Abstract
Aim
To examine the feasibility of a food‐based, low‐energy, low‐carbohydrate diet with behavioural support delivered by practice nurses for patients with type 2 diabetes.
Materials and Methods
People with type 2 diabetes and a body mass index (BMI) of ≥30 kg/m2 were randomized 2:1 to intervention or control (usual care) and assessed at 12 weeks. The intervention comprised an 800–1000 kcal/day, food‐based, low‐carbohydrate (<26% energy) diet for 8 weeks, followed by a 4‐week weight maintenance period and four 15‐20‐minute appointments with a nurse. Primary outcomes were feasibility of recruitment, fidelity of intervention delivery and retention of participants at 12 weeks. Secondary outcomes included change in weight and HbA1c. Focus groups explored the intervention experience.
Results
Forty‐eight people were screened, 33 enrolled and 32 followed‐up. Mean (±SD) weight loss in the intervention group was 9.5 kg (± 5.4 kg) compared with 2 kg (± 2.5 kg) in the control group (adjusted difference − 7.5 kg [−11.0 to −4.0, P < 0.001]). Mean reduction in HbA1c in the intervention group was 16.3 mmol/mol (± 13.3 mmol/mol) compared with 0.7 mmol/mol (±4.5 mmol/mol) in the control group (difference − 15.7 mmol/mol [−24.1 to −7.3, P < 0.001]).
Conclusions
It is feasible to recruit participants to a food‐based, low‐energy, low‐carbohydrate intervention, for practice nurses to deliver the programme in primary care, and to retain participants in both groups. There is evidence of clinically significant short‐term improvements in weight and glycaemic control.
Keywords: clinical trial, glycaemic control, primary care, randomized trial, type 2 diabetes, weight control
1. INTRODUCTION
The paradigm for diabetes management is changing.1 What was previously thought to be a lifelong progressive condition to be managed primarily with escalating doses of medications, may instead be put into remission if treated with intensive weight loss support, such as total diet replacement (TDR) programmes.2, 3 TDR programmes provide nutritionally complete formula diets, usually soups, shakes or bars, and exclude virtually all usual food. TDRs provide ~ 800 kcal/day leading to a mean 10 kg weight loss at 1 year. But these products are not routinely available in most healthcare systems and very few patients are offered this type of treatment.
The majority of patients with type 2 diabetes rely on general dietary advice from primary care professionals. While there is considerable uncertainty regarding the optimal diet composition for people with type 2 diabetes4 there is a growing interest from patients, practitioners and the general public in low carbohydrate diets.5, 6 Removing most carbohydrate from the diet and rigorous portion control of other foods offers the opportunity to achieve an energy intake comparable with TDR programmes. By focusing on simple rules, this approach has the potential to be delivered by generalist practitioners in routine settings to support patients to change their usual dietary habits and may offer a more sustainable intervention than TDR.
In 2017, a UK priority‐setting group comprising patients and clinicians identified the role of carbohydrates, dietary change and how best to support people to achieve these changes, as three of the top 10 research priorities in type 2 diabetes.7 We investigated the feasibility of practice nurses delivering a food‐based, low‐energy, low‐carbohydrate intervention in routine primary care to promote weight loss and improved glycaemic control in patients with type 2 diabetes.
2. MATERIALS AND METHODS
2.1. Study design and participants
This feasibility study was a pragmatic, individually randomized controlled trial, with a practice nurse allocating participants to a low‐energy, low‐carbohydrate diet or routine support over 12 weeks. The protocol was reviewed and approved by the South Central Oxford B REC Committee (ref: 18/SC/0071) and prospectively registered on the ISRCTN (62452621) and published following peer review.8
We recruited participants from three primary care practices in Oxfordshire, UK. General practitioners searched electronic health records for adults with type 2 diabetes and a body mass index (BMI) of at least 30 kg/m2 who had attended digital retinopathy screening in the last 12 months, and invited them by letter to participate. Major exclusion criteria were: history of an eating disorder; current use of insulin or sodium‐glucose co‐transporter‐2 inhibitor therapy; HbA1c ≥93 mmol/mol (10.5%); or non‐proliferative retinopathy level R2 or more severe, proliferative diabetic retinopathy or maculopathy (recognizing concerns that sudden normalization in retinal blood flow, associated with restoration of normoglycaemia, may result in deterioration of established retinopathy9) (see the supporting information, Appendix S1). After telephone screening by researchers, eligible patients were booked for a baseline appointment with a nurse at their local practice, where nurses confirmed consent and verified inclusion.
2.2. Randomization
An independent researcher produced a computer‐generated randomization list with 2:1 allocation (intervention: control) using random permuted blocks, stratified by general practice. Allocation was concealed in sequentially numbered sealed opaque envelopes, opened after enrolment. Because of the nature of the intervention, it was not possible to blind participants, clinicians or some of the researchers after treatment allocation.
