Summary
Uncertainty exists regarding the relation of body size and weight change with dementia risk. As populations continue to age and the global obesity epidemic shows no sign of waning, reliable quantification of such associations is important. We examined the relationship of body mass index, waist circumference, and annual percent weight change with risk of dementia and its subtypes by pooling data from 19 prospective cohort studies and four clinical trials using meta‐analysis. Compared with body mass index–defined lower‐normal weight (18.5‐22.4 kg/m2), the risk of all‐cause dementia was higher among underweight individuals but lower among those with upper‐normal (22.5‐24.9 kg/m2) levels. Obesity was associated with higher risk in vascular dementia. Similarly, relative to the lowest fifth of waist circumference, those in the highest fifth had nonsignificant higher vascular dementia risk. Weight loss was associated with higher all‐cause dementia risk relative to weight maintenance. Weight gain was weakly associated with higher vascular dementia risk. The relationship between body size, weight change, and dementia is complex and exhibits non‐linear associations depending on dementia subtype under scrutiny. Weight loss was associated with an elevated risk most likely due to reverse causality and/or pathophysiological changes in the brain, although the latter remains speculative.
Abbreviations
- BMI
body mass index
- CI
confidence intervals
- HR
hazard ratio
- WC
waist circumference
1. INTRODUCTION
Dementia, a disease primarily of aging, affects an estimated 47 million people globally.1 It is a heterogeneous condition chiefly comprising Alzheimer disease (60‐70% of cases) and vascular dementia accounting for about 15% of cases, although the two subtypes frequently co‐occur.1, 2 Aging, family history, and sarcopenia are important risk factors for dementia, and there is growing evidence that vascular risk factors, such as diabetes, may also confer increased risk, particularly for vascular dementia, although findings are inconsistent.3, 4
Excess body weight, typically defined as having a high body mass index (BMI), has been causally linked to a large number of chronic conditions, particularly vascular disease.5 While the relationship between BMI and dementia has been the subject of several large‐scale epidemiological studies, the findings have been inconsistent: some studies have reported that only individuals at the extreme ends of the body size spectrum (ie, underweight and obese) experience an increased dementia risk,6, 7 while others have documented positive8 and even inverse9 associations. Measures of central obesity, such as waist circumference (WC), have been argued to be more informative measures of obesity‐related risk compared with BMI. Currently, little is known about the association between central obesity and dementia risk.
As nonvascular and vascular dementia have different pathophysiologies, any association with body size may similarly differ according to endpoint. Distinguishing between possible dementia subtypes in any analysis with measures of body size may, therefore, prove informative in explaining some of the observed heterogeneity. Further, whether sex differences exist, or if the association between body size and dementia risk differs in middle and later life remains unclear.10, 11
In this meta‐analysis, we examined the relationships of body size with all‐cause dementia and possible vascular and nonvascular dementia by sex and baseline age in participants free of dementia who had their body size assessed at baseline and were later followed up on dementia status. Using repeat measures, where available, we explored the association between standardized annual weight change during follow‐up with subsequent dementia risk.
2. METHODS
The investigators of studies that were either identified from previous systematic reviews and meta‐analyses,6, 7, 12, 13, 14 or who were known to Dementia Pooling Project15 collaborators, were contacted and asked to contribute results for their studies (Figure S1). Seven of the 14 studies from the Dementia Pooling Project contributed data that were included in the analyses. Additional relevant data from 28 studies were identified from five previous systematic reviews and meta‐analyses of the association. Fourteen studies that had not contributed to previous overviews were identified through a PUBMED search by one investigator (CMYL). This was limited to human subjects and the period up to 1 September 2017, based on the search strategy: ([body mass index OR body weight OR obesity OR waist circumference] AND dementia) OR (clinical trial AND incident dementia). Studies were deemed eligible for inclusion if BMI or WC were collected at baseline and dementia status was available at follow‐up. Subsequently, 42 invitations were issued inviting study investigators to collaborate in this pooling study. Of these, 10 studies contributed results. Collaborators provided information on a further eight studies that had not been identified by the literature search, or which had not been included in previous overviews, as the data were unpublished; six of these studies provided data. A total of 23 studies responded (n = 2 790 753).
BMI was calculated as weight in kilogrammes divided by height in metres squared. Directly measured (n = 2 760 602; 98.9%) and self‐report (n = 30 151; 1.1%) height and weight were used in the calculation. WC was measured either at the midpoint between rib and iliac crest (n = 217 051; 29.9%), narrowest waist (n = 9768; 1.7%), umbilicus (n = 12 428; 1.3%), or narrowest waist or umbilicus (n = 486 275; 67.0%). Annual percent weight change was calculated as follows:
Five BMI categories were distinguished: underweight: less than 18.5 kg/m2; lower‐normal weight: 18.5‐22.4 kg/m2; upper‐normal weight: 22.5‐24.9 kg/m2; overweight: 25.0‐29.9 kg/m2; and obese: greater than or equal to 30.0 kg/m2.
Dementia endpoints were defined by investigators of each individual study. Ascertainment of dementia was by medical examination in 12 studies (Table 1). These studies classified dementia based solely on the Diagnostic and Statistical Manual of Method Disorders criteria38, 39 or in combination with the National Institute of Neurological and Communication Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association criteria for Alzheimer disease40 and the National Institute of Neurological Disorders and Stroke and the Association Internationale pour la Recherche et l'Enseignement en Neurosciences criteria for vascular dementia.41 Other methods used in dementia ascertainment included health records (two studies), death records (five studies), and death and health or hospitalization records (four studies). Ten of these studies that used other methods to ascertain dementia classified the disorder based on the International Classification of Diseases and one study used Read Codes42 to classify dementia (Table 1).
Table 1.
