Abstract
In Vietnam, Foot‐and‐mouth disease (FMD) is endemic, but no nationwide studies have been conducted to assess the monthly variations and space‐time clusters of FMD. The main objective was to identify the temporal patterns and space‐time clusters of FMD from 2007 to 2017 using national surveillance data in Vietnam. A total of 163,733 cases were reported from 2007 to 2017. Among them, the proportion of buffaloes (43.31% of total reported cases; 70,909 cases) was highest followed by cattle (30.11%; 49,306 cases), pigs (26.67%; 43,662 cases) and sheep/goats (0.41%; 675 cases). The serotype O was widely distributed across the country while serotype A was observed in Northeast, Central and Southern part of Vietnam while Asia 1 has been not identified since 2007. For monthly variations, most cases were observed during the dry season (from November to March) except Central Highlands. Under the spatial window was set at 50%, a total of seven clusters were identified. The primary cluster was observed from Dec 2009 to Dec 2010 in the northwest (radius: 101.67 km), showing a ratio of 3.75. The secondary cluster was detected in the northeast region (radius: 76.54 km) with a ratio of 3.53 in Feb 2017. The 3rd cluster was the largest with a radius of 176.69 km and located in the southern part of Vietnam. Interestingly, the most temporal clusters included between December and March during the study period. Our findings provide better insight into the temporal patterns and distribution of clusters of FMD in Vietnam. This study provides useful information to policymakers on the hotspot areas and timing of outbreaks. It also identifies when and where national surveillance and control programmes could be implemented more efficiently for the prevention and control of FMD.
Keywords: FMD, national surveillance data, space-time cluster analysis, temporal patterns, Vietnam
1. INTRODUCTION
Foot‐and‐mouth disease (FMD) is highly contagious and one of the most important transboundary diseases in cloven‐hoofed animals and affects more than 70 domestic and wild species (Coetzer, Thomson, & Tustin, 1994; Grubman & Baxt, 2004). The major clinical signs are pyrexia, lameness, shivering, drooling and vesicular lesions of the tongue, feet and teats (Alexandersen, Zhang, Donaldson, & Garland, 2003). FMD causes serious economic losses due to reduced production and trade restrictions at the local and international level (OIE 2013). The disease is caused by the FMD virus of the genus Aphthovirus within the family Picornaviridae (Belsham, 1993). Seven serotypes (namely: A, O, C, Asia1, South African Territories (SAT) 1, SAT 2 and SAT 3) have been identified with intraserotype antigenic variations around the world (Beck & Strohmaier, 1987; OIE, 2013). Recovery from infection or prophylactic vaccination for one serotype does not confer cross‐protection against other serotypes (Kitching, Knowles, Samuel, & Donaldson, 1989). Therefore, the characterization of serotypes is needed to select the appropriate vaccine to control FMD outbreaks (Jamal & Belsham, 2013).
In Southeast Asia, FMD is endemic and serotypes O, A and Asia 1 have been identified in pigs, cattle and buffaloes as circulating serotypes (Gleeson, 2002; Le et al., 2010). In Vietnam, FMD constitutes a huge economic burden for large‐ and small‐scale producers (Pham et al., 2017). It is a notifiable disease, and circulation of the serotypes O, A and Asia 1 has been reported across the country since 1999 (Le et al., 2010). Serotypes O and A are the most prevalent (Gleeson, 2002).
Since 2006, Vietnam has a national control programme in place which includes vaccination, movement control, modified stamping out and disinfection. A national FMD control and prevention programme has been established and runs in phases of 5 years. The current programme runs from 2016 to 2020 and includes vaccination, post‐vaccination monitoring, movement control, establishment of free zone with vaccination, surveillance (mainly, clinical investigation), outbreak investigation and response and communication. The FMD vaccination has been applied for cattle and buffalo in control and buffer zone, while vaccination for other livestock (pigs and small ruminants) has been applied at the owner's expenses. When there is an outbreak of FMD, suppressive vaccination is often applied to affected and surrounding premises. A total of 26 provinces (mostly national boundary provinces with Cambodia, Laos and China) are targeted by vaccination programmes, which provide free vaccination with twice per year, every 6 months for cattle and buffaloes. For pigs, vaccination is mainly used among large‐scale pig farms at their own expense, but it is not common in small‐scale farms, which account for 70%–75% of the total pig production in Vietnam. The main reason for this is the lack of subsidy and incentives for pig smallholders.
To our knowledge, no nationwide studies have been conducted to assess the monthly variations and space‐time clusters of FMD in Vietnam. To better understand infection dynamics, the main objective of this study was to identify the temporal patterns and space‐time clusters of FMD in pigs, cattle, buffaloes and sheep/goats from 2007 to 2017 using national surveillance data in Vietnam.