2.3. Interventions
The DIAMOND programme (DIetary Approaches to the Management Of type 2 Diabetes) is described elsewhere.8 In brief, the diet consisted of a low‐energy, low‐carbohydrate diet, comprising 800–1000 kcal/day, with <26% of daily energy intake from carbohydrates and a minimum of 60 g protein/day, for 8 weeks. Dietary advice focused on excluding sugary and starchy foods high in carbohydrates entirely from the diet (with the exception of dairy and limited fruit intake), strict portion control and minimal use of fats and oils. Participants were advised to eat fresh vegetables or salad and small amounts of lean meat and fish. After 8 weeks, participants were advised to gradually increase their energy intake, increasing portion size one meal at a time or adding one serving of high fibre carbohydrate, until weight stabilized, but with guidance on maintaining a sustainable lower carbohydrate diet in the longer term.
The programme was designed to be delivered by practice nurses across four 15‐20‐minute appointments at baseline and weeks 2, 4 and 8, providing support and motivation, including advice on goal‐setting and self‐monitoring, and problem‐solving strategies. Participants received a self‐help booklet with sample menus and recipes. Participants saw a GP in one 10‐minute appointment at baseline to review medication for diabetes and hypertension8 (Appendix S2).
For the comparator, participants saw their practice nurse for usual care dietary advice at baseline, following English guidance, which advocates “healthy balanced eating”. They received the DiabetesUK information booklet, “What is a healthy balanced diet for diabetes?“10 Diabetes treatment could be adjusted in either group following normal practice guidelines if warranted.
2.4. Procedures
Height was measured at baseline, and all other measurements at baseline and 12 weeks. In addition, for intervention group participants, weight and blood pressure were measured at 2, 4 and 8 weeks, blood samples were repeated at 8 weeks, and a brief dietary recall questionnaire was completed at 2 and 8 weeks. A digital scale was used to measure weight and body fat. Blood pressure was measured three times after 5 minutes of seated rest, and the mean of the last two readings was recorded. The questionnaires assessed participants’ quality of life,11 dietary intake (reporting the number of portions of different food groups over the preceding 24 hours), self‐reported motivation and perceptions across domains of diet, health and diabetes control, and self‐reported adherence to the intervention. Fasting blood samples were collected to measure HbA1c, glucose, insulin, liver function tests and lipid profile. The nurse‐delivered intervention sessions were audio‐recorded for assessment of the fidelity of intervention delivery.
2.5. Outcome measures
The primary outcomes were prespecified feasibility criteria to progress to a full trial: (i) that at least 60% of allocated intervention group participants attempted the dietary intervention after randomization; (ii) fidelity of intervention delivery (ie, that healthcare professionals conducted the intervention delivery session with at least 60% of essential elements present); and (iii) that 60% of participants attended the final follow‐up session.
Secondary outcomes included changes in clinical indicators of effect of the intervention between baseline and 12 weeks (body weight, glycaemic control and insulin sensitivity [HBA1c, fasting glucose, fasting insulin and all HOMA measures ‐ as fasting glucose and fasting insulin are used to compute the HOMA score so all are interrelated], [homeostatic model assessment {HOMA} measurement of insulin resistance {HOMA‐IR}, β cell function {HOMA‐B} and insulin sensitivity {HOMA‐S}12], blood pressure, lipid profile, and liver function, change in diabetic and antihypertensive medications [from baseline {prior to intervention visits} to 12 weeks], change in diagnostic range of HbA1c [<42, 42–47 or ≥ 48 mmol/mol, on or off medications], and change in Problem Areas In Diabetes [PAID] score), as well as study process measures (Appendix S3).
Qualitative data about the experience of the intervention were collected from focus groups with participants and healthcare professionals after intervention completion.
An additional exploratory outcome of change in calculated 10‐year risk of cardiovascular disease (QRISK)‐3–2018 score was added after publication of the protocol but before analysis.13
2.6. Statistical analyses
Sample size was based on the progression criteria. We expected at least 75% to achieve the progression criteria with 95% confidence intervals (CI) to exclude 60%. This required a sample of 30 participants allocated in a 2:1 ratio, intervention:control. The study was not powered to detect a statistically significant difference in efficacy between the arms.
We followed a statistical analysis plan finalized prior to database locking, and conducted analyses using Stata version 14.1 SE for Windows (StataCorp LP, College Station, TX, USA). Primary outcomes are presented as descriptive summary statistics, with 95% CI around proportions calculated using OpenEpi software14 with Wilson score corrected for population size. Fidelity of intervention delivery was assessed against six prespecified essential criteria: dietary principles of (i) reducing energy intake to 800–1000 kcal/day, (ii) reducing carbohydrate intake, and (iii) what to eat (ie, food choices); and behavioural principles of (iv) goal‐setting, (v) self‐monitoring, and (vi) problem‐solving or specified behavioural change techniques (eg, use of a planning tool). Fidelity was assessed by two independent reviewers (M. N. and E. M.) and discrepancies were resolved by discussion. “Attempting the diet” was assessed by (i) a nurse reporting on the case report form that the patient had directly stated they were willing to attempt the intervention after the intervention delivery visit, (ii) audio‐recordings of this visit, and (iii) a nurse report at the week‐2 patient follow‐up, at which all participants described their attempts at the intervention and received guidance and support with any elements of it they had found challenging.