Characteristics of included studies
| Study | Country | Baseline Year | Baseline Age (years) | N (% female) | Dementia Cases | Dementia Ascertainment | Dementia Criteria | Anthropometric Measurement (waist protocol) |
|---|---|---|---|---|---|---|---|---|
| Adult Changes in Thought study16 | USA | 1994‐1996 | ≥65 | 4343 (58.3) | 1096 | Medical examination | DSM‐IV, NINCDS‐ADRDA | Measured (narrowest waist) |
| Action in Diabetes and Vascular Disease Preteraz and Diamicron MR Controlled Evaluation trial17 | International | 2001‐2003 | 55‐88 | 11 136 (42.5) | 109 | Medical examination | DSM‐IV | Measured (midpoint between rib and iliac crest) |
| Aging Multidisciplinary Investigation (AMI) cohort18 | France | 2007 | ≥65 | 563 (38.7) | 65 | Medical examination | DSM‐III‐R, NINCDS‐ADRDA, NINDS‐AIREN | Measured (midpoint between rib and iliac crest) |
| The Copenhagen City Heart Study19 | Denmark | 1976‐1978 | ≥20 | 9037 (55.7) | 969 | Health record | ICD‐8, ICD‐10 | Measured (umbilicus) |
| Cache County Memory Study20 | USA | 1995 | ≥65 | 3185 (57.4) | 507 | Medical examination | DSM‐III‐R, NINCDS‐ADRDA, NINDS‐AIREN | Self‐report |
| The Copenhagen General Population Study21 | Denmark | 2003 | ≥20 | 10 4506 (55.2) | 1906 | Health record | ICD‐8, ICD‐10 | Measured (midpoint between rib and iliac crest) |
| Clinical Practice Research Datalink9 | UK | 1992‐2007 | ≥40 | 19 58191 (54.8) | 45507 | Health and death record | Read Codes | Measured |
| Finnish Twin Cohort22 | Finland | 1975 | ≥18 | 25 814 (51.1) | 960 | Death record | ICD‐8, ICD‐9, ICD‐10 | Self‐report |
| Framingham Heart Study23 | USA | 1992‐1996, 1998‐2001 | ≥60 | 2232 (56.0) | 289 | Medical examination | DSM‐IV, NINCDS‐ADRDA, NINDS‐AIREN | Measured (umbilicus) |
| General Post Office Study24 | UK | 1966‐1967 | 35‐70 | 1385 (37.0) | 18 | Death record | ICD‐8, ICD‐9, ICD‐10 | Measured |
| Hisayama Study25 | Japan | 1988 | ≥60 | 1192 (58.3) | 350 | Medical examination | DSM‐III‐R, NINCDS‐ADRDA, NINDS‐AIREN | Measured (umbilicus) |
| Health Survey for England and Scottish Health Survey26 | UK | 1995‐2008 | 16‐99 | 90 685 (54.7) | 524 | Death record | ICD‐9, ICD‐10 | Measured (midpoint between rib and iliac crest) |
| Hypertension in the Very Elderly Trial27 | International | 2000 | ≥80 | 3337 (60.4) | 263 | Medical examination | DSM‐IV | Measured |
| Norwegian Counties Study28 | Norway | 1974‐1978 | 35‐50 | 40 978 (50.6) | 1173 | Death record | ICD‐9, ICD‐10 | Measured |
| Origins of Variance in the Old‐Old29 | Sweden | 1963 | 45‐65 | 1152 (69.0) | 312 | Health record and interview | DSM‐III‐R, NINCDS‐ADRDA, NINDS‐AIREN | Self‐report |
| Prevention of Dementia by Intensive Vascular Care trial30 | The Netherlands | 2006‐2009 | 70‐78 | 3526 (54.4) | 233 | Medical examination | DSM‐IV | Measured (midpoint between rib and iliac crest) |
| Primary Prevention Study31 | Sweden | 1970‐1973 | 45‐55 | 7394 (0) | 788 | Death and hospitalization records | ICD‐8, ICD‐9, ICD‐10 | Measured |
| The Perindopril Protection Against Recurrent Stroke Study32 | International | 1995‐1997 | 26‐91 | 5865 (29.7) | 380 | Medical examination | DSM‐IV | Measured |
| Study of Osteoporotic Fractures33 | USA | 1986‐1988 | ≥65 | 1019 (100) | 232 | Medical examination | DSM‐IV | Measured (narrowest waist) |
| Three City Study34 | France | 1999‐2000 | ≥65 | 6721 (61.4) | 832 | Medical examination | DSM‐IV | Measured (midpoint between rib and iliac crest) |
| UK Biobank35 | UK | 2006‐2010 | 39‐74 | 48 6275 (54.6) | 344 | Death and hospitalization records | ICD | Measured (narrowest waist or umbilicus) |
| Whitehall I Study36 | UK | 1967‐1969 | 40‐69 | 17 167 (0) | 288 | Death record | ICD‐8, ICD‐9, ICD‐10 | Measured |
| Whitehall II Study37 | UK | 1985‐1988 | 35‐55 | 5050 (28.3) | 149 | Death and hospitalization records | ICD‐10 | Measured (narrowest waist) |
Abbreviations: DSM‐III, Diagnostic and Statistical Manual of Mental Disorders third edition criteria; DMS‐III‐R, Diagnostic and Statistical Manual of Mental Disorders third edition revised criteria; DSM‐IV, Diagnostic and Statistical Manual of Mental Disorders fourth edition criteria; ICD‐8, International Classification of Diseases eighth revision; ICD‐9, International Classification of Diseases ninth revision; ICD‐10, International Classification of Diseases tenth revision; and Related Health Problems; NINCDS‐ADRDA, National Institute of Neurological and Communication Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association criteria; NINDS‐AIREN, National Institute of Neurological Disorders and Stroke and Association Internationale pour la Recherche et l'Enseignement en Neurosciences criteria;
2.1. Data analysis
Sex‐specific hazard ratios (HRs) and 95% confidence intervals (CIs) were obtained for all‐cause dementia in relation to (a) each of five BMI categories, with lower‐normal weight as the referent group; (b) each fifth of WC, with the first fifth as the referent group; and (c) each of three annual percent weight change categories (greater than or equal to 0.5% annual weight loss, less than 0.5% annual weight change, and greater than or equal to 0.5% annual weight gain), with less than 0·5% annual weight change as the referent group. Following a pre‐specified common analytic protocol, effect estimates were adjusted for age (model 1); age, smoking, and education or socio‐economic status (model 2; which was the primary model—used in the reporting of outcomes herein); and age, smoking, education or socio‐economic status, diabetes, systolic blood pressure, total cholesterol, blood pressure–lowering medication, cholesterol‐lowering medication, and glucose‐lowering medication where available (model 3). For studies with information on possible dementia subtype, study‐specific estimates were requested for possible vascular dementia and possible nonvascular dementia.
A random effects meta‐analysis was used to combine study‐specific log HR to obtain an overall summary estimate and associated 95% CIs for BMI and WC in relation to all dementia endpoints investigated. Analyses were conducted for women and men combined and then separately. Heterogeneity between studies was quantified using the I 2 statistic.
Sensitivity analyses were conducted by excluding the largest study and by excluding the studies that did not calculate BMI using objective measures of height and weight. To assess the potential effect of reverse causality, in the same studies, we compared data that were non‐left censored with those that were. As the studies varied by the length of follow‐up, maximum periods of left censoring requested (3, 5, or 10 years) differed between studies. We also compared the associations by study design, by method of dementia ascertainment, and by study baseline mean age. All statistical analyses were performed using Stata/SE 14.0 (Stata Corp LP., USA).
3. RESULTS
Results from 23 studies comprised 2 790 753 participants (54% women) in whom 57 294 cases of all‐cause dementia were accumulated during a mean of 9.6 years of disease surveillance. Of these cases, 6792 were classed as nonvascular dementia, and 1214 were recorded as vascular dementia. Of the studies included, information from 14 cohort studies (n = 728 959; 26.1%) were previously unpublished, and four were from clinical trial populations (n = 23 864; 0.9%). Most studies were from Europe (n = 2 758 444; 98.8%), with one study9 contributing more than 70% of participants (79% cases of all‐cause dementia). Study characteristics are shown in Tables S1 and S2. In brief, mean age at baseline ranged from 36 to 83 years in men and 37 to 84 years in women. Mean duration of follow‐up varied from 4 to 38 years, with an overall median of 9 years. Mean BMIs at study baseline were 21.9 to 28.2 kg/m2 in men and 22.4 to 28.8 kg/m2 in women; for WC, the corresponding results were 96.9 to 100.7 and 80.7 to 97.5 cm.
