Abstract
Recently, a novel sex‐determination system was identified in the silkworm (Bombyx mori) in which a piwi‐interacting RNA (piRNA) encoded on the female‐specific W chromosome silences a Z‐linked gene (Masculinizer) that would otherwise initiate male sex‐determination and dosage compensation. Masculinizer provides various opportunities for developing improved genetic pest management tools. A pest lepidopteran in which a genetic pest management system has been developed, but which would benefit greatly from such improved designs, is the diamondback moth, Plutella xylostella. However, Masculinizer has not yet been identified in this species. Here, focusing on the previously described ‘masculinizing’ domain of B. mori Masculinizer, we identify P. xylostella Masculinizer (PxyMasc). We show that PxyMasc is Z‐linked, regulates sex‐specific alternative splicing of doublesex and is necessary for male survival. Similar results in B. mori suggest this survival effect is possibly through failure to initiate male dosage compensation. The highly conserved function and location of this gene between these two distantly related lepidopterans suggests a deep role for Masculinizer in the sex‐determination systems of the Lepidoptera.
Keywords: diamondback moth, Plutella xylostella, masculinizer, sex determination, gene drive, doublesex, dosage compensation
We have identified a Masculinizer homologue (PxyMasc) in the diamondback moth, Plutella xylostella.
As in Bombyx mori, this gene controls doublesex splicing and male viability suggesting deep conservation of this sex‐determination mechanism.
PxyMasc functions early (3–6 h) in embryonic development to initiate male‐form doublesex splicing, but its transcript is rapidly (6–24 h) eliminated in females.
Introduction
Sex‐determination systems are of fundamental biological interest and also provide targets and components for genetic pest management systems, including gene drives (Burt, 2003; KaramiNejadRanjbar et al., 2018). To date, molecular characterization of lepidopteran sex determination systems has been limited to the relatively closely related Bombycidae (Kiuchi et al., 2014; Lee et al., 2015) and Crambidae (Fukui et al., 2015; Fukui et al., 2018) families. As typical for lepidopterans, silkworm (Bombyx mori) males are homogametic (ZZ) whilst females are heterogametic (WZ). Recent evidence has shown that the sex‐determination cascade of B. mori originates in piRNA loci (Feminizer, Fem) located on the W chromosome (Kiuchi et al., 2014) – an example of a ‘dominant W’ sex‐determination system (Traut et al., 2007). In ZZ males, ie in the absence of Fem, Masculinizer (Masc, located on the Z chromosome) directs the male‐specific splicing of doublesex (dsx), leading to masculine terminal somatic differentiation. Masc is further involved in regulating dosage compensation of Z‐linked genes in B. mori as short‐interfering RNA (siRNA)‐based disruption of Masc messenger RNA (mRNA) in eggs led to male‐specific embryonic lethality. Fem piRNA silences Masc, leading to female‐type splicing of dsx mRNA. Transgenic expression of a Masc coding sequence (CDS)recoded to be resistant to Fem silencing resulted in a degree of female‐to‐male sex conversion and female‐specific lethality (Sakai et al., 2016) with in vivo CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats)/Cas9 studies suggesting truncated Masc protein may still provide a similar effect (Kiuchi et al., 2019). Thus, disruption or manipulation of Masc activity potentially provides a range of phenotypes desirable for genetic population suppression technologies.
The Lepidoptera include some of the most damaging pests of agriculture and forestry. The diamondback moth (DBM) Plutella xylostella (L.) (Lepidoptera: Plutellidae), a specialist pest of cruciferous crops, causes an estimated $5 billion dollars in damage and control measures each year (Furlong et al., 2013). The primary means of control is through the spraying of broad‐spectrum synthetic chemical insecticides, to which DBM is widely resistant (Talekar and Shelton, 1993). As such, development of alternative pest management strategies, particularly those that are robust to resistance (Alphey et al., 2011) and environmentally benign (Black et al., 2011), is needed. Previously, we reported a novel genetic pest management system that utilized a dsx splicing cassette from pink bollworm (Pectinophora gossypiella, PBW) to engineer female‐specific lethality into DBM (Jin et al., 2013). Subsequently, this system was also shown to work effectively in B. mori (Tan et al., 2013). For this system to function across such broad phylogenetic distances, splicing sites within the PBW dsx cassette must be recognized by endogenous splicing regulators within each of these two distantly related lepidopteran species. We therefore hypothesized that homologues of the key regulator of this splicing in silkworm, Masc, would also be present in DBM – a species with a confirmed WZ/ZZ sex chromosome arrangement (Dalikova et al., 2017). Previous research on B. mori Masc identified the amino acid region between 304 and 310 aa and particularly the two cysteine residues (cysteine‐cysteine domain) as being necessary for promoting male‐specific splicing of dsx (Katsuma et al., 2015). This region shows a relatively high degree of homology across the rather divergent putative Masc homologues identified, albeit from a restricted phylogenetic range within the Macroheterocera and Papilionoidea. We thus concentrated efforts on investigating putative DBM homologues that showed particular conservation in this region.
Here we describe the identification of a Masc homologue (PxyMasc) in the globally important crop pest DBM. Rapid amplification of cDNA ends (RACE) confirmed the existence of two previously unrecognized 5′ exons, one of which encodes the Masc characteristic CCCH‐tandem zinc finger domains: a feature that has probably hindered identification of the DBM homologue to date. A single unannotated 3′ exon was also identified. Through RNA interference (RNAi) knockdown in DBM embryos we demonstrate that PxyMasc expression is required for male survival and controls the male‐specific splicing of dsx. As the sex‐alternative splicing of DBM dsx has yet to be elucidated we further took the opportunity to characterize this. Through temporal profiling of paired PxyMasc and dsx expression/splicing in individual embryos we demonstrate that PxyMasc expression begins in embryos prior to terminal sex differentiation (as measured through dsx splicing patterns); however, PxyMasc expression is transient in female embryos and only sustained in males, a pattern consistent with Masc expression in B. mori. Finally, we demonstrate through genomic copy number quantitative PCR (qPCR) that PxyMasc is syntenic with Masc (ie is Z‐linked). Identification of PxyMasc extends the conservation of this unique sex‐determination system across the Lepidoptera and provides a target both for further research into upstream DBM sex‐determination components, as well as for building gene drive population suppression systems.
Results
Identification of Masc homologues in DBM
Using the B. mori Masc sequence as a query, two significant hits were identified in the DBM genome, which corresponded to genes LOC105386064 (annotated as cytokinesis protein SepA‐like = from here on SepA‐like) and LOC105388743 (annotated as zinc finger CCCH domain‐containing protein 10‐like = from here on CCCH). Reciprocal BlastP of these sequences against the B. mori genome gave Masc as the top hit for SepA‐like and an extremely similar gene as the top hit for CCCH; uncharacterized B. mori gene LOC101740889. Further BlastP searches using amino acid sequences of SepA‐like and CCCH as queries but not limiting search results to B. mori provided similar results, with SepA‐like showing significant homology to other putatively annotated Masc homologues in Danaus plexippus and Ostrinia furnacalis and CCCH showing high sequence similarity to a variety of lepidopteran zinc‐finger CCCH domain‐containing protein 10‐like genes from D. plexippus, Helicoverpa armigera, Spodoptera littoralis, Pieris rapae and Heliothis virescens. Interestingly, although all other identified lepidopteran Masc homologues are characterized by the presence of N‐terminus CCCH‐tandem zinc finger domains, the annotated SepA‐like CDS did not encode such domains.