We used linear regression models (ANCOVA) to test for a difference in change between groups in secondary outcomes from baseline to 12‐week follow‐up, using intention‐to‐treat analysis, adjusted for centre, as this was a stratification variable. To avoid over‐fitting the model, the change score was used as the outcome to incorporate adjustment for baseline values. In separate analyses, we adjusted for any variables that showed meaningful imbalance between groups, and assessed the sensitivity of the results to missing data using different imputation methods including baseline observation carried forward and completer‐only analysis. We conducted prespecified exploratory subgroup analyses to assess whether treatment effects on weight or HbA1c change differed by gender, glycaemic control at baseline (HbA1c <53 vs. ≥53 mmol/mol) or duration of diabetes (<6 and ≥6 years).
We analyzed qualitative data transcribed from focus groups following a thematic approach using NVivo (version 11) qualitative data analysis software.
2.7. Patient and public involvement
We convened two panels of patients with type 2 diabetes prior to ethical submission, one consisting of members who had recently tried variations of low‐energy, low‐carbohydrate diets. They informed and advised on the dietary and behavioural support components of the intervention, the patient materials and the perceived benefits or burdens of the study for patients. One patient member subsequently joined the trial management group.
3. RESULTS
Participants were recruited between April 3, 2018 and October 30, 2018. Of 422 participants invited by letter, 60 (15%) responded to the invitation and were screened by telephone, 48 were screened in person, 33 were eligible for enrolment and 21 were randomly allocated to the DIAMOND programme and 12 to usual care. Follow‐up was completed on February 19, 2019.
At baseline, the average age was 67 years (SD 11), 55% were women and 94% were white British. BMI was 35.4 kg/m2 (SD 4.7) and HbA1c was 61 mmol/mol (SD 13) (Tables 1A and 1B). All available participants were followed up at 12 weeks (n = 32); one participant in the control group died of an unrelated cause (Figure 1).
Table 1A.
Baseline characteristics of participants assigned to the DIAMOND programme (n = 21) or usual care (n = 12). Values are mean (standard deviation) unless stated otherwise
| Intervention (n = 21) | Control (n = 12) | All (n = 33) | |
|---|---|---|---|
| Age, years | 69 (10) | 64 (13) | 67 (11) |
| Gender, femalea | 9 (43) | 9 (75) | 18 (55%) |
| Body mass index, kg/m2 | 34.8 (3.4) | 36.4 (6.3) | 35.4 (4.7) |
| Weight, kg | 103.0 (16.7) | 97.6 (13.2) | 101.0 (15.6) |
| Height, m | 1.7 (0.1) | 1.6 (0.1) | 1.7 (0.1) |
| HbA1c, mmol/mol | 63.2 (14.8) | 57.1 (8.3) | 61.0 (13) |
| Systolic blood pressure, mmHg | 144 (20) | 132 (14) | 140 (19) |
| Diastolic blood pressure, mmHg | 84 (10) | 78 (7) | 82 (9) |
| Duration of diabetes, years | 9.0 (5.2) | 9.5 (7.9) | 9.2 (6.1) |
| HbA1c above target at baseline (HbA1c >53 mmol/mol) † | 14 (67) | 8 (67) | 22 (67) |
| Ethnic group a | |||
| White | 21 (100) | 10 (83) | 31 (94) |
| Black or Asian | 0 (0) | 0 (0) | 0 (0) |
| Mixed or other | 0 (0) | 2 (17) | 2 (6) |
| Education a | |||
| No formal qualifications | 8 (38) | 4 (33) | 12 (36) |
| Secondary education | 9 (43) | 3 (25) | 12 (36) |
| Higher education | 4 (19) | 5 (42) | 9 (27) |
| Relevant medications | |||
| Number of diabetes medications | 1.4 (0.9) | 1.1 (0.9) | 1.3 (0.9) |
| Number of antihypertensive medications | 1.4 (1.5) | 1.4 (1.1) | 1.4 (1.3) |
number of participants (%).
Table 1B.
Baseline characteristics, for additional variables, of participants assigned to the DIAMOND programme (n = 21) or usual care (n = 12). Values are mean (standard deviation) unless stated otherwise
| Outcome measure | Intervention group (mean ± SD) | Control group (mean ± SD) |
|---|---|---|
| Glycaemic control | ||
| Fasting glucose, mmol/L | 10.0 (3.4) | 8.3 (1.6) |
| Fasting insulin, pmol/L | 95.1 (28.6) | 92.6 (52.2) |
| HOMA | ||
| HOMA‐%B | 49.3 (26.9) | 56.2 (27.88) |
| HOMA‐%S | 51.7 (14.3) | 63.7 (24.7) |
| HOMA‐IR | 2.1 (0.6) | 1.9 (1.1) |
| Lipid profile | ||
| Total cholesterol, mmol/L | 4.3 (1.0) | 4.3 (1.0) |
| HDL, mmol/L | 1.1 (0.2) | 1.3 (0.3) |
| Triglycerides, mmol/L | 2.1 (1.2) | 1.7 (0.6) |
| Non‐HDL cholesterol, mmol/L | 2.4 (1.0) | 2.3 (0.9) |
| Total cholesterol: HDL ratio | 3.8 (1.0) | 3.5 (1.1) |
| Liver function tests | ||
| Bilirubin, μmol/L | 15.6 (7.7) | 14.3 (6.1) |
| AST, IU/L | 27.4 (12.0) | 23.6 (9.4) |
| ALT, IU/L | 34.1 (22.0) | 28.8 (19.6) |
| ALP, IU/L | 77.4 (23.1) | 83.9 (18.5) |
| Albumin, g/L | 39.1 (2.0) | 39.3 (2.4) |
| GGT, IU/L | 50.2 (26.8) | 59.8 (90.0) |
| AST:ALT ratio | 1.0 (0.3) | 0.9 (0.2) |
| QRISK | 28.2 (15.6) | 20.8 (10.1) |
| PAID score | 14.4 (13.4) | 20.7 (15.8) |
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma‐glutamyl transferase; HDL, high density lipoprotein; HOMA, homeostatic model assessment; HOMA‐IR, insulin resistance; HOMA‐β, steady state β cell function; HOMA‐S, insulin sensitivity; PAID, Problem Areas In Diabetes score; QRISK, calculated 10‐year risk of cardiovascular disease.