A non‐linear association between BMI with all‐cause dementia was observed: compared with the referent group (BMI: 18.5‐22.4 kg/m2) those who were underweight had a one‐quarter greater risk of dementia (HR: 1.26, 95% CI, 1.20‐1.31; Figure 1). This association remained similar after excluding the first 3, 5, or 10 years (median 10 years) of follow‐up in a subset of studies (1.35 [1.24‐1.46]; Figure S2a; Table S3). Relative to the referent group, individuals with upper‐normal BMI, overweight or obesity had a 10% to 15% lower dementia risk (Figure 1). Similar results were obtained after adjustment for age (Figures S3‐S6). Additional adjustment for cardiometabolic risk factors did not materially alter the relationship (model 3; Table S4). Neither were results significantly different in a range of sensitivity analyses (Table S5; Figure S7). Further, the associations were comparable between studies with baseline mean age younger than 60 and 60 years and older (Figure S8) and before and after exclusion of the first 3, 5, or 10 years (median 10 years; Figure S2a; Table S3).
Figure 1.
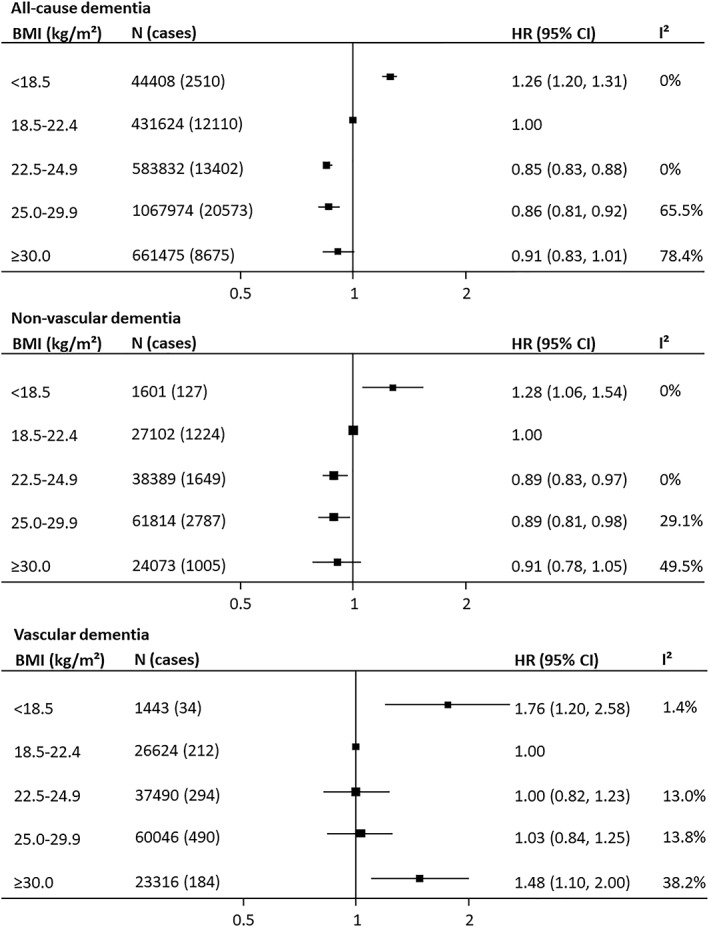
Association between body mass index (BMI) and incident fatal and nonfatal dementia and its major subtypes. Hazard ratios (HRs) and 95% confidence intervals (CIs) adjusted for age, smoking, and education or socio‐economic status
As with all‐cause dementia, the association between BMI and nonvascular dementia risk was non‐linear. Relative to lower‐normal BMI, individuals categorized as underweight were at approximately one‐quarter increased dementia risk (1.28 [1.06‐1.54]; Figure 1; Table S4). Adjustment for cardiometabolic risk factors did not materially affect the association (Table S4). The findings were similarly robust when restricting the analysis to studies that used measured height and weight. Findings were also consistent between clinical trials and observational studies and between studies with baseline mean age younger 60 and 60 years and older (Figures S8 and S9). There was no evidence of a sex difference in the association (Figure 2). Relative to the referent group, individuals with upper‐normal BMI, overweight, or obesity had 10% lower dementia risk (Figure 1). After excluding the first few years of follow‐up (median 7.5 years), the increased risk of nonvascular dementia in the underweight category remained, but the association was no longer significant in the overweight and obese categories (Figure S2b; Table S3).
Figure 2.
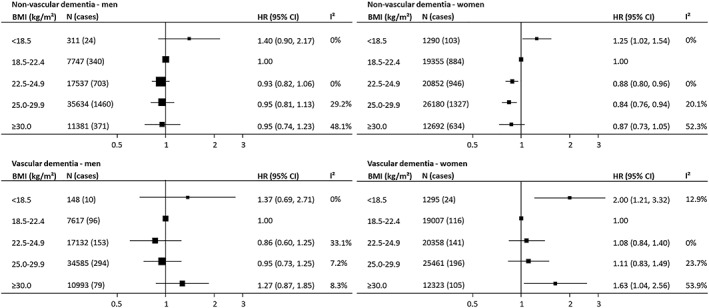
Associations between body mass index (BMI) and incident fatal and nonfatal dementia subtypes by sex. Hazard ratios (HRs) and 95% confidence intervals (CIs) adjusted for age, smoking, and education or socio‐economic status
Both individuals with underweight or obesity were at increased vascular dementia risk. Compared with the referent group, those who were underweight had an approximate 80% greater dementia risk, and for those in the obese category, the risk was approximately 50% higher (Figure 1). The excess risk among the obese group was largely mediated by cardiometabolic risk factors (HR for model 3: 1.26 [0.87‐1.84]; Table S4). Sensitivity analyses indicated findings compatible with the main result (Figure S9). There was no evidence of a sex difference in the association between BMI and vascular dementia risk (Figure 2). The association was broadly similar in studies with baseline mean age younger than 60 and 60 years and older (Figure S8). Excluding the first few years of follow‐up (median 7.5 years) did not materially influence the relationship (Figure S2c; Table S3).
A non‐linear association was observed between WC and all‐cause dementia among the 13 studies (725 522 individuals; 54.5% women; 7057 cases) that contributed to the analysis. Compared with individuals in the lowest fifth of WC, individuals with larger WC had 15% to 22% lower all‐cause dementia risk (Figure 3). Similar results were obtained when adjusted for age or after adjustment for cardiometabolic risk factors (Figures S10‐S13; Table S6). The estimates tended to be larger for studies that used death records to ascertain dementia status rather than those that used medical examination (Figure S14). Data from clinical trials produced similar results to those from nontrial populations (Figure S15). The estimate of effect was more pronounced for studies with baseline mean age younger than 60 than 60 years and older especially at higher WC categories (Figure S16).
Figure 3.
