Abstract
Background
Circulating cell‐free DNA (cfDNA) is not found in healthy subjects, but is readily detected after thermal injury and may contribute to the risk of multiple organ failure. The hypothesis was that a postburn reduction in DNase protein/enzyme activity could contribute to the increase in cfDNA following thermal injury.
Methods
Patients with severe burns covering at least 15 per cent of total body surface area were recruited to a prospective cohort study within 24 h of injury. Blood samples were collected from the day of injury for 12 months.
Results
Analysis of blood samples from 64 patients revealed a significant reduction in DNase activity on days 1–28 after injury, compared with healthy controls. DNase protein levels were not affected, suggesting the presence of an enzyme inhibitor. Further analysis revealed that actin (an inhibitor of DNase) was present in serum samples from patients but not those from controls, and concentrations of the actin scavenging proteins gelsolin and vitamin D‐binding protein were significantly reduced after burn injury. In a pilot study of ten military patients with polytrauma, administration of blood products resulted in an increase in DNase activity and gelsolin levels.
Conclusion
The results of this study suggest a novel biological mechanism for the accumulation of cfDNA following thermal injury by which high levels of actin released by damaged tissue cause a reduction in DNase activity. Restoration of the actin scavenging system could therefore restore DNase activity, and reduce the risk of cfDNA‐induced host tissue damage and thrombosis.
Circulating cell‐free DNA (cfDNA) is not found in healthy subjects but is readily detected after thermal injury and may contribute to the risk of multiple organ failure. The authors hypothesized that a postburn reduction in DNase protein/enzyme activity could contribute to the increase in cfDNA after thermal injury. The results of this study suggest a novel biological mechanism for the accumulation of cfDNA following thermal injury whereby high levels of actin released by damaged tissue cause a reduction in DNase activity. Restoration of the actin scavenging system could therefore restore DNase activity, and reduce the risk of cfDNA‐induced host tissue damage and thrombosis. NET, neutrophil extracellular trap.
DNAse activity reduced following thermal injury, likely driven in part by raised circulating actin
Antecedentes
El ADN libre de las células circulantes (circulating cell‐free DNA, cfDNA) no se encuentra en sujetos sanos, pero se detecta fácilmente después de una lesión térmica y puede contribuir al riesgo de fallo multiorgánico. La hipótesis fue que una disminución en la actividad de la proteína/enzima ADNasa tras la lesión térmica podría contribuir a la elevación del cfDNA que ocurre tras la misma.
Métodos
Los pacientes con quemaduras graves con una extensión ≥ 15% del área de superficie corporal total (total body surface area, TBSA) se incluyeron en un estudio prospectivo de cohortes durante las primeras 24 horas posteriores a la lesión. Se recogieron muestras de sangre desde el día de la lesión hasta los 12 meses posteriores a la misma.
Resultados
El análisis de muestras de sangre de 64 pacientes reveló una reducción significativa de la actividad de la ADNasa en los días 1 a 28 después de la lesión, en comparación con los controles sanos. Los niveles de proteína ADNasa no se vieron afectados, lo que sugiere la presencia de un inhibidor enzimático. Un análisis adicional reveló que la actina (un inhibidor de la ADNasa) estaba presente en las muestras de suero de los pacientes, pero no en los controles, y las concentraciones de la gelsolina, proteína que causa la disociación de la actina, y la proteína de unión a la vitamina D se redujeron significativamente después de la lesión térmica. En un estudio piloto de 10 pacientes con politrauma por lesiones militares, la administración de hemoderivados produjo un aumento en la actividad de la ADNasa y de los niveles de gelsolina.
Conclusión
Este estudio sugiere un nuevo mecanismo biológico para la acumulación de cfDNA después de una lesión térmica, por el cual los altos niveles de actina liberada por el tejido dañado causarían una reducción en la actividad de la ADNasa. La restauración del sistema eliminador de actina podría, por lo tanto, restaurar la actividad de la ADNasa y reducir el riesgo de daño tisular y trombosis en el huésped inducido por el cfDNA.
Introduction
Although advances in burn care have improved patient outcomes1, the prevalence of sepsis remains significant2, and failure to diagnose and treat sepsis early leads to multiple organ failure (MOF).
Major thermal injury and severe blunt trauma cause global genomic changes in both the innate and acquired immune pathways3. This response is often pronounced and prolonged in patients who do not achieve clinical recovery. Although MOF is well characterized clinically, the mechanisms mediating organ damage and mortality remain poorly understood4.
Quantification of cell‐free DNA (cfDNA) in blood from injured patients has shown potential to predict sepsis, septic shock and mortality5, 6, 7, 8, 9, 10. DNA from neutrophil extracellular trap (NET) formation has been implicated in immunothrombosis11. NETs provide a bridge between innate immunity and the haemostatic system12, and are capable of perturbing blood flow through capillary plexi13, which may manifest as tissue hypoxia. Recent evidence suggests that intact chromatin is released from NETs following both sterile injury and sepsis in a burn injury/infection model14. This chromatin is highly thrombogenic and implicated in the pathogenesis of MOF13.
A previous study15 reported an increase in cfDNA up to 28 days after severe thermal injury. This included NET‐derived DNA which, in combination with immune parameters, identified burned patients who developed sepsis15. Deoxyribonuclease (DNase) is responsible for the breakdown of circulating chromatin and DNA16, 17. DNase protein levels have been reported to be normal or abnormal in patients with traumatic injury and sepsis respectively18. Although a reduction in total DNase activity19 and genetic mutations in the DNase isoform DnaseIL320 have been shown to be associated with the development of autoimmune diseases and thrombotic microangiopathies21, DNase levels following thermal injury have yet to be studied.
DNase 1 activity can be inhibited by actin released from damaged cells, but enzyme activity is normally protected by the actin scavenging system proteins, gelsolin and vitamin D‐binding protein (VDBP), which together bind to and prevent build‐up of circulating actin19, 22, 23. However, the inter‐relationships between all these proteins is complex owing to their dynamic nature and interactions that ultimately control their individual plasma concentrations24, 25. Given the extensive tissue damage following severe thermal injury, it is highly probable that actin is released into the circulation, causing dysregulation of the actin scavenging system, inhibition of DNase 1 activity and accumulation of cfDNA.
The aim of this prospective longitudinal cohort study was to evaluate the effect of thermal injury on circulating cfDNA levels and DNase activity, and to identify potential mechanisms involved in altered DNase activity. In a separate follow‐on pilot study, the impact of prehospital resuscitation with plasma, which contains gelsolin and VDBP, on DNase activity in military patients with severe polytrauma was studied.
