Abstract
Chronic diarrhoea is a frequent complaint in canine practice and the diagnostic path is often characterised by numerous diagnostic tests and stepwise empirical treatments, often applied before gastrointestinal endoscopy/mucosal biopsies. These include dietary interventions (novel protein, hydrolysed protein diet), parasiticides and still, in many cases, antibacterials. Indiscriminate use of antibacterial drugs risks detrimental consequences for both the individual patient (antimicrobial resistance, long‐term disruption of intestinal bacterial populations, potential worsening of gastrointestinal signs) and the general public. For that reason, in this Perspective essay we advocate use of antibacterials only after histopathologic evaluation of gastrointestinal biopsies or, for those cases in which endoscopy is not possible, after other therapeutic trials, such as diet/pre‐probiotics or anti‐inflammatory drugs have proven unsuccessful. They should be reserved, after appropriate dietary trials, for those canine chronic diarrhoeic patients with signs of true primary infection (i.e. signs of systemic inflammatory response syndrome or evidence of adherent‐invasive bacteria) that justify antibacterial use.
The standard diagnostic investigation of chronic diarrhoea usually includes a variety of laboratory investigations, such as complete blood count, serum biochemistry panel, urinalysis, faecal exams, evaluation of pancreatic function and inflammation, endocrine assays (i.e. adrenal gland function), as well as diagnostic imaging procedures (e.g. abdominal radiographs/ultrasound) and GI endoscopy, including mucosal biopsies for histopathological examination. Empirical treatment is often trialled and can include dietary interventions, parasiticides and antibacterials (ABs) such as metronidazole or tylosin (Jergens et al. 2003, Kilpinen et al. 2015, Allenspach et al. 2016). These empirical treatments are often administered before GI endoscopy/mucosal biopsy because they may represent effective strategies for managing canine chronic enteropathy (Volkmann et al. 2017, Heilmann et al. 2018). Corticosteroids and other immunosuppressants are needed in some cases to control clinical signs, but are generally recommended after other treatment strategies have been exhausted and after mucosal biopsies have been obtained, which are necessary to diagnose intestinal inflammation and other GI disorders such as (primary) lymphangiectasia, infectious agents (e.g. fungal infections, adherent‐invasive Escherichia coli) and neoplastic infiltration (e.g. lymphoma).
Antibiotic‐responsive diarrhoea is recognised as one form of chronic enteropathy. Its clinical presentation is indistinguishable from other types of chronic enteropathy; it is associated with intestinal microbiota dysbiosis (Hall 2011) and responds exquisitely well to administration of ABs, while it often recurs as soon as they are withdrawn (Hall 2011, Westermarck 2016). Most often, tylosin (tylosin‐responsive diarrhoea) (Westermarck et al. 2005), metronidazole (Allenspach et al. 2016) and oxytetracycline (Hall 2011) have been used as therapy. Diagnosis is based on a positive response to ABs after exclusion of other conditions as outlined above. In antibiotic‐responsive diarrhoea, histopathology of intestinal biopsies, if acquired, frequently shows no or only mild non‐specific inflammatory infiltrates or abnormalities (Hall 2011, Volkmann et al. 2017). However, although ABs constitute an empirical therapy instituted by many clinicians in dogs with chronic diarrhoea, this might lead to unnecessary administration or overuse of ABs, as not all of these dogs will eventually be diagnosed with antibiotic‐responsive diarrhoea. This practice is of even greater concern considering recent reports in which antibiotic‐responsive diarrhoea was retrospectively reported in only 11 of 136 (8%) (Volkmann et al. 2017) and 33 of 203 (16.2%) (Allenspach et al. 2016) of dogs with chronic diarrhoea, respectively. In addition, a study by Jergens et al. (2010) is noteworthy, because it shows oral prednisone alone to be clinically as effective as prednisone plus metronidazole in dogs suffering from inflammatory bowel disease, suggesting that the use of antibiotics might not always be necessary.
Based on our experience and on the evidence in the literature reported here, the aim of this report is to make a strong argument against the empirical use of ABs when routinely managing dogs with suspected chronic enteropathy. The use of ABs should be reserved for patients in which all other conditions are excluded and other empirical treatments have been exhausted. The following paragraphs will elucidate potential detrimental effects of ABs on individual gut health and public health.