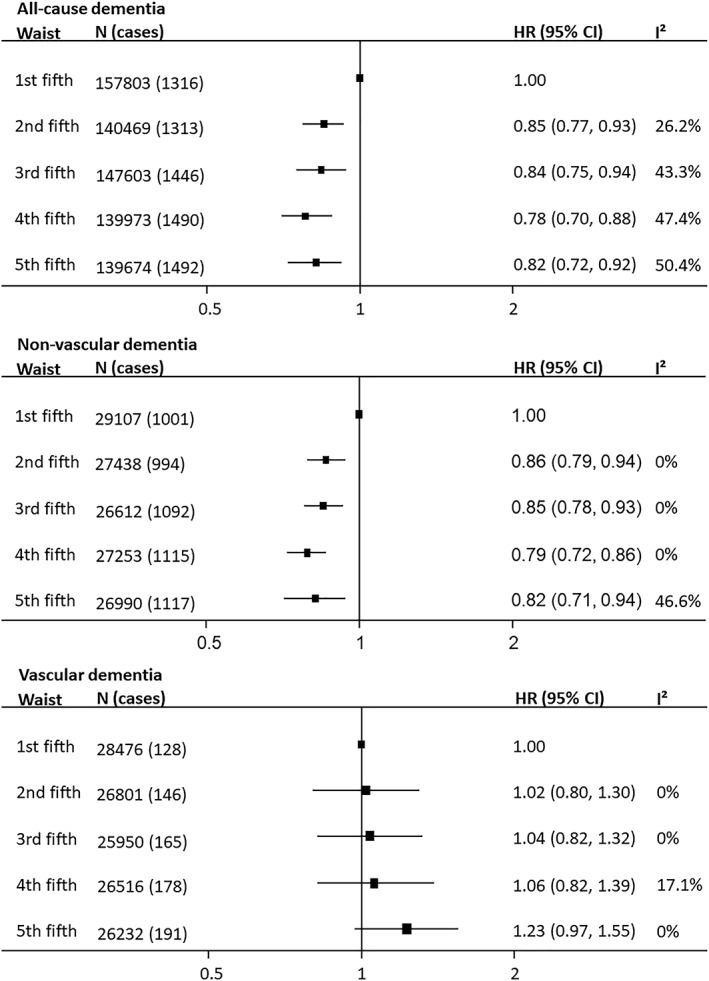
Association between waist circumference and incident fatal and nonfatal dementia and its major subtypes. Hazard ratios (HRs) and 95% confidence intervals (CIs) adjusted for age, smoking, and education or socio‐economic status
Pooling data from ten studies (5319 nonvascular dementia cases) indicated that, compared with the lowest fifth, all WC categories were associated with lower nonvascular dementia risk (Figure 3; Table S6). Adjustment for cardiometabolic risk factors did not alter the association (Table S6). Data from clinical trials produced a similar pattern (Figure S15), and there was little evidence of a sex difference in the association (Figure 4).
Figure 4.
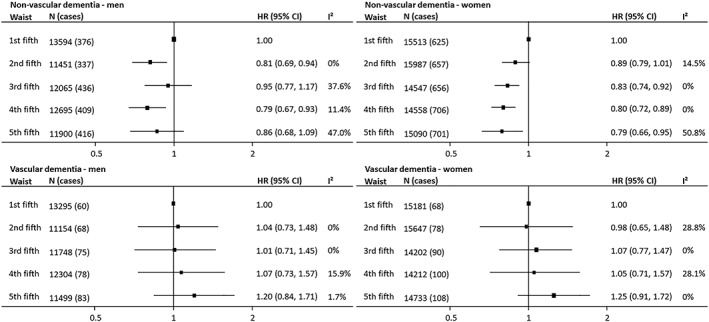
Associations between waist circumference and incident fatal and nonfatal dementia subtypes by sex. Hazard ratios (HRs) and 95% confidence intervals (CIs) adjusted for age, smoking, and education or socio‐economic status
For vascular dementia (808 cases), compared with the referent category, individuals in the highest fifth of WC had roughly one‐quarter higher risk (1.23 [0.97‐1.55]) that was attenuated after further adjustment for cardiometabolic risk factors (Figure 3; Table S6). No sex difference was evident (Figure 4). Data from clinical trial participants contained too few cases to draw meaningful conclusions (Figure S15). Only the highest WC category in studies with baseline mean age 60 years and older was associated with increased vascular dementia risk (Figure S16).
In the analysis of weight change (111 620 individuals; 44.8% female; 5626 dementia cases), compared with individuals who maintained a relatively stable weight during follow‐up, individuals with greater than or equal to 0.5% annual weight loss had approximately one‐third greater all‐cause dementia risk (1.32 [1.18‐1.47]; Table 2). In contrast, greater than or equal to 0.5% annual weight gain was not associated with dementia risk (1.00 [0.89‐1.12]; Table 2). The results remained unchanged for models 1 and 3, and when studies were stratified by baseline mean age (Figures S17‐S19; Table 2). For greater than or equal to 0.5% annual weight loss, the estimate of effect was more pronounced for studies that ascertained dementia by medical examination than studies that used death records (Figure S20).
Table 2.
Adjusted hazard ratios (HR) with 95% confidence intervals (CI) of dementia by annual percent weight change
| Dementia Type | ≥0.5% Weight Loss per Year | Reference | ≥0.5% Weight Gain per Year | |
|---|---|---|---|---|
| All‐cause dementia, 15 studies | ||||
| N (cases) | 23 590 (1668) | 44 425 (2254) | 43 605 (1704) | |
| Model 2a | HR (95% CI) | 1.32 (1.18‐1.47) | 1.00 | 1.00 (0.89‐1.12) |
| I 2 (%) | 46.2 | 54.0 | ||
| Model 3b | HR (95% CI) | 1.28 (1.15‐1.42) | 1.00 | 0.99 (0.89‐1.11) |
| I 2 (%) | 41.1 | 48.8 | ||
| Nonvascular dementia, 10 studies | ||||
| N (cases) | 8106 (898) | 10 929 (932) | 9926 (652) | |
| Model 2a | HR (95% CI) | 1.41 (1.19‐1.67) | 1.00 | 0.99 (0.83‐1.19) |
| I 2 (%) | 51.2 | 51.3 | ||
| Model 3b | HR (95% CI) | 1.36 (1.15‐1.61) | 1.00 | 0.98 (0.81, 1.17) |
| I 2 (%) | 49.0 | 51.0 | ||
| Vascular dementia, 7 studies | ||||
| N (cases) | 6000 (151) | 9034 (225) | 8275 (181) | |
| Model 2a | HR (95% CI) | 1.11 (0.88‐1.39) | 1.00 | 1.21 (0.98‐1.49) |
| I 2 (%) | 0 | 0 | ||
| Model 3b | HR (95% CI) | 1.09 (0.87‐1.36) | 1.00 | 1.13 (0.92‐1.40) |
| I 2 (%) | 0 | 0 | ||
Hazard ratio adjusted for age, smoking, education/socio‐economic status.
Hazard ratios adjusted for age, smoking, education/socio‐economic status, diabetes, systolic blood pressure, total cholesterol, blood pressure lowering medication, cholesterol lowering medication, and glucose lowering medication.
As with all‐cause dementia, greater than or equal to 0.5% annual weight loss was associated with higher nonvascular dementia risk (1.41 [1.19‐1.67]), whereas greater than or equal to 0.5% annual weight gain was not associated with risk (0.99 [0.83‐1.19]; Table 2). For vascular dementia, opposing trends were observed: a trend towards increased risk was observed in those with greater than or equal to 0.5% annual weight gain (1.21 [0.98‐1.49]) but only in those with baseline mean age younger than 60 years (Figure S19). Weight loss was not associated with increased vascular dementia risk (Table 2).
4. DISCUSSION
This is the largest study to characterize the relationship between measures of body size and weight change with dementia outcomes. We demonstrated that the relationship between body size, weight change, and subsequent risk varies by dementia subtype. When considering all‐cause dementia risk, and hence its major subtype of nonvascular dementia, there was no evidence that excess body weight (measured by either BMI or WC) conferred higher risk. Rather, levels of BMI greater than or equal to 22.5 kg/m2 (and higher WCs) were associated with a slightly lower dementia risk in later life. Conversely, and in agreement with previous findings,6, 7, 14 individuals who were categorized as underweight had higher all‐cause dementia risk compared with those with lower‐normal BMI. For vascular dementia, only the highest levels of BMI and WC were associated with increased risk relative to lower‐normal BMI and the lowest fifth of WC.