Methods
Patients with thermal injury and study design
Patients with a burn affecting at least 15 per cent of the total body surface area (TBSA) were recruited into a prospective longitudinal study within 24 h of injury and followed up for 12 months. Eighteen healthy controls were included in the study. Blood samples were collected into BD Vacutainer® tubes (Becton Dickinson, Oxford, UK) containing either z‐serum clotting activator or a 1/10 volume of 3·2 per cent trisodium citrate. Blood samples were collected at ten time points after injury: day 1 (24 h or less), day 3 (± 1 day), day 7 (± 1 day), day 14 (± 3 days), day 21 (± 3 days), day 28 (± 3 days), month 2 (± 3 days), month 3 (± 7 days), month 6 (± 7 days) and month 12 (± 7 days). Ethical approval was granted by a UK national research ethics committee (Reference 12/EM/0432). The Abbreviated Burn Severity Index (ABSI)26 and the revised Baux score27 were calculated for each patient. The variables include: sex and age of patient, presence of inhalation injury, presence of full‐thickness burn and percentage of TBSA burned26. The revised Baux score is a clinical scoring system that can predict mortality following thermal injury. It is calculated using the age of the patient, percentage of TBSA burned and presence of inhalation injury27. A diagnosis of sepsis was made when at least three of the sepsis trigger criteria agreed by the American Burn Association were met along with either a positive bacterial culture or when a clinical response to antibiotics was observed28.
Patients with polytrauma study design
A separate prospective cohort observational study was undertaken. All trauma casualties requiring full trauma team activation who presented to Camp Bastion, Afghanistan, between November 2011 and August 2013 were eligible for inclusion in this study. A full trauma team activation occurs for any patient triaged before hospital admission as T1 (the most severe triage category) or meeting the activation criteria (Table S1 , supporting information). All injured patients aged over 18 years were considered for the study and the mechanism of injury was explosion. Each patient was assessed against the inclusion and exclusion criteria (Table S2 , supporting information).
Because of the nature of major and massive haemorrhage after military trauma, a formal ethics submission was not required by the Ministry of Defence Research Ethics Committee as analysis was performed using leftover plasma (waste), which is a negligible amount in relation to the amount of clinical blood loss. The US ethical chain, however, granted ethical approval (log number M‐10242). Informed consent was not required as this represented a minimal risk study.
Preparation of platelet‐free plasma and serum
Citrate anticoagulated blood was centrifuged at 2000 g for 20 min at 4°C. Plasma was then centrifuged at 13 000 g for 20 min. For serum, blood samples were collected into BD Vacutainer® tubes containing z‐serum clotting activator and allowed to clot for 30 min at room temperature. Samples were centrifuged at 1500 g for 10 min at room temperature, after which the serum was removed and stored at –80°C pending analysis.
Preparation of plasma from patients injured in explosions
Some 4·5 ml blood was collected into Vacutainer® sodium citrate collection tubes, with a final ratio of blood to anticoagulant of 9 : 1. Blood was centrifuged at 1500 g for 20 min (Heraeus® Megafuge 16 series®; ThermoScientific, Altrincham, UK). The plasma was removed and frozen at –30°C. Frozen samples were transported to the UK and stored at –80°C.
Fluorometric analysis of plasma cell‐free DNA levels
Levels of cfDNA were measured by fluorometric assay using SYTOX® Green dye (Life Technologies, Warrington, UK). Some 10 μl plasma was incubated with 5 μmol/l SYTOX® Green dye for 10 min and fluorescence was measured using a BioTek® Synergy 2 fluorometric plate reader (NorthStar Scientific, Potton, UK) with excitation and emission set at 485 and 528 nm respectively. For calibration of samples, a λ‐DNA (Fisher Scientific, Loughborough, UK) standard curve was used. The interassay and intra‐assay coefficients of variation were 5·3 and 5·1 per cent respectively.
Quantification of DNase activity in serum and plasma samples
DNase activity was quantified as described previously19. In all experiments, 5 per cent serum or plasma was used for calibration of DNase activity assays, and NET degradation by pooled serum from nine healthy controls was used to define 100 per cent activity. Fluorescence was measured using a BioTek® Synergy 2 fluorometric plate reader with excitation and emission set at 485 and 528 nm respectively.
Visualization of neutrophil extracellular trap degradation by fluorescence microscopy
Isolated neutrophils (5 × 104) were seeded on to glass coverslips (VWR International, Lutterworth, UK) and stimulated with 25 nmol/l phorbol myristate acetate (PMA) for 3 h (37°C and 5 per cent carbon dioxide atmosphere) to generate NETs. Following stimulation, neutrophils were incubated for 6 h with 10 units/ml recombinant human DNase 1 (ThermoScientific), or 5 per cent serum from healthy controls or patients with thermal injuries. After incubation, cells were fixed in 4 per cent paraformaldehyde (37°C and 5 per cent carbon dioxide atmosphere), permeabilized with 0·1 per cent Triton X‐100, and the DNA stained with 1 μmol/l SYTOX® Green dye. Once stained, slides were mounted in Fluoromount™ (Sigma‐Aldrich, Poole, UK) medium and visualized using a Leica DMI 6000 B microscope (Leica, Newcastle Upon Tyne, UK) with a × 20 objective.
Quantification of gelsolin and vitamin D‐binding protein
Gelsolin levels in serum were quantified using a LSBio™ Human GSN/Gelsolin enzyme‐linked immunosorbent assay (ELISA) kit (LifeSpan BioSciences, Nottingham, UK). VDBP levels in serum were quantified using a VDBP ELISA kit (ImmunDiagnostik, Bensheim, Germany). DNase 1 levels were quantified in plasma using a human DNASE1/DNase I ELISA kit (LifeSpan BioSciences).
Detection of actin in platelet‐free plasma
Actin in platelet‐free plasma (PFP) was detected by western blotting using rabbit antihuman primary antibody against actin (A2103; Sigma‐Aldrich) and horseradish peroxidase‐linked antirabbit IgG secondary antibody (GE Healthcare Life Sciences, Amersham, UK) for 1 h. PFP samples were diluted 1 : 5 before analysis. Antigens were detected using enhanced chemiluminescence (GE Healthcare Life Sciences) and visualized using ChemiDoc™ technology (Bio‐Rad, Watford, UK).
Statistical analysis
Data were checked for normality using the Shapiro–Wilk test. Continuous variables were compared using Mann–Whitney U test or unpaired t test, with Bonferroni correction for multiple comparisons. The χ2 test was used for analysis of categorical variables. A one‐way ANOVA followed by Bonferroni's post hoc test was performed when comparing patient data to normal controls.
