Abstract
Background
Aromatherapy is a non‐pharmacological therapy for the improvement of dementia symptoms. This study aimed to assess the effects of aroma oil as a bath salt on cognitive function, olfactory function and sleep quality.
Methods
This was a randomised controlled trial. Overall, 49 patients were able to provide consent, and 35 were finally analysed (Alzheimer's disease: 10, mild cognitive impairment: 25). The patients were randomly assigned to use 0.1%, 0.5% or 1% aroma bath salt. During daily bathing, bath salt was added to the bath water, and the subjects remained in the bathroom for ≥10 min. The intervention period was 24 weeks, and the observation periods were 4 weeks before and after using the aroma bath salt. We performed the Touch Panel‐type Dementia Assessment Scale (TDAS), the Odour Stick Identification Test for Japanese (OSIT‐J) and the Japanese version of the Pittsburgh Sleep Quality Index (PSQI‐J) five times during the before and after observation periods and after the 12‐week intervention.
Results
There were no significant changes in the TDAS, OSIT‐J and PSQI‐J scores before and after the intervention in all groups. Moreover, there were no significant differences in the TDAS, OSIT‐J and PSQI‐J scores between the groups before and after the intervention. In the correlation analysis of changes in the TDAS and other tests during the intervention period, significant associations between TDAS and sleep latency and sleep disturbances, which are sub‐items of PSQI‐J, were observed in the use of 0.1% aroma bath salt group.
Conclusions
The use of aroma bath salt was not associated with improvement in cognitive function, olfactory function or sleep quality. However, sleep‐related aspects were associated with changes in cognitive function before and after use of aroma bath salt, which suggested that there is a link between improvements in sleep and that in cognitive function.
Keywords: Alzheimer disease, aromatherapy, cognitive function, mild cognitive impairment, olfactory perception, sleep
INTRODUCTION
In recent years, there have been several studies on the prevention of dementia onset and cognitive decline through physical activity interventions,1 cognitive training interventions,2 supplementary interventions3 and reduction of behavioural psychological symptoms of dementia (BPSD) by providing cognitive behaviour therapy,4 caregivers' education5 and care services.6 Non‐pharmacological approaches have received much attention, and aromatherapy is considered a non‐pharmacological therapy. In our previous study,7 we reported a therapeutic improvement effect of aromas spread using a diffuser, which used rosemary camphor and lemon essential oil in the morning and true lavender and sweet orange essential oils in the evening, on cognitive function in patients with dementia. Moreover, other studies have reported that aromatherapy had effects on agitation and emotional functioning,8 and improved the symptoms of sleep disturbance in patients with dementia.9 Therefore, aromatherapy is expected to improve both cognitive symptoms and BPSD.
Some patients with dementia exhibit olfactory disorders, and there are several reports on olfactory disorders, particularly in patients with Alzheimer's disease (AD) and those with dementia with Lewy bodies (DLB).10, 11 Furthermore, it has been reported that patients with mild cognitive impairment (MCI), which is a pre‐dementia state, exhibited olfactory disorders12, 13, 14 and that MCI patients with severe olfactory disorder exhibited poor memory performance.15 In addition, it has been reported that amyloid ß, phosphorylated tau and alpha synuclein, which are proteins associated with the pathogenesis of AD or DLB, were deposited in olfactory‐related areas.16, 17, 18, 19, 20 Therefore, it is considered that the decreases in olfactory test scores are caused by neurodegeneration and cognitive disorder. In an animal study, the ability of olfactory precursor cells to generate new neurons was found to decrease with ageing,21 and in an autopsy study, mitral cells in the olfactory bulb were shown to decrease with age.22 Therefore, ageing is also considered to be involved in the decline of olfactory function. Previous studies have reported that olfactory training performed in patients exposed twice daily to four intense odours (phenyl ethyl alcohol, eucalyptol, citronellal and eugenol) increased the olfactory bulb volume estimated using magnetic resonance imaging (MRI) in healthy participants (age range: 19–43 years)23 and olfactory function in approximately 30% of patients with olfactory loss (age range: 23–79 years).24 Although the findings in patients with dementia remain unknown, it is speculated that stimulating the sense of smell with aromatherapy may enhance olfactory function.
