne et al. 2010; Park et al. 2012). Heat stress is one of the factors responsible for a decrease in Paneth cell number, as we have demonstrated this in a previous study (Ducray et al. 2019). We did not observe a decrease in the number of goblet cells in PEx rats compared to sedentary controls. This finding is consistent with prior studies which have demonstrated no changes in goblet cell number after physical activity (Remedio et al. 2012) or high‐intensity training (Gong et al. 2012).
Notwithstanding, goblet cells significantly increased in rats administered BSB3 prior to treadmill running. Goblet cells are a major producer of mucin, which maintains the integrity of protective mucus barrier, and is a first line of innate defence (Kim and Ho 2010). We suggest that BSB3 influence in elevation of goblet cell number to prevent damaging effect of exhaustive running on gut morphology. Increase in goblet cells and healthy changes in the gut after probiotic supplementation have been reported by other authors (Desantis et al. 2019). Thus, the mechanisms responsible for goblet cell proliferation with various probiotic strains warrant further investigation.
Our data showed significant decrease in expression of all assayed TJ proteins in forced running rats, pretreated with PBS (PEx group), in comparison with other groups. Conversely, BSB3 administration prior to treadmill running prevented the downregulation of all assayed TJ proteins. Efficacy of B. subtilis probiotic in protection of TJ proteins expression during immunological stress has been reported in chickens (Gadde et al. 2017). The TJ is a protein complex, including occludin, claudins, zonula occludens (ZO) and junctional adhesion molecules (JAM), which maintain the integrity of the intestinal epithelial barrier by sealing adjacent epithelial cells. Previously, we showed damaging effect of heat stress on expression of TJ proteins in rats (Ducray et al. 2019). The decrease expression of TJ proteins indicates impaired gut barrier function, accompanied by intestinal permeability (Pastorelli et al. 2013; Bischoff et al. 2014). It was reported that disruption of intestinal epithelial integrity results in elevation of serum LPS (Van Houten et al. 2015) and the release of I‐FABP into serum indicating the presence of enterocyte injury (van Wijck et al. 2012).
We found significant increase in serum LPS and I‐FABP only in PEx group of rats, which directly coincided with a lower presence of TJ proteins in this group. Our observations are in line with results of other authors, who observed elevated levels of LPS, similar to florid sepsis occures in ultramarathon runners (Bosenberg et al. 1988; Walsh et al. 2011) and increase in serum I‐FABP level after high‐intensity exercise (Pugh et al. 2017; Sheahen et al. 2018). However, BSB3 treatment prevented damage of intestinal barrier and release of LPS and I‐FABP into circulation after exercise. Several research efforts have been launched to identify preventative strategies to minimize gastrointestinal function decrements in athletes (van Wijck et al. 2012). Probiotics have been proposed as one of the promising approaches to maintain gut health during exercise and physical activity (Pyne et al. 2015), but only few data show efficacy of probiotics in prevention of intestinal barrier integrity. For instance, Lamprecht et al. (2012) reported, that 14 days of multi‐strain probiotic supplementation by athletes increased ZO‐1 expression after exercise. However, these authors did not assess other TJ proteins, and alterations in the gut microbiota were not studied. Our finding that short‐term of B. subtilis BSB3 consumption significantly protected gut integrity by preventing the loss of TJ proteins following excessive exercise is novel, and we posit that this effect was modulated through positive alterations in the gut microbiota.
Gut microbiota play a significant role in maintaining gastrointestinal barrier integrity (Bischoff et al. 2014). Various diseases and disorders are accompained with dysfunctional intestinal barrier and gut dysbiosis (Konig et al. 2016; Wang et al. 2017). Culture‐based analysis of the gut microbiota in our study revealed significant elevation of haemolytic bacteria, yeasts and Bacteroides sp. only in rats from PEx group. We have previously reported that haemolytic bacteria are elevated in rats after exposure to heat stress (Ducray et al. 2019). Additionally, others have reported that elevations in haemolytic bacteria and yeasts signify the occurrence of dysbiosis in weanling pigs (Poulsen et al. 2017), patients with inflammatory bowel disease (Sokol et al. 2017) and individuals with autism spectrum disorders (Iovene et al. 2017). Bacteroides sp. is a component of the normal gut microbiota, but overrepresentation of these bacteria is associated with different pathological conditions, such as colorectal cancer (Zou et al. 2018), bacteraemia (Lassmann et al. 2007) and septic arthritis (Wexler 2007). We also found significant increase in Lactobacillus sp. and Bifidobacterium sp. in rats from the PEx group compare to other groups. These data are in accordance with results of Queipo‐Ortuno et al. (Queipo‐Ortuno et al. 2013) about elevation the number of Lactobacillus sp. and Bifidobacterium sp. in rats after exercise, although other authors reported decrease in relative abundance of Lactobacillus bacteria after strenuous physical activity (Chaves et al. 2018). Increase in Lactobacillus sp. in rats after heat stress was demonstrated in our previous work (Ducray et al. 2019). We can speculate that increase in Lactobacillus and Bifidobacterium in PEx rats is a response of the gut microbiota to a significant elevation of conditionally pathogenic bacteria, as an attempt to normalize microbial balance.
