Abstract
Optogenetics uses light‐sensitive proteins, so‐called optogenetic tools, for highly precise spatiotemporal control of cellular states and signals. The major limitations of such tools include the overlap of excitation spectra, phototoxicity, and lack of sensitivity. The protein characterized in this study, the Japanese lamprey parapinopsin, which we named UVLamP, is a promising optogenetic tool to overcome these limitations. Using a hybrid strategy combining molecular, cellular, electrophysiological, and computational methods we elucidated a structural model of the dark state and probed the optogenetic potential of UVLamP. Interestingly, it is the first described bistable vertebrate opsin that has a charged amino acid interacting with the Schiff base in the dark state, that has no relevance for its photoreaction. UVLamP is a bistable UV‐sensitive opsin that allows for precise and sustained optogenetic control of G protein‐coupled receptor (GPCR) pathways and can be switched on, but more importantly also off within milliseconds via lowintensity short light pulses. UVLamP exhibits an extremely narrow excitation spectrum in the UV range allowing for sustained activation of the Gi/o pathway with a millisecond UV light pulse. Its sustained pathway activation can be switched off, surprisingly also with a millisecond blue light pulse, minimizing phototoxicity. Thus, UVLamP serves as a minimally invasive, narrow‐bandwidth probe for controlling the Gi/o pathway, allowing for combinatorial use with multiple optogenetic tools or sensors. Because UVLamP activated Gi/o signals are generally inhibitory and decrease cellular activity, it has tremendous potential for health‐related applications such as relieving pain, blocking seizures, and delaying neurodegeneration.
Keywords: computational chemistry, electrophysiology, integrative modeling, mutagenesis, optogenetics, structural biology
The Japanese lamprey parapinopsin (“UVLamP”) serves as a minimally invasive, narrow‐bandwidth, bistable next‐generation optogenetic probe for controlling the Gi/o pathway. A millisecond UV light pulse allows sustained pathway activation that can be switched off with a millisecond blue light pulse on demand. The first structural model of parapinopsin in the dark state reveals novel interaction partners shedding light on mechanisms responsible for opsin bistability.
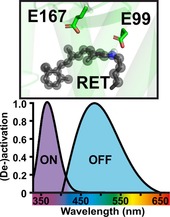
Using light to control cellular signals has already been suggested 40 years ago in 1979 by Crick, who imagined that neuronal excitability could be controlled via light with much more spatiotemporal precision than with pharmacological or electrical approaches. Starting with the expression of microbial and animal opsins in neurons, the field of optogenetics has been developed in the last two decades.1 Optogenetics uses the combination of optical, genetic and viral methods to achieve control of cellular states, function and signaling with unmatched spatial and temporal precision.2 Often so‐called opsins, a class of light‐sensitive proteins, are used to optogenetically control cellular functions ranging from in vitro assays to control of complex behavioral tasks in freely moving animals. Typically, these opsins are expressed in genetically precisely targeted cell populations, for example, by viral transduction and can then be used to exclusively modulate the desired cell population in multiple ways via application of light pulses. Opsins can be classified into type I (microbial) and type II (animal) opsins with type I opsins being employed by prokaryotes, fungi and algae and type II opsins being found in animals.3 Type II opsins belong to the seven‐transmembrane‐domain (7TM) GPCR superfamily, with most being typical light‐sensitive GPCRs, consisting of a 7TM protein moiety (opsin) and a light‐sensing non‐protein moiety (the chromophore retinal).4 The retinal itself is bound to the protein moiety through a protonated Schiff base (SB) linkage. This binding site is energetically unstable and has to be stabilized by a counterion in the protein interior. There are two tentative sites that can serve as a counterion: E113 in transmembrane helix 3 and E181 in extracellular loop 2 (positions for bovine rhodopsin). For monostable (commonly vertebrate) opsins this counterion is typically E113, whereas bistable (commonly invertebrate) opsins usually employ the more ancestral E181 as their counterion.3, 5 Interestingly, in the dark state structure of both vertebrates6 and invertebrates6 the analogues to positions 113 interact with the SB. It is assumed that within the photo cycle rearrangements in extracellular loop 2 lead to a change in the interaction network of the SB.7
Here we biophysically characterized the Japanese lamprey (Lethenteron camtschaticum) parapinopsin, a natively Gt‐coupled, UV‐sensitive, vertebrate nonvisual type II opsin. We reveal its capability to control the Gi/o signaling pathway with unseen unique features making it an ideal optogenetic tool for multiple applications in particular in the brain.