According to the results of our previous research,7 because the mean score of a cognitive function test deteriorated after discontinuing aromatherapy in test subjects, we believe that it is important to continue this type of therapy. Therefore, we considered that bathing, which is familiar to Japanese individuals and easy to incorporate into everyday life, would be a good method. In this study, as a primary outcome, we evaluated the effects of bath salt with aromas of true lavender and sweet orange essential oils, which had been confirmed effect on cognitive function in our previous research,7 on cognitive function. As a secondary outcome, we evaluated the effects of aroma oil as a bath salt on olfactory function and sleep quality. The aim of the present research was to establish a new aromatherapy method that could improve cognitive function, olfactory function and sleep quality.
METHODS
Subjects
Between February 2016 and April 2018, 49 subjects were recruited from Shinsei Hospital (Kurayoshi, Japan) outpatient clinic. The inclusion criteria were AD that met the diagnostic criteria of the Diagnostic and Statistical Manual of Mental Disorders, fifth edition25 or MCI that met Petersen's diagnostic criteria.26, 27 The exclusion criteria were a diagnosis of allergic dermatitis and/or of olfactory disturbance and/or cutis symptoms > grade 2 in hand and foot syndrome. Finally, 43 subjects participated in the study.
The design of this study was approved by the ethics committee of Tottori University (approval number: 1508B012) and followed the Declaration of Helsinki. The study was registered in the UMIN Clinical Trials Registry (UMIN000019044). The research protocol was explained to the patients and guardians who provided informed consent for participation.
Procedures
This was a randomised controlled trial. The schedule of the study is shown in Figure 1. The first examination was conducted after obtaining consent. Following a 4‐week observation period, the second examination was performed. We carried out block randomisation and the patients were randomly assigned to use 0.1%, 0.5% or 1% aroma bath salts, as presented in Table 1. The aroma oil added to the bath salt was a 2:1 blend of true lavender and sweet orange, which had been confirmed to improve cognitive function in our previous research.7 The intervention period was 24 weeks. Bathing was performed after 18:00 hours. One bag of 1% or 0.5% or 0.1% of the aroma oil/bath salt in the volumes shown in Table 1 was added to the bath, and the subjects were asked to stay in the bathroom for ≥10 min. The daily usage of bath salt was recorded in a diary by the patient or guardian. The third examination was performed 12 weeks after the initiation of intervention, and the fourth examination was performed 24 weeks after the initiation of intervention. Following a 4‐week observation period, the fifth examination was performed. Assessments included: the Touch Panel‐type Dementia Assessment Scale (TDAS) (Nihon Kohden Corporation, Tokyo, Japan)28; the Odor Stick Identification Test for Japanese (OSIT‐J)29 (Daiichi Yakuhin Sangyo Co., Ltd., Tokyo, Japan) and the Japanese version of the Pittsburgh Sleep Quality Index (PSQI‐J).30, 31
Figure 1.
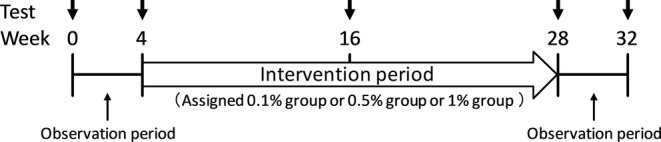
Schedule of this study.
Table 1.
Components of the bath salt
| Component | Daily dose (g) |
|---|---|
| 0.1% perfume (total: 30 g) | |
| Anhydrous sodium sulphate | 18 |
| Sodium hydrogen carbonate | 11.67 |
| Light anhydrous silicic acid | 0.21 |
| Riboflavin | 0.09 |
| Aroma oil | 0.03 |
| 0.5% perfume (total: 30 g) | |
| Anhydrous sodium sulphate | 18 |
| Sodium hydrogen carbonate | 11.55 |
| Light anhydrous silicic acid | 0.21 |
| Riboflavin | 0.09 |
| Aroma oil | 0.15 |
| 1% perfume (total: 30 g) | |
| Anhydrous sodium sulphate | 18 |
| Sodium hydrogen carbonate | 11.40 |
| Light anhydrous silicic acid | 0.21 |
| Riboflavin | 0.09 |
| Aroma oil | 0.30 |
Cognitive function test
To assess cognitive function, we used the TDAS.28 The TDAS is a modified version of the Alzheimer's disease Assessment Scale‐Cognitive Subscale (ADAS‐cog)32, 33 in which subjects enter their answers directly into a touch panel‐type computer following instructions. The nine examination items included ‘word recognition’, ‘following a command’, ‘visual–spatial perception’, ‘accuracy of the order of a process’, ‘naming fingers’, ‘orientation’, ‘money calculation’, ‘object recognition’ and ‘clock time recognition’. Scores ranged from 0 (all correct answers) to 101 points (all incorrect answers). The validity and reliability of the TDAS has previously been tested and the results showed a significant association between the TDAS and ADAS‐cog.28 Another study confirmed the results of performing the TDAS twice in patients with MCI.34