Results of pyrosequencing showed that Firmicutes, Bacteroidetes and Actinobacteria are the dominant phyla in all groups of rats, which correspond to observations by other authors (Byerley et al. 2017). No difference in phyla presentation was found in different groups, but the Firmicutes to Bacteroidetes ratio was highest in the BEx group. Analysis of the gut microbiota on the genus level revealed a substantial increase in Firmicutes (Allobaculum, Olsenella and Faecalibacterium) in rats from the BEx group in comparison with sedentary PCont rats. These bacteria are known to be effective producers of short‐chain fatty acids (SCFA) (Shing et al. 2014; Kubasova et al. 2018), particularly butyrate, which is the primary energy source for colonocytes, and can thus protect intestinal barrier (Donohoe et al. 2011). Allobaculum, Olsenella and Faecalibacterium have been associated with a reduction in gut inflammation and promotion of intestinal health (Wong et al. 2013; Rosa et al. 2018). In clinical trials intake of probiotic by runners significantly increased the abundance of Faecalibacterium and resulted in relief of stress‐associated symptoms (Sawada et al. 2019). Treatment with a probiotic, containing Lactobacillus acidophilus, Bifidobacterium longum and Enterococcus faecalis resulted in the improvement of the gut microbiota in obese mice with substantial elevation of Allobaculum and Olsenella (Kong et al. 2019). Data, obtained in our study, demonstrate that all tested parameters of gut health in rats from BEx group were similar to sedentary controls, and these parameters were generally more improved in BEx vs PEx rats. We also observed a substantial decrease in Flavonifractor bacteria in BEx rats. Positive associations have been reported between Flavonifractor and inflammatory markers in patients with pemphigus vulgaris disease (Huang et al. 2019). An increased abundance of Flavonifractor has also been reported in patients with systemic lupus erythematosus and bipolar disorder (He et al. 2016; Coello et al. 2019). Flavonifractor has been identified as pathogen for cholecystitis and a causative for infections in immunosuppressed patients (Berger et al. 2018). Thus, the effects BSB3 had in preventing increases in intestinal Flavonifractor levels following a strenuous exercise may promote widespread health benefits in individuals who chronically engage in high levels of physical activity.
Unlike beneficial changes in microbiota in BEx animals, pathogenic bacteria were dominant in the microbiota of PEx rats. In this regard, a significant elevation of Enterorhabdus, and appearance of Facklamia and Veillonella was found in this group of rats. Enterorhabdus has been implicated in the development of obesity (Wang et al. 2016), autism spectrum disorders (de Theije et al. 2014), and is predominant in prediabetic patients (Yang et al. 2015). Facklamia, alpha‐haemolytic bacteria, have been shown to cause bacteraemia (Rahmati et al. 2017), acute cystitis and sepsis (Mostafa et al. 2019). Veillonella species have been reported to be aetiological factor of endocarditis (Saladi et al. 2017) and associated with Crohn’s disease (Kugathasan et al. 2017). Significant increases in these bacteria have been observed in the gut microbiota of children with inflammatory bowel syndrome (Rigsbee et al. 2012) and in patients with primary sclerosing cholangitis (Kummen et al. 2017). Obtained data about predominance of pathogenic bacteria in the gut microbiota of PEx rats agree with the results of our culture‐based analysis and with our previous finding, which indicated significant elevation of pathogens in the gut microbiota of rats after heat stress (Ducray et al. 2019). Our results showed considerable decrease in C. arthromitus in rats after running (groups PEx and BEx) in comparison with sedentary group PCont. Candidatus arthromitus is associated with healthy gut, maturation of the immune system, and protection against pathogens (Hooda et al. 2016; Hedblom et al. 2018). Reduction in C. arthromitus in treadmill‐exercised rats indicates specific depressive effect of endurance running on this bacterium.
Analysis of the gut microbiota in BCont rats revealed significant elevation of Olsenella bacteria, the same as in BEx group. We conclude that increase in these bacteria in rats, pretreated with BSB3, is a result of beneficial stimulation by this strain. Additionally, abundance of Parasporobacterium in BCont group decreased. This is a positive change in the gut microbiota, given that the predominance of Parasporobacterium is associated with Down syndrome (Biagi et al. 2014) and with inflammatory bowel syndrome (Shankar et al. 2015).
