Abstract
Arbuscular mycorrhizal fungi (AMF) form symbioses with most crops, potentially improving their nutrient assimilation and growth. The effects of cultivar and atmospheric CO2 concentration ([CO2]) on wheat–AMF carbon‐for‐nutrient exchange remain critical knowledge gaps in the exploitation of AMF for future sustainable agricultural practices within the context of global climate change. We used stable and radioisotope tracers (15N, 33P, 14C) to quantify AMF‐mediated nutrient uptake and fungal acquisition of plant carbon in three wheat (Triticum aestivum L.) cultivars. We grew plants under current ambient (440 ppm) and projected future atmospheric CO2 concentrations (800 ppm). We found significant 15N transfer from fungus to plant in all cultivars, and cultivar‐specific differences in total N content. There was a trend for reduced N uptake under elevated atmospheric [CO2]. Similarly, 33P uptake via AMF was affected by cultivar and atmospheric [CO2]. Total P uptake varied significantly among wheat cultivars and was greater at the future than current atmospheric [CO2]. We found limited evidence of cultivar or atmospheric [CO2] effects on plant‐fixed carbon transfer to the mycorrhizal fungi. Our results suggest that AMF will continue to provide a route for nutrient uptake by wheat in the future, despite predicted rises in atmospheric [CO2]. Consideration should therefore be paid to cultivar‐specific AMF receptivity and function in the development of climate smart germplasm for the future.
Keywords: arbuscular mycorrhizal fungi, carbon, climate change, CO2, nitrogen, phosphorus, sustainable agriculture, wheat
Arbuscular mycorrhizal fungi (AMF) form symbioses with most crops, potentially improving their nutrient assimilation with possible applications in future sustainable agriculture. However, the effects of cultivar and increasing CO2 on wheat mycorrhizal function remain unknown. Using isotope tracers, we tracked mycorrhizal function in three wheat cultivars across two CO2 scenarios. Our results suggest AMF could provide a route of nutrient uptake to wheat in the future, despite predicted rises in atmospheric CO2. Consideration should be paid to cultivar‐specific AMF receptivity and function in the development of climate smart future crops.
1. INTRODUCTION
The agricultural ‘green revolution’ of the 1950s brought dramatic increases in worldwide crop productivity, driven largely by the development and application of novel pesticides and fertilizers, coupled to advances in plant breeding. Such crop improvements are exemplified by the long‐term increase in UK wheat yields since the 1950s (Mackay et al., 2011). More recently, wheat yields have started to decline despite increasing application of nitrogen‐ and phosphorus‐based fertilizers—a widespread trend observed across many other key crop species across the globe (Grassini, Eskridge, & Cassman, 2013; Ray, Ramankutty, Mueller, West, & Foley, 2012). An ever‐increasing human population (Gerland et al., 2014), depletion of natural resources such as rock phosphate (Cordell, Drangert, & White, 2009) and rising energy prices are making fertilizer and pesticide production unsustainable. In the context of global climate change, future food security is far from assured (Godfray et al., 2010).
In recent years, there has been increasing agronomic interest in exploiting the symbiotic associations formed between crop plants and arbuscular mycorrhizal fungi (AMF; Chen, Arato, Borghi, Nouri, & Reinhardt, 2018; Sosa‐Hernandez, Leifheit, Ingraffia, & Rillig, 2019; Thirkell, Charters, Elliott, Sait, & Field, 2017). The roots of around 75% of all vascular plant species, including many cereals (Smith & Smith, 2011) form associations with the obligately biotrophic fungi of the subphyllum Glomeromycotina (Brundrett & Tedersoo, 2018; Spatafora et al., 2016; van der Heijden, Martin, Selosse, & Sanders, 2015). Host plants may allocate up to 20% of recently‐fixed carbon (C) to their AMF symbionts (Bago, Pfeffer, & Shachar‐Hill, 2000; Douds, Pfeffer, & Shachar‐Hill, 2000; Soudzilovskaia et al., 2015). On a global scale, such transfer of carbohydrates and fatty acids (Keymer et al., 2017; Luginbuehl et al., 2017) from plants to fungal partners comprises up to 5 billion tons of C annually (Bago et al., 2000), representing an important input to soil carbon stocks. In return, AMF may facilitate the acquisition of up to 80% of plant phosphorus (P; Bucher, 2007; Sawers et al., 2017; Smith, Smith, & Jakobsen, 2004), in addition to potentially making contributions towards plant nitrogen (N; Hodge, Campbell, & Fitter, 2001; Leigh, Hodge, & Fitter, 2009; Thirkell, Cameron, & Hodge, 2016) and micronutrient demand (Smith & Read, 2008). Associating with AMF may confer further benefits on host plants beyond improving access to soil nutrients, such as improving plant growth, water uptake (Ruiz‐Lozano et al., 2016) and priming of host plant defence responses (Cameron, Neal, Wees, & Ton, 2013), leading to increased tolerance and/or resistance to pests and diseases (Berdeni et al., 2018; Jung, Martinez‐Medina, Lopez‐Raez, & Pozo, 2012).
Taking consideration of AMF in widescale agricultural management decisions requires changes in current practice, although it has been argued that sufficient data corroborating the nutritional benefit of AMF in agricultural crops to warrant these shifts are currently lacking (Rillig et al., 2019; Ryan & Graham, 2018). A prevailing assertion is that cereals are generally negatively or neutrally affected by AMF colonization (Rillig et al., 2019; Smith & Smith, 2011); the fungi are assumed to offer little nutritional benefit to plants selectively bred for fine and dense root architecture optimized for nutrient‐acquisition efficiency, especially under high‐nutrient environments (Smith & Smith, 2011; Wen et al., 2019; Zheng et al., 2018). Despite two meta‐analyses suggesting an overall benefit of AMF to crop nutrient uptake and grain yield (Lekberg & Koide, 2005; Zhang, Lehmann, Zheng, You, & Rillig, 2019), a sceptical view remains in the literature with regard to the utility of AMF in modern and future agriculture (e.g. Ryan & Graham, 2018).
The functional response of plants to AMF colonization is highly diverse (Hoeksema et al., 2010) in terms of both inter‐ and intraspecificity (Johnson, Martin, Cairney, & Anderson, 2015; Jones & Smith, 2004; Mensah et al., 2015; Munkvold, Kjoller, Vestberg, Rosendahl, & Jakobsen, 2004; Watts‐Williams et al., 2019) and given the ubiquity of AMF in most agricultural soils, arable crops are far more likely to be mycorrhizal than nonmycorrhizal (Smith & Smith, 2011). As such, determining the conditions under which AMF positively influence crop nutrient uptake must remain a research priority. Plant and fungal genotype (Klironomos, 2003; Munkvold et al., 2004), the availability of mineral nutrients (Johnson, 2010; Johnson, Wilson, Wilson, Miller, & Bowker, 2015) and atmospheric conditions (Field et al., 2012) all mediate plant responses to AMF colonization.
Atmospheric CO2 concentrations ([CO2]) have increased rapidly because of anthropogenic activities since preindustrial times, from 280 ppm in 1750 to concentrations in excess of 400 ppm today (Meinshausen et al., 2011). Climate model projections suggest that atmospheric [CO2] will continue to rise, potentially reaching 800 ppm atmospheric [CO2] by the end of the century (Meinshausen et al., 2011) if steps to curb emissions are not taken. The ‘carbon fertilisation effect’ is responsible for increased rates of carbon fixation under elevated atmospheric [CO2] (hereafter eCO2), especially among C3 species in temperate zones (Ainsworth & Long, 2005; McGrath & Lobell, 2013; O'Leary et al., 2015) which include some of the world's most economically and socially important plants. As photosynthesis is not currently carbon‐limited at ambient atmospheric [CO2] (hereafter aCO2; Fitzgerald et al., 2016), plants grown at eCO2 generally show reduced photorespiratory losses and increased net photosynthetic rates. The extent to which increasing atmospheric [CO2] will impact crop–AMF associations remains unclear (Cotton, 2018). Given the key role of atmospheric [CO2] in regulating photosynthetic rate (van der Kooi, Reich, Low, Kok, & Tausz, 2016) and subsequent C metabolism, how AMF might ameliorate or accentuate any atmospheric [CO2]‐driven changes to crop growth and nutrition warrants further investigation.
As obligate symbionts, AMF are entirely reliant on their plant hosts for carbon (C) thus high atmospheric [CO2] could directly affect C allocation to mycorrhizas. Increased C acquisition by AMF has been demonstrated in a number of plant and fungal species when under eCO2 (Alberton, Kuyper, & Gorissen, 2005; Drigo et al., 2013; Field et al., 2012; Treseder, 2004). Furthermore, recent evidence even suggests that AMF carbon acquisition from host plants might directly increase rates of carbon fixation (Gavito, Jakobsen, Mikkelsen, & Mora, 2019), potentially by ameliorating end‐product inhibition of photosynthesis (Arp, 1991). Greater C acquisition by AMF may enable further hyphal proliferation through soil and thus increase their assimilation of mineral nutrients and subsequently increase transfer to host plants. However, whether this hypothetical positive feedback is realized in AMF–plant symbioses is not clearly supported by the available data (Cotton, 2018).
The nature and extent of atmospheric [CO2] effects on AMF are complex (Cotton, 2018). Increased plant N uptake via AMF under eCO2 has been demonstrated both in wild grasses, such as Avena fatua (Cheng et al., 2012) and in domesticated crop plants, including wheat Triticum aestivum L. (Zhu, Song, Liu, & Liu, 2016). In contrast, AMF‐mediated P uptake in vascular plants appears to be less affected by changes in atmospheric [CO2]. Mycorrhizal P uptake was not increased by eCO2 in Pisum sativum (Gavito, Bruhn, & Jakobsen, 2002; Gavito, Schweiger, & Jakobsen, 2003), Medicago truncatula or Brachypodium distachyon (Jakobsen et al., 2016). Similarly, Plantago lanceolata showed decreased 33P acquisition via AMF per unit of plant‐fixed carbon allocated to the fungi in eCO2 conditions (Field et al., 2012). Host plant genotype must also be considered when investigating the effect of environmental perturbation on symbiotic functioning between crops and AMF; intraspecific diversity is an important driver of variation in these interactions (Johnson, Martin, et al., 2015). As a result of intensive crop breeding to promote various economically important traits, modern crop cultivars vary in their receptiveness to colonization by AMF (Lehnert, Serfling, Enders, Friedt, & Ordon, 2017; Lehnert, Serfling, Friedt, & Ordon, 2018) and therefore potentially also vary in carbon‐for‐nutrient exchange between symbiotic partners in both aCO2 and eCO2 atmospheric conditions.
Here we address the critical research question, “How do eCO2 and plant host genotype affect carbon‐for‐nutrient exchange between wheat and arbuscular mycorrhizas?” Using 15N, 33P and 14C isotope tracers across three modern wheat (T. aestivum L.) cultivars, we determined (a) the extent to which AMF contribute to assimilation of N and P from soil, and (b) the extent to which wheat transfers C to extraradical mycelia of their fungal symbionts in three modern wheat (T. aestivum L.) cultivars at aCO2 (440 ppm) and eCO2 (800 ppm), to simulate the predicted increase in atmospheric [CO2] over the next 80 years (Meinshausen et al., 2011). Specifically, we tested the hypotheses that (a) AMF would acquire greater amounts of plant‐fixed C under future climate eCO2 scenarios, and (b) increased C allocation would increase transfer and assimilation of 15N and 33P tracers from the AMF to the plant across all cultivars tested.
