Abstract
Background and Aims
Although European‐wide data on the new psychoactive substances (NPS) drug market are available, country‐specific data are limited. We studied recent NPS trend data relative to all recreational drugs on the Dutch drug market.
Design
National observational study.
Setting
The Netherlands.
Data sources
Three national indicators were used between 2013 and 2017: (1) forensic drug samples offered to the Netherlands Forensic Institute (NFI); (2) drug samples submitted by consumers to the Drugs Information and Monitoring System (DIMS); and (3) exposures in which the Dutch Poisons Information Center (DPIC) was consulted.
Measurements
Overall NPS incidence rate was the primary outcome. Numbers and specific categories of NPS were also studied. Changes in NPS incidence rates over time were analyzed using Poisson regression analyses [year effect expressed as incidence rate ratios (IRR)].
Findings
From 2013 to 2017, NPS were involved in 1892 forensic samples, 6316 consumer samples and 481 poisons center exposures. In 2013, NPS incidence rates were 2.5, 7 and 4% versus 3, 11 and 11% in 2017, respectively, in the NFI, DIMS and DPIC samples/exposures. NPS incidence rates increased significantly in consumer samples between 2013 and 2016 [IRR = 1.23; 95% confidence interval (CI) = 1.18, 1.29] and in poisons center exposures between 2013 and 2017 (IRR = 1.19; 95% CI = 1.06, 1.35), while the trend in forensic samples appeared more stable. Phenethylamines were the largest class and were detected in 58, 80 and 63% of NFI, DIMS and DPIC samples/exposures, respectively. Detected phenethylamines mainly involved 4‐fluoroamphetamine and 2C‐x derivatives. The second largest class were cathinones, which were detected in 21, 11 and 16% of NFI, DIMS and DPIC samples/exposures, respectively.
Conclusions
Analysis of forensic drug samples, consumer drug samples and exposures reported to poison centers from 2013 to 2017 shows the constant presence of new psychoactive substances on the Dutch drug market and its use by the Dutch population. The two largest classes present in the Netherlands were phenethylamines and cathinones.
Introduction
During the last decade, new psychoactive substances (NPS) have emerged on the drug market within the European Union (EU). The number of newly detected NPS has increased annually, with the highest reported number of 101 NPS in 2014 compared with fewer than 20 NPS reported for the first time to the EU Early Warning System (EWS) in 2007. The pace at which NPS appear for the first time on the market has been slowing since 2015, but they are still reported at a rate of approximately one per week 1, 2, 3. By the end of 2018, 730 NPS were monitored by the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) 4.
With their widespread availability, it is not surprising that 4% of all 15–16‐year‐old European school students had used at least one NPS in 2014 3 and 8% of young adults (aged 15–34 years) in 2015 5. In high‐risk drug users, prevalence of use rises to 26% 6. NPS use may pose a risk for public health, including poisonings and fatalities associated with their use. As a consequence, more than 70 risk communications (health alerts) were issued by the EU EWS between 2013 and 2017 7, 8, 9, 10 [7–9, Andrew Cunningham, personal communication]. Therefore, insight into the presence and use of NPS and their associated health risks is of vital importance. The EMCDDA collects data from more than 30 European countries on NPS seizures, use and related harms via the EU EWS, and provides a thorough insight into the European drug situation. However, so far no EU Member State has reported the correlation between NPS‐related forensic seizures, use and harm on a national level. Also, the relative size of NPS compared with more traditional substances is not apparent.
Data acquired from forensic drug seizures provide information on the presence of NPS in a specific country. However, the presence itself provides little information on the actual use of NPS by the local population. For example, for some drugs a specific country might only serve as a transit country. Even when drugs are manufactured in a specific country, they could be intended for export only. Therefore, additional data are needed to determine whether drugs detected in forensic seizures are also used in that same country. Some countries offer drug‐checking services to potential consumers. While these services are controversial, they can provide an exceptional insight into which drugs are actually used. Also, these services can detect and warn for more toxic contaminants such as para‐methoxymethamphetamine (PMMA), which has been present in tablets sold as ecstasy 11, 12, 13. In addition, poisons centers conduct toxico‐surveillance and can identify and monitor the emergence of drugs that are a possible threat to public health. Through analysis of poisons center inquiries, data on adverse health effects can be obtained for specific drugs 14, 15. Combining data from these different sources provides a balanced overview of the presence, use and health risks of NPS in a specific country.
The objectives of this study are to investigate to what extent NPS have entered the Dutch drug market between 2013 and 2017 by studying recent NPS trend data relatively to all recreational drugs on the Dutch drug market, and to identify the NPS categories most often notified. To obtain a broad overview, we used three national indicators: forensic data, data from consumer drug‐checking services and poisons center data.
Methods
Seized drugs: Netherlands Forensic Institute database (NFI)
The NFI analyses suspected drug samples (street samples, dealer samples and drug import or export samples) offered by the Dutch (military) police and other authorities engaged in investigation of criminal offenses. Analysis should determine the presence of substances controlled by the Dutch Opium Act or Misuse of chemicals act.
Laboratory analysis of the drug samples typically involved Fourier transform infrared (FTIR) spectroscopy, gas chromatography–mass spectrometry (GC–MS) and/or ultra‐high‐performance liquid chromatography‐quadrupole time‐of‐flight MS (UHPLC‐Q‐TOF‐MS). Occasionally, nuclear magnetic resonance spectroscopy (NMR) was used for structure elucidation of new NPS. For details, see Supporting information methods.
All drug‐related samples offered to the team Illicit Drugs of the NFI from 2013 to 2017 were included into this study. One sample can contain more than one drug and/or NPS.