This study demonstrated that forced treadmill running results in significant pathological changes in morphology and function of the gut, as well as in the gut microbiota. Our results for the first time showed that short‐term pretreatment of animals with B. subtilis BSB3 beneficially modulates the gut microbiota and as a result, prevents adverse effects of excessive exercise. Indeed, our data are in rodents following an acute bout of unaccustomed treadmill exercise. We believe that BSB3 has a great potential for the development of a valid approach for prevention of excessive exercise‐induced adverse effects.
Conflict of Interest
No conflict of interest declared.
Acknowledgements
This work was supported by School of Kinesiology, Auburn University, Auburn, AL, USA.
References
- Allen, J.M. , Miller, M.E.B. , Pence, B.D. , Whitlock, K. , Nehra, V. , Gaskins, H.R. , White, B.A. , Fryer, J.D. et al (2015) Voluntary and forced exercise differentially alters the gut microbiome in C57BL/6J mice. J Appl Physiol 118, 1059–1066. [DOI] [PubMed] [Google Scholar]
- Allen, J.M. , Mailing, L.J. , Niemiro, G.M. , Moore, R. , Cook, M.D. , White, B.A. , Holscher, H.D. and Woods, J.A. (2018) Exercise alters gut microbiota composition and function in lean and obese humans. Med Sci Sports Exerc 50, 747–757. [DOI] [PubMed] [Google Scholar]
- Aureli, P. , Capurso, L. , Castellazzi, A.M. , Clerici, M. , Giovannini, M. , Morelli, L. , Poli, A. , Pregliasco, F. et al (2011) Probiotics and health: an evidence‐based review. Pharmacol Res 63, 366–376. [DOI] [PubMed] [Google Scholar]
- Benno, Y. and Mitsuoka, T. (1992) Impact of Bifidobacterium longum on human fecal microflora. Microbiol Immunol 36, 683–694. [DOI] [PubMed] [Google Scholar]
- Berger, F.K. , Schwab, N. , Glanemann, M. , Bohle, R.M. , Gartner, B. and Groesdonk, H.V. (2018) Flavonifractor (Eubacterium) plautii bloodstream infection following acute cholecystitis. IDCases 14, e00461– 10.1016/j.idcr.2018.e00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevins, C.L. and Salzman, N.H. (2011) Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol 9, 356–368. [DOI] [PubMed] [Google Scholar]
- Biagi, E. , Candela, M. , Centanni, M. , Consolandi, C. , Rampelli, S. , Turroni, S. , Severgnini, M. , Peano, C. et al (2014) Gut microbiome in down syndrome. PLoS ONE 9, 10.1371/journal.pone.0112023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff, S.C. , Barbara, G. , Buurman, W. , Ockhuizen, T. , Schulzke, J.D. , Serino, M. , Tilg, H. , Watson, A. et al (2014) Intestinal permeability ‐ a new target for disease prevention and therapy. BMC Gastroenterol 14, 25 10.1186/s12876-014-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosenberg, A.T. , Brockutne, J.G. , Gaffin, S.L. , Wells, M.T.B. and Blake, G.T.W. (1988) Strenuous exercise causes systemic endotoxemia. J Appl Physiol 65, 106–108. [DOI] [PubMed] [Google Scholar]
- Byerley, L.O. , Samuelson, D. , Blanchard, E. , Luo, M. , Lorenzen, B.N. , Banks, S. , Ponder, M.A. , Welsh, D.A. et al (2017) Changes in the gut microbial communities following addition of walnuts to the diet. J Nutr Biochem 48, 94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani, P.D. (2018) Human gut microbiome: hopes, threats and promises. Gut 67, 1716–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castell, L.M. , Nieman, D.C. , Bermon, S. and Peeling, P. (2019) Exercise‐induced illness and inflammation: can immunonutrition and iron help? Int J Sport Nutr Exerc Metab 29, 181–188. [DOI] [PubMed] [Google Scholar]
- Chaves, F.M. , Baptista, I.L. , Simabuco, F.M. , Quaresma, P.G.F. , Pena, F.L. , Bezerra, R.M.N. , Pauli, J.R. , Da Cunha, D.T. et al (2018) High‐intensity‐exercise‐induced intestinal damage is protected by fermented milk supplemented with whey protein, probiotic and pomegranate (Punica granatum L.). Br J Nutr 119, 896–909. [DOI] [PubMed] [Google Scholar]