2. MATERIALS AND METHODS
2.1. Wheat pregermination and AMF inoculation
Seeds of bread wheat (T. aestivum L., cv. ‘Avalon’, ‘Cadenza’, ‘Skyfall’; RAGT Seeds, Cambridgeshire, UK) were surface sterilized using Cl2 gas (Method S1) and incubated on moistened filter paper for 5 days to germinate. Avalon and Cadenza were selected as they are parent lines of a reference population currently used as a basis for improving European wheat germplasm (Ma et al., 2015), and Skyfall is currently among the United Kingdom's most commonly planted wheat cultivars. Healthy seedlings were selected and transferred to 1.5 L plant pots containing a 3:1 mix of agricultural top soil (collected on 7 December 2016 from Leeds University Farm; 53°52′30.1″N, 1°19′15.8″W) and heat‐sterilized (120 min at <120°C) soft sand (Figure S1).
To supplement the naturally occurring AMF inoculum in the field soil, an inoculum of the generalist mutualistic AMF species Rhizophagus irregularis (Kiers et al., 2011) was also added (Method S1). Homogenized inoculum was added to the sterilized sand immediately prior to mixing with the soil, with each pot receiving 10 ml of the inoculum. Spore density was quantified at 1,300 ± 100 spores per ml, such that each plant was inoculated with an additional 13,000 ± 1,000 R. irregularis spores.
2.2. Plant growth conditions
Plants were maintained in controlled environment growth cabinets (Snijder Labs) on a light cycle of 15 hr daytime (20°C and 70% humidity) and 9 hr night‐time (at 15°C and 70% humidity). Daytime PAR, supplied by LED lighting was 225 µmol m−2 s−1 at canopy level. CO2 concentrations were 440 and 800 ppm. Atmospheric [CO2] was monitored using a Vaisala sensor system (Vaisala), maintained throughout the addition of gaseous CO2. Plants were transferred between growth cabinets every 4 weeks to mitigate any cabinet effects. After 4 weeks, plants were given weekly doses of 40 ml of a low‐P preparation (containing 25% of the original P quantity) of Long Ashton solution (Smith, Johnston, & Cornforth, 1983), prepared using the nitrate formulation (Table S1). Plants were watered with tap water, as required.
2.3. 33P and 15N isotope tracing
Arbuscular mycorrhizal fungi‐mediated N and P assimilation was quantified using an approach adapted from Johnson, Leake, and Read (2001) using mesh‐walled cores, into which the 33P and 15N tracers were added. Briefly, each pot contained two mesh cores constructed from PVC tubing (length 80 mm, diameter 18 mm), with windows (approx. 50 mm × 12 mm) cut in each side (Figure S2). These windows and the bottom of each core were covered in a 20 µm nylon mesh which prevents root access but permits hyphal growth into the core contents. Nylon mesh was attached to PVC cores using Tensol® adhesive (Bostik Ltd). Two of the cores were filled with the same soil and sand substrate as the bulk soil, plus 3 g/L crushed basalt (particle size <1 mm), to act as a fungal ‘bait’ (Quirk et al., 2012). Each pot also contained a third mesh‐windowed core, loosely packed with glass wool (Acros Organics) and then the top sealed with a SubaSeal® (Perkin Elmer). This created an airtight septum through which gas sampling can be conducted with a hypodermic syringe, in order to measure belowground respiration throughout the course of the experiment.
To ensure only symbiotic fungal‐mediated tracer movement was measured, one of the mesh‐windowed soil cores in each pot was gently rotated immediately prior to isotope tracer additions, 10 weeks postplanting. This rotation severed the fungal connections between the plant and the core contents, preventing direct transfer of the isotope tracers to the host plants via extraradical mycorrhizal fungal mycelium. Core rotation was conducted every 48 hr until the end of the experiment. The second core in each pot remained static, thereby preserving the hyphal connections between the core contents and the host plant. After 10 weeks of growth, 100 µl labelling solution, containing 1 MBq 33P (as H3 33PO4, specific activity = 111 TBq/mmol; Perkin Elmer) and 46.26 µg 15N (as >98 atom% 15NH4Cl; Sigma Aldrich) was introduced to each pot. Labelling solution was added via pierced capillary tubing running down the centre of the core to ensure even distribution of tracer within the core. In half of microcosms (n = 6 per cultivar), labelling solution was added to the static core, and in the remaining microcosms (n = 6 per cultivar), to the rotated core. Cores which did not receive tracer solution were given 100 µl autoclaved distilled H2O. By subtracting the quantity of isotope tracers detected in plants from pots with severed hyphal connections to the isotope core (rotated isotope core treatment) from those where the AMF mycelium remained intact (static isotope core treatment), we were able to account for movement of isotopes caused by dissolution and diffusion and/or alternative soil microbial nutrient cycling processes.
2.4. Plant‐to‐fungus carbon transfer
Two weeks after 33P and 15N tracer additions, plants were prepared for 14CO2 labelling, to allow movement of carbon from plant to fungus to be quantified. A 110 µl solution of NaH14CO3 (Perkin Elmer) containing 1.0175 MBq 14C (specific activity = 1.621 GBq/mmol) was added to a cuvette in each pot. The tops of all mesh‐windowed cores were sealed using gas‐tight rubber septa (SubaSeal) to minimize diffusion of 14CO2 into the cores. 14CO2 gas was liberated from the NaH14CO3 by addition of 10% lactic acid, generating a 1.0175 MBq pulse of 14CO2. Samples of 1 ml above‐ground gas and 1 ml below‐ground gas (via the glass wool‐filled core) were taken 1 hr after release of 14CO2 and every 4 hr thereafter to monitor the drawdown, respiration and flux of 14C through the plant–AMF network. Gas samples were injected into gas‐evacuated scintillation vials containing 10 ml Carbosorb® (Perkin Elmer), a carbon‐trapping compound. To this, 10 ml Permafluor scintillation cocktail (Perkin Elmer) was added, and 14C content of each sample was quantified by liquid scintillation counting (Tricarb 3100TR scintillation counter; Perkin Elmer).
Pots were maintained under cabinet conditions until detection of maximum below‐ground 14C flux (20–22 hr after 14CO2 liberation) at which point 3 ml 2 M KOH was added to cuvettes within each microcosm to capture remaining gaseous 14CO2.
2.5. Harvest, sample preparation and analysis
All plant shoots, roots, bulk and core soil samples were separated, cleaned (roots only) and weighed before being immediately frozen and freeze‐dried (Scanvac Cool‐Safe freeze‐dryer; LaboGeneApS) within 24 hr. Shoot, root and soil samples were homogenized and subsamples of core and bulk soils were collected for quantification of hyphal length density. Subsections of roots were separated before freezing for quantification of mycorrhizal colonization using acidified ink (Vierheilig, Coughlan, Wyss, & Piche, 1998). Root colonization by AMF and the presence of arbuscules and vesicles was quantified by light microscopy using the protocol of McGonigle, Miller, Evans, Fairchild, and Swan (1990).
Plant phosphorus (nonradioactive) concentration was quantified by spectrophotometer assay following sulphuric acid digest. Sample P concentration was then calculated from a calibration curve constructed using known concentration of sodium dihydrogen orthophosphate. Briefly, plant root and shoot samples of known weight (30 ± 5 mg) were heated in a dry block heater (Grant Instruments) to 365°C in 1 ml 96% (v/v) sulphuric acid for 15 min. Once samples had cooled to 25°C, 0.25 ml 35% (v/v) hydrogen peroxide was added, at which point the samples turned colourless. Samples were again left to cool to 25°C. A 0.5 ml sample of this digest product was transferred to a 4 ml spectrophotometry cuvette, together with 0.2 ml 0.1 M l‐ascorbic acid (C6H8O6), 0.2 ml 3.44 M NaOH to neutralize acidity and 0.5 ml of a developer solution. The developer solution was prepared by dissolving 4.8 g of ammonium molybdate ((NH4)6Mo7O24.4H2O) and 0.1 g antimony potassium tartrate (C6H4O7SbK) in 250 ml 2 M H2SO4, which was then diluted to 500 ml with distilled water. The volume of sample in the cuvette was made up to 3.8 ml and samples were kept in the dark for 45 min, after which absorbance was measured at 882 nm using a Jenway 6300 spectrophotometer (Cole‐Palmer).
2.6. Quantification of carbon‐for‐nutrient exchange between plants and AMF symbionts
Shoot and root 33P content was quantified using aliquots of the digest product described above. About 1 ml aliquots of this digested product were mixed with 10 ml Emulsifier‐Safe (Perkin Elmer) and 33P was quantified by liquid scintillation counting. About 4 mg (±2 mg) of shoot and root tissue from all plants was weighed for analysis for 15N content by continuous‐flow mass spectrometry (PDZ Europa 2020 Isotope Ratio Mass Spectrometer coupled to PDZ ANCA GSL preparation unit). Data were collected as atom% 15N and %N using unlabelled plants for background detection. Quantification of plant 15N was calculated following the methods of Cameron, Leake, and Read (2006). About 15 mg (±2 mg) of shoot and root tissue, and (40 ± 5 mg) soil from static and rotated cores, and the bulk soil was subsampled for 14C quantification by liquid scintillation counting, following combustion using a sample oxidizer (Packard 307 Sample Oxidiser; Perkin‐Elmer).
Following the methods of Cameron, Johnson, Read, and Leake (2008), total C fixed by the plant and subsequently acquired by the fungus was calculated as a function of total CO2 volume in the labelling chamber and the proportion of the 14CO2 which was fixed by wheat plants over the labelling period (Figure S1). Comparing 14C quantities in static versus rotated cores for each pot allows calculation of C acquisition by the fungi, controlling for 14C detected due to root exudation or respiration, or alternative microbial carbon cycling processes.
2.7. Statistics
Statistical analyses were carried out using ‘R’ statistical software, version 3.4.3. (R Core Team, 2017), implemented within the RStudio graphical user interface (RStudio Team, 2015). Data were tested by two‐way ANOVA, where the cultivar and atmospheric [CO2] were used as predictor variables. Where ANOVA gave p < .05 for the main effects, Tukey post hoc tests were used to identify statistical differences between groups. Prior to running analyses, data were tested for normality using Shapiro–Wilk test and by visual inspection of residual plots. Where data did not pass assumptions of normality and homogeneity of variance, data were log10 transformed. Following results from Akaike information criterion (AIC) testing which showed better model fit, data were log‐transformed prior to statistical analysis.
3. RESULTS
3.1. Elevated [CO2] increases above‐ground wheat growth and frequency of intraradical mycorrhizal structures
Plants grown under eCO2 (800 ppm) had on average 14% greater shoot biomass than those grown in aCO2 (440 ppm; Figure 1a; F 5,71 = 16.33, p < .001), although among cultivars this response was only significant for Skyfall (Tukey test: p = .009). Mean cultivar shoot biomass ranged from 1.15 ± 0.04 g in Avalon grown at ambient [CO2] to 1.86 ± 0.30 g in Skyfall grown at eCO2. Root biomass did not respond to atmospheric [CO2] or cultivar (Figure 1b).