GPCRs coupling to the Gi/o signaling pathways in the brain are inhibitory. They are therefore gatekeepers of brain function by reducing neuronal excitability and keeping the brain in balance during emotion and arousal, contributing to neuronal plasticity during development, learning and memory formation. The main and modulatory transmitter systems such as glutamate, GABA, serotonin, dopamine, Ach, adrenaline and noradrenaline rely on negative feedback mechanisms using GPCRs coupling to the Gi/o pathway and therefore represent major drug targets of the pharmaceutical industry.8 These pathways can now be controlled in a highly precise manner using parapinopsin.
Japanese lamprey parapinopsin (hereafter: UVLamP or parapinopsin) is a homologue of the first identified catfish parapinopsin, which was detected in the catfish pineal complex.9 Parapinopsin homologues have been found in the pineal organ and its related organs of lower vertebrates.10 They have been found to couple to the Gt pathway and to be involved in extraretinal photoreception, especially in color discrimination between UV and visible light due to their bistable nature, with the optogenetic potential of parapinopsin being first proposed in 2015.11, 12
Recently, we characterized mouse and human homologues of melanopsin, another non‐visual opsin that can be found in intrinsically photosensitive retinal ganglion cells (ipRGCs) of the vertebrate retina and is involved in multiple physiological processes, for example, the circadian rhythm. We could show that melanopsin is a bistable/tristable opsin and a unique optogenetic tool that can be switched on/off with blue/green light pulses and can precisely control the Gi/o and Gq pathway in different expression systems like the mouse brain.13, 14 Despite its advantageous characteristics, like high light sensitivity, bistable switching between sustained active and inactive states with short light pulses and strong expression in different systems, two drawbacks remained: First, melanopsin is activated/deactivated in the visible spectrum ranging from blue for activation to green/yellow for deactivation, therefore overlapping with most other optogenetic tools. Second, depending on the expression system, melanopsin is capable of activating two different G protein pathways, namely the Gi/o and the Gq pathway, making it potentially imprecise for specific pathway control experiments. Herein, we show that UVLamP overcomes these drawbacks making it an ideal next generation optogenetic tool, especially for applications involving multi tool expression and highly precise control of distinct G protein pathways.
Lamprey parapinopsin induces a light‐dependent hyperpolarization response in photoreceptor cells of the pineal organ and inhibits adenylyl cyclase‐dependent cAMP responses in HEK 293S cells,11, 12 thus suggesting the activation of the Gi/o pathway. Therefore, we characterized the light‐dependent responses on Gi/o‐mediated activation and deactivation of GIRK currents, using HEK 293 cells that stably express G protein‐coupled inwardly rectifying K+ (GIRK) channels,15 which has been shown to be an ideal system for precise characterization of light‐induced Gi/o pathway activation and deactivation13, 15, 16, 17 GIRK channels are modulated through the Gi/o pathway via fast direct interaction with G protein βγ‐subunits leading to the hyperpolarization of the cell membrane in brain and heart.16 We found that, like other Gt coupled photoreceptors derived from rods and cones, UVLamP effectively activates Gi/o‐mediated GIRK channels. UVLamP induces sustained Gi/o‐dependent GIRK currents (Figures 1 and 3 A) through a 100 ms UV light pulse (360 nm, 0.5 mW m−2) that can be completely deactivated with another 100 ms blue light pulse (470 nm, 0.5 mW m−2; Figure 1 A). Thus, UVLamP demands much shorter light pulses for switching between active and inactive states in comparison for example to bistable mouse melanopsin that needs several seconds of constant light stimulation for a full deactivation.13 We used this direct GIRK channel modulation to electrophysiologically characterize the action spectrum of UVLamP rather than biochemically measuring the absorption spectrum. Characterizing the action spectrum is much more sensitive and involves functional GPCR pathway activation as a readout.18 We found that UVLamP displays a strongly UV‐shifted and narrow excitation (action) spectrum as it is exclusively activated by UV light below 410 nm with a maximum activation efficiency between 360–370 nm and can be deactivated with blue/green light between 440–570 nm with a maximum deactivation efficiency in the blue range between 470–510 nm (Figure 1 B). Additionally, UVLamP can be repetitively activated with minimal decline in response amplitude (Figure 1 D).