The main novel finding, however, relates to how weight change appears to influence dementia risk in later life. Relative to weight maintenance, weight loss over follow‐up was associated with approximately 30% and 40% increased risk of all‐cause dementia and nonvascular dementia, respectively. The association between weight loss and nonvascular dementia may reflect the subclinical expression of prodromal dementia (ie, where an individual has early cognitive impairment but remains functionally independent), or in epidemiological terms, reverse causality. In support of this argument, weight loss has been observed in the preclinical stage of autosomal dominant Alzheimer disease, suggesting that decreasing BMI could be a consequence, rather than a risk factor, of dementia.43
An additional, albeit more speculative, explanation may be pathophysiological such as weight loss–induced cortical thinning, as cerebral atrophy is a characteristic of dementia. In a cohort of healthy elderly individuals, faster cognitive decline and accelerated atrophy rate were observed in those with relative weight loss greater than or equal to 5% (equivalent to greater than or equal to 1.2% annual loss) compared with those with relative weight loss less than 5% (equivalent to less than 1.2% annual loss).44 Similarly, a Norwegian study that assessed percent change in BMI in midlife reported that while greater than or equal to 5% loss (equivalent to approximately greater than or equal to 0.6% annual loss) was associated with increased risk of dementia‐related mortality, a gain of greater than or equal to 20% (equivalent to approximately greater than or equal to 2.2% annual gain) was associated with reduced risk.45
Conversely, for vascular dementia, weight gain was associated with a modest 20% increased risk but only in those aged younger than 60 years at study baseline. This is consistent not only with what we know about weight gain being a risk factor for other vascular conditions such as coronary heart disease46, 47 but also with the diminution of the strength in the association between vascular risk factors such as diabetes and blood pressure with vascular risk at older ages.48 Moreover, data from animal studies have indicated that weight gain is associated with increased vascular dementia risk.49
For all‐cause dementia, and its major subtype, that of nonvascular dementia, there was no evidence that carrying excess body weight (either in general or more centrally) conferred increased risk. Rather, individuals with a BMI of greater than or equal to 22.5 kg/m2 (and higher WCs) had a slightly lower risk of dementia in later life. Conversely, and in agreement with some previous findings,6, 7, 14 individuals who were categorized as underweight had a one‐quarter greater risk of developing all‐cause dementia compared with those with lower‐normal BMI. When we attempted to exclude those individuals who may have had undetected signs of cognitive impairment at study baseline by excluding the first few years of follow‐up, the relationship remained.
To date, evidence from Mendelian randomization studies has provided little support of a relationship between low levels of BMI with future risk of Alzheimer disease, implying that reverse causality or residual confounding may be driving the observed effect in observational studies. However, as Mendelian randomization studies do not use methods that are suited to capturing non‐linear associations, the potential for other explanations, other than reverse causality, to explain the observed association remains.50
For vascular dementia, the relationship with body size was similar to other vascular conditions with both underweight and obesity conferring increased risk. Underweight individuals had 75% greater vascular dementia risk, which was unaltered by excluding the first 5 years of data. Individuals with obesity had 50% greater risk, possibly due to the adverse effects of high levels of BMI on other vascular risk factors as the relationship was significantly attenuated after adjustment for vascular risk factors. An increased vascular dementia risk was observed for WC but only among those with the highest levels of central obesity.
Recent studies have suggested that the association between BMI and dementia risk is dependent on the age when BMI was assessed.37, 51 We investigated the association separately for midlife and late life by stratifying studies by their baseline mean age. Aside from the effect of weight gain on vascular dementia risk, our results indicated that there was no evidence of any difference between the two age groups, although the crude dichotomization of age precludes us from definitely concluding that there is no evidence of an age effect in the association between body size and dementia risk. Similarly, this study found no evidence to suggest that the associations reported herein differed between women and men. In addition to its large sample size and number of dementia cases, key strengths of the study included the ability to look at the effect of a measure of central obesity and the influence of weight change on the association between body size and dementia outcomes. BMI and WC were divided into five categories to allow the study of the relationship with dementia in more detail. The lowest fifth of WC was chosen as referent as four studies already included women with abdominal obesity in this group. Nevertheless, using the second or third fifth as referent would not have changed the relationship between WC and dementia. Limitations included the between‐study differences in design and methodologies used in the ascertainment of dementia as well as different lengths of study follow‐up. In regard to the latter, we attempted to address the potential for reverse causality by excluding the first 3, 5, or 10 years of follow‐up (depending on the data set). However, given the often long lag period between prodromal dementia until dementia onset, this may not have been a sufficiently long enough period of time to fully exclude the potential for reverse causality. Previous reports have indicated that it is necessary to exclude up to 20 years of follow‐up in order to fully negate the effects of inadvertently including individuals with early cognitive impairment at study baseline.11 In our analysis of weight change, we were unable to distinguish between intentional (eg, because of a diagnosis of hypertension or diabetes in midlife) versus unintentional weight loss (ie, because of pre‐existing disease), which may have diluted the observed associations. Studies that contributed to the weight change analysis also varied in the length of time between first and last weight measurement. We standardized weight change across studies by calculating annual percent weight change; however, this method assumes a consistent weight change over time, which may not be valid. In addition, we attempted to distinguish between vascular dementia and nonvascular dementia separately, but the dichotomization is problematic as the two subtypes frequently co‐occur.52 Finally, as body size (both underweight and overweight) is related to a wide range of chronic illnesses, and dementia is mainly a disease of aging, individuals may have died before they had the opportunity to develop dementia. We were unable to apply competing risks methodology in the current analysis, which may have resulted in an underestimation of the relationship between body size and dementia risk. It potentially could also explain why above normal levels of BMI and WC were not associated with increased all‐cause dementia risk.
Excess body weight was not associated with risk of all‐cause dementia and its major subtype of nonvascular dementia, whereas it was positively associated with risk of vascular dementia. Underweight was related to increased risk of all‐cause dementia and both its subtypes, possibly due to reverse causality. Weight loss in midlife to late life was associated with an increased risk of developing dementia and nonvascular dementia. Future studies should focus on examining the basis between weight loss and increased nonvascular dementia risk to determine if it has a pathophysiological basis or is due to limitations in the epidemiology. Given the known adverse effects of excess body weight on a wide range of health outcomes, from a public health perspective, maintaining a healthy body weight and minimizing weight fluctuation in adult life should continue to be widely promoted.
CONFLICT OF INTEREST
MW has received personal fees from Amgen and Kirin outside the submitted work; JC has received grants from Idorsia outside the submitted work; EDA has received grants from NHS Blood and Transplant, British Heart Foundation, UK Medical Research Council, and National Institute for Health Research outside the submitted work; JK has received grants from Academy of Finland during the conduct of the study; MK has received grants from the Medical Research Council (MR/R024227/1), NIH National Institute on Aging (R01AG056477), Academy of Finland (311492), and Helsinki Institute of Life Sciences during the conduct of the study; EBL has received grants from NIH during the conduct of the study and personal fees from Up to Date outside the submitted work; ESL reported grants from Merck Inc outside the submitted work; NQ reported other from pharmaceutical industry outside the submitted work; EV reported grants from The Academy of Finland during the conduct of the study; KY serves on DSMBs for Takeda and Eli Lily outside the submitted work and is a member of the Beeson Scholars in Aging Scientific Advisory Board and of the Senate of the German Center for Neurodegenerative Diseases.