2. MATERIALS AND METHODS
2.1. Study location and description of data
Vietnam is located on the Eastern Indochinese peninsula and is a long, narrow nation with an estimated human population of 92.7 million in 2016 (GSO 2019). Weather differs significantly from one region to another due to the length of the country, resulting in significant variations in the distribution of livestock production systems. Livestock sector contributes about 15%–20% of total income of farmers, which are mostly smallholders (accounting for 70% of livestock production in Vietnam). These small farms have a low level of awareness in biosecurity, which would allow animal movements without restriction. In addition, lack of access to information on livestock management and veterinary services has been identified as a major issue that constrains their livestock production activity. The national surveillance data of FMD from 2007 to 2017 were obtained from the Department of Animal Health (DAH) in the Ministry of Agriculture and Rural Development (MARD). The national data include the number of cases, animal species (pigs, cattle, buffaloes and goats/sheep), identified serotypes, estimated outbreak dates and locations (commune, district and provincial level). The national surveillance data were collected via passive surveillance on a daily/weekly basis by the local sub‐DAH(s). Among reported cases, most of cases were diagnosed on clinical grounds only by local animal health professionals while some cases were confirmed by national laboratories. In addition, provincial‐level data on livestock populations (except sheep and goat) in 2017 were obtained from the General Statistics Office of Vietnam (Figure 1).
Figure 1.
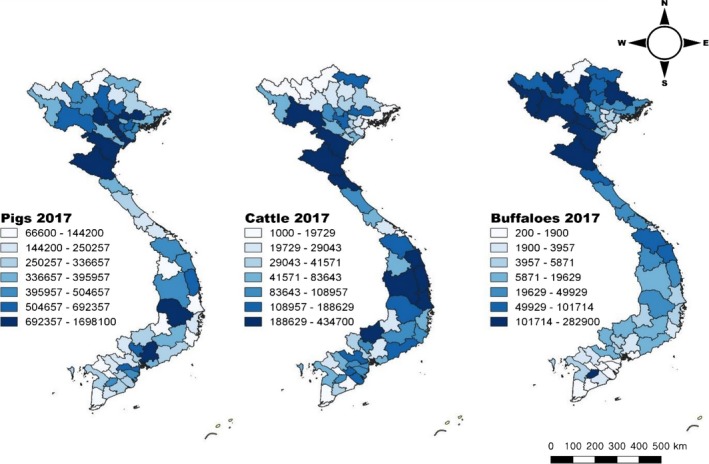
Livestock population by each species in 2017 (sheep and goat populations are not available at provincial level). Source: General Statistics office of Vietnam: https://www.gso.gov.vn/default_en.aspx?tabxml:id=778 [Colour figure can be viewed at http://wileyonlinelibrary.com]
2.2. Data analysis
Vietnam is officially divided into three administrative units: provinces (total: 63), districts (total: 724) and communes (total: 11,181), and Vietnamese government groups these 63 provinces into eight regions based on similarities of geographical conditions and weather conditions (Figure 2). Our surveillance data were collated at regional level (eight regions), and monthly cumulative cases of FMD were calculated for the study period.
Figure 2.
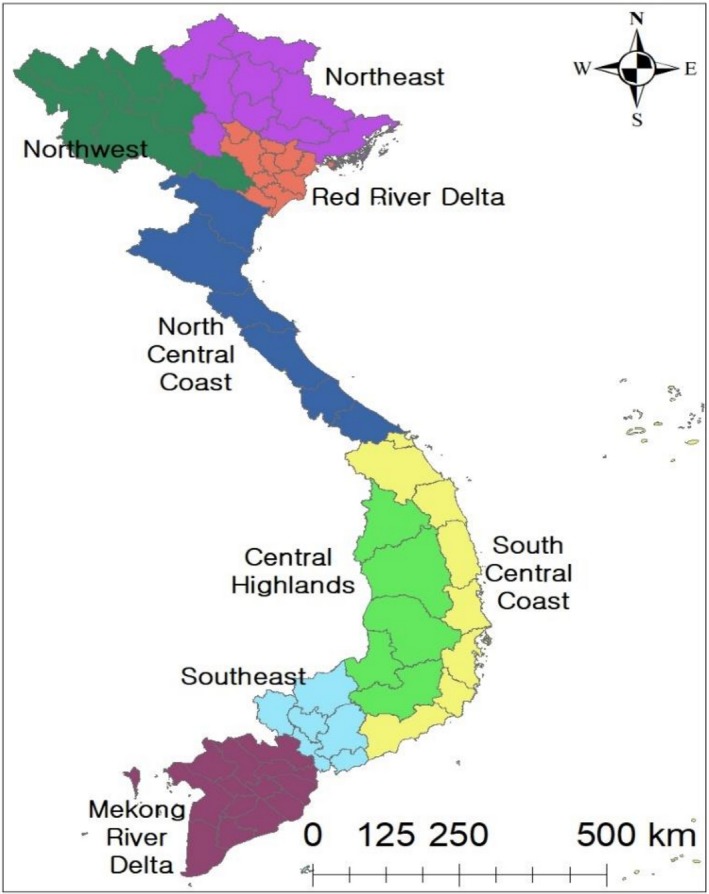
List of regions in Vietnam [Colour figure can be viewed at http://wileyonlinelibrary.com]
Space‐ time cluster analysis was implemented using the SaTScan (version 9.5 free available, http://www.satscan.org), which is commonly used to determine space and space‐time clusters in public health fields (Kulldorff, 2001; Mostashari, Kulldorff, Hartman, Miller, & Kulasekera, 2003). There was a discrepancy of surveillance data hierarchy between cases and livestock population as cases were recorded at commune level (total: 11,181) while livestock population (except sheep and goats) were available at provincial level, which is the largest administrative unit in Vietnam (total: 63). It is difficult to rationalize that the entire livestock population in a province is at risk if an FMD outbreak occurs at commune level. Therefore, a space‐time permutation model was used to assess the space‐time cluster occurrence as only reported cases were available, but not the livestock‐at‐risk population