Results
Demographic and clinical data for patients with thermal injury
Some 64 adult patients with burns were included in the study, with a mean age of 43 (range 16–88) years and mean burn size of 35 (15–95) per cent TBSA. Eighteen healthy controls were included with a mean age of 45 (20–96) years (Table 1). The incidence of sepsis in this cohort of burned patients was 59 per cent, with 38 patients experiencing one or more septic episodes during the hospital stay. The mean number of septic episodes per patient was 3. The mean time to the first episode was 5 (range 3–70) days after injury, and the time to the last episode was 23 (3–130) days following injury. The incidence of MOF in the cohort was 30 per cent (19 of 64). The mean number of independent episodes of MOF was 2 per patient, with the mean time to the first and last episodes of 5 (2–15) and 15 (2–56) days respectively following injury. All patients received standardized burn resuscitation protocols according to the Parkland formula and as such received equivalent fluid resuscitation (mean(s.d.) 5·4(2·1) ml per kg per cent TBSA). Twenty‐one patients received fresh frozen plasma (FFP) when required clinically. The time between injury and the first unit received ranged from 1 to 57 (median 3) days; patients did not receive sustained administration. Fifty‐eight patients with burns (91 per cent) had surgical necrotomies starting at a median 2 days after admission to the burns centre. Of these, 54 (93 per cent) had complete excision of all deep burns by a median 7 days after admission. The remaining four patients did not survive until full excision of deep burns.
Table 1.
Characteristics of patients with thermal injury and healthy controls
| Patients with thermal injury (n = 64) | Healthy controls (n = 18) | |
|---|---|---|
| Age (years)* | 43 (16–88) | 45 (20–96) |
| Sex ratio (M : F) | 43 : 21 | 9 : 9 |
| %TBSA burned* | 35 (15–95) | |
| ABSI score* | 8 (4–14) | |
| Survived | 44 | |
| Sepsis | 38 | |
| Multiple organ failure | 19 |
Values are mean (range). TBSA, total body surface area; ABSI, Abbreviated Burn Severity Index.
DNase activity in patients with thermal injury
On days 1–28 after burn injury, there was a significant reduction in serum DNase activity, relative to that in healthy controls (64 patients, P < 0·001) (Fig. 1 a). Reduced DNase activity was confirmed by fluorescence microscopy; chromatin remained visible when treated with serum from burned patients but not that from healthy controls (Fig. 1 c). DNase activity on the day of injury did not correlate with the size of thermal injury (per cent TBSA), ABSI or revised Baux score (Fig. S1 , supporting information). Levels of DNase protein were quantified to investigate whether the reduction in DNase activity was mediated by a reduction in circulating DNase protein. Levels were quantified in 24 patients who had reduced DNase activity (below 50 per cent of that in healthy controls) within 24 h of injury. There was no significant difference in DNase protein levels on days 1–3 after injury, but a significant increase was detected on days 7–14 and on day 28 after injury compared with levels in healthy controls (P < 0·010) (Fig. 1 b).
Figure 1.
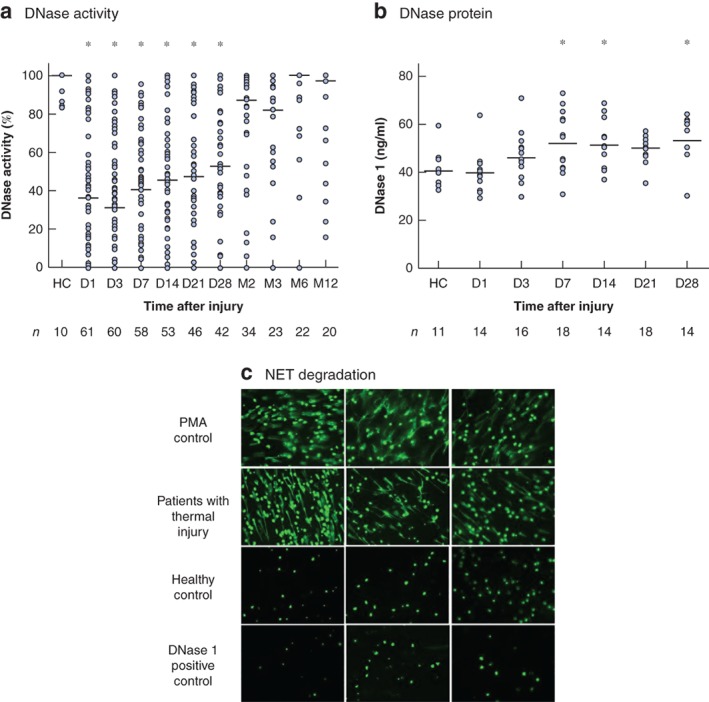
Thermal injury results in reduced DNase activity in the presence of stable or increased antigen levels a DNase activity in serum taken from 64 patients on day (D) 1 to month (M) 12 after thermal injury, and from healthy controls (HC). Median (horizontal bars) and individual values are shown. *P < 0·001 versus HC (1‐way ANOVA followed by Kruskal–Wallis test). b DNase 1 antigen levels in serum taken from 24 patients on D1 to D28 after thermal injury, and from HC. Median (horizontal bars) and individual values are shown. *P < 0·010 versus HC (1‐way ANOVA followed by Bonferroni's multiple comparison test). c Fluorescence microscopic imaging of neutrophil extracellular trap (NET) degradation with phorbol myristate acetate (PMA)‐induced NET DNA stained with SYTOX® Green for untreated control (N = 3), and NETS treated with serum from patients with thermal injuries (N = 3), serum from healthy controls (N = 3) and DNAse 1 positive controls (N = 3) (original magnification ×20).
Actin in serum following thermal injury
Circulating actin was detected in 16 of 20 samples taken from patients within 24 h of injury, but not in plasma from five healthy controls. Western blot data for plasma actin in nine representative patient samples taken within 24 h of injury and two healthy controls are shown in Fig. 2 a (full‐length blots are available in Fig. S2 , supporting information). Actin was also measured at later time points in five patients to assess its persistence in the circulation. Actin was detectable up to day 23 after injury in these patients (Fig. 2 b; Fig. S3 , supporting information). Longitudinal western blot data are shown for a patient with a burn affecting 66 per cent of TBSA who developed sepsis and MOF. Actin was detected within 24 h, and on days 3 and days 20–23 after thermal injury in this patient (Fig. 2 b).
Figure 2.