- Clevers, H.C. and Bevins, C.L. (2013) Paneth cells: maestros of the small intestinal crypts. Annu Rev Physiol 75, 289–311. 10.1146/annurev-physiol-030212-183744. [DOI] [PubMed] [Google Scholar]
- Coello, K. , Hansen, T.H. , Sorensen, N. , Munkholm, K. , Kessing, L.V. , Pedersen, O. and Vinberg, M. (2019) Gut microbiota composition in patients with newly diagnosed bipolar disorder and their unaffected first‐degree relatives. Brain Behav Immun 75, 112–118. [DOI] [PubMed] [Google Scholar]
- Coenen, P. , Huysmans, M.A. , Holtermann, A. , Krause, N. , Van Mechelen, W. , Straker, L.M. and Van Der Beek, A.J. (2018) Do highly physically active workers die early? A systematic review with meta‐analysis of data from 193 696 participants. Br J Sports Med 52, 1320–1326. [DOI] [PubMed] [Google Scholar]
- De Oliveira, E.P. and Burini, R.C. (2009) The impact of physical exercise on the gastrointestinal tract. Curr Opin Clin Nutr Metab Care 12, 533–538. [DOI] [PubMed] [Google Scholar]
- De Theije, C.G.M. , Wopereis, H. , Ramadan, M. , Van Eijndthoven, T. , Lambert, J. , Knol, J. , Garssen, J. , Kraneveld, A.D. et al (2014) Altered gut microbiota and activity in a murine model of autism spectrum disorders. Brain Behav Immun 37, 197–206. [DOI] [PubMed] [Google Scholar]
- Desantis, S. , Mastrodonato, M. , Accogli, G. , Rossi, G. and Crovace, A.M. (2019) Effects of a probiotic on the morphology and mucin composition of pig intestine. Histol Histopath 34, 1037–1050. [DOI] [PubMed] [Google Scholar]
- Di Sabatino, A. , Miceli, E. , Dhaliwal, W. , Biancheri, P. , Salerno, R. , Cantoro, L. , Vanoli, A. , De Vincenzi, M. et al (2008) Distribution, proliferation, and function of Paneth cells in uncomplicated and complicated adult celiac disease. Am J Clin Pathol 130, 34–42. [DOI] [PubMed] [Google Scholar]
- Dicks, L.M.T. , Geldenhuys, J. , Mikkelsen, L.S. , Brandsborg, E. and Marcotte, H. (2018) Our gut microbiota: a long walk to homeostasis. Benef Mirbobes 9, 3–19. [DOI] [PubMed] [Google Scholar]
- Donohoe, D.R. , Garge, N. , Zhang, X.X. , Sun, W. , O'connell, T.M. , Bunger, M.K. and Bultman, S.J. (2011) The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab 13, 517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd, S.E. , Callaway, T.R. , Wolcott, R.D. , Sun, Y. , Mckeehan, T. , Hagevoort, R.G. and Edrington, T.S. (2008) Evaluation of the bacterial diversity in the feces of cattle using 16S rDNA bacterial tag‐encoded FLX amplicon pyrosequencing (bTEFAP). BMC Microbiol 8, 1471–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducray, H.A.G. , Globa, L. , Pustovyy, O. , Reeves, S. , Robinson, L. , Vodyanoy, V. and Sorokulova, I. (2016) Mitigation of heat stress‐related complications by a yeast fermentate product. J Therm Biol 60, 26–32. [DOI] [PubMed] [Google Scholar]
- Ducray, H.A.G. , Globa, L. , Pustovyy, O. , Morrison, E. , Vodyanoy, V. and Sorokulova, I. (2019) Yeast fermentate prebiotic improves intestinal barrier integrity during heat stress by modulation of the gut microbiota in rats. J Appl Microbiol 127, 1192–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estienne, M. , Claustre, J. , Clain‐Gardechaux, G. , Paquet, A. , Tache, Y. , Fioramonti, J. and Plaisancie, P. (2010) Maternal deprivation alters epithelial secretory cell lineages in rat duodenum: role of CRF‐related peptides. Gut 59, 744–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadde, U.D. , Oh, S. , Lee, Y. , Davis, E. , Zimmerman, N. , Rehberger, T. and Lillehoj, H.S. (2017) Dietary Bacillus subtilis‐based direct‐fed microbials alleviate LPS‐induced intestinal immunological stress and improve intestinal barrier gene expression in commercial broiler chickens. Res Vet Sci 114, 236–243. [DOI] [PubMed] [Google Scholar]
- Gong, M. , Ren, W.K. , Qi, H.J. , Yin, Y.L. , Wang, D.H. , Liu, G. and Wu, G.Y. (2012) Effect of movement training on the amino acids distribution and intestines morphosis in rats. J Anim Vet Adv 11, 3000–3007. [Google Scholar]