data for FMD was available (Kulldorff, Heffernan, Hartman, Assunção, & Mostashari, 2005). The scan statistic imposes a cylindrical window with a circular geographical base and height corresponding to time, indicating a space‐time cluster. This circular window moves across each centroid of communes and then calculates the expected cases within the window under the assumption they are randomly distributed in space. Clusters are identified by observed/expected cases under the null hypothesis of no clustering. For our analysis, the spatial and temporal window sizes were set to a maximum of 50%. The test statistic of the identified clusters was calculated by a maximum likelihood ratio function, and p‐value was obtained by Monte Carlo simulation with 999 replications of the dataset under the null hypothesis. A p‐value of less than .05 was considered statistically significant. All maps were generated using QGIS (Quantum GIS development Team 2018, QGIS version number 3.4.4) to visualize spatial‐temporal clusters and outbreak locations. Surveillance and livestock data were recorded in the Microsoft Excel 2016 and analysed using STATA version 14.0 (Stata Corp).
3. RESULTS
3.1. Regional distributions and temporal patterns of FMD cases
A total of 163,733 cases were reported from 2007 to 2017. The highest cases were reported in 2011 followed by 2010. Among them, the proportion of buffaloes (43.31% of total reported cases; 70,909 cases) was highest followed by cattle (30.11%; 49,306 cases), pigs (26.67%; 43,662 cases) and sheep/goats (0.41%; 675 cases) (Table 1). Only 19.56% of cases were identified with serotypes (A, O or A and O). The serotype O was widely distributed across the country while serotype A was observed in Northeast, Central and Southern part of Vietnam while Asia 1 has been not identified since 2007 (Figure 3). For monthly variations, the Northeast region (52,208 cases; 31.89%) displayed the highest proportion of reported cases followed by South Central Coast region (25,137 cases; 15.35%) whereas the Red River Delta (1,076 cases; 0.66%) and Southeast (2,709 cases; 1.65%) had the lowest reported cases. In the Northwest/East and Red River Delta regions, cases were predominantly observed between November and February (Figure 4). In the North/South Central Coast, the highest cases were reported between October and November followed by February and March. In the Central Highlands, relatively highest cases were observed in February followed by January, December and July. The Southeast showed that January had the highest cases followed by October and February. In the Mekong River Delta region, most of the cases were reported from November to March. Overall, most cases were observed during the dry season (from November to March) except Central Highlands.
Table 1.
Annual reported cases by species from 2007 to 2017, Vietnam
| Species | Pigs | Cattle | Buffaloes | Sheep and goats | Total |
|---|---|---|---|---|---|
| Year | |||||
| 2007 | 4.289 | 2.561 | 3.320 | 0 | 10.170 |
| 2008 | 64 | 855 | 1.804 | 0 | 2.723 |
| 2009 | 923 | 4.981 | 4.235 | 0 | 10.139 |
| 2010 | 3.576 | 5.982 | 27.006 | 145 | 36.709 |
| 2011 | 27.175 | 16.811 | 28.278 | 433 | 72.697 |
| 2012 | 2.246 | 161 | 222 | 0 | 2.629 |
| 2013 | 2.593 | 5.566 | 1.606 | 4 | 9.769 |
| 2014 | 387 | 1.778 | 1.280 | 33 | 3.478 |
| 2015 | 885 | 3.217 | 831 | 48 | 4.981 |
| 2016 | 333 | 2.164 | 986 | 7 | 3.490 |
| 2017 | 1.191 | 5.230 | 522 | 5 | 6.948 |
| Total | 43.662 | 49.306 | 70.909 | 675 | 163.733 |
Figure 3.