Figure 1.
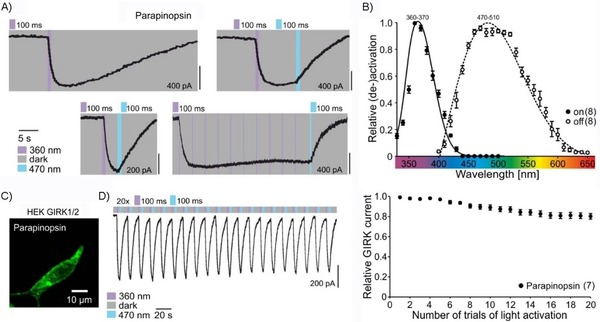
In vitro characterization of Japanese lamprey parapinopsin (“UVLamP”) via whole‐cell patch‐clamp recordings of GIRK currents in HEK GIRK 1/2 cells. a) UVLamP induces sustained Gi/o‐mediated GIRK currents via millisecond UV light stimulation that can be deactivated via millisecond blue light stimulation. b) Action spectra depicting the wavelength dependence of UVLamP activation and deactivation. c) Expression of UVLamP (L. camtschaticum parapinopsin‐eGFP) in HEK cells. d) Repetitive (de‐)activation of UVLamP.
Figure 3.
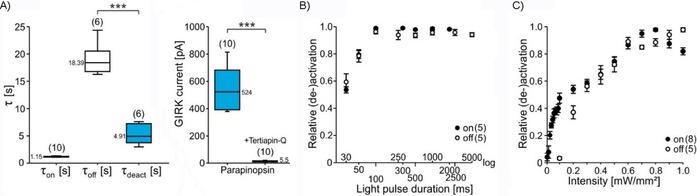
a) Comparison of UV‐light‐induced activation (τ on), unstimulated dark‐adapted inactivation (τ off) and blue‐light‐induced deactivation (τ deact) time constants for UVLamP (left). Light‐induced GIRK currents with and without addition of GIRK channel blocker Tertiapin‐Q (right). b) Light pulse duration dependence of UVLamP activation and deactivation. c) Light intensity dependence of UVLamP activation and deactivation.
So far, the 3D structure of the Japanese lamprey parapinopsin (UVLamP) has not been determined. Here, we constructed in silico an atomistic model of membrane inserted solvated parapinopsin in complex with GDP bound Gi/o protein, reflecting the dark state structure. To this end, we employed the modeling concept we recently developed to build and validate an atomic model of melanopsin14 as outlined under Model construction and Model validation in the Supporting Information. The X‐ray structure of bovine rhodopsin (PDB ID: https://www.rcsb.org/structure/1U19)19 served as a basis to build parapinopsin. The X‐ray structures of the heterotrimeric Gi/o protein with PDB IDs https://www.rcsb.org/structure/1GP2 20 and https://www.rcsb.org/structure/1BOF 21 were used to complete the binary complex. The sequence alignment shown in Figure S2 in the Supporting Information reveals that the similarity between parapinopsin and bovine rhodopsin is sufficient to build a reliable model exhibiting a similarity of 66 % within the modeled sequence region (Table S1).