- He, Z.X. , Shao, T.J. , Li, H.C. , Xie, Z.J. and Wen, C.P. (2016) Alterations of the gut microbiome in Chinese patients with systemic lupus erythematosus. Gut Pathog 8, 10.1186/s13099-016-0146-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedblom, G.A. , Reiland, H.A. , Sylte, M.J. , Johnson, T.J. and Baumler, D.J. (2018) Segmented filamentous bacteria ‐ metabolism meets immunity. Front Microbiol 9, 10.3389/fmicb.2018.01991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooda, S. , Levesque, C.L. , Lepp, D. , Yu, H. , Gong, J. and De Lange, C.F.M. (2016) Ileal mucosa and digesta associated microbiota of starter pigs and changes linked to time postweaning and dietary interventions. J Anim Sci 94, 344–348. [Google Scholar]
- Huang, S.L. , Mao, J. , Zhou, L. , Xiong, X. and Deng, Y.Q. (2019) The imbalance of gut microbiota and its correlation with plasma inflammatory cytokines in pemphigus vulgaris patients. Scand J Immunol 90, 10 10.1111/sji.12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iovene, M.R. , Bombace, F. , Maresca, R. , Sapone, A. , Iardino, P. , Picardi, A. , Marotta, R. , Schiraldi, C. et al (2017) Intestinal dysbiosis and yeast isolation in stool of subjects with autism spectrum disorders. Mycopathologia 182, 349–363. [DOI] [PubMed] [Google Scholar]
- Kang, S.S. , Jeraldo, P.R. , Kurti, A. , Miller, M.E.B. , Cook, M.D. , Whitlock, K. , Goldenfeld, N. , Woods, J.A. et al (2014) Diet and exercise orthogonally alter the gut microbiome and reveal independent associations with anxiety and cognition. Mol Neurodegener 9, 12 10.1186/1750-1326-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, Y.S. and Ho, S.B. (2010) Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr Gastroenterol Rep 12, 319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong, C. , Gao, R.Y. , Yan, X.B. , Huang, L.S. and Qin, H.L. (2019) Probiotics improve gut microbiota dysbiosis in obese mice fed a high fat or high‐sucrose diet. Nutrition 60, 175–184. [DOI] [PubMed] [Google Scholar]
- Konig, J. , Wells, J. , Cani, P.D. , Garcia‐Rodenas, C.L. , Macdonald, T. , Mercenier, A. , Whyte, J. , Troost, F. et al (2016) Human intestinal barrier function in health and disease. Clin Transl Gastroenterol 7, 13 10.1038/ctg.2016.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus, W.E. , Powell, K.E. , Haskell, W.L. , Janz, K.F. , Campbell, W.W. , Jakicic, J.M. , Troiano, R.P. , Sprow, K. et al (2019) Physical activity, all‐cause and cardiovascular mortality, and cardiovascular disease. Med Sci Sports Exerc 51, 1270–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruk, J. and Czerniak, U. (2013) Physical activity and its relation to cancer risk: updating the evidence. Asian Pac J Cancer Prev 14, 3993–4003. [DOI] [PubMed] [Google Scholar]
- Kubasova, T. , Davidova‐Gerzova, L. , Babak, V. , Cejkova, D. , Montagne, L. , Le‐Floc'h, N. and Rychlik, I. (2018) Effects of host genetics and environmental conditions on fecal microbiota composition of pigs. PLoS ONE 13, 10.1371/journal.pone.0201901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugathasan, S. , Denson, L.A. , Walters, T.D. , Kim, M.O. , Marigorta, U.M. , Schirmer, M. , Mondal, K. , Liu, C. et al (2017) Prediction of complicated disease course for children newly diagnosed with Crohn's disease: a multicentre inception cohort study. Lancet 389, 1710–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummen, M. , Holm, K. , Anmarkrud, J.A. , Nygard, S. , Vesterhus, M. , Hoivik, M.L. , Troseid, M. , Marschall, H.U. et al (2017) The gut microbial profile in patients with primary sclerosing cholangitis is distinct from patients with ulcerative colitis without biliary disease and healthy controls. Gut 66, 611–619. [DOI] [PubMed] [Google Scholar]
- Lambert, G.P. , Gisolfi, C.V. , Berg, D.J. , Moseley, P.L. , Oberley, L.W. and Kregel, K.C. (2002) Selected contribution: hyperthermia‐induced intestinal permeability and the role of oxidative and nitrosative stress. J Appl Physiol 92, 1750–1761. [DOI] [PubMed] [Google Scholar]