Drug‐checking service: Drugs Information and Monitoring System (DIMS)
DIMS consists of a nation‐wide network of testing facilities that offer drug‐checking to potential users. In brief, laboratory analysis of the drug samples involved the use of the Marquis reagent test, thin‐layer chromatography (TLC), gas chromatography nitrogen phosphorous detection (GC‐NPD), GC–MS and liquid chromatography coupled with diode array detection (LC‐DAD). For a detailed description of laboratory methods, see Brunt et al. 2017 11. All drug samples submitted to the DIMS from 2013 to 2017 were included into this study.
Poisons center data: Dutch Poisons Information Center (DPIC) database
The DPIC provides a 24/7 telephone service on the management of (suspected) poisonings to health‐care professionals only. All inquiries are logged for monitoring purposes, and during inquiry, anonymous data are collected on patient and exposure characteristics as well as on symptoms present prior to or at the time of the inquiry. All inquiries on human NPS exposures from 2013 to 2017 were queried. An inquiry may concern a patient with more than one drug exposure and, in this study, exposures were analyzed. For the analysis of the reported symptoms after NPS use, only patients without concomitant exposures were included. Therapeutic doses of medication and ≤ 2 units of alcohol were not considered as concomitant exposures. The observed severity of poisoning in patients without concomitant exposures was graded into five levels, using the poisoning severity score (PSS): none, mild, moderate, severe and fatal 16. The PSS was assigned based on symptoms reported during the telephone inquiry.
Data analysis
Overall NPS incidence rates were calculated by dividing the number of samples or exposures containing NPS by the number of samples or exposures containing recreational drugs, per year. ‘Traditional' recreational drugs included, for example, cannabis, cocaine, 3,4‐methylenedioxymethamphetamine (MDMA), (meth)amphetamine, gamma‐hydroxybutyric acid, (GHB), ketamine, lysergic acid diethylamide (LSD) and heroin. Poisson analyses were performed with the number of samples or exposures containing NPS as outcome variable, the year as predictor and the annual total number of samples or exposure as an offset variable. Data were checked for over‐dispersion and robust estimators of the standard errors were used.
To study specific categories of NPS, samples or exposures involving more than one NPS were included in the analysis of frequencies of occurrence for each specific NPS. NPS were categorized into the following classes: phenethylamines, cathinones, tryptamines, piperazines, arylcyclohexylamines, synthetic cannabinoid receptor agonists (SCRAs), designer benzodiazepines and new synthetic opioids (Supporting information, Tables S1–S3). Additional Poisson analyses were performed with the number of specific NPS as outcome variable, the year as predictor and the annual total of NPS as an offset variable.
Results are presented as numbers, proportions and incidence rate ratios (IRR) with 95% confidence intervals (CI) where appropriate. Data were collected in MS Excel 2010 and SPSS version 25.0 for Windows. Statistical analyses were executed using SPSS, R (version 3.6.0) and R studio (version 1.2).
The analysis was not pre‐registered and the results should be considered exploratory.
Results
NPS occurrence on the Dutch drug market
Between 2013 and 2017, NPS were involved in 1892 forensic samples, 6316 consumer samples and 481 poisons center exposures. Recreational drugs were detected in 66 365 forensic samples, 55 839 consumer samples and 5421 exposures reported to the DPIC (Table 1). Between 2013 and 2017, the NPS incidence rate varied between 2 and 15% (Fig. 1). The NPS incidence rate of consumer drug samples submitted to the DIMS significantly increased between 2013 and 2016, as did the incidence rate of NPS exposures reported to the DPIC between 2013 and 2017 (Table 1).
Table 1.
Presence of “traditional” recreational drugs and NPS in different data sources
| Forensic samples (NFI) | Consumer samples (DIMS) | Poison Center exposures (DPIC) | ||
|---|---|---|---|---|
| Number of samples and exposures | ||||
| 2013 | NPS | 362 | 754 | 39 |
| Drugs | 14689 | 10125 | 955 | |
| IR | 2,5% | 7,4% | 4,1% | |
| 2014 | NPS | 574 | 1161 | 88 |
| Drugs | 14699 | 10623 | 1035 | |
| IR | 3,9% | 10,9% | 8,5% | |
| 2015 | NPS | 288 | 1357 | 97 |
| Drugs | 13929 | 11914 | 1078 | |
| IR | 2,1% | 11,4% | 9,0% | |
| 2016 | NPS | 338 | 1694 | 126 |
| Drugs | 12312 | 11215 | 1120 | |
| IR | 2,7% | 15,1% | 11,3% | |
| 2017 | NPS | 330 | 1350 | 131 |
| Drugs | 10736 | 11962 | 1233 | |
| IR | 3,1% | 11,3% | 10,6% | |
| Total | NPS | 1892 * | 6316 | 481 |
| Drugs | 66365 | 55839 | 5421 | |
| IR | 2,9% | 11,3% | 8,9% | |
| Poisson regression models | ||||
| Period of time | 2013‐2017 | 2013‐2016 | 2013‐2017 | |
| IRR estimate | 0.99 | 1.23 | 1.19 | |
| 95% CI | 0.90‐1.10 | 1.18‐1.29 | 1.06‐1.35 | |
NFI: Netherlands Forensic Institute; DIMS: Drugs Information and Monitoring System; DPIC: Dutch Poisons Information Center; IR: incidence rate, IRR: Incidence Rate Ratio; CI: confidence interval.
111 samples contained more than one NPS. For further analyses to determine the frequencies of NPS classes and specific NPS, these samples were counted 2 to 4 times depending on the number of NPS per sample, resulting in 2,012 forensic samples.
Figure 1.