EFFECTS OF ANTIBACTERIALS ON GI MICROBIOTA
Although the effect of AB administration on the faecal microbiota still needs to be defined further, some aspects have been investigated in humans (Rizzatti et al. 2018) and both healthy and diseased animals. It is known that AB administration causes changes in the composition and richness of the intestinal microbiota in dogs and cats and that this dysbiosis can be detrimental to overall host health (Suchodolski 2016), similarly to those presenting with inflammatory bowel disease (Minamoto et al. 2015). Specifically, the administration of oral tylosin (20 to 22 mg/kg SID for 14 days) to healthy dogs was associated with changes in the proportions of jejunal bacteria (e.g. increases in Enterococcus‐like organisms and Pasteurella spp.). Microbiota alterations (i.e. increase of Escherichia coli‐like organisms) were still observed 14 days after withdrawal of tylosin (Suchodolski et al. 2009). The phylogenetic composition of the microbiota following tylosin withdrawal was comparable to day 0 in only two of five dogs, while bacterial diversity was similar in three of five dogs, suggesting a possible long‐term adverse effect in some animals (Suchodolski et al. 2009). Very recently, as found in healthy dogs, it was shown that the administration of tylosin (20 mg/kg PO BID) induced dysbiosis‐ and eubiosis was not restored by 56 days following tylosin discontinuation, leading the authors to conclude that in these patients “… reestablishment of the native microbiota is possible but not guaranteed.” (Manchester et al. 2019). Faecal bacterial diversity was also reduced when administering oral metronidazole for 14 days (12.5 mg/kg BID) to healthy dogs (Igarashi et al. 2014). Similarly, oral administration of amoxicillin (10 mg/kg BID for 7 days) to healthy dogs resulted in differences in faecal bacterial composition before and after administration, with many faecal E. coli isolates showing increased resistance to multiple ABs during and after treatment (Grønvold et al. 2010). Interestingly, in dogs with tylosin‐responsive diarrhoea, there were increases in faecal Enterococcus spp. and other potentially probiotic bacteria (including lactic acid bacteria) (Kilpinen et al. 2015). It was speculated that there could be a possible indirect probiotic effect of tylosin by exerting a selection pressure, resulting in a relative increase in tylosin‐resistant enterococci (Kilpinen et al. 2015). Although these results are interesting, there are still concerns that antibacterial resistance could be passed horizontally from commensal bacteria or alleged probiotics to pathogenic bacteria sharing the same intestinal environment (von Wintersdorff et al. 2016).
Furthermore, the metabolic pathways through which ABs may alter gut homeostasis are still under investigation. For example, a recent paper suggests that the administration of a cocktail of antibiotics in mice (antibiotic‐induced microbiome depletion) in addition to modifying the abundance of some bacterial species, also altered glucose homeostasis and luminal short‐chain fatty acid concentration (enterocytes use glucose instead of butyrate, which is reduced in the intestinal lumen) and bile acid metabolism (Zarrinpar et al. 2018).
Additionally, it is anecdotally reported that select antimicrobials may have immunomodulatory or anti‐inflammatory actions in treating chronic enteropathy; in particular, metronidazole has recognised immunosuppressive and anti‐inflammatory properties (Shakir et al. 2011). In one small pilot study, the effects of metronidazole, tylosin and conjugated linoleic acid on immune function were evaluated in healthy dogs (Jergens et al. 2007). In this study, peripheral blood mononuclear cells were isolated, incubated with graded doses of the antibiotics and conjugated linoleic acid, and immune responses were investigated using MTT cytotoxicity assay, immunostaining and flow cytometry of B and T lymphocyte subpopulations and in vitro mitogen‐induced lymphocyte proliferation (3H‐thymidine incorporation). Results indicated that metronidazole and tylosin, at different dosages, were not successful in arresting mitogen‐stimulated proliferation of lymphocytes; in contrast, conjugated linoleic acid was shown to directly inhibit peripheral blood mononuclear cell blastogenesis. Moreover, it must also be remembered that the use of antimicrobials for non‐antimicrobial effects is questionable and discouraged (Weese et al. 2015).