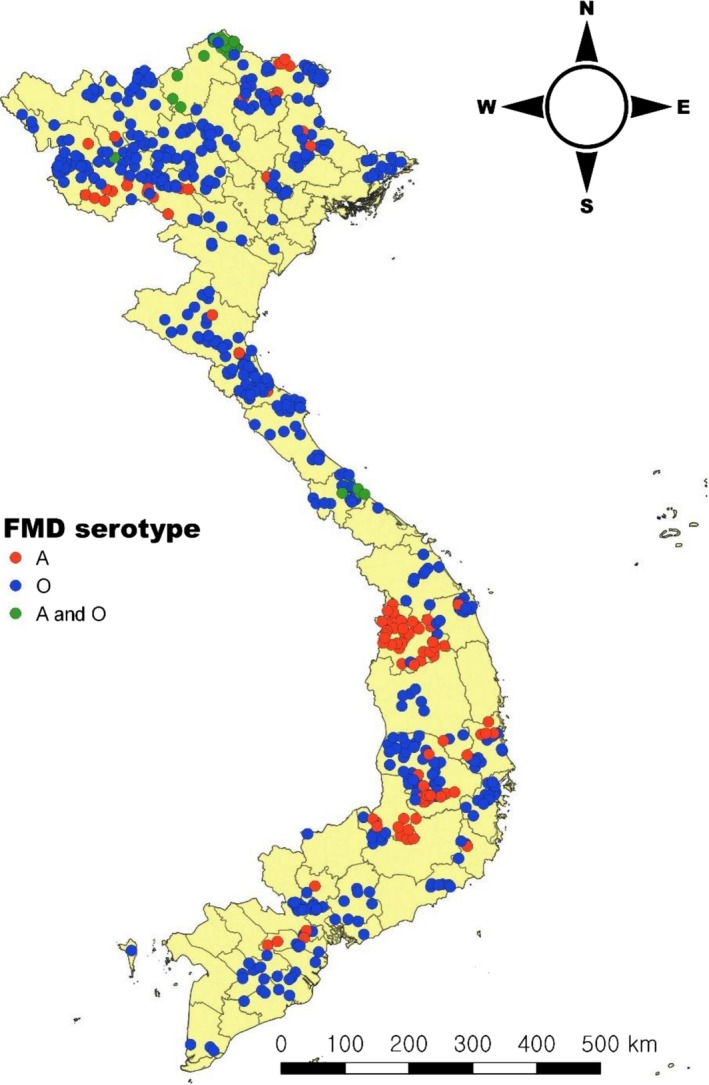
Distribution of identified FMD serotype from 2007 to 2017 in Vietnam [Colour figure can be viewed at http://wileyonlinelibrary.com]
Figure 4.
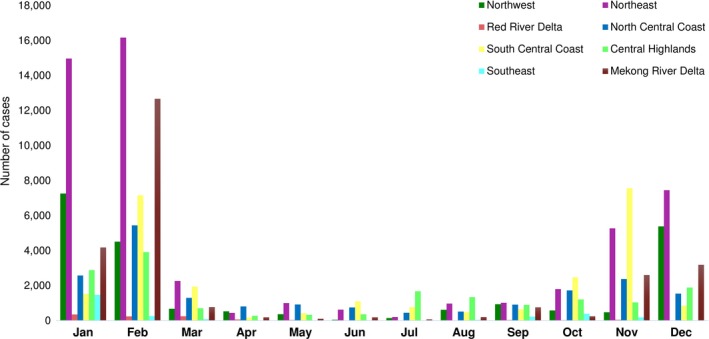
Monthly cumulative cases (regardless of species) of FMD from 2007 to 2017 at region level in Vietnam [Colour figure can be viewed at http://wileyonlinelibrary.com]
3.2. Space‐time cluster analysis
Under the spatial window was set at 50%, a total of seven clusters were identified (Figure 5). The primary cluster (most likely cluster) is least likely to have occurred by chance. SaTScan also identifies the secondary and other clusters in the data set in addition to the most likely cluster and orders them according to their likelihood ratio test statistic. The primary cluster was observed from Dec 2009 to Dec 2010 in the northwest (radius: 101.67 km), showing ratios , 3.75 (19,087/5,095) (Table 2). The secondary cluster was detected in the northeast region (radius: 76.54 km) with a ratio of 3.53 in Jan 2011. The 5th cluster showed the highest ratio (43.32) followed by the 6th cluster (26.68). The 3rd cluster was the largest with a radius of 176.69 km and located over the 12 provinces and Cambodia while the smallest size was the 4th cluster (radius: 43.95 km) in Long An and Tien Giang provinces. Interestingly, the most temporal clusters included between December and March during the study period.
Figure 5.