Figure 1.
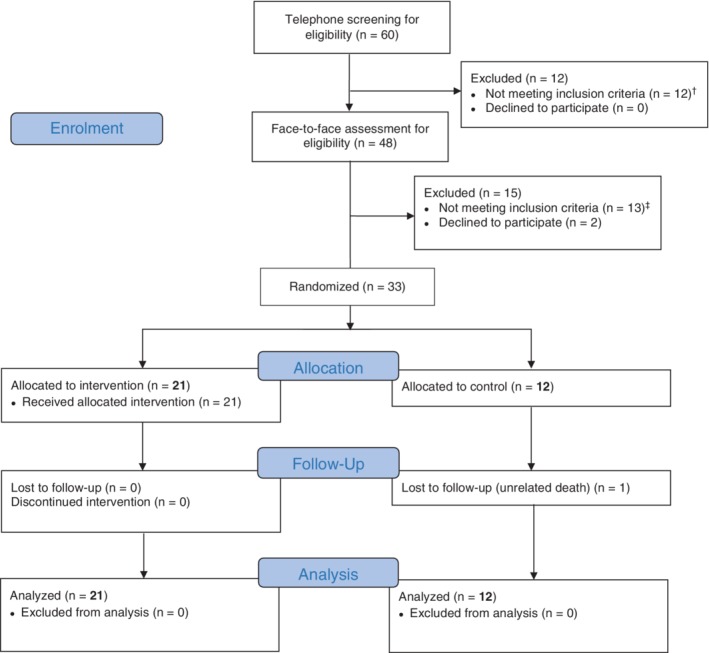
Consort flowchart. † Exclusions at telephone screening: body mass index (BMI) <30 kg/m2 (n = 6), eye screening results (significant retinopathy or maculopathy, n = 3), other (bariatric surgery, n = 1; use of sodium‐glucose co‐transporter‐2 inhibitor; n = 1; medication for epilepsy, n = 1). ‡ Exclusions at baseline visit: BMI <30 kg/m2 (n = 8), no longer active diagnosis of diabetes/diabetes in remission (n = 5)
3.1. Primary outcomes
Feasibility was shown and progression criteria achieved for all three primary outcome measures. All participants randomized to the intervention group attempted the intervention (95% CI 85% to 100%) and attended the final follow‐up session at 12 weeks (95% CI 89% to 100%; excluding the control group participant who died). Nineteen of the 21 intervention sessions were audio‐recorded and available for assessment of fidelity. Of the available records, 100% (95% CI 85% to 100%) showed each of the six prespecified essential criteria.
3.2. Secondary outcomes
3.2.1. Weight
Mean weight change at 12 weeks was −9.5 kg (SD 5.4 kg) in the intervention group and −2 kg (SD 2.5 kg) in the usual care group, adjusted difference −7.5 kg (95% CI −11.0 to −4.0 kg, P < 0.001).
3.2.2. Glycaemic control, insulin sensitivity and diabetes diagnostic thresholds
Mean change in HbA1c at 12 weeks was −16.3 mmol/mol (SD 13.3) in the intervention group and −0.7 (SD 4.5) in the usual care group, adjusted difference −15.7 mmol/mol (−24.1 to −7.3, P < 0.001). There was no evidence of an effect of duration of diabetes or change in the number of diabetes medications. There was a significantly greater improvement in the intervention than the control arm in all measures of glucose regulation except for steady state beta cell function (HOMA‐%B) (Table 2).
Table 2.