RNAi knockdown of potential Masc homologues
Investigating effects on sex‐specific survival
In B. mori, RNAi‐mediated knockdown of Masc was shown to cause male‐specific embryonic lethality owing to incomplete dosage compensation of Z‐linked genes. Unlike in B. mori, we are unable to sex DBM individuals at the egg stage but could test for this phenotype by assessing whether hatch rates of eggs injected with double‐stranded RNA (dsRNA) targeting the two putative Masc homologues diverged significantly from control injections (dsRNA against Anemonia majano Cyan (AmCyan) CDS) – if all male embryos died owing to the treatment, the hatch rate would be half that of control injections. However, no significant difference in hatch rates was observed between treatments (Χ 2 = 2.90, df = 2, P = 0.24 – Table 1).
Table 1.
Phenotypes resulting from RNA interference‐mediated knockdown of putative Masculinizer (Masc) homologues. Hatch rates, pupal sex ratios and number of eggs exhibiting male‐isoform doublesex (dsx) splicing are shown of diamondback moth eggs following injection with double‐stranded RNA targeting either cytokinesis protein SepA‐like (SepA‐like; Plutella xylostella Masc), zinc finger CCCH domain‐containing protein 10‐like (CCCH) or an Anemonia majano Cyan (AmCyan) control. Hatch rates did not differ significantly between treatments (Χ 2 = 2.90, df = 2, P = 0.24). However, the effect of treatment on pupal sex ratio was significant (Χ 2 = 22.8, df = 2, P < 0.001) with SepA‐like being significantly different (a bias towards females) to both other treatments (P < 0.001), which themselves did not differ significantly (P > 0.05). A similar pattern was observed in dsx splicing in 48 h embryos with SepA‐like injected eggs showing a significant deviation from the AmCyan treatment (Χ 2 = 16.25, df = 1, P < 0.001) representing a significant shift towards female‐isoform splicing. The AmCyan treatment itself did not significantly differ from the expected 50:50 ratio (Χ 2 = 0.924, df = 1, P > 0.05)
| SepA‐like | CCCH | AmCyan | |
|---|---|---|---|
| Eggs hatched | 73/115 | 85/125 | 102/139 |
| Number of pupae that were male | 7/54 | 40/79 | 46/95 |
| Number of eggs exhibiting male dsx splicing | 1/39 | NA | 17/39 |
Larvae from each of the three above dsRNA treatments were reared until pupation, which in DBM is the first stage that can be reliably sexed by eye. An omnibus Χ 2 test identified a highly significant effect of treatment (ie dsRNA target) on pupal sex ratio (Χ 2 = 22.8, df = 2, P < 0.001), which further post‐hoc testing showed was the result of a significant deviation of SepA‐like injected pupal sex from either AmCyan (P < 0.001) or CCCH pupae (P < 0.001), which themselves did not differ significantly (P > 0.05) (Table 1). Given the strong effect on male survival observed in these experiments, we putatively identified SepA‐like as the DBM homologue of Masc and refer to it as PxyMasc from here on.
Characterization of sex‐specific dsx splicing
Using cDNA from male and female pupal samples, sequencing of cloned dsx bands revealed a single male (M) and at least four female sex‐specific splicing variants (F1–F4) (Fig. 1A, B). As in other lepidopterans (Jin et al., 2013; Wang et al., 2014), the male transcript included exons 2 and 5 with internal sequences excised. Female splice variants F2, F3 and F4 utilized alternative splicing sites to the predominant female splice variant F1, leading to longer variants of exons 3 and 4, with altered coding potential. Interestingly, the alternative acceptor sites within exon 4 used in F2, F3 and F4 coincided with previously identified (in PBW) highly conserved intronic sequences (Jin et al., 2013; see Supporting Information Figure S2), confirming a pattern also observed in Helicoverpa armigera and O. furnacalis (Wang et al., 2014).
Figure 1.
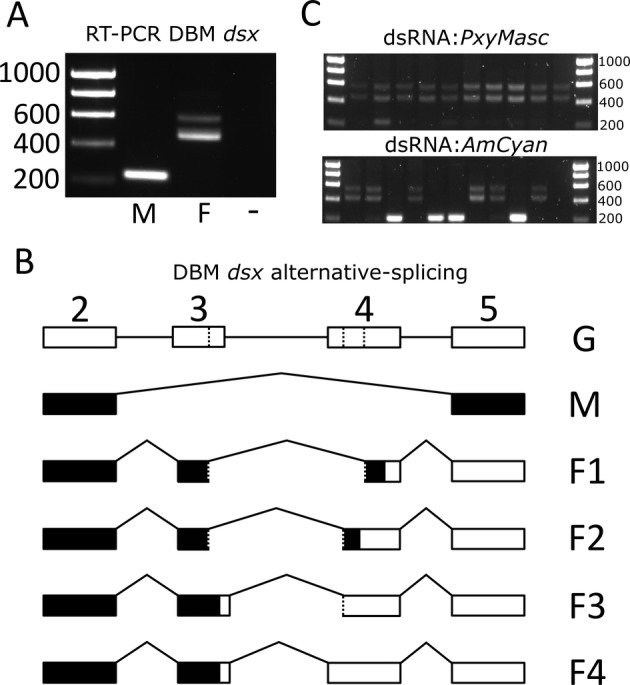
Characterizing sex‐alternative splicing of doublesex (dsx) in the diamondback moth (DBM). (A) Results of Reverse‐Transcription‐PCR (RT‐PCR) conducted on male and female pupal cDNA using primers specific to the exons flanking the sex‐alternatively spliced region (exons 2 and 5 in B) of dsx. No template control (NTC) shown by (−). Visible bands were cloned and sequenced identifying a single male‐specific transcript (M) and four female transcripts (F1–F4) (B). Intron/exon structure of the dsx gene within this region, revealed by sequencing RT‐PCR products from A, relative to genomic sequence (G). Dotted lines represent alternative splice sites within exons. The male transcript includes the shared exons (2 and 5) but excludes the internal female‐specific exons (3 and 4). Female transcripts all contained both the shared exons and various combinations of the alternatively spliced female‐specific exons. For transcripts, coding region is shown in black and 3′ untranslated region in white. Sequences of each transcript are available in the Supporting Information Figure S1. Panel C shows dsx splicing patterns in individual embryos following injection with double‐stranded RNA (dsRNA) targeting either the Plutella xylostella Masculinizer (PxyMasc) transcript (upper row) or the Anemonia majano Cyan (AmCyan) control (lower row). Second‐from‐right lane in the AmCyan row is the NTC. Panel C consists of two cropped images from a single gel. For reference, the uncropped image can be found in the Supporting Information Figure S4. PCR images shown are of representative samples from each treatment.