Supporting information
Table S1: Study characteristics of male participants
Table S2: Study characteristics of female participants
Table S3: Adjusted hazard ratios with 95% confidence intervals (CI) of dementia by body mass index categories before and after left censoring of data
Table S4: Adjusted hazard ratios with 95% confidence intervals (CI) of dementia by body mass index categories for studies with all covariates listed in model 3
Table S5: Age, smoking, and education or socioeconomic status adjusted hazard ratios with 95% confidence intervals (CI) of all‐cause dementia by body mass index categories and subgroup
Table S6: Adjusted hazard ratios* with 95% confidence intervals of dementia by fifths of waist circumference for studies with all covariates listed in model 3
Fig. S1: Flow diagram
Fig. S2: Comparison of the association between body mass index (BMI) at study baseline with fatal and non‐fatal dementia and its major subtypes during follow‐up between non‐left censored and longest available left censored data (by 3, 5, or 10 years)
Fig. S3: Random effects pooled age adjusted hazard ratios with 95% confidence intervals of all‐cause dementia for body mass index <18.5 kg/m2 with body mass index 18.5–22.4 kg/m2 as referent
Fig. S4: Random effects pooled age adjusted hazard ratios with 95% confidence intervals of all‐cause dementia for body mass index 22.5–24.9 kg/m2 with body mass index 18.5–22.4 kg/m2 as referent.
Fig. S5: Random effects pooled age adjusted hazard ratios with 95% confidence intervals of all‐cause dementia for body mass index 25.0–29.9 kg/m2 with body mass index 18.5–22.4 kg/m2 as referent
Fig. S6: Random effects pooled age adjusted hazard ratios with 95% confidence intervals of all‐cause dementia for body mass index ≥30.0 kg/m2 with body mass index 18.5–22.4 kg/m2 as referent
Fig. S7: Associations between all‐cause dementia and body mass index by dementia ascertainment
Fig. S8: Associations between dementia and body mass index by study baseline mean age
Fig. S9: Associations between dementia and body mass index by study design
Fig. S10: Random effects pooled age adjusted hazard ratios with 95% confidence intervals of all‐cause dementia for second fifth of waist circumference with the first fifth of waist circumference as referent
Fig. S11: Random effects pooled age adjusted hazard ratios with 95% confidence intervals of all‐cause dementia for third fifth of waist circumference with the first fifth of waist circumference as referent
Fig. S12: Random effects pooled age adjusted hazard ratios with 95% confidence intervals of all‐cause dementia for fourth fifth of waist circumference with the first fifth of waist circumference as referent
Fig. S13: Random effects pooled age adjusted hazard ratios with 95% confidence intervals of all‐cause dementia for last fifth of waist circumference with the first fifth of waist circumference as referent
Fig. S14: Associations between all‐cause dementia and waist circumference by dementia ascertainment
Fig. S15: Associations between dementia and waist circumference by study design
Fig. S16: Associations between dementia and waist circumference by study baseline mean age
Fig. S17: Random effects pooled age adjusted hazard ratios with 95% confidence intervals of all‐cause dementia for ≥0.5% weight loss per year with <0.5% weight change has referent
Fig. S18: Random effects pooled age adjusted hazard ratios with 95% confidence intervals of all‐cause dementia for ≥0.5% weight gain per year with <0.5% weight change has referent
Fig. S19: Associations between all‐cause dementia and annual percent weight change by study baseline mean age
Fig. S20: Associations between all‐cause dementia and annual percent weight change by dementia ascertainment
ACKNOWLEDGEMENTS
Adult Changes in Thought Study was funded by a National Institutes of Health Grant U01 AG0006781. The Cache County Memory Study was funded by NIA Grants R01 AG011380 and R01 AG018712; Dr Hayden's effort on this project was supported by NIA R01 AG042633. J. Kaprio acknowledges support for the Finnish Twin Cohort by the Academy of Finland (Grants 265240, 263278, 308248, and 312073). This work was supported by the dedication of the Framingham Heart Study participants. This work and the investigators received grant support from the National Heart, Lung, and Blood Institute's Framingham Heart Study (contracts no. N01‐HC‐25195 and HHSN268201500001I) and grants from the National Institute of Neurological Disorders and Stroke (NS17950 and UH2 NS100605), and the National Institute on Aging (R01 AG054076, R01 AG049607, R01 AG033193, U01 AG049505, and U01 AG052409). The Study of Osteoporotic Fractures (SOF) is supported by National Institutes of Health funding. The National Institute on Aging (NIA) provides support under the following grant numbers: R01 AG005407, R01 AR35582, R01 AR35583, R01 AR35584, R01 AG005394, R01 AG027574, and R01 AG027576. The Three‐City Study is conducted under a partnership agreement between the INSERM, the ISPED of the University of Bordeaux, and Sanofi‐Aventis. The Foundation pour la Recherche Médicale funded the preparation and initiation of the study. TheThree‐City Study is also supported by the Caisse Nationale Maladie des Travailleurs Salariés, Direction Générale de la Santé, Mutuelle Générale de l'Education Nationale, Institut de la Longévité, Conseils Régionaux of Aquitaine and Bourgogne, Fondation de France, and Ministry of Research‐INSERM Programme « Cohortes et collections de données biologiques », French National Research Agency COGINUT ANR‐06‐PNRA‐005, COGICARE ANR Longvie (LVIE‐003‐01), the Fondation Plan Alzheimer (FCS 2009‐2012), and the Caisse Nationale pour la Solidarité et l'Autonomie. This research has been conducted using the UK Biobank Resource under Application number 7439. SBell and EDA are supported by core funding from NIHR Blood and Transplant Research Unit in Donor Health and Genomics (NIHR BTRU‐2014‐10024), UK Medical Research Council (MR/L003120/1), British Heart Foundation (RG/13/13/30194), and the NIHR Cambridge BRC. GDB is supported by the UK Medical Research Council (MR/P023444/1) and the US National Institute on Aging (1R56AG052519‐01; 1R01AG052519‐01A1). Whitehall II was supported by the Medical Research Council (MR/R024227/1), the NIH National Institute on Aging (R01AG056477), and British Heart Foundation (32334). EVuoksimaa was supported by the Academy of Finland (Grants 314639 and 320109)
Lee CM, Woodward M, Batty GD, et al. Association of anthropometry and weight change with risk of dementia and its major subtypes: A meta‐analysis consisting 2.8 million adults with 57 294 cases of dementia. Obesity Reviews. 2020;21:e12989 10.1111/obr.12989
The copyright line for this article was changed on 6 February 2020 after original online publication.
REFERENCES
- 1. World Health Organization . Dementia 2017. Available from http://www.who.int/news-room/fact-sheets/detail/dementia. Assessed 31 May 2018.