ANTIBACTERIAL RESISTANCE
Antibiotic resistance represents one of the most serious and imminent health‐related problems worldwide (WHO 2017); the last joint EFSA/ECDC report Antimicrobial resistance in Europe, (http://www.efsa.europa.eu/it/interactive_pages/AMR_Report_2016) based on 2016 data reflects on this alarming situation. The problem is also recognised in companion animals (Peter et al. 2017), with serious potential concerns for human health associated with methicillin‐resistant Staphylococcus aureus (MRSA) (Rossi et al. 2017). More specifically, for complaints related to the digestive tract, the gut microbiota has been considered as a dynamic reservoir of antibiotic resistance, termed the (“gut resistome”), which can be affected by the administration of ABs (Rizzatti et al. 2018). A recent report showed that 54% of isolates of Clostridium perfringens from dogs with acute diarrhoea, which had not been treated with antibiotics, presented with a decreased susceptibility to metronidazole (Gobeli et al. 2012). Moreover, dogs are also considered a possible reservoir of antibiotic resistant strains potentially dangerous for human patients. This includes the discovery of epidemic ribotypes of multiresistant Clostridioides (Clostridium) difficile (Nagy 2018) in dogs with GI disorders (Orden et al. 2017).
ALTERNATIVE INTESTINAL MICROBIOTA MODULATION
There is growing interest and clinical evidence supporting alternative treatments to modulate bacterial populations, which could include the administration of prebiotics, probiotics or synbiotics. Several studies show that probiotics are likely beneficial in cases of diarrhoea, even in dogs suffering from inflammatory bowel disease (Rossi et al. 2014, White et al. 2017, Rossi et al. 2018). Another promising modality is faecal microbiota transplantation. It has been recommended for recurrent Clostridioides (Clostridium) difficile infections in humans, a hospital‐acquired infection that usually develops after extensive AB treatment (Cammarota et al. 2015,Cammarota et al. 2017, Quraishi et al. 2017). Even though an equivalent of this condition is lacking in dogs (it is not comparable to an infection with Clostridioides [Clostridium] difficile sometimes seen in dogs with both acute and chronic diarrhoea) (Marks et al. 2011), it is reasonable to assume that dysbiosis caused by inappropriate AB treatment should be avoided in canine patients. Results from faecal microbiota transplantation in human subjects suggest that the attempt to “restore” a physiological microbiota could be more effective than ABs alone in some specific cases (Cammarota et al. 2017, Quraishi et al. 2017). Unfortunately, large scale studies on the clinical effect (short‐ and long‐term effects) or the efficacy of restoring eubiosis by administration of faecal microbiota transplantation are currently lacking. The optimal donor screening and indication, as well as the best modality and frequency of administration of faecal microbiota transplantation in dogs, is currently unknown, because only a few studies are available (Chaitman et al. 2016, Burton et al. 2016, Pereira et al. 2018). We are still a far way away from suggesting its use as a routine treatment in dogs with acute and/or chronic diarrhoea, because scientific evidence from appropriately designed prospective studies is lacking. However, these data underline that ABs may not be the best option to treat some forms of infectious diarrhoea; on the contrary, alternative attempts to modulate and restore intestinal microbiota should be considered in diarrhoeic dogs.
PROPOSAL FOR RATIONAL USE OF ANTIBACTERIALS IN THE DIAGNOSIS AND TREATMENT OF CHRONIC DIARRHOEA IN DOGS
The necessity of avoiding empirical and injudicious use of antibacterials in diarrhoeic dogs has previously been emphasised (Marks et al. 2011, Heilmann et al. 2018). Furthermore, considering the global concern for rising antibiotic resistance, the dysbiosis associated with indiscriminate use of ABs, and the potentially associated worsening of GI signs, the risks for both the individual patient and potential harm to the general public of using ABs in chronic GI diseases needs to be fully appreciated by clinicians.
Clinicians should consider using ABs only after appropriate dietary trials have been unsuccessful and only after histopathologic evaluation of GI biopsies in all cases in which it is possible. In cases in which biopsies cannot be acquired we recommend use of ABs only after other empirical therapeutic trials are unsuccessful. In short, after having excluded all conditions (Fig 1) that would not benefit from administration of ABs, they can be considered for those canine chronic diarrhoeic patients with signs of true primary infection that justify AB usage or in those patients in which endoscopy is not possible (or histopathology is not conclusive) and that are not responsive to any other treatment. They may be an option in those cases showing signs of systemic inflammatory response syndrome (e.g. pyrexia, inflammatory left‐shifted leucogram or leucopenia) or in cases of acute infection with a known enteric bacterial pathogen that is not self‐limiting or responding to symptomatic treatment. However, considering that there are few indications for using ABs in chronic diarrhoea, because primary bacterial agents causing non‐self‐limiting disease are rare (Marks et al. 2011), it means that these treatments should be reserved for specific diseases and in accordance with appropriate AB resistance testing panels, avoiding the empirical use of broad spectrum ABs such as amoxicillin/clavulanic acid or fluoroquinolones.