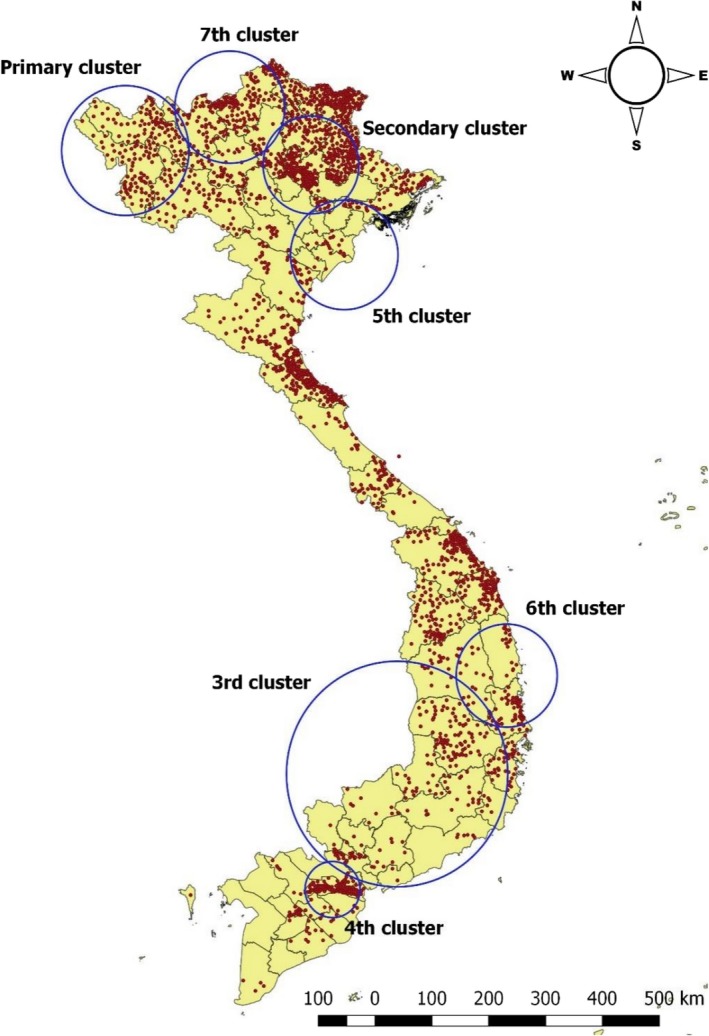
Space‐time cluster analysis of FMD outbreaks (in red, regardless of species) from 2007 to 2017 in Vietnam (50% at risk) [Colour figure can be viewed at http://wileyonlinelibrary.com]
Table 2.
Space‐time clusters of FMD for all species from 2007 to 2017 in Vietnam (space window: 50% at risk)
| Cluster No. | Time year/month | Obs/exp = ratio | Radius (km) | p‐value |
|---|---|---|---|---|
| Primary | Dec/2009–Dec/2010 | 19.087/5.095.07 = 3.75 | 101.67 | <.001 |
| Secondary | Jan/2011–Jan/2011 | 10.735/3.037.57 = 3.53 | 76.54 | <.001 |
| 3rd | Aug/2015–Oct/2017 | 6.885/1.370.59 = 5.02 | 176.69 | <.001 |
| 4th | Feb/2011–Feb/2011 | 10.389/3.006.69 = 3.46 | 43.95 | <.001 |
| 5th | Jan/2012–Mar/2012 | 1.905/43.98 = 43.32 | 85.87 | <.001 |
| 6th | Mar/2007–Mar/2007 | 1.367/51.23 = 26.68 | 80.7 | <.001 |
| 7th | Feb/2011–Mar/2011 | 8.411/3653.14 = 2.30 | 87.83 | <.001 |
4. DISCUSSION
This study was the first attempt to evaluate the temporal patterns and space‐time clusters of FMD using national surveillance data in Vietnam. More buffalo and cattle cases were reported than pigs per head of population throughout the study period. As of October 2017, estimated livestock populations for pigs, cattle and buffaloes were 27 million, 5.6 million and 2.5 million, respectively (GSO 2019). It was possible that FMD cases in pigs were underestimated compared to other livestock. The main explanation is that small‐scale farms (accounting for 70%–75% of pig population in Vietnam) lacked knowledge about livestock diseases (including FMD) and were less accessible to professional animal health workers. In addition, all pigs should be culled when a new outbreak is confirmed on the farm, which may have resulted in being hesitant when it comes to reporting to authorities. These multiple factors may have led to relatively lower reported cases of FMD in pigs. However, in endemic areas, the authority allows farmers to keep cattle and buffaloes with FMD unless new serotypes and serious epidemic are reported, which makes it difficult to control and eradicate FMD in Vietnam.
The primary cluster was detected in the Northeast region where highest cattle and buffalo populations are raised (Figure 1). The 6th cluster was observed in the South Central Coast region where highest cattle population is raised. A recent study supported that 22% of tested samples in cattle and water buffalo had been previously infected with FMDV, and 10.8% of them were found to be asymptomatic carriers in Vietnam (de Carvalho Ferreira et al., 2017). In addition, some studies have suggested that FMDV transmission is possible from cattle to buffaloes or vice versa (Gomes, Ramalho, & Mello, 1997; Madhanmohan et al., 2014). According to the OIE report (Smith et al., 2015), evaluating the animal movements in the Greater Mekong Subregion. It has been suggested that cattle and buffalo movements seemed to occur from Northeast and Southcentral to other parts of the regions. These regions may play a critical role in FMD prevention and control in Vietnam. The secondary cluster was observed in the middle of the Red River Delta and the Northwest regions. The Red River Delta region is the main area for intensive pig farms where most of piglets are produced and transported to other provinces and countries (e.g. China, Cambodia and Laos) (Dietze, Pinto, Wainwright, Hamilton, & Khomenko, 2011). One study has suggested that pigs play a role in the transmission of FMDV to cattle as pigs are commonly raised with other livestock in rural households in Vietnam (Brito et al., 2017). However, our study was not able to evaluate the possible transmission of FMDV between species. Therefore, further investigations are needed to better understand the route of FMDV transmission in Vietnam and from Vietnam to other countries.