Investigating effects on dsx splicing
As we did not observe an effect on male survival in dsRNA injections targeting CCCH it was excluded from further experiments. In a separate experiment, dsRNA targeting PxyMasc and AmCyan were again injected into DBM eggs. RNA was extracted 48 h postinjection and PCR run on cDNA from individual samples (eggs) to identify patterns of dsx splicing. Whereas AmCyan dsRNA injected eggs showed dsx splicing patterns not significantly different from an expected 50:50 male : female ratio (Χ 2 = 0.924, df = 1, P > 0.05), patterns in PxyMasc injected eggs showed a significant bias towards females with only 1/39 individuals showing a male splice pattern (Χ 2 = 16.25, df = 1, P < 0.001) (Fig. 1C). These results suggest that PxyMasc (as Masc in B. mori) functions upstream of sex‐alternative dsx splicing. To elucidate the relationship between PxyMasc and dsx further we next assessed the temporal expression patterns of these two genes through early embryonic development (ie coinciding with the sex‐determination stage).
Temporal expression pattern of PxyMasc and dsx
Individual eggs laid within a 5‐min window were collected at 3‐, 6‐ and 24‐h time points postlaying and used to assess patterns of PxyMasc/dsx expression and splicing. At 3 h postlaying, dsx transcript in all eggs assayed (n = 25) was spliced in an exclusively female‐specific manner (Fig. 2A), as has been found in other lepidopterans prior to sex‐determination systems being activated (Chen et al., 2019). During this early period, no PxyMasc expression could be detected in these individuals (Fig. 2B). At 6 h postlaying, the majority of eggs assayed (n = 26) showed both male and female dsx splicing indicating that the sexual differentiation process had begun in these individuals. The remaining four eggs at this time point remained in a female splicing pattern. Concurrently, those eggs that had begun the sexual differentiation process (ie showed a male dsx band) also expressed PxyMasc. Finally, at 24 h postlaying, the majority of eggs had completed their sexual differentiation process [ie were splicing dsx in an exclusively male (n = 10) or female (n = 14) pattern], with a small number (n = 2) still showing both isoforms, albeit in both cases with one form being significantly stronger than the other. During this stage, PxyMasc expression was observed exclusively in those individuals that were strongly ‘male’ in terms of dsx splicing.
Figure 2.
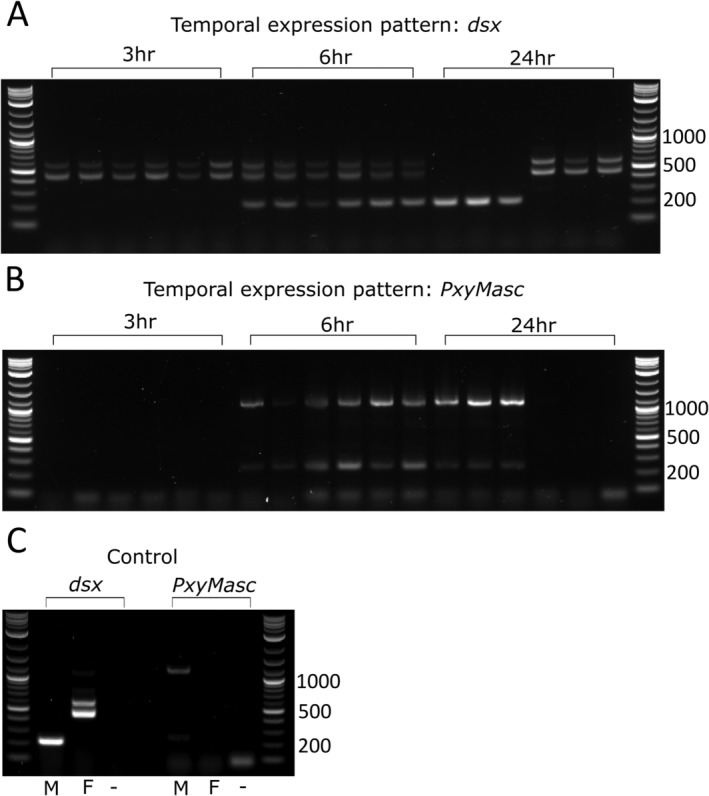
Temporal analysis of Plutella xylostella Masculinizer (PxyMasc) and doublesex (dsx) expression/splicing patterns in early eggs. Individual eggs were collected within 5 min of being laid and allowed to develop for 3, 6 and 24 h before being sampled (frozen in liquid nitrogen, RNA extracted, cDNA synthesized). Samples were used as template for paired PxyMasc/dsx PCR. A minimum of 25 eggs were obtained at each time point with the images above displaying representative samples from each group. Panel A shows results of dsx PCR, panel B shows results of PxyMasc PCR (PCRs in the same lanes in these two panels were run using the same sample, ie cDNA from the same egg), panel C shows controls run using sexed male (M) and female (F) pupal cDNA as well as a no template control (−). At 3 h postlaying, all eggs show female dsx splicing and no PxyMasc transcript is observable. At 6 h postlaying all individuals are showing both male (c. 200 bp) and female (c. 600 & 450 bp) dsx bands and all show full PxyMasc expression (c. 1200 bp) as well as a smaller PxyMasc transcript (c. 200 bp, Fig. 3B). At 24 h postlaying, samples showed either male dsx splicing (first three samples) or female dsx splicing (second three samples) with PxyMasc expression only remaining in those individuals showing male dsx bands.
Characterization of PxyMasc gene structure
5′ RACE of PxyMasc revealed two previously unannotated exons, including one within the CDS. As noted above, the annotated PxyMasc mRNA sequence (https://www.ncbi.nlm.nih.gov/nuccore/XM_011556550.1) does not include the N‐terminal CCCH‐tandem zinc finger domains that are a conserved feature of other Masc homologues. We found regions homologous to these zinc finger domains in the coding exon uncovered by RACE (Fig. 3C). Reverse Trascription‐PCR (RT‐PCR) confirmed the presence of 13 exons, 11 of which were coding (Fig. 3A). Furthermore, this assay revealed an alternatively spliced PxyMasc transcript (Fig. 3B), which sequencing showed had spliced directly between exons 4 and 13, excising the cysteine‐cysteine domain as well as the majority of the coding sequence, while extending the CDS into the 3′ untranslated region (UTR; exon 13). This smaller transcript is visible in PxyMasc RT‐PCR images in Fig. 2B as a c. 200‐bp band. Analysis of the full coding region showed that areas of the PxyMasc mRNA are extremely GC rich, particularly the first coding exon (exon 2), with large stretches >80% GC (Fig. S5). Overall the PxyMasc CDS showed 63% GC, above the average for DBM (53%, http://ensembl.lepbase.org/Plutella_xylostella_dbmfjv1x1/Info/Index). Comparison of the cysteine‐cysteine ‘masculinizing’ domain found in PxyMasc exon 5 with previously reported lepidopteran Masc homologues (Fig. 3D) showed that while the two cysteine residues as well as a downstream glutamine are highly conserved, there is significant divergence between DBM and the other identified lepidopteran sequences in the remainder of this area. Alignment of the full amino acid sequence of PxyMasc and other putative and confirmed lepidopteran Masc homologues was used to build a phylogenetic tree using clustal omega (https://www.ebi.ac.uk/Tools/msa/clustalo/; Fig. S6). Results somewhat matched the phylogenetic distances between the respective species with members of the Bombycoidea (B. mori and Trilocha varians) grouping together. However, beyond this clade, relationships aligned less well with other homologues all relatively divergent from each other. Within this outer group, PxyMasc remained the most distantly related. These results suggest that away from the established Masculinizing and zinc finger domains, the Masc protein has seen significant sequence divergence over evolutionary time.