The resulting structural model shown in Figure 2 A was then used to initiate a 475 ns molecular mechanics (MM) simulation. The protein backbone forming Cα atom positions converged after 350 ns as reflected by their root‐mean‐square deviation (RMSD) shown in Figure S7. Thus, we consider the obtained equilibrated trajectory as stable and reliable conformation, reflecting the dynamic interaction network of dark state parapinopsin. Next, we compared the dynamics of the interaction network of parapinopsin in detail with those derived from melanopsin MD simulations. The comparison of a representative dark state structure of the converged retinal binding pockets of parapinopsin and melanopsin is shown in Figure 2 B,C. Interestingly, the models show that despite their high identity within the retinal binding pocket the key binding partner of the retinal SB is different. In parapinopsin a glutamate (E99 (E113 for bovine rhodopsin)) interacts with the SB in the dark state, in contrast to melanopsin where it is a tyrosine (Y145 (E113 for bovine rhodopsin)). Within both proteins the E99 or Y145 interact with the SB over the whole simulation trajectory which indicates very strong binding. This is of particular interest because for other bistable vertebrate opsins except for encephalopsin,22 position 99 (113 for bovine rhodopsin) is typically occupied by neutral amino acid residues such as Y, F, M. Yet, parapinopsin employs glutamate at both positions 99 (113 for bovine rhodopsin) and 167 (181 for bovine rhodopsin) similar to monostable pigments like bovine rhodopsin.3, 10, 23
Figure 2.
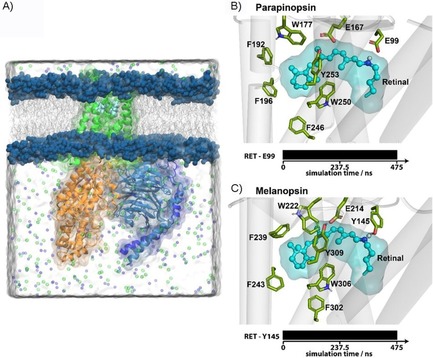
In silico characterization of parapinopsin in comparison with melanopsin. a) Simulation system of a membrane inserted solvated parapinopsin (green) Gi/o protein complex. Illustrated at right are the representative structures of the retinal (cyan) binding pocket of b) parapinopsin and c) melanopsin of the converged MD simulation. Amino acids interacting with the retinal are highlighted as sticks. The contact pattern over the simulation time of the interaction partners of the SB is shown below as bar.
To identify the counterion, we created and electrophysiologically characterized mutations of the potential counterion sites at position E99 (E113 for bovine rhodopsin) and position E167 (E181 for bovine rhodopsin; Figure S1). Point mutations at both positions (E99A/H/Y, E167A/H/Y) showed that altering/ removing the potential counterion at position E99 does not interfere with UVLamP functionality, whereas alterations at position E167 completely abolished its functionality (Figure S1). That E99 does not impact parapinopsin function is in contrast to melanopsin, where the mutation of the E99 analogue Y145 is functionally important. Mutation of Y145, despite not being a potential counterion, led to a total loss of melanopsin function,24 while for encephalopsin the functional relevance of its analogous aspartate is still unknown.12 The parapinopsin results show that E167 is the sole counterion for the photoproduct of UVLamP.10 Thus, comparable with mouse melanopsin, the Japanese lamprey parapinopsin (UVLamP), despite belonging to the group of vertebrate opsins, still uses the ancestral invertebrate opsin counterion.
In conclusion, our electrophysiological data show that parapinopsin is bistable. Parapinopsin uses E167 (E181 for bovine rhodopsin) as a sole counterion, at least for the photoproduct, with mutations at position E99 having no impact on parapinopsin functionality. Complementary analysis of the contact network (Figure 2) within biomolecular simulations shows that E99 is strongly bound to the SB in the dark state. Therefore, our data imply that critical interaction of the amino acid residue at this position, in contrast to melanopsin, cannot be of relevance for parapinopsin bistability or functionality.
We further characterized the properties of light‐induced Gi/o signaling via UVLamP in detail in vitro and found that its light‐induced activation and deactivation time constants with 1.15 s for activation and 4.95 s for deactivation (Figure 3 A). These time constants are even faster than those of wild‐type mouse melanopsin (≈1.4 and ≈8.5 s, respectively13) and comparable with the recently engineered Y211F mutant.14 We next investigated the minimal light pulse duration for activation and deactivation of GIRK channels using UVLamP. We found that 100 ms light pulses (360/470 nm) are sufficient for full activation and deactivation efficiency with half‐maximal (de‐)activation already occurring at 30 ms (Figure 3 B), outperforming other GPCR‐based optogenetic tools. Also optogenetically beneficial is the very high light sensitivity of UVLamP (Figure 3 C), which reaches maximal activation and deactivation by 100 ms light pulses at intensities of 0.7 mW m−2 with half‐maximal activation already occurring at 0.1 mW m−2.