- Lamprecht, M. , Bogner, S. , Schippinger, G. , Steinbauer, K. , Fankhauser, F. , Hallstroem, S. , Schuetz, B. and Greilberger, J.F. (2012) Probiotic supplementation affects markers of intestinal barrier, oxidation, and inflammation in trained men; a randomized, double‐blinded, placebo‐controlled trial. J Int Soc Sport Nutr 9, 13 10.1186/1550-2783-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson, E.B. , Wang, L. , Bowen, J.D. , Mccormick, W.C. , Teri, L. , Crane, P. and Kukull, W. (2006) Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med 144, 73–81. [DOI] [PubMed] [Google Scholar]
- Lassmann, B. , Gustafson, D.R. , Wood, C.M. and Rosenblatt, J.E. (2007) Reemergence of anaerobic bacteremia. Clin Infect Dis 44, 895–900. [DOI] [PubMed] [Google Scholar]
- Leite, G.S.F. , Resende, A.S. , West, N.P. and Lancha, A.H. (2019) Probiotics and sports: a new magic bullet? Nutrition 60, 152–160. [DOI] [PubMed] [Google Scholar]
- Martin, D. (2011) Physical activity benefits and risks on the gastrointestinal system. South Med J 104, 831–837. [DOI] [PubMed] [Google Scholar]
- Moore, T. , Globa, L. , Pustovyy, O. , Vodyanoy, V. and Sorokulova, I. (2014) Oral administration of Bacillus subtilis strain BSB3 can prevent heat stress‐related adverse effects in rats. J Appl Microbiol 117, 1463–1471. [DOI] [PubMed] [Google Scholar]
- Mostafa, H.H. , Taffner, S.M. , Wang, J. , Malek, A. , Hardy, D.J. and Pecora, N.D. (2019) Genome sequence of a Facklamia hominis isolate from a patient with urosepsis. Microbiol Resour Announc 8, 10.1128/mra.00100-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naseribafrouei, A. , Hestad, K. , Avershina, E. , Sekelja, M. , Linlokken, A. , Wilson, R. and Rudi, K. (2014) Correlation between the human fecal microbiota and depression. Neurogastroenterol Motil 26, 1155–1162. [DOI] [PubMed] [Google Scholar]
- O'toole, P.W. and Cooney, J.C. (2008) Probiotic bacteria influence the composition and function of the intestinal microbiota. Interdiscip Perspect Infect Dis 2008, 175285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pane, M. , Amoruso, A. , Deidda, F. , Graziano, T. , Allesina, S. and Mogna, L. (2018) Gut microbiota, probiotics, and sport from clinical evidence to agonistic performance. J Clin Gastroenterol 52, S46–S49. [DOI] [PubMed] [Google Scholar]
- Park, S.W. , Kim, M. , Kim, J.Y. , Ham, A. , Brown, K.M. , Mori‐Akiyama, Y. , Ouellette, A.J. , Dagati, V.D. et al (2012) Paneth cell‐mediated multiorgan dysfunction after acute kidney injury. J Immunol 189, 5421–5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastorelli, L. , De Salvo, C. , Mercado, J.R. , Vecchi, M. and Pizarro, T.T. (2013) Central role of the gut epithelial barrier in the pathogenes of chronic intestinal inflammation: lessons learned from animal models and human genetics. Front. Immunol 4, 22 10.3389/fimmu.2013.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham, T.A.N. and Law, T.D. (2014) Emerging insights on intestinal dysbiosis during bacterial infections. Curr Opin Microbiol 17, 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires, W. , Veneroso, C.E. , Wanner, S.P. , Pacheco, D.A.S. , Vaz, G.C. , Amorim, F.T. , Tonoli, C. , Soares, D.D. et al. (2017) Association between exercise‐induced hyperthermia and intestinal permeability: a systematic review. Sports Med 47, 1389–1403. [DOI] [PubMed] [Google Scholar]
- Poulsen, A.R. , De Jonge, N. , Sugiharto, S. , Nielsen, J.L. , Lauridsen, C. and Canibe, N. (2017) The microbial community of the gut differs between piglets fed sow milk, milk replacer or bovine colostrum. Br J Nutr 117, 964–978. [DOI] [PubMed] [Google Scholar]
- Przyborowski, K. , Kassassir, H. , Wojewoda, M. , Kmiecik, K. , Sitek, B. , Siewiera, K. , Zakrzewska, A. , Rudolf, A.M. et al. (2017) Effects of a single bout of strenuous exercise on platelet activation in female ApoE/LDLR‐/‐ mice. Platelets 28, 657–667. [DOI] [PubMed] [Google Scholar]