Secondary outcomes by group allocation, of participants assigned to the DIAMOND programme (n = 21) or usual care (n = 12)
| Outcome measure | Intervention group change, baseline to 12 weeks (mean ± SD) | Control group change, baseline to 12 weeks (mean ± SD) | Adjusted between group difference (mean difference, 95% CI) | P |
|---|---|---|---|---|
| Weight | ||||
| Weight, kg | −9.5 (5.4) | −2.0 (2.5) | −7.5 (−11.0 to −4.0) | <0.001* |
| Glycaemic control | ||||
| HbA1c, mmol/mol | −16.3 (13.3) | −0.7 (4.5) | −15.7 (−24.1 to −7.3) | 0.001* |
| Fasting glucose, mmol/L | −1.80 (3.0) | 0.40 (0.9) | −2.3 (−4.1 to −0.4) | 0.020* |
| Fasting insulin, pmol/L | −22.2 (25.6) | 6.60 (21.2) | −28.9 (−48.2 to −9.6) | 0.005* |
| HOMA | ||||
| HOMA‐%B | 5.6 (29.8) | −1.4 (11) | 7.3 (−12.1 to 26.6) | 0.449 |
| HOMA‐%S | 26.0 (29.8) | −2.7 (16.8) | 28.9 (8.5 to 49.5) | 0.007* |
| HOMA‐IR | −0.6 (0.6) | 0.1 (0.4) | −0.8 (−1.2 to −0.4) | 0.001* |
| Blood pressure | ||||
| Systolic blood pressure, mmHg | −9.6 (16.2) | 4.8 (10.6) | −14.4 (−25.8 to −3.0) | 0.010* |
| Diastolic blood pressure, mmHg | −5.3 (11.0) | 0.5 (8.8) | −6.0 (−13.7 to 1.8) | 0.130 |
| Lipid profile | ||||
| Total cholesterol, mmol/L | 0.05 (0.6) | 0.03 (0.4) | 0.02 (−0.4 to 0.5) | 0.900 |
| HDL, mmol/L | 0.06 (0.2) | −0.04 (0.2) | 0.09 (−0.04 to 0.22) | 0.160 |
| Triglycerides, mmol/L | −0.49 (0.7) | 0.09 (0.6) | −0.58 (−1.09 to −0.06) | 0.030* |
| Non‐HDL cholesterol, mmol/L | 0.08 (0.5) | 0.03 (0.2) | 0.06 (−0.25 to 0.37) | 0.700 |
| Total cholesterol: HDL ratio | −0.17 (0.7) | 0.10 (0.3) | −0.26 (−0.70 to 0.17) | 0.230 |
| Liver function tests | ||||
| Bilirubin, μmol/L | −1.4 (4.0) | −0.7 (2.6) | −0.7 (−3.5 to 2.2) | 0.635 |
| AST, IU/L | −5.6 (7.7) | −2.2 (6.8) | −3.0 (−8.9 to 2.8) | 0.296 |
| ALT, IU/L | −12.8 (16.0) | −3.5 (10.7) | −9.1 (−20.6 to 2.3) | 0.114 |
| ALP, IU/L | −1.5 (10.8) | −3.3 (8.1) | 1.9 (−6.3 to 10.0) | 0.470 |
| Albumin, g/L | 0.4 (3.0) | −0.5 (1.1) | 0.9 (−1.2 to 2.9) | 0.379 |
| GGT, IU/L | −17.2 (17.1) | −22.6 (81.8) | 5.8 (−34.7 to 46.3) | 0.771 |
| AST:ALT ratio | 0.1 (0.3) | 0.1 (0.3) | 0.03 (−0.19 to 0.25) | 0.764 |
| QRISK, % † | −2.6 (3.9) | 1.0 (1.8) | −3.6 (−6.2 to −1.0) | 0.008* |
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma‐glutamyl transferase; HDL, high density lipoprotein; HOMA, homeostatic model assessment; HOMA‐IR, insulin resistance; HOMA‐β, steady state β cell function; HOMA‐S, insulin sensitivity; QRISK, calculated 10‐year risk of cardiovascular disease.
P < 0.05.
exploratory outcome measure.
No participants in the control group changed their diabetes diagnostic status over the 12 weeks. In the intervention group, 62% of participants improved their diagnostic range of HbA1c over the 12 weeks (Appendix S3).
3.2.3. Medication changes
In the intervention group, seven participants stopped one or more diabetic medications and seven stopped one or more hypertensive medications over the 12‐week study period. There was no change in either class of drug in the control group. The adjusted difference in the number of diabetes medications was −0.4 (−0.8 to −0.001, P = 0.051) and for antihypertensive medication changes it was −0.5 (−1.0 to −0.04, P = 0.035).
3.2.4. Additional secondary outcomes
Other secondary outcomes showed somewhat more favourable changes in cardiovascular risk factors for the intervention rather than the control group, not all of which were significant (Table 2). There were no significant differences in PAID score (Table 2). Process measures are shown in Appendix S3.
3.2.5. Exploratory analyses
There was a significant improvement in QRISK3 score at 12 weeks in participants in the intervention group (adjusted between‐group difference −3.6% [−6.2 to −1.0%, P = 0.008]).
There were no statistically significant interactions between gender or diabetes control at baseline and the effect of the intervention on HbA1c (P = 0.15 and 0.07, respectively) or weight change (P = 0.32 for gender) at 12 weeks. There was a statistically significant interaction between duration of diabetes and effect of the intervention on HbA1c change at 12 weeks (P = 0.01). In the intervention group, longer duration of diabetes was associated with a smaller reduction in HbA1c (r = 0.56, P = 0.008) (Figure 2).
Figure 2.