- 2. O'Brien JT, Thomas A. Vascular dementia. Lancet. 2015;386:1698‐1706. [DOI] [PubMed] [Google Scholar]
- 3. Harrison SL, Ding J, Tang EYH, et al. Cardiovascular disease risk models and longitudinal changes in cognition: a systematic review. PLoS ONE. 2014;9(12):e114431 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4257686/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Batty GD, Galobardes B, Starr JM, Jeffreys M, Davey Smith G, Russ TC. Examining if being overweight really confers protection against dementia: sixty‐four year follow‐up of participants in the Glasgow University alumni cohort study. J Negat Results Biomed. 2016;15(1):19 https://www.ncbi.nlm.nih.gov/pubmed/?term=Examining+if+being+overweight+really+confers+protection+against+dementia:+sixty-four+year [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. The Global BMI Mortality Collaboration . Body‐mass index and all‐cause mortality: individual‐participant‐data meta‐analysis of 239 prospective studies in four continents. Lancet. 2016;388(10046):776‐786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beydoun MA, Beydoun HA, Wang Y. Obesity and central obesity as risk factors for incident dementia and its subtypes: a systematic review and meta‐analysis. Obes Rev. 2008;9(3):204‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Anstey KJ, Cherbuin N, Budge M, Young J. Body mass index in midlife and late‐life as a risk factor for dementia: a meta‐analysis of prospective studies. Obes Rev. 2011;12:426‐437. [DOI] [PubMed] [Google Scholar]
- 8. Whitmer RA, Gunderson EP, Barrett‐Connor E, Quesenberry CP Jr, Yaffe K. Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. BMJ. 2005;330(7504):1360 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC558283/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Qizilbash N, Gregson J, Johnson ME, et al. BMI and risk of dementia in two million people over two decades: a retrospective cohort study. Lancet Diabetes Endocrinol. 2015;3(6):431‐436. [DOI] [PubMed] [Google Scholar]
- 10. Legdeur N, Heymans MW, Comijs HC, Huisman M, Maier AB, Visser PJ. Age dependency of risk factors for cognitive decline. BMC Geriatr. 2018;18(1):187 https://www.ncbi.nlm.nih.gov/pubmed/30126373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kivimäki M, Luukkonen R, Batty GD, et al. Body mass index and risk of dementia: analysis of individual‐level data from 1.3 million individuals. Alzheimers Dement. 2018;14(5):601‐609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gorospe EC, Dave JK. The risk of dementia with increased body mass index. Age Ageing. 2007;36(1):23‐29. [DOI] [PubMed] [Google Scholar]
- 13. Pedditizi E, Peters R, Beckett N. The risk of overweight/obesity in mid‐life and late life for the development of dementia: a systematic review and meta‐analysis of longitudinal studies. Age Ageing. 2016;45:14‐21. [DOI] [PubMed] [Google Scholar]
- 14. Albanese E, Launer LJ, Egger M, et al. Body mass index in midlife and dementia: systematic review and meta‐regression analysis of 589,649 men and women followed in longitudinal studies. Alzheimers Dement (Amst). 2017;8:165‐178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chatterjee S, Peters SA, Woodward M, et al. Type 2 diabetes as a risk factor for dementia in women compared with men: a pooled analysis of 2.3 million people comprising more than 100,000 cases of dementia. Diabetes Care. 2016;39(2):300‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Crane PK, Walker R, Hubbard RA, et al. Glucose levels and risk of dementia. N Engl J Med. 2013;369(6):540‐548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Batty GD, Li Q, Huxley R, et al. Oral disease in relation to future risk of dementia and cognitive decline: prospective cohort study based on the ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified‐Release Controlled Evaluation) trial. Eur Psychiatry. 2013;28(1):49‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Peres K, Matharan F, Allard M, et al. Health and aging in elderly farmers: the AMI cohort. BMC Public Health. 2012;12:558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rasmussen KL, Tybjærg‐Hansen A, Nordestgaard BG, Frikke‐Schmidt R. Plasma apolipoprotein E levels and risk of dementia—a mendelian randomization study of 106,562 individuals. Alzheimers Dement. 2018;14(1):71‐80. [DOI] [PubMed] [Google Scholar]
- 20. Hayden KM, Zandi PP, Lyketsos CG, et al. Vascular fisk factors for incident Alzheimer disease and vascular dementia: the cache county Study. Alzheimer Dis Assoc Disord. 2006;20(2):93‐100. [DOI] [PubMed] [Google Scholar]
- 21. Nordestgaard LT, Tybjaerg‐Hansen A, Nordestgaard BG, Frikke‐Schmidt R. Body mass index and risk of Alzheimer disease: a Mendelian randomization study of 399,536 individuals. J Clin Endocrinol Metab. 2017;102(7):2310‐2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Iso‐Markku P, Waller K, Kujala UM, Kaprio J. Physical activity and dementia: long‐term follow‐up study of adult twins. Ann Med. 2015;47(2):81‐87. [DOI] [PubMed] [Google Scholar]
- 23. Satizabal CL, Beiser AS, Chouraki V, Chene G, Dufouil C, Seshadri S. Incidence of dementia over three decades in the Framingham Heart Study. N Engl J Med. 2016;374(6):523‐532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ferrie JE, Singh‐Manoux A, Kivimaki M, et al. Cardiorespiratory risk factors as predictors of 40‐year mortality in women and men. Heart. 2009;95(15):1250‐1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yoshitake T, Kiyohara Y, Kato I, et al. Incidence and risk factors of vascular dementia and Alzheimer's disease in a defined elderly Japanese population: The Hisayama Study. Neurology. 1995;45(6):1161‐1168. [DOI] [PubMed] [Google Scholar]
- 26. Batty GD, Russ TC, Starr JM, Stamatakis E, Kivimaki M. Modifiable cardiovascular disease risk factors as predictors of dementia death: pooling of ten general population‐based cohort studies. J Negat Results Biomed. 2014;13(1):8 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4036694/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Peters R, Beckett N, Fagard R, et al. Increased pulse pressure linked to dementia: further results from the Hypertension in the Very Elderly Trial – HYVET. J Hypertens. 2013;31(9):1868‐1875. [DOI] [PubMed] [Google Scholar]
- 28. Strand BJ, Langballe EM, Hjellvik V, et al. Midlife vascular risk factors and their association with dementia deaths: results from a Norwegian prospective study followed up for 35 years. J Neurol Sci. 2013;324(1‐2):124‐130. [DOI] [PubMed] [Google Scholar]
- 29. Hassing LB, Dahl AK, Thorvaldsson V, et al. Overweight in midlife and risk of dementia: a 40‐year follow‐up study. Int J Obes (Lond). 2009;33(8):893‐898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moll van Charante EP, Richard E, Eurelings LS, et al. Effectiveness of a 6‐year multidomain vascular care intervention to prevent dementia (preDIVA): a cluster‐randomised controlled trial. Lancet. 2016;388(10046):797‐805. [DOI] [PubMed] [Google Scholar]
- 31. Rosengren A, Skoog I, Gustafson D, Wilhelmsen L. Body mass index, other cardiovascular risk factors, and hospitalization for dementia. Arch Intern Med. 2005;165(3):321‐326. [DOI] [PubMed] [Google Scholar]
- 32. The PROGRESS Collaborative Group . Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch Intern Med. 2003;163:1069‐1075. [DOI] [PubMed] [Google Scholar]
- 33. LeBlanc ES, Rizzo JH, Pedula KL, et al. Weight trajectory over 20 years and likelihood of mild cognitive impairment or dementia among older women. J Am Geriatr Soc. 2017;65(3):511‐519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. 3C Study Group . Vascular factors and risk of dementia: design of the Three‐City Study and baseline characteristics of the study population. Neuroepidemiology. 2003;22:316‐325. [DOI] [PubMed] [Google Scholar]