Olfactory test
We performed the OSIT‐J as an olfactory test.29 The experimenter applied an odorous semisolid cream from an odour stick to a 2‐cm circle on a thin paraffin paper, folded the paper in half, rubbed it to grind the microcapsules and passed it to the patient. The patient then opened and sniffed the paper and chose one of six possible answers: four items plus ‘detectable but not recognised’ and ‘no smell detected’. There were 12 kinds of smells familiar to Japanese (India ink, wood, perfume, menthol, Japanese orange, curry, cooking gas, rose, Japanese cypress, fermented beans/sweaty socks, condensed milk and roasted garlic). A score of 12 points indicated that the patient answered all questions correctly, and a score of 0 points indicated that the patient's answers were all incorrect. As for the validity and reliability of the OSIT‐J, a previous study showed a significant association with the OSIT‐J and cross‐cultural smell identification test and examined test–retest reliability.29
The validity and reliability of the OSIT‐J was previously tested, and the results showed a significant association between the OSIT‐J and the cross‐cultural smell identification test and determined test–retest reliability.29
Sleep questionnaire
To assess sleep quality, we used the PSQI‐J.30, 31 The PSQI‐J is a self‐administered questionnaire consisting of 18 questions. The self‐rated items of the PSQI generate seven component scores (range of subscale scores, 0–3): sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbance, use of sleeping medication and daytime dysfunction. The sum of these seven component scores yields one global score of subjective sleep quality (range, 0–21), with higher scores representing poorer subjective sleep quality. In a test of the validity and reliability of the PSQI‐J, a previous study showed that PSQI‐J scores were significantly higher in patients with primary insomnia and the PSQI‐J demonstrated a high degree of internal homogeneity.31
Statistical analysis
The sample size was originally targeted to be 60 patients (20 in each arm) on the basis of feasibility considerations. However, because of difficulties in the recruitment process, the sample size was reduced to 43. In our previous study7 that used the TDAS to examine the effect of aromatherapy on cognitive function, 17 patients with AD were studied. Therefore, we considered that the effect on cognitive function could be proven by including 20 patients in each group. We were unable to infer the effect size in the calculation of the sample size because of the lack of previous research that had evaluated the effect of aroma oil bath salt on cognitive symptoms. Therefore, we did not perform a power analysis in this study.
SPSS statistical software (version 25, IBM Japan, Tokyo, Japan) was used for statistical analyses. The Shapiro–Wilk test was performed to assess the normal distributions of the data. Differences in the baseline demographics and background characteristics of the subjects were assessed by using one‐way analysis of variance, the Kruskal–Wallis test or the Chi‐squared test. The fluctuation of each test result before and after use of the aroma bath salt in each group was evaluated by one‐way repeated measures analysis of variance or the Friedman test. Mean changes from pre‐intervention for each measured outcome were compared between groups by using one‐way analysis of variance or the Kruskal–Wallis test. In the multiple comparisons, we used the Tukey method or Bonferroni correction. Pearson's correlation coefficient or Spearman's correlation coefficient was used to evaluate the correlations between TDAS scores and the results of the other tests. All statistical significance tests were two‐sided, and an alpha‐level of 0.05 was considered as indicative of statistical significance.
RESULTS
Subjects and baseline characteristics
Figure 2 shows the patient flow in this study. Tests were performed on 43 patients. The patients were assigned to 0.1%, 0.5% or 1% aroma bath salt groups, and 35 patients underwent all tests. As a breakdown, there were 13 patients in the 0.1% aroma bath salt use group (three with AD, 10 with MCI), 11 patients in the 0.5% aroma bath salt use group (two with AD, nine with MCI) and 11 patients in the 1% aroma bath salt use group (five with AD, six with MCI). The characteristics of the patients are summarised in Table 2. The groups did not differ in terms of age, sex, TDAS scores, OSIT‐J scores or PSQI‐J scores. A survey of bathing salt usage days from a self‐reported diary, showed that no patients used bath salts for less than two‐thirds of the 24‐week intervention period.
Figure 2.