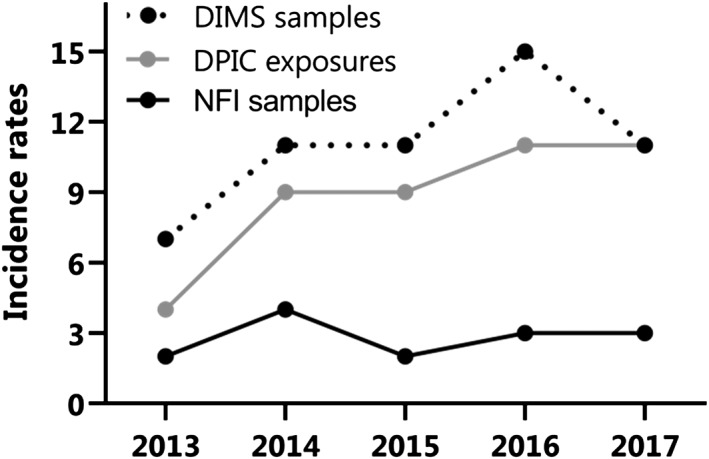
Incidence rates of new psychoactive substances (NPS) in the Netherlands between 2013 and 2017. Incidence rates of NPS relative to all recreational drugs in forensic drug samples submitted to the Netherlands Forensic Institute (NFI, black solid line), consumer drug samples submitted to the Drugs Information and Monitoring System (DIMS, black dotted line) and exposures reported to the Dutch Poisons Information Center (DPIC grey solid line)
Seized drugs: NFI database
Of the 1892 samples containing NPS, 111 samples (6%) contained more than one NPS. To determine frequencies of NPS classes and specific NPS, these samples were counted for each NPS present, resulting in 2012 forensic samples. Most samples contained phenethylamines (n = 1158, 58%) and cathinones (n = 415, 21%) (Table 2). Over time, the distribution between classes fluctuated (Fig. 2a–c), although phenethylamines were always the largest class. New synthetic opioids were detected in only two samples from 2013 to 2016, while in 2017 they were detected in 13 samples.
Table 2.
Annual number of samples and inquiries involving ‘traditional' recreational drugs and new psychoactive substances (NPS).
| 2013 | 2014 | 2015 | 2016 | 2017 | Total | |
|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Forensic samples (NFI) | ||||||
| NPS total | 385 (100) | 614 (100) | 308 (100) | 351 (100) | 354 (100) | 2012 (100) |
| PE (% of NPS) | 198 (51) | 328 (53) | 172 (56) | 253 (72) | 207 (58) | 1158 (58) |
| 4‐FA (% of PE) | 35 (18) | 71 (22) | 44 (26) | 89 (35) | 67 (32) | 306 (26) |
| 2C‐X (% of PE) | 57 (29) | 109 (33) | 67 (39) | 111 (44) | 95 (46) | 439 (38) |
| 4‐MA (% of PE) | 58 (29) | 63 (19) | 8 (5) | 2 (1) | 0 (0) | 131 (11) |
| Cathinones (% of NPS) | 61 (16) | 169 (28) | 57 (19) | 57 (16) | 71 (20) | 415 (21) |
| Consumer samples (DIMS) | ||||||
| NPS total | 754 (100) | 1161 (100) | 1357 (100) | 1694 (100) | 1350 (100) | 6316 (100) |
| PE (% of NPS) | 524 (69) | 920 (79) | 1111 (82) | 1458 (86) | 1064 (79) | 5077 (80) |
| 4‐FA (% of PE) | 105 (20) | 277 (30) | 613 (55) | 953 (65) | 547 (51) | 2495 (49) |
| 2C‐X (% of PE) | 88 (17) | 194 (21) | 283 (25) | 297 (20) | 382 (36) | 1244 (25) |
| 4‐MA (% of PE) | 87 (17) | 48 (5) | 13 (1) | 2 (0) | 5 (0) | 155 (3) |
| Cathinones (% of NPS) | 85 (11) | 123 (11) | 130 (10) | 169 (10) | 215 (16) | 722 (11) |
| Poison Center exposures (DPIC) | ||||||
| NPS total | 39 (100) | 88 (100) | 97 (100) | 126 (100) | 131 (100) | 481 (100) |
| PE (% of NPS) | 22 (56) | 61 (69) | 66 (68) | 80 (63) | 73 (56) | 302 (63) |
| 4‐FA (% of PE) | 11 (50) | 27 (44) | 44 (67) | 50 (63) | 39 (53) | 171 (57) |
| 2C‐X (% of PE) | 4 (18) | 25 (41) | 16 (24) | 22 (28) | 24 (33) | 91 (30) |
| 4‐MA (% of PE) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Cathinones (% of NPS) | 8 (21) | 10 (11) | 11 (11) | 23 (18) | 23 (18) | 75 (16) |
NFI = Netherlands Forensic Institute; DIMS = Drugs Information and Monitoring System; DPIC = Dutch Poisons Information Center.
PE = phenethylamines; 4‐FA = 4‐fluoroamphetamine; 2C‐x = 2,5‐dimethoxy‐4‐x‐phenethylamine derivatives; 4‐MA = 4‐methylamphetamine.
Figure 2.