Figure 3.
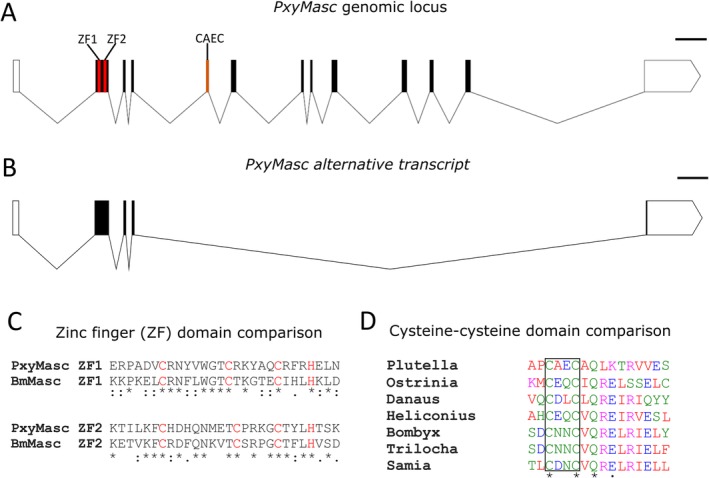
Characterization of Plutella xylostella Masculinizer (PxyMasc) gene structure. PxyMasc gene structure (panel A) was determined through 5′ and 3′ rapid amplification of cDNA ends from exons 5 and 12/13, respectively, followed by RT‐PCR of the intervening region. These analyses revealed the presence of the CCCH‐tandem (Cysteine‐Cysteine‐Cysteine‐Histidine) zinc finger motifs (ZF1 and ZF2) and the masculinizing ‘cysteine‐cysteine domain’ (CAEC) indicative of other Masc homologues (details provided in panel D). RT‐PCR revealed a secondary PxyMasc transcript (panel B) where exons 5–12 were excised (exons 4–13 directly joined). This allowed a small region within exon 13 to be included within the coding sequence. Putative coding regions are shown in black, untranslated regions in white. Analysis revealed that the previously unannotated coding exon 2 contained amino acid sequences homologous to the CCCH‐tandem zinc finger motifs identified in Bombyx mori Masc (alignment shown in panel C). Here, the CCCH conserved amino acids are highlighted in red. Panel D shows alignment of the integral masculinizing cysteine‐cysteine domain (found in exon 5) from lepidopterans where Masc homologues have been identified (sequences other than Plutella adapted from Katsuma et al., 2015). The integral cysteine‐cysteine region is outlined in black. Full transcript sequence is given in Fig. 3.
Assessing PxyMasc genomic copy number
As B. mori Masc is located on the Z chromosome, and this chromosome is reported to be highly conserved within the Lepidoptera (Fraisse et al., 2017), we were interested to assess whether our putative Masc homologue was also located here. As such, we designed and performed a qPCR assay for PxyMasc using genomic DNA (gDNA) from sexed pupae as sample template (Fig. 4). As DBM males are ZZ and DBM females are WZ, copy levels in female DBM should be, on average, half that of males, if PxyMasc was indeed Z‐linked. A positive control was included in the previously confirmed Z‐linked gene kettin (Belousova et al., 2019). PCR efficiency (E) was experimentally determined for each qPCR primer set [PxyMasc (114.4%; R 2 = 0.999), defensin (110.2%; R 2 = 0.991) and kettin (95.5%; R 2 = 0.994)]. The specificity of each qPCR primer set was confirmed by visually inspecting the melt curve profiles of each reaction for a single peak at the expected temperature. Profiles for each primer set showed a single sharp peak indicating that a single product was amplified, and no primer dimers were produced. Each primer set produced amplicons of the expected size when visualized on a 2% agarose gel. The relative quantification (2−ΔΔCq) ratio between males (n = 10) and females (n = 10) was close to 2 for both PxyMasc (2.1) and the known Z‐linked positive control kettin (2.3). This suggests that PxyMasc is Z‐linked in DBM, as is consistent with Masc in B. mori (Kiuchi et al., 2014).
Figure 4.
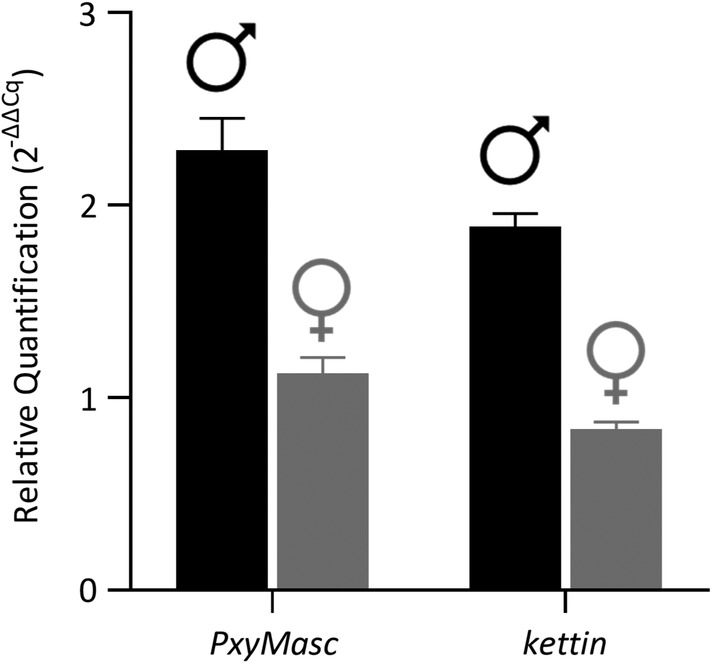
Genomic copy number of Plutella xylostella Masculinizer (PxyMasc) in male and female diamondback moths (DBM) estimated by quantitative PCR (qPCR) on genomic DNA template. Relative quantification (2−ΔΔCq) of PxyMasc and a known Z‐linked positive control (kettin) in male (black bars; n = 10) and female (grey bars; n = 10) DBM pupae. Bars represent mean (2−ΔΔCq) values and error bars represent standard error. A known autosomal gene (defensin) was used as a reference gene during analysis.