We next investigated the pathway specificity of UVLamP to verify its use as a Gi/o specific optogenetic probe. Three main GPCR pathways are distinguished, that is, Gi/o, Gq11/12/13 and Gs.25 We monitored the Gq/11 pathway by observing Gq/11‐induced rise in intracellular Ca2+ using co‐expression of genetically encoded calcium indicators (GECIs) in HEK tsA 201 cells26 and compared mouse melanopsin with UVLamP (GCamP6m and jRCaMP1b, respectively). We found that blue light stimulation of mouse melanopsin induces a fast rising sustained Ca2+ signal, again confirming its capability of modulating the Gq/11 pathway,13 whereas UV light stimulation of UVLamP does not lead to a change in intracellular Ca2+ (Figure 4 A). We also looked at the Gs pathway via Gs‐induced rise in intracellular cAMP27 using co‐expression of a red fluorescent protein‐based cAMP indicator (Pink Flamindo).28 We found that UV (or blue) light stimulation of UVLamP did not lead to a rise in intracellular cAMP, whereas direct stimulation of the cAMP producing adenylyl cyclase via Forskolin29 lead to a fast cAMP increase (Figure 4 B), that is blocked by activation of UVLamP following compound washout (Figure S8). These results suggest that UVLamP exclusively modulates the Gi/o but not the Gq or Gs pathway.
Figure 4.
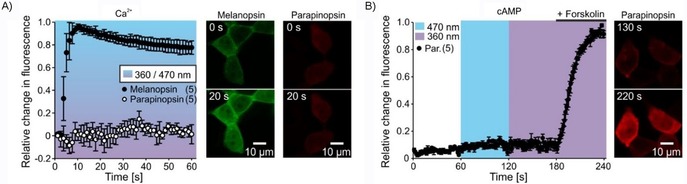
a) Light‐induced Gq‐mediated Ca2+ responses in HEK tsA201 cells for mouse melanopsin (blue light, GCaMP6m, green) and UVLamP (UV light, jRCaMP1b, red). b) Light‐induced Gs‐mediated intracellular cAMP increase in HEK tsA201 cells for UVLamP (blue and UV light, Pink Flamindo, red). Expressing cells were stimulated with forskolin (activator of adenylyl cyclase) in a final step.
In summary, we present the first experimental prove for the high optogenetic potential of the Japanese lamprey parapinopsin (UVLamP), establishing it as a unique next generation optogenetic tool. We used a hybrid strategy to characterize the biophysical properties of UVLamP and construct its first structural model. Therefore, we combined molecular, cellular, electrophysiological and computational procedures.
In contrast to most other frequently used optogenetic tools, UVLamP enables precise and exclusive control of the Gi/o pathway by using millisecond low intensity light pulses for switching this pathway on and most importantly also off. Importantly, UVLamP shows a strongly UV‐shifted narrow activation spectrum, enabling combinatory experimental designs with blue‐, green‐, or red‐shifted tools with minimal crosstalk. (Note, red‐shifted tools are in principle preferable over blue shifted tools because of the lower energy and deeper tissue penetration of red light.30) To our knowledge, UVLamP is the first bistable vertebrate opsin with a positively charged amino acid interacting with the SB in the dark state with this interaction being not relevant for its photoreaction. Additionally, UVLamP allows for long‐term activation of the Gi/o pathway with millisecond, low intensity light pulses. Due to its bistable nature, UVLamP can also be switched off with a millisecond light pulse in the blue spectrum on demand, leading to highly reduced cellular phototoxicity. UVLamP′s unique potential of pathway activation and deactivation by millisecond light pulses is essential for minimizing cellular photodamage due to the high energy UV light.31 Thus, UVLamP enables new minimally invasive experimental procedures to elucidate GPCR (dis‐)function in health and disease.