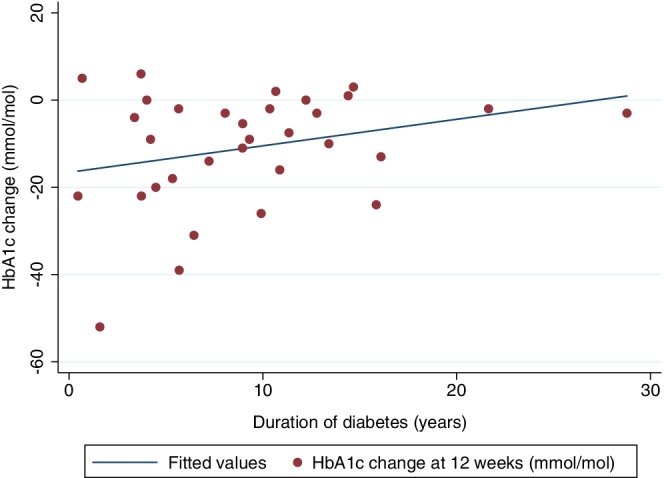
Association between duration of diabetes (years) and HbA1c change (mmol/mol) in intervention group participants (r = 0.56, P = 0.008)
3.2.6. Qualitative findings
Seven participants from the intervention group and four healthcare professionals participated in two focus groups. The key themes and data are presented in Appendix S4.
All participants found the intervention content and delivery acceptable and positive. First perceptions ranged from doubts about possible success through to enthusiasm. Participants and healthcare professionals reported growing engagement, confidence and motivation after seeing the initial rapid results. Participants reported positive impacts on their emotional and psychological well‐being and influence within their social circle.
Participants were keen to discuss their experiences with the dietary aspects of the programme, and unanimously expressed that they would not have thought, or talked, so much about individual foods, and what they were eating and cooking, prior to participating in the programme, which they reported to be a new and positive development. Key initial barriers to the real food approach were “unknown educational unknowns” (“I thought…I mean, I know how to eat healthily…[W]hat's good food and what's bad food”), or their baseline dietary and lifestyle patterns (“They gave me this recipe, you may as gave me something foreign. Because cooking and me do not go together”). However, they reported that the structured written materials, and range of strategies (from how to read food labels, and key foods to avoid or to try, through to lists of ingredients and recipes), made the programme easy to follow despite their different backgrounds and baseline dietary intake. They described integrating practical changes into their lifestyles, including shopping patterns, meal planning, or engaging partners and family members in their dietary needs.
Participants reported sustained behavioural change. They placed a high value on (i) the role of social support in their home and personal life, (ii) the role of the healthcare professionals in providing external motivation and support, and (iii) the importance of personal accountability in making the required changes. Healthcare professionals considered the follow‐up contact was popular and motivating for participants and also improved their own motivation and engagement with the intervention, and their belief that it could improve patient outcomes. However, participants and healthcare professionals recognized the necessary balance between provision of support and the use of NHS resources.
Participants described the importance of both internal motivation (wanting to change) and external motivation (stimuli from healthcare professionals and family). Healthcare professionals initially perceived that participants needed to be internally motivated to engage with the study, but also reported positive results in those who were initially externally motivated. Participants derived continuing motivation from observing measurable changes, whether objective (eg, a reduction in glucose or blood pressure) or subjective (eg, perceived changes in activity or appearance). Healthcare professionals and participants perceived that the potential to reduce or stop medications was a strong motivator for initial engagement and sustained adherence.
3.2.7. Adverse events
There were no unexpected and related serious adverse events. One death was reported in the control group, but was judged by both the site Principal Investigator and central study team to be unrelated to the study procedures.
4. DISCUSSION
In this randomized trial, we met feasibility criteria on recruitment, intervention fidelity and patient acceptability. Secondary outcomes showed a 7.5 kg and 16 mmol/mol greater improvement in weight and HbA1c, respectively, in the intervention rather than the control group. There was patient and practitioner enthusiasm for pursuing this approach.
4.1. Strengths and limitations of this study
To our knowledge, this is the first trial to attempt energy restriction to a level similar to that used in TDR programmes (~ 800 kcal/day), with real food rather than meal‐replacement products, supported by generalists in routine care. Some effective programmes, such as the Diabetes Prevention Program, have not had the same impact as observed in the trials when delivered in primary care.15, 16 We therefore spent a considerable time developing the DIAMOND programme, working with professionals and people with diabetes to draw on their experiences to refine the training we offered professionals and the materials for participants. The programme made extensive use of recipes supported by shopping cards, food label advice and portion size guidance to facilitate adherence and obviate the need for professionals to have detailed nutritional knowledge. This approach appears to have been effective and acceptable, producing high engagement from practitioners, and drew on the strong bond between patients and the healthcare professionals who deliver routine care to produce good follow‐up and adherence to the intervention. However, it remains to be seen whether the DIAMOND programme delivers these results when implemented more widely in the definitive trial that is needed to test the long‐term impact in people with diabetes.
It is impossible to blind most behavioural interventions. This could mean that some of the effect achieved was a result of the novelty of the approach, which may fade over time or if this programme ever became an accepted part of diabetes treatment. One consequence of lack of blinding was that three of 12 participants in the control group reported a low‐carbohydrate diet at follow‐up, perhaps prompted by the participant information sheet, which indicated this was the diet under test. A future trial may need to amend this to reduce this source of contamination, which would otherwise underestimate between‐group differences. We did not assess for any subgroup effect of background dietary intake prior to the study on the effect of the intervention because of the small sample size. National survey data suggest the proportion of carbohydrate in the adult diet is 46% total energy (±7.5%),17 although low‐carbohydrate diets are gaining popularity among people with type 2 diabetes and some people may have already made changes to their diet. A larger study would be needed to assess whether the response to the intervention varied by habitual diet composition. We reported on change in diabetes status as an exploratory outcome, but a longer‐term trial is needed to assess whether this approach can achieve the remission seen in TDR‐based programmes.