Discussion
Here we identify and characterize the function of a Masc homologue (PxyMasc) in the globally important crop pest P. xylostella. As in other lepidopterans, PxyMasc was found to be involved in regulating dsx splicing as well as male‐specific survival – silencing of this gene resulted in male‐specific lethality. Unlike in B. mori (Kiuchi et al., 2014), however, this lethality was not observed at the egg stage (as assessed by hatch rates), but occurred in early larvae. In B. mori, where genomic resources are considerably more advanced than DBM, this male‐specific lethality has been attributed to the failure of Z‐linked dosage compensation initiation. Similarly, in O. furnacalis, it has been shown that the intracellular bacterium Wolbachia achieves male‐specific lethality in early embryos through reduction of Masc transcript, resulting in failure of Z‐linked dosage compensation (Fukui et al., 2015). Within the context of these results, it is tempting to speculate that our reported male‐specific lethality is also attributable to a dosage compensation effect, although we did not assess this directly and unlike these previous results, we inferred this lethality effect in early larvae rather than observing it in embryos. Similarly ‘late’ effects on males were also observed when CRISPR/Cas9 was used to create Masc knockout chimeras in Agrotis ipsilon (Wang et al., 2019). The difference in timing of these effects may possibly be a result of differing levels of knockdown/knockout between our experiments and those reported previously, or the differing mechanics of siRNA (used previously) and dsRNA (used here) based RNAi. However, as Masc clearly functions upstream of important male terminal differentiation signals our results cannot rule out a dosage compensation independent effect.
Profiling the temporal expression profiles of PxyMasc and dsx in the early embryo suggests a role for PxyMasc similar to that of Masc in B. mori. PxyMasc expression coincided with the onset of male‐form splicing of dsx, which was apparent in almost all individuals – assumed to be a mix of both males and females ‐ assayed at 6 h postlaying. By 24 h postlaying, however, PxyMasc expression was no longer observed in individuals that did not show male dsx splicing (ie in females). In B. mori, knockdown of Masc is initiated via expression of the W‐specific Fem piRNA and although our study did not seek to elucidate the upstream regulators of PxyMasc, our results provide a temporal window in which such a regulator is likely to be active.
Previous studies in which phylogenetic analysis was used to identify Masc homologues in lepidopterans did not include a DBM homologue (Kiuchi et al., 2014; Fukui et al., 2015), despite the economic importance of this pest and the existence of a high quality genome sequence. In characterizing PxyMasc we found that the N‐terminal Masc‐characteristic CCCH‐tandem zinc finger domains are present, but not included in the genome annotated coding region – probably contributing to the difficulty in correctly identifying this homologue to date. Although previous research into the function of B. mori Masc has shown that these zinc finger domains are not necessary for the masculinizing ability of Masc protein (Katsuma et al., 2015) or its dosage compensation effects (Kiuchi et al., 2019), they are a common feature of other lepidopteran Masc homologues (Kiuchi et al., 2014; Fukui et al., 2015; Lee et al., 2015) as well as the reported homologue from the brine shrimp Artemia franciscana (Li et al., 2017). We also identified an alternatively spliced PxyMasc transcript, which lacked the cysteine‐cysteine domain required for masculinizing activity in B. mori (Katsuma et al., 2015), as well as the majority of the coding sequence. It remains to be determined what function this transcript may play in DBM sex determination.
With the split between the Yponomeutoidea (including DBM) and the Apodytrisia [containing B. mori as well as all other lepidopteran species in which Masc homologues have been functionally characterized (Fukui et al., 2015, Lee et al., 2015)] estimated at c. 140 mya (Mitter et al., 2017), our finding that PxyMasc is Z‐linked and therefore syntenic with B. mori Masc confirms the deep conservation of this chromosome across the Lepidoptera (Fraisse et al., 2017). Further work will seek to explore whether the upstream components of the sex‐determination cascade (of which only one example – Fem – has currently been identified in lepidopterans), which probably control the levels of PxyMasc in female DBM early in embryonic development, are equally conserved.
The involvement of PxyMasc in controlling sexual differentiation and sex‐specific viability opens opportunities to utilize this gene in improved genetic pest management strategies aimed at population suppression. Currently, female‐specific release of insects carrying a dominant lethal (fsRIDL) strategies targeting DBM utilize a PBW dsx splicing cassette to allow female‐specific, tetracycline repressible, expression of a tetracycline‐repressible transactivator (tTAV) in a lethal positive feedback loop (Jin et al., 2013). Whilst this strategy has been demonstrated as effective at the population level (Harvey‐Samuel et al., 2015), fitness costs to transgenic males were significant (Jin et al., 2013; Harvey‐Samuel et al., 2014) leading to probable increased deployment costs, were this technology to be used commercially. While alternative genomic integration sites and the utilization of a splicing cassette based on the DBM dsx gene described here may reduce these fitness costs, a degree of transgene‐based fitness reduction in males may be unavoidable as the default lepidopteran splicing pattern of dsx in both sexes is the female splicing isoform (Kiuchi et al., 2014; Fukui et al., 2015; Lee et al., 2015), so the expression profile of tTAV in such designs is probably not sex‐specific in very early embryos. In B. mori, transgenic expression of a Fem‐resistant Masc transcript led to both female‐specific lethality and partial female‐to‐male sex reversal (Sakai et al., 2016). Although it remains to be determined whether a similar piRNA‐based system operates in DBM, one might speculate that expression of an analogous, nucleotide recoded, PxyMasc transcript in DBM females would act as a dominant female‐specific lethal effector for an fsRIDL system – probably with fewer fitness costs to transgenic males as this is an endogenously expressed gene in this sex. If a gene drive was used to spread such a dominant female lethal at a neutral locus this would constitute a ‘RIDL‐with‐Drive’ population suppression strategy (Thomas et al., 2000): a design that has been previously modelled as significantly more powerful at population reduction than current self‐limiting systems (Thomas et al., 2000; Backus and Gross, 2016; Prowse et al., 2017; Burt and Deredec, 2018). The inherent self‐limiting nature of a RIDL‐with‐Drive system may be preferable for a pest such as DBM, which is capable of extremely long distance migrations (up to 1000 km per day; Talekar and Shelton, 1993) and for which less controllable ‘global drive’ designs may therefore be less appropriate (Esvelt et al., 2014).
The above designs assume a ‘dominant W’ sex determination system in DBM – ie the existence of a Fem orthologue – or functionally similar upstream signal. However, evidence suggests that the ancestral ‘Z counting’ mechanism – where sex is determined by dosage of Z‐linked genes ‐ may be present even in lepidopterans with a WZ/ZZ sex chromosome arrangement (Yoshido et al., 2016). Although the ultimate sex‐determination signals in DBM have yet to be identified, use of Masc is not dependent on the dominant W system. This flexibility further emphasizes the utility of designing genetic pest management systems around upstream sex‐determination components such as Masc.