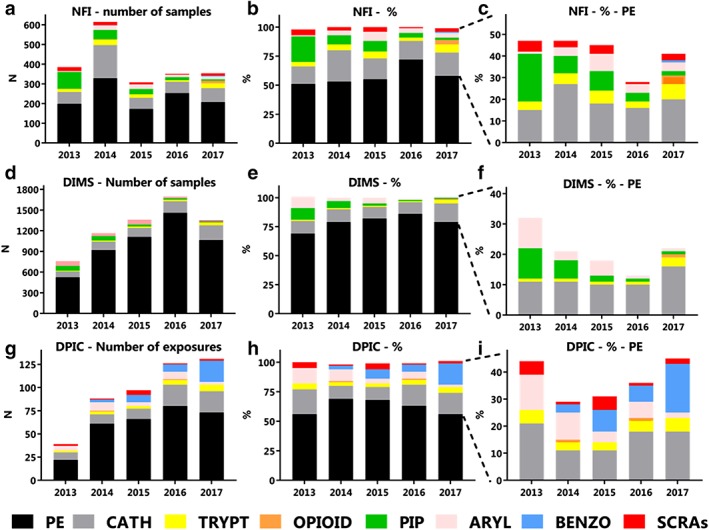
Detection of different new psychoactive substances (NPS) classes in the Netherlands between 2013 and 2017 in frequency and proportion. Drug samples offered to the Netherlands Forensic Institute (NFI, a–c), consumer drug samples offered to the Drug Information and Monitoring System (DIMS, d–f) and exposures in which the Dutch Poisons Information Center was consulted (DPIC, g–i) involving NPS are presented. Absolute numbers (a,d,g), the proportion (b,e,h) and the proportion without the phenethylamines (c,f,i) are presented.PE = phenethylamines; CATH = cathinones; TRYPT = tryptamines; OPIOID = new synthetic opioids; PIP = piperazines; ARYL = arylcyclohexylamines; BENZO = designer benzodiazepines; SCRAs = synthetic cannabinoid receptor agonists. For specific NPS within NPS classes present in NFI, DIMS or DPIC data, see Supporting information, Tables S1, S2 or S3, respectively. [Colour figure can be viewed at http://wileyonlinelibrary.com]
From 2013 to 2017, 35 different phenethylamines and 47 different cathinones were detected (Supporting information, Table S1). For specific NPS that were detected in at least two of three data sources, see Table 3. 2C‐x derivatives were the most frequently detected phenethylamines (29–46% of all phenethylamines). Their proportion within all phenethylamines increased between 2013 and 2017 (year effect estimate = 1.13, 95% CI = 1.10–1.15, Fig. 3a, Table 2). 4‐Methylamphetamine was as frequently observed as 2C‐x derivatives in 2013, but decreased in successive years and was absent in 2017 (year effect estimate = 0.39, 95% CI = 0.29–0.51). 4‐Fluoroamphetamine was the second most frequently detected phenethylamine (18–35% of all phenethylamines), and its proportion increased between 2013 and 2017 (year effect estimate = 1.18, 95% CI = 1.12–1.25). 4‐MEC was the most detected cathinone in 2013 and 2014, while 3‐MMC was the most detected cathinone from 2014 to 2017 (Fig. 3d).
Table 3.
Specific new psychoactive substances (NPS) by drug class detected in two of three data sources: forensic drug samples, consumer drug samples or poisons center exposures.
| 2013 | 2014 | 2015 | 2016 | 2017 | |
|---|---|---|---|---|---|
| Phenethylamines | 2C‐B, 4‐FA, 4‐MA, 5/6‐APB, 25B‐NBOMe, 25I‐NBOMe, DOC, PMA, PMMA | 2,5‐DMA, 2C‐B, 2C‐D, 2C‐E, 2C‐I, 2C‐P, 2‐FMA, 3,4‐DMMA, 4‐FA, 4‐MA, 5/6‐APB, 25B‐NBOMe, 25C‐NBOMe, 25I‐NBOMe, DOC, MDAI, PMMA | 2C‐B, 2C‐E, 2C‐I, 3,4‐DMMA, 4‐FA, 4‐MA, 5/6‐APB, 5‐MAPB, 25I‐NBOMe, DOC, PEA, PMMA | 2C‐B, 2C‐E, 2C‐I, 4‐FA, 4‐FMA, 5/6‐APB,25B‐NBOMe, 25C‐NBOMe, 25I‐NBOMe, DOC, DOB, PEA, PMA, PMMA | 2C‐B, 2C‐C, 2C‐B‐fly, 2C‐E, 2‐FMA, 4‐FA, 4‐FMA, 5/6‐APB, 25B‐NBOMe, 25C‐NBOMe, 25I‐NBOMe, 25H‐NBOMe, DOC, PEA, PMA, PMMA |
| Synthetic cathinones | 3‐MMC, 4‐MEC, 4‐MMC, methylone, MDPV, pentedrone | 3‐MMC, 3,4‐DMMC, 4‐MEC, 4‐MMC, butylone, methylone, MDPV, pentedrone | 3‐MMC, 3,4‐DMMC, 4‐MEC, 4‐MMC, α‐PVP, ethylone, methylone, MDPV | 3‐FMC, 3‐MMC, 3,4‐DMMC, 4‐MEC, 4‐MMC, α‐PVP, MDPV, methylone, pentedrone | 3‐FMC, 3‐MMC, 3,4‐DMMC, 4‐CEC, 4‐chloro‐α‐PVP, 4‐CMC, 4‐fluoro‐pentedrone, 4‐MEAP, 4‐MEC, α‐PVP, ethylpentylone, iso‐mephedrone, iso‐pentedrone, mephedrone, methylone, MPHP, N‐ethylhexedrone, pentedrone, pentylone, pyrovalerone |
| Synthetic cannabinoids | XLR‐11 | AM‐2201 | 5F‐AKB48 | ‐ | AMB‐FUBINACA, AM‐2201 |
| Tryptamines | DMT | 5‐MeO‐DMT, 5‐MeO‐MiPT, AMT, DMT | 5‐MeO‐DALT, 5‐MeO‐MiPT, DMT | 4‐AcO‐DMT, 4‐HO‐MET, 5‐MeO‐MiPT, DMT | 5‐MeO‐MiPT, 5‐MeO‐DMT, DMT |
| Arylcyclohexylamines | MXE | MXE | Deschloroketamine, MXE | MXE | 2‐FDCK, 3‐methoxy‐PCP, deschloroketamine, MXE |
| Piperazines | mCPP, TFMPP | mCPP | mCPP | mCPP | mCPP |
| New synthetic opioids | ‐ | ‐ | ‐ | U‐47700 | Furanylfentanyl, U‐47700 |
| Designer benzodiazepines | ‐ | Diclazepam | Etizolam | Etizolam | Clonazolam, diclazepam, etizolam, flunitrazolam |
NPS that were present in at least two of three data sources are listed. Data sources were: samples from the Netherlands Forensic Institute (NFI), consumer samples offered to the Drugs Information and Monitoring System (DIMS), and exposures reported to the Dutch Poisons Information Center (DPIC). For NPS present in NFI, DIMS or DPIC data, see Supporting information, Tables S1, S2 or S3, respectively.