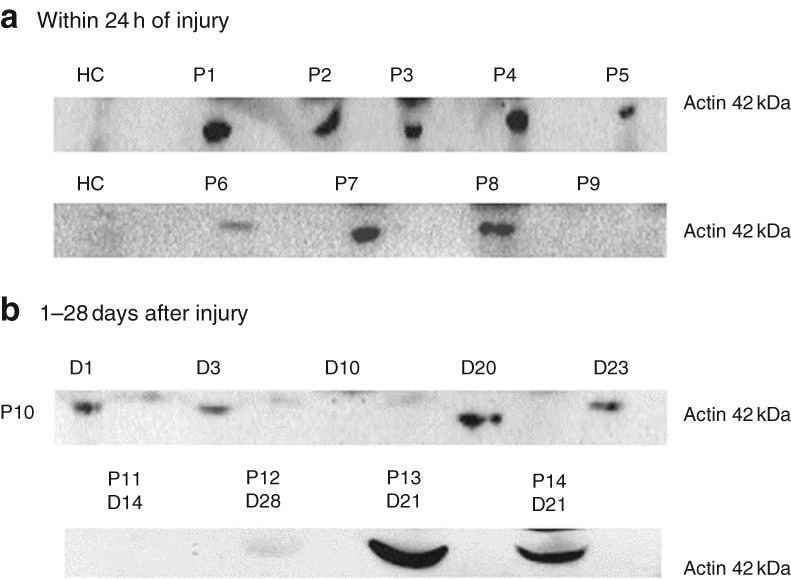
Thermal injury causes release of actin into the blood a Western blot of actin in plasma samples from nine patients (P1–P9) taken within 24 h of injury. Actin was not detected in plasma from healthy controls (HC). Data are from two independent western blots. b Western blot for a patient (P10) with a burn affecting 66 per cent of the total body surface area who developed multiple organ failure (MOF). Actin was detected within 24 h and up to day (D) 3 after thermal injury. Actin was detectable at later time points, day 20–23, corresponding to the later development of MOF. A second western blot for four independent patients (P11–14) shows actin at later time points.
Effect of thermal injury on circulating vitamin D‐binding protein and gelsolin levels
Thermal injury resulted in a rapid and significant reduction in VDBP levels from day 1 to day 3 after injury compared with levels in healthy controls (P < 0·001), with values returning to control levels by day 7 (Fig. 3 a). Levels of VDBP weakly correlated with DNase activity across all time points (r = 0·15, P = 0·013). Thermal injury also resulted in a significant reduction in gelsolin from day 1 to day 21 after injury compared with levels in healthy controls (P < 0·010), with values returning to control levels thereafter (Fig. 3 b). Levels of gelsolin also correlated weakly with DNase activity across all time points (r = 0·13, P = 0·0058).
Figure 3.
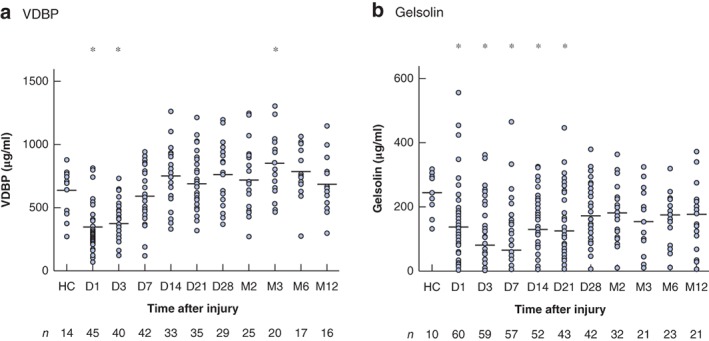
Levels of vitamin D‐binding protein and gelsolin are reduced after thermal injury Levels of a vitamin D‐binding protein (VDBP) in 50 patients and b gelsolin in 64 patients from day (D) 1 to month (M) 12 after thermal injury, and in healthy controls (HC). Median (horizontal bars) and individual values are shown. *P < 0·010 versus HC (1‐way ANOVA followed by Bonferroni's multiple comparison test).
Impact of fresh frozen plasma on gelsolin levels and DNase activity in military patients with polytrauma
Levels of gelsolin, VDBP and DNase activity were quantified in plasma from patients with polytrauma admitted to hospital following injuries sustained in explosions. This cohort of military patients was split into patients who had (5) or had not (5) received FFP before admission to hospital. There was no significant difference in Injury Severity Score, New Injury Severity Score or time to admission after injury between the two groups (Table 2). The decision whether to administer blood products was determined by resources available and not the mechanism or severity of injury. Patients received an mean of 3 units of blood products before hospital admission.
Table 2.
Data for patients with polytrauma injury
| No FFP before admission (n = 5) | FFP before admission (n = 5) | P * | |
|---|---|---|---|
| Injury Severity Score | 27 (17–59) | 22 (16–42) | 0·579 |
| New Injury Severity Score | 36 (18–75) | 35 (16–66) | 0·999 |
| Interval between injury and admission (min) | 75 (30–135) | 83 (50–130) | 0·571 |
Values are mean (range). The mechanism of injury was explosion. FFP, fresh frozen plasma.
Mann–Whitney U test.
Levels of DNase activity (Fig. 4 a) and gelsolin (Fig. 4 b) were significantly higher in patients who received FFP than in those who did not (P = 0·028 and P = 0·032 respectively). There was no difference between the two groups in circulating VDBP or cfDNA levels (Fig. 4 c,d).
Figure 4.
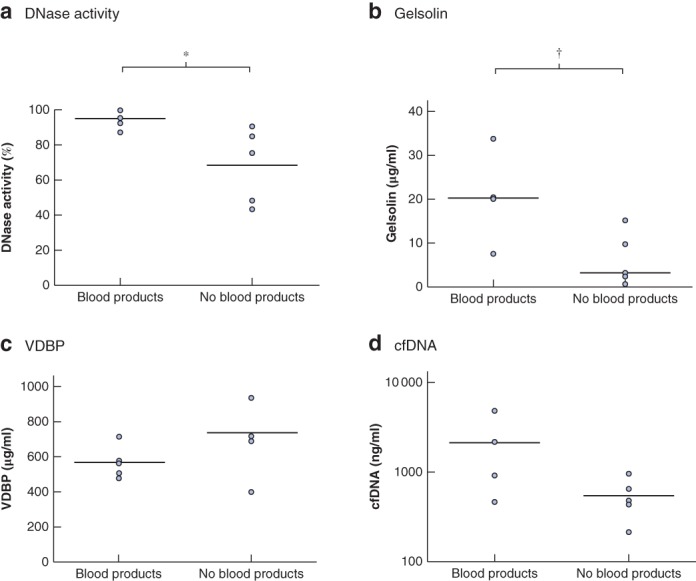
Blood products increase circulating gelsolin levels and protect against inhibition of DNase activity in patients with polytrauma Comparison of a DNase activity, b gelsolin, c vitamin D‐binding protein (VDBP) and d cell‐free (cf) DNA levels at hospital admission between five patients who had previously received blood products (fresh frozen plasma) and five who had not. Median (horizontal bars) and individual values are shown. *P = 0·028, †P = 0·032 (Mann–Whitney U test).