4.2. Comparison with other studies
In a randomized controlled trial of a TDR programme for people with obesity, mean weight loss at 3 months in the TDR arm was 13.3 kg (between‐group difference −9.6 kg [95% CI −11 to −8.2 kg] when compared with usual care).3 The slightly greater weight loss than observed in our food‐based programme may reflect the greater difficulties people may experience in adhering to the rigorous portion size restriction when preparing their own food. The HbA1c changes appear greater than observed with weight loss achieved using TDR in patients with type 2 diabetes in the DiRECT study,2 which reported a 9.6 mmol/mol reduction in HbA1c with a 10 kg weight change at 12 months. However, this may be a consequence of taking the sample at the end of the energy restriction phase, rather than at 1 year in DiRECT, when participants were, on average, regaining weight.
The short‐term reductions in HbA1c and weight shown in our study compare favourably with other food‐based interventions, which typically have provided more intensive or specialist support. A recent systematic review of low‐carbohydrate diets for patients with type 2 diabetes reported a 2.5 kg greater weight loss, and −0.19% (−2 mmol/mol) greater reduction in HbA1c, in low‐carbohydrate, rather than high‐carbohydrate diets, at 3 months.18 The interventions were heterogeneous with most not restricting total energy in the low‐carbohydrate diets, or suggesting only moderate energy restriction, and most achieved much smaller differences in energy and carbohydrate intake between intervention and control than planned. The greater average reductions in both weight and HbA1c achieved in our study probably reflect the combined focus on achieving a low‐energy and low‐carbohydrate diet and the usual care comparator.
The extent of weight loss achieved here, in a programme delivered by generalists in routine care, is especially notable given the existing evidence that weight loss advice delivered by primary care teams for people with obesity is often not effective,19 and weight losses achieved in diabetes structured education programmes are often small.20
4.3. Implications of this research
This study shows that this food‐based, low‐energy, low‐carbohydrate dietary intervention programme is feasible and practical for practice nurses to deliver in primary care. With tailored and focused participant and professional materials it is possible for a primary care‐led dietary intervention to support patients to achieve clinically significant changes in weight, at least in the short term. The next step is to design a trial with a longer‐term outcome to assess the effects on weight and glycaemic control, and cardiovascular risk factors, in people with type 2 diabetes. It will be important to try to achieve a balance between a sustainable lower carbohydrate dietary regimen and ensuring diet quality (in particular, adequate dietary fibre, given its association with cardiovascular disease outcomes).21
In conclusion, it is feasible and acceptable for practice nurses to support a food‐based, low‐energy, low‐carbohydrate dietary programme for people with type 2 diabetes in primary care with short‐term evidence of marked improvements in weight and HbA1c. This suggests a full‐scale trial is feasible and worthwhile.
CONFLICT OF INTEREST
P. A. and S. A. J. have received research grant funding but no personal remuneration from commercial weight loss companies, but none of these companies have interests in the programme described here. C. B. is the author of The 8‐week blood sugar diet recipe book and The Fast 800 recipe book, and part‐owner of thefast800.com. P. D. is a member (unpaid) of the Joint SACN/NHS England/Diabetes UK Working Group to review the evidence on lower carbohydrate diets compared with current government advice for adults with type 2 diabetes.
AUTHOR CONTRIBUTIONS
E. M., P. A. and S. A. J. developed the concept for the study and wrote the first draft of the protocol. E. M., P. A., S. A. J. and P. D. prepared the study documents and coordinated the HRA and ethics application. E. M. drafted the manuscript for publication, with input from P. A. and S. A. J. All of the authors were involved in the detailed design of the study, and approved the final protocol and manuscript.
DISCLAIMER
This report is independent research by the National Institute for Health Research. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the University of Oxford, the NIHR or the Department of Health and Social Care.
Supporting information
Appendix S1: Supporting information
ACKNOWLEDGMENTS
The authors would like to thank the members of the DIAMOND study patient participation panels for their valuable input and advice throughout all stages of study design. We also thank Sara Ryan (University of Oxford) for her advice on qualitative study design and Kathy Hoffman for her input into the study protocol.
E. M. was funded by an NIHR In‐Practice Fellowship and is currently funded by a Wellcome Trust Doctoral Research Fellowship. P. A. and S. A. J. are National Institute for Health Research (NIHR) Senior Investigators and funded by NIHR Oxford Applied Research Centre. P. A., S. A. J., G. D. T., P. D. and M. N. are supported by the NIHR Oxford Biomedical Research Centre (BRC). This work was supported by the NIHR BRC and the School for Primary Care Research (SPCR) (grant reference number 404).
Morris E, Aveyard P, Dyson P, et al. A food‐based, low‐energy, low‐carbohydrate diet for people with type 2 diabetes in primary care: A randomized controlled feasibility trial. Diabetes Obes Metab. 2020;22:512–520. 10.1111/dom.13915
Peer Review The peer review history for this article is available at https://publons.com/publon/10.1111/dom.13915.