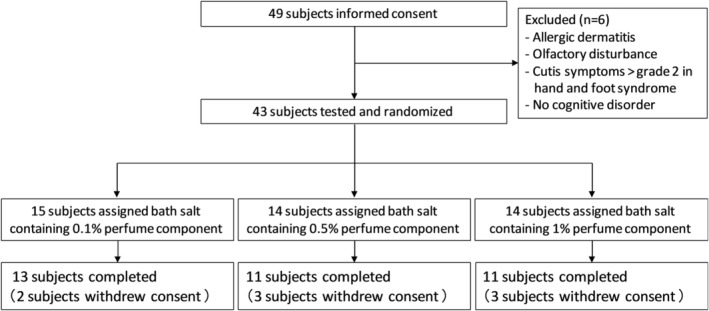
Flow chart of the subjects of this study.
Table 2.
Baseline demographic characteristics of the subjects
| Bath salt containing an aroma component | ||||
|---|---|---|---|---|
| Variable | 0.1% group | 0.5% group | 1% group | P‐value |
| Number (n) | 13 | 11 | 11 | ‐ |
| Age, years | 76 (75–81) | 78 (75.5–81) | 82 (78.5–84) | 0.181 |
| Sex (M:F) | 9:4 | 4:7 | 4:7 | 0.171 |
| Type of disease | ||||
| AD, n/% | 3/23.1 | 2/18.2 | 5/45.5 | ‐ |
| MCI, n/% | 10/76.9 | 9/81.8 | 6/54.5 | ‐ |
| TDAS | 6 (1–17) | 5 (4.5–11) | 12 (9–18.5) | 0.185 |
| OSIT‐J | 3 (1–6) | 5 (4.5–6.5) | 2 (1–3.5) | 0.051 |
| PSQI‐J | 3 (2–5) | 4 (1–5.5) | 3 (2–3) | 0.626 |
Data presented as median (interquartile range).
AD, Alzheimer's disease; MCI, mild cognitive impairment; TDAS, Touch Panel‐type Dementia Assessment Scale; OSIT‐J, Odour Stick Identification Test for Japanese; PSOI‐J, Japanese version of the Pittsburgh Sleep Quality Index.
Changes in the TDAS, OSIT‐J and PSQI‐J scores
The results of the TDAS, OSIT‐J and PSQI‐J scores are shown in Figures 3a–i. There were no significant changes in the TDAS, OSIT‐J and PSQI‐J scores during the study period in all groups. Moreover, there were no significant differences in the changes in the TDAS, OSIT‐J and PSQI‐J scores from pre‐intervention (week 4) to intermediate intervention (week 16) or post‐intervention (week 28) between the groups (Table 3). When the cut‐off point of OSIT‐J was set to ≤7.5 points in reference to the previous research,12 31 (88.6%) patients had olfactory disorders at baseline. When the cut‐off point of PSQI‐J was set to ≥5.5 points in reference to the previous research,31 six (17.1%) patients showed declines in subjective sleep quality from baseline values.
Figure 3.
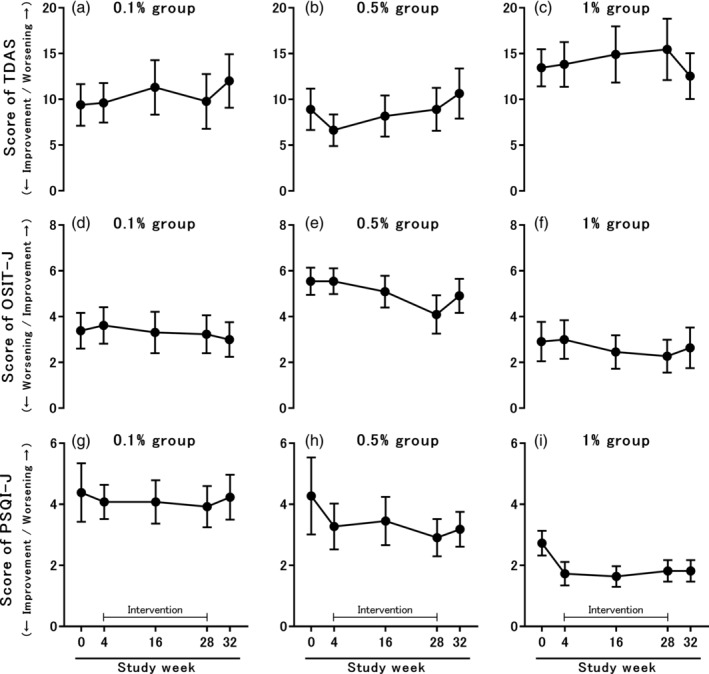
Changes in scores of the TDAS (a–c), OSIT‐J (d–f) and PSQI‐J (g–i). All data are presented as the mean ± standard error. In all groups, changes in the TDAS, OSIT‐J and PSQI‐J scores did not differ significantly throughout the study period. TDAS, Touch Panel‐type Dementia Assessment Scale; OSIT‐J, Odour Stick Identification Test for Japanese; PSOI‐J, Japanese version of the Pittsburgh Sleep Quality Index.