Discussion
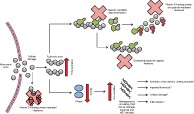
This study showed a reduced ability of postburn injury serum to degrade cfDNA ex vivo, with concomitant high levels of cfDNA in the circulation15. Deficiency in DNase activity will predispose patients to accumulation of circulating cfDNA released following tissue injury15, and during infection. A novel mechanistic link between the initial traumatic injury and the pathogenesis of thrombosis and MOF is suggested (Fig. 5). Furthermore, a preliminary study highlighted the potential benefit of targeting the actin scavenging system and reduced DNase activity following major trauma using human blood products.
Figure 5.
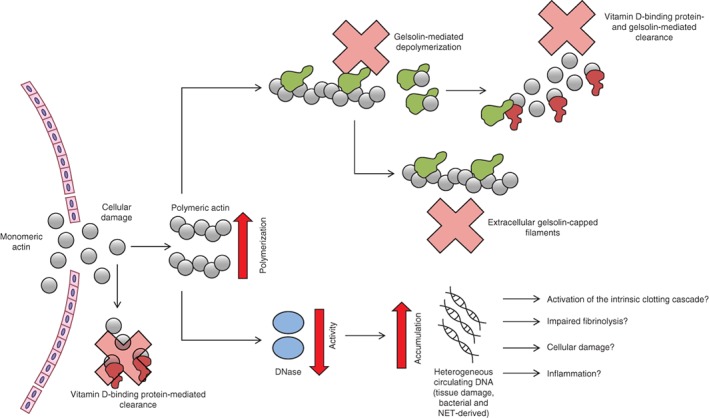
Hypothesis model suggesting how severe thermal injury results in disruption of the actin scavenging system which predisposes to DNase activity inhibition and accumulation of cell‐free DNA Following severe thermal injury, polymerized and monomeric actin is released, which immediately reduces vitamin D‐binding protein and gelsolin levels. The polymerized actin can then bind to DNase and inhibit its activity. Injury‐ and immune cell‐derived DNA can accumulate and potentially cause activation of the intrinsic clotting cascade, impaired fibrinolysis, bind to platelets, and cause cellular damage and/or inflammation. NET, neutrophil extracellular trap.
There was a reduction in total DNase activity from day 1 to day 28 after injury, a time frame paralleled by an increase in circulating cfDNA levels from analysis of the same patient samples15. Measurement of cfDNA is not straightforward as it can be released by necrosis and apoptosis as well as from NETs, resulting in significant differences in size. For example, chromatin released from NETs is largely intact, in contrast to the heterogeneous/random size of DNA released by necrosis and small/uniform DNA by apoptosis29. In the present cohort, the rapid increase in cfDNA correlated with measurements of burn size and severity15. Therefore, although the initial increase in circulating DNA most likely originated from tissue damage caused by injury, the exact origin is difficult to determine. Although PCR confirmed that the cfDNA was derived from the nucleus15, these measurements cannot distinguish between intact chromatin or oligonucleotides derived by DNase degradation14. Circulating NETs originate from neutrophils after induction of tissue injury and sepsis, and are largely comprised of intact chromatin14. Owing to the cytotoxic/prothrombotic nature of DNA30, 31, the initial increase in chromatin may therefore contribute to the immediate host tissue and organ damage. The inability to clear chromatin may result in an increased risk of thrombosis, host tissue damage, occlusion of capillary plexi and MOF13, 31. Importantly, thrombotic effects of DNA and NETs are abolished by DNase32, 33, 34. Hence, reduced DNase may predispose patients to host tissue damage, thrombosis and organ damage mediated by NET‐derived DNA released following a burn injury. Although a reduction in DNase activity was shown, it was not possible to determine accurately a level of DNase activity inhibition at which this mechanism became important clinically.
Actin exists in a balance between monomeric and filamentous forms35, 36, and acts as a damage‐associated molecular pattern37. Circulating actin was detected in patients with severe burns for up to 28 days after injury. The immediate release of actin most likely resulted from the extensive tissue damage, with further release caused by surgery, infection and/or MOF. Indeed, 58 of 64 patients in this study had surgical necrotomies starting a median of 2 days after admission, with 54 of these having complete excision of deep burns by a median of 7 days. Lee and colleagues38 have also reported increased levels of actin in a cohort of patients with sepsis38.
Many of the detrimental effects of actin are normally controlled by the proteins gelsolin and VDBP23. Levels of both proteins decreased for up to 21 days after injury in the present study. Decreased levels of gelsolin and VDBP have also been reported in a number of disease pathologies associated with tissue damage39, 40, 41, 42, 43, 44, and have been suggested to be good prognostic markers of outcome/organ damage following severe trauma45, 46, 47, 48, 49, 50. Of note, the initial reduction in VDBP and gelsolin levels occurred before the onset of sepsis and MOF in the present cohort. It is probable that excess actin released from the injury caused the immediate reduction in their circulating levels. Therefore, all patients with severe thermal injuries are predisposed to a reduction in DNase activity, and the potential complications associated with the accumulation of cfDNA (Fig. 5). Although this study has provided supporting evidence for the potential effect of actin on the consumption of the actin scavengers gelsolin and VDBP, and subsequent loss of protection of the inhibition of DNase activity, the dynamic balance of their individual circulating concentrations will be determined by their rate of biosynthesis, half‐life and consumption; this is complex in the context of severe injury owing to surgery, treatment with blood products, vessel wall leakiness and dilution caused by resuscitation.