Figure 3.
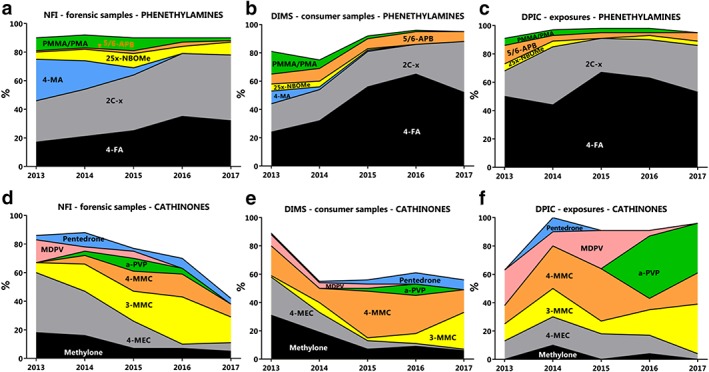
The proportion of specific new psychoactive substances (NPS) within classes from 2013 to 2017The proportion of specific phenethylamines (a–c) and cathinones (d–f) present in drug samples offered to the Netherlands Forensic Institute (NFI, a,d), consumer drug samples offered to the Drug Information and Monitoring System (DIMS, b,e) and exposures in which the Dutch Poisons Information Center was consulted (DPIC, c,f), are presented. For specific NPS of the 2C‐x and 25x‐NBOMe group present in NFI, DIMS or DPIC data, see Supporting information, Tables S1, S2 or S3, respectively. [Colour figure can be viewed at http://wileyonlinelibrary.com]
NPS that were detected in samples with the highest number of tablets varied over the years: m‐chlorophenylpiperazine (mCPP) in 2013 and 2015 (61 266 and 7377 tablets, respectively), 2C‐B in 2014 and 2017 (69 972 and 12 751 tablets, respectively) and PMMA in 2016 (43 824 tablets).
Drug‐checking service: DIMS
Most of the 6316 consumer samples involving NPS contained phenethylamines (n = 5077, 80%) and cathinones (n = 722, 11%) (Table 2). Fluctuations between classes were observed over time (Fig. 2d–f), although phenethylamines have always been the largest class (69–86%). Samples containing NPS of nearly all classes were observed annually, although new synthetic opioids and designer benzodiazepines were detected for the first time in 2015 (one sample each).
From 2013 to 2017, 49 different phenethylamines and 41 different cathinones were detected (Supporting information, Table S2). 4‐Fluoroamphetamine was the most frequently detected phenethylamine throughout this time‐period (20–65% of all phenethylamines), followed by 2C‐x derivatives (17–36%, Fig. 3b, Table 2).
The percentage of samples containing 4‐fluoroamphetamine and 2C‐x derivatives significantly increased between 2013 and 2016 (year effect estimate = 1.45, 95% CI = 1.35, 1.56; and year effect estimate = 1.16, 95% CI = 1.05, 1.28, respectively).
Of the cathinones, methylone and 4‐MEC were the most detected in 2013 and 2014 (methylone = 31% (n = 26) and 19% (n = 23), 4‐MEC = 27% (n = 23) and 14% (n = 17) of all cathinones), while 4‐MMC was the most detected in 2015 and 2016 (34% (n = 44) and 27% (n = 46). In 2017, 3‐MMC was the most detected cathinone (26%, n = 56) (Fig. 3f).
Poisons center data: DPIC database
From 2013 to 2017, the DPIC received inquiries on 447 patients with a NPS exposure, involving 481 NPS exposures (see Supporting information, Table S4 for patient and exposure characteristics). Most exposures involved phenethylamines (n = 302, 63%) and cathinones (n = 75, 16%) (Fig. 2g–i, Table 2). The proportion of phenethylamine exposures remained relatively stable through the years (56–69%). The second largest group varied during this period, most often being the cathinones (11–21%), although in 2017 the proportion of cathinone and designer benzodiazepine exposures was equal (18%, n = 23). Exposures to NPS of most classes were reported annually, with the exception of piperazine exposures that were not reported at all from 2013 to 2017. In addition, new synthetic opioids were only reported twice (once in 2014 and once in 2016) and designer benzodiazepines were only reported from 2014 onwards.
Between 2013 and 2017, exposures to nine different phenethylamines and 11 different cathinones were reported (Supporting information, Table S3). The most frequently occurring phenethylamine was 4‐fluoroamphetamine (Fig. 3c, 44–67% of all phenethylamines), followed by 2C‐x derivatives (14–33%).
Most cathinones were reported each year, although in different proportions (Fig. 3f). However, α‐PVP was only reported in 2016 (n = 10, 43% of all cathinones) and 2017 (n = 8, 35%) and MDPV was mainly reported from 2013 to 2015. Only one 3‐MMC exposure was reported in 2015, while eight were reported in 2017 (9 and 35% of all cathinone exposures).