- Pugh, J.N. , Impey, S.G. , Doran, D.A. , Fleming, S.C. , Morton, J.P. and Close, G.L. (2017) Acute high‐intensity interval running increases markers of gastrointestinal damage and permeability but not gastrointestinal symptoms. Appl Physiol Nutr Metab 42, 941–947. [DOI] [PubMed] [Google Scholar]
- Pyne, D.B. , West, N.P. , Cox, A.J. and Cripps, A.W. (2015) Probiotics supplementation for athletes ‐ clinical and physiological effects. Eur J Sport Sci 15, 63–72. [DOI] [PubMed] [Google Scholar]
- Queipo‐Ortuno, M.I. , Seoane, L.M. , Murri, M. , Pardo, M. , Gomez‐Zumaquero, J.M. , Cardona, F. , Casanueva, F. and Tinahones, F.J. (2013) Gut microbiota composition in male rat models under different nutritional status and physical activity and its association with serum leptin and ghrelin levels. PLoS ONE 8, 11 10.1371/journal.pone.0065465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmati, E. , Martin, V. , Wong, D. , Sattler, F. , Petterson, J. , Ward, P. , Butler‐Wu, S.M. and She, R.C. (2017) Facklamia species as an underrecognized pathogen. Open Forum Infect Dis 4, 10.1093/ofid/ofw272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remedio, R.N. , Castellar, A. , Barbosa, R.A. , Gomes, R.J. and Caetano, F.H. (2012) Morphological analysis of colon goblet cells and submucosa in type I diabetic rats submitted to physical training. Microsc Res Tech 75, 821–828. [DOI] [PubMed] [Google Scholar]
- Rieder, R. , Wisniewski, P.J. , Alderman, B.L. and Campbell, S.C. (2017) Microbes and mental health: a review. Brain Behav Immun 66, 9–17. [DOI] [PubMed] [Google Scholar]
- Rigsbee, L. , Agans, R. , Shankar, V. , Kenche, H. , Khamis, H.J. , Michail, S. and Paliy, O. (2012) Quantitative profiling of gut microbiota of children with diarrhea‐predominant irritable bowel syndrome. Am J Gastroenterol 107, 1740–1751. [DOI] [PubMed] [Google Scholar]
- Rosa, B.A. , Supali, T. , Gankpala, L. , Djuardi, Y. , Sartono, E. , Zhou, Y.J. , Fischer, K. , Martin, J. et al (2018) Differential human gut microbiome assemblages during soil‐transmitted helminth infections in Indonesia and Liberia. Microbiome 6, 10.1186/s40168-018-0416-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saladi, L. , Zeana, C. and Singh, M. (2017) Native valve endocarditis due to Veillonella species: a case report and review of the literature. Case Rep Infect Dis 3, 10.1155/2017/4896186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada, D. , Kuwano, Y. , Tanaka, H. , Hara, S. , Uchiyama, Y. , Sugawara, T. , Fujiwara, S. , Rokutan, K. et al (2019) Daily intake of Lactobacillus gasseri CP2305 relieves fatigue and stress‐related symptoms in male university Ekiden runners: a double‐blind, randomized, and placebo‐controlled clinical trial. J Funct Food 57, 465–476. [Google Scholar]
- Shankar, V. , Reo, N.V. and Paliy, O. (2015) Simultaneous fecal microbial and metabolite profiling enables accurate classification of pediatric irritable bowel syndrome. Microbiome 3, 10.1186/s40168-015-0139-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahen, B.L. , Fel, J.W. , Zadow, E.K. , Hartley, T.F. and Kitic, C.M. (2018) Intestinal damage following short‐duration exercise at the same relative intensity is similar in temperate and hot environments. Appl Physiol Nutr Metab 43, 1314–1320. [DOI] [PubMed] [Google Scholar]
- Shing, C.M. , Peake, J.M. , Lim, C.L. , Briskey, D. , Walsh, N.P. , Fortes, M.B. , Ahuja, K.D.K. and Vitetta, L. (2014) Effects of probiotics supplementation on gastrointestinal permeability, inflammation and exercise performance in the heat. Eur J Appl Physiol 114, 93–103. [DOI] [PubMed] [Google Scholar]
- Sokol, H. , Leducq, V. , Aschard, H. , Pham, H.P. , Jegou, S. , Landman, C. , Cohen, D. , Liguori, G. et al (2017) Fungal microbiota dysbiosis in IBD. Gut 66, 1039–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer, F. and Backhed, F. (2013) The gut microbiota ‐ masters of host development and physiology. Nat Rev Microbiol 11, 227–238. [DOI] [PubMed] [Google Scholar]