FFP is widely used in trauma and burns as an effective resuscitation/coagulopathy therapy. Given the complexity of FFP, it is difficult to assess which of its many components provide benefit. By definition, FFP contains high levels of gelsolin and VDBP, which may explain some of the therapeutic potential in the context of traumatic injury51. To investigate the potential therapeutic benefit of FFP, the authors undertook a preliminary analysis of samples acquired from military patients who had received FFP before admission to hospital following severe polytrauma. Early administration of FFP significantly increased gelsolin levels immediately after severe trauma, which was also accompanied by a significant increase in DNase activity, though cfDNA levels were not reduced. In 2005, Chhabra and co‐workers52 showed that the N‐terminal fragment of gelsolin could bind to and disrupt actin–DNase complexes, in turn restoring enzymatic activity52. FFP is not only effective in treating trauma but was shown previously to attenuate extracellular nucleosome levels and depletion of DNase53, 54, and to provide neuroprotection in models of traumatic brain injury55, 56, 57.
Given the extensive literature and debate on the value of FFP in trauma58, 59, 60, it may be more beneficial to use gelsolin in isolation to scavenge excess actin and restore DNase activity. Indeed, low plasma gelsolin levels on admission to hospital following trauma have been associated with poor outcome49. Data generated in a rat burn model showed that administration of gelsolin before burn injury protected against pulmonary microvasculature dysfunction61. Although the underlying mechanism was not confirmed, protection of DNase activity will improve the clearance of circulating chromatin and cfDNA.
A major limitation of the DNA measurements in both the trauma and burns cohorts in the present study is that the size of chromatin/cfDNA was not determined14. Another limitation is the relatively small sample size as this study was designed to be exploratory in nature. As such, no attempt was made to investigate whether levels of DNase activity, gelsolin or VDBP differed between patients with and without MOF. The fact that only some patients received FFP, coupled with the sporadic timing and lack of sustained administration, means that the effect it may have exerted on DNase activity and the actin scavenging system cannot be determined.
A model of postinjury complications has been described, in which DNase activity was reduced following thermal injury, driven most likely by raised circulating actin and acute reductions in levels of the actin scavengers gelsolin and VDBP. The reduced DNase activity and accumulation of chromatin/cfDNA may have contributed to organ damage, thrombosis, inflammation and impaired fibrinolysis (Fig. 5). The results support the possible use of therapeutic agents including not only DNase itself but FFP and gelsolin, which can also restore and protect DNase activity in severe injury.
Supporting information
Table S1. Trauma team activation criteria according to Clinical Guidelines for Operations. JSP 999.
Table S2. Inclusion and Exclusion Criteria for polytrauma patients.
Fig. S1. DNase activity within 24 hours of injury does not correlate with severity and size of injury. A, Correlation of DNase activity and total body surface area (TBSA) burn (%) (n = 64) B, Correlation of DNase activity and abbreviated burn severity score index (ABSI) (n = 64). C, Correlation of DNase activity and revised Baux score (rBaux) (n = 64). All measurements were taken within 24 hours of injury. Data was compared by Spearmans rank.
Fig. S2. Full length western blots for Figure 2 A. Western blots are independent experiments and calibrated using a molecular weight marker.
Fig. S3. Full length western blots for Figure 2 B. Western blots are independent experiments and calibrated using a molecular weight marker.
Acknowledgements
The authors thank the nursing team at the Birmingham Burns Centre for their assistance with sample collection. They acknowledge the Scar Free Foundation and the National Institute for Health Research Surgical Reconstruction and Microbiology Research Centre (NIHR‐SRMRC), a partnership between University Hospitals Birmingham NHS Foundation Trust, the University of Birmingham and the Royal Centre for Defence Medicine. This work was funded by the Scar Free Foundation and NIHR‐SRMRC.
Disclosure: The authors declare no conflict of interest.
References
- 1. Jackson PC, Hardwicke J, Bamford A, Nightingale P, Wilson Y, Papini R et al Revised estimates of mortality from the Birmingham Burn Centre, 2001–2010: a continuing analysis over 65 years. Ann Surg 2014; 259: 979–984. [DOI] [PubMed] [Google Scholar]
- 2. Mann EA, Baun MM, Meininger JC, Wade CE. Comparison of mortality associated with sepsis in the burn, trauma, and general intensive care unit patient: a systematic review of the literature. Shock 2012; 37: 4–16. [DOI] [PubMed] [Google Scholar]
- 3. Xiao W, Mindrinos MN, Seok J, Cuschieri J, Cuenca AG, Gao H et al; Inflammation and Host Response to Injury Large‐Scale Collaborative Research Program. A genomic storm in critically injured humans. J Exp Med 2011; 208: 2581–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cohen J, Vincent JL, Adhikari NK, Machado FR, Angus DC, Calandra T et al Sepsis: a roadmap for future research. Lancet Infect Dis 2015; 15: 581–614. [DOI] [PubMed] [Google Scholar]
- 5. Dwivedi DJ, Toltl LJ, Swystun LL, Pogue J, Liaw KL, Weitz JI et al; Canadian Critical Care Translational Biology Group . Prognostic utility and characterization of cell‐free DNA in patients with severe sepsis. Crit Care 2012; 16: R151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saukkonen K, Lakkisto P, Pettila V, Varpula M, Karlsson S, Ruokonen E et al; Finnsepsis Study Group . Cell‐free plasma DNA as a predictor of outcome in severe sepsis and septic shock. Clin Chem 2008; 54: 1000–1007. [DOI] [PubMed] [Google Scholar]
- 7. Rhodes A, Wort SJ, Thomas H, Collinson P, Bennett ED. Plasma DNA concentration as a predictor of mortality and sepsis in critically ill patients. Crit Care 2006; 10: R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shoham Y, Krieger Y, Perry ZH, Shaked G, Bogdanov‐Berezovsky A, Silberstein E et al Admission cell free DNA as a prognostic factor in burns: quantification by use of a direct rapid fluorometric technique. Biomed Res Int 2014; 2014: 306580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lo YM, Rainer TH, Chan LY, Hjelm NM, Cocks RA. Plasma DNA as a prognostic marker in trauma patients. Clin Chem 2000; 46: 319–323. [PubMed] [Google Scholar]