Figure 1.
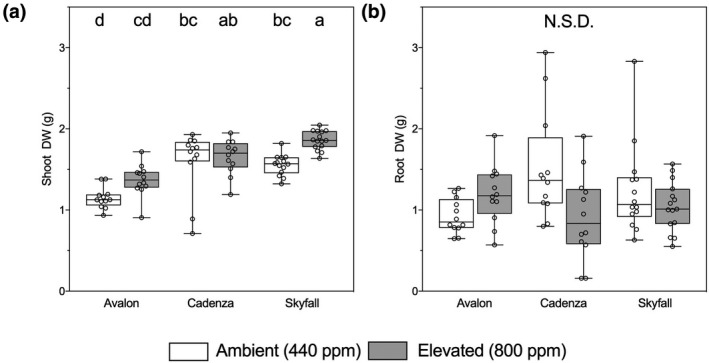
Root (a) and shoot (b) dry weight (g) of wheat (Triticum aestivum L., cv. Avalon, Cadenza, Skyfall) grown at ambient (440 ppm, white boxes) and elevated (800 ppm, grey boxes) CO2. Bars sharing letters are not significantly different, where p > .05 (ANOVA, Tukey post hoc test). Data were log10 transformed where data assumptions were not met. N.S.D., not significantly different
All plants were colonized by AMF, with significant variation among cultivars in terms of per cent root length colonized (RLC; Figure 2a; F 2,74 = 5.024, p < .01). Cadenza had significantly lower mean RLC (58.5 ± 3.5%) than Avalon (71.5 ± 2.2%), while mean Skyfall colonization (62.5 ± 2.8%) was not significantly different from either of the other cultivars (Tukey test: p > .05). The frequency of arbuscules was not affected by cultivar or atmospheric [CO2] although there was a significant interaction between these factors (Table S3), driven by reduced arbuscule frequency at eCO2 in Skyfall (Tukey: p < .001; Figure 2b). There is a trend towards greater vesicle abundance in wheat–AMF symbioses at 800 ppm than 440 ppm [CO2] across cultivars (Figure 2c), although this is not statistically significant.
Figure 2.
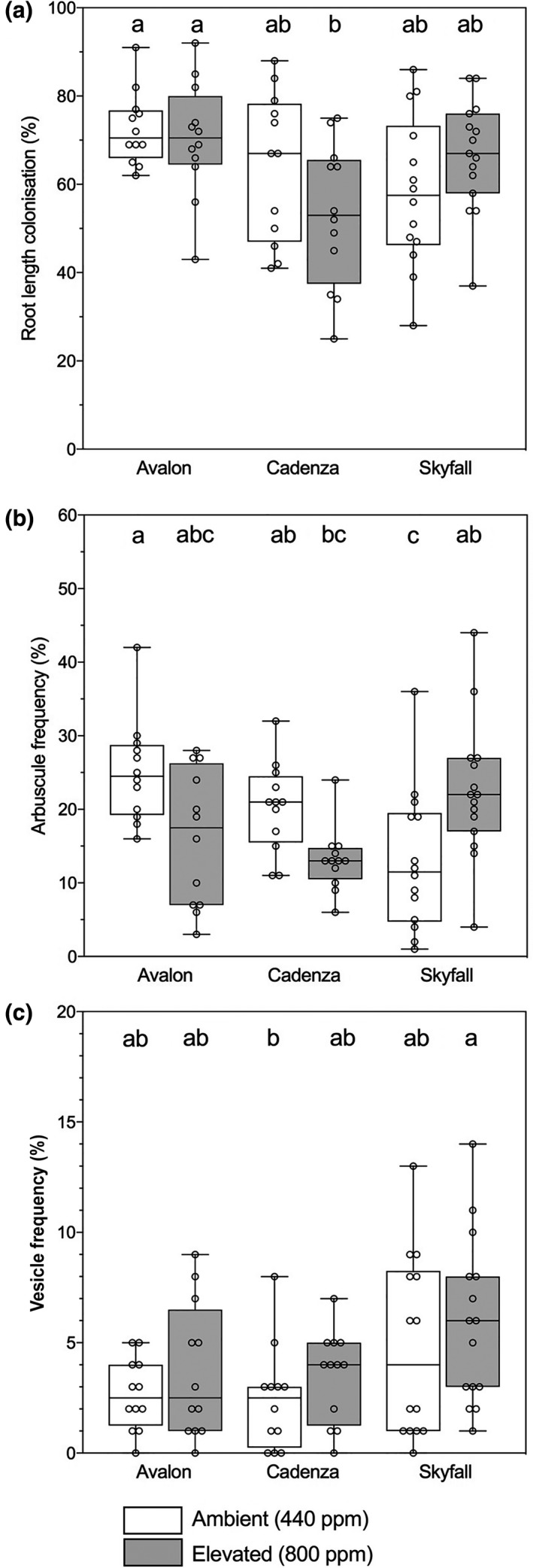
Root colonization (a), arbuscule frequency (b) and vesicle frequency (c) in roots of wheat (Triticum aestivum L., cv. Avalon, Cadenza, Skyfall) collected at harvest. Plants were grown at ambient (440 ppm, white boxes) and elevated (800 ppm, grey boxes) CO2. Bars sharing letters are not significantly different, where p > .05 (ANOVA, Tukey post hoc test). Data were log10 transformed where data assumptions were not met
3.2. Cultivar and aCO2 drive differences in plant P and mycorrhizal‐acquired 33P
There are strong effects of cultivar and atmospheric [CO2] effects on P content in shoots (F 5,70 = 38.96, p < .001; Figure 3a). P content in Cadenza shoots was 196% greater than in Avalon shoots, and 137% higher than in Skyfall shoots. Similarly, P concentration in Cadenza shoots was 186% higher than in Avalon shoots, and 153% higher than in Skyfall shoots. Cadenza plants grown at eCO2 had the highest shoot P content and concentration of all cultivars for both atmospheric [CO2] treatments (Figure 3a,b). Root P content varied significantly by cultivar (F 2,73 = 9.935, p < .001) but not CO2 concentration (p > .05). Combining data for CO2 treatments, Cadenza had the highest root P content (4.58 ± 0.49 mg), compared to Skyfall (3.33 ± 0.24 mg) and Avalon (2.34 ± 0.19 mg). Similarly, root P concentration was not affected by atmospheric [CO2], but varied by cultivar (F 2,73 = 42.68, p < .001; Table S1). Avalon has significantly lower P concentration (2.08 ± 0.10 mg/g DW) in the roots than Skyfall (3.04 ± 0.12 mg/g DW) or Cadenza (3.86 ± 0.16 mg/g DW).
Figure 3.
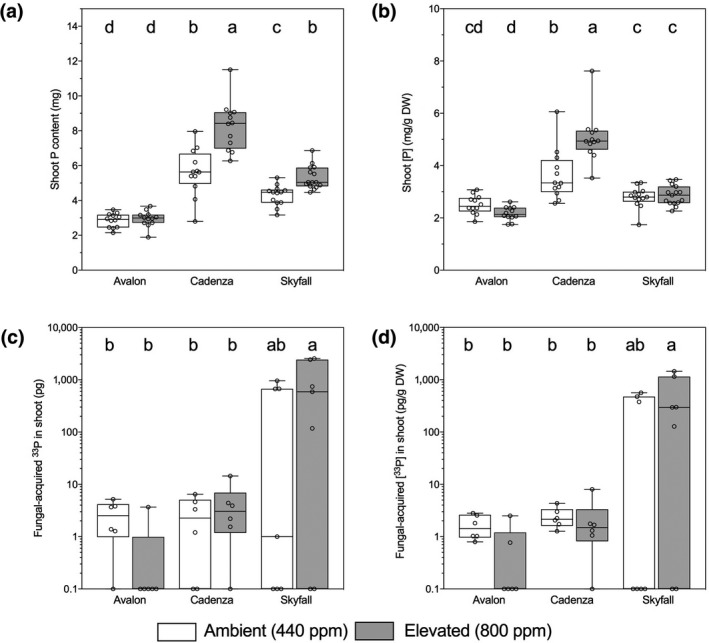
Shoot phosphorus (P) content (a) and concentration (b) of wheat (Triticum aestivum L., cv. Avalon, Cadenza, Skyfall) grown at ambient (440 ppm, white boxes) and elevated (800 ppm, grey boxes) CO2. Shoot content (c) and concentration (d) of fungal‐acquired 33P. Plants were grown at ambient (440 ppm, white boxes) and elevated (800 ppm, grey boxes) CO2. Bars sharing letters are not significantly different, where p > .05 (ANOVA, Tukey post hoc test). Data were log10 transformed where data assumptions were not met
Plant assimilation of fungal‐acquired 33P tracer in cultivars Avalon and Cadenza (content and concentration; Figure 3c,d; Table S3) was reduced in eCO2 treatment, but slightly increased in Skyfall, although these trends were not statistically significant. There was high variability in 33P tracer uptake by Skyfall, requiring log10 transformation of the data to meet the assumptions of ANOVA. There were clear differences between cultivars in terms of 33P acquisition via mycorrhizas. Combining data from eCO2 and aCO2, Skyfall acquired 570 times more 33P tracer than Avalon and 225 times more than Cadenza (Figure 3c,d).
3.3. Cultivar‐specific differences in plant‐acquired N, but not mycorrhizal‐acquired 15N tracer
Elevated atmospheric [CO2] significantly decreased shoot N content in Cadenza (Tukey: p < .001; Figure 4a; Table S3) but not in Avalon and Skyfall. Cultivars also showed significant variation in shoot N content (Figure 4a; Table S3). Avalon shoots contained significantly lower N content than Cadenza (Tukey: p < .001) and Skyfall (Tukey: p < .001), while Cadenza and Skyfall did not significantly differ (p > .05). eCO2 also had a significant negative effect on shoot N concentration (F 1,74 = 11.09, p = .001; Figure 4b), driven by large decreases in shoot N concentration in Cadenza (Tukey: p < .01) and Skyfall (Tukey: p < .01).
Figure 4.