Clinical features
Nearly all patients experienced adverse effects at the time of the inquiry (96%). Of the 224 patients without concomitant exposures, 95% experienced symptoms, mainly involving neurological and cardiovascular effects. During inquiry, 43% of the patients experienced a moderate to severe poisoning. No large differences were observed between different classes of NPS (Table 4).
Table 4.
NPS exposures reported to the Dutch Poisons Information Center: clinical features during inquiry.
| All NPS exposures | Phenethyl‐amines | Cathinones | Arylcyclo‐hexylamines | Tryptamines | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n = 224 | n = 147 | n = 29 | n = 17 | n = 14 | ||||||
| n | % | n | % | n | % | n | % | n | % | |
| Symptoms | 212 | 95 | 139 | 95 | 28 | 97 | 17 | 100 | 12 | 86 |
| PSS | ||||||||||
| None | 3 | 1 | 1 | 1 | 1 | 3 | 0 | 0 | 0 | 0 |
| Mild | 117 | 52 | 79 | 54 | 12 | 41 | 8 | 47 | 6 | 43 |
| Moderate | 80 | 36 | 51 | 35 | 14 | 48 | 6 | 35 | 6 | 43 |
| Severe | 15 | 7 | 9 | 6 | 2 | 7 | 3 | 18 | 0 | 0 |
| Unknown | 9 | 4 | 7 | 5 | 0 | 0 | 0 | 0 | 2 | 14 |
| Cardiovascular | ||||||||||
| Asystole | 1 | 0.4 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tachycardia | 51 | 23 | 36 | 24 | 7 | 24 | 3 | 18 | 3 | 21 |
| Hypertension | 23 | 10 | 13 | 9 | 6 | 21 | 2 | 12 | 2 | 14 |
| Chest pain | 14 | 6 | 7 | 5 | 4 | 14 | 2 | 12 | 0 | 0 |
| Neurological, other | ||||||||||
| Cerebral hemorrhage | 1 | 0.4 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Coma | 5 | 2 | 1 | 1 | 1 | 3 | 2 | 12 | 0 | 0 |
| Convulsion | 6 | 3 | 4 | 3 | 1 | 3 | 0 | 0 | 0 | 0 |
| Headache | 26 | 12 | 23 | 16 | 2 | 7 | 1 | 6 | 0 | 0 |
| Agitation | 45 | 20 | 24 | 16 | 8 | 28 | 4 | 24 | 5 | 36 |
| Aggression | 12 | 5 | 7 | 5 | 3 | 10 | 0 | 0 | 2 | 14 |
| Anxiety | 38 | 17 | 27 | 18 | 3 | 10 | 3 | 18 | 5 | 36 |
| Hallucination | 33 | 15 | 24 | 16 | 3 | 10 | 3 | 18 | 3 | 21 |
| Psychosis | 12 | 5 | 3 | 2 | 4 | 14 | 3 | 18 | 1 | 7 |
| Mydriasis | 26 | 12 | 20 | 14 | 3 | 10 | 0 | 0 | 2 | 14 |
| Temperature ≥ 37.8°C | 13 | 6 | 10 | 7 | 2 | 7 | 0 | 0 | 1 | 7 |
Only patients without concomitant exposures were included for the analysis of symptoms.
Tachycardia = heart rate > 100 beats per minute (b.p.m.); tachycardia = heart rate < 60 b.p.m.; hypertension = systolic blood pressure > 140 mmHg. PSS = Poisoning Severity Score 16].
Discussion
Our analysis of forensic drug samples, consumer drug samples and exposures reported to the poisons center clearly shows the constant presence of NPS on the Dutch drug market and its use by the Dutch population. Our data also provided the opportunity to determine the relative share of NPS in comparison to more ‘traditional' recreational drugs. NPS incidence rates show a fair share of NPS on the Dutch drug market (2–15%, Fig. 1, Table 1). Increases in rates were observed in consumer samples offered to the DIMS between 2013 and 2016 and in exposures reported to the DPIC between 2013 and 2017 (Table 1).
Phenethylamines were the largest class in all three data sources. Forty‐two per cent of the forensic samples concerned NPS of classes which were different from phenethylamines, compared to approximately 44% of poisons center exposures and 21% of consumer samples in 2017 (Fig. 2c,f,i). In contrast to the Dutch market, NPS of the phenethylamine class are only a small class Europe‐wide, representing 3% of the seizures and approximately 10% of the NPS notified for the first time to the EWS. The main classes Europe‐wide and globally are cathinones and SCRAs 3, 17. Cathinones are the second largest class in our Dutch data, while SCRAs are hardly encountered, especially not by drug‐checking services (Fig. 2f). Although the proportion of SCRAs in Dutch consumer samples is below 1% (n = 16 between 2013 and 2017), their presence in forensic samples (Fig. 2c) and in NPS exposures reported to the Dutch poisons center (Fig. 2i) is slightly higher, between 1–5% (n = 63 and n = 11 between 2013–2017). This indicates that SCRAs appear to be used on a small scale in the Netherlands. The difference between the Dutch and the global NPS market is due most probably to the liberal policy of tolerance regarding cannabis that applies in the Netherlands and hence the easy availability of cannabis in the so‐called coffee shops.
In addition, Europe‐wide data recently showed a strong increase in the number of new synthetic opioids reported for the first time, as well as their number of seizures. Although their share on the European drug market is still small (2% of all seizures), new synthetic opioids pose a severe risk of fatal poisoning as they have a high potency to activate opioid receptors. Only a small amount is needed to synthetize thousands of doses, and therefore new synthetic opioids are easy to conceal and smuggle 18. In our data, new synthetic opioids are hardly observed. However, in 2017, 12 forensic samples contained new synthetic opioids, in which 11 different opioids were detected (Supporting information, Table S1), while none were detected in 2015 and 2016. In drug consumer samples opioids were also detected mainly in 2017 (seven of nine samples).