- 10. Naumann DN, Hazeldine J, Dinsdale RJ, Bishop JR, Midwinter MJ, Harrison P et al Endotheliopathy is associated with higher levels of cell‐free DNA following major trauma: a prospective observational study. PLoS One 2017; 12: e0189870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol 2013; 13: 34–45. [DOI] [PubMed] [Google Scholar]
- 12. Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM et al Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med 2007; 13: 463–469. [DOI] [PubMed] [Google Scholar]
- 13. Boneschansker L, Inoue Y, Oklu R, Irimia D. Capillary plexuses are vulnerable to neutrophil extracellular traps. Integr Biol (Camb) 2016; 8: 149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Otawara M, Roushan M, Wang X, Ellett F, Yu YM, Irimia D. Microfluidic assay measures increased neutrophil extracellular traps circulating in blood after burn injuries. Sci Rep 2018; 8: 16983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hampson P, Dinsdale RJ, Wearn CM, Bamford AL, Bishop JRB, Hazeldine J et al Neutrophil dysfunction, immature granulocytes, and cell‐free DNA are early biomarkers of sepsis in burn‐injured patients: a prospective observational cohort study. Ann Surg 2017; 265: 1241–1249. [DOI] [PubMed] [Google Scholar]
- 16. Napirei M, Wulf S, Eulitz D, Mannherz HG, Kloeckl T. Comparative characterization of rat deoxyribonuclease 1 (Dnase1) and murine deoxyribonuclease 1‐like 3 (Dnase1l3). Biochem J 2005; 389: 355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Napirei M, Ludwig S, Mezrhab J, Klockl T, Mannherz HG. Murine serum nucleases – contrasting effects of plasmin and heparin on the activities of Dnase1 and Dnase1‐like 3 (Dnase1l3). FEBS J 2009; 276: 1059–1073. [DOI] [PubMed] [Google Scholar]
- 18. Meng W, Paunel‐Görgülü A, Flohé S, Witte I, Schädel‐Höpfner M, Windolf J et al Deoxyribonuclease is a potential counter regulator of aberrant neutrophil extracellular traps formation after major trauma. Mediators Inflamm 2012; 2012: 149560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hakkim A, Fürnrohr BG, Amann K, Laube B, Abed UA, Brinkmann V et al Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci U S A 2010; 107: 9813–9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Al‐Mayouf SM, Sunker A, Abdwani R, Abrawi SA, Almurshedi F, Alhashmi N et al Loss‐of‐function variant in DNASE1L3 causes a familial form of systemic lupus erythematosus. Nat Genet 2011; 43: 1186–1188. [DOI] [PubMed] [Google Scholar]
- 21. Jimenez‐Alcazar M, Napirei M, Panda R, Kohler EC, Kremer Hovinga JA, Mannherz HG et al Impaired DNase1‐mediated degradation of neutrophil extracellular traps is associated with acute thrombotic microangiopathies. J Thromb Haemost 2015; 13: 732–742. [DOI] [PubMed] [Google Scholar]
- 22. Lazarides E, Lindberg U. Actin is the naturally occurring inhibitor of deoxyribonuclease I. Proc Natl Acad Sci U S A 1974; 71: 4742–4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee WM, Galbraith RM. The extracellular actin‐scavenger system and actin toxicity. N Engl J Med 1992; 326: 1335–1341. [DOI] [PubMed] [Google Scholar]
- 24. Kwiatkowski DJ, Janmey PA, Yin HL. Identification of critical functional and regulatory domains in gelsolin. J Cell Biol 1989; 108: 1717–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yin HL, Iida K, Janmey PA. Identification of a polyphosphoinositide‐modulated domain in gelsolin which binds to the sides of actin filaments. J Cell Biol 1988; 106: 805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tobiasen J, Hiebert JM, Edlich RF. The abbreviated burn severity index. Ann Emerg Med 1982; 11: 260–262. [DOI] [PubMed] [Google Scholar]
- 27. Osler T, Glance LG, Hosmer DW. Simplified estimates of the probability of death after burn injuries: extending and updating the Baux score. J Trauma 2010; 68: 690–697. [DOI] [PubMed] [Google Scholar]
- 28. Greenhalgh DG, Saffle JR, Holmes JH IV, Gamelli RL, Palmieri TL, Horton JW et al; American Burn Association Consensus Conference on Burn Sepsis and Infection Group . American Burn Association consensus conference to define sepsis and infection in burns. J Burn Care Res 2007; 28: 776–790. [DOI] [PubMed] [Google Scholar]
- 29. Thierry AR, El Messaoudi S, Gahan PB, Anker P, Stroun M. Origins, structures, and functions of circulating DNA in oncology. Cancer Metastasis Rev 2016; 35: 347–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bhagirath VC, Dwivedi DJ, Liaw PC. Comparison of the proinflammatory and procoagulant properties of nuclear, mitochondrial, and bacterial DNA. Shock 2015; 44: 265–271. [DOI] [PubMed] [Google Scholar]
- 31. Kaplan MJ, Radic M. Neutrophil extracellular traps: double‐edged swords of innate immunity. J Immunol 2012; 189: 2689–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD Jr et al Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A 2010; 107: 15 880–15 885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fuchs TA, Brill A, Wagner DD. Neutrophil extracellular trap (net) impact on deep vein thrombosis. Arterioscler Thromb Vasc Biol 2012; 32: 1777–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gupta AK, Joshi MB, Philippova M, Erne P, Hasler P, Hahn S et al Activated endothelial cells induce neutrophil extracellular traps and are susceptible to NETosis‐mediated cell death. FEBS Lett 2010; 584: 3193–3197. [DOI] [PubMed] [Google Scholar]
- 35. Mogilner A, Oster G. Cell motility driven by actin polymerization. Biophys J 1996; 71: 3030–3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ananthakrishnan R, Ehrlicher A. The forces behind cell movement. Int J Biol Sci 2007; 3: 303–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Srinivasan N, Gordon O, Ahrens S, Franz A, Deddouche S, Chakravarty P et al Actin is an evolutionarily‐conserved damage‐associated molecular pattern that signals tissue injury in Drosophila melanogaster . Elife 2016; 5: pii: e19662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee PS, Patel SR, Christiani DC, Bajwa E, Stossel TP, Waxman AB. Plasma gelsolin depletion and circulating actin in sepsis: a pilot study. PLoS One 2008; 3: e3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lind SE, Smith DB, Janmey PA, Stossel TP. Depression of gelsolin levels and detection of gelsolin–actin complexes in plasma of patients with acute lung injury. Am Rev Respir Dis 1988; 138: 429–434. [DOI] [PubMed] [Google Scholar]