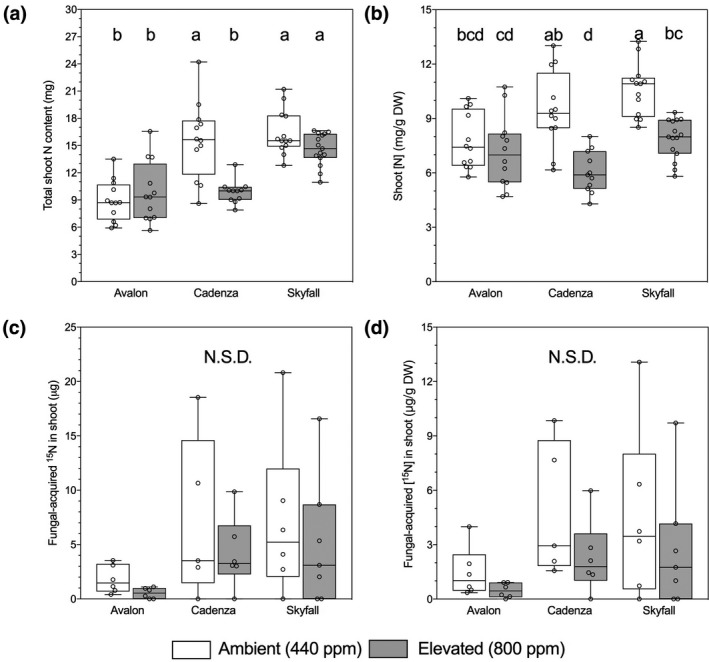
Shoot nitrogen (N) content (a) and concentration (b) of wheat (Triticum aestivum L., cv. Avalon, Cadenza, Skyfall) grown at ambient (white boxes) and elevated CO2 (black boxes). Shoot content (c) and concentration (d) of fungal‐acquired 15N. Plants were grown at ambient (440 ppm, white boxes) and elevated (800 ppm, grey boxes) CO2. Bars sharing letters are not significantly different, where p > .05 (ANOVA, Tukey post hoc test). Data were log10 transformed where data assumptions were not met. N.S.D., not significantly different
There were no significant differences among cultivars in total mycorrhizal‐acquired 15N tracer or concentration in shoots (Figure 4c,d), although there was a trend (not significant) across all three cultivars for greater 15N content in plants grown at aCO2 compared to those grown under the eCO2 treatment. 15N content and concentration of roots was not affected by atmospheric [CO2] or cultivar (Table S3).
3.3.1. Cultivar‐specific carbon allocation to fungal partners
All plants in both atmospheric [CO2] treatments transferred small amounts of carbon to the extraradical mycelium of their fungal symbionts (Figure 5a,b). The amounts were not significantly different between atmospheric [CO2] treatments in terms of per cent of carbon fixed during the labelling period allocated to the symbiotic fungi within the soil core (Figure 5a) or total amount of C transferred to extraradical fungal mycelium (ERM) within the pot (Figure 5b). However, there were trends suggestive of cultivar‐specific responses to eCO2 with per cent allocation of recent photosynthate and total amount of C transferred to fungal partners being greater at eCO2 than at aCO2 in cv. Avalon, lower in cv. Cadenza and unchanged in cv. Skyfall (Figure 5a,b). The hyphal length density in the bulk soil showed significant variation between cultivars (Tables S2 and S3; F 2,31 = 15.79, p < .001); Avalon supported significantly less extraradical mycelium than Skyfall.
Figure 5.
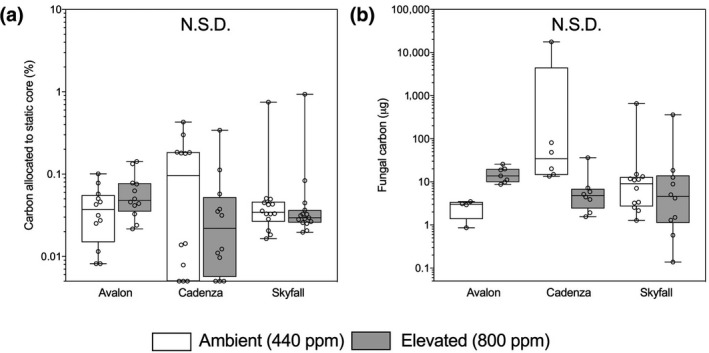
Total carbon transferred from wheat (Triticum aestivum L., cv. Avalon, Cadenza, Skyfall) to fungal mycelium during the course of 14C labelling experiment (a), and per cent of carbon fixed during the labelling period which was recovered in the static core at harvest (%) (b). Plants were grown at ambient (440 ppm, white boxes) and elevated (800 ppm, grey boxes). Bars sharing letters are not significantly different, where p > .05 (ANOVA, Tukey post hoc test). Data were log10 transformed where data assumptions were not met. N.S.D., not significantly different
3.3.2. Carbon‐for‐nutrient transfer between wheat and AMF
Carbon for nutrient transfer between plants and AMF was tested using Spearman's rank correlation coefficient (Figure S3). Overall, there was no correlation between fungal carbon acquisition and fungal transfer of 33P (r s(34) = 0.025, p = .89) or 15N (r s(34) = 0.067, p = .070) to host plants. There was also no correlation between the amounts of N and P transferred to host plants by AMF (r s(34) = 0.18), p = .30). Spearman rank tests were also carried out on subset data, grouped by CO2 concentration, cultivar and factorial permutations of these. In no cases were there correlations between the nutrients transferred (data not shown).
4. DISCUSSION
Global atmospheric [CO2] is predicted to increase through the 21st century, and the effects of this change on crops remains uncertain. Maximizing the physiological benefits eCO2 may bring, such as increased photosynthetic rates (Ainsworth & Long, 2005), while minimizing deleterious effects such as reduced plant tissue nutrient concentration, presents a significant challenge. How far AMF may be useful in tackling this challenge, and their utility in wider agriculture generally, remains unclear (Cotton, 2018; Rillig et al., 2019; Ryan & Graham, 2018; Ryan, Graham, Morton, & Kirkegaard, 2019).
Significant variation in growth responses to colonization by AMF has previously been identified across cereal varieties (Hetrick, Wilson, & Cox, 1992; Lehnert et al., 2018; Watts‐Williams et al., 2019). Such genotypic differences in growth resulting from AMF symbioses are likely to be linked not only to the receptivity to fungal colonization, but also to the physiological function of the AMF associations, particularly the degree to which the fungal symbionts represent a carbon sink (Walder et al., 2012) and nutrient source (Watts‐Williams et al., 2019) for their host plants. The stoichiometry of the bidirectional exchange of plant carbon for fungal‐acquired nutrients characteristic of AM symbioses between cereals and AMF has, until now, remained unquantified.
4.1. Carbon outlay by wheat to AMF is unaffected by atmospheric [CO2]
In our experiments, plant biomass increased in eCO2 (Figure 1b). However, the C transferred to the extraradical mycelium, in terms of both total amounts, and per cent of recently fixed photosynthate, was not affected (Figure 5). This suggests that transfer of plant C to fungal symbionts in our experiments was not limited by availability of plant‐fixed C and that allocation of C to AMF by wheat is independent of its own C demand for growth. Plant photosynthates are used by AMF symbionts to build fungal structures both inside and outside their host plant cells. ERM is formed using carbon resources throughout the growth of both plant and fungal symbionts, and so the extent of fungal mycelium may be used to indicate the relative C allocation to fungal symbionts across a longer time period than the isotope tracing alone. We found no differences in ERM density between atmospheric [CO2] treatments (Tables S2 and S3) which supports our finding that atmospheric [CO2] does not affect wheat C allocation to fungal mycelium and that this is true across cultivars. However, there are strong effects of cultivar (Table S3) with greater C allocation to mycorrhizal fungi by Avalon compared to Cadenza or Skyfall plants over the course of the experiment.
Intracellular plant–fungal interfaces are formed, and degenerate, throughout the lifetime of the symbiosis. As such, the abundance of these structures, particularly those believed to serve fungal storage organs, may be used to infer relative plant carbon investment (Müller, Ngwene, Peiter, & George, 2017) over a longer period of time than the instantaneous measurements made through the isotope tracing approach used here. The frequency of vesicles, as fungal lipid stores, may be indicative of AMF carbon acquisition (Smith, Grace, & Smith, 2009). In our experiments, vesicle frequency did not differ between atmospheric [CO2] treatments (Figure 2c). Thus, it appears that there was no ‘carbon fertilisation effect’ of eCO2 for wheat‐associated AMF (Alberton et al., 2005). The lack of atmospheric [CO2] response in terms of AMF C acquisition observed in our experiments runs counter to the trends observed in meta‐analyses (Alberton et al., 2005; Treseder, 2004) and other experimental studies (Field et al., 2012). Intensive modern breeding programmes which have given rise to elite wheat cultivars such as those used in our experiments may be responsible for the lack of atmospheric [CO2] effect on AMF C acquisition. To maximize nutrient uptake efficiency in systems where fertilizer nutrients are applied in readily available forms (Good & Beatty, 2011), modern elite cereals are bred to have reduced root‐to‐shoot ratios compared to older cultivars (Siddique, Belford, & Tennant, 1990). Those cultivars with large root systems where nutrients are easily acquired could be viewed by breeders as C‐inefficient, as C allocated to below‐ground growth could be retained above‐ground. To this end, the allocation of C to mycorrhizas and ERM may have been inadvertently selected against in the breeding of modern cereal cultivars. Alternatively, the apparent lack of atmospheric [CO2] response observed here may be partly due to plant and fungal C allocation to AMF spores not being quantified in the present investigation; it is possible that under eCO2 the AMF produced greater number of spores than in aCO2. This would not have been quantified in our experiment given the relatively short 14CO2 labelling period, and might also account for a significant fraction of fungal C. In addition, AMF hyphal turnover is thought to be rapid (Staddon, Ramsey, Ostle, Ineson, & Fitter, 2003) and may represent a significant source of C input to soils (Godbold et al., 2006). Respiratory losses of hyphal‐derived C would not be quantifiable in our experimental approach. How atmospheric [CO2] affects hyphal turnover in AMF associated with crop plants remains to be determined.
The amounts of C allocated to AMF by the wheat cultivars in these experiments are similar to those recorded in comparable experiments with noncrop vascular plants (Field et al., 2012). However, only a small fraction of the total C fixed during the experimental period by the various wheat cultivars here was allocated to their fungal mycelium (Figure 5b), regardless of the availability of C in the atmosphere. Adding 14CO2 to an enclosed system, such as the labelling chamber in our experiments, inevitably leads to an increased CO2 concentration which would impact plant physiology. However, the addition of 1.1 MBq of 14CO2 to our labelling chambers increased the concentration of atmospheric [CO2] within the chambers by 1.24% in aCO2 and 0.36% in eCO2 treatments. This slight increase in atmospheric [CO2] is unlikely to have elicited a substantial physiological response in the plants used in our experiment. Given that our plants were only able to fix and assimilate 14CO2 for one photoperiod, it is likely that the amount of C measured by the isotope tracing was not reflective of total plant carbon allocation to symbiotic fungi across the life cycle of the plant; this warrants further investigation. Despite this, our experiment provides valuable insights into the allocation of recently fixed C to fungal symbionts of wheat during a period of rapid plant growth and high nutrient demand.