Table 3.
Change in the TDAS, OSIT‐J and PSQI‐J scores from pre‐intervention
| 0.1% group | 0.5% group | 1% group | P‐value | |
|---|---|---|---|---|
| TDAS | ||||
| Week 16–Week 4 | 1.7 (2.0) | 1.5 (1.1) | 1.1 (0.9) | 0.962 |
| Week 28–Week 4 | 0.2 (2.5) | 2.3 (1.9) | 1.6 (1.4) | 0.727 |
| OSIT‐J | ||||
| Week 16–Week 4 | −0.3 (0.5) | −0.5 (0.4) | −0.5 (0.5) | 0.906 |
| Week 28–Week 4 | −0.4 (0.5) | −1.5 (0.5) | −0.7 (0.5) | 0.353 |
| PSQI‐J | ||||
| Week 16–Week 4 | 0 (0.4) | 0.2 (0.3) | 0.1 (0.3) | 0.874 |
| Week 28–Week 4 | −0.2 (0.5) | −0.4 (0.2) | 0.1 (0.3) | 0.712 |
Data presented as mean (standard error).
TDAS, Touch Panel‐type Dementia Assessment Scale; OSIT‐J, Odour Stick Identification Test for Japanese; PSOI‐J, Japanese version of the Pittsburgh Sleep Quality Index.
Correlations between the TDAS and other outcomes
Table 4 shows the results of correlation analysis between the changes in the TDAS scores and other test results from pre‐intervention (week 4) to intermediate intervention (week 16) or post‐intervention (week 28). In the correlation analysis of the pre‐intervention to intermediate intervention results, the 0.1% aroma bath salt group showed a significant association with sleep latency and sleep disturbances, which are sub‐items of the PSQI‐J (r = 0.660, r = 0.618, respectively). In contrast, in the 0.5% aroma bath salt group and 1% aroma bath salt group, no significant correlation was observed between the TDAS scores and other test results. In the correlation analysis of the pre‐intervention to post‐intervention results, there were no significant correlations observed between the TDAS scores and the other test results in all groups.
Table 4.
Correlation between TDAS and other tests prior to and after the intervention
| Change from week 4 to week 16 | Change from week 4 to week 28 | ||||||
|---|---|---|---|---|---|---|---|
| TDAS vs | 0.1% group | 0.5% group | 1% group | 0.1% group | 0.5% group | 1% group | |
| OSIT‐J | r | 0.259 | 0.832 | 0.030 | 0.276 | −0.190 | 0.152 |
| PSQI‐J component | |||||||
| Sleep quality | r | −0.211 | −0.075 | −0.350 | 0.237 | 0.139 | −0.149 |
| Sleep latency | r | 0.660* | −0.102 | −0.437 | 0.462 | −0.169 | 0.068 |
| Sleep duration | r | 0.536 | −0.413 | −0.562 | 0.054 | ‐ | −0.439 |
| Sleep efficiency | r | ‐ | ‐ | ‐ | 0.325 | ‐ | ‐ |
| Sleep disturbances | r | 0.618* | 0.204 | 0.301 | −0.090 | −0.100 | 0.301 |
| Sleep medication use | r | 0.310 | ‐ | 0.502 | 0.497 | ‐ | ‐ |
| Daytime dysfunction | r | −0.115 | ‐ | 0.402 | −0.122 | −0.187 | 0.401 |
| Total score | r | 0.529 | −0.119 | −0.316 | 0.318 | −0.091 | −0.091 |
P < 0.05.
‘–’ means that correlation analysis could not be performed because there was no change prior to or after the intervention.
r, correlation; TDAS, Touch Panel‐type Dementia Assessment Scale; OSIT‐J, Odour Stick Identification Test for Japanese; PSOI‐J, Japanese version of the Pittsburgh Sleep Quality Index.
Safety
There were no serious adverse events in any group. Minor adverse events, including complaints of redness and itching of the skin, were reported: one patient with skin itching in the 0.1% aroma bath salt group, two patients with skin redness in the 0.5% aroma bath salt group and one patient with skin itching in the 1% aroma bath salt group.
DISCUSSION
The study results showed there were no improvements in cognitive function, olfactory function or sleep quality associated with the use of 0.1%, 0.5% or 1% aroma bath salt during a 24‐week period. In addition, no differences in the effects on cognitive function, olfactory function and sleep quality due to the differences in aroma oil concentrations were observed.