Because GPCRs coupling to the Gi/o pathway are important drug targets for various diseases including anxiety, depression, epilepsy and pain and importantly contribute to synaptic plasticity and neuronal development,32 our next goals are to engineer UVLamP variants for altered trafficking into disease relevant GPCR domains in the mammalian brain. Because of the unique, blue‐shifted, narrow bandwidth features of UVLamP it will now be possible, in combination with other optogenetic tools and genetically encoded sensors, to shed light onto important, unresolved questions in neurobiology, that is, how differently shaped GPCR signals such as fast, transient, long lasting or sustained signals contribute to brain function and change during disease states.14, 33
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
This work was supported by Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) grants He2471/23‐1, He2471/21‐1, He2471/19‐1, Priority Program (SPP1926) and GE 599/19‐1 (to S.H. and K.G.), SFB874 (DFG project no. 122679504) (to S.H.), SFB1280 (DFG project no. 316803389) (to S.H.), Ma5806/2‐1 (to M.D.M.), the Individual Research Grant no. 321722360 (GE 599/20‐1; to K.G.) and scholarships by Studienstiftung des deutschen Volkes (to D.E.) and Friedrich‐Ebert‐Stiftung (to R.K.). Further support was provided by the Protein Research Unit Ruhr within Europe (PURE) funded by the Ministry of Innovation, Science and Research (MIWF) of North‐Rhine Westphalia, Germany (to K.G.).
D. Eickelbeck, T. Rudack, S. A. Tennigkeit, T. Surdin, R. Karapinar, J.-C. Schwitalla, B. Mücher, M. Shulman, M. Scherlo, P. Althoff, M. D. Mark, K. Gerwert, S. Herlitze, ChemBioChem 2020, 21, 612.
Contributor Information
Dennis Eickelbeck, Email: dennis.eickelbeck@rub.de.
Prof. Dr. Klaus Gerwert, Email: gerwert@bph.rub.de.
References
- 1.
- 1a. Zemelman B. V., Lee G. A., Ng M., Miesenböck G., Neuron 2002, 33, 15; [DOI] [PubMed] [Google Scholar]
- 1b. Li X., Gutierrez D. V., Hanson M. G., Han J., Mark M. D., Chiel H., Hegemann P., Landmesser L. T., Herlitze S., Proc. Natl. Acad. Sci. USA 2005, 102, 17816; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1c. Herlitze S., Landmesser L. T., Curr. Opin. Neurobiol. 2007, 17, 87. [DOI] [PubMed] [Google Scholar]
- 2. Deisseroth K., Nat. Methods 2011, 8, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ernst O. P., Lodowski D. T., Elstner M., Hegemann P., Brown L. S., Kandori H., Chem. Rev. 2014, 114, 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Terakita A., Genome Biol. 2005, 6, 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shichida Y., Matsuyama T., Philos. Trans. R. Soc. London Ser. B 2009, 364, 2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Murakami M., Kouyama T., Nature 2008, 453, 363. [DOI] [PubMed] [Google Scholar]
- 7. Yan E. C. Y., Kazmi M. A., Ganim Z., Hou J.-M., Pan D., Chang B. S. W., Sakmar T. P., Mathies R. A., Proc. Natl. Acad. Sci. USA 2003, 100, 9262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Oliveira P. G., Ramos M. L. S., Amaro A. J., Dias R. A., Vieira S. I., Front. Aging Neurosci. 2019, 11, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Blackshaw S., Snyder S. H., J. Neurosci. 1997, 17, 8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Terakita A., Koyanagi M., Tsukamoto H., Yamashita T., Miyata T., Shichida Y., Nat. Struct. Mol. Biol. 2004, 11, 284. [DOI] [PubMed] [Google Scholar]
- 11.