NPS from most classes were observed in all three Dutch data sources. However, piperazines were observed only in forensic samples and consumer samples, and not in exposures reported to the poisons center. This could indicate that exposure to piperazines is less likely to result in serious adverse health effects for which users seek medical advice or that users choose not to take the drug after piperazines were confirmed in a sample.
With respect to specific NPS detected in the Netherlands, 4‐fluoroamphetamine (4‐FA) was the most observed NPS in all three data sources investigated, although in 2017 the 4‐FA incidence rates relative to all phenethylamines appeared to decrease (Fig. 3a–c, Table 2). Also, preliminary unpublished data shows a decline in consumer samples and poisons center exposures. In addition, the number of 4‐FA incidents decreased at medical and first‐aid services at dance festivals, as well as the number of 4‐FA posts on internet forums 19, 20. This decrease is due possibly to several reports of severe toxicity after 4‐FA use 14, 21, which made Dutch headlines in early September 2016. This led to a national risk assessment and subsequently listing of 4‐FA as a Schedule 1 drug on 25 May 2017. The decline in the presence of 4‐FA appears to be partly compensated by an increase in 2C‐x derivatives and cathinones (Fig. 3a–c, Table 2). The frequencies of specific cathinones that are observed also vary strongly. Methylone and 4‐MEC were mainly observed in 2013–14, but this shifted to 4‐MMC in 2014–16 and to 3‐MMC in 2017 (Fig. 3d–f). Possibly, these trends are influenced by changing legislation. Methylone and 4‐MEC have been Schedule 1 drugs in the Netherlands since 2016 and 2018, respectively, while 3‐MMC is not scheduled. However, 4‐MMC has already been scheduled since 2012. The search for new legal highs that are mainly traded on‐line might be more sensitive to legal prohibition, even though implementing regulatory measures for the use of conventional drugs (non‐NPS) have shown little association with drug use prevalence 22.
Limitations
Not all forensic samples will be offered to the NFI for analysis due to the legal status of most NPS in the Netherlands. Furthermore, forensic data cannot discriminate between samples intended for the Dutch drug market or for export. The main limitation of drug‐checking services is selection bias, as only offered samples can be monitored. Additionally, mainly ‘party drugs' are offered, which may result in under‐reporting of new synthetic opioids and designer benzodiazepines. Although it is highly likely that samples submitted to DIMS were meant for personal use only, there is no absolute guarantee. The main limitation of the poisons center data is also selection bias. The DPIC is only contacted by physicians and mainly on NPS users who already experience adverse effects, and mainly when physicians are unfamiliar with the symptoms or the treatment of NPS exposure. Only information obtained during the inquiry on poisoning was included, while additional clinical information could have been obtained during follow‐up. Furthermore, reported exposures were not analytically confirmed.
External factors, such as changing legislation and media reports on health consequences, may have influenced the occurrence of (specific) NPS between 2013 and 2017. Finally, customs data 23, data from waste water analysis 24, 25, emergency department visits (such as the Euro‐Den database 26) and data from fatal poisonings 27 could supplement the presented data. However, databases for such sources are not always available or do not represent the whole country.
Conclusion
Combining data on NPS from forensic samples, consumer samples offered to drug‐checking services and exposures reported to the poisons center within the same country provides an exceptional insight from three different perspectives into the national drug market and local drug use. Registration and analysis over time allows for monitoring trends and offers opportunities to protect public health.
Declaration of interests
All authors have completed the ICMJE uniform disclosure form at http://www.icmje.org/coi_disclosure.pdf and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Supporting information
Table S1 Specific NPS by drug class detected in forensic samples of the Netherlands Forensic Institute (NFI).
Table S2 Specific NPS by drug class detected in Dutch user samples offered to the Drugs Information and Monitoring System (DIMS).
Table S3 Specific NPS by drug class reported to the Dutch Poisons Information Center.
Table S4 Patient and exposure characteristics of NPS exposures reported to the Dutch Poisons Information Center.
Hondebrink, L. , Nugteren‐van Lonkhuyzen, J. J. , Hunault, C. C. , van den Berg, J. , van der Gouwe, D. , and van Riel, A. J. H. P. (2020) New psychoactive substances (NPS) in the Netherlands: occurrence in forensic drug samples, consumer drug samples and poisons center exposures between 2013 and 2017. Addiction, 115: 716–725. 10.1111/add.14868.
References
- 1. European Monitoring Centre for Drugs and Drug Addiction . New psychoactive substances in Europe. An update from the EU Early Warning System (March 2015). Luxembourg: Publications Office of the European Union; 2015.
- 2. European Monitoring Centre for Drugs and Drug Addiction . European Drug Report 2017: Trends and Developments. Luxembourg: Publications Office of the European Union; 2017. [Google Scholar]
- 3. European Monitoring Centre for Drugs and Drug Addiction . European Drug Report 2018: Trends and Developments. Luxembourg: Publications Office of the European Union; 2018. [Google Scholar]
- 4. European Monitoring Centre for Drugs and Drug Addiction . European Drug Report 2019: Trends and Developments. Luxembourg: Publications Office of the European Union; 2019. [Google Scholar]
- 5. European Monitoring Centre for Drugs and Drug Addiction . European Drug Report 2016: Trends and Developments. Luxembourg: Publications Office of the European Union; 2016. [Google Scholar]
- 6. European Monitoring Centre for Drugs and Drug Addiction . High‐risk drug use and new psychoactive substances, EMCDDA Rapid Communication. Luxembourg: Publications Office of the European Union; 2017. [Google Scholar]
- 7. European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). Europol 2013 Annual Report on the implementation of Council Decision 2005/387/JHA. Luxembourg: Publications Office of the European Union. 10.2810/51986. [DOI]
- 8. European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). Europol 2014 Annual Report on the implementation of Council Decision 2005/387/JHA. Luxembourg: Publications Office of the European Union. doi: 10.2810/112317. [DOI]
- 9. European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). Europol 2015 Annual Report on the implementation of Council Decision 2005/387/JHA. Luxembourg: Publications Office of the European Union. doi: 10.2810/932574. [DOI]
- 10. European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). Personal communication. 26‐11‐2018 email Andrew Cunningham, Head of sector Markets, Crime and supply reduction of the EMCDDA. Risk communications in 2016 and 2017.