Experimental procedures
Insect rearing
DBM were reared on beet armyworm artificial diet (Frontier Biosciences, Germantown, Maryland, USA) under a 16:8 h light : dark cycle, 25 °C and 50% relative humidity.
Identification of putative DBM Masc homologues
The B. mori Masc amino acid sequence (accession number: AB840788.1) was used as a BLASTp query against the available DBM genome (http://iae.fafu.edu.cn/DBM/). Genes showing significant hits were further searched for evidence of homology to the 304–310 aa region of B. mori Masc, a highly conserved sequence containing a cysteine‐cysteine domain known to be required for masculinizing ability (Katsuma et al., 2015).
RNAi injections
The e‐rnai online program (https://www.dkfz.de/signaling/e-rnai3/) was used to identify suitable target regions in putative DBM Masc homologues (450 bp in SepA‐like and 498 bp in CCCH) as well as the AmCyan fluorescent protein CDS (456 bp) used as a negative control. AmCyan was chosen as an inert control as it did not show significant sequence similarity when used as a BLASTn query against the DBM genome. Pooled third‐instar total cDNA was used as template for SepA‐like owing to the presence of introns in the suitable region. For CCCH, gDNA was used while AmCyan was amplified from a plasmid template. Primers were designed to amplify these regions while adding a T7 promoter sequence to the 5′ and 3′ ends of the amplicon. Each amplicon was used as template in a Megascript T7 in vitro transcription kit (Ambion, Austin, Texas, USA), then cleaned up with a Megaclear kit (Ambion). dsRNA integrity was confirmed by running aliquots on a 1.5% agarose gel prior to injection. Primer sequences used were as follows with T7 sequences underlined.
SepA.target+T7F:TAATACGACTCACTATAGGAGAGTAAACAACTCATTGAACAATCAATAAAC.
SepA.target+T7R: TAATACGACTCACTATAGCGTACCCGTTGTTCACTTCATG.
CCCH‐like.target+T7F: TAATACGACTCACTATAGCGAGGATCAGTTCTACTGCAC.
CCCH‐like.target+T7R: TAATACGACTCACTATAGGGTCATACTCACAGTGGCAAG.
AmCyan‐RNAi +T7F: TAATACGACTCACTATAGATCTTCTCGAAGGAGGGGTCC.
AmCyan‐RNAi +T7R: TAATACGACTCACTATAGTATGGCCCTGTCCAACAAGTTCATC.
To assess the ability of the putative homologues to disrupt male dosage compensation, each of the three dsRNA mixes was individually injected into DBM eggs c. 2–3 h after being laid. Each dsRNA was injected at approx. 5 μM using injection procedures previously described (Martins et al., 2012). Injected eggs were incubated in a humidified Petri dish and assessed for hatch rates, pupation rates, eclosion rates and sex ratios of pupae (the first life stages that can be reliably sexed by eye).
In a separate experiment to assess the ability of the putative homologues to disrupt male dsx splicing, dsRNA mixes for SepA‐like and AmCyan were injected into 2–3‐h‐old DBM eggs as above. Eggs were allowed to develop in a humidified Petri dish and 50 from each injection cohort were individually frozen in liquid nitrogen 48 h postinjection for subsequent RNA extractions.
RNA extractions and dsx PCR
To characterize the sex‐alternate splicing of DBM dsx we utilized partial dsx sequences available from the DBM genome (http://iae.fafu.edu.cn/DBM/), combined with our previous experience from identifying conserved dsx exonic regions in the pink bollworm P. gossypiella (Jin et al., 2013) to design primers specific to the exons flanking the sex‐alternatively spliced region using Q5 polymerase (New England Biolabs, Beverly, MA, USA) and primers Dsxshared.F: CGGTGAACATCGAGAACCTGGT + Dsxshared.R: GCAGCACAGCGAGTACGTGTCC. PCR programme = 98 °C – 30 s, 35 cycles of 98 °C – 15 s, 70 °C – 30 s, 72 °C – 30 s, final extension 72 °C – 2 min. Exon numbering is based on homology to dsx genes characterized in other lepidopterans (Jin et al., 2013; Wang et al., 2014). Reading frame is as the conserved reading frame in available DBM genome resources (http://iae.fafu.edu.cn/DBM/ and http://ensembl.lepbase.org/Plutella_xylostella_pacbiov1/Info/Index). Total RNA was extracted from manually sexed male and female DBM pupae using a RNeasy Mini kit (Qiagen, Valencia, CA, USA) and cDNA generated using a High Capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA, USA). This cDNA was used as a template for PCR with dsx primers; after gel electrophoresis of the PCR products visible bands were cloned into a Clonejet PCR cloning kit (Thermo Fisher Scientific, Waltham, Massachusetts, USA) and sequenced to determine sex‐alternative splicing patterns in males and females. Sequenced cDNA sequences are provided in Fig. S1.
To assess whether knockdown of putative DBM Masc homologues affected dsx splicing, RNA from individual injected eggs was extracted 48 h postinjection (see above) using a RNeasy Micro kit (Qiagen). Total RNA was then used for cDNA synthesis using the High Capacity cDNA reverse transcription kit. Dsx splicing was assessed by PCR of these individual cDNA samples using the primers/programme listed above and rates of ‘male’ and ‘female’ specific splicing patterns recorded by analysing characteristic band sizes on 1% agarose gels.
5′ and 3′ RACE of SepA‐like
Male pupal total RNA was extracted using the RNeasy Mini kit. 5′ and 3′ RACE was performed on this RNA using a SMARTer 5′/3′ RACE kit (Takara Bio, Kyoto, Japan). Two gene specific primers were chosen in CDS regions of SepA‐like downstream of the cysteine‐cysteine motif (for 5′ RACE) SepA.5′RACE.Nest1: GTGGGTCTCCTTTTGCACGACGGGCGTGCCCTC, SepA.5′RACE.Nest2: TTTACTCTCAACAACTCTTGTCTTGAGCTGAGCAC and in the final annotated exon for 3′ RACE.
SepA.3′RACE.Nest1: GGAGTGTGCCAGAACATGTCCTCATCCTACCACTACACCACG, SepA.3′RACE.Nest2: CTCTCCATTCCCCTTTGGTACAAAGAATATCAGCTCCAGAAATTTGATTGTCTGATGCAG. Nested reactions were performed according to the manufacturerʼs instructions and generated bands cloned into the Clonejet PCR cloning kit, sequenced and aligned against available DBM genome resources (http://iae.fafu.edu.cn/DBM/ and http://ensembl.lepbase.org/Plutella_xylostella_pacbiov1/Info/Index). Once UTR ends had been confirmed, two‐step RT‐PCR (LunaScript RT Mastermix Kit, New England Biolabs) was conducted to confirm the transcript sequence between these regions using Q5 polymerase (New England Biolabs) and primers SepA CDS.F: GCATATTATGGTCCTGTCCCACACCCCACCTACAC + SepA CDS.R: CGTTGCCCAACACTTACAAAATATTGAGATCTACGTGTTTGTG. PCR programme = 98 °C – 30 s, 35 cycles of 98 °C – 15 s, 72 °C – 30 s, 72 °C – 1 min, final extension 72 °C – 2 min. The full mRNA transcript is provided in the Supporting Information Figure S3.