Our previous study7 reported that aromatherapy provided by diffusing different odours in the morning and evening in patients with dementia improved cognitive function evaluated by TDAS. Although the mechanism has not been elucidated yet, we speculated that stimulation of the sense of smell was projected to the cerebral limbic system and activated those areas, including the hippocampus and amygdala. In the present study, we tried to stimulate the sense of smell by inhaling odorous molecules that spread from the bath waters throughout the bathroom and examined the effect on cognitive function, but the expected effect was not obtained. The reason for these different results could be that, unlike in the previous research, only the blended smells of true lavender and sweet orange were used, the implemented method and time of the aromatherapy were different, and the study included MCI patients with relatively mild cognitive decline.
It is well known that olfactory sensory neurons are replaced; that is, new neurons are made continuously to replace the old ones.35 Furthermore, sensory input to the olfactory bulb plays a key role in the survival of newly generated granule cells.36 Previous studies have reported that olfactory training with odorants have increased the olfactory bulb volume estimated by MRI in healthy participants23 and olfactory function in patients with olfactory loss.24 Therefore, we speculated that olfactory function may be enhanced by stimulating the sense of smell with aromatherapy, but when aromatherapy with bath salt was performed for elderly people with dementia in this study, there was no improvement in olfactory function. Therefore, although results may differ depending on the methodology, in the method of this study we observed no improvement in the olfactory function of elderly dementia patients with olfactory dysfunction due to neurodegeneration.
It has been reported that inhalation aromatherapy using essential oils, such as linalool, santalol, cedrol and piperonal, that are also contained in essential oils blended with true lavender and sweet oranges improved symptoms of sleep disturbance in the elderly with dementia.9 Therefore, aromatherapy may have the effect of improving sleep quality. In addition, according to the results of animal experiments, the concentration of amyloid β protein in brain interstitial fluid, which is one of the causal proteins in AD, decreased approximately 25% during sleep relative to that after awakening.37 Additionally, a human study found that the amount of amyloid β protein in cerebrospinal fluid changed daily by increasing during wakefulness and decreasing during sleep.38 Furthermore, in a longitudinal study in which non‐demented participants aged >65 years were followed for 3 years, sleep inadequacy and increased daytime sleepiness were risk factors for dementia in older adults,39 which suggested that dementia and sleep are closely related. Although the improvement in sleep quality could not be proved in this study, a significant correlation was found between the variation in the PSQI‐J sub‐item and the variation in the TDAS results during the intervention period in the 0.1% aroma bath salt use group. Although it is not possible to identify which, change in sleep or cognitive function, preceded the other, the results of the previous research discussed above and odour components indicate that subjects whose sleep quality was improved by the use of aroma bath salts also tended to show improved cognitive function. However, similar results were not obtained in the 0.5% and 1% aroma bath salt use groups, and there was no correlation in the total PSQI‐J score in the 0.1% aroma bath salt use group. We think that it is necessary to increase the number of subjects and to evaluate outcomes longitudinally whether or not these positive results can be confirmed.
Several limitations of this study should be considered. The first is the small number of subjects. Recruitment was difficult because people who bathed at a day‐service facility or had a hot spring at their home (as is unique to the area) could not use the bath salts. As the target number of cases was not reached, we believe it would be worthwhile to increase the number of subjects and to analyse further. The second limitation is the concentration of the aroma bath salts. Because the subjects used the bath salts at home, the sizes of the bathrooms varied, and the density of the vaporised aroma components in the bathroom might have been different.
In this study, we could not find any effect on cognitive function, olfactory function or sleep quality by using aroma bath salt. A previous descriptive analysis of randomised clinical trials showed that results were different because of differences in the aroma therapeutic method used.40 Therefore, to obtain the effects of aromatherapy, we thought that it was necessary to consider the kinds of odour, the method of operation, the exposure time and selection of appropriate subjects. However, despite our main negative finding, our results suggest that there is room for a further investigation to evaluate the relationship between improvement in sleep quality and improved cognitive function by using aroma bath salts. There are few existing reports on the effects of aromatherapy on cognitive function, olfactory function and sleep quality for patients with dementia. Although we did not obtain the expected results in this study, in future it will be necessary to collect further data and to examine the mechanism of the effect at the molecular level.
DISCLOSURE
Minoru Kouzuki has no conflict of interest to declare. Katsuya Urakami owns a patent on the TDAS and receives royalties from Nihon Kohden Corporation. Satoshi Kitao and Takeshi Kaju are employed by Fuji Sangyo Co., Ltd.