Funding information This study was supported by the NIHR Oxford Biomedical Research Centre (BRC) and NIHR School for Primary Care Research (SPCR).
REFERENCES
- 1. Morris E, Jebb S, Aveyard P. Type 2 diabetes: treating not managing. Lancet Diabetes Endocrinol. 2019;7(5):326‐327. [DOI] [PubMed] [Google Scholar]
- 2. Lean MEJ, Leslie WS, Barnes AC, et al. Primary care‐led weight management for remission of type 2 diabetes (DiRECT): an open‐label, cluster‐randomised trial. Lancet. 2018;391:541‐551. [DOI] [PubMed] [Google Scholar]
- 3. Astbury NM, Aveyard P, Nickless A, et al. Doctor Referral of Overweight People to Low Energy total diet replacement Treatment (DROPLET): pragmatic randomised controlled trial. BMJ. 2018;362:k3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Forouhi NG, Misra A, Mohan V, Taylor R, Yancy W. Dietary and nutritional approaches for prevention and management of type 2 diabetes. BMJ. 2018;361:k2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Murdoch C, Unwin D, Cavan D, Cucuzzella M, Patel M. Adapting diabetes medication for low carbohydrate management of type 2 diabetes: a practical guide. BMJ. 2019;69:360‐361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Unwin M, Unwin J. Low carbohydrate diet to achieve weight loss and improve HbA1c in type 2 diabetes and pre‐diabetes: experience from one general practice. Pract Diabetes. 2014;31:76‐79. [Google Scholar]
- 7. James Lind Alliance . James Lind Alliance Priority Setting partnerships: Diabetes (Type 2) Top 10. http://wwwjlanihracuk/priority-setting-partnerships/diabetes-type-2/top-10-uncertaintieshtm. Accessed March 2019.
- 8. Morris E, Aveyard P, Dyson P, et al. Dietary Approaches to the Management Of type 2 Diabetes (DIAMOND): protocol for a randomised feasibility trial. BMJ Open. 2019;e026460:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arun CS, Pandit R, Taylor R. Long‐term progression of retinopathy after initiation of insulin therapy in type 2 diabetes: an observational study. Diabetologia. 2004;47:1380‐1384. [DOI] [PubMed] [Google Scholar]
- 10.DiabetesUK. What is a healthy, balanced diet for diabetes? https://wwwdiabetesorguk/guide-to-diabetes/enjoy-food/eating-with-diabetes/what-is-a-healthy-balanced-diet. Accessed September 2016.
- 11. Polonsky WH, Anderson BJ, Lohrer PA, et al. Assessment of diabetes‐related distress. Diabetes Care. 1995;18:754‐760. [DOI] [PubMed] [Google Scholar]
- 12. Levy JC, Matthews DR, Hermans MP. Correct Homeostasis Model Assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21:2191‐2192. [DOI] [PubMed] [Google Scholar]
- 13. Hippisley‐Cox J, Coupland C, Brindle P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: prospective cohort study. BMJ. 2017;357:j2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dean A, Sullivan K, OpenEpi Soe M.: Open Source Epidemiologic Statistics for Public Health, Version 3.0, updated April 2013. http://www.openepi.com. Accessed 5 September, 2019.
- 15. Dunkley AJ, Bodicoat DH, Greaves CJ, et al. Diabetes prevention in the real world: effectiveness of pragmatic lifestyle interventions for the prevention of type 2 diabetes and of the impact of adherence to guideline recommendations. Diabetes Care. 2014;37:922‐933. [DOI] [PubMed] [Google Scholar]
- 16. Vermunt PW, Milder IE, Wielaard F, de Vries JH, van Oers HA, Westert GP. Lifestyle counseling for type 2 diabetes risk reduction in Dutch primary care: results of the APHRODITE study after 0.5 and 1.5 years. Diabetes Care. 2011;34:1919‐1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Public Health England. National Diet and Nutrition Survey Results from Years 5 and 6 (combined) of the Rolling Programme (2012/2013 – 2013/2014), Public Health England and the Food Standards Agency. https://assetspublishingservicegovuk/government/uploads/system/uploads/attachment_data/file/551352/NDNS_Y5_6_UK_Main_Textpdf. Accessed November 2019.
- 18. Sainsbury E, Kizirian NV, Partridge SR, Gill T, Colagiuri S, Gibson AA. Effect of dietary carbohydrate restriction on glycemic control in adults with diabetes: A systematic review and meta‐analysis. Diabetes Res Clin Pract. 2018;139:239‐252. [DOI] [PubMed] [Google Scholar]
- 19. Jolly K, Lewis A, Beach J, et al. Comparison of range of commercial or primary care led weight reduction programmes with minimal intervention control for weight loss in obesity: lighten up randomised controlled trial. BMJ. 2011;343:d6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Davies MJ, Heller S, Skinner TC, et al. Effectiveness of the diabetes education and self management for ongoing and newly diagnosed (DESMOND) programme for people with newly diagnosed type 2 diabetes: cluster randomised controlled trial. BMJ 2008;336:491‐495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reynolds A, Mann J, Cummings J, Winter N, Mete E, Te Morenga L. Carbohydrate quality and human health: a series of systematic reviews and meta‐analyses. Lancet. 2019;393:434‐445. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting information