Temporal expression patterns of dsx and SepA‐like
Initial collection: DBM were allowed to lay eggs on a cabbage‐juice painted piece of Parafilm (Bemis Company, Inc., Neenah, Wisconsin, USA) during a 5 min period. 50 eggs were individually collected and frozen in liquid nitrogen at each of 3, 6 and 24 h after this initial 5‐min collection period. A minimum of 25 eggs from each collection point were used for subsequent RNA extraction (RNeasy Micro kit, Qiagen) and two‐step RT‐PCR (LunaScript RT Mastermix Kit, New England Biolabs). cDNA from individual embryos was immediately used as template in PCR (Q5 polymerase, New England Biolabs) to assess the splicing/expression patterns of SepA‐like and dsx at each of these time points. Primers and programmes for these PCRs given above.
DNA extractions and genomic copy number qPCR
DBM pupae were manually sexed and gDNA was extracted from fresh individual males and females using a NucleoSpin® Tissue kit (Macherey‐Nagel, Düren, Germany) following homogenization of whole pupae with a mortar and pestle. DNA yield and purity for each sample were quantified using a NanoDrop 2000 Spectrophotometer (Thermo Fisher Scientific).
To determine if SepA‐like was Z‐linked we used sequences available from the DBM genome (http://iae.fafu.edu.cn/DBM/) in conjunction with sequences obtained from 5′ RACE and subsequent RT‐PCR of PxyMasc to design qPCR primers that amplified a 75‐bp region of the PxyMasc CDS. Primers used were as follows: PxyMasc_qCNV_F: GGTACCACCTCAGTGCCTCATC and PxyMasc_qCNV_R: AACTATGTGACTTACTGGGCCGA.
Defensin, a known autosomal gene, was used as a reference gene using the previously published primers Def‐qF:CCAACCGGTCAACAGTCAAAATG and Def‐qR: TCTCGGGTAACACAAAGCACTCG (Belousova et al., 2019). Kettin, a known Z‐linked gene with a conserved sequence across Lepidoptera, was used as a positive control using primers ketU‐F: TACAGCCAGCTCGCGAATC and ketU‐R: GCCCGTAGGTGCATGATGTT, again previously published (Belousova et al., 2019).
To assess the efficiency of each primer set we constructed standard curves using a four‐point dilution series (230–0.2 ng) of pooled male and female DNA. PCR efficiencies (E) were constructed on quantstudio design and analysis software v. 1.3.1 (Thermo Fisher Scientific) using the following formula: E = 10−1/slope − 1.
Each reaction was run on the QuantStudio3 PCR system (Applied Biosystems) on ‘Fast’ cycling settings using SYBR green chemistry. Each 10‐μl reaction was run on a 96‐well plate and included 1x Luna® Universal qPCR Master Mix (New England BioLabs), a final concentration of 500 nM of both forward and reverse primers, nuclease‐free molecular grade water, and 2 ng template. The cycling conditions were as follows: an initial denaturation step of 95 °C for 20 s followed by 40 cycles of 95 °C for 1 s and 60 °C for 20 s, followed by a final dissociation stage for melt curve analysis of 95 °C for 1 s, 60 °C for 20 s, and 95 °C for 1 s. 10 biological replicates were included for each group (ie 10 males and 10 females). Each reaction was run in triplicate, and each plate included no template controls for each primer set and an inter‐run calibrator sample. The melt curve profile for each reaction was assessed to check for specificity, and products were visualized on a 2% agarose gel to confirm a single product of the expected size. Relative quantification was calculated using the 2−ΔΔCq method to estimate genomic copy numbers of PxyMasc in male and female DBM.
Statistics
Sex ratio data, hatch rate and double sex splicing rates were compared between treatments using a chi‐squared analysis. Post‐hoc tests, where applicable, used the sequential Bonferroni correction. All analyses and figures were carried out in R (R Core Team, 2018, v. 3.5.0, https://www.r-project.org) and prism (graphpad, v. 8.1.2, http://www.graphpad.com). Details of replicates and individual test results are provided in text.
Supporting information
Appendix S1 Supporting Information
Acknowledgements
Thanks to Dan Olmstead for providing permission to use the graphical abstract image. T.H.‐S., V.C.N., R.C., E.L. and L.A. are supported by European Union H2020 Grant nEUROSTRESSPEP (634361). This project was supported through strategic funding from the UK Biotechnology and Biological Sciences Research Council (BBSRC) to the Pirbright Institute (BBS/E/I/00007033, BBS/E/I/00007038 and BBS/E/I/00007039).