4.2. Cultivar‐specific wheat nutrient gains via mycorrhizas
All cultivars assimilated 15N and 33P via their mycorrhizal symbionts, with the amounts of each tracer varying according to the cultivar. Skyfall assimilated the most mycorrhizal‐acquired 33P tracer compared to cv. Avalon and Cadenza (Figure 4c,d). This pattern of nutrient gain from AMF is not reflected in the total nutrient content or concentration of plant tissues across cultivars (Figure 4a,b). Cadenza contains the most P, both fungal‐ and plant‐acquired, in its above‐ground tissues (Figure 4a,b) but it is cv. Skyfall that acquires the most 33P tracer via AMF symbionts. This pattern may be reflective of variation in nutrient acquisition strategies across the cultivars tested. Cadenza has the greatest P concentration of above‐ground tissues (Figure 4b), but lower AMF‐assimilated tracer content (Figure 4c) and concentration (Figure 4d) than other cultivars and thus appears to operate a more effective plant P assimilation pathway than cv. Skyfall, which appears to rely more heavily on the mycorrhizal pathway for nutrient acquisition (Smith, Smith, & Jakobsen, 2003; Smith et al., 2004). With the highest levels of AMF colonization (Figure 2a) and extraradical mycelial density (Table S2), but the lowest AMF contribution to 33P uptake (Figure 3c,d) and lowest above‐ground dry mass (Figure 1a), it appears that Avalon forms a less nutritionally mutualistic interaction with AMF than the other two culitvars tested, potentially resulting in suppression of growth. This observation is unlikely a result of the AMF exerting an excessive carbon “drain” given that cv. Avalon does not allocate more C to its AMF than the other cultivars tested (Figure 5), and that the percentage of C allocated to AMF by wheat is low compared to other plants (e.g. Field et al., 2012). Instead, it is possible that downregulation of plant phosphate transporters following AMF colonization may be partly responsible, and as a result, plant P uptake is reduced relative to the nonmycorrhizal counterpart (Li, Smith, Dickson, Holloway, & Smith, 2008). As we do not have nonmycorrhizal treatments to compare nutrient acquisition and growth in these cultivars against, it is not possible to determine whether AMF suppress growth of cv. Avalon but this certainly warrants further research.
Mycorrhiza‐mediated uptake of 33P and 15N tracers was not significantly influenced by atmospheric [CO2] in any of the cultivars tested (Figures 3c,d and 4c,d). This finding is counter to some modelling predictions (Bever, 2015) and some experimental data (Field et al., 2012) but is broadly in agreement with experiments conducted in Pisum (Gavito et al., 2002, 2003), Brachypodium and Medicago (Jakobsen et al., 2016) which also showed little effect of atmospheric [CO2] on AMF‐acquired plant nutrient assimilation. Increased total P content (i.e. plant‐ and mycorrhizal‐acquired) at eCO2 compared to aCO2 treatment in shoots of cvs. Skyfall and Cadenza is counter to the general observation that P, like N, is usually relatively diluted in plant tissues at eCO2 owing to increased plant biomass (Jakobsen et al., 2016). Increased P uptake at eCO2 is not unprecedented, however (Campbell & Sage, 2002), it may be due to changes in root morphology (Nie, Lu, Bell, Raut, & Pendall, 2013). Our 33P labelling suggests that the AMF were not responsible for this increased P uptake (Figure 4c,d).
Plant tissue N content and concentration may be reduced when plants are grown in eCO2 conditions, as a result of increasing plant biomass (Cotrufo, Ineson, & Scott, 1998; Hogy & Fangmeier, 2008; Taub, Miller, & Allen, 2008). This trend is apparent in cv. Cadenza and Skyfall plants in our experiments, although not in Avalon (Figure 3a,b). The phenomenon of reduced N content of arable crops has potentially serious implications for the nutritional quality of grain and grain‐based food products (Pleijel & Uddling, 2012). Here we show that symbiotic fungal contributions to plant N assimilation are similar across atmospheric [CO2] treatments and cultivars, suggesting that AMF‐acquired N in wheat may not increase as atmospheric [CO2] increases in the future. Alarmingly, a recent study suggests that although increased N fertilizer application is capable of increasing yields in elevated [CO2] atmospheres, it is incapable of maintaining N concentrations of plant tissues comparable to those achieved at aCO2 (Pleijel, Broberg, Hogy, & Uddling, 2019).
Our data support plant/cultivar identity as an important driver of mycorrhizal benefit to plant hosts (Field & Pressel, 2018; Klironomos, 2003; Walder & van der Heijden, 2015). However, our data do not support the notion that carbon for nutrient exchange between wheat and AMF are governed by a linear, ‘reciprocal rewards’ model of mutualism (Bever, 2015; Fellbaum et al., 2012; Kiers et al., 2011) as previously shown using Petri dish‐based microcosm systems (e.g. Kiers et al., 2011) or single AMF species inoculation (e.g. Fellbaum et al., 2012). In our systems, where plants were grown in a nonsterile soil‐based substrate, inoculation with R. irregularis from root organ cultures is likely to have resulted in a mixed intraradical AMF community of multiple species, probably dominated by R. irregularis. As such, our experimental strategy does not permit us to comment on the influence of fungal identity on wheat–AMF function beyond there being a mixed AMF community present here.
There was no correlation between assimilation of fungal‐acquired nutrients in wheat in our experimental microcosms and C transfer to fungal partners across all cultivars tested, regardless of the availability of CO2 for photosynthesis. The exchange of wheat carbon for fungal‐acquired nutrients observed here may be better explained by differences in plant–fungal receptiveness and compatibility (Walder & van der Heijden, 2015). Given that our experiments were conducted using a nonsterilised agricultural soil, there were likely additional interactions and feedbacks with soil microbes and fungi that may have influenced carbon‐for‐nutrient exchange dynamics with factors such as soil microbial community composition playing an influential role.
Inter‐ and intraspecific genetic variation in plants and their AMF symbionts has been identified as sources of functional diversity in arbuscular mycorrhizal symbiosis (Johnson, Martin, et al., 2015; Watts‐Williams et al., 2019). In complex systems such as these, disentangling the causes of variation in plant–fungal environment interactions can prove challenging. For instance, Watts‐Williams et al. (2019) demonstrated that the expression of a suite of assorted genes in Sorghum bicolor was dependent not only upon AM fungal identity but also S. bicolor cultivar identity. Furthermore, these effects were not seen exclusively in genes involved directly in symbiosis; there was altered expression in genes linked to defence response, stress response and maturation onset (Watts‐Williams et al., 2019). Further crop traits which are variable among cultivars, such as phosphorus use efficiency, may also determine the extent to which AMF are beneficial for host plants (Smith & Smith, 2011). Perhaps surprisingly, root architecture traits may have limited effects on a plant's nutritional and growth response to mycorrhization (Maherali, 2014). Inter‐ and intraspecific functional diversity is also present in AMF species (Jones & Smith, 2004; Mensah et al., 2015; Munkvold et al., 2004; Watts‐Williams et al., 2019). By using unsterilized soil in our experiment, our experimental plants are likely to have been colonized by a mixed community of AMF, where the relative contributions of individual species or isolates cannot be ascertained. As AMF community structure is understood to impact symbiotic function (Frew, 2019; Smith et al., 2004; van der Heijden et al., 1998), this is of great potential agronomic interest. Understanding the role of genetic variability in plant–fungal interactions to the point where it can begin to help informing agriculture will likely prove to be a substantial, but ultimately worthwhile, undertaking (Johnson, Martin, et al., 2015). Metagenomic techniques should identify species and intraspecific diversity of the AMF present within field‐crop plant roots, combined with functional studies to determine the role these fungi play in crop nutrient uptake or other non‐nutritional beneficial roles. As illustrated by the present investigation, further factors to consider include the effects of abiotic factors on AMF community structure and diversity. Recent field‐scale atmospheric [CO2] manipulation has shown how CO2 enrichment can affect AMF community composition (Cotton, Fitter, Miller, Dumbrell, & Helgason, 2015; Maček et al., 2019). How these atmospheric [CO2]‐driven community changes might influence the stoichiometry of carbon‐for‐nutrient exchange between symbionts in the field remains to be determined (Cotton, 2018).
4.3. Future perspectives
Our results, and those of other studies investigating mycorrhizal responses to eCO2, must be contextualized with the likelihood that climate change will encompass shifts in multiple abiotic variables. Factors such as N deposition, warming and drought are at least as important an influence on AMF as atmospheric [CO2] (Kivlin, Emery, & Rudgers, 2013). Our data demonstrate that AMF will continue to provide N and P nutrition to their plant hosts under eCO2 and that there is no evidence for significant C drain from the fungi. Whether these trends are seen following simultaneous perturbations of temperature, water availability and N deposition in crop plants is not clear, as experimental testing of such scenarios is lacking.
While AMF may not prove to be the silver bullet, ‘sustainable saviours’ for agricultural intensification (Thirkell et al., 2017), our experiments have demonstrated that AMF do have the potential to contribute to cereal nutrient assimilation. As such, AMF could have an important role to play in reducing application of N‐ and P‐based fertilizers as part of a wider strategy for sustainable soil management. We echo calls for further field scale experimentation of the function of AMF in crop plants to determine what role, nutritional or otherwise, AMF might be playing in crop growth in situ (Lekberg & Helgason, 2018; Rillig et al., 2019). To date, very little work has been carried out on crop breeding to optimize mycorrhizal benefit. Given the potential influence of AMF on plant nutrient uptake and growth (Klironomos, 2003) and their ubiquity in farm systems (Oehl, Laczko, Oberholzer, Jansa, & Egli, 2017; Sale et al., 2015) it appears remiss that AMF should not be considered in breeding programmes. Recent steps have been taken to investigate the genetic basis for mycorrhizal colonization (Lehnert et al., 2017) as well as mycorrhizal “benefit” and drought response in wheat (Lehnert et al., 2018), while similar efforts in other crop species have been in progress for several years (De Vita et al., 2018; Galvan et al., 2011; Kaeppler et al., 2000). Better understanding of the mechanisms underlying plant–microbial interactions remains important in the future‐proofing and sustainable intensification of agriculture.
Supporting information
ACKNOWLEDGEMENTS
We thank Michael Charters, Ashleigh Elliott, Grace Hoysted and Bev Merry for assistance with plant harvesting and sample preparation, Richard Summers and RAGT for supply of seeds, and Heather Walker at the University of Sheffield for performing IRMS analysis. This research was funded by a BBSRC Translational Fellowship award (BB/M026825/1) and a Rank Prize Funds New Lecturer Award to KJF. We thank N8 Agrifood for support to KJF and TT. We thank two anonymous reviewers and the editor for their constructive comments during peer review.