Figure 1.
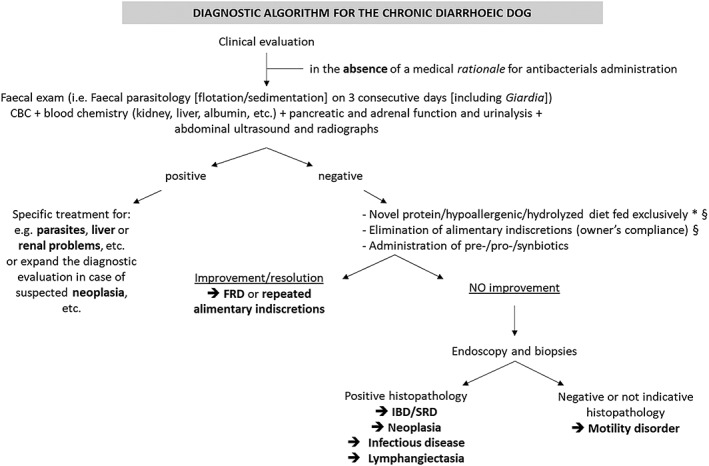
Diagnostic algorithm for the diarrhoeic dog. This algorithm is suggested by our combined clinical experience, but different parts are also variably widely reported in literature, including (but not limited to): Jergens et al. 2003, Allenspach et al. 2007, Cerquetella et al. 2010, Washabau et al. 2010, Allenspach et al. 2016, Westermarck et al. Westermarck 2016, Erdmann & Heilmann 2017, Volkmann et al. 2017, Cerquetella et al. 2018, Heilmann et al. 2018. It is recommended to use (targeted, not broad spectrum) ABs in the chronic diarrhoeic dog (when there is no clinical evidence of a medical rationale for their immediate use), only at the end of the diagnostic protocol, once GI biopsies are performed, and with evidence of infectious causes. *In some cases more than one diet change may be needed, and for this reason duration of diet trial may vary. §Depending on the severity of clinical condition, these trials can be postponed going forward with the algorithm. FRD Food‐responsive diarrhoea, IBD Inflammatory bowel disease, SRD Immunosuppressive/steroid‐responsive diarrhoea
Conflict of interest
None of the authors of this article has a financial or personal relationship with other people or organisations that could inappropriately influence or bias the content of the paper.
References
- Allenspach, K. , Wieland, B. , Gröne, A. , et al (2007) Chronic enteropathies in dogs: evaluation of risk factors for negative outcome. Journal of Veterinary Internal Medicine 21, 700‐708 [DOI] [PubMed] [Google Scholar]
- Allenspach, K. , Culverwell, C. & Chan, D. (2016) Long‐term outcome in dogs with chronic enteropathies: 203 cases. Veterinary Record 178, 368 [DOI] [PubMed] [Google Scholar]
- Antimicrobial resistance in Europe (n.d.). http://www.efsa.europa.eu/en/interactive_pages/AMR_Report_2016. Accessed November 27, 2018
- Burton, E. N. , O'Connor, E. , Ericsson, A. C. , et al (2016) Evaluation of fecal microbiota transfer as treatment for postweaning diarrhea in research‐colony puppies. Journal of the American Association for Laboratory Animal Science 55, 582‐587 [PMC free article] [PubMed] [Google Scholar]
- Cammarota, G. , Masucci, L. , Ianiro, G. , et al (2015) Randomised clinical trial: faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Alimentary Pharmacology & Therapeutics 41, 835‐843 [DOI] [PubMed] [Google Scholar]
- Cammarota, G. , Ianiro, G. , Tilg, H. , et al (2017) European consensus conference on faecal microbiota transplantation in clinical practice. Gut 66, 569‐580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerquetella, M. , Spaterna, A. , Laus, F. , et al (2010) Inflammatory bowel disease in the dog: differences and similarities with humans. World Journal of Gastroenterology 16, 1050‐1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerquetella, M. , Rossi, G. , Spaterna, A. , et al (2018) Is irritable bowel syndrome also present in dogs? Tierarztliche Praxis Ausgabe K: Kleintiere/Heimtiere 46, 176‐180 [DOI] [PubMed] [Google Scholar]
- Chaitman, J. , Jergens, A. E. , Gaschen, F. , et al (2016) Commentary on key aspects of fecal microbiota transplantation in small animal practice. Veterinary Medicine: Research and Reports 7, 71‐74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann, C. & Heilmann, R. M. (2017) Diagnostic and therapeutic approach to chronic inflammatory enteropathies in dogs. Tierarztliche Praxis Ausgabe K: Kleintiere/Heimtiere 45, 317‐327 (in German) [DOI] [PubMed] [Google Scholar]
- Gobeli, S. , Berset, C. , Burgener, I. , et al (2012) Antimicrobial susceptibility of canine Clostridium perfringens strains from Switzerland. Schweizer Archiv für Tierheilkunde 154, 247‐250 [DOI] [PubMed] [Google Scholar]