FUNDING
This study was performed with support from Fuji Sangyo Co., Ltd.
ACKNOWLEDGMENTS
We thank the patients and their families who participated in this study. We also thank the staff at Shinsei Hospital (Kurayoshi, Japan) and the graduate students at Tottori University (Yonago, Japan) who co‐operated in this study.
Disclosure: The authors have no potential conflicts of interest to disclose.
REFERENCES
- 1. Brasure M, Desai P, Davila H et al Physical activity interventions in preventing cognitive decline and Alzheimer‐type dementia: a systematic review. Ann Intern Med 2018; 168: 30–38. [DOI] [PubMed] [Google Scholar]
- 2. Butler M, McCreedy E, Nelson VA et al Does cognitive training prevent cognitive decline?: a systematic review. Ann Intern Med 2018; 168: 63–68. [DOI] [PubMed] [Google Scholar]
- 3. Butler M, Nelson VA, Davila H et al Over‐the‐counter supplement interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer‐type dementia: a systematic review. Ann Intern Med 2018; 168: 52–62. [DOI] [PubMed] [Google Scholar]
- 4. Tay KW, Subramaniam P, Oei TP. Cognitive behavioural therapy can be effective in treating anxiety and depression in persons with dementia: a systematic review. Psychogeriatrics 2019; 19: 264–275. [DOI] [PubMed] [Google Scholar]
- 5. Terayama H, Sakurai H, Namioka N et al Caregivers' education decreases depression symptoms and burden in caregivers of patients with dementia. Psychogeriatrics 2018; 18: 327–333. [DOI] [PubMed] [Google Scholar]
- 6. Suzuki Y, Kazui H, Yoshiyama K et al Advantages of different care services for reducing neuropsychiatric symptoms in dementia patients. Psychogeriatrics 2018; 18: 252–258. [DOI] [PubMed] [Google Scholar]
- 7. Jimbo D, Kimura Y, Taniguchi M, Inoue M, Urakami K. Effect of aromatherapy on patients with Alzheimer's disease. Psychogeriatrics 2009; 9: 173–179. [DOI] [PubMed] [Google Scholar]
- 8. Strøm BS, Ytrehus S, Grov EK. Sensory stimulation for persons with dementia: a review of the literature. J Clin Nurs 2016; 25: 1805–1834. [DOI] [PubMed] [Google Scholar]
- 9. Takeda A, Watanuki E, Koyama S. Effects of inhalation aromatherapy on symptoms of sleep disturbance in the elderly with dementia. Evid Based Complement Alternat Med 2017; 2017: 1902807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alves J, Petrosyan A, Magalhães R. Olfactory dysfunction in dementia. World J Clin Cases 2014; 2: 661–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Williams SS, Williams J, Combrinck M, Christie S, Smith AD, McShane R. Olfactory impairment is more marked in patients with mild dementia with Lewy bodies than those with mild Alzheimer disease. J Neurol Neurosurg Psychiatry 2009; 80: 667–670. [DOI] [PubMed] [Google Scholar]
- 12. Kouzuki M, Suzuki T, Nagano M et al Comparison of olfactory and gustatory disorders in Alzheimer's disease. Neurol Sci 2018; 39: 321–328. [DOI] [PubMed] [Google Scholar]
- 13. Roalf DR, Moberg MJ, Turetsky BI et al A quantitative meta‐analysis of olfactory dysfunction in mild cognitive impairment. J Neurol Neurosurg Psychiatry 2017; 88: 226–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tabert MH, Liu X, Doty RL et al A 10‐item smell identification scale related to risk for Alzheimer's disease. Ann Neurol 2005; 58: 155–160. [DOI] [PubMed] [Google Scholar]
- 15. Makizako M, Makizako H, Doi T et al Olfactory identification and cognitive performance in community‐dwelling older adults with mild cognitive impairment. Chem Senses 2014; 39: 39–46. [DOI] [PubMed] [Google Scholar]
- 16. Thal DR, Rüb U, Orantes M, Braak H. Phases of a beta‐deposition in the human brain and its relevance for the development of AD. Neurology 2002; 58: 1791–1800. [DOI] [PubMed] [Google Scholar]
- 17. Serrano‐Pozo A, Frosch MP, Masliah E, Hyman BT. Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med 2011; 1: a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease‐associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 2006; 112: 389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hubbard PS, Esiri MM, Reading M, McShane R, Nagy Z. Alpha‐synuclein pathology in the olfactory pathways of dementia patients. J Anat 2007; 211: 117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Silveira‐Moriyama L, Holton JL, Kingsbury A et al Regional differences in the severity of Lewy body pathology across the olfactory cortex. Neurosci Lett 2009; 453: 77–80. [DOI] [PubMed] [Google Scholar]