Some studies have suggested that we cannot ignore the possible role of FMDV transmission from wild boars to domestic livestock (Breithaupt et al., 2012; Valdazo‐González et al., 2012). However, no studies have been conducted to assess the epidemiological investigation of wild boars in Vietnam. The North and Central regions are high mountainous areas, which are a good habitat for wild boars, implying that there is a higher chance of direct/indirect contact between domestic livestock and wild boars. Especially, buffaloes are commonly raised in the Northern part of Vietnam and play an important role in Vietnam agro‐economics. For instance, they are often used for ploghing rice fields and carrying crops/luggage. In addition, they provide milk, meats and leather for local people. The buffaloes are commonly raised under free ranging systems in Vietnam, which may provide more opportunities to come into contact with wild boars. One study found that higher prevalence of FMD was observed among buffaloes as compared to dairy and beef cattle (de Carvalho Ferreira et al., 2017). Therefore, strengthened monitoring and surveillance programmes are necessary to better understand the role of wild boars and their interaction with livestock.
The number of reported cases has decreased since 2011, but it was difficult to evaluate through our study whether the reduction in reported cases is linked to the FMD control and prevention programme. We found the similar monthly patterns of reported cases across regions during the study period. More cases were reported from November to April, which may be mainly attributed to cultural factors and the national vaccination control programme. For instance, human and animal movement are increasing before and after the Tet holiday (Vietnamese New Year, normally in February), which may lead to a higher chance of FMDV transmission. After that, the first round of vaccination programme takes place in March until early April, reducing the number of new cases and thus resulting in less reported cases between March and September. The booster vaccination programme begins after 6 months, but the farmers have a tendency to avoid the second vaccination due to side effect (e.g. abortion etc.), which may result in slightly higher reported case numbers from late September and October. Therefore, it is important to educate farmers about the importance of biosecurity management (especially, during the Tet holiday) and national vaccination programme (especially booster vaccination).
Current animal surveillance showed that less than 20% of cases were identified with serotypes. The main reason was due to delayed reporting for laboratory analysis or livestock owners have a tendency to use alternative medicine (e.g. lemon juice, starfruit and methylene blue) for treatment, which may kill live virus in the specimens. The serotype Asia 1 was reported to the OIE in 2007, since then this serotype has not been recorded under the national surveillance programmes. However, it is difficult to determine whether this serotype really is no longer circulating in the country. In general, the identification of FMD virus strains is regularly conducted by national veterinary laboratories and OIE reference laboratories, which are transferred to local Vietnamese commercial pharmaceutical companies for local production of vaccines. In addition, imported FMD vaccines are available depending on the demand of farmers. Currently, mono‐ and bi‐valent vaccines (serotype O and A) are in use for FMD control in Vietnam. However, it is important to conduct extensive molecular epidemiological investigation for identification of the new circulating serotypes across the country on a regular basis, otherwise current vaccine may not able to offer full protection.
Our study had some limitations, mainly related to data quality. Firstly, it was likely that FMD cases were underreported due to lack of awareness, professional animal health workers and medical facilities in rural areas. It is also related to a compensation policy for livestock under the natural disaster and diseases. The government compensates VND 38,000 (USD 1.62) per kg for pigs and VND 45,000 (USD 1.92) per kg for cattle, buffaloes, sheep and goats, which are approximately 80% of market price in Vietnam. However, most of farmers are not aware of this policy or reluctant to claim as the compensate rates are relatively low. In addition, it takes at least several months (expert opinion) to be compensated due to prolonged administrative procedures even if they request it. Secondly, some reported cases of FMD were misdiagnosed with other diseases (e.g. swine vesicular diseases and bovine viral diarrhoea virus) (Duong, Alenius, Huong, & Bjorkman, 2008; Liu, Li, Liang, Li, & Cui, 2019; Reid, Ferris, Hutchings, King, & Alexandersen, 2004; Vu et al., 2017) as approximately only 20%–30% of cases (expert opinion) were confirmed by national laboratories. Therefore, it is necessary to strengthen the national surveillance system with laboratory confirmation. Lastly, the centroids of each commune were used for our cluster analysis as the coordinates or address of affected farms was not recorded in the national surveillance data. This may have resulted in inaccurate results as the negative impact of inaccuracies in spatial event data have been reported by other studies (Burra, Jerrett, Burnett, & Anderson, 2002; DeLuca & Kanaroglou, 2008). Moreover, it was assumed that the livestock population (pigs, buffaloes, cattle and sheep/goats) did not dramatically change during the study period, which is crucial for a space‐time permutation model as it was not able to distinguish at increase/decrease risk of disease due to a population vs. disease increase/decrease. It is possible that our detected clusters may have been influenced by livestock population at risk if the background population dramatically increased or decreased in one area compared to another. However, from available data and experience, it seems as if the total livestock population by each species has not changed dramatically (4% increase in pigs, 13% decrease in cattle, 11% decrease in buffaloes and 6% increase in sheep/goats) from 2007 to 2017, with a slight increase/decrease overall, minimizing risks of bias in our findings.
Our findings provide better insight into the temporal patterns and distribution of clusters of FMD in Vietnam. This study provides useful information to policymakers on the hotspot areas and timing of outbreaks. It also identifies when and where national surveillance and control programmes could be implemented more efficiently for the prevention and control of FMD. It is important to raise public awareness on biosecurity management and national vaccination programme to prevent outbreaks and onward transmission.