- Grønvold, A. M. , L'abée‐Lund, T. M. , Sørum, H. , et al (2010) Changes in fecal microbiota of healthy dogs administered amoxicillin. FEMS Microbiology Ecology 71, 313‐326 [DOI] [PubMed] [Google Scholar]
- Hall, E. J. (2011) Antibiotic‐responsive diarrhea in small animals. Veterinary Clinics of North America: Small Animal Practice. 41, 273‐286 [DOI] [PubMed] [Google Scholar]
- Heilmann, R. M. , Berghoff, N. , Mansell, J. , et al (2018) Association of fecal calprotectin concentrations with disease severity, response to treatment, and other biomarkers in dogs with chronic inflammatory enteropathies. Journal of Veterinary Internal Medicine 32, 679‐692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi, H. , Maeda, S. , Ohno, K. , et al (2014) Effect of oral administration of metronidazole or prednisolone on fecal microbiota in dogs. PLoS One 9, e107909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jergens, A. E. , Schreiner, C. A. , Frank, D. E. , et al (2003) A scoring index for disease activity in canine inflammatory bowel disease. Journal of Veterinary Internal Medicine 17, 291‐297 [DOI] [PubMed] [Google Scholar]
- Jergens, A. E. , Doran, A. , Boll, A. , et al (2007) The effects of selected antibiotics and nutraceuticals on mitogen‐stimulated lymphocyte proliferation in healthy dogs. Journal of Veterinary Internal Medicine 21, 654‐655 [Google Scholar]
- Jergens, A. E. , Crandell, J. , Morrison, J. A. , et al (2010) Comparison of oral prednisone and prednisone combined with metronidazole for induction therapy of canine inflammatory bowel disease: a randomized‐controlled trial. Journal of Veterinary Internal Medicine 24, 269‐277 [DOI] [PubMed] [Google Scholar]
- Kilpinen, S. , Rantala, M. , Spillmann, T. , et al (2015) Oral tylosin administration is associated with an increase of faecal enterococci and lactic acid bacteria in dogs with tylosin‐responsive diarrhoea. The Veterinary Journal 205, 369‐374 [DOI] [PubMed] [Google Scholar]
- Manchester, A. C. , Webb, C. B. , Blake, A. B. , et al (2019) Long‐term impact of tylosin on fecal microbiota and fecal bile acids of healthy dogs. Journal of Veterinary Internal Medicine, 33, 2605‐2617. 10.1111/jvim.15635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks, S. L. , Rankin, S. C. , Byrne, B. A. , et al (2011) Enteropathogenic bacteria in dogs and cats: diagnosis, epidemiology, treatment, and control. Journal of Veterinary Internal Medicine 25, 1195‐1208 [DOI] [PubMed] [Google Scholar]
- Minamoto, Y. , Otoni, C. C. , Steelman, S. M. , et al (2015) Alteration of the fecal microbiota and serum metabolite profiles in dogs with idiopathic inflammatory bowel disease. Gut Microbes 6, 33‐47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy, E. (2018) What do we know about the diagnostics, treatment and epidemiology of Clostridioides (Clostridium) difficile infection in Europe? Journal of Infection and Chemotherapy 24, 164‐170 [DOI] [PubMed] [Google Scholar]
- Orden, C. , Blanco, J. L. , Álvarez‐Pérez, S. , et al (2017) Isolation of Clostridium difficile from dogs with digestive disorders, including stable metronidazole‐resistant strains. Anaerobe 43, 78‐81 [DOI] [PubMed] [Google Scholar]
- Pereira, G. Q. , Gomes, L. A. , Santos, I. S. , et al (2018) Fecal microbiota transplantation in puppies with canine parvovirus infection. Journal of Veterinary Internal Medicine 32, 707‐711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter, R. , Demuth, D. , Müntener, C. , et al (2017) AntibioticScout.Ch: a decision supporting tool for antimicrobial stewardship: application to companion animal medicine. Schweizer Archiv für Tierheilkunde 159, 525‐533 [DOI] [PubMed] [Google Scholar]
- Quraishi, M. N. , Widlak, M. , Bhala, N. , et al (2017) Systematic review with meta‐analysis: the efficacy of faecal microbiota transplantation for the treatment of recurrent and refractory Clostridium difficile infection. Alimentary Pharmacology & Therapeutics 46, 479‐493 [DOI] [PubMed] [Google Scholar]