Contributor Information
T. Harvey‐Samuel, Email: tim.harvey-samuel@pirbright.ac.uk.
L. Alphey, Email: luke.alphey@pirbright.ac.uk.
References
- Alphey, N. , Bonsall, M.B. and Alphey, L. (2011) Modeling resistance to genetic control of insects. Journal of Theoretical Biology, 270, 42–55. [DOI] [PubMed] [Google Scholar]
- Backus, G.A. and Gross, K. (2016) Genetic engineering to eradicate invasive mice on islands: modelling the efficiency and ecological impacts. Ecosphere, 7, e01589. [Google Scholar]
- Belousova, I. , Ershov, N. , Pavlushin, S. , Ilinsky, Y. and Martemyanov, V. (2019) Molecular sexing of Lepidoptera. Journal of Insect Physiology, 114, 53–56. [DOI] [PubMed] [Google Scholar]
- Black, W.C. , Alphey, L. and James, A.A. (2011) Why RIDL is not SIT. Trends in Parasitology, 27, 362–370. [DOI] [PubMed] [Google Scholar]
- Burt, A. (2003) Site‐specific selfish genes as tools for the control and genetic engineering of natural populations. Proceedings of the Royal Society B: Biological Sciences, 270, 921–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt, A. and Deredec, A. (2018) Self‐limiting population genetic control with sex‐linked genome editors. Proceedings of the Royal Society B: Biological Sciences, 285, 20180776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X. , Cao, Y. , Zhan, S. , Tan, A. , Palli, S.R. and Huang, Y. (2019) Disruption of sex‐specific doublesex exons results in male‐ and female‐specific defects in the black cutworm, Agrotis ipsilon . Pest Management Science, 75, 1697–1706. [DOI] [PubMed] [Google Scholar]
- Dalikova, M. , Zrzava, M. , Hladova, I. , Nguyen, P. , Sonsky, I. , Flegrova, M. et al (2017) New insights into the evolution of the W chromosome in Lepidoptera. Journal of Heredity, 108, 709–719. [DOI] [PubMed] [Google Scholar]
- Esvelt, K.M. , Smidler, A.L. , Catteruccia, F. and Church, G.M. (2014) Concerning RNA‐guided gene drives for the alteration of wild populations. Elife, 3, e03401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraisse, C. , Picard, M.A.L. and Vicoso, B. (2017) The deep conservation of the Lepidoptera Z chromosome suggests a non‐canonical origin of the W. Nature Communications, 8, 1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui, T. , Kawamoto, M. , Shoji, K. , Kiuchi, T. , Sugano, S. , Shimada, T. et al (2015) The endosymbiotic bacterium Wolbachia selectively kills male hosts by targeting the masculinizing gene. PLoS Pathogens, 11, e1005048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui, T. , Kiuchi, T. , Shoji, K. , Kawamoto, M. , Shimada, T. and Katsuma, S. (2018) In vivo masculinizing function of the Ostrinia furnacalis Masculinizer gene. Biochemical and Biophysical Research Communications, 503, 1768–1772. [DOI] [PubMed] [Google Scholar]
- Furlong, M.J. , Wright, D.J. and Dosdall, L.M. (2013) Diamondback moth ecology and management: problems, progress, and prospects. Annual Review of Entomology, 58, 517–541. [DOI] [PubMed] [Google Scholar]
- Harvey‐Samuel, T. , Ant, T. , Gong, H. , Morrison, N.I. and Alphey, L. (2014) Population‐level effects of fitness costs associated with repressible female‐lethal transgene insertions in two pest insects. Evolutionary Applications, 7, 597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey‐Samuel, T. , Morrison, N.I. , Walker, A.S. , Marubbi, T. , Yao, J. , Collins, H.L. et al (2015) Pest control and resistance management through release of insects carrying a male‐selecting transgene. BMC Biology, 13, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, L. , Walker, A.S. , Fu, G. , Harvey‐Samuel, T. , Dafaʼalla, T. , Miles, A. et al (2013) Engineered female‐specific lethality for control of pest Lepidoptera. ACS Synthetic Biology, 2, 160–166. [DOI] [PubMed] [Google Scholar]
- KaramiNejadRanjbar, M. , Eckermann, K.N. , Ahmed, H.M.M. , Sanchez, C.H.M. , Dippel, S. , Marshall, J.M. et al (2018) Consequences of resistance evolution in a Cas9‐based sex conversion‐suppression gene drive for insect pest management. Proceedings of the National Academy of Sciences of the United States of America, 115, 6189–6194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuma, S. , Sugano, Y. , Kiuchi, T. and Shimada, T. (2015) Two conserved cysteine residues are required for the masculinizing activity of the silkworm Masc protein. Journal of Biological Chemistry, 290, 26114–26124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiuchi, T. , Koga, H. , Kawamoto, M. , Shoji, K. , Sakai, H. , Arai, Y. et al (2014) A single female‐specific piRNA is the primary determiner of sex in the silkworm. Nature, 509, 636–636. [DOI] [PubMed] [Google Scholar]
- Kiuchi, T. , Sugano, Y. , Shimada, T. and Katsuma, S. (2019) Two CCCH‐type zinc finger domains in the Masc protein are dispensable for masculinization and dosage compensation in Bombyx mori . Insect Biochemistry and Molecular Biology, 104, 30–38. [DOI] [PubMed] [Google Scholar]
- Lee, J. , Kiuchi, T. , Kawamoto, M. , Shimada, T. and Katsuma, S. (2015) Identification and functional analysis of a Masculinizer orthologue in Trilocha varians (Lepidoptera: Bombycidae). Insect Molecular Biology, 24, 561–569. [DOI] [PubMed] [Google Scholar]
- Li, D.R. , Ye, H.L. , Yang, J.S. , Yang, F. , Wang, M.R. , De Vos, S. et al (2017) Identification and characterization of a Masculinizer (Masc) gene involved in sex differentiation in Artemia . Gene, 614, 56–64. [DOI] [PubMed] [Google Scholar]
- Martins, S. , Naish, N. , Walker, A.S. , Morrison, N.I. , Scaife, S. , Fu, G. et al (2012) Germline transformation of the diamondback moth, Plutella xylostella L., using the piggyBac transposable element. Insect Molecular Biology, 21, 414–421. [DOI] [PubMed] [Google Scholar]
- Mitter, C. , Davis, D.R. and Cummings, M.P. (2017) Phylogeny and evolution of Lepidoptera. Annual Review of Entomology, 62(62), 265–283. [DOI] [PubMed] [Google Scholar]
- Prowse, T.A.A. , Cassey, P. , Ross, J.V. , Pfitzner, C. , Wittmann, T.A. and Thomas, P. (2017) Dodging silver bullets: good CRISPR gene‐drive design is critical for eradicating exotic vertebrates. Proceedings of the Royal Society B‐Biological Sciences, 284, 20170799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai, H. , Sumitani, M. , Chikami, Y. , Yahata, K. , Uchino, K. , Kiuchi, T. et al (2016) Transgenic expression of the piRNA‐resistant Masculinizer gene induces female‐specific lethality and partial female‐to‐male sex reversal in the silkworm, Bombyx mori . PLoS Genetics, 12, e1006203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talekar, N.S. and Shelton, A.M. (1993) Biology, ecology, and management of the diamondback moth. Annual Review of Entomology, 38, 275–301. [DOI] [PubMed] [Google Scholar]
- Tan, A. , Fu, G. , Jin, L. , Guo, Q. , Li, Z. , Niu, B. et al (2013) Transgene‐based, female‐specific lethality system for genetic sexing of the silkworm, Bombyx mori . Proceedings of the National Academy of Sciences of the United States of America, 110, 6766–6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, D.D. , Donnelly, C.A. , Wood, R.J. and Alphey, L.S. (2000) Insect population control using a dominant, repressible, lethal genetic system. Science, 287, 2474–2476. [DOI] [PubMed] [Google Scholar]
- Traut, W. , Sahara, K. and Marec, F. (2007) Sex chromosomes and sex determination in Lepidoptera. Sexual Development, 1, 332–346. [DOI] [PubMed] [Google Scholar]
- Wang, X.Y. , Zheng, Z.Z. , Song, H.S. and Xu, Y.Z. (2014) Conserved RNA cis‐elements regulate alternative splicing of Lepidopteran doublesex . Insect Biochemistry and Molecular Biology, 44, 1–11. [DOI] [PubMed] [Google Scholar]
- Wang, Y.H. , Chen, X.E. , Yang, Y. , Xu, J. , Fang, G.Q. , Niu, C.Y. et al (2019) The Masc gene product controls masculinization in the black cutworm, Agrotis ipsilon . Insect Science, 26, 1037–1044. [DOI] [PubMed] [Google Scholar]
- Yoshido, A. , Marec, F. and Sahara, K. (2016) The fate of W chromosomes in hybrids between wild silkmoths, Samia cynthia ssp.: no role in sex determination and reproduction. Heredity, 116, 424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information