ETHICAL APPROVAL
This study was approved by the Hanoi University of Public Health Review Board (No. 186/2018/YTCC‐HD3), Vietnam.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
All authors contributed to the conception and design of this study. HSL designed and ran the statistical analyses, wrote the draft of the manuscript and prepared all the figures and tables presented; TLP collected the data; all authors (TLP and BW) contributed to the editing and comprehensive revision of this manuscript.
ACKNOWLEDGEMENTS
This study was funded by the Consortium of International Agricultural Research Centers (CGIAR) Research Program on Livestock.
Lee H, Pham TL, Wieland B. Temporal patterns and space‐time cluster analysis of foot‐and‐mouth disease (FMD) cases from 2007 to 2017 in Vietnam. Transbound Emerg Dis. 2020;67:584–591. 10.1111/tbed.13370
DATA AVAILABILITY STATEMENT
All datasets supporting our findings are available from the corresponding author on reasonable request.
REFERENCES
- Alexandersen, S. , Zhang, Z. , Donaldson, A. I. , & Garland, A. J. M. (2003). The pathogenesis and diagnosis of foot‐and‐mouth disease. Journal of Comparative Pathology, 129, 1–36. 10.1016/S0021-9975(03)00041-0 [DOI] [PubMed] [Google Scholar]
- Beck, E. , & Strohmaier, K. (1987). Subtyping of European foot‐and‐mouth disease virus strains by nucleotide sequence determination. Journal of Virology, 61, 1621–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsham, G. J. (1993). Distinctive features of foot‐and‐mouth disease virus, a member of the picornavirus family; aspects of virus protein synthesis, protein processing and structure. Progress in Biophysics and Molecular Biology, 60, 241–260. 10.1016/0079-6107(93)90016-D [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breithaupt, A. , Depner, K. , Haas, B. , Alexandrov, T. , Polihronova, L. , Georgiev, G. , … Beer, M. (2012). Experimental infection of wild boar and domestic pigs with a foot and mouth disease virus strain detected in the southeast of Bulgaria in December of 2010. Veterinary Microbiology, 159, 33–39. 10.1016/j.vetmic.2012.03.021 [DOI] [PubMed] [Google Scholar]
- Brito, B. , Pauszek, S. J. , Eschbaumer, M. , Stenfeldt, C. , de Carvalho Ferreira, H. C. , Vu, L. T. , … Arzt, J. (2017). Phylodynamics of foot‐and‐mouth disease virus O/PanAsia in Vietnam 2010–2014. Veterinary Research, 48, 24 10.1186/s13567-017-0424-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burra, T. , Jerrett, M. , Burnett, R. T. , & Anderson, M. (2002). Conceptual and practical issues in the detection of local disease clusters: A study of mortality in Hamilton, Ontario. The Canadian Geographer/Le Géographe Canadien, 46, 160–171. 10.1111/j.1541-0064.2002.tb00737.x [DOI] [Google Scholar]
- Coetzer, J. A. W. , Thomson, G. R. , & Tustin, R. C. (1994). Infectious diseases of livestock with special reference to. Southern Africa.
- de Carvalho Ferreira, H. C. , Pauszek, S. J. , Ludi, A. , Huston, C. L. , Pacheco, J. M. , Le, V. T. , … Arzt, J. (2017). An integrative analysis of foot‐and‐mouth disease virus carriers in Vietnam achieved through targeted surveillance and molecular epidemiology. Transboundary and Emerging Diseases, 64, 547–563. 10.1111/tbed.12403 [DOI] [PubMed] [Google Scholar]
- DeLuca, P. F. , & Kanaroglou, P. S. (2008). Effects of alternative point pattern geocoding procedures on first and second order statistical measures. Journal of Spatial Science, 53, 131–141. 10.1080/14498596.2008.9635141 [DOI] [Google Scholar]
- Dietze, K. , Pinto, J. , Wainwright, S. , Hamilton, C. , & Khomenko, S. (2011). Porcine reproductive and respiratory syndrome (PRRS). FAO Emergency Prevention System, 1, 1–8. [Google Scholar]
- Duong, M. C. , Alenius, S. , Huong, L. T. T. , & Bjorkman, C. (2008). Prevalence of Neospora caninum and bovine viral diarrhoea virus in dairy cows in Southern Vietnam. Veterinary Journal, 175, 390–394. 10.1016/j.tvjl.2006.01.016 [DOI] [PubMed] [Google Scholar]
- GSO . (2019). General Statistics of Vietnam Retrieved from https://www.gso.gov.vn/default_en.aspx?tabxml:id=778 (accessed on 02/02/2019)
- Gleeson, L. J. (2002). A review of the status of foot and mouth disease in South‐East Asia and approaches to control and eradication. Revue Scientifique Et technique‐Office International Des Épizooties, 21, 465–472. 10.20506/rst.21.3.1346 [DOI] [PubMed] [Google Scholar]