- 21. Watanabe K, Kondo K, Takeuchi N, Okano H, Yamasoba T. Musashi‐1 expression in postnatal mouse olfactory epithelium. Neuroreport 2007; 18: 641–644. [DOI] [PubMed] [Google Scholar]
- 22. Sama‐ul‐Haq, Tahir M, Lone KP. Age and gender‐related differences in mitral cells of olfactory bulb. J Coll Physicians Surg Pak 2008; 18: 669–673. [PubMed] [Google Scholar]
- 23. Negoias S, Pietsch K, Hummel T. Changes in olfactory bulb volume following lateralized olfactory training. Brain Imaging Behav 2017; 11: 998–1005. [DOI] [PubMed] [Google Scholar]
- 24. Hummel T, Rissom K, Reden J, Hähner A, Weidenbecher M, Hüttenbrink KB. Effects of olfactory training in patients with olfactory loss. Laryngoscope 2009; 119: 496–499. [DOI] [PubMed] [Google Scholar]
- 25. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, 5th edn. Arlington, TX: American Psychiatric Association, 2013. [Google Scholar]
- 26. Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med 2004; 256: 183–194. [DOI] [PubMed] [Google Scholar]
- 27. Petersen RC. Clinical practice. Mild cognitive impairment. N Engl J Med 2011; 364: 2227–2234. [DOI] [PubMed] [Google Scholar]
- 28. Inoue M, Jimbo D, Taniguchi M, Urakami K. Touch panel‐type dementia assessment scale: a new computer‐based rating scale for Alzheimer's disease. Psychogeriatrics 2011; 11: 28–33. [DOI] [PubMed] [Google Scholar]
- 29. Saito S, Ayabe‐Kanamura S, Takashima Y et al Development of a smell identification test using a novel stick‐type odor presentation kit. Chem Senses 2006; 31: 379–391. [DOI] [PubMed] [Google Scholar]
- 30. Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res 1989; 28: 193–213. [DOI] [PubMed] [Google Scholar]
- 31. Doi Y, Minowa M, Uchiyama M et al Psychometric assessment of subjective sleep quality using the Japanese version of the Pittsburgh sleep quality index (PSQI‐J) in psychiatric disordered and control subjects. Psychiatry Res 2000; 97: 165–172. [DOI] [PubMed] [Google Scholar]
- 32. Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry 1984; 14: 1356–1364. [DOI] [PubMed] [Google Scholar]
- 33. Homma A, Fukuzawa K, Tsukada Y, Ishii T, Hasegawa K. Development of a Japanese version of Alzheimer's disease assessment scale (ADAS). Jpn J Geriatr Psychiatry 1992; 3: 647–655. (in Japanese). [Google Scholar]
- 34. Saito J, Inoue M, Kitaura M et al Assessment of new subject selection methods and evaluation methods for dementia prevention classes. Dementia Jpn 2005; 19: 177–186. (in Japanese with English abstract). [Google Scholar]
- 35. Farbman AI. Olfactory neurogenesis: genetic or environmental controls? Trends Neurosci 1990; 13: 362–365. [DOI] [PubMed] [Google Scholar]
- 36. Yamaguchi M, Mori K. Critical period for sensory experience‐dependent survival of newly generated granule cells in the adult mouse olfactory bulb. Proc Natl Acad Sci U S A 2005; 102: 9697–9702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kang JE, Lim MM, Bateman RJ et al Amyloid‐beta dynamics are regulated by orexin and the sleep‐wake cycle. Science 2009; 326: 1005–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Huang Y, Potter R, Sigurdson W et al Effects of age and amyloid deposition on Aβ dynamics in the human central nervous system. Arch Neurol 2012; 69: 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tsapanou A, Gu Y, Manly J et al Daytime sleepiness and sleep inadequacy as risk factors for dementia. Dement Geriatr Cogn Dis Extra 2015; 5: 286–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Press‐Sandler O, Freud T, Volkov I, Peleg R, Press Y. Aromatherapy for the treatment of patients with behavioral and psychological symptoms of dementia: a descriptive analysis of RCTs. J Altern Complement Med 2016; 22: 422–428. [DOI] [PubMed] [Google Scholar]