- 11. Brunt T. M., Nagy C., Bucheli A., Martins D., Ugarte M., Beduwe C., et al Drug testing in Europe: monitoring results of the Trans European Drug Information (TEDI) project. Drug Test Anal 2017; 9: 188–198. [DOI] [PubMed] [Google Scholar]
- 12. Brunt T. M., Niesink R. J. The drug information and monitoring system (DIMS) in the Netherlands: implementation, results, and international comparison. Drug Test Anal 2011; 3: 621–634. [DOI] [PubMed] [Google Scholar]
- 13. Bardwell G., Kerr T. Drug checking: a potential solution to the opioid overdose epidemic? Subst Abuse Treat Prev Policy 2018; 13: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hondebrink L., Nugteren‐van Lonkhuyzen J. J., Rietjens S. J., Brunt T. M., Venhuis B., Soerdjbalie‐Maikoe V., et al Fatalities, cerebral hemorrhage, and severe cardiovascular toxicity after exposure to the new psychoactive substance 4‐Fluoroamphetamine: a prospective cohort study. Ann Emerg Med 2018; 71: 294–305. [DOI] [PubMed] [Google Scholar]
- 15. Palamar J. J., Su M. K., Hoffman R. S. Characteristics of novel psychoactive substance exposures reported to new York City poison center, 2011–2014. Am J Drug Alcohol Abuse 2016; 42: 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Persson H. E., Sjöberg G. K., Haines J. A., et al Poisoning severity score. Grading of acute poisoning. J Toxicol Clin Toxicol 1998; 36: 205–213. [DOI] [PubMed] [Google Scholar]
- 17. United Nations. World Drug Report 2018. Sales no. E.18.XI.9. New York: United Nations Publications; 2018.
- 18. European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) . Fentanils and synthetic cannabinoids: driving greater complexity into the drug situation. An update from the EU Early Warning System (June 2018). Luxembourg: Publications Office of the European Union; 2018. [Google Scholar]
- 19. Lameijer M., Wijers L., Croes E., de Ruiter N., Valkenberg H. Monitor Drugsincidenten: Factsheet 2017; 2018. Available at: https://assets.trimbos.nl/docs/92d66803-a73a-4fbe-9834-9a6e65cefb25.pdf (accessed 15 January 2019).
- 20. Blankers M., van der Gouwe D., van Laar M. 4‐Fluoramphetamine in the Netherlands: text‐mining and sentiment analysis of internet forums. Int J Drug Policy 2018; 64: 34–39. [DOI] [PubMed] [Google Scholar]
- 21. Wijers C. H. W., van Litsenburg R. T. H., Hondebrink L., Niesink R. J. M., Croes E. A. Acute toxic effects related to 4‐fluoroamphetamine. Lancet 2017; 389: 600. [DOI] [PubMed] [Google Scholar]
- 22. Hughes B., Matias J., Griffiths P. Inconsistencies in the assumptions linking punitive sanctions and use of cannabis and new psychoactive substances in Europe. Addiction 2018; 113: 2155–2157. [DOI] [PubMed] [Google Scholar]
- 23. Johnson C. S., Copp B. R., Lewis A. New psychoactive substances detected at the New Zealand border, 2014–2018. Drug Test Anal 2019; 11: 341–346. [DOI] [PubMed] [Google Scholar]
- 24. Archer J. R., Dargan P. I., Lee H. M., Hudson S., Wood D. M. Trend analysis of anonymised pooled urine from portable street urinals in Central London identifies variation in the use of novel psychoactive substances. Clin Toxicol 2014; 52: 160–165. [DOI] [PubMed] [Google Scholar]
- 25. Bade R., Stockham P., Painter B., Celma A., Bijlsma L., Hernandez F., et al Investigating the appearance of new psychoactive substances in South Australia using wastewater and forensic data. Drug Test Anal 2019; 11: 250–256. [DOI] [PubMed] [Google Scholar]
- 26. Dines A. M., Wood D. M., Yates C., Heyerdahl F., Hovda K. E., Giraudon I., et al Acute recreational drug and new psychoactive substance toxicity in Europe: 12 months data collection from the European drug emergencies network (euro‐DEN). Clin Toxicol 2015; 53: 893–900. [DOI] [PubMed] [Google Scholar]
- 27. Kronstrand R., Guerrieri D., Vikingsson S., Wohlfarth A., Green H. Fatal poisonings associated with new psychoactive substances. Handb Exp Pharmacol 2018; 252: 495–541. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Specific NPS by drug class detected in forensic samples of the Netherlands Forensic Institute (NFI).
Table S2 Specific NPS by drug class detected in Dutch user samples offered to the Drugs Information and Monitoring System (DIMS).
Table S3 Specific NPS by drug class reported to the Dutch Poisons Information Center.
Table S4 Patient and exposure characteristics of NPS exposures reported to the Dutch Poisons Information Center.