- 35. Sudlow C, Gallacher J, Allen N, et al. UK Biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4380465/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kivimaki M, Singh‐Manoux A, Shipley MJ, Elbaz A. Does midlife obesity really lower dementia risk? Lancet Diabetes Endocrinol. 2015;3(7):498 https://www.ncbi.nlm.nih.gov/pubmed/26138161 [DOI] [PubMed] [Google Scholar]
- 37. Singh‐Manoux A, Dugravot A, Shipley M, et al. Obesity trajectories and risk of dementia: 28 years of follow‐up in the Whitehall II Study. Alzheimers Dement. 2018;14(2):178‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Diagnostic and Statistical Manual of Mental Disorders. 3rd ed. .‐rev. Washington DC: American Psychiatric Association; 1987. [Google Scholar]
- 39. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington DC: American Psychiatric Association; 1994. [Google Scholar]
- 40. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS‐ADRDA work group under the auspices of Department of Health and human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939‐944. [DOI] [PubMed] [Google Scholar]
- 41. Roman GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS‐AIREN International Workshop. Neurology. 1993;43(2):250‐260. [DOI] [PubMed] [Google Scholar]
- 42. NHS Digital . Read codes. Available from: https://digital.nhs.uk/services/terminology-and-classifications/read-codes
- 43. Müller S, Preische O, Sohrabi HR, et al. Decreased body mass index in the preclinical stage of autosomal dominant Alzheimer's disease. Sci Rep. 2017;7(1):1225 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5430642/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jimenez A, Pegueroles J, Carmona‐Iragui M, et al. Weight loss in the healthy elderly might be a non‐cognitive sign of preclinical Alzheimer's disease. Oncotarget. 2017;8(62):104706‐104716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Strand BH, Wills AK, Langballe EM, Rosness TA, Engedal K, Bjertness E. Weight change in midlife and risk of mortality from dementia up to 35 years later. J Gerontol A Biol Sci Med Sci. 2017;72:855‐860. [DOI] [PubMed] [Google Scholar]
- 46. Rosengren A, Wedel H, Wilhelmsen L. Body weight and weight gain during adult life in men in relation to coronary heart disease and mortality. A prospective population study. Eur Heart J. 1999;20(4):269‐277. [PubMed] [Google Scholar]
- 47. Cui R, Iso H, Tanabe N, Watanabe Y, Tamakoshi A, JACC Study Group . Association between weight change since 20 years of age with mortality from myocardial infarction and chronic heart failure in the Japanese Collaborative Cohort (JACC) Study. Circ J. 2014;78(3):649‐655. [DOI] [PubMed] [Google Scholar]
- 48. Asia Pacific Cohort Studies Collaboration . Blood pressure indices and cardiovascular disease in the Asia pacific region. Hypertension. 2003;42:69‐75. [DOI] [PubMed] [Google Scholar]
- 49. Cope EC, LaMarca EA, Monari PK, et al. Microglia play an active role in obesity‐associated cognitive decline. J Neurosci. 2018;38(41):8889‐8904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nordestgaard LT, Tybjærg‐Hansen A, Nordestgaard BG, Frikke‐Schmidt R. Body mass index and risk of Alzheimer's disease: a Mendelian randomization study of 399,536 individuals. J Clin Endocrinol Metab. 2017;102(7):2310‐2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Strand BH, Langballe EM, Rosness TA, Engedal K, Bjertness E. Does midlife obesity really lower dementia risk? Lancet Diabetes Endocrinol. 2015;3(7):498‐499. [DOI] [PubMed] [Google Scholar]
- 52. Langa KM, Foster N, Larson EB. Mixed dementia: emerging concepts and therapeutic implications. JAMA. 2004;292(23):2901‐2908. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Study characteristics of male participants
Table S2: Study characteristics of female participants
Table S3: Adjusted hazard ratios with 95% confidence intervals (CI) of dementia by body mass index categories before and after left censoring of data
Table S4: Adjusted hazard ratios with 95% confidence intervals (CI) of dementia by body mass index categories for studies with all covariates listed in model 3
Table S5: Age, smoking, and education or socioeconomic status adjusted hazard ratios with 95% confidence intervals (CI) of all‐cause dementia by body mass index categories and subgroup
Table S6: Adjusted hazard ratios* with 95% confidence intervals of dementia by fifths of waist circumference for studies with all covariates listed in model 3
Fig. S1: Flow diagram
Fig. S2: Comparison of the association between body mass index (BMI) at study baseline with fatal and non‐fatal dementia and its major subtypes during follow‐up between non‐left censored and longest available left censored data (by 3, 5, or 10 years)
Fig. S3: Random effects pooled age adjusted hazard ratios with 95% confidence intervals of all‐cause dementia for body mass index <18.5 kg/m2 with body mass index 18.5–22.4 kg/m2 as referent
Fig. S4: Random effects pooled age adjusted hazard ratios with 95% confidence intervals of all‐cause dementia for body mass index 22.5–24.9 kg/m2 with body mass index 18.5–22.4 kg/m2 as referent.
Fig. S5: Random effects pooled age adjusted hazard ratios with 95% confidence intervals of all‐cause dementia for body mass index 25.0–29.9 kg/m2 with body mass index 18.5–22.4 kg/m2 as referent
Fig. S6: Random effects pooled age adjusted hazard ratios with 95% confidence intervals of all‐cause dementia for body mass index ≥30.0 kg/m2 with body mass index 18.5–22.4 kg/m2 as referent
Fig. S7: Associations between all‐cause dementia and body mass index by dementia ascertainment
Fig. S8: Associations between dementia and body mass index by study baseline mean age
Fig. S9: Associations between dementia and body mass index by study design
Fig. S10: Random effects pooled age adjusted hazard ratios with 95% confidence intervals of all‐cause dementia for second fifth of waist circumference with the first fifth of waist circumference as referent
Fig. S11: Random effects pooled age adjusted hazard ratios with 95% confidence intervals of all‐cause dementia for third fifth of waist circumference with the first fifth of waist circumference as referent
Fig. S12: Random effects pooled age adjusted hazard ratios with 95% confidence intervals of all‐cause dementia for fourth fifth of waist circumference with the first fifth of waist circumference as referent
Fig. S13: Random effects pooled age adjusted hazard ratios with 95% confidence intervals of all‐cause dementia for last fifth of waist circumference with the first fifth of waist circumference as referent
Fig. S14: Associations between all‐cause dementia and waist circumference by dementia ascertainment
Fig. S15: Associations between dementia and waist circumference by study design
Fig. S16: Associations between dementia and waist circumference by study baseline mean age
Fig. S17: Random effects pooled age adjusted hazard ratios with 95% confidence intervals of all‐cause dementia for ≥0.5% weight loss per year with <0.5% weight change has referent
Fig. S18: Random effects pooled age adjusted hazard ratios with 95% confidence intervals of all‐cause dementia for ≥0.5% weight gain per year with <0.5% weight change has referent
Fig. S19: Associations between all‐cause dementia and annual percent weight change by study baseline mean age
Fig. S20: Associations between all‐cause dementia and annual percent weight change by dementia ascertainment