- Rizzatti, G. , Ianiro, G. & Gasbarrini, A. (2018) Antibiotic and modulation of microbiota: a new paradigm? Journal of Clinical Gastroenterology 52 Suppl 1, 1‐4. 10.1097/MCG.0000000000001069 [DOI] [PubMed] [Google Scholar]
- Rossi, G. , Pengo, G. , Caldin, M. , et al (2014) Comparison of microbiological, histological, and immunomodulatory parameters in response to treatment with either combination therapy with prednisone and metronidazole or probiotic VSL#3 strains in dogs with idiopathic inflammatory bowel disease. PLoS One 9, e94699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi, G. , Cerquetella, M. & Attili, A. R. (2017) Amphixenosic aspects of Staphylococcus aureus infection in man and animals. Current Topics in Microbiology and Immunology 409, 297‐323 [DOI] [PubMed] [Google Scholar]
- Rossi, G. , Cerquetella, M. , Scarpona, S. , et al (2018) Effects of probiotic bacteria on mucosal polyamines levels in dogs with IBD and colonic polyps: a preliminary study. Beneficial Microbes 9, 247‐255 [DOI] [PubMed] [Google Scholar]
- Shakir, L. , Javeed, A. , Ashraf, M. , et al (2011) Metronidazole and the immune system. Pharmazie 66, 393‐398 [PubMed] [Google Scholar]
- Suchodolski, J. S. (2016) Diagnosis and interpretation of intestinal dysbiosis in dogs and cats. The Veterinary Journal 215, 30‐37 [DOI] [PubMed] [Google Scholar]
- Suchodolski, J. S. , Dowd, S. E. , Westermarck, E. , et al (2009) The effect of the macrolide antibiotic tylosin on microbial diversity in the canine small intestine as demonstrated by massive parallel 16S rRNA gene sequencing. BMC Microbiology 9, 210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmann, M. , Steiner, J. M. , Fosgate, G. T. , et al (2017) Chronic Diarrhea in dogs – retrospective study in 136 cases. Journal of Veterinary Internal Medicine 31, 1043‐1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Wintersdorff, C. J. , Penders, J. , van Niekerk, J. M. , et al (2016) Dissemination of antimicrobial resistance in microbial ecosystems through horizontal gene transfer. Frontiers in Microbiology 7, 173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washabau, R. J. , Day, M. J. , Willard, M. D. , et al (2010) Endoscopic, biopsy, and histopathologic guidelines for the evaluation of gastrointestinal inflammation in companion animals. Journal of Veterinary Internal Medicine 24, 10‐26 [DOI] [PubMed] [Google Scholar]
- Weese, J. S. , Giguère, S. , Guardabassi, L. , et al (2015) ACVIM consensus statement on therapeutic antimicrobial use in animals and antimicrobial resistance. Journal of Veterinary Internal Medicine 29, 487‐498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermarck, E. (2016) Chronic diarrhea in dogs: what do we actually know about it? Topics in Companion Animal Medicine 31, 78‐84 [DOI] [PubMed] [Google Scholar]
- Westermarck, E. , Skrzypczak, T. , Harmoinen, J. , et al (2005) Tylosin‐responsive chronic diarrhea in dogs. Journal of Veterinary Internal Medicine 19, 177‐186 [DOI] [PubMed] [Google Scholar]
- White, R. , Atherly, T. , Guard, B. , et al (2017) Randomized, controlled trial evaluating the effect of multi‐strain probiotic on the mucosal microbiota in canine idiopathic inflammatory bowel disease. Gut Microbes 8, 451‐466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2017) Global Antimicrobial Resistance Surveillance System (GLASS) Report: Early Implementation 2016‐2017. World Health Organization, Geneva, Switzerland: pp 1‐164 [Google Scholar]
- Zarrinpar, A. , Chaix, A. , Xu, Z. Z. , et al (2018) Antibiotic‐induced microbiome depletion alters metabolic homeostasis by affecting gut signaling and colonic metabolism. Nature Communications 9, 2872 [DOI] [PMC free article] [PubMed] [Google Scholar]