- Gomes, I. , Ramalho, A. K. , & de Mello, P. A. (1997). Infectivity assays of foot‐and‐mouth disease virus: Contact transmission between cattle and buffalo (Bubalus bubalis) in the early stages of infection. Veterinary Record, 140, 43–47. 10.1136/vr.140.2.43 [DOI] [PubMed] [Google Scholar]
- Grubman, M. , & Baxt, B. (2004). Foot‐and‐mouth disease. Clinical Microbiology Reviews, 10.1128/CMR.17.2.465-493.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal, S. M. , & Belsham, G. J. (2013). Foot‐and‐mouth disease: Past, present and future. Veterinary Research, 10.1186/1297-9716-44-116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitching, R. P. , Knowles, N. J. , Samuel, A. R. , & Donaldson, A. I. (1989). Development of foot‐and‐mouth disease virus strain characterisation‐A review. Tropical Animal Health and Production, 21(3), 153–166. 10.1007/BF02250825 [DOI] [PubMed] [Google Scholar]
- Kulldorff, M. (2001). Prospective time periodic geographical disease surveillance using a scan statistic. Journal of the Royal Statistical Society: Series A (Statistics in Society), 10.1111/1467-985X.00186 [DOI] [Google Scholar]
- Kulldorff, M. , Heffernan, R. , Hartman, J. , Assunção, R. , & Mostashari, F. (2005). A space‐time permutation scan statistic for disease outbreak detection. PLoS Medicine, 2(3), e59 10.1371/journal.pmed.0020059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le, V. P. , Nguyen, T. , Park, J. H. , Kim, S. M. , Ko, Y. J. , Lee, H. S. , … Lee, K. N. (2010). Heterogeneity and genetic variations of serotypes O and Asia 1 foot‐and‐mouth disease viruses isolated in Vietnam. Veterinary Microbiology, 10.1016/j.vetmic.2010.04.005 [DOI] [PubMed] [Google Scholar]
- Liu, C. , Li, X. , Liang, L. , Li, J. , & Cui, S. (2019). Isolation and phylogenetic analysis of an emerging Senecavirus A in China, 2017. Infection, Genetics and Evolution, 68, 77–83. 10.1016/j.meegid.2018.12.009 [DOI] [PubMed] [Google Scholar]
- Madhanmohan, M. , Yuvaraj, S. , Nagendrakumar, S. B. , Srinivasan, V. A. , Gubbins, S. , Paton, D. J. , & Parida, S. (2014). Transmission of foot‐and‐mouth disease virus from experimentally infected Indian buffalo (Bubalus bubalis) to in‐contact naive and vaccinated Indian buffalo and cattle. Vaccine, 32, 5125–5130. [DOI] [PubMed] [Google Scholar]
- Mostashari, F. , Kulldorff, M. , Hartman, J. J. , Miller, J. R. , & Kulasekera, V. (2003). Dead bird clusters as an early warning system for West Nile virus activity. Emerging Infectious Diseases, 9, 641 10.3201/eid0906.020794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- OIE (2013). Foot and mouth diseases. Retrieved from http://www.oie.int/fileadmin/home/eng/animal_health_in_the_world/docs/pdf/disease_cards/foot_and_mouth_disease.pdf (accessed on 15/03/2019).
- Pham, H. T. T. , Antoine‐Moussiaux, N. , Grosbois, V. , Moula, N. , Truong, B. D. , Phan, T. D. , … Peyre, M. (2017). Financial impacts of priority swine diseases to pig farmers in Red River and Mekong River Delta, Vietnam. Transboundary and Emerging Diseases, 64, 1168–1177. 10.1111/tbed.12482 [DOI] [PubMed] [Google Scholar]
- Reid, S. M. , Ferris, N. P. , Hutchings, G. H. , King, D. P. , & Alexandersen, S. (2004). Evaluation of real‐time reverse transcription polymerase chain reaction assays for the detection of swine vesicular disease virus. Journal of Virological Methods, 116, 169–176. 10.1016/j.jviromet.2003.11.007 [DOI] [PubMed] [Google Scholar]
- Smith, P. , Bourgeois Lüthi, N. , Li, H. , Kyaw, N. O. , Phonvisay, A. , Premashthira, S. , … Miller, C. (2015). Movement pathways and market chains of large ruminants in the Greater Mekong Sub‐region. Paris, France: World Organisation for Animal Health. [Google Scholar]
- Valdazo‐González, B. , Polihronova, L. , Alexandrov, T. , Normann, P. , Knowles, N. J. , Hammond, J. M. , … King, D. P. (2012). Reconstruction of the transmission history of RNA virus outbreaks using full genome sequences: Foot‐and‐mouth disease virus in Bulgaria in 2011. PLoS One, 7, e49650 10.1371/journal.pone.0049650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu, L. T. , Long, N. T. , Brito, B. , Stenfeldt, C. , Phuong, N. T. , Hoang, B. H. , … Arzt, J. (2017). First detection of foot‐and‐mouth disease virus O/Ind‐2001d in Vietnam. PLoS One, 12, e0177361 10.1371/journal.pone.0177361 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All datasets supporting our findings are available from the corresponding author on reasonable request.