Thirkell TJ, Pastok D, Field KJ. Carbon for nutrient exchange between arbuscular mycorrhizal fungi and wheat varies according to cultivar and changes in atmospheric carbon dioxide concentration. Glob Change Biol. 2020;26:1725–1738. 10.1111/gcb.14851
REFERENCES
- Ainsworth, E. A. , & Long, S. P. (2005). What have we learned from 15 years of free‐air CO2 enrichment (FACE)? A meta‐analytic review of the responses of photosynthesis, canopy. New Phytologist, 165(2), 351–371. 10.1111/j.1469-8137.2004.01224.x [DOI] [PubMed] [Google Scholar]
- Alberton, O. , Kuyper, T. W. , & Gorissen, A. (2005). Taking mycocentrism seriously: Mycorrhizal fungal and plant responses to elevated CO2 . New Phytologist, 167(3), 859–868. 10.1111/j.1469-8137.2005.01458.x [DOI] [PubMed] [Google Scholar]
- Arp, W. J. (1991). Effects of source‐sink relations on photosynthetic acclimation to elevated CO2 . Plant Cell and Environment, 14(8), 869–875. 10.1111/j.1365-3040.1991.tb01450.x [DOI] [Google Scholar]
- Bago, B. , Pfeffer, P. E. , & Shachar‐Hill, Y. (2000). Carbon metabolism and transport in arbuscular mycorrhizas. Plant Physiology, 124(3), 949–957. 10.1104/pp.124.3.949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdeni, D. , Cotton, T. E. A. , Daniell, T. J. , Bidartondo, M. I. , Cameron, D. D. , & Evans, K. L. (2018). The effects of arbuscular mycorrhizal fungal colonisation on nutrient status, growth, productivity, and canker resistance of apple (Malus pumila). Frontiers in Microbiology, 9, 1461 10.3389/fmicb.2018.01461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bever, J. D. (2015). Preferential allocation, physio‐evolutionary feedbacks, and the stability and environmental patterns of mutualism between plants and their root symbionts. New Phytologist, 205(4), 1503–1514. 10.1111/nph.13239 [DOI] [PubMed] [Google Scholar]
- Brundrett, M. C. , & Tedersoo, L. (2018). Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytologist, 220(4), 1108–1115. 10.1111/nph.14976 [DOI] [PubMed] [Google Scholar]
- Bucher, M. (2007). Functional biology of plant phosphate uptake at root and mycorrhiza interfaces. New Phytologist, 173(1), 11–26. 10.1111/j.1469-8137.2006.01935.x [DOI] [PubMed] [Google Scholar]
- Cameron, D. D. , Johnson, I. , Read, D. J. , & Leake, J. R. (2008). Giving and receiving: Measuring the carbon cost of mycorrhizas in the green orchid, Goodyera repens . New Phytologist, 180(1), 176–184. 10.1111/j.1469-8137.2008.02533.x [DOI] [PubMed] [Google Scholar]
- Cameron, D. D. , Leake, J. R. , & Read, D. J. (2006). Mutualistic mycorrhiza in orchids: Evidence from plant‐fungus carbon and nitrogen transfers in the green‐leaved terrestrial orchid Goodyera repens . New Phytologist, 171(2), 405–416. 10.1111/j.1469-8137.2006.01767.x [DOI] [PubMed] [Google Scholar]
- Cameron, D. D. , Neal, A. L. , van Wees, S. C. M. , & Ton, J. (2013). Mycorrhiza‐induced resistance: More than the sum of its parts? Trends in Plant Science, 18(10), 539–545. 10.1016/j.tplants.2013.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell, C. D. , & Sage, R. F. (2002). Interactions between atmospheric CO2 concentration and phosphorus nutrition on the formation of proteoid roots in white lupin (Lupinus albus L.). Plant Cell and Environment, 25(8), 1051–1059. 10.1046/j.1365-3040.2002.00883.x [DOI] [Google Scholar]
- Chen, M. , Arato, M. , Borghi, L. , Nouri, E. , & Reinhardt, D. (2018). Beneficial services of arbuscular mycorrhizal fungi – From ecology to application. Frontiers in Plant Science, 9, 1270 10.3389/fpls.2018.01270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, L. , Booker, F. L. , Tu, C. , Burkey, K. O. , Zhou, L. , Shew, H. D. , … Hu, S. (2012). Arbuscular mycorrhizal fungi increase organic carbon decomposition under elevated CO2 . Science, 337(6098), 1084–1087. 10.1126/science.1224304 [DOI] [PubMed] [Google Scholar]
- Cordell, D. , Drangert, J. O. , & White, S. (2009). The story of phosphorus: Global food security and food for thought. Global Environmental Change‐Human and Policy Dimensions, 19(2), 292–305. 10.1016/j.gloenvcha.2008.10.009 [DOI] [Google Scholar]
- Cotrufo, M. F. , Ineson, P. , & Scott, A. (1998). Elevated CO2 reduces the nitrogen concentration of plant tissues. Global Change Biology, 4(1), 43–54. 10.1046/j.1365-2486.1998.00101.x [DOI] [Google Scholar]
- Cotton, T. E. A. (2018). Arbuscular mycorrhizal fungal communities and global change: An uncertain future. FEMS Microbiology Ecology, 94(11). 10.1093/femsec/fiy179 [DOI] [PubMed] [Google Scholar]
- Cotton, T. E. A. , Fitter, A. H. , Miller, R. M. , Dumbrell, A. J. , & Helgason, T. (2015). Fungi in the future: Interannual variation and effects of atmospheric change on arbuscular mycorrhizal fungal communities. New Phytologist, 205(4), 1598–1607. 10.1111/nph.13224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vita, P. , Avio, L. , Sbrana, C. , Laidò, G. , Marone, D. , Mastrangelo, A. M. , … Giovannetti, M. (2018). Genetic markers associated to arbuscular mycorrhizal colonization in durum wheat. Scientific Reports, 8(1). 10.1038/s41598-018-29020-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douds, D. D. , Pfeffer, P. E. , & Shachar‐Hill, Y. (2000). Application of in vitro methods to study carbon uptake and transport by AM fungi. Plant and Soil, 226(2), 255–261. 10.1104/pp.120.2.587 [DOI] [Google Scholar]
- Drigo, B. , Kowalchuk, G. A. , Knapp, B. A. , Pijl, A. S. , Boschker, H. T. S. , & van Veen, J. A. (2013). Impacts of 3 years of elevated atmospheric CO2 on rhizosphere carbon flow and microbial community dynamics. Global Change Biology, 19(2), 621–636. 10.1111/gcb.12045 [DOI] [PubMed] [Google Scholar]
- Fellbaum, C. R. , Gachomo, E. W. , Beesetty, Y. , Choudhari, S. , Strahan, G. D. , Pfeffer, P. E. , … Buecking, H. (2012). Carbon availability triggers fungal nitrogen uptake and transport in arbuscular mycorrhizal symbiosis. Proceedings of the National Academy of Sciences of the United States of America, 109(7), 2666–2671. 10.1073/pnas.1118650109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field, K. J. , Cameron, D. D. , Leake, J. R. , Tille, S. , Bidartondo, M. I. , & Beerling, D. J. (2012). Contrasting arbuscular mycorrhizal responses of vascular and non‐vascular plants to a simulated Palaeozoic CO2 decline. Nature Communications, 3, 835 10.1038/ncomms1831 [DOI] [PubMed] [Google Scholar]
- Field, K. J. , & Pressel, S. (2018). Unity in diversity: Structural and functional insights into the ancient partnerships between plants and fungi. New Phytologist, 220(4), 996–1011. 10.1111/nph.15158 [DOI] [PubMed] [Google Scholar]
- Fitzgerald, G. J. , Tausz, M. , O'Leary, G. , Mollah, M. R. , Tausz‐Posch, S. , Seneweera, S. , … Norton, R. (2016). Elevated atmospheric CO2 can dramatically increase wheat yields in semi‐arid environments and buffer against heat waves. Global Change Biology, 22(6), 2269–2284. 10.1111/gcb.13263 [DOI] [PubMed] [Google Scholar]
- Frew, A. (2019). Arbuscular mycorrhizal fungal diversity increases growth and phosphorus uptake in C3 and C4 crop plants. Soil Biology and Biochemistry, 135, 248–250. 10.1016/j.soilbio.2019.05.015 [DOI] [Google Scholar]
- Galvan, G. A. , Kuyper, T. W. , Burger, K. , Keizer, L. C. P. , Hoekstra, R. F. , Kik, C. , & Scholten, O. E. (2011). Genetic analysis of the interaction between Allium species and arbuscular mycorrhizal fungi. Theoretical and Applied Genetics, 122(5), 947–960. 10.1007/s00122-010-1501-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavito, M. E. , Bruhn, D. , & Jakobsen, I. (2002). Phosphorus uptake by arbuscular mycorrhizal hyphae does not increase when the host plant grows under atmospheric CO2 enrichment. New Phytologist, 154(3), 751–760. 10.1046/j.1469-8137.2002.00404.x [DOI] [PubMed] [Google Scholar]
- Gavito, M. E. , Jakobsen, I. , Mikkelsen, T. N. , & Mora, F. (2019). Direct evidence for modulation of photosynthesis by an arbuscular mycorrhiza‐induced carbon sink strength. New Phytologist, 223(2), 896–907. 10.1111/nph.15806 [DOI] [PubMed] [Google Scholar]
- Gavito, M. E. , Schweiger, P. , & Jakobsen, I. (2003). P uptake by arbuscular mycorrhizal hyphae: Effect of soil temperature and atmospheric CO2 enrichment. Global Change Biology, 9(1), 106–116. 10.1046/j.1365-2486.2003.00560.x [DOI] [Google Scholar]
- Gerland, P. , Raftery, A. E. , Sevcikova, H. , Li, N. , Gu, D. , Spoorenberg, T. , … Wilmoth, J. (2014). World population stabilization unlikely this century. Science, 346(6206), 234–237. 10.1126/science.1257469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbold, D. L. , Hoosbeek, M. R. , Lucak, M. , Cotrufo, M. F. , Janssens, I. A. , Ceulemans, R. , … Peressotti, A. (2006). Mycorrhizal hyphal turnover as a dominant process for carbon input into soil organic matter. Plant and Soil, 281, 15–24. 10.1007/s11104-005-3701-6 [DOI] [Google Scholar]
- Godfray, H. C. J. , Beddington, J. R. , Crute, I. R. , Haddad, L. , Lawrence, D. , Muir, J. F. , … Toulmin, C. (2010). Food security: The challenge of feeding 9 billion people. Science, 327(5967), 812–818. 10.1126/science.1185383 [DOI] [PubMed] [Google Scholar]
- Good, A. G. , & Beatty, P. H. (2011). Fertilizing nature: A tragedy of excess in the commons. PLOS Biology, 9(8), e1001124 10.1371/journal.pbio.1001124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassini, P. , Eskridge, K. M. , & Cassman, K. G. (2013). Distinguishing between yield advances and yield plateaus in historical crop production trends. Nature Communications, 4, 2918 10.1038/ncomms3918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetrick, B. A. D. , Wilson, G. W. T. , & Cox, T. S. (1992). Mycorrhizal dependence of modern wheat‐varieties, landraces, and ancestors. Canadian Journal of Botany, 70(10), 2032–2040. 10.1139/b92-253 [DOI] [Google Scholar]