- Sommer, F. , Anderson, J.M. , Bharti, R. , Raes, J. and Rosenstiel, P. (2017) The resilience of the intestinal microbiota influences health and disease. Nat Rev Microbiol 15, 630–638. [DOI] [PubMed] [Google Scholar]
- Sorokulova, I. , Globa, L. , Pustovyy, O. and Vodyanoy, V. (2016) Prevention of heat stress adverse effects in rats by Bacillus subtilis strain. J Vis Exp 10.3791/54122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens, A. (1990) The haematoxylins In Theory and Practice of Histological Techniques ed. Bancrorft J.D., Stevens A. and Turner D.R. Edinburg, London, Melbourne and New York: Churchill Livingstone. [Google Scholar]
- Sudo, N. , Chida, Y. , Aiba, Y. , Sonoda, J. , Oyama, N. , Yu, X.N. , Kubo, C. and Koga, Y. (2004) Postnatal microbial colonization programs the hypothalamic‐pituitary‐adrenal system for stress response in mice. J Physiol‐London 558, 263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevizan, A.R. , Vicentino‐Vieira, S.L. , Watanabe, P.D. , Gois, M.B. , De Melo, G.D.N. , Garcia, J.L. , Araujo, E.J.D. and Sant'ana, D.D.G. (2016) Kinetics of acute infection with Toxoplasma gondii and histopathological changes in the duodenum of rats. Exp Parasitol 165, 22–29. [DOI] [PubMed] [Google Scholar]
- Vainrub, A. , Pustovyy, O. and Vodyanoy, V. (2006) Resolution of 90 nm (lambda/5) in an optical transmission microscope with an annular condenser. Opt Lett 31, 2855–2857. [DOI] [PubMed] [Google Scholar]
- Van Houten, J.M. , Wessells, R.J. , Lujan, H.L. and Dicarlo, S.E. (2015) My gut feeling says rest: Increased intestinal permeability contributes to chronic diseases in high‐intensity exercisers. Med Hypo 85, 882–886. [DOI] [PubMed] [Google Scholar]
- Van Wijck, K. , Lenaerts, K. , Grootjans, J. , Wijnands, K.A.P. , Poeze, M. , Van Loon, L.J.C. , Dejong, C.H.C. and Buurman, W.A. (2012) Physiology and pathophysiology of splanchnic hypoperfusion and intestinal injury during exercise: strategies for evaluation and prevention. Am J Physiol‐Gastroint Liver Physiol 303, G155–G168. [DOI] [PubMed] [Google Scholar]
- Walsh, N.P. , Gleeson, M. , Pyne, D.B. , Nieman, D.C. , Dhabhar, F.S. , Shephard, R.J. , Oliver, S.J. , Bermon, S. et al (2011) Position statement part two: maintaining immune health. Exerc Immunol Rev 17, 64–103. [PubMed] [Google Scholar]
- Wang, J.J. , Tang, H. , Wang, X.X. , Zhang, X. , Zhang, C.H. , Zhang, M.H. , Zhao, Y.F. , Zhao, L. et al (2016) The structural alteration of gut microbiota in low‐birth‐weight mice undergoing accelerated postnatal growth. Sci Rep 6, 13 10.1038/srep27780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, B.H. , Yao, M.F. , Lv, L.X. , Ling, Z.X. and Li, L.J. (2017) The human microbiota in health and disease. Engineering 3, 71–82. [Google Scholar]
- Wexler, H.M. (2007) Bacteroides: the good, the bad, and the nitty‐gritty. Clin Microbiol Rev 20, 593–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, V.W.S. , Tse, C.H. , Lam, T.T.Y. , Wong, G.L.H. , Chim, A.M.L. , Chu, W.C.W. , Yeung, D.K.W. , Law, P.T.W. et al (2013) Molecular characterization of the fecal microbiota in patients with nonalcoholic steatohepatitis ‐ a longitudinal study. PLoS ONE 8, 10.1371/journal.pone.0062885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, J.P. , Summanen, P.H. , Henning, S.M. , Hsu, M. , Lam, H. , Huang, J.J. , Tseng, C.H. , Dowd, S.E. et al (2015) Xylooligosaccharide supplementation alters gut bacteria in both healthy and prediabetic adults: a pilot study. Front Physiol 6, 11 10.3389/fphys.2015.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou, S.M. , Fang, L.K. and Lee, M.H. (2018) Dysbiosis of gut microbiota in promoting the development of colorectal cancer. Gastroenterol Rep 6, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]