- 40. Ito H, Kambe H, Kimura Y, Nakamura H, Hayashi E, Kishimoto T et al Depression of plasma gelsolin level during acute liver injury. Gastroenterology 1992; 102: 1686–1692. [DOI] [PubMed] [Google Scholar]
- 41. Huang LF, Yao YM, Li JF, Dong N, Liu C, Yu Y et al Reduction of plasma gelsolin levels correlates with development of multiple organ dysfunction syndrome and fatal outcome in burn patients. PLoS One 2011; 6: e25748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Suhler E, Lin W, Yin HL, Lee WM. Decreased plasma gelsolin concentrations in acute liver failure, myocardial infarction, septic shock, and myonecrosis. Crit Care Med 1997; 25: 594–598. [DOI] [PubMed] [Google Scholar]
- 43. Osborn TM, Verdrengh M, Stossel TP, Tarkowski A, Bokarewa M. Decreased levels of the gelsolin plasma isoform in patients with rheumatoid arthritis. Arthritis Res Ther 2008; 10: R117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hu Y, Li H, Li WH, Meng HX, Fan YZ, Li WJ et al The value of decreased plasma gelsolin levels in patients with systemic lupus erythematosus and rheumatoid arthritis in diagnosis and disease activity evaluation. Lupus 2013; 22: 1455–1461. [DOI] [PubMed] [Google Scholar]
- 45. Dahl B, Schiødt FV, Nielsen M, Kiaer T, Williams JG, Ott P. Admission level of Gc‐globulin predicts outcome after multiple trauma. Injury 1999; 30: 275–281. [DOI] [PubMed] [Google Scholar]
- 46. Dahl B, Schiødt FV, Ott P, Wians F, Lee WM, Balko J et al Plasma concentration of Gc‐globulin is associated with organ dysfunction and sepsis after injury. Crit Care Med 2003; 31: 152–156. [DOI] [PubMed] [Google Scholar]
- 47. Dahl B, Schiødt FV, Rudolph S, Ott P, Kiaer T, Heslet L. Trauma stimulates the synthesis of Gc‐globulin. Intensive Care Med 2001; 27: 394–399. [DOI] [PubMed] [Google Scholar]
- 48. Dahl B, Schiødt FV, Kiaer T, Ott P, Bondesen S, Tygstrup N. Serum Gc‐globulin in the early course of multiple trauma. Crit Care Med 1998; 26: 285–289. [DOI] [PubMed] [Google Scholar]
- 49. Mounzer KC, Moncure M, Smith YR, Dinubile MJ. Relationship of admission plasma gelsolin levels to clinical outcomes in patients after major trauma. Am J Respir Crit Care Med 1999; 160: 1673–1681. [DOI] [PubMed] [Google Scholar]
- 50. DiNubile MJ. Plasma gelsolin as a biomarker of inflammation. Arthritis Res Ther 2008; 10: 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mitra B, Mori A, Cameron PA, Fitzgerald M, Paul E, Street A. Fresh frozen plasma (FFP) use during massive blood transfusion in trauma resuscitation. Injury 2010; 41: 35–39. [DOI] [PubMed] [Google Scholar]
- 52. Chhabra D, Nosworthy NJ, dos Remedios CG. The N‐terminal fragment of gelsolin inhibits the interaction of DNase I with isolated actin, but not with the cofilin–actin complex. Proteomics 2005; 5: 3131–3136. [DOI] [PubMed] [Google Scholar]
- 53. Sillesen M, Johansson PI, Rasmussen LS, Jin G, Jepsen CH, Imam A et al Fresh frozen plasma resuscitation attenuates platelet dysfunction compared with normal saline in a large animal model of multisystem trauma. J Trauma Acute Care Surg 2014; 76: 998–1007. [DOI] [PubMed] [Google Scholar]
- 54. Watts S, Nordmann G, Brohi K, Midwinter M, Woolley T, Gwyther R et al Evaluation of prehospital blood products to attenuate acute coagulopathy of trauma in a model of severe injury and shock in anesthetized pigs. Shock 2015; 44(Suppl 1): 138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Halaweish I, Bambakidis T, Nikolian VC, Georgoff P, Bruhn P, Piascik P et al Early resuscitation with lyophilized plasma provides equal neuroprotection compared with fresh frozen plasma in a large animal survival model of traumatic brain injury and hemorrhagic shock. J Trauma Acute Care Surg 2016; 81: 1080–1087. [DOI] [PubMed] [Google Scholar]
- 56. Georgoff PE, Nikolian VC, Halaweish I, Chtraklin K, Bruhn PJ, Eidy H et al Resuscitation with lyophilized plasma is safe and improves neurological recovery in a long‐term survival model of swine subjected to traumatic brain injury, hemorrhagic shock, and polytrauma. J Neurotrauma 2017; 34: 2167–2175. [DOI] [PubMed] [Google Scholar]
- 57. Imam AM, Jin G, Sillesen M, Duggan M, Jepsen CH, Hwabejire JO et al Early treatment with lyophilized plasma protects the brain in a large animal model of combined traumatic brain injury and hemorrhagic shock. J Trauma Acute Care Surg 2013; 75: 976–983. [DOI] [PubMed] [Google Scholar]
- 58. Zhang LM, Li R, Zhao XC, Zhang Q, Luo XL. Increased transfusion of fresh frozen plasma is associated with mortality or worse functional outcomes after severe traumatic brain injury: a retrospective study. World Neurosurg 2017; 104: 381–389. [DOI] [PubMed] [Google Scholar]
- 59. Mesar T, Larentzakis A, Dzik W, Chang Y, Velmahos G, Yeh DD. Association between ratio of fresh frozen plasma to red blood cells during massive transfusion and survival among patients without traumatic injury. JAMA Surg 2017; 152: 574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hagiwara A, Kushimoto S, Kato H, Sasaki J, Ogura H, Matsuoka T et al Can early aggressive administration of fresh frozen plasma improve outcomes in patients with severe blunt trauma? – A report by the Japanese Association for the Surgery of Trauma. Shock 2016; 45: 495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rothenbach PA, Dahl B, Schwartz JJ, O'Keefe GE, Yamamoto M, Lee WM et al Recombinant plasma gelsolin infusion attenuates burn‐induced pulmonary microvascular dysfunction. J Appl Physiol (1985) 2004; 96: 25–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Trauma team activation criteria according to Clinical Guidelines for Operations. JSP 999.
Table S2. Inclusion and Exclusion Criteria for polytrauma patients.
Fig. S1. DNase activity within 24 hours of injury does not correlate with severity and size of injury. A, Correlation of DNase activity and total body surface area (TBSA) burn (%) (n = 64) B, Correlation of DNase activity and abbreviated burn severity score index (ABSI) (n = 64). C, Correlation of DNase activity and revised Baux score (rBaux) (n = 64). All measurements were taken within 24 hours of injury. Data was compared by Spearmans rank.
Fig. S2. Full length western blots for Figure 2 A. Western blots are independent experiments and calibrated using a molecular weight marker.
Fig. S3. Full length western blots for Figure 2 B. Western blots are independent experiments and calibrated using a molecular weight marker.