- Hodge, A. , Campbell, C. D. , & Fitter, A. H. (2001). An arbuscular mycorrhizal fungus accelerates decomposition and acquires nitrogen directly from organic material. Nature, 413(6853), 297–299. 10.1038/35095041 [DOI] [PubMed] [Google Scholar]
- Hoeksema, J. D. , Chaudhary, V. B. , Gehring, C. A. , Johnson, N. C. , Karst, J. , Koide, R. T. , … Umbanhowar, J. (2010). A meta‐analysis of context‐dependency in plant response to inoculation with mycorrhizal fungi. Ecology Letters, 13(3), 394–407. 10.1111/j.1461-0248.2009.01430.x [DOI] [PubMed] [Google Scholar]
- Hogy, P. , & Fangmeier, A. (2008). Effects of elevated atmospheric CO2 on grain quality of wheat. Journal of Cereal Science, 48(3), 580–591. 10.1016/j.jcs.2008.01.006 [DOI] [Google Scholar]
- Jakobsen, I. , Smith, S. E. , Smith, F. A. , Watts‐Williams, S. J. , Clausen, S. S. , & Gronlund, M. (2016). Plant growth responses to elevated atmospheric CO2 are increased by phosphorus sufficiency but not by arbuscular mycorrhizas. Journal of Experimental Botany, 67(21), 6173–6186. 10.1093/jxb/erw383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, D. , Leake, J. R. , & Read, D. J. (2001). Novel in‐growth core system enables functional studies of grassland mycorrhizal mycelial networks. New Phytologist, 152(3), 555–562. 10.1046/j.0028-646X.2001.00273.x [DOI] [PubMed] [Google Scholar]
- Johnson, D. , Martin, F. , Cairney, J. W. G. , & Anderson, I. (2015). The importance of individuals: Intraspecific diversity of mycorrhizal plants and fungi in ecosystems. New Phytologist, 194, 614–628. 10.1111/j.1469-8137.2012.04087.x [DOI] [PubMed] [Google Scholar]
- Johnson, N. C. (2010). Resource stoichiometry elucidates the structure and function of arbuscular mycorrhizas across scales. New Phytologist, 185(3), 631–647. 10.1111/j.1469-8137.2009.03110.x [DOI] [PubMed] [Google Scholar]
- Johnson, N. C. , Wilson, G. W. T. , Wilson, J. A. , Miller, R. M. , & Bowker, M. A. (2015). Mycorrhizal phenotypes and the law of the minimum. New Phytologist, 205(4), 1473–1484. 10.1111/nph.13172 [DOI] [PubMed] [Google Scholar]
- Jones, M. D. , & Smith, S. E. (2004). Exploring functional definitions of mycorrhizas: Are mycorrhizas always mutualisms? Canadian Journal of Botany, 82(8), 1089–1109. 10.1139/b04-110 [DOI] [Google Scholar]
- Jung, S. C. , Martinez‐Medina, A. , Lopez‐Raez, J. A. , & Pozo, M. J. (2012). Mycorrhiza‐induced resistance and priming of plant defenses. Journal of Chemical Ecology, 38(6), 651–664. 10.1007/s10886-012-0134-6 [DOI] [PubMed] [Google Scholar]
- Kaeppler, S. M. , Parke, J. L. , Mueller, S. M. , Senior, L. , Stuber, C. , & Tracy, W. F. (2000). Variation among maize inbred lines and detection of quantitative trait loci for growth at low phosphorus and responsiveness to arbuscular mycorrhizal fungi. Crop Science, 40(2), 358–364. 10.2135/cropsci2000.402358x [DOI] [Google Scholar]
- Keymer, A. , Pimprikar, P. , Wewer, V. , Huber, C. , Brands, M. , Bucerius, S. L. , … Gutjahr, C. (2017). Lipid transfer from plants to arbuscular mycorrhiza fungi. Elife, 6, e29107 10.7554/eLife.29107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiers, E. T. , Duhamel, M. , Beesetty, Y. , Mensah, J. A. , Franken, O. , Verbruggen, E. , … Bucking, H. (2011). Reciprocal rewards stabilize cooperation in the mycorrhizal symbiosis. Science, 333(6044), 880–882. 10.1126/science.1208473 [DOI] [PubMed] [Google Scholar]
- Kivlin, S. N. , Emery, S. M. , & Rudgers, J. A. (2013). Fungal symbionts alter plant responses to global change. American Journal of Botany, 100(7), 1445–1457. 10.3732/ajb.1200558 [DOI] [PubMed] [Google Scholar]
- Klironomos, J. N. (2003). Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecology, 84(9), 2292–2301. 10.1890/02-0413 [DOI] [Google Scholar]
- Lehnert, H. , Serfling, A. , Enders, M. , Friedt, W. , & Ordon, F. (2017). Genetics of mycorrhizal symbiosis in winter wheat (Triticum aestivum). New Phytologist, 215(2), 779–791. 10.1111/nph.14595 [DOI] [PubMed] [Google Scholar]
- Lehnert, H. , Serfling, A. , Friedt, W. , & Ordon, F. (2018). Genome‐wide association studies reveal genomic regions associated with the response of wheat (Triticum aestivum L.) to mycorrhizae under drought stress conditions. Frontiers in Plant Science, 9, 1728 10.3389/fpls.2018.01728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh, J. , Hodge, A. , & Fitter, A. H. (2009). Arbuscular mycorrhizal fungi can transfer substantial amounts of nitrogen to their host plant from organic material. New Phytologist, 181(1), 199–207. 10.1111/j.1469-8137.2008.02630.x [DOI] [PubMed] [Google Scholar]
- Lekberg, Y. , & Helgason, T. (2018). In situ mycorrhizal function – Knowledge gaps and future directions. New Phytologist, 220(4), 957–962. 10.1111/nph.15064 [DOI] [PubMed] [Google Scholar]
- Lekberg, Y. , & Koide, R. T. (2005). Is plant performance limited by abundance of arbuscular mycorrhizal fungi? A meta‐analysis of studies published between 1988 and 2003. New Phytologist, 168(1), 189–204. 10.1111/j.1469-8137.2005.01490.x [DOI] [PubMed] [Google Scholar]
- Li, H. Y. , Smith, F. A. , Dickson, S. , Holloway, R. E. , & Smith, S. E. (2008). Plant growth depressions in arbuscular mycorrhizal symbioses: Not just caused by carbon drain? New Phytologist, 178(4), 852–862. 10.1111/j.1469-8137.2008.02410.x [DOI] [PubMed] [Google Scholar]
- Luginbuehl, L. H. , Menard, G. N. , Kurup, S. , Van Erp, H. , Radhakrishnan, G. V. , Breakspear, A. , … Eastmond, P. J. (2017). Fatty acids in arbuscular mycorrhizal fungi are synthesized by the host plant. Science, 356(6343), 1175–1178. 10.1126/science.aan0081 [DOI] [PubMed] [Google Scholar]
- Ma, J. , Wingen, L. U. , Orford, S. , Fenwick, P. , Wang, J. , & Griffiths, S. (2015). Using the UK reference population Avalon × Cadenza as a platform to compare breeding strategies in elite Western European bread wheat. Molecular Breeding, 35, 70 10.1007/s11032-015-0268-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maček, I. , Clark, D. R. , Šibanc, N. , Moser, G. , Vodnik, D. , Müller, C. , & Dumbrell, A. J. (2019). Impacts of long-term elevated CO2 concentrations on communities of arbuscular mycorrhizal fungi. Molecular Ecology, 28(14), 3445–3458. 10.1111/mec.15160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay, I. , Horwell, A. , Garner, J. , White, J. , McKee, J. , & Philpott, H. (2011). Reanalyses of the historical series of UK variety trials to quantify the contributions of genetic and environmental factors to trends and variability in yield over time. Theoretical and Applied Genetics, 122(1), 225–238. 10.1007/s00122-010-1438-y [DOI] [PubMed] [Google Scholar]
- Maherali, H. (2014). Is there an association between root architecture and mycorrhizal growth response? New Phytologist, 204(1), 192–200. https://doi.org/10.1111.nph.12927 [DOI] [PubMed] [Google Scholar]
- McGonigle, T. P. , Miller, M. H. , Evans, D. G. , Fairchild, G. L. , & Swan, J. A. (1990). A new method which gives an objective‐measure of colonization of roots by vesicular arbuscular mycorrhizal fungi. New Phytologist, 115(3), 495–501. 10.1111/j.1469-8137.1990.tb00476.x [DOI] [PubMed] [Google Scholar]
- McGrath, J. M. , & Lobell, D. B. (2013). Regional disparities in the CO2 fertilization effect and implications for crop yields. Environmental Research Letters, 8(1), 014054 10.1088/1748-9326/8/1/014054 [DOI] [Google Scholar]
- Meinshausen, M. , Smith, S. J. , Calvin, K. , Daniel, J. S. , Kainuma, M. L. T. , Lamarque, J.‐F. , … van Vuuren, D. P. (2011). The RCP greenhouse gas concentrations and their extensions from 1765 to 2300. Climatic Change, 109(1–2), 213–241. 10.1007/s10584-011-0156-z [DOI] [Google Scholar]
- Mensah, J. A. , Koch, A. M. , Antunes, P. M. , Kiers, E. T. , Hart, M. , & Bucking, H. (2015). High functional diversity within species of arbuscular mycorrhizal fungi is associated with differences in phosphate and nitrogen uptake and fungal phosphate metabolism. Mycorrhiza, 27(7), 533–546. 10.1007/s00572-015-0631-x [DOI] [PubMed] [Google Scholar]
- Müller, A. , Ngwene, B. , Peiter, E. , & George, E. (2017). Quantity and distribution of arbuscular mycorrhizal fungal storage organs within dead roots. Mycorrhiza, 27(3), 201–210. 10.1007/s00572-016-0741-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munkvold, L. , Kjoller, R. , Vestberg, M. , Rosendahl, S. , & Jakobsen, I. (2004). High functional diversity within species of arbuscular mycorrhizal fungi. New Phytologist, 164(2), 357–364. 10.1111/j.1469-8137.2004.01169.x [DOI] [PubMed] [Google Scholar]
- Nie, M. , Lu, M. , Bell, J. , Raut, S. , & Pendall, E. (2013). Altered root traits due to elevated CO2: A meta‐analysis. Global Ecology and Biogeography, 22(10), 1095–1105. 10.1111/geb.12062 [DOI] [Google Scholar]
- Oehl, F. , Laczko, E. , Oberholzer, H. R. , Jansa, J. , & Egli, S. (2017). Diversity and biogeography of arbuscular mycorrhizal fungi in agricultural soils. Biology and Fertility of Soils, 53(7), 777–797. 10.1007/s00374-017-1217-x [DOI] [Google Scholar]
- O'Leary, G. J. , Christy, B. , Nuttall, J. , Huth, N. , Cammarano, D. , Stockle, C. , … Asseng, S. (2015). Response of wheat growth, grain yield and water use to elevated CO2 under a Free‐Air CO2 Enrichment (FACE) experiment and modelling in a semi‐arid environment. Global Change Biology, 21(7), 2670–2686. 10.1111/gcb.12830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleijel, H. , Broberg, M. C. , Hogy, P. , & Uddling, J. (2019). Nitrogen application is required to realize wheat yield stimulation by elevated CO2 but will not remove the CO2‐induced reduction in grain protein concentration. Global Change Biology, 25(5), 1868–1876. 10.1111/gcb.14586 [DOI] [PubMed] [Google Scholar]