- 11a. Kawano-Yamashita E., Koyanagi M., Wada S., Tsukamoto H., Nagata T., Terakita A., PLoS One 2015, 10, e0141280; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11b. Wada S., Shen B., Kawano-Yamashita E., Nagata T., Hibi M., Tamotsu S., Koyanagi M., Terakita A., Proc. Natl. Acad. Sci. USA 2018, 115, 11310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Koyanagi M., Kawano E., Kinugawa Y., Oishi T., Shichida Y., Tamotsu S., Terakita A., Proc. Natl. Acad. Sci. USA 2004, 101, 6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Spoida K., Eickelbeck D., Karapinar R., Eckhardt T., Mark M. D., Jancke D., Ehinger B. V., König P., Dalkara D., Herlitze S., et al., Curr. Biol. 2016, 26, 1206. [DOI] [PubMed] [Google Scholar]
- 14. Tennigkeit S. A., Karapinar R., Rudack T., Dreier M.-A., Althoff P., Eickelbeck D., Surdin T., Grömmke M., Mark M. D., Spoida K., et al., ChemBioChem 2019, 20, 1766. [DOI] [PubMed] [Google Scholar]
- 15. Masseck O. A., Spoida K., Dalkara D., Maejima T., Rubelowski J. M., Wallhorn L., Deneris E. S., Herlitze S., Neuron 2014, 81, 1263. [DOI] [PubMed] [Google Scholar]
- 16. Mark M. D., Herlitze S., Eur. J. Biochem. 2000, 267, 5830. [DOI] [PubMed] [Google Scholar]
- 17. Hille B., Trends Neurosci. 1994, 17, 531. [DOI] [PubMed] [Google Scholar]
- 18.
- 18a. Emanuel A. J., Do M. T. H., Neuron 2015, 85, 1043; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18b. Govardovskii V. I., Fyhrquist N., Reuter T., Kuzmin D. G., Donner K., Visual Neurosci. 2000, 17, 509. [DOI] [PubMed] [Google Scholar]
- 19. Okada T., Sugihara M., Bondar A.-N., Elstner M., Entel P., Buss V., J. Mol. Biol. 2004, 342, 571. [DOI] [PubMed] [Google Scholar]
- 20. Wall M. A., Coleman D. E., Lee E., Iñiguez-Lluhi J. A., Posner B. A., Gilman A. G., Sprang S. R., Cell 1995, 83, 1047. [DOI] [PubMed] [Google Scholar]
- 21. Coleman D. E., Sprang S. R., Biochemistry 1998, 37, 14376. [DOI] [PubMed] [Google Scholar]
- 22. Blackshaw S., Snyder S. H., J. Neurosci. 1999, 19, 3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nakagawa M., Iwasa T., Kikkawa S., Tsuda M., Ebrey T. G., Proc. Natl. Acad. Sci. USA 1999, 96, 6189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rodgers J., Peirson S. N., Hughes S., Hankins M. W., Cell. Mol. Life Sci. 2018, 75, 3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Offermanns S., Prog. Biophys. Mol. Biol. 2003, 83, 101. [DOI] [PubMed] [Google Scholar]
- 26.
- 26a. Partridge A. H., Smith I. E., Rumble R. B., JOP 2015, 11, 42; [Google Scholar]
- 26b. Chen T.-W., Wardill T. J., Sun Y., Pulver S. R., Renninger S. L., Baohan A., Schreiter E. R., Kerr R. A., Orger M. B., Jayaraman V., et al., Nature 2013, 499, 295; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26c. Dana H., Mohar B., Sun Y., Narayan S., Gordus A., Hasseman J. P., Tsegaye G., Holt G. T., Hu A., Walpita D., et al., eLife 2016, 5, e12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hanoune J., Defer N., Annu. Rev. Pharmacol. Toxicol. 2001, 41, 145. [DOI] [PubMed] [Google Scholar]
- 28. Harada K., Ito M., Wang X., Tanaka M., Wongso D., Konno A., Hirai H., Hirase H., Tsuboi T., Kitaguchi T., Sci. Rep. 2017, 7, 7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alasbahi R. H., Melzig M. F., Pharmazie 2012, 67, 5. [PubMed] [Google Scholar]
- 30. Ash C., Dubec M., Donne K., Bashford T., Lasers Med. Sci. 2017, 32, 1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Slominski A., Pawelek J., Clin. Dermatol. 1998, 16, 503. [DOI] [PubMed] [Google Scholar]
- 32. Hauser A. S., Attwood M. M., Rask-Andersen M., Schiöth H. B., Gloriam D. E., Nat. Rev. Drug Discovery 2017, 16, 829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.
- 33a. Spoida K., Masseck O. A., Deneris E. S., Herlitze S., Proc. Natl. Acad. Sci. USA 2014, 111, 6479; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33b. Eickelbeck D., Karapinar R., Jack A., Suess S. T., Barzan R., Azimi Z., Surdin T., Grömmke M., Mark M. D., Gerwert K., et al., Commun. Biol. 2019, 2, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