- Pleijel, H. , & Uddling, J. (2012). Yield vs. quality trade‐offs for wheat in response to carbon dioxide and ozone. Global Change Biology, 18(2), 596–605. 10.1111/j.1365-2486.2011.2489.x [DOI] [PubMed] [Google Scholar]
- Quirk, J. , Beerling, D. J. , Banwart, S. A. , Kakonyi, G. , Romero‐Gonzalez, M. E. , & Leake, J. R. (2012). Evolution of trees and mycorrhizal fungi intensifies silicate mineral weathering. Biology Letters, 8(6), 1006–1011. 10.1098/rsbl.2012.0503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . (2017). R: A language and environment for statistical computing. Vienna, Austria: https://www.R-project.org/ [Google Scholar]
- Ray, D. K. , Ramankutty, N. , Mueller, N. D. , West, P. C. , & Foley, J. A. (2012). Recent patterns of crop yield growth and stagnation. Nature Communications, 3, 1293 10.1038/ncomms2296 [DOI] [PubMed] [Google Scholar]
- Rillig, M. C. , Aguilar‐Trigueros, C. A. , Camenzind, T. , Cavagnaro, T. R. , Degrune, F. , Hohmann, P. , … Yang, G. (2019). Why farmers should manage the arbuscular mycorrhizal symbiosis. New Phytologist, 222(3), 1171–1175. 10.1111/nph.15602 [DOI] [PubMed] [Google Scholar]
- RStudio Team . (2015). RStudio: Integrated development for R. Boston, MA: RStudio Inc; http://www.rstudio.com/ [Google Scholar]
- Ruiz‐Lozano, J. M. , Aroca, R. , Zamarreno, A. M. , Molina, S. , Andreo‐Jimenez, B. , Porcel, R. , … Lopez‐Raez, J. A. (2016). Arbuscular mycorrhizal symbiosis induces strigolactone biosynthesis under drought and improves drought tolerance in lettuce and tomato. Plant Cell and Environment, 39(2), 441–452. 10.1111/pce.12631 [DOI] [PubMed] [Google Scholar]
- Ryan, M. H. , & Graham, J. H. (2018). Little evidence that farmers should consider abundance or diversity of arbuscular mycorrhizal fungi when managing crops. New Phytologist, 220(4), 1092–1107. 10.1111/nph.15308 [DOI] [PubMed] [Google Scholar]
- Ryan, M. H. , Graham, J. H. , Morton, J. B. , & Kirkegaard, J. A. (2019). Research must use a systems agronomy approach if management of the arbuscular mycorrhizal symbiosis is to contribute to sustainable intensification. New Phytologist, 222(3), 1176–1178. 10.1111/nph.15600 [DOI] [PubMed] [Google Scholar]
- Sale, V. , Aguilera, P. , Laczko, E. , Mader, P. , Berner, A. , Zihlmann, U. , … Oehl, F. (2015). Impact of conservation tillage and organic farming on the diversity of arbuscular mycorrhizal fungi. Soil Biology & Biochemistry, 84, 38–52. 10.1016/j.soilbio.2015.02.005 [DOI] [Google Scholar]
- Sawers, R. J. H. , Svane, S. F. , Quan, C. , Grønlund, M. , Wozniak, B. , Gebreselassie, M.‐N. , … Paszkowski, U. (2017). Phosphorus acquisition efficiency in arbuscular mycorrhizal maize is correlated with the abundance of root‐external hyphae and the accumulation of transcripts encoding PHT1 phosphate transporters. New Phytologist, 214(2), 632–643. 10.1111/nph.14403 [DOI] [PubMed] [Google Scholar]
- Siddique, K. H. M. , Belford, R. K. , & Tennant, D. (1990). Root‐shoot ratios of old and modern, tall and semidwarf wheats in a mediterranean environment. Plant and Soil, 121(1), 89–98. 10.1007/BF00013101 [DOI] [Google Scholar]
- Smith, F. A. , Grace, E. J. , & Smith, S. E. (2009). More than a carbon economy: Nutrient trade and ecological sustainability in facultative arbuscular mycorrhizal symbioses. New Phytologist, 182(2), 347–358. 10.1111/j.1469-8137.2008.02753.x [DOI] [PubMed] [Google Scholar]
- Smith, F. A. , & Smith, S. E. (2011). What is the significance of the arbuscular mycorrhizal colonisation of many economically important crop plants? Plant and Soil, 348(1–2), 63–79. 10.1007/s11104-011-0865-0 [DOI] [Google Scholar]
- Smith, G. S. , Johnston, C. M. , & Cornforth, I. S. (1983). Comparison of nutrient solutions for growth of plants in sand culture. New Phytologist, 94(4), 537–548. 10.1111/j.1469-8137.1983.tb04863.x [DOI] [Google Scholar]
- Smith, S. E. , & Read, D. J. (2008). Mycorrhizal symbiosis. London: Academic Press. [Google Scholar]
- Smith, S. E. , Smith, F. A. , & Jakobsen, I. (2003). Mycorrhizal fungi can dominate phosphate supply to plants irrespective of growth responses. Plant Physiology, 133(1), 16–20. 10.1104/pp.103.024380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S. E. , Smith, F. A. , & Jakobsen, I. (2004). Functional diversity in arbuscular mycorrhizal (AM) symbioses: The contribution of the mycorrhizal P uptake pathway is not correlated with mycorrhizal responses in growth or total P uptake. New Phytologist, 162(2), 511–524. 10.1111/j.1469-8137.2004.01039.x [DOI] [Google Scholar]
- Sosa‐Hernandez, M. A. , Leifheit, E. F. , Ingraffia, R. , & Rillig, M. C. (2019). Subsoil arbuscular mycorrhizal fungi for sustainability and climate‐smart agriculture: A solution right under our feet? Frontiers in Microbiology, 10, 744 10.3389/fmicb.2019.00744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soudzilovskaia, N. A. , van der Heijden, M. G. A. , Cornelissen, J. H. C. , Makarov, M. I. , Onipchenko, V. G. , Maslov, M. N. , … van Bodegom, P. M. (2015). Quantitative assessment of the differential impacts of arbuscular and ectomycorrhiza on soil carbon cycling. New Phytologist, 208(1), 280–293. 10.1111/nph.13447 [DOI] [PubMed] [Google Scholar]
- Spatafora, J. W. , Chang, Y. , Benny, G. L. , Lazarus, K. , Smith, M. E. , Berbee, M. L. , … Stajich, J. E. (2016). A phylum‐level phylogenetic classification of zygomycete fungi based on genome‐scale data. Mycologia, 108(5), 1028–1046. 10.3852/16-042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staddon, P. L. , Ramsey, C. B. , Ostle, N. , Ineson, P. , & Fitter, A. H. (2003). Rapid turnover of mycorrhizal fungi detemined by AMF microanalysis of 14C. Science, 300(5622), 1138–1140. 10.1126/science.1084269 [DOI] [PubMed] [Google Scholar]
- Taub, D. R. , Miller, B. , & Allen, H. (2008). Effects of elevated CO2 on the protein concentration of food crops: A meta‐analysis. Global Change Biology, 14(3), 565–575. 10.1111/j.1365-2486.2007.01511.x [DOI] [Google Scholar]
- Thirkell, T. J. , Cameron, D. D. , & Hodge, A. (2016). Resolving the 'nitrogen paradox' of arbuscular mycorrhizas: Fertilization with organic matter brings considerable benefits for plant nutrition and growth. Plant, Cell & Environment, 39(8), 1683–1690. 10.1111/pce.12667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirkell, T. J. , Charters, M. D. , Elliott, A. J. , Sait, S. M. , & Field, K. J. (2017). Are mycorrhizal fungi our sustainable saviours? Considerations for achieving food security. Journal of Ecology, 105(4), 921–929. 10.1111/1365-2745.12788 [DOI] [Google Scholar]
- Treseder, K. K. (2004). A meta‐analysis of mycorrhizal responses to nitrogen, phosphorus, and atmospheric CO2 in field studies. New Phytologist, 164(2), 347–355. 10.1111/j.1469-8137.2004.01159.x [DOI] [PubMed] [Google Scholar]
- van der Heijden, M. G. A. , Klironomos, J. N. , Ursic, M. , Moutoglis, P. , Streitwolf‐Engel, R. , Boller, T. , … Sanders, I. R. (1998). Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature, 396(6706), 69–72. 10.1038/23932 [DOI] [Google Scholar]
- van der Heijden, M. G. A. , Martin, F. M. , Selosse, M.‐A. , & Sanders, I. R. (2015). Mycorrhizal ecology and evolution: The past, the present, and the future. New Phytologist, 205(4), 1406–1423. 10.1111/nph.13288 [DOI] [PubMed] [Google Scholar]
- van der Kooi, C. J. , Reich, M. , Low, M. , De Kok, L. J. , & Tausz, M. (2016). Growth and yield stimulation under elevated CO2 and drought: A meta‐analysis on crops. Environmental and Experimental Botany, 122, 150–157. 10.1016/j.envexpbot.2015.10.004 [DOI] [Google Scholar]
- Vierheilig, H. , Coughlan, A. P. , Wyss, U. , & Piche, Y. (1998). Ink and vinegar, a simple staining technique for arbuscular‐mycorrhizal fungi. Applied and Environmental Microbiology, 64(12), 5004–5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walder, F. , Niemann, H. , Natarajan, M. , Lehmann, M. F. , Boller, T. , & Wiemken, A. (2012). Mycorrhizal networks: Common goods of plants shared under unequal terms of trade. Plant Physiology, 159(2), 789–797. 10.1104/pp.112.195727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walder, F. , & van der Heijden, M. G. A. (2015). Regulation of resource exchange in the arbuscular mycorrhizal symbiosis. Nature Plants, 1(11), 7 10.1038/nplants.2015.159 [DOI] [PubMed] [Google Scholar]
- Watts‐Williams, S. J. , Emmett, B. D. , Levesque‐Tremblay, V. , MacLean, A. M. , Sun, X. P. , Satterlee, J. W. , … Harrison, M. J. (2019). Diverse Sorghum bicolor accessions show marked variation in growth and transcriptional responses to arbuscular mycorrhizal fungi. Plant Cell and Environment, 42(5), 1758–1774. 10.1111/pce.13509 [DOI] [PubMed] [Google Scholar]
- Wen, Z. H. , Li, H. B. , Shen, Q. , Tang, X. M. , Xiong, C. Y. , Li, H. G. , … Shen, J. B. (2019). Tradeoffs among root morphology, exudation and mycorrhizal symbioses for phosphorus‐acquisition strategies of 16 crop species. New Phytologist, 223(2), 882–895. 10.1111/nph.15833 [DOI] [PubMed] [Google Scholar]
- Zhang, S. J. , Lehmann, A. , Zheng, W. S. , You, Z. Y. , & Rillig, M. C. (2019). Arbuscular mycorrhizal fungi increase grain yields: A meta‐analysis. New Phytologist, 222(1), 543–555. 10.1111/nph.15570 [DOI] [PubMed] [Google Scholar]
- Zheng, Z. , Ma, P. F. , Li, J. , Ren, L. F. , Bai, W. M. , Tian, Q. Y. , … Zhang, W. H. (2018). Arbuscular mycorrhizal fungal communities associated with two dominant species differ in their responses to long‐term nitrogen addition in temperate grasslands. Functional Ecology, 32(6), 1575–1588. 10.1111/1365-2435.13081 [DOI] [Google Scholar]
- Zhu, X. C. , Song, F. B. , Liu, S. Q. , & Liu, F. L. (2016). Arbuscular mycorrhiza improve growth, nitrogen uptake, and nitrogen use efficiency in wheat grown under elevated CO2 . Mycorrhiza, 26(2), 133–140. 10.1007/s00572-015-0654-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


